Reproductive performance in goats and causes of perinatal mortality: a review
S. M. Robertson A B F , T. Atkinson C , M. A. Friend B D , M. B. Allworth A B and G. Refshauge E
A B F , T. Atkinson C , M. A. Friend B D , M. B. Allworth A B and G. Refshauge E
A School of Animal and Veterinary Sciences, Charles Sturt University, Boorooma Street, Wagga Wagga, NSW 2678, Australia.
B Graham Centre for Agricultural Innovation (Charles Sturt University and NSW Department of Primary Industries), Albert Pugsley Place, Wagga Wagga, NSW 2650, Australia.
C New South Wales Department of Primary Industries, Trangie Agricultural Research Centre, Trangie, NSW 2823, Australia.
D Charles Sturt University, Boorooma Street, Wagga Wagga, NSW 2678, Australia.
E New South Wales Department of Primary Industries, Cowra Agricultural Research and Advisory Station, Cowra, NSW 2794, Australia.
F Corresponding author. Email: surobertson@csu.edu.au
Animal Production Science 60(14) 1669-1680 https://doi.org/10.1071/AN20161
Submitted: 16 March 2020 Accepted: 13 August 2020 Published: 3 September 2020
Journal compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Goat meat production is an expanding industry in Australia. However, there is limited data quantifying the levels of reproductive performance, particularly under extensively grazed rangeland conditions, which would inform interventions to improve performance. This review aimed to quantify the levels of reproduction, time and causes of reproductive wastage in goats. It considers the levels of fertility, fecundity, embryonic loss, fetal loss and post-natal survival reported under Australian conditions, and comparisons are made with international reports. Key management factors that may contribute to reproductive performance include breed, seasonality, nutritional conditions, and weather conditions at kidding. While goats are potentially prolific breeders, in Australia, the variation in weaning rate (kids/doe joined) among properties is large (51–165%), although the causes of this variation are not well defined. Generally, conception and kidding rates are high, although fetal loss associated with undernutrition is more likely in goats than sheep. As with sheep, perinatal losses are generally the largest source of wastage, with an average 20% kid mortality, but this level is influenced by litter size and appears to be higher under extensive rangeland systems. The causes of perinatal kid loss under Australian conditions are similar to those in sheep, with starvation–mismothering–exposure and dystocia or stillbirth the key causes. Studies are needed to accurately quantify the level and causes of reproductive wastage in commercial herds, including a range of management situations, to enable effective interventions to be developed.
Additional keywords: conception, litter size, reproduction, survival.
Introduction
In 2018, the goat industry in Australia slaughtered 1.65 million goats, worth AU$182.6 million in carcass exports, with a further 21 600 live goats exported worth AU$7.7 million (MLA 2019). The Australian domestic market consumed 2400 t of goat carcass in 2018, worth an estimated AU$12.3 million. While the industry has historically been based on harvesting of wild populations, there is increasing interest in managing goats. The industry aims to increase managed goat numbers to improve the stability of meat supply, and, therefore, markets and meat prices (Anon. 2015).
Increasing the rate of reproduction is a common strategy used to improve livestock production and, for goat meat enterprises, weaning percentage is a key determinant of profitability (Norton 2004). Goats are produced in many of the same areas of Australia as are sheep, yet, while there is a comprehensive knowledge of the reproductive performance of sheep, there are limited reports for goats under Australian conditions. The lack of industry benchmarks hampers identification of suboptimal performance and the ability to develop and apply management interventions to improve the reproductive rate. Of particular interest is the observation that perinatal mortality was not noted as a priority disease for goats in a recent industry survey (Lane et al. 2015), despite mean kid losses of up to 33% in some regions (Nogueira et al. 2016). Perinatal mortality is the largest source of reproductive wastage in sheep (Kleemann and Walker 2005). Estimates suggest that only 10% of Australian goat producers pregnancy scan (Nogueira et al. 2016), and this, combined with the extensive nature of many operations, limits the ability to quantify the extent of reproductive wastage.
The aim of the present review is to investigate the level of reproductive wastage in the Australian and international meat goat industry. Reproductive wastage includes failure to conceive, embryonic and fetal loss, neonatal and post-weaning mortality, and these components are considered. Comparisons with sheep are made, since they are similar small ruminants often produced in the same regions.
Goat production systems in Australia
Meat goat enterprises dominate the Australian goat industry. Meat goats are widely distributed, with over 70% of the herd in western New South Wales (NSW), and others concentrated in central southern Queensland (Qld) and in South Australia (SA), south of the dog fence, and Western Australia (WA) (Pople and Froese 2012). In 2018, 1.65 million goats were slaughtered in Australia, predominantly for supplying goat meat export markets (MLA 2019). The majority (90%) of meat goats are sourced from the semiarid or arid rangelands, where they are either harvested from free-roaming wild populations or produced in extensively managed production systems (MLA 2006b). The wild goat population in the southern rangelands of Australia was estimated as 4.1 million in 2018 (Waters et al. 2018), but it is difficult to distinguish wild from managed goats in the aerial surveys used (Pople and Froese 2012). The Australian rangeland goat is a composite incorporating dairy, fibre and meat goat breeds, that has evolved over the past 200 years (MLA 2006b). In managed production systems, rangeland does can be joined with selected or introduced bucks, including rangeland, Boer or Kalahari Red breeds (Ferrier and McGregor 2002; Nogueira et al. 2016). Goat meat is also produced in higher-rainfall regions, where the production systems are typically more intensively managed than are the rangeland production systems.
The reproductive management of goats in Australia varies with production system. In rangeland systems, mating is typically not controlled, with bucks remaining with does, and kids may not be weaned by management interventions (Nogueira et al. 2016). However, in some rangeland enterprises, reproduction is managed including the time of mating, buck control and nutrition of does (Plumbe et al. 2019). In higher-rainfall managed systems, the mating period is more likely to be controlled, with peak mating estimated to occur during autumn (Ferrier and McGregor 2002). The range in weaning rates (51–165%) indicates potential for management to influence reproduction in these systems (Ferrier and McGregor 2002). More detailed data are needed to determine the time and cause of reproductive wastage if recommendations are to be provided to improve weaning rates.
Reproductive potential in goats
A herds’ reproductive rate is determined by ovulation rate (number of ova shed/ewe), fertility (ability to conceive), fertilisation rate (ova fertilised by sperm), conception rate (pregnant of those mated or given the opportunity to mate), and losses during embryonic (to Day 40 of gestation), fetal (from Day 41 to term), perinatal (immediately before, during and within 7 days of birth) and later periods. These, and the factors contributing to these, will be considered.
Seasonal breeders
Goats are seasonal breeders and the degree of seasonality varies among breeds and with latitude (Fatet et al. 2011). Ovulations occur between April and August in rangeland does in north-eastern NSW, but the breeding season is extended to between March and September, with either continual or part-time exposure to bucks (Restall 1992). This contrasts with NSW and Qld producer perceptions of the breeding season as being between December and May (Nogueira et al. 2016), which may reflect differences in climate, nutrition or management practices. Good pasture conditions can allow rangeland does to enter oestrus year-round (SCA 1982). Other environmental factors may also be involved in the timing of doe breeding activity, since oestrus seems to be triggered by monsoon rain (Fatet et al. 2011), or rain and subsequent pasture growth (Nogueira et al. 2016). It is possible that an improvement in pasture following rainfall, and hence nutrition of does, may increase doe liveweights and induce oestrus, particularly in pastoral areas where nutrition is more likely to be limiting. Thermal comfort may also improve reproductive activity, since the percentage of Creole does in oestrus was shown to be negatively correlated with minimum temperatures and maximum humidity (Chemineau 1986).
Out-of-season cycling in does can be induced by use of melatonin implants (Zarazaga et al. 2009). Introducing bucks to non-cycling does can also induce ovulation. The first ovulation is often not accompanied by oestrus behaviour (40% of does), so does do not mate at this ovulation, but is followed by either a short (75% of does) or normal-length (18–22 days, average 21 days) oestrous cycle, with does then displaying oestrus (12–36 h duration) and improved fertility with the next ovulation, peaking at 6–8 days or 25–27 days after bucks are introduced (Chemineau 1987). However, sexually active bucks are required to induce oestrus behaviour and ovulation in seasonally anoestrus does (Delgadillo et al. 2002; Martínez-Alfaro et al. 2014). Does do not need to be isolated from bucks for the introduction or presence of bucks to stimulate ovulation (Véliz et al. 2006a). However, does with lower liveweights are less likely to respond (Véliz et al. 2006b). The continual presence of active bucks can prevent does entering seasonal anoestrus, but anoestrus will occur if bucks are not active (Delgadillo et al. 2015). Buck activity is also a limitation for out-of-season breeding.
Bucks as well as does are influenced by photoperiod, becoming sexually inactive when daylength increases (Delgadillo et al. 2015). Delgadillo et al. (2015) showed that an artificial reduction in daylength induces sexual activity in bucks at low latitudes. Melatonin implants in bucks are also effective (Zarazaga et al. 2019), so may have potential as a management tool. Exposure of bucks to oestrus does can lengthen the period when bucks are active, and an increase in nutrition is likely to increase buck activity (Walkden-Brown et al. 1994).
The seasonality of goat breeding can, therefore, be a limitation to maximising the rate of reproduction where desired mating dates differ from the natural breeding season, such as with accelerated mating systems. The management of bucks will contribute to variation in the breeding pattern of does and level of mating activity. Seasonality of breeding patterns can also present challenges in matching nutritional supply from forage to critical reproductive periods.
Age of puberty, ovulation rate and fecundity
The age of puberty in does is dependent upon weight, level of nutrition, season of birth and breed (Greyling 2000). It can occur between 5 and 12 months of age, at a minimum of ~15-kg liveweight in rangeland goats (SCA 1982), or an average of 28 kg in Boer does (Greyling 2000).
Ovulation rate sets the upper limit to fecundity (the number of kids born per pregnant doe). Goats are prolific breeders and high rates of fecundity have been reported in numerous countries and breeds, including in Kalahari Red, 1.6 kids/doe (Oderinwale et al. 2017), and Boer, 2.0 kids/doe (Erasmus 2000). However, fecundity can be low with reports of 95% of does producing single kids (Aldomy et al. 2009). Australian rangeland does can have a high fecundity, such as 2.17 kids/pregnant doe (Allan et al. 1991) or 1.96 kids/doe (Goodwin and Norton 2004). High litter sizes (≥3) are common in Boer goats (Đuričić et al. 2012), and, in Australia, stud Boer herds are reported as having a fecundity of 1.6 kids/doe, with 22% singles, 65% twins and 13% triplet births (Nogueira et al. 2016).
Several factors influence ovulation rate, including age (parity), breed, season, liveweight and nutrition. Does in their first pregnancy are likely to have lower fecundity than those of higher parity (Mellado et al. 2006), although this may be largely associated with lower liveweights of younger does (Ritar et al. 1990). Breed has an influence, with Norton (2004) recording similar fecundity (1.5–1.7 kids/doe) in mixed-age Boer cross, Saanen cross and rangeland does, while Angora and Angora cross does had a lower fecundity and produced only 1.3–1.4 kids/doe. Ovulation rate is 10–40% higher at the peak of the natural breeding season than outside the breeding season (Restall 1992). Ovulation rate can be increased by up to 60% by improved nutrition in the days before ovulation (Mani et al. 1992; De Santiago-Miramontes et al. 2008), or by a higher condition score or liveweight of does when fed to maintenance requirement (condition score 2.7 had 1.9 ovulations; condition score 1.9 had 1.6 ovulations; De Santiago-Miramontes et al. 2009). The percentage of twin ovulations has been reported to increase by ~4.8% per kilogram liveweight for Angora does above 25 kg (Shelton and Groff 1984). Management of nutrition is, therefore, a key means of altering ovulation rate and potential fecundity.
Conception rate
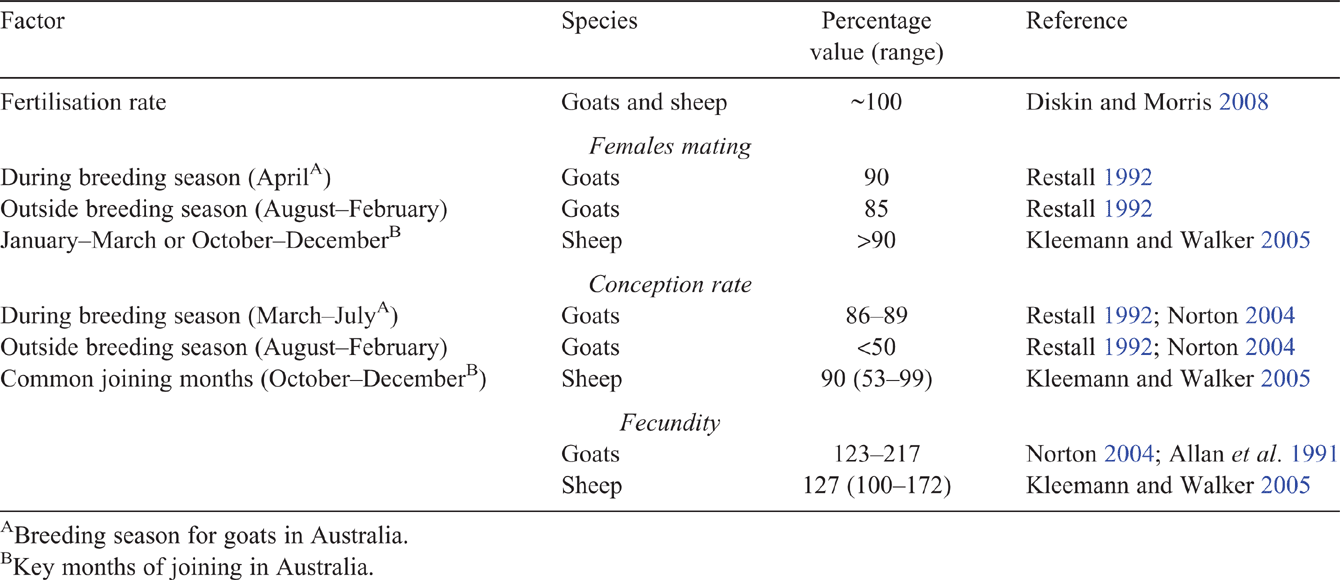
Fertilisation rates in goats are expected to be close to 100% (Diskin and Morris 2008). However, while conception rates in Australian commercial herds can exceed 93%, rates of 60% have also been reported (Nogueira et al. 2016), and suboptimal conception rates have been identified as a cause of poor reproductive rates (Ferrier and McGregor 2002).
Several factors contribute to variation in conception rate, including season, nutrition, age and breed. The fertility of does that do mate out of season (the August to February period in Australia) may be low (<50% kidding; Restall 1992; Norton 2004). Lower levels of nutrition at mating are well documented to reduce conception rates (Mani et al. 1992; Mellado et al. 2004; Urrutia-Morales et al. 2012). A body condition score of 3 (1–5 scale) at mating is recommended for does to optimise reproductive performance (MLA 2006a). In Mexico, body condition at mating as low as 1.5 (1–5 scale) did not reduce the proportion of does conceiving (Mellado et al. 2004), but there appears to be little literature examining the relationship between body condition score in does and the reproductive outcomes. Does of first parity (younger and lighter) are likely to have lower conception rates than those of parity two to five, although conception rates also decline in does above parity of five (Mellado et al. 2006). Kids that were lighter at birth or at 25 days of age were 20% less likely to conceive as adults (Mellado et al. 2006), indicating possible long-term nutritional or genetic influences.
The activity and fertility of bucks are also critical to adequate conception rates in does, and individual or seasonal variation in buck activity and sperm production may contribute. Higher nutritional levels for bucks have been reported to increase buck activity, and conception rates in does by 24% on Day 10 of mating (Walkden-Brown et al. 1993). Breed of buck may be limiting conception rates in Australian rangeland herds, due, in part, to poorer survival of Boer than rangeland bucks (Jones 2012). Bucks should be acclimatised after purchase, preferably at a young age, in addition to providing more care to manage the greater susceptibility of Boer goats to nutritional stress and internal parasites (Plumbe et al. 2019). Boer does in Tennessee (USA) have also been reported to have higher intestinal parasite burdens and higher death rates than Kiko or Spanish does (Browning et al. 2011), so further breed comparisons are advised.
Pseudopregnancy is a condition that is characterised by the persistence of a corpus luteum in the absence of pregnancy, and, so, it will reduce fertility in goats. In international studies, it has been reported to occur at levels of between 3% and 20% (Fatet et al. 2011), although there do not appear to be any reports for this in Australian herds.
Vitamin and mineral imbalances have the potential to reduce conception rates, although their occurrence is likely to be location or situation specific in grazing livestock. Conception rates in ewes may be reduced with zinc or manganese deficiency (Egan 1972). The role of minerals in reproduction is reviewed elsewhere (Hurley and Doane 1989; Haenlein and Anke 2011).
A summary of the expected conception rate of does is given in Table 1, which highlights the risk due to out-of-season joinings, which is not evident in sheep. Factors affecting conception rates that may be investigated experimentally include the following: the role of cryptorchids in stimulating oestrus without conception for does joined outside the breeding season; the effect of seasonality in central northern Australian goat herds; and the potential for melatonin implants to improve out-of-season breeding performance.
|
Embryonic and fetal losses
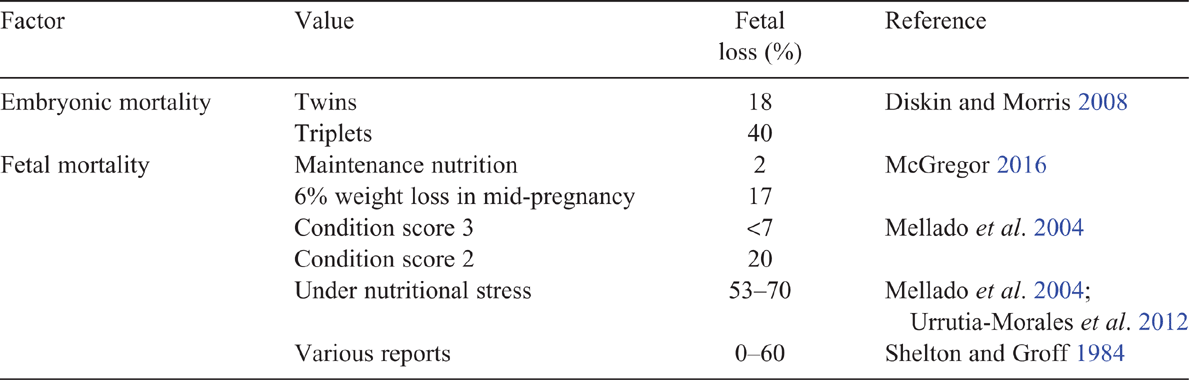
Embryonic mortality is a source of reproductive wastage in goats and the levels (18% for twin ovulations, 40% ≥ triplets) appear to be similar to that in sheep (Diskin and Morris 2008). In contrast, fetal losses appear to be higher than in sheep, and may present as abortion storms at 90–120 days of gestation, with inadequate nutrition being a key factor implicated in these losses (Shelton and Groff 1984). Less commonly, does abort in sequential pregnancies.
A summary of key literature is shown in Table 2, but liveweight loss as little as 25 g/day has been shown to increase fetal loss in does (Urrutia-Morales et al. 2012). In Australian Angora goats, a 2-kg weight loss (6%) during mid-pregnancy has been shown to result in 17% fetal losses, compared with 2% fetal loss in does provided with their maintenance requirement (McGregor 2016). Nutritional stress during late pregnancy has been associated with very high levels of fetal loss, namely, 53% of does (Urrutia-Morales et al. 2012) and 70% of does (Mellado et al. 2004). Stress may be induced by both limited pasture and negative effects of social status on food intake (Pretorius 1970; Urrutia-Morales et al. 2012). In contrast, mid-pregnancy restriction down to 0.3 times the maintenance requirements results in small increases in fetal loss in ewes (Kelly et al. 1989), while undernutrition during late pregnancy is likely to result in pregnancy toxaemia and death of the ewe and fetus (Besier et al. 2010).
|
A low condition score at mating increases the risk of fetal loss, leading to recommendations that does should have a condition score of >2 at mating (Mellado et al. 2004). Estimates have indicated a fetal loss of <7% for does with a condition score of 3, increasing to 20% for does with a condition score of 2 at mating (Mellado et al. 2004), being consistent with higher fetal losses with lower liveweights in other reports (Shelton and Groff 1984). Nutrition early in life may also be critical, as does with growth rates of <136 g/day between birth and 25 days of age are more likely to abort as mature animals (Mellado et al. 2006). The literature, therefore, indicates that management of nutrition is a key factor in preventing fetal losses in does.
An interaction between age (parity) and nutrition influences fetal losses. Does in their first pregnancy have a higher fetal loss than do mature goats (Mellado et al. 2004). Does in parities two to five were half as likely to abort as did does in their first or greater than fifth pregnancy, although the overall level of fetal loss was only 3.5% (Mellado et al. 2006). Similarly, Rattner et al. (1994) reported 11% fetal loss in first-parity does, compared with 5% in adults. However, inadequate growth and nutrition of young does may be the primary cause of their higher rates of fetal loss (Shelton and Groff 1984), rather than age or parity per se.
Disease may be a factor where fetal losses are lower in first-parity than in mature does (Aldomy et al. 2009). Infectious disease including brucellosis and bluetongue are endemic in some regions, but are not present in Australia (Givens and Marley 2008). In housed Norwegian dairy herds with a history of a high fetal loss (>15%), infectious causes were not implicated (Engeland et al. 1997). Rather, factors including previous fetal loss, more than two fetuses, low social status and pregnancy from the third or later oestrus opportunity all indicated at least twice the risk of loss.
Polled goats may be more likely to abort than those with horns (Mellado et al. 2004), although this was not observed in a study using dairy breeds (Engeland et al. 1997). A Mexican study found that does mated during summer were less likely to abort than did those mated in autumn (Mellado et al. 2006), although a higher level of fetal loss has been reported for winter-mated does in Israel (Rattner et al. 1994). These differences may be due to different nutritional or environmental pressures or genetic differences.
The level of fetal loss in Australian herds is poorly documented (Ferrier and McGregor 2002; McGregor 2016). Breed of buck has been reported to influence doe conception rate (Jones 2012), but this was not found under experimental conditions (Norton 2004). However, crossbred Boer × rangeland does had an 18% lower kidding rate than did rangeland does (62.5% vs 80.4%; Norton 2004). Kidding rates of 88–89% have been recorded for April-mated Australian rangeland goats in experimental conditions (Eady and Rose 1988; Restall 1992), and the conception or kidding rates of stud Boer herds in high-rainfall areas of NSW and Qld have been reported as 94% (Nogueira et al. 2016). However, Victorian producers have cited low doe fertility as a key issue (Ferrier and McGregor 2002); so, fetal loss may be a contributing factor.
The literature indicates that high levels of fetal loss due to nutritional stress have the potential to be a significant cause of reproductive wastage in Australian goats. While this does not appear to be a major loss in closely managed herds, further investigation is needed from commercial herds, particularly in the lower-rainfall and pastoral regions, where nutritional stress may be more likely. Walk-over-weighing systems may offer a low-cost method to examine variation in liveweight change (Brown et al. 2015) and relationships with fetal loss.
General patterns of kid survival from birth and post-weaning
Perinatal loss is a major source of reproductive wastage in goats, although there are limited data for Australian commercial herds. Data from 16 050 records in KIDPLAN (the Australian genetic recording scheme) indicate that mortality to weaning averaged 20% (Aldridge et al. 2015). However, 14 stud herds in high-rainfall areas reported a mortality rate (mean ± s.d.) of kids (0–3 months) of 11.6% ± 9.9, which was much lower than the 33.3% ± 23 reported for three commercial herds in pastoral regions (Nogueira et al. 2016). Under experimental grazing conditions, mortality to ≤16 days has varied between 2.9% and 21% (Allan et al. 1991; Goodwin and Norton 2004), and 6.4–21% for goats kidding in pens (Eady and Rose 1988; McGregor 2016).
The key factors associated with perinatal kid mortality are shown in Table 3. Each factor potentially influences maternal or kid behaviour, and physiology, as mechanisms to cause of death. Regional differences may be mediated by intensity of management system, climate and nutrition (Sherman 1987). Sex of kid does not appear to influence survival (Rattner et al. 1994; Snyman 2010). Breed of goat may also contribute, and Angora kids have lower survival rates than do various meat breeds and crosses (Norton 2004). While the mortality of Boer and Boer-cross kids (~10%) was similar to that of rangeland goats under experimental conditions (Norton 2004), the Boer goat has clearly been shown to have a lower kid survival than do some other breeds in South Africa (Lehloenya et al. 2005) and the United States (Browning et al. 2011). The contrast among studies may reflect different breed comparison, different genetics or environmental conditions.

|
The timing of kid loss is not well documented in the available literature, but it is clear that large losses occur perinatally, with most losses occurring within days of birth (Bajhau and Kennedy 1990; Allan et al. 1991). However, losses at later ages may be increased due to disease under housed conditions (Sherman 1987), and post-weaning losses can also be high in some situations (Rattner et al. 1994; Nogueira et al. 2016). In the survey by Nogueira et al. (2016), the reported mortality rates from 4 to 12 months were also usually lower in the high-rainfall regions (6% ± 5.1) than in the rangeland regions (15.7% ± 17). Therefore, it is critical to understand the timing of kid loss for a particular herd if effective intervention strategies are to be devised.
Causes of perinatal mortality
The key causes of perinatal mortality for kids and goats are similar, and the causes of perinatal lamb (Dwyer 2008; Hinch and Brien 2014; Dwyer et al. 2016) and kid mortality (Sherman 1987) in Australia and, elsewhere, have been reviewed previously. Further information may be available in veterinary laboratory or departmental internal reports, but such reports are difficult to access. The lack of information for goats highlights a need to quantify the causes of death and their relative importance under the various management regimes, such that appropriate management interventions may be devised.
The relative importance of various causes of perinatal lamb and kid mortality under Australian extensive grazing conditions are shown in Table 4. Dystocia or stillbirth (including birth injury) and the starvation–mismothering–exposure (SME) complex are the pre-dominant causes of death in Australian lambs and kids, being responsible for ~80% of perinatal deaths. However, any of the causes may increase in importance on particular properties or years (Refshauge et al. 2016).
The SME complex as the major cause of perinatal kid mortality in Australia is consistent with reports for extensively grazed goats in Texas (Shelton and Groff 1984), but differs from some international reports. The large South African study of Snyman (2010) indicated that predation and weak kids were the major causes, but that study included only kids born alive, and cause of death was determined only for 29.8% of those dying. A New Zealand study (Buddle et al. 1988) reported various causes for different ages, but a random sampling method was not used. The relative importance of various death classifications can be expected to vary with intensity of management, climate, and exposure to causative factors, including predators.
Dystocia or stillbirth
Dystocia includes newborns where the mother requires assistance to deliver the term fetus, as well as those born naturally but either born dead or compromised due to a prolonged or difficult delivery. Stillbirth includes newborns dying during parturition that are born dead. However, terminology is often not defined or has contrasting definitions in the literature; so, both terms are used in this review to encapsulate the meaning of the individual reports.
While dystocia is a common cause of lamb death (Table 4), there are few reports on the incidence of dystocia in goats. Dystocia has been identified as an important source of loss in grazing rangeland goats (Bajhau and Kennedy 1990; Allan et al. 1992b), and for pen-fed Angora goats (McGregor 2016). For dairy breed goats, Mellado et al. (2006) recorded 4.2% stillbirths. The rate of kid stillbirths is higher in triplets (15.5%) than in singles or twins (≤7.1%; Rattner et al. 1994), and is more than double the incidence in does of more than five parities compared with younger does (Mellado et al. 2006). In contrast, several studies do not note dystocia or stillbirth as a cause of kid loss (Shelton and Groff 1984; Lehloenya et al. 2005; Aldomy et al. 2009; Nogueira et al. 2016; Kouri et al. 2018). A high level of supervision at kidding may be the reason why dystocia or stillbirth was not mentioned in reports of intensive studies. Alternatively, on large rangeland properties, kidding is not observed; so, the cause of death is not determined.
A high birthweight is a large risk factor for death from dystocia in sheep, where the relationship between birthweight and survival is quadratic (Geenty et al. 2014). For goats, the relationship appears to be curvilinear, without a reduction in survival at higher weights (Rattner et al. 1994; Lehloenya et al. 2005; Snyman 2010), including for Boer goats on the Australian KIDPLAN database (Aldridge et al. 2015). However, further investigation under commercial management conditions is warranted. The propensity for multiple fetuses in goats perhaps reduces the likelihood for excessive birthweights compared with sheep. Given the strong relationship among litter size, birthweight and survival of kids (Shelton and Groff 1984; Allan et al. 1992b; Awemu et al. 1999; Lehloenya et al. 2005), management strategies need to be devised to improve the survival of low-birthweight twins and higher-order births.
Genetic selection is another potential method to reduce deaths from dystocia. The heritability of birthweight in Boer goats is 0.53, and there is a low positive genetic correlation with kid survival (0.19, s.e. not reported; Aldridge et al. 2019). Currently, survival is not reduced at the highest birthweights, but selection for higher birthweights may increase losses from dystocia in the future (Aldridge et al. 2015). This is in contrast to sheep, where the genetic correlation between birthweight and lamb survival to 3 days of life has been estimated at –0.32 ± 0.23 (Brien et al. 2010), and the genetic correlation between birthweight and lamb ease was –0.56 ± 0.27. The genetic correlation among all classes of dystocia and lamb ease (where 1 = no assistance during parturition and 5 = veterinary assistance) was estimated at 0.26 ± 0.12 (Brown et al. 2014). Selection for survival directly is more effective than selecting for birthweight to increase survival, since it considers mortality from all causes. However, the heritability of survival is negligible in both kids (0.08; Aldridge et al. 2019) and lambs (0.01–0.03; Safari et al. 2005; Brien et al. 2010, 2014), meaning that other strategies are required.
Starvation, mismothering and exposure
Starvation, mismothering and exposure (SME) are often inter-related, and, with dystocia, are the other predominant cause of death in Australian lambs (Table 4) and kids (Eady and Rose 1988; Bajhau and Kennedy 1990; Allan et al. 1991, 1992b). SME is also a major cause of perinatal kid mortality internationally (Shelton and Groff 1984; Snyman 2010).
Hyperthermia has caused the death of kids (Kouri et al. 2018) and lambs (Rose 1972), although the importance of this cause will depend on location and month of birthing. Hypothermia is a large risk during the colder months. Primary exposure occurs when bodily heat loss exceeds heat generation. Twins are more susceptible than are singles due to their lower birthweights and a larger surface area to weight ratio, with hypothermia being probable in some lambs at temperatures of 23°C under windy conditions (Alexander 1962b). Secondary hypothermia occurs where the levels of chill alone are insufficient to cause death. Any factor that reduces the ability of the lamb or kid to consume sufficient milk will result in a failure to generate sufficient heat, and hypothermia develops. Such factors include a difficult birth, resulting in delay in suckling (Dwyer et al. 1996), a reduced ability to thermoregulate (Darwish and Ashmawy 2011) and impaired maternal behaviour (Shelley 1970; Dwyer et al. 1996), insufficient milk supply or damaged teats, competition from litter-mates and mismothering.
Kids are susceptible to low temperatures (Shelton and Groff 1984; Buddle et al. 1988; Mellado et al. 2000; Aldomy et al. 2009), with kids of low birthweight being at a higher risk of hypothermia (Allan et al. 1992b). At Condobolin, 62% of all perinatal kid losses in Australian rangeland and crossbred goats occurred when the chill index was above 950 kJ/m2.h (Bajhau and Kennedy 1990). In semi-intensively managed Sirohi goats in India, winter kidding has been associated with 2.2 times higher risk of mortality than kidding in other seasons (Chauhan et al. 2019). Birthing in milder months will reduce the risk of mortality associated with exposure (Broster et al. 2012).
Birthweight is a key factor associated with low vigour and risk of death from SME. The approximate birthweight of Australian rangeland kids in the pastoral zone is 2.5–3 kg (SCA 1982). Lighter than average lambs (Geenty et al. 2014) and kids (Allan et al. 1992b; Rattner et al. 1994) have a lower survival rate. Across various breeds, very high levels of kid mortality are reported for the lowest birthweights of ≤1.5 kg (44%; Awemu et al. 1999), ≤1.5 kg (20%; Rattner et al. 1994), <2 kg (50%; Lehloenya et al. 2005), <2 kg (>50%; Allan et al. 1992b), and <4 kg (80%; Shelton and Groff 1984). Boer stud kids in Australia show a similar trend, although generally a lower mortality, with birthweights of <2 kg having more than 30% mortality, compared with 15% for 2–2.4 kg, and ~5% for birthweights 3–6.4 kg (Aldridge et al. 2015). However, overfeeding ewes or does in an attempt to increase the average birthweight may reduce survival due to the increased risk of dystocia (Bajhau and Kennedy 1990; Hatcher et al. 2009).
For lambs, the management of nutrition is key to minimising deaths from SME, through achieving optimum birthweight (Oldham et al. 2011), adequate milk production (McCance and Alexander 1959), optimal maternal behaviour (Dwyer 2014) and ensuring that lambs have high energy reserves at birth (Alexander 1962a). Similar patterns occur in goats (Idamokoro et al. 2017). The timing of nutrition can be important. A mid-pregnancy weight loss of 6% has not reduced perinatal kid survival in pen-fed goats (McGregor 2016). However, a submaintenance level of nutrition, compared with maintenance, from the last month of pregnancy increased kid mortality from 5.5% to 33.3% at Condobolin (Bajhau and Kennedy 1990). Pre-weaning kid mortality differed among seasons and years by up to 60% (10–50%) in a Nigerian study (Awemu et al. 1999), and it is expected that differences in nutritional level and weather contributed to this variation. Goat producers in the pastoral regions of Australia have cited poor nutrition as a key cause of mortality (Nogueira et al. 2016); so, it is probable that nutrition contributes to the high among-herd and among-year variation in weaning rates reported at least partially through impacts on perinatal kid survival.
Even short-term increases in nutrition can potentially reduce kid deaths from SME. Supplementation with 360 g/day of a grain and meal mixed ration for 2 weeks before the start of kidding reduced mortality from 17.8% to 2.9% in cashmere does in Qld (Goodwin and Norton 2004). Similarly, a 17-day period of above-maintenance compared with submaintenance supplementation (lupin plus oat grain) from 1 week before kidding (does were mated over 24 days) has reduced kid mortality (Allan et al. 1991).
Mismothering causes death due to starvation, as both lambs and kids are reliant on milk for the first month of life. Mismothering is recognised as a cause of some kid mortality in both extensive and intensive conditions (Shelton and Groff 1984; Eady and Rose 1988; McGregor 2016). Improved nutrition promotes desirable maternal behaviour in does (Ramírez-Vera et al. 2012). However, the factors that result in poor mothering in does have not been fully investigated. Breed differences and the effects of nutrition on mothering ability is an area requiring further research.
Starvation does occur in the absence of mismothering. Teat problems have been implicated in perinatal deaths of kids (Snyman 2010). Teat and udder problems are more common in older does, and shearing cuts may cause a higher rate of faults in Angora does than in dairy and meat breeds that are not shorn (Shelton and Groff 1984). Where practical, annual examinations and culling of does with faulty udders may promote kid survival.
Primary predation
In Australia, predation by wild dogs, foxes, pigs and wedge-tailed eagles has been implicated in kid losses, with producers perceiving predation as a key cause of perinatal mortality (Ferrier and McGregor 2002; MLA 2006b). Quantitative data indicate that primary predation typically causes less than 10% of all lamb deaths (Rowley 1970; Dennis 1974; Greentree et al. 2000), and the limited studies available suggest a similar incidence in kids (Allan et al. 1992b). However, there are instances where predation is significant (Smith 1960; Rowley 1970; Jordan and Le Feuvre 1989). Due to the need to differentiate primary from secondary predation, caution should be exercised in attributing high levels of loss to predation, without appropriate evidence.
Infection
Australian studies have indicated that infection is generally a minor cause of perinatal death in lambs (≤6%; Refshauge et al. 2016). For kids, infection has not been noted as a cause in the limited Australian studies where cause of death was reported (Eady and Rose 1988; Bajhau and Kennedy 1990; Allan et al. 1992a; McGregor 2016). Toxoplasma gondii has been found in Qld goats (SCA 1982), although it is unclear whether this disease is significantly affecting kid survival rates. The importance of infection as a cause of death appears to be higher internationally than in Australia, with intensive (housed) or semi-intensive management systems being a contributing factor (Sherman 1987; Aldomy et al. 2009; Snyman 2010). Clostridial diseases are reported as contributing to post-natal kid losses, with regional variation in the risk and use of preventive vaccines (Nogueira et al. 2016).
Other
Congenital abnormalities typically occur at a low level in dead lambs (<2%; Table 1) and kids (≤4%; Snyman 2010). There is potential for mineral deficiencies in some areas to contribute to perinatal mortality and preventive strategies are recommended in regions where deficiencies are known to occur (Caple and McDonald 1983; Buddle et al. 1988). Goats have higher requirements for some minerals than do sheep, including iodine and copper (SCA 1982), and calcium in late lactation (NRC 2007). Iodine may be of high relevance to goats due to its role in thermoregulation (SCA 1990), and the importance of cold exposure as a cause of perinatal death. Symptoms of iodine deficiency in kids are enlarged thyroid glands (>0.4 g/kg liveweight), lack of hair, stillbirth and abortion (SCA 1990). Goats are more susceptible to iodine deficiency than are sheep, partly due to their browse habit and trend for winter pregnancies, when soil iodine intake is lower than at other times of the year (Hosking et al. 1986). Hence, an assessment of iodine status may be important for goat producers. Selenium deficiency resulting in white muscle disease has been reported for kids, but lambs appear to be more commonly affected (Hosking et al. 1986).
Causes of post-weaning mortality
Estimates of post-weaning kid mortality in Australia are limited. For Boer studs in high-rainfall areas, 4–15% has been reported, but higher mortality rates (15%) have been reported in pastoral regions among varying breeds (Nogueira et al. 2016), and similar values have been reported for weaner sheep (Campbell et al. 2014). A rate of 3.2% was estimated in the study by Eady and Rose (1988). In Israel, post-weaning mortalities are also significant, being higher for kids of low birthweight, and varying with the kidding season (Rattner et al. 1994). These studies have indicated that maternal and post-weaning nutrition is likely to be a key factor in post-weaning survival of kids, as with lambs (Campbell 2010). Intestinal parasites occur in rangeland and more intensively managed goats and have the potential to cause high post-weaning mortality, but their importance varies among locations (SCA 1982; Ferrier and McGregor 2002; Nogueira et al. 2016).
Conclusions
Goats have the potential for high weaning percentages, and some commercial Australian herds are weaning in excess of 160%/doe joined. However, there is large variation among properties, indicating potential for gains. It remains unclear whether poor fertility due to out of season mating, or buck management, are significant contributors to low weaning rates. There are clear data to indicate that pregnancy rate and kidding rates are at expected levels (>90%) in intensively managed herds and that in these herds, perinatal loss is typically the largest source of kid loss. The known impact of low nutrition indicates that this as a potentially important source of both fetal and perinatal kid loss in goats under seasonally variable pasture conditions, which may be important in the pastoral regions. However, there is little data for herds in pastoral regions, nor detailed information from commercial non-stud herds, to explain the wide variation in weaning rates among properties, nor to identify the timing and cause of poor performance. However, this variation is similar to that reported for commercial sheep flocks, where perinatal mortality is widely recognised as the most important cause of loss. The main causes of perinatal kid loss are similar to those in sheep, and, generally, are SME and dystocia or stillbirth. Further studies, including the use of sentinel herds, are recommended to more clearly define the time of reproductive failure on commercial farms in different regions. Attempts to quantify the relative importance of the different causes of perinatal and post-weaning losses in different regions, and the factors associated with their prevalence, appear warranted. This information would allow regionally relevant recommendations to be provided to reduce potential kid loss.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the financial support of Meat & Livestock Australia in funding this review, and the constructive comments from reviewers that have improved the manuscript.
References
Aldomy F, Hussein NO, Sawalha L, Khatatbeh K, Aldomy A (2009) A national survey of perinatal mortality in sheep and goats in Jordan. Pakistan Veterinary Journal 29, 102–106.Aldridge MN, Brown DJ, Pitchford WS (2015) Genetic and phenotypic relationships between kid survival and birth weight in Australian meat goats. Proceedings of the Association for the Advancement of Animal Breeding and Genetics. 21, 350–353.
Aldridge MN, Pitchford WS, Brown DJ (2019) Current progress on developing a selection index for Australian meat goats. Proceedings of the Association for the Advancement of Animal Breeding and Genetics. 23, 51–54.
Alexander G (1962a) Energy metabolism in the starved new-born lamb. Australian Journal of Agricultural Research 13, 144–164.
| Energy metabolism in the starved new-born lamb.Crossref | GoogleScholarGoogle Scholar |
Alexander G (1962b) Temperature regulation in the new-born lamb. V. Summit metabolism. Australian Journal of Agricultural Research 13, 100–121.
| Temperature regulation in the new-born lamb. V. Summit metabolism.Crossref | GoogleScholarGoogle Scholar |
Allan CJ, Holst PJ, Hinch GN (1991) Behaviour of parturient Australian bush goats. I. Doe behaviour and kid vigour. Applied Animal Behaviour Science 32, 55–64.
| Behaviour of parturient Australian bush goats. I. Doe behaviour and kid vigour.Crossref | GoogleScholarGoogle Scholar |
Allan CJ, Hinch GN, Holst PJ (1992a) Comparison of techniques for determination of early post-natal deaths of kids. In ‘5th international conference on goats’, 2–8 March 1992, New Delhi, India. Abstract 309. (International Goat Association: Little Rock, AR, USA)
Allan CJ, Holst PJ, Hinch GN (1992b) Factors affecting survival of Australian bush goat kids. In ‘V international conference on goats’, 2–8 March 1992. Abstract 310. (International Goat Association: Little Rock, AR, USA)
Anon. (2015) ‘Goatmeat and livestock industry strategic plan 2020.’ Available at https://www.goatindustrycouncil.com.au/wp-content/uploads/2019/06/GoatStrategicPlan_WEB.pdf [Verified 26 June 2019]
Awemu EM, Nwakalor LN, Abubakar BY (1999) Environmental influences on preweaning mortality and reproductive performance of Red Sokoto does. Small Ruminant Research 34, 161–165.
| Environmental influences on preweaning mortality and reproductive performance of Red Sokoto does.Crossref | GoogleScholarGoogle Scholar |
Bajhau HS, Kennedy JP (1990) Influence of pre- and postpartum nutrition on growth of goat kids. Small Ruminant Research 3, 227–236.
| Influence of pre- and postpartum nutrition on growth of goat kids.Crossref | GoogleScholarGoogle Scholar |
Besier B, Jacobsen C, Woodgate R, Bell K (2010) Sheep health. In ‘International sheep and wool handbook’. (Ed. DJ Cottle) pp. 471–488. (Nottingham University Press: Nottingham, UK)
Brien FD, Hebart ML, Smith DH, Hocking Edwards JE, Greeff JC, Hart KW, Refshauge G, Bird-Gardiner TL, Gaunt G, Behrendt R, Robertson MW, Hinch GN, Geenty KG, van der Werf JHJ (2010) Opportunities for genetic improvement of lamb survival. Animal Production Science 50, 1017–1025.
| Opportunities for genetic improvement of lamb survival.Crossref | GoogleScholarGoogle Scholar |
Brien FD, Cloete SWP, Fogarty NM, Greeff JC, Hebart ML, Hiendleder S, Hocking Edwards JE, Kelly JM, Kind KL, Kleemann DO, Plush KL, Miller DR (2014) A review of the genetic and epigenetic factors affecting lamb survival. Animal Production Science 54, 667–693.
| A review of the genetic and epigenetic factors affecting lamb survival.Crossref | GoogleScholarGoogle Scholar |
Broster JC, Robertson SM, Dehaan RL, King BJ, Friend MA (2012) Evaluating seasonal risk and the potential for windspeed reductions to reduce chill index at six locations using GrassGro. Animal Production Science 52, 921–928.
| Evaluating seasonal risk and the potential for windspeed reductions to reduce chill index at six locations using GrassGro.Crossref | GoogleScholarGoogle Scholar |
Brown DJ, Jones RM, Hinch GN (2014) Genetic parameters for lamb autopsy traits. Animal Production Science 54, 736–744.
| Genetic parameters for lamb autopsy traits.Crossref | GoogleScholarGoogle Scholar |
Brown DJ, Savage DB, Hinch GN, Hatcher S (2015) Monitoring liveweight in sheep is a valuable management strategy: a review of available technologies. Animal Production Science 55, 427–436.
| Monitoring liveweight in sheep is a valuable management strategy: a review of available technologies.Crossref | GoogleScholarGoogle Scholar |
Browning R, Leite-Browning ML, Byars M (2011) Reproductive and health traits among Boer, Kiko, and Spanish meat goat does under humid, subtropical pasture conditions of the southeastern United States. Journal of Animal Science 89, 648–660.
| Reproductive and health traits among Boer, Kiko, and Spanish meat goat does under humid, subtropical pasture conditions of the southeastern United States.Crossref | GoogleScholarGoogle Scholar | 21036938PubMed |
Buddle BM, Herceg M, Ralston MJ, Pulford HD, Millar KR, Elliott DC (1988) A goat mortality study in the southern North Island. New Zealand Veterinary Journal 36, 167–170.
| A goat mortality study in the southern North Island.Crossref | GoogleScholarGoogle Scholar | 16031483PubMed |
Campbell AJD (2010) Weaner management. In ‘International sheep and wool handbook’. (Ed. DJ Cottle) pp. 277–293. (Nottingham University Press: Nottingham, UK)
Campbell AJD, Broekhuizen A, Curtis K, Croker KP, Behrendt R, Thompson AN (2014) A survey of post-weaning mortality of sheep in Australia and its association with farm and management factors. Animal Production Science 54, 783–790.
| A survey of post-weaning mortality of sheep in Australia and its association with farm and management factors.Crossref | GoogleScholarGoogle Scholar |
Caple IW, McDonald JW (1983) Trace mineral nutrition. In ‘Sheep production and preventive medicine. Proceedings of a refresher course for veterinarians’. pp. 236–264. (The Post-Graduate Committee in Veterinary Science, University of Sydney: Sydney, NSW, Australia)
Chauhan IS, Misra SS, Kumar A, Gowane GR (2019) Survival analysis of mortality in pre-weaning kids of Sirohi goat. Animal 13, 2896–2902.
| Survival analysis of mortality in pre-weaning kids of Sirohi goat.Crossref | GoogleScholarGoogle Scholar | 31317862PubMed |
Chemineau P (1986) Sexual behaviour and gonadal activity during the year in the tropical Creole meat goat. 1. Female oestrous behaviour and gonadal activity. Reproduction, Nutrition, Development 26, 441–452.
| Sexual behaviour and gonadal activity during the year in the tropical Creole meat goat. 1. Female oestrous behaviour and gonadal activity.Crossref | GoogleScholarGoogle Scholar |
Chemineau P (1987) Possibilities for using bucks to stimulate ovarian and oestrous cycles in anovulatory goats: a review. Livestock Production Science 17, 135–147.
| Possibilities for using bucks to stimulate ovarian and oestrous cycles in anovulatory goats: a review.Crossref | GoogleScholarGoogle Scholar |
Darwish RA, Ashmawy TAM (2011) The impact of lambing stress on post-parturient behaviour of sheep with consequences on neonatal homeothermy and survival. Theriogenology 76, 999–1005.
| The impact of lambing stress on post-parturient behaviour of sheep with consequences on neonatal homeothermy and survival.Crossref | GoogleScholarGoogle Scholar | 21719079PubMed |
De Santiago-Miramontes MA, Rivas-Muñoz R, Muñoz-Gutiérrez M, Malpaux B, Scaramuzzi RJ, Delgadillo JA (2008) The ovulation rate in anoestrous female goats managed under grazing conditions and exposed to the male effect is increased by nutritional supplementation. Animal Reproduction Science 105, 409–416.
| The ovulation rate in anoestrous female goats managed under grazing conditions and exposed to the male effect is increased by nutritional supplementation.Crossref | GoogleScholarGoogle Scholar | 18178343PubMed |
De Santiago-Miramontes MA, Malpaux B, Delgadillo JA (2009) Body condition is associated with a shorter breeding season and reduced ovulation rate in subtropical goats. Animal Reproduction Science 114, 175–182.
| Body condition is associated with a shorter breeding season and reduced ovulation rate in subtropical goats.Crossref | GoogleScholarGoogle Scholar | 18930360PubMed |
Delgadillo JA, Flores JA, Véliz FG, Hernández HF, Duarte G, Vielma J, Poindron P, Chemineau P, Malpaux B (2002) Induction of sexual activity in lactating anovulatory female goats using male goats treated only with artificially long days. Journal of Animal Science 80, 2780–2786.
| Induction of sexual activity in lactating anovulatory female goats using male goats treated only with artificially long days.Crossref | GoogleScholarGoogle Scholar | 12462243PubMed |
Delgadillo JA, Flores JA, Hernández H, Poindron P, Keller M, Fitz-Rodríguez G, Duarte G, Vielma J, Fernández IG, Chemineau P (2015) Sexually active males prevent the display of seasonal anestrus in female goats. Hormones and Behavior 69, 8–15.
| Sexually active males prevent the display of seasonal anestrus in female goats.Crossref | GoogleScholarGoogle Scholar | 25497417PubMed |
Dennis SM (1974) Perinatal lamb mortality in Western Australia. 1. General procedures and results. Australian Veterinary Journal 50, 443–449.
| Perinatal lamb mortality in Western Australia. 1. General procedures and results.Crossref | GoogleScholarGoogle Scholar | 4447527PubMed |
Dennis SM (1975) Perinatal lamb mortality in Western Australia 7. Congenital defects. Australian Veterinary Journal 51, 80–82.
| Perinatal lamb mortality in Western Australia 7. Congenital defects.Crossref | GoogleScholarGoogle Scholar | 1172430PubMed |
Diskin MG, Morris DG (2008) Embryonic and early foetal losses in cattle and other ruminants. Reproduction in Domestic Animals 43, 260–267.
| Embryonic and early foetal losses in cattle and other ruminants.Crossref | GoogleScholarGoogle Scholar | 18638133PubMed |
Đuričić D, Grizelj J, Dobranić T, Harapin I, Vince V, Kočila P, Folnožić I, Lipar M, Gregurić Gračner G, Samardžija M (2012) Reproductive performance of Boer goats in a moderate climate zone. Veterinarski Arhiv 82, 351–358.
Dwyer CM (2008) Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. Journal of Animal Science 86, E246–E258.
| Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management.Crossref | GoogleScholarGoogle Scholar | 17709772PubMed |
Dwyer CM (2014) Maternal behaviour and lamb survival: from neuroendocrinology to practical application. Animal 8, 102–112.
| Maternal behaviour and lamb survival: from neuroendocrinology to practical application.Crossref | GoogleScholarGoogle Scholar | 24103485PubMed |
Dwyer CM, Lawrence AB, Brown HE, Simm G (1996) Effect of ewe and lamb genotype on gestation length, lambing ease and neonatal behaviour of lambs. Reproduction, Fertility and Development 8, 1123–1129.
| Effect of ewe and lamb genotype on gestation length, lambing ease and neonatal behaviour of lambs.Crossref | GoogleScholarGoogle Scholar |
Dwyer CM, Conington J, Corbiere F, Holmøy IH, Muri K, Nowak R, Rooke J, Vipond J, Gautier JM (2016) Invited review: improving neonatal survival in small ruminants: science into practice. Animal 10, 449–459.
| Invited review: improving neonatal survival in small ruminants: science into practice.Crossref | GoogleScholarGoogle Scholar | 26434788PubMed |
Eady SJ, Rose M (1988) Reproductive performance of cashmere goats in south western Queensland. Proceedings of the Australian Society of Animal Production 17, 182–185.
Egan A (1972) Reproductive responses to supplemental zinc and manganese in grazing Dorset Horn ewes. Australian Journal of Experimental Agriculture 12, 131–135.
| Reproductive responses to supplemental zinc and manganese in grazing Dorset Horn ewes.Crossref | GoogleScholarGoogle Scholar |
Engeland IV, Waldeland H, Andresen Ø, Tverdal A (1997) Foetal loss in dairy goats: an epidemiological study in 515 individual goats. Animal Reproduction Science 49, 45–53.
| Foetal loss in dairy goats: an epidemiological study in 515 individual goats.Crossref | GoogleScholarGoogle Scholar | 9458949PubMed |
Erasmus JA (2000) Adaptation to various environments and resistance to disease of the Improved Boer goat. Small Ruminant Research 36, 179–187.
| Adaptation to various environments and resistance to disease of the Improved Boer goat.Crossref | GoogleScholarGoogle Scholar | 10760454PubMed |
Fatet A, Pellicer-Rubio M-T, Leboeuf B (2011) Reproductive cycle of goats. Animal Reproduction Science 124, 211–219.
| Reproductive cycle of goats.Crossref | GoogleScholarGoogle Scholar | 20888155PubMed |
Ferrier GR, McGregor BA (2002) Benchmarks of Victorian commercial goat meat enterprises. Animal Production in Australia 24, 65–68.
Geenty KG, Brien FD, Hinch GN, Dobos RC, Refshauge G, McCaskill M, Ball AJ, Behrendt R, Gore KP, Savage DB, Harden S, Hocking-Edwards JE, Hart K, van der Werf JHJ (2014) Reproductive performance in the Sheep CRC Information Nucleus using artificial insemination across different sheep-production environments in southern Australia. Animal Production Science 54, 715–726.
| Reproductive performance in the Sheep CRC Information Nucleus using artificial insemination across different sheep-production environments in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Givens MD, Marley MSD (2008) Infectious causes of embryonic and fetal mortality. Theriogenology 70, 270–285.
| Infectious causes of embryonic and fetal mortality.Crossref | GoogleScholarGoogle Scholar | 18502494PubMed |
Goodwin N, Norton BW (2004) Improving doe nutrition immediately prior to kidding increases kid survival. Animal Production in Australia 25, 251
Greentree C, Saunders G, Mcleod L, Hone J (2000) Lamb predation and fox control in south-eastern Australia. Journal of Applied Ecology 37, 935–943.
| Lamb predation and fox control in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Greyling JPC (2000) Reproduction traits in the Boer goat doe. Small Ruminant Research 36, 171–177.
| Reproduction traits in the Boer goat doe.Crossref | GoogleScholarGoogle Scholar |
Haenlein GFW, Anke M (2011) Mineral and trace element research in goats: a review. Small Ruminant Research 95, 2–19.
| Mineral and trace element research in goats: a review.Crossref | GoogleScholarGoogle Scholar |
Hatcher S, Atkins KD, Safari E (2009) Phenotypic aspects of lamb survival in Australian Merino sheep. Journal of Animal Science 87, 2781–2790.
| Phenotypic aspects of lamb survival in Australian Merino sheep.Crossref | GoogleScholarGoogle Scholar | 19502501PubMed |
Hinch GN, Brien F (2014) Lamb survival in Australian flocks: a review. Animal Production Science 54, 656–666.
| Lamb survival in Australian flocks: a review.Crossref | GoogleScholarGoogle Scholar |
Hosking WJ, Caple IW, Halpin CG, Brown AJ, Paynter DI, Conley DN, North-Coombes DL (1986) ‘Trace elements for pastures and animals in Victoria.’ (Victorian Government Printing Office, on behalf of the Department of Agriculture and Rural Affairs: Melbourne, Vic., Australia)
Hurley WL, Doane RM (1989) Recent developments in the roles of vitamins and minerals in reproduction. Journal of Dairy Science 72, 784–804.
| Recent developments in the roles of vitamins and minerals in reproduction.Crossref | GoogleScholarGoogle Scholar | 2654228PubMed |
Idamokoro EM, Muchenje V, Masika PJ (2017) Peri- and post-parturient consequences of maternal undernutrition of free ranging does: a review. Livestock Research for Rural Development 29, 202
Jones A (2012) ‘Rangeland goat production in Western NSW.’ (NSW Department of Primary Industries: Orange, NSW, Australia)
Jordan DJ, Le Feuvre AS (1989) The extent and cause of perinatal lamb mortality in 3 flocks of Merino sheep. Australian Veterinary Journal 66, 198–201.
| The extent and cause of perinatal lamb mortality in 3 flocks of Merino sheep.Crossref | GoogleScholarGoogle Scholar | 2775061PubMed |
Kelly R, Wilkins J, Newnham J (1989) Fetal mortality from day 30 of pregnancy in Merino ewes offered different levels of nutrition. Australian Journal of Experimental Agriculture 29, 339–342.
| Fetal mortality from day 30 of pregnancy in Merino ewes offered different levels of nutrition.Crossref | GoogleScholarGoogle Scholar |
Kleemann DO, Walker SK (2005) Fertility in South Australian commercial Merino flocks: sources of reproductive wastage. Theriogenology 63, 2075–2088.
| Fertility in South Australian commercial Merino flocks: sources of reproductive wastage.Crossref | GoogleScholarGoogle Scholar | 15826674PubMed |
Kouri A, Charallah S, Kouri F, Amirat Z, Khammar F (2018) Reproductive performances and abortion etiologies of native Bedouin goats in the arid zones of Algeria. Livestock Research for Rural Development 30, 7–15.
Lane J, Jubb T, Shephard R, Webb-Ware J, Fordyce G (2015) Final report B.AHE.0010. Priority list of endemic diseases for the red meat industries. Meat & Livestock Australia, Sydney, NSW, Australia.
Lehloenya KC, Greyling JPC, Schwalbach LMJ (2005) Reproductive performance of South African indigenous goats following oestrous synchronisation and AI. Small Ruminant Research 57, 115–120.
| Reproductive performance of South African indigenous goats following oestrous synchronisation and AI.Crossref | GoogleScholarGoogle Scholar |
Mani AU, McKelvey WAC, Watson ED (1992) The effects of low level of feeding on response to synchronization of estrus, ovulation rate and embryo loss in goats. Theriogenology 38, 1013–1022.
| The effects of low level of feeding on response to synchronization of estrus, ovulation rate and embryo loss in goats.Crossref | GoogleScholarGoogle Scholar | 16727199PubMed |
Martínez-Alfaro JC, Hernández H, Flores JA, Duarte G, Fitz-Rodríguez G, Fernández IG, Bedos M, Chemineau P, Keller M, Delgadillo JA, Vielma J (2014) Importance of intense male sexual behavior for inducing the preovulatory LH surge and ovulation in seasonally anovulatory female goats. Theriogenology 82, 1028–1035.
| Importance of intense male sexual behavior for inducing the preovulatory LH surge and ovulation in seasonally anovulatory female goats.Crossref | GoogleScholarGoogle Scholar | 25139756PubMed |
McCance I, Alexander G (1959) The onset of lactation in the Merino ewe and its modification by nutritional factors. Australian Journal of Agricultural Research 10, 699–719.
| The onset of lactation in the Merino ewe and its modification by nutritional factors.Crossref | GoogleScholarGoogle Scholar |
McGregor BA (2016) The effects of nutrition and parity on the development and productivity of Angora goats: 1. Manipulation of mid pregnancy nutrition on energy intake and maintenance requirement, kid birth weight, kid survival, doe live weight and mohair production. Small Ruminant Research 145, 65–75.
| The effects of nutrition and parity on the development and productivity of Angora goats: 1. Manipulation of mid pregnancy nutrition on energy intake and maintenance requirement, kid birth weight, kid survival, doe live weight and mohair production.Crossref | GoogleScholarGoogle Scholar |
Mellado M, Vera T, Meza-Herrera C, Ru ÍZF (2000) A note on the effect of air temperature during gestation on birth weight and neonatal mortality of kids. The Journal of Agricultural Science 135, 91–94.
| A note on the effect of air temperature during gestation on birth weight and neonatal mortality of kids.Crossref | GoogleScholarGoogle Scholar |
Mellado M, Valdez R, Lara LM, García JE (2004) Risk factors involved in conception, abortion, and kidding rates of goats under extensive conditions. Small Ruminant Research 55, 191–198.
| Risk factors involved in conception, abortion, and kidding rates of goats under extensive conditions.Crossref | GoogleScholarGoogle Scholar |
Mellado M, Valdéz R, García JE, López R, Rodríguez A (2006) Factors affecting the reproductive performance of goats under intensive conditions in a hot arid environment. Small Ruminant Research 63, 110–118.
| Factors affecting the reproductive performance of goats under intensive conditions in a hot arid environment.Crossref | GoogleScholarGoogle Scholar |
MLA (2006a) ‘Going into goats Module 6 husbandry.’ Available at https://www.mla.com.au/globalassets/mla-corporate/generic/extension-training-and-tools/gig-husbandry.pdf [Verified 18 September 2019]
MLA (2006b) ‘Going into goats: a practical guide to producing goats in the rangelands.’ Available at http://www.rangelandgoats.com.au [Verified 25 June 2019]
MLA (2019) ‘Global snapshot goatmeat.’ Available at https://www.mla.com.au/globalassets/mla-corporate/prices–markets/documents/os-markets/red-meat-market-snapshots/2019-mla-ms_global-goatmeat-final.pdf [Verified 26 June 2019]
Nogueira DM, Gummow B, Gardiner CP, Cavalieri J, Fitzpatrick LA, Parker AJ (2016) A survey of the meat goat industry in Queensland and New South Wales. 2. Herd management, reproductive performance and animal health. Animal Production Science 56, 1533–1544.
| A survey of the meat goat industry in Queensland and New South Wales. 2. Herd management, reproductive performance and animal health.Crossref | GoogleScholarGoogle Scholar |
Norton BW (2004) The role of the Boer goat in the development of the Australian goat meat industry. Final report project TR.012. Meat & Livestock Australia, Sydney, NSW, Australia.
NRC (2007) ‘Nutrient requirements of small ruminants: sheep, goats, cervids and New World camelids.’ (National Research Council, National Academies Press: Washington, DC, USA)
Oderinwale OA, Oluwatosin BO, Sowande OS, Bemji MN, Amosu SD, Sanusi GO (2017) Concentrate supplementations of grazing pregnant Kalahari Red goats: effects on pregnancy variables, reproductive performance, birth types and weight of kids. Tropical Animal Health and Production 49, 1125–1133.
| Concentrate supplementations of grazing pregnant Kalahari Red goats: effects on pregnancy variables, reproductive performance, birth types and weight of kids.Crossref | GoogleScholarGoogle Scholar | 28597193PubMed |
Oldham CM, Thompson AN, Ferguson MB, Gordon DJ, Kearney GA, Paganoni BL (2011) The birthweight and survival of Merino lambs can be predicted from the profile of liveweight change of their mothers during pregnancy. Animal Production Science 51, 776–783.
| The birthweight and survival of Merino lambs can be predicted from the profile of liveweight change of their mothers during pregnancy.Crossref | GoogleScholarGoogle Scholar |
Plumbe M, Atkinson T, Turnbull G (2019) ‘Rangeland goat production in western NSW: where are they now?’ Available at https://western.lls.nsw.gov.au/__data/assets/pdf_file/0004/853411/Rangeland-goat-production-in-Western-NSW.pdf [Verified 20 August 2020]
Pople T, Froese J (2012) Distribution, abundance and harvesting of goats in the Australian rangelands 1984–2011. Final report to the ACRIS Management Committee, Queensland Government, Brisbane, Qld, Australia.
Pretorius PS (1970) Effect of aggressive behaviour on production and reproduction in the Angora. goat (Copra hircus angoraensis). Agroanimalia 2, 161–164.
Ramírez-Vera S, Terrazas A, Delgadillo JA, Flores JA, Serafín N, Vielma J, Duarte G, Fernández IG, Fitz-Rodríguez G, Hernández H (2012) Inclusion of maize in the grazing diet of goats during the last 12 days of gestation reinforces the expression of maternal behaviour and selectivity during the sensitive period. Livestock Science 148, 52–59.
| Inclusion of maize in the grazing diet of goats during the last 12 days of gestation reinforces the expression of maternal behaviour and selectivity during the sensitive period.Crossref | GoogleScholarGoogle Scholar |
Rattner D, Riviere J, Bearman JE (1994) Factors affecting abortion, stillbirth and kid mortality in the Goat and Yaez (Goat × ibex). Small Ruminant Research 13, 33–40.
| Factors affecting abortion, stillbirth and kid mortality in the Goat and Yaez (Goat × ibex).Crossref | GoogleScholarGoogle Scholar |
Refshauge G, Brien FD, Hinch GN, van de Ven R (2016) Neonatal lamb mortality: factors associated with the death of Australian lambs. Animal Production Science 56, 726–735.
| Neonatal lamb mortality: factors associated with the death of Australian lambs.Crossref | GoogleScholarGoogle Scholar |
Restall BJ (1992) Seasonal variation in reproductive activity in Australian goats. Animal Reproduction Science 27, 305–318.
| Seasonal variation in reproductive activity in Australian goats.Crossref | GoogleScholarGoogle Scholar |
Ritar A, Ball P, O’May P (1990) Artificial insemination of Cashmere goats: effects on fertility and fecundity of intravaginal treatment, method and time of insemination, semen freezing process, number of motile spermatozoa and age of females. Reproduction, Fertility and Development 2, 377–384.
| Artificial insemination of Cashmere goats: effects on fertility and fecundity of intravaginal treatment, method and time of insemination, semen freezing process, number of motile spermatozoa and age of females.Crossref | GoogleScholarGoogle Scholar |
Rose M (1972) Vital statistics for an experimental flock of Merino sheep in north west Queensland. Proceedings of the Australian Society of Animal Production 9, 48–54.
Rowley I (1970) Lamb predation in Australia: incidence, predisposing conditions, and the identification of wounds. CSIRO Wildlife Research 15, 79–123.
| Lamb predation in Australia: incidence, predisposing conditions, and the identification of wounds.Crossref | GoogleScholarGoogle Scholar |
Safari E, Fogarty NM, Gilmour AR (2005) A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livestock Production Science 92, 271–289.
| A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep.Crossref | GoogleScholarGoogle Scholar |
SCA (1982) Goats for meat and fibre in Australia. Standing Committee on Agriculture technical report, series no. 11, Canberra, ACT, Australia.
SCA (Ed.) (1990) ‘Feeding standards for Australian livestock: ruminants.’ (CSIRO: Melbourne, Vic., Australia)
Shelley L (1970) Interrelationships between the duration of parturition, post-natal behaviour of ewes and lambs and the incidence of neonatal mortality. Proceedings of the Australian Society of Animal Production 8, 348–352.
Shelton M, Groff J (1984) ‘Improving reproductive efficiency in Angora goats.’ (Texas Agricultural Experiment Station: San Angelo, TX, USA)
Sherman DM (1987) Causes of kid morbidity and mortality: an overview. In ‘Proceedings of the IV international conference on goats’, 14–19 July 1987, Brasilia, Brazil. pp. 335–355. (International Goat Association: Little Rock, AR, USA)
Smith ID (1960) The effect of the physical environment upon ovine reproduction in tropical Queensland. Proceedings of the Australian Society of Animal Production 3, 115–119.
Snyman MA (2010) Factors affecting pre-weaning kid mortality in South African Angora goats. South African Journal of Animal Science 40, 54–64.
| Factors affecting pre-weaning kid mortality in South African Angora goats.Crossref | GoogleScholarGoogle Scholar |
Urrutia-Morales J, Meza-Herrera CA, Tello-Varela L, Díaz-Gómez MO, Beltrán-López S (2012) Effect of nutritional supplementation upon pregnancy rates of goats under semiarid rangelands and exposed to the male effect. Tropical Animal Health and Production 44, 1473–1477.
| Effect of nutritional supplementation upon pregnancy rates of goats under semiarid rangelands and exposed to the male effect.Crossref | GoogleScholarGoogle Scholar | 22311376PubMed |
Véliz FG, Poindron P, Malpaux B, Delgadillo JA (2006a) Maintaining contact with bucks does not induce refractoriness to the male effect in seasonally anestrous female goats. Animal Reproduction Science 92, 300–309.
| Maintaining contact with bucks does not induce refractoriness to the male effect in seasonally anestrous female goats.Crossref | GoogleScholarGoogle Scholar | 16084676PubMed |
Véliz FG, Poindron P, Malpaux B, Delgadillo JA (2006b) Positive correlation between the body weight of anestrous goats and their response to the male effect with sexually active bucks. Reproduction, Nutrition, Development 46, 657–661.
| Positive correlation between the body weight of anestrous goats and their response to the male effect with sexually active bucks.Crossref | GoogleScholarGoogle Scholar | 17169312PubMed |
Walkden-Brown SW, Restall BJ, Henniawati (1993) The male effect in the Australian cashmere goat. 3. Enhancement with buck nutrition and use of oestrous females. Animal Reproduction Science 32, 69–84.
| The male effect in the Australian cashmere goat. 3. Enhancement with buck nutrition and use of oestrous females.Crossref | GoogleScholarGoogle Scholar |
Walkden-Brown SW, Restall BJ, Norton BW, Scaramuzzi RJ (1994) The ‘female effect’ in Australian cashmere goats: effect of season and quality of diet on the LH and testosterone response of bucks to oestrous does. Reproduction (Cambridge, England) 100, 521–531.
| The ‘female effect’ in Australian cashmere goats: effect of season and quality of diet on the LH and testosterone response of bucks to oestrous does.Crossref | GoogleScholarGoogle Scholar |
Waters C, Reseigh-O’Brien J, Pahl L, Atkinson T, Burnside D, Revell D (2018) ‘Addressing feed supply and demand through total grazing pressure management.’ (NSW Department of Primary Industries: Orange, NSW, Australia)
Zarazaga LA, Gatica MC, Celi I, Guzmán JL, Malpaux B (2009) Effect of melatonin implants on sexual activity in Mediterranean goat females without separation from males. Theriogenology 72, 910–918.
| Effect of melatonin implants on sexual activity in Mediterranean goat females without separation from males.Crossref | GoogleScholarGoogle Scholar | 19631371PubMed |
Zarazaga LA, Gatica MC, Hernández H, Chemineau P, Delgadillo JA, Guzmán JL (2019) Photoperiod-treated bucks are equal to melatonin-treated bucks for inducing reproductive behaviour and physiological functions via the ‘male effect’ in Mediterranean goats. Animal Reproduction Science 202, 58–64.
| Photoperiod-treated bucks are equal to melatonin-treated bucks for inducing reproductive behaviour and physiological functions via the ‘male effect’ in Mediterranean goats.Crossref | GoogleScholarGoogle Scholar | 30717994PubMed |



