Does generic entry lower the prices paid for pharmaceuticals in Australia? A comparison before and after the introduction of the mandatory price-reduction policy
Jean Spinks A C , Gang Chen B and Lara Donovan AA Centre for Health Economics, Monash University, Clayton, Vic. 3800, Australia. Email: lara.donovan@bms.com
B Flinders Health Economics Group, Flinders University, Daw Park, SA 5041, Australia. Email: gang.chen@flinders.edu.au
C Corresponding author. Email: jean.spinks@monash.edu
Australian Health Review 37(5) 675-681 https://doi.org/10.1071/AH13024
Submitted: 7 August 2012 Accepted: 19 August 2013 Published: 28 October 2013
Journal Compilation © AHHA 2013
Abstract
Objective We investigated the relationship between the number of generic medicines and pharmaceutical prices over time in Australia.
Methods A dataset was utilised containing 76 items for 4 years (2003–2007) on the national subsidy scheme – the Pharmaceutical Benefits Scheme (PBS) – for which a generic brand is available. The PBS price was used as the dependent variable, and the number of generics available the key explanatory variable. The ordinary least-squares estimator was adopted for estimation. In the robustness analysis, an instrumental-variables method was used to account for potential endogeneity.
Results Results suggested that the effect of increased generic medicine sellers on reducing the prices paid for generics is marginal but statistically significant.
Conclusions It is suggested that structural changes to the way generic prices are determined needs to be reconsidered by the Australian government if the policy aim of using increased ‘competition’ to lower prices is to be maximised.
What is known about the topic? There is scant empirical evidence that supports the notion that increased generic availability for pharmaceuticals, in heavily price-regulated markets such as Australia, has a significant effect on lowering the prices paid over time. Despite this, Australia has adopted a policy that promotes increased generic ‘competition’ as a means of controlling prices, without establishing if this policy has, or is likely to be, successful in the longer term.
What does this paper add? Using longitudinal data from Medicare Australia, this paper quantifies the relationship between the number of branded and generic items of a given drug molecule and formulation, and prices paid over time, controlling for other explanatory variables.
What are the implications for practitioners? The results suggest that although increased generic entry may lower prices over time in the Australian context, the price reduction gained is likely to be very small. Therefore, whilst generic entry should be encouraged, it is important not to assume that this price-lowering effect is realised without question and that the magnitude of such an effect is comparable with other price-regulated countries.
Additional keywords: competition, cost containment, generic pharmaceuticals, generic pricing, Pharmaceutical Benefits Scheme (PBS).
Introduction
To date, empirical evidence of the effect of increased generic entry in price-regulated pharmaceutical markets, such as Australia, has been surprisingly scarce given the importance of the topic.1 One of the most relevant studies2 quantified the effect of an increased number of generic sellers on prices paid overall. A strong negative correlation was shown between increased numbers of generic sellers and lower prices in markets with relatively free pricing (such as the United States (US), United Kingdom (UK), Germany and Canada), whereas there was either no effect or a positive effect on the prices paid with strict price regulation (such as France, Italy and Japan). Other literature on pharmaceutical pricing tends to concentrate on price differentials between the original product on the market, and the first or subsequent generic sellers of the same molecule.3–8 Most of these analyses show that branded prices and generic prices respond differently to generic entry.3–6 A ‘segmented market’ may exist, whereby some consumers continue to buy the branded products after generic entry, whilst others convert to a generic medication once it becomes available.7 Other findings suggest that generic entry leads to an initial sharp fall in price, and a more gradual price decline with additional generic sellers.8 More recently, analysis of the Finnish experience of the introduction of generic substitution found that prices fell on average more than 10% in the first year, although this effect was not consistent across all items.9
Recent studies have suggested that Australia is paying too much for many pharmaceuticals, particularly generics.10–13 As such, it is timely to assess the relative success or otherwise of pricing policies over time. The ‘Minimum Pricing Policy’14, introduced in December 1990, had little impact in increasing price competition between generic manufacturers until the subsequent generic or ‘Brand Substitution Policy’ was introduced in 1994.14 The latter allowed pharmacists to substitute between interchangeable brands of the same medication (with consumer and prescriber consent). A brand premium exists when a manufacturer chooses a price above the benchmark price paid by the government, with the resulting difference in price passed on to the consumer. Thus, unless the manufacturer believes that brand loyalty to a particular product exists, there is little incentive to charge a price to the government substantially above the benchmark price. Indeed, it has been argued that current government policy actually impedes any competition between firms that may lead to lower prices in less regulated markets.11 Proposed price reductions by either a new or existing manufacturer that lower the benchmark price for the next pricing cycle are disclosed to all competitors, providing no incentive to lower prices as a way of increasing market share.11 In contrast, there is evidence that significant volume discounts are offered to pharmacies, by both generic and branded manufacturers, in order to influence the substitution habits of the dispensing pharmacist, with a view to increasing market share.15 Thus, despite stimulation of generic entry for well over 10 years, the Australian government has not benefitted to the same extent as other countries with different price regulation strategies. On 1 August 2005, the government initiated a one-off 12.5% ‘mandatory price reduction’ for the benchmark price, triggered by any new listing of a generic medicine.16,A The price reduction was applied once to the first generic brand of any Pharmaceutical Benefits Scheme (PBS)-listed item, and the price reduction was designed to ‘flow-on’ to all brands of that medicine, including different forms and strengths, as well as any other medicines linked in the same reference pricing group (if applicable). In this analysis, we specifically test the effect of the introduction of this mandatory price-reduction policy on the prices paid by government. As only some items were affected during the panel, analysis is undertaken separately for the time period before and after the policy implementation.
Aim
The aim of this study is to test the hypothesis that an increase in generic sellers in the highly price-regulated Australian market reduces the average price paid for pharmaceuticals by the government over time. In addition, we test the effect of introducing a mandatory price-reduction policy.16
Methods and data
The dataset utilised for this analysis contains national-level data for all prescriptions dispensed under the PBS scheme, excluding those prescriptions that were under the co-payment level.B Obtained from Medicare Australia, it contains monthly data for the 4-year period of July 2003 to June 2007. The dataset includes the number of prescriptions dispensed for each item code (within the PBS, each strength of a particular pharmaceutical has an item code for each approved indication) by trade name. Both brand and generic trade names could be identified from the data. Drugs were selected for inclusion in the database if, as of December 2007, a generic bio-equivalent was offered. From this larger dataset, a subset was selected that contained all PBS items for which the first generic entered the market within the 4-year timeframe specified. This resulted in identification of 30 unique compounds with 76 items (different strengths and formulations).C Items for 9 of the 14 Anatomical Therapeutic Classification (ATC) groups were included in this subset (see Table 1).
The number of generic sellers is assumed to influence the rate of change in price over time. More generic sellers might be expected to reduce the price more over time compared with a drug with less generic sellers (deemed to be substitutable under the PBS). To empirically investigate the effects of generic sellers and the mandatory price-reduction policy, the following equation is used:
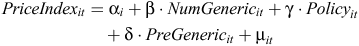
where the dependent variable, PriceIndex, is defined as the ratio of the real (benchmark) price of an item i at time t to the (benchmark) price of the item when the first generic competitor entered the market; α is an item-specific constant term; NumGeneric is the main explanatory variable of interest – number of generic sellers for a given item code; Policy is a dummy variable that coded as 1 if the item was effected by the mandatory price-reduction policy implemented from August 2005, 0 otherwise; PreGeneric is a time dummy representing 3 months before the competition commencementD and is used to account for any anticipatory price changes;3 µit is the error term; β, γ and δ are the coefficients to be estimated.
This method of price standardisation for the dependent variable, PriceIndex, is similar to Lexchin19 and Aalto-Setälä9 and was used for two reasons: (1) when used in regression analysis, the coefficients are readily interpretable; and (2) to account for the large variation in real prices between different items.E Prices used in the model are presented in 2007 Australian dollar prices adjusted by consumer price index.20 Two main explanatory variables are the number of generic sellers (NumGeneric) and the policy dummy. If generic ‘competition’ on the price of brand drugs is evident, we expect the estimated coefficient β would be significantly negative. Considering the effect of the number of generic sellers might be non-linear,7 we also include the squared term of the number of generic drugs in the equation. Similarly, we expect the estimated coefficient γ to be significantly negative.
Ordinary least-squares (OLS) regression has been used to estimate the equation. However, the number of generic sellers might be endogenous in this context, that is, the number of generic sellers can be affected by profit possibilities (i.e. drug price) in the market.2,3,9,21 In this context, reverse causality will cause the OLS results to underestimate the true effect of generic competition.2,21 Therefore, in the robustness analysis, we employ an instrumental variable (IV) approach and use the two-stage least-square (2SLS) estimator to deal with this problem.F Similar to Caves et al.,3 we use the quantity sold (mean total sales) for each item 3 months before generic entry as the IV for the number of competitors. The underlying assumption is that for a particular drug, the market size could present the general level of demand. However, the quantity sold before generic entry will not directly impact on the price index after generic entry.
We performed the regression analyses separately for the time period before and after the August 2005 policy was introduced, which is roughly halfway through the panel. All analyses were performed in Stata version 12.1 (StataCorp LP, College Station, TX, USA).
Results
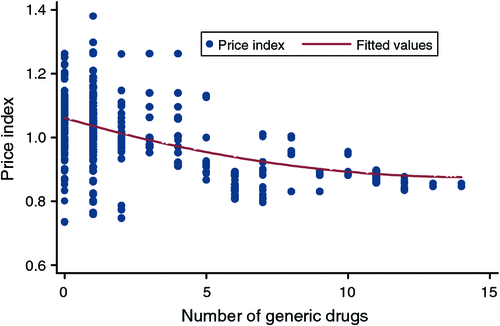
In terms of the overall changes in mean benchmark prices over the 4-year panel, 70 of the 76 items were available in July 2003 (start of the panel), with a mean real price of $49.30 (median $26.97, standard deviation (s.d.) 49.66, 95% confidence interval (CI) $8.28–$176.99, range $6.89–$208.72). In June 2007, all 76 items were available with a mean price of $42.72 (median $25.05, s.d. 41.00, 95% CI $8.64–$141.94, range $7.61–$176.45). The mean number of generic sellers has increased from 0.19 (median 0, s.d. 0.39, 95% CI 0–1, range 0–1) to 3.71 (median 2, s.d. 3.37, 95% CI 1–12, range 1–14) over the panel. Therefore, overall summary statistics indicate that benchmark prices have decreased over time, as generic entry has increased, as illustrated in Fig. 1. Although, for nine items, the benchmark price increased when generic entry began.
|
The descriptive statistics can also be analysed by breaking the panel into pre and post policy periods. Pre-policy (July 2003 to July 2005), the mean of the variable PriceIndex was calculated to be 1.06 (median 1.00, s.d. 0.08, 95% CI 0.98–1.24, range 0.73–1.26) and the mean number of generic sellers is 0.81 (median 0, s.d. 1.44, 95% CI 0–3, range 0–8) in the same period. Post-policy (Aug 2005 to June 2007), the mean of PriceIndex fell to 0.97 (median 0.98, s.d. 0.10, 95% CI 0.81–1.13, range 0.67–1.40) and the mean number of generic sellers changed to 2.85 (median 2, s.d. 2.85, 95% CI 0–9, range 0–14). This indicates that prices do seem to have fallen in response to the policy as expected.
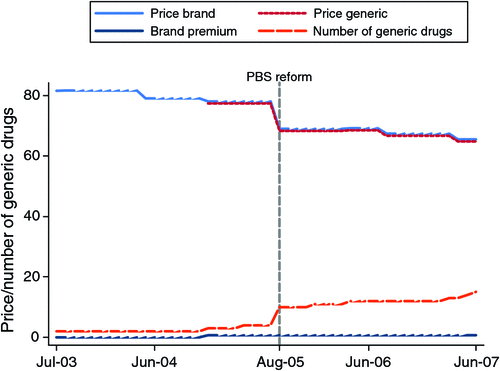
Changes in price and generic seller numbers over time can also be illustrated by tracking single items in the panel. Using the specific example of simvastatin 40 mg, Fig. 2 illustrates the fall in the price of both the branded and generic products with increasing number of generic sellers.
|
From this example, it appears that benchmark prices are falling with increasing numbers of generic sellers. However, this does not account for the policy change (mandatory price reduction) in August 2005, and whether any effect of competition above and beyond the mandated price reduction is present. The regression results with price index as the dependent variable are reported in Table 2.
Regression results are presented in a stepwise fashion, and reported separately for the two subsamples (July 2003–July 2005 and August 2005–June 2007), as the mandatory 12.5% price-reduction policy was implemented in August 2005. Three columns of results for each subsample are presented, with the Policy dummy only included in the second subsample to capture the policy effect, as only some items (71% of the dataset) were affected during the panel.
Column 1 in Table 2 suggests that the number of generic sellers has significantly reduced the standardised price index (–0.007, P < 0.01). The relationship is consistent when a time dummy (which used to account for any anticipatory price changes)3 is included in Column 2. In Column 3, the potential non-linear effect of the number of generic sellers is investigated by including the squared term of the number of generic sellers. As can be seen, although the quadric term is statistically significant, the original term becomes insignificant.
The estimates using the second subsample are reported in Columns 4–6. Specifically, the policy dummy has been included in the analyses and results suggest that the mandatory price-reduction policy has shown the anticipated effect – it has significantly reduced the standardised price index. Coefficients on the key variable of interest – the number of generic sellers – are consistently negative and significant. The non-linear effect of the number of generic sellers is reported in Column 6 and it is significant in the second subsample. Combining the coefficients of the linear and quadric terms, it can be concluded that an increased number of generic sellers has a negative but decreasing marginal impact on the standardised price index. The time dummy is insignificant, suggesting that no anticipatory price effect was seen just before generic entry.
These results were then compared with the IV approach using a 2SLS estimator for robustness.G Full results are available upon request from the authors. The magnitudes of 2SLS estimates are larger than the corresponding OLS estimates reported in Table 2. For example, the 2SLS estimate on the number of competitors is –0.010 (P < 0.01) corresponding to the –0.005 (P < 0.01) in Column 4 of Table 2. This is consistent with the hypothesis that the analysis using OLS may underestimate the true effect of generic competition. The robustness results, along with the main results reported in Table 2, confirm that an increased number of generic sellers has led to a small but significant decrease in the standardised price (–0.4% to –1% per competitor, assuming the effect is linear based on OLS or 2SLS estimators respectively). Allowing for the non-linear effect, we estimate that the marginal effect of generic competition on price reduction disappears when the number of competitors reaches between 10 (2SLS) and 12 (OLS).
Finally, to assess the robustness of the results to the specification of the dependent variable, following Danzon and Chao2 and Wang,21 we also use the log (benchmark) price for item i at time t as the dependent variable (see Appendix 1). The results are comparable with those discussed above and conclusions are unchanged.
Discussion
Using 4 years of national-level data from Medicare Australia, this is the first study to empirically investigate the effect of the number of generic sellers on benchmark drug prices in Australia, allowing for the concurrent effect of the mandatory price-reduction policy implemented from August 2005.
It is concluded from the results that the number of generic sellers does appear to be a factor in driving prices down over time in this highly regulated market, contrary to the findings by Danzon et al.,2 but this effect is small. It must be remembered that this analysis concentrates on the price paid by the monopsonistic buyer in this instance (the government), and the results do not exclude the possibility of heightened competition at the retail pharmacy level (where items are reimbursed to the benchmark price but may be bought at significantly reduced prices from manufacturers and wholesalers).
Interestingly, the introduction of the first generic seller led to price rises by the branded product in many cases. This effect has also been seen in Canada, where price regulation is similar to Australia, and may be a compensatory mechanism to account for the expected loss in market share,19,22 although other factors may be important.23,24
There are several limitations to this study. It is unknown what effect ‘ultra-generics,’ or generic copies manufactured by the branded company, may have on competition and prices and an attempt to capture this has not been made. There is also no measure of consumers’ perceived difference in quality between branded and generic products. A data limitation exists whereby information on prescriptions that fall under the co-payment set by the government is not collected. This may be as high as 34% of prescriptions nationally.H,25 If the magnitude of generic competition is larger in expensive drugs, then the results of this study may overestimate the true effect.9 Additionally, no information has been included regarding the time between patent expiry and the time of entry of a generic seller onto the PBS market. Although this is not directly relevant to the question this paper seeks to address, it may provide a proxy measure of the regulatory barriers to entry of generic sellers.
There are several opportunities for future research. These include extending the current dataset sample to better understand the relationship between the number of generic sellers and the benchmark price over a longer time period and factoring in more recent policy implementation, such as the mandatory price disclosure arrangements.26 Another interesting research question would be to quantify the effect of the number of generic sellers in the market to the price paid by pharmacy over the same time period.
Finally, any price effect of increasing the number of generic sellers should not be considered in isolation from the potentially negative impact on patient outcomes. Swapping patients between an increasing number of generic alternatives may increase confusion and potentially lead to adverse events.27 In 2008 it was estimated that medication-related hospital admissions in Australia cost $660 million per annum;28 however, disentangling patient-confusion errors with other prescribing or administration errors, or adverse events, is difficult. Regardless, the economic impact of an increasing number of negative medication events related to generic substitution is worthy of further consideration.
Conclusions
Given that one of the stated policy aims of government is to increase generic competition as a way of decreasing pharmaceutical prices,14 it is important not to assume that this effect is realised without question. The average effect of a 0.4–1% price reduction per generic seller as suggested by these results on the overall PBS budget may provide significant budget savings, although the estimation of the magnitude of this effect is beyond the scope of this paper. Regardless, comparisons with the prices paid for pharmaceuticals in other countries still suggest Australia is paying more than other countries10–13 and therefore, simply relying on the effect of increasing the number of generic sellers alone to lower prices may be insufficient to produce maximal savings.
Thus, it may be prudent for the government to closely monitor the number of generic sellers in the PBS market to gauge the extent of ‘competition’, and the effect on benchmark prices over time, in order to inform future policy decisions. Further, the experience of other regulated markets, where consumers pay the full-price of medicines whose prices have fallen significantly below the benchmark price (thus effectively removing the co-payment so that competition can occur at the consumer level), is also worthy of consideration.29
From a policy perspective, it is timely to evaluate this research in light of possible future policy directions. The effect of the mandatory price disclosure arrangements, implemented in 2008,26 is still being evaluated; however, there have been suggestions that the 16% decrease when a patent expires is insufficient to achieve the prices paid in other countries.32 This may depend, to some extent, on whether structural changes can be successfully implemented to encourage generic companies to compete on the price paid by government (or directly by consumers), rather than at the pharmacy level.
Competing interests
The authors declare there are no competing interests.
Acknowledgement
We would like to acknowledge the advice provided by Prof Brett Inder in early versions of this manuscript.
References
[1] Puig-Junoy J. Impact of European pharmaceutical price regulation on generic price competition: a review. Pharmacoeconomics 2010; 28 649–63.| Impact of European pharmaceutical price regulation on generic price competition: a review.Crossref | GoogleScholarGoogle Scholar | 20515079PubMed |
[2] Danzon PM, Chao LW. Cross-national price differences for pharmaceuticals: how large, and why? J Health Econ 2000; 19 159–95.
| Cross-national price differences for pharmaceuticals: how large, and why?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FgsVGmuw%3D%3D&md5=13d81118461b56dd2da300d33a1aeec3CAS | 10947575PubMed |
[3] Caves RE, Whinston MD, Hurwitz MA. Patent expiration, entry, and competition in the U.S. pharmaceutical industry. Brookings Pap Econ Act 1991; 1–66.
[4] Grabowski HG, Vernon JM. Brand loyalty, entry, and price competition in pharmaceuticals after the 1984 Drug Act. J Law Econ 1992; 35 331–50.
| Brand loyalty, entry, and price competition in pharmaceuticals after the 1984 Drug Act.Crossref | GoogleScholarGoogle Scholar |
[5] Grabowski HG, Vernon JM. Longer patents for increased generic competition in the US: the Waxman–Hatch Act after one decade. Pharmacoeconomics 1996; 10 110–23.
| Longer patents for increased generic competition in the US: the Waxman–Hatch Act after one decade.Crossref | GoogleScholarGoogle Scholar |
[6] Frank RG, Salkever DS. Generic entry and the pricing of pharmaceuticals. J Econ Manage Strategy 1997; 6 75–90.
| Generic entry and the pricing of pharmaceuticals.Crossref | GoogleScholarGoogle Scholar |
[7] Frank RG Salkever DS 1992 Pricing, Patent Loss and the Market for Pharmaceuticals. Southern Economic Journal 59 2 165
[8] Wiggins SN, Maness R. Price competition in pharmaceuticals: the case of anti-infectives. Econ Inq 2004; 42 247–63.
| Price competition in pharmaceuticals: the case of anti-infectives.Crossref | GoogleScholarGoogle Scholar |
[9] Aalto-Setälä V. The impact of generic substitution on price competition in Finland. Eur J Health Econ 2008; 9 185–91.
| The impact of generic substitution on price competition in Finland.Crossref | GoogleScholarGoogle Scholar | 17508226PubMed |
[10] Searles A, Jefferys S, Doran E, Henry DA. Reference pricing, generic drugs and proposed changes to the Pharmaceutical Benefits Scheme. Med J Aust 2007; 187 236–9.
| 17564580PubMed |
[11] Bulfone L. High prices for generics in Australia – more competition might help. Aust Health Rev 2009; 33 200–14.
| High prices for generics in Australia – more competition might help.Crossref | GoogleScholarGoogle Scholar | 19563309PubMed |
[12] Clarke PM, Fitzgerald EM. Expiry of patent protection on statins: effects on pharmaceutical expenditure in Australia. Med J Aust 2010; 192 633–6.
| 20528715PubMed |
[13] Spinks J, Richardson J. Paying the right price for pharmaceuticals: a case study of why the comparator matters. Aust Health Rev 2011; 35 267–72.
| Paying the right price for pharmaceuticals: a case study of why the comparator matters.Crossref | GoogleScholarGoogle Scholar | 21871185PubMed |
[14] McManus P, Birkett DJ, Dudley J, Stevens A. Impact of the Minimum Pricing Policy and introduction of brand (generic) substitution into the Pharmaceutcial Benefits Scheme in Australia. Pharmacoepidemiol Drug Saf 2001; 10 295–300.
| Impact of the Minimum Pricing Policy and introduction of brand (generic) substitution into the Pharmaceutcial Benefits Scheme in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FjsVeksg%3D%3D&md5=ef89c1636e92a8b780cd25b8cb99ee7eCAS | 11760489PubMed |
[15] Löfgren H. Reshaping Australian drug policy: the dilemmas of generic medicines policy. Aust New Zealand Health Policy 2007; 4 11
| Reshaping Australian drug policy: the dilemmas of generic medicines policy.Crossref | GoogleScholarGoogle Scholar | 17543116PubMed |
[16] Department of Health and Ageing. PBS pricing. Canberra: Commonwealth Government of Australia; 2005. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-pricing-adminguide [verified 20 July 2012].
[17] Department of Health and Ageing. Pharmaceutical Benefits Scheme (PBS) reform. Commonwealth Government of Australia, Canberra; 2006. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/pbs_reform_02feb07.htm [Verified 20 July 2012].
[18] de Boer R. PBS Reform – a missed opportunity? Aust Health Rev 2009; 33 176–85.
| PBS Reform – a missed opportunity?Crossref | GoogleScholarGoogle Scholar | 19563306PubMed |
[19] Lexchin J. The effect of generic competition on the price of brand-name drugs. Health Policy 2004; 68 47–54.
| The effect of generic competition on the price of brand-name drugs.Crossref | GoogleScholarGoogle Scholar | 15033552PubMed |
[20] Australian Bureau of Statistics. Consumer price index, Australia. 2011. Available from: http://abs.gov.au/ausstats/abs@.nsf/mf/6401.0/ [Verified 9 October 2011]
[21] Wang YR. Price competition in the Chinese pharmaceutical market. Int J Health Care Finance Econ 2006; 6 119–29.
| Price competition in the Chinese pharmaceutical market.Crossref | GoogleScholarGoogle Scholar | 16783505PubMed |
[22] Kong Y. The price premium of generic to brand-names and pharmaceutical price index. Appl Econ 2004; 36 731–7.
| The price premium of generic to brand-names and pharmaceutical price index.Crossref | GoogleScholarGoogle Scholar |
[23] Doran E, Alexander HD. Australian pharmaceutical policy: price control, equity and drug innovation in Australia. J Public Health Policy 2008; 29 106–20.
| Australian pharmaceutical policy: price control, equity and drug innovation in Australia.Crossref | GoogleScholarGoogle Scholar | 18368023PubMed |
[24] Lofgren H. Generic drugs: international trends and policy developments in Australia, Working Paper No.10, Pharmaceutical Industry Project, Equity, Sustainability and Industry development; 2002. Available from: http://www.cfses.com/documents/pharma/10-Role_of_generics.PDF [Verified 20 July 2012]
[25] Department of Health and Ageing. Australian statistics on medicines, 2009. Canberra: Commonwealth Government of Australia; 2009. Available from: http://www.health.gov.au/internet/main/Publishing.nsf/Content/pbs-asm-2009 [Verified 5 January 2013]
[26] Department of Health and Ageing. Policies, procedures and methods used in the recommendations for pricing of pharmaceutical products; 2009. Canberra: Commonwealth Government of Australia. Available from: http://www.health.gov.au/internet/publications/publishing.nsf/Content/health-pbs-pbpa-pricing-policiesdoc~attachF-further [Verified 2 August 2012]
[27] Kalisch L, Roughead E, Gilbert A. Brand substitution or multiple switches per patient? An analysis of pharmaceutical brand substitution in Australia. Pharmacoepidemiol Drug Saf 2008; 17 620–5.
| Brand substitution or multiple switches per patient? An analysis of pharmaceutical brand substitution in Australia.Crossref | GoogleScholarGoogle Scholar | 18324613PubMed |
[28] Roughead E, Semple S. Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008. Aust New Zealand Health Policy 2009; 6 18
| Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008.Crossref | GoogleScholarGoogle Scholar | 19671158PubMed |
[29] Godman B, Hayxoc A, Schwabe U, Joppi R, Garattini S. Having your cake and eating it: office of fair trading proposal for funding new drugs to benefits patients and innovative companies. Pharmacoeconomics 2008; 26 91–8.
| Having your cake and eating it: office of fair trading proposal for funding new drugs to benefits patients and innovative companies.Crossref | GoogleScholarGoogle Scholar | 18198930PubMed |
[30] Wooldridge JM. Econometric analysis of cross section and panel data. Cambridge, MA: The MIT Press; 2002.
[31] Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica 1997; 65 557–86.
| Instrumental variables regression with weak instruments.Crossref | GoogleScholarGoogle Scholar |
[32] Duckett SJ, Breadon P, Ginnivan L, Venkataraman P. Australia’s bad drug deal: high pharmaceutical prices. Melbourne: Grattan Institute; 2013.
A AThis was superseded on 1 August 2007 with a more complex price reduction scheme, known as ‘PBS Reform’. The thrust of this initiative is that the government is aware of the cost savings being offered to pharmacies by manufacturers and wholesalers, which are thought not to be flowing on to reduced prices paid by government.17,18
B BData are not collected nationally for these items.
C CWith 76 items, spanning 48 months, we expect 3648 observations. However, as 5 medicines appear for the first time on the PBS after the start of the panel, there are 3592 observations available for analysis.
D DFour months was chosen as the appropriate time frame as earlier time periods of the panel saw PBS price changes only three or four times a year.
E ELog price may also be used following Danzon and Chao2 and Wang.20 We test the robustness of results to the specification of the dependent variable.
F FThe 2SLS provides consistent parameter estimates and it involves two steps: first, the endogenous variable (the number of generic sellers) is regressed on the instrument variable (market size) and all other exogenous variables in the equation; second, the predicted number of generic sellers from the first step is used to replace the observed number of generic sellers s in the equation. OLS estimator is used in both two steps and standard errors in the second step are adjusted. A detailed explanation of the theoretical background to the model can be found in Wooldridge.29 When the squared term of endogenous variable is also included, we need at least one more IV to help identify the equation. Following Wooldridge,29 we use the squared term of the predicted number of generic sellers in the first step as an additional IV.
G GThe first-stage F-statistics of instrument is 21.26, higher than 10, the Staiger–Stock rule of thumb,31 suggesting the IV is not weak. We test for regressor endogeneity by performing a Hausman test following Wooldridge.30 The test statistic suggests that number of competitors is endogenous.
H HIn 2009 (the latest data publically available), it was estimated by survey that 34% of all prescriptions were classified either as under co-payment, repatriation or private.24 As disaggregated figures for each of these subcategories were not provided in the report, it is unclear what percentage falls under co-payment alone (repatriation falls under a separate budget and private prescriptions are a consumer out of pocket expense).





