Genetics of steer daily and residual feed intake in two tropical beef genotypes, and relationships among intake, body composition, growth and other post-weaning measures
S. A. Barwick A B E , M. L. Wolcott A B , D. J. Johnston A B , H. M. Burrow A C and M. T. Sullivan A DA Cooperative Research Centre for Beef Genetic Technologies, Armidale, NSW 2351, Australia.
B Animal Genetics and Breeding Unit1, University of New England, Armidale, NSW 2351, Australia.
C CSIRO Livestock Industries, Rockhampton, Qld 4702, Australia.
D Queensland Department of Primary Industries and Fisheries, PO Box 1333, Mount Isa, Qld 4825, Australia.
E Corresponding author. Email: steve.barwick@dpi.nsw.gov.au
Animal Production Science 49(6) 351-366 https://doi.org/10.1071/EA08249
Submitted: 8 October 2008 Accepted: 24 February 2009 Published: 13 May 2009
Abstract
Genetic parameters for Brahman (BRAH) and Tropical Composite (TCOMP) cattle were estimated for steer production traits recorded at weaning (WEAN), 80 days post-weaning (POSTW), feedlot entry (ENTRY) and after ∼120 days feedlot finishing (EXIT). The TCOMP was 50% Bos indicus, African Sanga or other tropically adapted Bos taurus, and 50% non-tropically adapted Bos taurus. Data involved 2216 steers, comprising 1007 BRAH by 53 sires and 1209 TCOMP by 50 sires. Individual daily feed intake (DFI) and residual feed intake (RFI) were assessed on 680 BRAH and 783 TCOMP steers over an ~70-day feedlot test. Other traits were liveweight (LWT), average daily gain (ADG), ultrasonically scanned rump (SP8) fat depth, rib (SRIB) fat depth, M. longissimus area (SEMA) and intra-muscular fat % (SIMF), body condition score (CS), hip height (HH), flight time (FT) and serum insulin-like growth factor-I concentration (IGF-I).
BRAH were significantly (P < 0.05) lighter at ENTRY and EXIT, and had lower DFI (10.8 v. 13.2 kg/day) and RFI (–0.30 v. 0.17 kg/day), greater SP8 (5.8 v. 5.1 mm) but similar SRIB at ENTRY, lower SRIB (8.2 v. 8.9 mm) but similar SP8 at EXIT, and greater HH than TCOMP. Heritabilities for DFI, RFI, LWT, ADG, scanned body composition, HH and IGF-I measures, across measurement times, were generally in the 20 to 60% range for both genotypes. Genetic variance for RFI was 0.19 (kg/day)2 in BRAH and 0.41 (kg/day)2 in TCOMP, suggesting a clear potential to genetically change RFI in both genotypes. Trait variances and genetic correlations often differed between the genotypes, supporting the use of genotype-specific parameters in genetic evaluation. The genotype differences may be associated with evolutionary changes that have occurred in B. indicus as a part of their adaptation to tropical environments.
Measures with potential to be used as genetic indicators of DFI were LWT measures in BRAH and TCOMP, ADG at ENTRY in TCOMP, and SP8 and SIMF at ENTRY in BRAH. Measures with potential to be genetic indicators of RFI were HH and ADG at ENTRY in BRAH, and IGF-I in both genotypes. Taller and faster-growing BRAH steers at ENTRY had genetically lower RFI. IGF-I was negatively genetically correlated with RFI whether IGF-I was measured at POSTW, ENTRY or EXIT. SRIB fatness at EXIT was strongly positively genetically correlated with RFI in TCOMP but only lowly correlated in BRAH. Fatness at ENTRY was lowly and negatively genetically correlated with RFI. The results emphasise the need for a population-specific understanding of trait relationships and of trait differences between measurement times if genetic indicator traits are to be utilised in genetic evaluation of RFI.
Additional keywords: adaptation, correlations, genotype × environment, heritability, IGF-I, variance components.
Introduction
The genetic knowledge available to support beef cattle breeding for tropical environments is currently inadequate in several respects. Genetic parameter estimates available are mostly for young animal growth (Kriese et al. 1991; Robinson and O’Rourke 1992; Eler et al. 1995; Johnston et al. 2003) and carcass traits (Moser et al. 1998; Riley et al. 2002; Reverter et al. 2003). There are few reports on feed intake in tropical cattle (Frisch and Vercoe 1969). Tropical environments are challenging because they are often characterised by heat, humidity, parasites and deficiencies of ‘dry’ season feed. The need for a greater understanding of trade-offs among traits is a key issue in these environments so that productivity gains can be made without compromising the ability of tropically-adapted animals to survive and reproduce (Burrow et al. 2003).
A study of young animal and cow performance traits was initiated in Brahman (BRAH) and Tropical Composite (TCOMP) cattle in northern Australia to better meet these needs. The TCOMP encompasses genotypes derived 50% from tropically adapted and 50% from non-tropically adapted breeds. This paper reports on genetic and phenotypic correlations, variances and means for live steer production traits including, as a particular focus, individual daily (DFI) and residual (RFI) feed intake. RFI is a measure of the efficiency of feed use that is being actively researched for use in genetic evaluation (Archer et al. 1999; Arthur et al. 2004; Herd et al. 2003). A further aim was to evaluate the potential of measures, including insulin-like growth factor-I concentration (IGF-I), as genetic indicators of RFI. Genetic indicators of feed efficiency are needed in virtually all species as a consequence of the cost and difficulty of measuring actual feed intake on individual animals. Moore et al. (2005) provided evidence that IGF-I could be a useful genetic indicator of RFI in temperate beef cattle. Robinson and Oddy (2004) showed fatness of the animal to be a potential indicator.
Materials and methods
Animals
BRAH and TCOMP steers were bred in northern Australia in 1999 through 2003 on seven cooperating properties (4 BRAH and 3 TCOMP) and the CSIRO ‘Belmont’ research station (both BRAH and TCOMP), using artificial insemination (AI) and natural service. At weaning, there were 2216 steers (1007 BRAH, 1209 TCOMP), representing 53 BRAH and 50 TCOMP sires. Steers that were born and raised together at Belmont represented 32 BRAH and 27 TCOMP sires. The use of AI to breed calves generated genetic linkage across properties of origin and years, and across AI and natural service calvings within a genotype. Additional linkage was generated by re-using natural service and AI backup sires across years. Linkage statistics, and the distribution of steers at weaning by genotype, property of origin and year weaned, are in Table 1.
On average, the 50% tropically adapted component of the TCOMP is about one-half derived from the Bos indicus Brahman and one-half from the African Sanga (Frisch et al. 1997) (24% Africander) or other adapted B. taurus (2% N’Dama, through the Senepol). The non-tropically adapted component derives from non-tropically adapted B. taurus. The majority (~58%) of the TCOMP population studied were a stabilised composite (F2 or later generation) that was either the Belmont Red or the ‘Alexandria’ composite of the North Australian Pastoral Co. The genetic composition of each of these is ~50% tropically adapted and 50% non-tropically adapted. The Belmont Red emanated from the Africander, Brahman, Shorthorn and Hereford breeds (Rudder et al. 1976; Turner 1977). Breeds in the Alexandria composite are the Brahman, Shorthorn, Belmont Red and Charolais. The remaining 42% (also 50% tropically adapted, 50% non-tropically adapted) were by Belmont Red, Alexandria composite, Australian Agricultural Co. composite and Belmont Red-infused sires from other composite and Belmont Red × Brahman dams, and were considered an F1 type. Breeds in the background to these F1 types include the Brahman, Belmont Red, Santa Gertrudis, Senepol, Charolais and Red Angus.
AI sires representing a range within each genotype were sourced from industry seedstock herds and from sires represented in earlier data (Newman et al. 2002; Johnston et al. 2003). Natural service sires were sourced either from industry seedstock herds or from sires bred or purchased by the cooperating properties.
Environment
Properties of origin were located throughout central and north-western Queensland and the Northern Territory in tropical and subtropical Australia. Following weaning, steers were allocated to other properties in central Queensland and northern New South Wales where they were grown to feedlot entry weight. Allocation of steers to post-weaning properties was on a within property of origin and sire basis to maintain genetic linkage, but otherwise at random. Steers that were born and raised together at Belmont were allocated similarly but also so that BRAH and TCOMP steers from Belmont were run together and managed similarly throughout life.
At each post-weaning property, steers weaned in the same year were defined as a cohort. The distribution of steers to cohorts (by genotype, property of origin and year), and linkage statistics for cohorts, are also in Table 1. Steer cohorts were managed as single groups throughout grow-out and finishing without drafting or culling, except for some splitting for allocation to groups of feedlot pens for finishing. At these times, care was taken to retain genetic links across newly-formed groups. Following grow-out, steers were trucked to the Beef Cooperative Research Centre (CRC) ‘Tullimba’ research feedlot west of Armidale in northern New South Wales for finishing.
The period from weaning to feedlot entry for cohorts averaged 15.5 months and ranged from 10.0 to 24.6 months. The growth rate of cohorts over this period averaged 0.55 kg/day and ranged from 0.35 to 0.89 kg/day. Because of drought, one of the initially-formed cohorts (cohort 3; Table 1) was supplementary fed a low energy ration for six weeks before feedlot entry. The growth rate of cohorts in the feedlot averaged 1.40 kg/day and ranged from 0.98 to 1.72 kg/day.
Management and treatment
On each property of origin, date of birth, calf sex, dam identification number and dam year of birth were recorded. Sire parentage was determined by DNA fingerprinting (Vankan 2005). Management of steers followed accepted industry practice. Steers were dehorned, branded and castrated before weaning. Weaning was at ~6.5 months of age, near the start of the annual tropical ‘dry’ season (June to November). Each year, weaned steers were assembled at central locations before being trucked to their allocated post-weaning properties. They were vaccinated twice against clostridial diseases in their first year, including at weaning, and then vaccinated annually. Steers were treated with Compudose 200 (Elanco, West Ryde, Australia; active ingredient oestradiol 17β) after arrival at post-weaning properties, and subsequently at ~200-day intervals. Over much of the ‘dry’ season each year, steers at pasture had access to a urea-based dry lick delivering ~75 g crude protein equivalent/day per 450 kg adult equivalent. One cohort of 97 steers, early-weaned in June 2002, was fed a commercial weaner meal between June and August 2002. Tick levels of steers were monitored, and steers were dipped with an acaracide only when this was required for movement into New South Wales.
Steers entered the feedlot when the average of their cohort was ~400 kg. At entry, steers were treated with a broad spectrum anthelmintic and with Compudose 100. Age at entry varied with season and with feedlot and feeder pen availability. The average age of cohorts at entry was 22.0 months and ranged from 16.5 to 29.0 months. Steers were fed for an average of 119 days to a finished average liveweight of 568 kg. They were given a 2–3 week introduction to the feedlot ration, over which time the proportion (by weight) of dry rolled barley grain in the diet was increased from 40% to 80% and roughage reduced from 50% to 10%. The standard finisher feedlot ration comprised of (by weight) 80% dry rolled barley grain, 10% milled sorghum hay, 8% Molafos (Ridley, Wacol, Qld, Australia, providing 0.8% urea and 25 mg/kg Monensin sodium, trace minerals and vitamins), 1% limestone, 0.5% ammonium sulfate and 0.5% sodium bicarbonate. The estimated energy density of the ration was 12.2 MJ ME /kg dry matter (DM), crude protein % (w/w) was 16.25, and DM was 87%.
Measurements
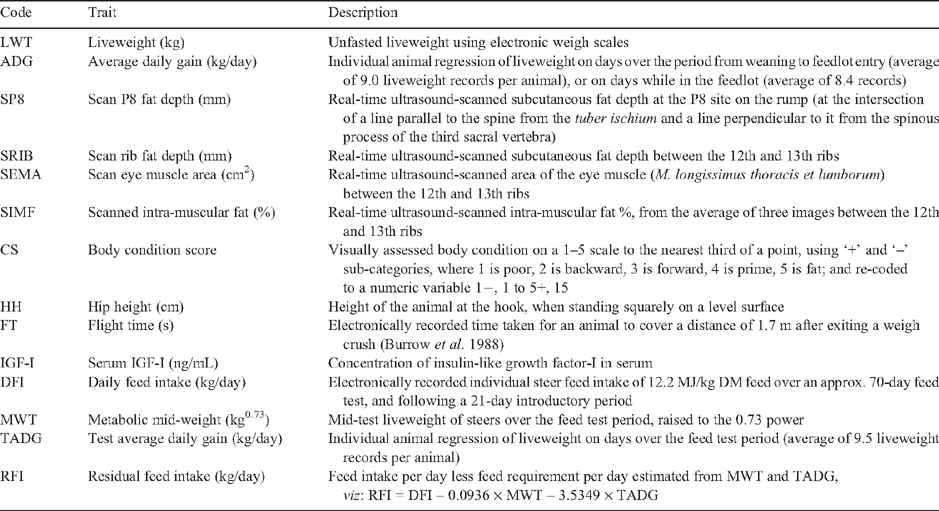
Details of measurements recorded on individual steers are in Table 2. In brief, measures of liveweight (LWT), ultrasonically scanned fat depth at the rump P8 site (SP8) and the 12/13th rib (SRIB), scanned area of M. longissimus thoracis et lumborum at the 12/13th rib (SEMA), scanned intra-muscular fat % (SIMF), body condition score (CS), hip height (HH), flight time (FT) (Burrow et al. 1988) and serum insulin-like growth factor-I concentration (IGF-I) were taken at intervals from weaning to feedlot exit. The measurement times reported on were at weaning (WEAN), ~80 days post-weaning (POSTW), feedlot entry (ENTRY) and feedlot exit (EXIT). Ultrasound measurements were taken by accredited technicians using a commercially available scanner (Pie Medical 200 SLC with 18 cm 3.5 MHz ASP-18 transducer; Pie Medical, Maastricht, The Netherlands). IGF-I was measured using a commercially available enzyme-linked immunosorbent assay and associated sampling, as described by Moore et al. (2005).
|
For a subset of steers, DFI was measured on individual steers in pens fitted with purpose-built automatic feeders. The ration for measurement of DFI was the same standard finisher feedlot ration (12.2 MJ ME/kg DM, 16.25 crude protein % (w/w), 87% DM) already described. Feeders were fitted with electronic data recording and there were usually 12 steers per pen. Steers were allowed a 3-week introduction to the feeders. Shy feeders were removed and given one additional chance to adjust before being removed from the feeding test. The recording of DFI was over an average 71.6-day (range 63 to 80 day) test period (FEEDTEST). Data were summarised into daily totals for amount of feed eaten. Steers in the feeder pens were weighed approximately weekly, with an average of 9.5 LWT records per steer over the test period. These weights were used to determine metabolic mid-test weight (MWT; i.e. mid-test LWT0.73) and test average daily LWT gain (TADG) for steers. TADG was calculated from individual steer regressions of LWT on days in the feed test. Mid-test LWT was calculated from LWT at the start of the test, TADG and the number of test days to mid-test. All other steers were bunk fed in standard feedlot pens, and LWT of these steers was recorded monthly.
Residual feed intake (RFI) was calculated as the difference between the daily feed intake (DFI) of steers and the expected feed requirement of steers based on their MWT and TADG (Table 2). The partial regression coefficients needed for estimating feed requirement were first assessed by multiple regression of DFI on MWT and TADG, in a model that also included genotype, other significant fixed effects (including cohort), and sire as a random effect. The partial regressions were thus determined after adjustment for genotype and other effects. The other significant fixed effects included were identified in the same manner as described below for all traits. The assessed partial regression coefficients were similar for BRAH and TCOMP, i.e. interactions of genotype with MWT and TADG were not significant (P > 0.05). Intercepts from the regression model were ignored in the definition of RFI as these have no effect on resulting estimates of variance components (Robinson and Oddy 2004). The mean intercept (a constant) was included for tabulating genotype means, with the trait then being denoted as RFI*. Values for RFI and RFI* differed only by this constant.
Average daily gain (ADG) was also assessed from weaning to feedlot entry, and over the whole feedlot period, from individual steer regressions. These gains were assessed for all steers, and were based on an average of 9.0 and 8.4 LWT records per steer, respectively.
Statistical analyses
Fixed effect modelling
Initial editing identified a small number of outlier records more than three standard deviations from their contemporary group mean. These usually affected less than five steers for a trait and were excluded from analyses. In addition, for DFI data, records on 19 BRAH and 6 TCOMP steers were excluded where TADG was <0.30 kg/day, following Robinson and Oddy (2004).
Fixed effect modelling for each trait was first carried out for BRAH and TCOMP individually, using PROC MIXED in SAS (SAS Institute, Cary, NC, USA), with all models including sire as a random effect. Main effects of property of origin, age of dam (in years), birth month, cohort, and all first-order interactions were initially fitted and non-significant effects (P > 0.05) then systematically removed. Birth month included effects of season as well as age effects. Cohort was formed by concatenating year and post-weaning location levels. For TCOMP analyses, sire group (6 levels), dam group within herd of origin, and their interaction were additional effects fitted to account for average differences among differing combinations of sire groups and dam groups.
Significant fixed effects for each trait were also identified for use in analyses of the data combined over genotypes. Models in this case initially included the fixed effects identified for individual genotypes along with the addition of genotype and all first-order interactions with genotype. Non-significant terms (P > 0.05) were systematically removed to identify the fixed effects for use in analyses of the combined genotypes.
Mean prediction
Model predicted means for BRAH and TCOMP, for each trait, were derived from final analyses of combined genotype data using the mean prediction procedure of ASReml, averaging over the other fixed effect levels present (Gilmour et al. 2004). The genotype means predicted were for those steers born and raised together at the ‘Belmont’ property of origin. Selected genotype × cohort means were also derived where this interaction was significant. The data for 15 BRAH and 2 TCOMP late-born steers were excluded from the mean predictions to avoid averaging over unequal or poorly represented birth months. Because there was a predominance of Belmont Red dams at Belmont, the means predicted for TCOMP are for a sample of the genotype where the contribution of Africander to the tropically adapted component is higher (40% Africander, 1% N’Dama, 10% Brahman) than applied in the whole data.
Variance component estimation
Restricted maximum likelihood estimates of BRAH-specific and TCOMP-specific variance components were derived for each trait from univariate analyses using ASReml (Gilmour et al. 1999). The basic model describing records was:

where y is an n × 1 vector of records, n is the number of records, b is a p × 1 vector of fixed effects, p is the number of levels of fixed effects, u is a q × 1 vector of random animal additive genetic effects, q is the number of levels of animal effects, c is an r × 1 vector of random common environmental effects of the dam, e is an n × 1 vector of random residual effects, X is a known incidence matrix of order n × p relating records to fixed effects, Z is a known incidence matrix of order n × q relating records to random animal effects, and W is a known incidence matrix of order n × r relating records to random common environmental effects of the dam. For TCOMP analyses, the inclusion of sire group, dam group, and their interaction, where these were significant from the fixed effect modelling, removed the average effect of any composite group with the potential to be expressing new heterosis. No other adjustment for heterosis was made. Relationships among the random animal additive genetic effects were described by the numerator relationship matrix (A) derived from pedigree. Other random effects were assumed to be uncorrelated.
Components estimated were the additive direct genetic variance (σ2A), common dam environmental variance (σ2C) and residual variance (σ2E), all components of the phenotypic variance (σ2P). All analyses were run both with the above base model and with the same model excluding the common dam environmental component. The best-fitting of these models was decided on a trait-by-trait basis by likelihood ratio test. Results are presented only for the best-fitting model.
Genetic and phenotypic correlations were derived from bivariate ASReml analyses. Components estimated were the additive genetic covariance (σA1,2) and residual covariance (σE1,2) between traits 1 and 2, and σ2A, σ2C and σ2E for each trait. The genetic and residual covariances sum to the phenotypic covariance (σP1,2), genetic correlations are determined as σA1,2/(σA1.σA2), and phenotypic correlations as σP1,2/(σP1.σP2). The models for the bivariate analyses were those identified from the univariate analyses except for analyses involving weaning weight. For those analyses, the common environmental effect of the dam was always included for both traits to aid convergence.
The bivariate analyses were undertaken separately for each genotype and then also for the combined genotypes. The genotype-specific estimates are presented where the estimates for BRAH and TCOMP differed by more than the sum of the standard errors of the individual estimates. In other cases, only the estimates for the combined genotypes are presented.
Results
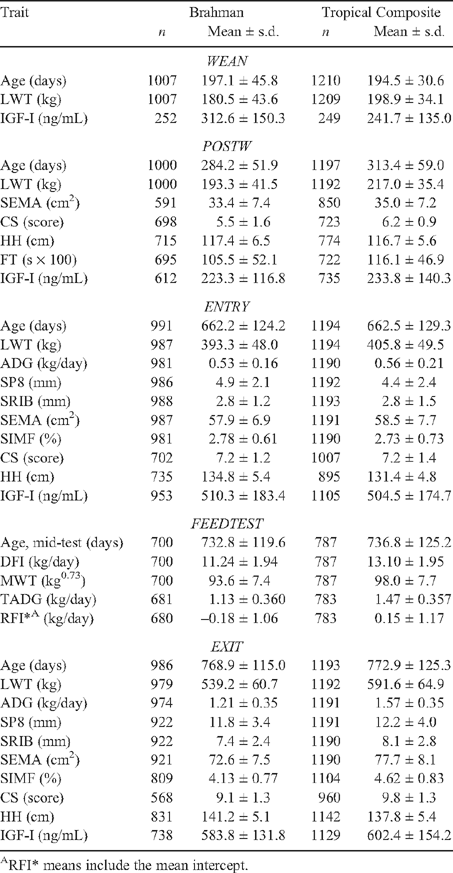
Means and standard deviations for the measures studied are in Table 3. There were insufficient FT records at ENTRY and EXIT for analysis, and mean fatness levels at WEAN and POSTW were too low to be informative. The feed consumed per kg of LWT gain (i.e. feed conversion ratio; not tabulated) over the feed test period averaged 10.1 kg (9.4 for TCOMP, 11.0 for BRAH), or 8.8 kg (8.2 for TCOMP, 9.5 for BRAH) on a DM basis.
|
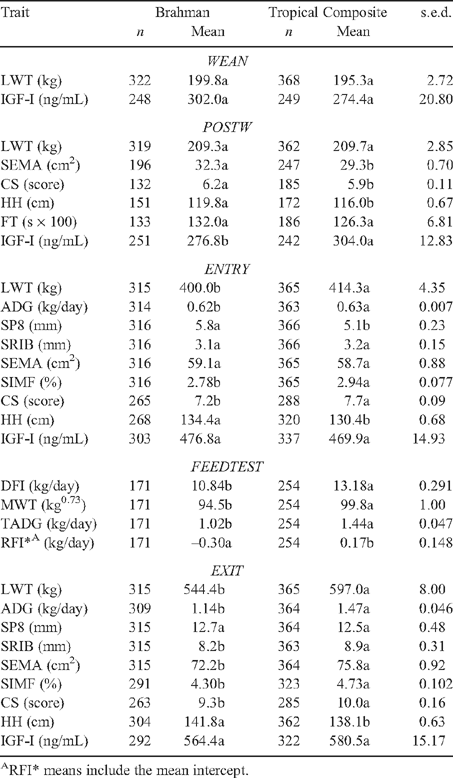
Model predicted means for BRAH and TCOMP are in Table 4. TCOMP was significantly (P < 0.05) heavier and faster growing than BRAH at ENTRY, FEEDTEST and EXIT, and had higher IGF-I at POSTW, CS at ENTRY and EXIT, DFI at FEEDTEST, and SEMA at EXIT. BRAH had greater CS and SEMA than TCOMP at POSTW, greater HH at all measurement times, and lower RFI (P < 0.05). At ENTRY, SP8 fatness was greater in BRAH than in TCOMP, while at EXIT SRIB fatness was greater in TCOMP than in BRAH (P < 0.05). SIMF was greater in TCOMP than in BRAH at both ENTRY and EXIT (P < 0.05).
|
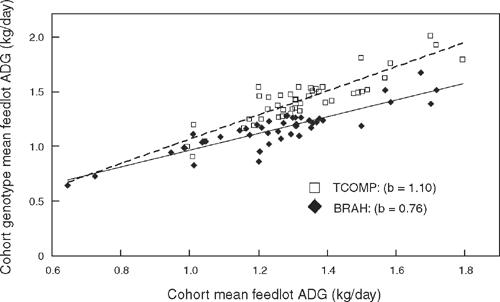
Genotype × cohort was significant (P < 0.05) for all ENTRY measures and for ADG at EXIT, and the effect interacted with birth month for ADG, SP8, SRIB, CS and HH at ENTRY. A plot of cohort genotype means against cohort means showed ADG at EXIT increased more rapidly with cohort level for TCOMP than for BRAH (Fig. 1). The effect of birth month was significant (P < 0.05) for all EXIT measures except ADG and IGF-I, for FEEDTEST measures except for TADG and RFI, and for all earlier measures.
|
Trait variances and variance ratios are in Table 5. Phenotypic variances and the percentage of the observed variance that the phenotypic variance represented (P%) both were generally smaller for BRAH than for TCOMP. P% tended to be lower (also indicating the percentage of the observed variance explained by fixed effects was correspondingly higher) for measures earlier in life, particularly in BRAH. Heritabilities for DFI, RFI, LWT, ADG, scanned body composition, HH and IGF-I measures were generally in the 20 to 60% range for both BRAH and TCOMP. Heritability of SEMA was sometimes low in BRAH (6% at POSTW, 10% at EXIT), while that for CS was low in BRAH at POSTW (7%), and low in both genotypes at ENTRY (7% in BRAH, 3% in TCOMP). In line with the trend in phenotypic variances, genetic variances tended to be lower in BRAH than in TCOMP. Exceptions were IGF-I measures, HH at ENTRY and EXIT, and some fatness measures at ENTRY. The common dam environmental effect was significant and hence included in the model for several growth traits and some fatness traits of TCOMP, and for SP8 at ENTRY for BRAH (Table 5).

|
Genetic and phenotypic correlations among steer measures at WEAN, POSTW and ENTRY are in Table 6. Estimates involving CS at POSTW and ENTRY are not presented because their standard errors were high. Correlations relating WEAN, POSTW and ENTRY measures to FEEDTEST and EXIT measures are in Tables 7 and 8. Similar correlations among FEEDTEST and EXIT measures are in Table 9. All of the results of Tables 6–9 are for combined genotypes. Genotype-specific correlations are in Table 10.

|

|

|

|

|
There was wide variation in the degree of correlation among WEAN, POSTW and ENTRY measures. For example, LWT and HH were highly genetically related, especially in TCOMP and at younger ages, whereas LWT and fatness were lowly and quite variably related (Tables 6 and 10). The high correlations suggest LWT at WEAN and POSTW, and HH at POSTW and ENTRY, are genetic and phenotypic indicators of LWT at ENTRY (Table 6). LWT at POSTW was less related to LWT at ENTRY in BRAH than in TCOMP; and greater HH at POSTW was more correlated with increased ADG to ENTRY in BRAH (Table 10). IGF-I at POSTW was genetically strongly and positively related to fat and muscle measures at ENTRY and unrelated to LWT at ENTRY (Table 6).
The capacities for WEAN, POSTW and ENTRY measures to be genetic and phenotypic indicators of FEEDTEST and EXIT measures are shown in the correlations in Tables 7 and 8. The measures most genetically correlated with DFI were LWT at each measurement time and ADG at ENTRY, the latter more so in TCOMP (0.75 ± 0.14) than in BRAH (0.25 ± 0.27) (Table 10). Fatness at ENTRY was negatively genetically correlated with DFI, especially in BRAH (Tables 7 and 10). The most genetically correlated indicators of RFI were ADG (–0.68 ± 0.30) and HH (–0.56 ± 0.26) at ENTRY in BRAH. Measures of IGF-I at POSTW and ENTRY were genetically negatively correlated (about –0.30) with both DFI and RFI (Table 7).
The best genetic indicator of EXIT traits tended to be the corresponding measure at ENTRY (Table 7). The genetic correlations involved, though high, quite often were different from unity (e.g. LWT 0.87 ± 0.04, ADG 0.64 ± 0.15 in TCOMP). The genetic correlation between ADG at ENTRY and EXIT in BRAH was low (0.10 ± 0.27) (Table 10). Genetic correlations between corresponding measures at POSTW and EXIT were lower (Table 7), in line with the greater age difference between POSTW and EXIT than between ENTRY and EXIT (Table 2). Genetic correlations between earlier HH measures and HH at EXIT were not different from unity (Table 7).
Of the measures assessed at or before ENTRY, the measure most phenotypically correlated with DFI was LWT (range from 0.31 to 0.42). Phenotypic correlations with RFI were low for all measures; and there was no phenotypic association between FT at POSTW and either DFI or RFI (Table 8). Trends for phenotypic correlations with EXIT measures closely followed those for genetic correlations, but with the phenotypic correlations being generally lower (Tables 7 and 8).
Genetic correlations among FEEDTEST and EXIT measures (Table 9) were mostly positive except for those involving IGF-I. The association between LWT and fatness (~0.40) was the only obvious antagonism. Among FEEDTEST measures (Table 9), DFI was genetically highly correlated with both MWT and TADG. Genetic correlations of RFI with MWT and TADG were low and of about equal size for the two components. Lower RFI was genetically associated with lower DFI as a consequence of RFI and DFI being positively genetically correlated (0.59 ± 0.12). Lower RFI was genetically associated with greater HH at EXIT especially in BRAH (–0.61 ± 0.23), with lower fatness at EXIT especially for SRIB in TCOMP (0.60 ± 0.18), and with higher IGF-I at EXIT (–0.56 ± 0.18) (Tables 9 and 10). Trends in phenotypic correlations among FEEDTEST and EXIT measures closely followed those for genetic correlations, but with the phenotypic correlations again being lower (Table 9).
Discussion
Mean performance of Brahman and Tropical Composite
The results of Table 4 suggest production advantages for TCOMP over BRAH steers under feedlot finishing. BRAH steers, however, consumed less feed, had a lower RFI, and deposited less fat. The lower RFI suggests a greater efficiency of feed use for BRAH for the same MWT and TADG, which is in contrast with the higher observed feed conversion ratio for BRAH. RFI and feed conversion ratio are quite different traits (Archer et al. 1999); Robinson and Oddy (2004) reported a genetic correlation between them of 0.41 in mixed breeds, and Arthur et al. (2001) found the correlation to be 0.66 in Angus. In the feed conversion ratio trait, there is no consideration of the feed required to maintain liveweight. Selection studies have also shown feed conversion ratio to reflect mostly genetic differences in the amount of gain (Mrode et al. 1990; Bishop et al. 1991).
In a small study in bulls and steers, Frisch and Vercoe (1969) found Brahmans consumed less feed at the same liveweight, and gained more liveweight at the same feed intake, than Africanders and Shorthorn × Herefords, and they suggested the Brahman may have a lower maintenance requirement. While we found BRAH consumed less feed for the same liveweight and gain, we did not find a significant difference between BRAH and TCOMP in the partial regressions of DFI on either MWT or TADG. The RFI difference between BRAH and TCOMP therefore exists despite the genotypes being similar in the amount of feed they require for change in metabolic weight at the same liveweight gain and for change in liveweight gain at the same metabolic weight.
There are numerous possible biological mechanisms for variation in efficiency of feed utilisation in beef cattle, reviewed by Herd et al. (2004). While the mechanisms for the present RFI difference between BRAH and TCOMP are not known, our other differing results for the genotypes could be pointers to some of the contributors. One of these is the difference in DFI between the genotypes. The observed 0.59 genetic correlation between DFI and RFI can be interpreted as indicating DFI explains 35% of the within-genotype genetic differences in RFI, so it is probable that it also accounts for some part of the RFI difference between the genotypes. The Bos indicus BRAH may have evolved an ability to restrict feed intake as a part of its adaptation to tropical environments. Reduced intake is a known means for lowering body heat production (Blackshaw and Blackshaw 1994), and Robinson and Oddy (2004) found BRAH, and to a lesser extent other tropically-adapted genotypes, ate more frequent and smaller meals than temperate genotypes. It should be noted that the DFI and RFI differences between the genotypes need not be causally related even if they are genetically associated, as both could have arisen from some more fundamental biological difference. Whatever the precise cause, the apparent efficiency difference between the genotypes is likely to have arisen as a part of the adaptations of B. indicus and Bos taurus to the very different environments they have encountered since their evolutionary divergence. Loftus et al. (1994) showed it is probably more than 200 000 years since cattle of Indian origin diverged from those of European and African origin, and that there may even have been independent domestications.
Greater growth was observed in TCOMP steers than in BRAH steers at pasture, as evidenced by TCOMP steers being 20 kg heavier at ENTRY (Table 4). In a study of related genotypes under conditions less suited to growth, Prayaga (2003) found no significant growth disadvantage for Brahman. Growth differences in the present study were more evident in the feedlot (TCOMP were 55 kg heavier at EXIT), especially reflecting the genotype difference in DFI (Table 4). The DFI and feedlot growth differences were also reflected in scanned body composition differences at EXIT, which were also generally in line with carcass trait differences from the same experiment (Wolcott et al. 2009).
The BRAH and TCOMP differences in SP8 and SRIB fatness, and in HH and LWT (Table 4), may also have their origins in evolutionary differences between B. indicus and B. taurus, and particularly in adaptations of B. indicus to tropical environments. Ledger (1959) observed that B. indicus deposit relatively more of their fat as intermuscular fat than B. taurus, and suggested the heat tolerance of B. indicus could be linked to a lesser subcutaneous fat deposition. Burrow et al. (1991a) showed some other fat depot differences to be genetically associated with rectal temperature, and Johnston et al. (2003) observed tropical genotypes had more subcutaneous rump P8 fat relative to rib fat than temperate genotypes. In the present study, there were differences between BRAH and TCOMP in both amount and distribution of subcutaneous fat. At EXIT, when fat levels were high, BRAH had less fat than TCOMP over the rib (P < 0.05), close to the heat producing organs and viscera, but similar fat over the rump, away from these sources of heat (Table 4). The greater HH and lower LWT of BRAH, taken together, could also indicate a greater capacity for heat loss in BRAH, as these effects are consistent with a possible difference in body surface area per unit of weight (Gaughan et al. 1999).
BRAH were also observed to have more fat over the rump and similar fat over the rib than TCOMP at ENTRY (Table 4), i.e. after an extended period grazing variable quality pasture. This suggests BRAH have a greater ability to store fat (on the rump) in the growing animal at pasture, a time when animals would otherwise be lean. This could have important ramifications for whole herd performance if it is also true of the breeding female, as BRAH and TCOMP cow herds have to perform in environments that are consistently more limiting and stressful than steers were exposed to here. These aspects will be examined in a later report.
Evidence for genotype × environment interaction
Genotype × environment interactions commonly occur when breeds or breed-crosses are compared across variable environments (Thompson et al. 1981; Darnell et al. 1987; Arthur et al. 1994), and in our study, they were indicated by the presence of significant genotype × cohort effects. The cohort mean level for a trait was taken as the measure of environment level. The fact that we observed significant genotype × cohort effects for many measures at and before ENTRY indicates that the BRAH and TCOMP differences we saw before feedlot finishing could change under differing environmental conditions.
Fig. 1 illustrates the genotype × cohort effect observed for ADG in the feedlot. It shows there was a significant tendency for ADG to increase more rapidly with environment level in TCOMP than in BRAH. It also shows the TCOMP advantage in feedlot ADG is expected to be greater when conditions favour greater growth rate, and less when growth conditions are poorer. Note that cohort effects within a genotype are better estimated in our study than are genotype effects across cohorts, as the genetic linkage between cohorts was greater than that between properties of origin (Table 1). Our ability to compare genotypes across cohorts derives from the complete ability to compare genotypes at the Belmont property of origin and the very good ability to compare cohorts within each of the genotypes.
Genetic correlations between growing and finishing
Genetic correlations between corresponding measures taken at ENTRY and EXIT often differed from unity (Tables 7 and 10), supporting the treatment of these as different traits where they are encountered in genetic evaluation. The trait difference between ENTRY and EXIT reflects both the differing growth stage of steers and the differing environments that were associated with these measurement times. Our genetic correlation results for combined genotypes were similar to those reported by Johnston et al. (2003) for mixed breeds, except for fat measures; results for fat measures between the start and end of finishing were lower than those of Johnston et al. (2003) (0.55 compared with 0.82 for SRIB, 0.70 compared with 0.84 for SP8).
The genetic correlation between ADG at ENTRY and ADG at EXIT was low in BRAH (0.10 ± 0.27) and 0.64 ± 0.15 in TCOMP. Mackinnon et al. (1991) observed extremely low genetic correlations between weight gains at different times (e.g. –0.02 between pre- and post-weaning weight gains) in stabilised zebu × B. taurus crosses of two genotypes. Robinson and O’Rourke (1992) suggested low correlations between growth rates, as compared with between liveweights, could be due to growth over an interval being only a small contributor to variation in a subsequent liveweight. Davis (1993) drew attention to the likelihood that selection of tropical cattle for high growth rate at pasture would not result in high growth rate in the feedlot. Our results support this conclusion and show that it applies more in BRAH than in TCOMP.
Liveweights are the primary criteria used for genetic evaluation of growth in both temperate and tropical genotypes and it is well established that selection for liveweight at one time will increase weights at other times (e.g. Burrow et al. 1991b; Robinson and O’Rourke 1992; Meyer et al. 1993). The genetic correlations among LWT measures in the present study were high (0.72 to 0.99; Tables 6, 7 and 10) in support of this usual finding.
Genetic parameter differences between genotypes
The generally lower trait variances for BRAH than for TCOMP, and the important differences in some genetic correlations (e.g. –0.25 ± 0.24 between SRIB and SEMA at ENTRY for BRAH, compared with 0.54 ± 0.22 for TCOMP; Table 10), support the use of separate parameter estimates for genetic evaluation of BRAH and TCOMP. While the capacity for these parameters to differ is already allowed for in genetic evaluation in Australia (Graser et al. 2005), there have previously been few genotype-specific genetic correlation estimates available for these genotypes. Our results are in broad agreement with the observation by Robinson and O’Rourke (1992) of lower phenotypic variances for Brahman, and with the lower genetic variances and genetic correlations for tropical compared with temperate breeds seen by Johnston et al. (2003) for growth and scanned body composition traits.
The way B. indicus have adapted to tropical conditions might also explain the different sized genetic correlations seen for BRAH than for TCOMP. Correlations further from unity signify a greater capacity for populations to change one trait without changing the other, and it may be that this capacity is important for functioning in tropical environments. Under this view, the lower genetic correlations in BRAH between growth rates at different times (Table 10) could be a part of their capacity to cope with extremely variable environments, as occurs, for example, between annual tropical ‘wet’ and ‘dry’ seasons; and the lower correlations between rump and rib fat depths (0.82 ± 0.08 in BRAH, 0.98 ± 0.03 in TCOMP at ENTRY) could reflect the greater ability of BRAH to vary where subcutaneous fat is deposited. The higher correlation between ENTRY and EXIT SRIB fatness for BRAH (0.70 ± 0.12, compared with 0.36 ± 0.20 for TCOMP) may indicate a correspondingly lesser adaptation of BRAH to feedlot finishing, and reflect a lower responsiveness of BRAH fat level to more plentiful conditions.
Potential genetic indicators of feed intake and residual feed intake
Our results are among the first genetic parameter estimates reported for RFI and DFI in specifically tropical genotypes, and they show there is clearly potential to change RFI genetically in each of BRAH and TCOMP (heritabilities 0.24 and 0.38; genetic variances 0.19 and 0.41 (kg/day)2, respectively). These estimates are slightly higher than were observed by Robinson and Oddy (2004) for mixed temperate and tropical breeds, where RFI had a heritability of 0.18 and a genetic variance of 0.139 (kg/day)2. Our animals were older on average (24.5 months v. 21 months at mid test) and slightly heavier than those studied by Robinson and Oddy (2004).
The consistently negative genetic association between IGF-I and RFI in our study differs from other reports. Johnston et al. (2002) reported IGF-I to be positively genetically correlated with RFI in two datasets, one of which included some tropical breeds; and Moore et al. (2005) found a positive genetic correlation (0.54 ± 0.31) between IGF-I measured post-weaning and RFI measured in a post-weaning feed test in Angus. In this study the genetic correlation between IGF-I and RFI was negative whether IGF-I was measured at POSTW (–0.38), ENTRY (–0.28) or EXIT (–0.56). The different results may be in part because we measured RFI on older steers and under feedlot conditions, compared with at post-weaning in the study of Moore et al. (2005). Others (Hoque and Oikawa 2004; Robinson and Oddy 2004) have noted that RFI is not the same trait at different stages of growth. The different results are consistent with other trait associations with IGF-I that were seen in each study. Johnston et al. (2002) found lower post-weaning IGF-I to be genetically associated (approximately –0.20) with greater ADG and with decreased or unchanged DFI, consistent with a lower RFI. Our results showed lower post-weaning IGF-I to be genetically associated with greater ADG (–0.10) but more strongly associated with an increased DFI (–0.32), consistent with a higher RFI.
Genetic associations between IGF-I and measures of fatness have previously been reported as both positive (Moore et al. 2005) and negative (Davis and Simmen 2000). Positive associations were taken by Moore et al. (2005) as supporting their positive association of IGF-I with RFI. We found the association of IGF-I with fatness to change with measurement time and genotype. The association was strongly positive (0.47 to 0.61) between POSTW IGF-I and fatness measures at ENTRY, but less so as either measure was taken later. When each was measured at EXIT, the association in TCOMP was negative (–0.48 ± 0.19 with SP8; Table 10). In support of Robinson and Oddy (2004) who suggested fatness as an indicator trait for RFI, fatness at EXIT was strongly positively genetically correlated with RFI in TCOMP (0.60 ± 0.18 with SRIB). However, it was only lowly correlated (0.16 ± 0.25; Table 10) in BRAH. Fatness measured earlier, at ENTRY, was only lowly and even negatively correlated with RFI for both SRIB and SP8. This latter result may be support for the low association between rib fat depth at around 400 days and post-weaning RFI reported by Arthur et al. (2001) in Angus.
From this study, measures with potential to be used as genetic indicators of DFI were LWT measures in both BRAH and TCOMP, ADG at ENTRY in TCOMP, and SP8 and SIMF at ENTRY in BRAH. The relationships with DFI were positive for the weight and gain measures and negative for the fatness measures. Measures with potential to be genetic indicators of RFI were IGF-I in both genotypes and HH and ADG at ENTRY in BRAH. Higher IGF-I was associated with lower RFI in combined genotypes. In BRAH, taller and faster growing steers at ENTRY had genetically lower RFI in the feedlot. The results emphasise the need for a population-specific understanding of trait relationships and of trait differences between measurement times when considering the use of genetic indicator traits for RFI.
Conclusions
BRAH and TCOMP steers differed in their mean growth, scanned body composition, DFI and RFI, and genetic parameter estimates differed between the genotypes. Variances and correlations for traits were generally lower in BRAH. Some of the differences may be associated with changes that have occurred in B. indicus for tropical adaptation. In BRAH, there is little genetic relationship between growth rate at pasture and growth rate in the feedlot. Feedlot RFI is lower in BRAH than in TCOMP, suggesting an advantage in efficiency of feed use for BRAH. Feedlot DFI is lower in BRAH than in TCOMP, which saves on feed but limits BRAH performance. There is clear potential to genetically change RFI in both BRAH and TCOMP. Separate genetic parameter estimates need to be utilised in genetic evaluation of BRAH and TCOMP, and ENTRY and EXIT measures need to be distinguished in genetic evaluation when these are encountered.
Measures with potential to be used as genetic indicators of DFI were LWT measures in BRAH and TCOMP, ADG at ENTRY in TCOMP, and SP8 and SIMF at ENTRY in BRAH. Measures with potential to be genetic indicators of RFI were IGF-I in both genotypes and HH and ADG at ENTRY in BRAH. In contrast with other reports, IGF-I was negatively genetically correlated with RFI whether IGF-I was measured at POSTW, ENTRY or EXIT. A population-specific understanding of trait relationships and of trait differences between measurement times is needed if reliance is to be placed on genetic indicator traits for genetic evaluation of RFI.
Acknowledgements
Financial or in-kind support was provided by the Commonwealth Cooperative Research Centre program, NSW Department of Primary Industries, CSIRO, Queensland Department of Primary Industries, University of New England, Meat and Livestock Australia, Australian Centre for International Agricultural Research, AgForce Queensland, Australian Agricultural Co., C. & R. Briggs, Consolidated Pastoral Co., J. & S.M. Halberstater, S. Kidman & Co., G.E. & J. McCamley, North Australian Pastoral Co., and Stanbroke Pastoral Co. Numerous staff assisted through cattle management, steer feeding, data collection, laboratory analyses and data handling. We especially acknowledge the skilled assistance of Warren Sim (data management), Paul Williams and Nick Corbett (ultrasound scanning), Reid Geddes (feeding management) and Andrew McCann (database management); and the assistance of the Managers and staff of CSIRO ‘Belmont’, QDPI ‘Toorak’ and ‘Brigalow’ Research Stations, and of the properties ‘Alcala’, ‘Alexandria’, ‘Beresford’, ‘Berrigurra’, ‘Cona Creek’, ‘Kiargathur’, ‘Mandalay’, ‘Mimong’, ‘Tartrus’, ‘Tullimba’, and ‘Weetalaba’.
Archer JA,
Richardson EC,
Herd RM, Arthur PF
(1999) Potential for selection to improve efficiency of feed use in beef cattle: a review. Australian Journal of Agricultural Research 50, 147–161.
| Crossref | GoogleScholarGoogle Scholar |
[Verified 19 March 2009]
Wolcott ML,
Johnston DJ,
Barwick SA,
Iker CL,
Thompson JM, Burrow HM
(2009) Genetics of meat quality and carcass traits and the impact of tenderstretching in two tropical beef genotypes. Animal Production Science 49, 383–398.
| Crossref | GoogleScholarGoogle Scholar |

1 Animal Genetics and Breeding Unit is a joint venture of New South Wales Department of Primary Industries and the University of New England.



