Separate feeding of calcium improves performance and ileal nutrient digestibility in broiler chicks
S. J. Wilkinson A C , P. H. Selle A , M. R. Bedford B and A. J. Cowieson AA Poultry Research Foundation, Faculty of Veterinary Science, The University of Sydney, Camden, NSW 2570, Australia.
B AB Vista Feed Ingredients, Marlborough, Wiltshire, SN84AN, UK.
C Corresponding author. Email: stuart.wilkinson@sydney.edu.au
Animal Production Science 54(2) 172-178 https://doi.org/10.1071/AN12432
Submitted: 18 December 2012 Accepted: 11 February 2013 Published: 17 April 2013
Abstract
A total of 144 Cobb 500 broilers were used to investigate if modern commercial broilers could regulate their calcium (Ca) intake using choice feeding and whether separating the delivery of a portion of the Ca from the mixed ration would be advantageous for performance and nutrient recovery. Birds were fed corn+soy-based diets formulated to contain 2.5, 5.0, 7.5 or 10.0 g/kg total Ca and all groups had access to a separate Ca source (CaCO3). The trial was conducted from Day 1 to Day 21 and birds had ad libitum access to both the experimental diets and a separate Ca source throughout. Total feed and separate Ca intake were monitored daily, weight gain and feed intake weekly and on Day 21, the apparent ileal digestibility of DM, nitrogen, selected minerals and amino acids were determined. Consumption of the separate Ca source increased (P < 0.05) with decreasing total Ca concentration of the mixed ration. No differences (P > 0.05) in toe ash were found. Increasing dietary Ca concentration negatively influenced the apparent ileal digestibility of DM, nitrogen, minerals and amino acids. It can be concluded that broilers can select and consume Ca from a separate source to broadly maintain their requirement. Feeding a separate source of Ca in combination with reduced dietary Ca in the mixed ration had beneficial effects on nutrient digestibility, phosphorus excretion and performance.
Additional keywords: choice feeding, phosphorus, phytate.
Introduction
High dietary Ca concentrations have been reported to impede the availability of minerals such as P, Mg, Mn and Zn as well as to reduce the efficacy of phytase through the formation of Ca-phytate complexes (Driver et al. 2005; Selle et al. 2009). Though reducing the concentration of dietary Ca has been reported to improve phytate-P availability, this has also been shown to reduce skeletal integrity (Selle et al. 2009). Thus decreasing dietary Ca concentrations may lead to improved growth performance and P digestibility but may have negative implications for the health and welfare of poultry through increased leg health problems and compromised skeletal integrity.
Mucosal phytases have been identified in the small intestine of poultry. However, their efficacy is thought to be limited by the high concentrations of Ca in poultry diets, which renders phytate insoluble at small intestinal pH (Tamim et al. 2004). As early as the 1940s it was proposed that poultry are able to utilise phytate-P (without exogenous phytases), however, as the level of dietary Ca increases, the extent of phytate destruction is reduced or may be completely prevented (Taylor 1965). Tamim et al. (2004) highlighted the influence of dietary Ca on the birds’ endogenous capacity to utilise phytate-P, reporting that birds were able to hydrolyse 69% of phytate-P from corn-soy based diets containing 0.2% total Ca but only 25% of phytate-P was liberated when birds were fed diets containing 0.5% Ca.
Poultry have been shown to possess specific appetites for nutrients and are able to select a diet from a variety of sources to meet their nutritional requirements. Seminal work in layer hens by Kempster (1916) and Rugg (1925) showed that hens produced more eggs when they were able to choice feed when compared with those fed a single mixed ration. Specific appetites in poultry have also been shown for lysine (Newman and Sands 1983), methionine (Steinruck et al. 1990), total protein (Forbes and Shariatmadari 1994), Se (Zuberbuehler et al. 2002) and Ca (Wood-Gush and Kare 1966; Hughes and Wood-Gush 1971; Joshua and Mueller 1979). Numerous studies in laying hens have demonstrated a specific appetite for Ca that has been shown to increase in the afternoon as well as on shell-forming when compared with non-shell-forming days (Hughes 1972; Gilbert 1983). Wood-Gush and Kare (1966) demonstrated in Ca-deficient birds that were offered a choice of two diets, which differed only in the presence or absence of added Ca that there was a preference for the Ca-supplemented diet.
Thus, as it has been previously noted that birds possess a Ca-specific appetite and also that feeding low Ca diets improves the digestibility of phytate-P, the present study aimed to feed low Ca diets and a separate Ca source and evaluate the effects on broiler performance and nutrient digestibility coefficients. The hypothesis was that removing a portion of the Ca from the mixed ration and providing it separately as a supplemental grit would simultaneously enhance P digestibility and meet the Ca requirements of the bird.
Materials and methods
All experimental procedures conducted in this study were in accordance with the University of Sydney Animal Ethics Committee and with the Australian code for the care and use of animals for scientific purposes (National Health and Medical Research Council 2004).
Animals and housing
A total of 144 Cobb 500-day-old male broilers were obtained from a commercial hatchery (Baiada Poultry Pty Ltd, Marsden Park, NSW, Australia), weighed and randomly allocated to one of four dietary treatments in a completely randomised design. The trial period was from day old to 22 days of age with each treatment replicated six times with six chicks per replicate cage. Broilers were kept at a temperature of 31°C for Days 1–4 and thereafter this was reduced by 0.5°C/day to 24°C. The lighting regime for the study consisted of 23L : 1D for the first 4 days and then 18L : 6D for the remainder of the experiment.
Diets and experimental procedures
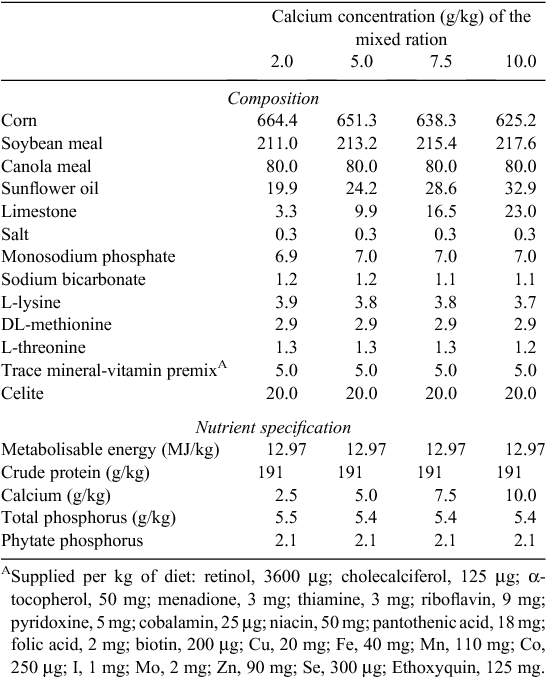
Mash diets were based on corn and soybean meal and were formulated to contain 2.5, 5.0, 7.5 or 10.0 g/kg total Ca (Table 1) while all other nutrients were kept constant. The diets were formulated to contain adequate concentrations of all nutrients with the exception of Ca and available P (NRC 1994). Available P was formulated to be 2.5 g/kg across all diets and was deliberately below the requirement for young broilers in order to allow the examination of the hypothesis that separate provision of Ca would enhance phytate-P availability. All birds had free access to the mixed ration, water and, in a second feed trough, the separate source of Ca (CaCO3 grit, 38% Ca and 2-mm mean particle size). Feed and Ca source intake were recorded daily and bodyweight weekly. To calculate the apparent ileal digestibility (AID) coefficients for crude protein, amino acids, energy and minerals, an indigestible marker (acid insoluble ash; AIA) (Celite 281, Filchem Australia Pty Ltd, Castle Hill, NSW, Australia) was added to diets at a concentration of 20 g/kg. The AIA concentration in the separate CaCO3 grit was analysed (Siriwan et al. 1993) and found to be below the detection limit of the assay (data not shown). The potential diluting effect of separate CaCO3 on total AIA intake was considered and though of negligible importance (diluting analysed AIA in ingesta from ~23.5 to 23.4 g/kg), this was included in the calculations. Similarly, the DM and Ca intakes from both the diet and the separate CaCO3 source were considered in the digestibility calculations. On Day 22, all birds were euthanised with an intravenous injection of a diluted sodium pentobarbitone solution (Lethabarb, Virbac Australia Pty Ltd, Milperra, NSW, Australia). The contents of the lower ileum were collected and pooled for each cage, immediately frozen and freeze-dried thereafter according to the methods of Ravindran et al. (2005). Toe ash measurements were recorded from samples that were obtained by severing the middle toe through the joint between the 2nd and 3rd tarsal bones from the distal end. Toes were collected from individual birds and pooled by treatment replicate. Samples were dried to a constant weight at 105°C and then ashed in a muffle furnace at 500°C for 12 h.
|
Chemical analyses
The gross energy (GE) and N contents of diets and ileal digesta were determined. The diets and digesta were also analysed for the minerals (P, Ca, Cu, K, Mg, Mn, Na, Sr, Fe and Zn). The GE of diets and excreta were determined using a Parr 1281 adiabatic bomb calorimeter (Parr Instrument Co., Moline, IL, USA) that was standardised with benzoic acid. Nitrogen concentration of samples was determined by the Dumas method using a FP-428 N analyser (LECO Corporation, St Joseph, MI, USA) as described by Sweeney (1989). Samples were wet acid digested using nitric acid and hydrogen peroxide (Peters et al. 2003) before the determination of mineral concentration by Inductively Coupled Plasma-Optical Emission Spectroscopy using a Perkin Elmer OPTIMA 7300 (Perkin Elmer Inc., Waltham, MA, USA). Phytate concentrations of the diets were determined using the Megazyme method (Megazyme International Ireland, Wicklow, Ireland). Amino acid concentrations in the diet and dried ileal digesta samples were determined using a Waters AccQTag Ultra amino acid analysis system following acid hydrolysis in 6M HCl at 110°C. The AIA component of dried diets and ileal digesta samples were determined according to the method of Siriwan et al. (1993). The AID coefficient of DM, energy, N, amino acids and minerals were calculated as per Ravindran et al. (2001). Taurine was not detected in experimental diets but is synthesised in vivo and excreted in biliary and urinary secretions (Lourenco and Camilo 2002). Concentrations of taurine determined in ileal digesta were deemed to be of endogenous origin and were used to estimate total endogenous losses. Similarly, hydroxyproline, a metabolite of proline commonly found in collagen was not detected in the experimental diets therefore concentrations detected in ileal digesta were used to calculate endogenous losses.
Statistical analyses
Data were analysed for significance by a one-way ANOVA model using JMP version 8.01 software (SAS Institute, Cary, NC, USA). Treatment differences were considered significant at P < 0.05. If significance was determined, a Tukey’s HSD was performed to differentiate between treatments. Linear and quadratic contrasts are reported where applicable to determine the relationship between dietary Ca and nutrient digestibility.
Results
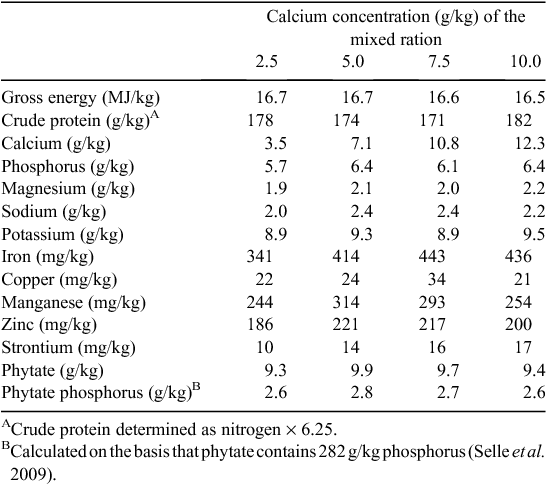
The determined composition of the experimental diets is presented in Table 2. The analysed concentration of Ca in diets was ~1–2 g/kg greater than expected across all diets. These determined concentrations were used in the subsequent calculations and considered in the interpretation of the results presented below. Phytate-P concentrations were slightly higher than expected based on formulated values and averaged 2.7 g/kg across the four diets (Table 2). Total P was determined to be ~6 g/kg, close to the expected values from the formulation.
|
Birds fed the diet containing 10 g/kg total Ca consumed less feed and weighed less at Day 21 compared with birds from the other treatment groups (P < 0.05) (Table 3). There was no effect (P > 0.05) of dietary treatment on the proportion of toe ash of broilers at Day 21.
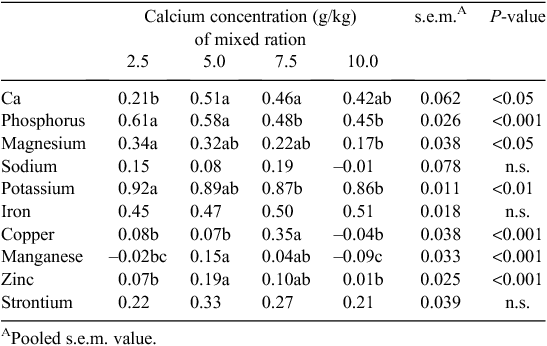
There was a significant relationship between Ca concentration of the mixed ration and the intake of the separate Ca source (P < 0.001) (Table 3). Birds fed diets formulated with 2.5 g/kg Ca consumed more of the separate Ca source when compared with birds fed the diets containing 5.0, 7.5 or 10.0 g/kg Ca (P < 0.05). Furthermore, birds fed the diet containing 5.0 g/kg Ca consumed more of the Ca source than birds offered the diets containing 7.5 and 10.0 g/kg Ca. Broilers fed the 2.5 g/kg Ca diet consumed less total Ca than the birds fed diets formulated with 10 g/kg Ca (0.45 vs 0.57 g/bird.day; P < 0.05). There was no difference in total Ca intake between birds fed the diets containing 5.0, 7.5 or 10.0 g/kg Ca. Total Ca intake as a proportion of the diet was 9.3 g/kg for birds fed the 2.5 g/kg Ca diet and this was less (P < 0.05) than birds from the 7.5 and 10.0 g/kg Ca treatment groups, which consumed 13.5 and 15.1 g/kg Ca, respectively. The Ca concentration of the diet influenced the proportion of Ca that was obtained from the separate Ca source (P < 0.001). Birds from the 2.5 g/kg Ca group obtained ~710 g/kg of their total Ca intake from the separate source of Ca, which was in contrast to birds fed the 10 g/kg Ca diet that derived only 220 g/kg of their Ca intake from the separate source (P < 0.05).
The influence of Ca concentration of the mixed ration on the AID of minerals is summarised in Table 4. Calcium digestibility was significantly lower for birds fed 2.5 g/kg Ca when compared with birds fed 5.0 and 7.5 g/kg Ca diets. Increasing dietary Ca concentrations resulted in a quadratic (P < 0.05) effect on the digestibility of Cu with birds fed 7.5 g/kg Ca showing a significantly greater digestibility coefficient than birds from the other three treatment groups. As dietary Ca concentration increased, the digestibility of P, K and Mg decreased (P < 0.001, 0.01 and 0.05, respectively). Manganese and Zn digestibility were significantly greater in birds that received the diet containing 5 g/kg Ca when compared with those formulated with 2.5 or 10 g/kg Ca. There was no effect of diet on the digestibility of Fe, Na and Sr.
|
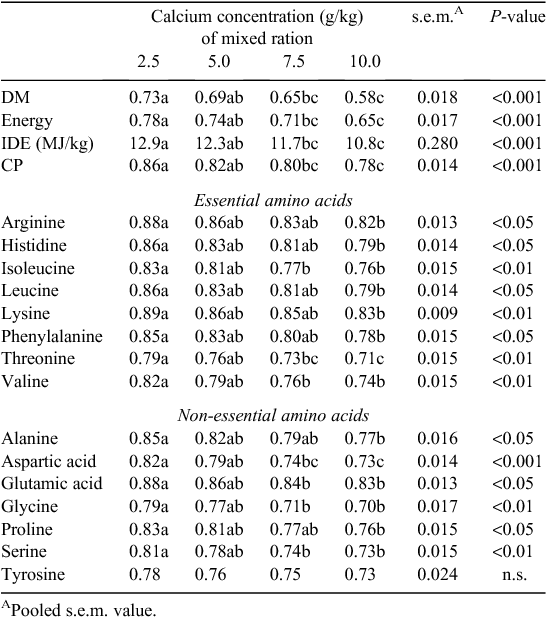
Reducing dietary Ca significantly, and so axiomatically, encouraging greater consumption of the separate CaCO3 source, increased the AID of DM (P < 0.001), N (P < 0.001) and all amino acids (P < 0.05) except for tyrosine (Table 5). Comparing the 2.5 and 10.0 g/kg diets directly, the average improvement in ileal amino acid digestibility associated with the reduced dietary Ca content was ~8% and up to 10% for threonine, aspartic acid and glycine Additionally, increasing dietary Ca concentrations from 2.5 to 10.0 g/kg resulted in a decrease (P < 0.001) in ileal digestible energy from 12.9 to 10.80 MJ/kg.
|
The effect of dietary Ca concentrations on the loss of endogenous taurine and hydroxyproline are presented in Table 6. Diets were analysed for the presence of taurine and hydroxyproline and both were below the detection limit of the assay (data not shown). There was no effect of dietary Ca concentration on the concentration of either compound in the ileal digesta (P > 0.05). However, reducing dietary Ca concentration from 10 to 2.5 g/kg resulted in a substantial (P < 0.01) reduction in the loss of these compounds from the ileum. Reducing dietary Ca resulted in a quadratic effect on the loss of hydroxyproline (P < 0.01) per bird each day. Birds from the 7.5 g/kg group lost ~46% more taurine than those fed 2.5 g/kg Ca. Daily losses of hydroxyproline were greater for birds fed 7.5 g/kg Ca compared with those fed 2.5 g/kg Ca diets (P < 0.05).
Discussion
Environmental and economic effects of excess P from manure are major issues for pig and poultry production (Letourneau-Montminy et al. 2011). The majority of P in plant materials is in the form of phytate-P, which is thought to be largely unavailable to poultry when high concentrations of dietary Ca are present (Cowieson et al. 2006). As pH increases above 1.1, phytate carries an increasingly stronger negative charge that allows the chelation of divalent cations such as Zn2+, Cu2+, Ni2+, Co2+, Mn2+, Fe2+ and Ca2+, rendering these less available to poultry (Maenz et al. 1999; Angel et al. 2002). Importantly, Angel et al. (2002) suggested that Ca, despite its lesser affinity with phytate, may play the most defining role in the formation of phytate-mineral complexes due to its disproportionately high dietary concentration, reducing the efficacy of both endogenous and exogenous phytases. In the present study, reducing the dietary Ca concentration of broiler diets while providing a separate source of Ca significantly increased the AID of DM, P, N and all the amino acids except tyrosine. Further, there was no effect on the proportion of toe ash between treatment groups suggesting that bone mineralisation was maintained. From the available literature, this is the first time that enhanced bodyweight gain and bone mineralisation have been achieved when feeding mixed rations with reduced Ca concentrations with a separate Ca source.
A key observation from this study was the innate ability of the birds fed diets with varying concentrations of Ca to consume the separate source of Ca in sufficiency to their requirement for bone mineralisation. The results of this study confirm that a contemporary broiler has retained a specific appetite for Ca, similar to previously reported (Joshua and Mueller 1979). Birds in this study that were fed diets formulated with 2.5 g/kg Ca consumed the greatest quantities of the separate Ca source in contrast to birds fed 10 g/kg Ca diets who consumed the least.
The AID of Ca was significantly lower for birds fed 2.5 g/kg Ca compared with birds fed 5.0 and 7.5 g/kg. However, uniquely for Ca in the present experiment, intake was from two sources and as ileal digesta were collected at one time point it is possible that digestibility coefficients, calculated as they were based on both Ca from the diet and Ca from the separate CaCO3 source, are erroneous. There was no difference between birds fed diets formulated with 5.0, 7.5 or 10.0 g/kg Ca in the AID of Ca. However, increasing dietary Ca concentration was negatively associated with the AID of P. This finding is in agreement with work in pigs and poultry whereby increased concentrations of dietary Ca did not influence the apparent total tract digestibility of Ca but decreased that of P (Stein et al. 2011). Inorganic sources of P are added to poultry diets to compensate for the lack of availability of phytate-bound P. However, poultry diets commonly use Ca from limestone, which may also interact with inorganic sources of P in the gut lumen-forming Ca-phosphates that become less soluble with increasing pH (Moore and Miller 1994; Selle et al. 2009). Limestone has been shown to increase the pH of digesta from 5.6 to 6.1 in the small intestine of poultry (Shafey 1999) and it is likely to promote the formation of Ca-phosphate precipitates.
All diets were formulated to contain the same amount of available P (2.5 g/kg). In the diet containing 10 g/kg Ca the ileal digestibility coefficient for P was 0.45 (Table 4), resulting in an approximate available P content of 2.7 g/kg assuming all diets contained ~6 g/kg total P (Table 2). However, the diet with 2.5 g/kg total Ca returned an ileal digestibility coefficient for P of 0.61, or 3.7 g/kg available P. Thus, reducing dietary Ca concentration from 10 to 2.5 g/kg resulted in an increase in available P of ~1.0 g/kg with no change in total P concentrations. This substantial change in available P concentration is in keeping with the work of Tamim and Angel (2003) and Tamim et al. (2004) and is likely to be primarily responsible for the advantageous effects on performance. However, unlike the earlier work of Tamim and colleagues, in the present study these benefits were associated not only with a low Ca feeding regime but with a separate Ca feeding system. This suggests that the digestibility of P can be enhanced not only by feeding less Ca per se but by consuming essentially the same amount of Ca separately from the mixed ration.
The effects of reduced dietary Ca concentrations on ileal amino acid digestibility are instructive (Table 5). Cowieson and Bedford (2009) reviewed the effects of both xylanases and phytases on ileal amino acid digestibility in pigs and poultry. In that review paper, the authors describe a consistent pattern of effect, both for phytase and xylanase, where certain amino acids are advantaged more than others. The addition of phytase to pig and poultry diets tends to disproportionately improve the ileal recovery of threonine, serine, proline, aspartic acid, glycine and cysteine compared with the more recalcitrant amino acids methionine, arginine, glutamic acid and lysine (Cowieson and Bedford 2009). This is partly due to a reduction, with phytase addition, in ileal endogenous amino acid flow (Cowieson and Ravindran 2007) and partly due to altered absorptive function (Liu et al. 2009). The separate feeding of Ca in the present study resulted in an improvement in ileal amino acid digestibility of ~8% (comparing the 2.5 g/kg diet with the 10 g/kg diet). This improvement was virtually identical to the use of exogenous phytase under standard Ca provision regimes and warrants further investigation.
Phytate, perhaps through the formation of binary and ternary complexes with protein has been shown to negatively influence the availability of protein for poultry (Cowieson et al. 2006). Binary protein-phytate complexes involve the interaction between basic amino acid residues in dietary or endogenous protein and phytate by salt-like linkages (Cosgrove 1966). Ternary protein-phytate complexes are formed in the small intestine with the major components linked by a cationic bridge, usually Ca2+, which is the most abundant divalent cation in digesta (Selle et al. 2012). Therefore, reducing the amount of dietary Ca in broiler diets would likely promote greater phytate destruction by endogenous phytases due to a reduction in ternary complex formation. Another possible outcome would be reduced substrate availability (Ca2+) used in the formation of ternary protein–phytate complexes. The results of this study support previous findings that dietary Ca concentration influences the digestibility of N and amino acids (Ravindran et al. 1999; Selle et al. 2009). In the present study, the improvement of apparent digestibility of amino acids was broadly in keeping with the results of Cowieson et al. (2006) who reported ingestion of fully phosphorylated phytate (IP6) adversely affected the true digestibility coefficients of alanine, serine, aspartic acid and threonine to a greater extent than other amino acids.
An interesting finding in the present study showed that increasing dietary Ca concentrations from 2.5 to 10 g/kg resulted in an increase (P < 0.001) in the flow of endogenous taurine and hydroxyproline by 66 and 35%, respectively. Taurine is an amino sulfonic acid, synthesised from methionine and its presence in the intestine is likely predominantly associated with bile (Tufft and Jensen 1992). Tufft and Jensen (1992) demonstrated that adding taurine to broiler and turkey diets resulted in modest improvements in fat digestibility in the neonate. It may be that reducing dietary Ca concentrations aids micelle formation in the avian gut, reducing the need for bile salt synthesis. This is partially evidenced by the improved ileal digestible energy values in the treatment groups where Ca was largely provided separately from the mixed ration (Table 5). Bile also contains substantial concentrations of glycine and so a reduced synthesis and/or loss of bile may also enhance the ileal digestibility of glycine. Indeed, in the present study reducing Ca concentration in the diet from 10 to 2.5 g/kg resulted in an improvement in AID of glycine of ~12% and this amino acid was the most advantaged of all the amino acids observed in this study. Further, exogenous phytase enhances the ileal digestibility of glycine considerably (Cowieson and Bedford 2009) and has been shown to reduce the antinutritional effect of phytate on endogenous glycine flow (Cowieson and Ravindran 2007). These effects suggest a link between the mechanism of action of phytase and axiomatically, phytate, and the impeding effects of consumption of limestone and/or Ca simultaneous with the mixed ration. Hydroxyproline together with proline forms ~30% of collagen proteins, which in turn represent ~30% of total body protein (Wu et al. 2011). Of possible significance is that hydroxyproline has been recently recognised as a substrate for the synthesis of glycine (Wu et al. 2011), suggesting possible links to the mechanisms presented above. Further, as hydroxyproline is involved in collagen synthesis, its increased presence in the lumen may be indicative of increased turnover of the epithelium and/or reduced gastrointestinal tract integrity. Notably, hydroxyproline has been used as an indicator of collagen breakdown and osteoporosis in humans (Russell 1995) and this may also prove useful for poultry.
It can be concluded that modern Cobb broilers have a Ca-specific appetite that promotes increased consumption of a separate Ca source when the basal diet is insufficient in supply. This innate ability may be commercially exploited to enhance P digestibility via separate feeding of Ca to broilers under commercial production systems, perhaps through post-pellet limestone grit addition. Post-pellet limestone (or equivalent) addition, perhaps in concert with higher concentrations of dietary phytase and vitamin D3, warrants further research to explore the boundaries of performance, phosphorus and amino acid retention in poultry.
Acknowledgements
The authors are grateful to the Australian Rural Industries Research and Development Corporation Chicken Meat Program for their financial support of this study.
References
Angel R, Tamim NM, Applegate TJ, Dhandu AS, Ellestad LE (2002) Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. Journal of Applied Poultry Research 11, 471–480.Cosgrove DJ (1966) Chemistry and biochemistry of inositol polyphosphates. Reviews of Pure and Applied Chemistry 16, 209–224.
Cowieson AJ, Bedford MR (2009) The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? World’s Poultry Science Journal 65, 609–624.
| The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action?Crossref | GoogleScholarGoogle Scholar |
Cowieson AJ, Ravindran V (2007) Effect of phytic acid and microbial phytase on the flow and amino acid composition of endogenous protein at the terminal ileum of growing broiler chickens. The British Journal of Nutrition 98, 745–752.
| Effect of phytic acid and microbial phytase on the flow and amino acid composition of endogenous protein at the terminal ileum of growing broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWhsrjO&md5=8a2080180754cf0a5b1d3064bdbe6c68CAS | 17524177PubMed |
Cowieson AJ, Acamovic T, Bedford MR (2006) Phytic acid and phytase: implications for protein utilization by poultry. Poultry Science 85, 878–885.
Driver JP, Pesti GM, Bakalli RI, Edwards HM (2005) Calcium requirements of the modern broiler chicken as influenced by dietary protein and age. Poultry Science 84, 1629–1639.
Forbes JM, Shariatmadari F (1994) Diet selection for protein by poultry. World’s Poultry Science Journal 50, 7–24.
| Diet selection for protein by poultry.Crossref | GoogleScholarGoogle Scholar |
Gilbert AB (1983) Calcium and reproductive function in the hen. The Proceedings of the Nutrition Society 42, 195–212.
| Calcium and reproductive function in the hen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltVCjsbY%3D&md5=3d34bec5dfff3c26b7d87aeadcd39cc9CAS | 6351080PubMed |
Hughes BO (1972) Circadian rhythm of calcium intake in domestic fowl. British Poultry Science 13, 485–493.
| Circadian rhythm of calcium intake in domestic fowl.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3s%2Fmt1Wmuw%3D%3D&md5=1ce785676485a5ea583c778c797bca51CAS | 4641261PubMed |
Hughes BO, Wood-Gush DGM (1971) Specific appetite for calcium in domestic chickens. Animal Behaviour 19, 490–499.
| Specific appetite for calcium in domestic chickens.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE387gtFansA%3D%3D&md5=c825e5f50174a763bed75d632e3efc08CAS | 5156615PubMed |
Joshua IG, Mueller WJ (1979) The development of a specific appetite for calcium in growing broiler chicks. British Poultry Science 20, 481–490.
| The development of a specific appetite for calcium in growing broiler chicks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhtV2msb0%3D&md5=f856ae8bd71191f854033ad5693964f9CAS |
Kempster HL (1916) Food selection by laying hens. Journal of the American Association of Instructors and Investigators in Poultry Husbandry 3, 26–28.
Letourneau-Montminy MP, Narcy A, Lescoat P, Magnin M, Bernier JF, Sauvant D, Jondreville C, Pomar C (2011) Modeling the fate of dietary phosphorus in the digestive tract of growing pigs. Journal of Animal Science 89, 3596–3611.
| Modeling the fate of dietary phosphorus in the digestive tract of growing pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqsrrI&md5=498ea1b5a5acbcbf58235698b9336ba5CAS | 21680789PubMed |
Liu N, Ru YJ, Li FD, Wang JP, Lei XQ (2009) Effect of dietary phytate and phytase on proteolytic digestion and growth regulation of broilers. Archives of Animal Nutrition 63, 292–303.
| Effect of dietary phytate and phytase on proteolytic digestion and growth regulation of broilers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1WisLvE&md5=434c03519c671266740719f313bafa5bCAS |
Lourenco R, Camilo ME (2002) Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutricion hospitalaria: organo oficial de la Sociedad Espanola de Nutricion Parenteral Enteral 17, 262–270.
Maenz DD, Engele-Schaan CM, Newkirk RW, Classen HL (1999) The effect of minerals and mineral chelators on the formation of phytase-resistant and phytase-susceptible forms of phytic acid in solution and in a slurry of canola meal. Animal Feed Science and Technology 81, 177–192.
| The effect of minerals and mineral chelators on the formation of phytase-resistant and phytase-susceptible forms of phytic acid in solution and in a slurry of canola meal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmsVWmur4%3D&md5=aba3fe21ac2da29d70e5108842600ff0CAS |
Moore PA, Miller DM (1994) Decreasing phosphorus solubility in poultry litter with aluminum, calcium and iron amendments. Journal of Environmental Quality 23, 325–330.
| Decreasing phosphorus solubility in poultry litter with aluminum, calcium and iron amendments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXisFagsbg%3D&md5=d62e7db67d01f55bdb9514071617d407CAS |
National Health and Medical Research Council (2004) ‘Australian code of practice for the care and use of animals for scientific purposes.’ 7th edn. (Commonwealth Government of Australia: Canberra)
Newman RK, Sands DC (1983) Dietary selection for lysine by the chick. Physiology & Behavior 31, 13–19.
| Dietary selection for lysine by the chick.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltVCitrg%3D&md5=a38a8eca975a48fbbbac5cecfa85edb7CAS |
NRC (1994) ‘Nutrient requirements of domestic animals. Nutrient requirements of poultry.’ 9th rev. edn. (National Research Council, National Academy Press: Washington, DC)
Peters J, Combs S, Hoskins B, Jarman J, Kovar J, Watson M, Wolf A, Wolf N (2003) ‘Recommended methods of manure analysis.’ (University of Wisconsin Cooperative Extension Publishing: Madison, WI)
Ravindran V, Cabahug S, Ravindran G, Bryden WL (1999) Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poultry Science 78, 699–706.
Ravindran V, Selle PH, Ravindran G, Morel PCH, Kies AK, Bryden WL (2001) Microbial phytase improves performance, apparent metabolizable energy, and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poultry Science 80, 338–344.
Ravindran V, Hew LI, Ravindran G, Bryden WL (2005) Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. Animal Science 81, 85–97.
| Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWqt7vK&md5=9cccc76b6be5ad2e31c725714893a4d0CAS |
Rugg WC (1925) Feeding experiments, free choice of feeds. Victoria Department of Agriculture Bulletin 54, 36–56.
Russell RGG (1995) The assessment of bone metabolism in-vivo using biochemical approaches. Nefrologia 15, 36–43.
Selle PH, Cowieson AJ, Ravindran V (2009) Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livestock Science 124, 126–141.
| Consequences of calcium interactions with phytate and phytase for poultry and pigs.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Cowieson AJ, Cowieson NP, Ravindran V (2012) Protein–phytate interactions in pig and poultry nutrition: a reappraisal. Nutrition Research Reviews 25, 1–17.
| Protein–phytate interactions in pig and poultry nutrition: a reappraisal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvVOisb0%3D&md5=084a7f0902ba399b55bcd5dd0b064231CAS | 22309781PubMed |
Shafey TM (1999) Effects of high dietary calcium and fat levels on the performance, intestinal pH, body composition and size and weight of organs in growing chickens. Asian-Australasian Journal of Animal Sciences 12, 49–55.
Siriwan P, Bryden WL, Mollah Y, Annison EF (1993) Measurement of endogenous amino acid losses in poultry. British Poultry Science 34, 939–949.
| Measurement of endogenous amino acid losses in poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFOgsLg%3D&md5=c52e168818d4fa01ed2ee31d68632af7CAS | 8156432PubMed |
Stein HH, Adeola O, Cromwell GL, Kim SW, Mahan DC, Miller PS, Swine NCCC (2011) Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but decreases digestibility of phosphorus by growing pigs. Journal of Animal Science 89, 2139–2144.
| Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but decreases digestibility of phosphorus by growing pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXoslCisL8%3D&md5=0df976128cb2169676f19574996b6aafCAS | 21335534PubMed |
Steinruck U, Roth FX, Kirchgessner M (1990) Selective feed-intake of broilers during methionine deficiency. Archiv fur Geflugelkunde 54, 173–183.
Sweeney RA (1989) Generic combustion method for determination of crude protein in feeds – collaborative study. Journal - Association of Official Analytical Chemists 72, 770–774.
Tamim NM, Angel R (2003) Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets. Journal of Agricultural and Food Chemistry 51, 4687–4693.
| Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVGgt7k%3D&md5=54d08b54f1a3e0a861a1f8945657cd3fCAS | 14705897PubMed |
Tamim NM, Angel R, Christman M (2004) Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poultry Science 83, 1358–1367.
Taylor TG (1965) The availability of calcium and phosphorus of plant materials for animals. The Proceedings of the Nutrition Society 24, 105–112.
| The availability of calcium and phosphorus of plant materials for animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF2M%2FotFCmtw%3D%3D&md5=fe74e490050c78040bd257d47c32c5daCAS | 14276887PubMed |
Tufft LS, Jensen LS (1992) Influence of dietary taurine on performance and fat retention in broilers and turkey poults fed varying levels of fat. Poultry Science 71, 880–885.
| Influence of dietary taurine on performance and fat retention in broilers and turkey poults fed varying levels of fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XktVKku70%3D&md5=2e3c136b0a7941cfd5ae6e2c4ab0a80aCAS | 1608883PubMed |
Wood-Gush DGM, Kare MR (1966) The behaviour of calcium-deficient chickens. British Poultry Science 7, 285–290.
| The behaviour of calcium-deficient chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXntlOhsA%3D%3D&md5=4c75c1273d7ba4fef0cb774a6d0fdb21CAS |
Wu GY, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40, 1053–1063.
| Proline and hydroxyproline metabolism: implications for animal and human nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFGjtLo%3D&md5=b2184504403ba87add63f55f7184aabdCAS |
Zuberbuehler CA, Messikommer RE, Wenk C (2002) Choice feeding of selenium-deficient laying hens affects diet selection, selenium intake and body weight. The Journal of Nutrition 132, 3411–3417.




