Regulation of post-mortem glycolysis in ruminant muscle
D. M. Ferguson A C and D. E. Gerrard BA CSIRO Division of Animal, Food and Health Sciences, Locked Bag 1, Armidale, NSW 2350, Australia.
B Department of Animal and Poultry Sciences, Virginia University, Blacksburg, VA 24061, USA.
C Corresponding author. Email: Drewe.Ferguson@csiro.au
Animal Production Science 54(4) 464-481 https://doi.org/10.1071/AN13088
Submitted: 8 March 2013 Accepted: 6 January 2014 Published: 11 March 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
As a tissue, muscle has the unique ability to switch its metabolic source of ATP, the energy currency underpinning muscle function. During oxygen debt, such as that occurring immediately following the death of animals, anaerobic metabolism is initiated in an attempt to restore homeostasis within the muscle. The cascade of biochemical events that are initiated is paramount in the context of meat quality. This review revisits this reasonably well-known subject but takes a new perspective by drawing on the understanding outside the traditional discipline of meat science. Our understanding of the intrinsic regulators of glycolytic flux has improved but knowledge gaps remain. Further efforts to understand how the glycolytic enzyme kinetics are influenced by both pre- and post-slaughter factors will be beneficial in the ongoing quest to maximise fresh meat quality.
Additional keywords: muscle, post-mortem glycolysis, ruminant.
Introduction
Transformation of muscle to meat involves several physiological and biochemical processes evoked by the animal and its tissues in a futile attempt to reinstate homeostatic control. The magnitude, extent, and timing of these responses before, during or post-slaughter can dramatically affect meat quality development. In order to ensure consistent production of the highest quality meat possible, those involved in the meat industry must understand these biological processes and implement management practices that optimise them.
Any effort to understand post-mortem muscle biochemistry should begin with an appreciation for how energy is managed in living muscle, especially under conditions where this highly specialised tissue functions. Muscle cells are uniquely organised and designed to convert chemical energy into movement. Muscle shortens in response to neuronal stimulations that ultimately cause calcium release in the sarcoplasm. Once calcium concentration eclipses a regulatory threshold, myosin and actin interact to create movement through consumption of ATP (via myofibrillar ATPase). Because of the immediate and synchronised nature of contraction and its sensitivity to calcium, ATP-mediated calcium pumps are strategically located throughout the muscle cell on membranous vesicles that rapidly sequester calcium after such a twitch or contraction. These ATPases, as well as those responsible for maintaining membrane potentials and those participating in a myriad of other cellular processes, must function continually; yet under normal resting conditions, energy consumption (ATP turnover) is rather modest, particularly in locomotive muscles, because the major motor proteins remain idle. By contrast, working muscle cells are capable of increasing ATP turnover 100-fold (Hochachka and McClelland 1997).
This highly efficient and robust means of providing ATP rapidly in working skeletal muscle cells is retained, at least in part, during the transformation of muscle to meat. Indeed, it is exactly these unique capabilities that spring into action at slaughter in muscle; however, due to circulatory failure, muscle tissues simply lack sufficient oxygen to maintain a high level of ATP generation. When the concentration of ADP increases in living muscle, glycolytic flux increases (Cheetham et al. 1986; Crowther et al. 2002), as it does in post-mortem muscle (Kastenschmidt et al. 1968). Conversely, in the presence of sufficient oxygen or at a steady-state level of exercise, pyruvate is directed to the mitochondria and is further metabolised (oxidation) in the matrix to support the hydrogen ion gradient between the inner and outer membranes, which in turn synthesises ATP (Conley et al. 2001). This process changes rapidly under non-steady-state exercise, where consumption of ATP exceeds the capacity of the cell to re-synthesise ATP. This is quite similar to that which occurs in post-mortem muscle (Scheffler et al. 2011). At this point, pyruvate no longer enters the mitochondria because the electron transport chain stops functioning, and lactate and hydrogen ions accumulate. In humans that stop exercising, lactate and hydrogen ions are removed from the cell by the monocarboxylate transporter and homeostasis is once again established (Sahlin 1978; Juel et al. 2004). This is not the case in post-mortem muscle. Rather, lactate and protons accumulate and muscle pH decreases ultimately to a level generally found in fresh meat (Briskey et al. 1966).
Clearly, much is known about post-mortem energy metabolism and the conversion of muscle to meat (e.g. see reviews by Bendall 1973; Pöso and Puolanne 2005; England et al. 2013). However, gaps still exist in our understanding of this critical process. Significant further insight can be garnered by taking advantage of the literature (e.g. exercise physiology) outside the discipline of meat science. Given the central importance of pH decline to meat quality development, the aim of this review was to re-examine this central dogma with a slightly different perspective, particularly with regard to the ruminant. We review the current knowledge of muscle energy metabolism, post-mortem biophysical changes and the relevance to meat quality. Further, we examine how animal-related factors and pre- and post-slaughter practices influence post-mortem glycolysis and subsequent meat quality.
Post-mortem energy metabolism and meat quality
There is substantial evidence to show that consumers rate tenderness as the most important of all palatability traits, especially for fresh beef (e.g. Huffman et al. 1996; Watson et al. 2008). Tenderness is also essential to lamb consumers but trails flavour/odour as the highest rated consumer attribute (Pethick et al. 2006). Post-mortem energy metabolism or glycolysis in muscle is highly relevant to ultimate meat quality, particularly tenderness. Typically, estimates of glycolytic rate in post-mortem muscle are obtained from measurements of pH over time. The rate of glycolysis post-mortem can profoundly influence two central mechanisms, which ultimately govern myofibrillar tenderness, notably, the degree of myofibrillar contraction and the rate and extent of proteolysis during ageing (Ferguson et al. 2001; Koohmaraie and Geesink 2006). Moreover, the combination of rapid glycolytic rates post-mortem at high muscle temperatures can compromise muscle protein integrity, which subsequently leads to losses in visual appeal and meat functionality (e.g. Simmons et al. 1996).
Although the rate of post-mortem glycolysis in both bovine (Butchers et al. 1998) and ovine (McGeehin et al. 2001) muscle can vary between animals, the regulatory mechanisms and extrinsic factors governing this variation are not well defined. Intrinsically, the kinetic properties of the individual ATPases govern post-mortem glycolytic rate (Bendall 1978). In muscle, several ATPases are central to muscle contraction and cell maintenance. However, the relative activities of the individual ATPases, aside from those directly responsible for calcium homeostasis, have not been investigated in detail with regard to rigor development.
After slaughter, the rate of glycolysis can be dramatically accelerated through the application of electrical stimulation, a technology specifically designed for rapid chilling of beef carcasses without the risk of cold shortening occurring. The interesting feature of electrical stimulation is that, in addition to the large initial rate change during stimulation, it also results in a faster rate of glycolysis subsequent to stimulation (Horgan and Kuypers 1985; Daly 1997).
Rigor biochemistry and associated biophysical changes in muscle
With the cessation of blood supply to the musculature, a complex cascade of biochemical events ensues, resulting in significant structural changes. The structural changes are biphasic, characterised by general stiffening or loss in inextensibility as the muscle enters rigor, followed by a phase of partial rigor attenuation. The extent and rate of these biochemical and subsequent structural changes are critical in terms of the visual appearance and/or palatability, in particular, the tenderness/toughness of meat.
The following summarises the primary biochemical and biophysical changes that occur during the conversion of muscle to meat. These are reviewed in more detail by Bendall (1973) and Tornberg (1996).
Rigor biochemistry
With respect to the biochemistry of rigor, much of our current knowledge stems from the pioneering work of Bendall and his colleagues (e.g. Bate-Smith and Bendall 1947; Bendall 1951; Bendall 1973), as well as the understanding of energy metabolism that occurs during exercise in living muscle (reviewed by Robergs et al. 2004). As noted above, after slaughter, anaerobic metabolism is initiated, at some point, in order to supply ATP for the continuation of cellular function. The high-energy-carrying ATP molecule forms the basis for the phosphagen system (Sahlin 1985; Kent-Braun et al. 1993). As for most biological reactions, hydrolysis of ATP (ATP + H2O ↔ ADP + Pi + H+) essentially provides the necessary energy (ΔGoʹ = 31kJ/mol) to regulate and drive muscle contraction. In living muscle, this energy value is nearly twice (60kJ/mol) as high because the steady-state metabolic state maintains ADP and Pi concentrations much lower than that at equilibrium (Kushmerick and Conley 2002). As mentioned above, bouts of exercise and events occurring in post-mortem muscle require vast amounts of energy in the form of ATP. The most immediate means of maintaining or restoring ATP levels in muscle involves phosphocreatine (PCr) as a critical ‘first response’ of the phosphagen system. Phosphocreatine contains a phosphate group that readily transfers to ADP via an enzyme known as creatine kinase (creatine phosphate + ADP + H+ ↔ creatine + ATP). It is important to understand that this reaction consumes a hydrogen ion and is at equilibrium because the change in free energy with this reaction is very close to zero in vivo. Therefore, any change on either side of the reaction will result in a compensation (balancing) by the opposite side of the reaction. This is particularly important during periods of recovery and subsequent re-establishment of the PCr pool. In exercising muscle, and certainly in the case of post-mortem muscle tissue, PCr reserves are exhausted quickly and ADP concentrations rise. There is evidence that the disappearance of PCr in muscle tissue early post-mortem may be especially relevant in development of aberrant meat quality, at least in the pig muscle (Copenhafer et al. 2006; Scheffler et al. 2011).
In non-steady-state conditions (Connett and Sahlin 1996), where consumption of ATP is greater than production, adenylate kinase (AK), or myokinase generates additional ATP through the reaction: ADP + ADP ↔ ATP + AMP. Generation of AMP is particularly important as will be discussed in some detail later, because AMP is a major regulator of energy metabolism in muscle cells (Winder and Thomson 2007). The final reaction of the phosphagen system is mediated by AMP deaminase (AMP + H+ ↔ IMP + NH4+) and is actually coupled to the previous reaction. Although the exact role of AMP deaminase is not known, it is important to understand that the latter two reactions occur mainly when mitochondrial respiration is incapable of supplying the necessary energy (ATP) demanded by the cells (Lowenstein 1990; Tullson and Terjung 1991; Tullson et al. 1996). In this case, the energy status of the muscle is compromised. Moreover, some suggest that AMP deaminase prevents AMP accumulation in the cell and, thus, helps facilitate ATP generation (Hellsten et al. 1999), thereby retarding fatigue in exercising muscle. Korzeniewski (2006) further modelled the role of these two reactions, and showed that the effective removal of ADP reduces proton production by anaerobic glycolysis and may help ‘delay’ a fatigue-induced termination of glycolysis during a heavy bout of exercise. Curiously, their models predicted that changes in AMP deaminase under hypoxic (low oxygen levels) conditions were capable of altering the ultimate pH of fatigued muscle by nearly 0.3 pH units—a truly remarkable change in muscle pH, especially considering the lack of variation surrounding the ultimate pH of meat. Regardless, these data outline the critical changes that occur in muscle in response to a functioning phosphagen system during a bout of exercise and show how these reactions may alter post-mortem metabolism.
As a consequence of the aforementioned reactions, reductions in muscle PCr and, in due course, ATP occur with a concomitant increase and decrease in lactate and muscle pH, respectively (Bendall 1973). The decline in muscle pH post-mortem or in exercising muscle has commonly been attributed to the increase in lactic acid, but this is a popular misconception, as the major source of H+ is clearly from ATP hydrolysis (see review by Robergs et al. 2004; discussed later). The resting pH of mammalian muscle ranges from 7.1 to 7.3 and will typically decline non-linearly to 5.5–5.6 in post-mortem muscle (Pearson and Young 1989). The attainment of ultimate pH coincides with the termination of glycolysis. This has been attributed to either a lack of available substrate (i.e. glycogen) or inactivation of one or more of the glycolytic enzymes due to the acidic conditions (Lawrie 1998), although the exact mechanism is yet unknown.
In his review, Bendall (1973) asserts that post-mortem pH decline is biphasic, characterised by an initial period of minimal change in both pH and ATP followed a phase of rapid decline in both. The initial period is termed the ‘delay phase’ and the conservation of ATP is attributed to the re-synthesis of ATP from PCr and ADP. As discussed previously, this reaction consumes a proton, and hence may buffer muscle pH decline. The resting concentrations of PCr vary depending on species, but a range of 18–23 μmol/g was reported by Bendall (1973). More recent estimates suggest that the concentration at slaughter may be much lower in beef (1–2 μmol/g; Hertzman et al. 1993) and sheep (3 μmol/g; Ferguson 2003) muscle. Given this and the lack of evidence to support a delay phase, mainly in beef m. longissimus (e.g. O’Halloran et al. 1997a; Butchers et al. 1998), the applicability and relevance of the delay phase has been questioned in conventionally slaughtered livestock (Ferguson 2003).
In many of the studies conducted by Bendall and his colleagues, the muscle relaxant myanesin was administered to the animal well before slaughter (Bendall 1973). Consequently, as Bendall (1973) observed, there was very little involuntary muscle contraction immediately following death. In commercial abattoirs, most animals endure some level of emotional stress and physical activity immediately before slaughter. It is well known that initiation of the adrenergic stress response causes elevations in cAMP, which allosterically activates glycogen phosphorylase and therefore glycolysis (Meyer and Foley 1996). Although β-adrenergic stimulation does not directly influence PCr hydrolysis (Ren and Hultman 1989), it is highly unlikely that PCr is spared during pre-slaughter handling, given its high catalytic capacity (Krause and Wegener 1996). Second, vigorous muscle contractions, particularly during the clonic phase after stunning, are evident in most, but not all animals. The absence of the delay phase in pH decline in conventionally slaughtered cattle and sheep is probably due to the combined actions of pre-slaughter stress and immediate post-stun muscle contractions, which deplete the PCr supply and rapidly activate glycolysis.
During the rapid phase, the ATP concentration declines markedly during rigor. Another key biochemical feature of the rapid phase is a rise in intracellular calcium concentration (Jeacocke 1993; Hopkins and Thompson 2001). Although there is consensus about this, the reported increases in concentration have varied considerably (Dransfield 1999), and this may in part be associated with accuracy of methods used to quantify cytosolic calcium concentration (Mickelson and Louis 1993). The increase in free calcium within the cytosol during and after rigor has been attributed to altered sarcoplasmic reticulum function specifically, leakage of calcium via ryanodine receptors, reduced activity of the calcium pumps due to depletion of ATP, and finally through disruption of membrane structures (Mickelson and Louis 1993).
The duration and rate of the rapid glycolytic phase, as is the case for most biochemical reactions, is temperature-dependent (Marsh 1954; Cassens and Newbold 1967a, 1967b; Newbold and Scopes 1967; Bendall 1973; Hertzman et al. 1993; Daly 1997; Ferguson 2003). However, it also important to recognise that variations in glycolytic rate can be observed, even at constant temperatures (Bendall 1978; Daly 1997; O’Halloran et al. 1997a). In the context of meat tenderness and other meat quality traits (e.g. colour, water-holding capacity), the interaction between post-mortem glycolysis and temperature in muscle is paramount.
Rigor biophysical changes
Myofibrillar shortening
Once the ATP concentration falls to ~50% of its resting level (5–6 μmol/g), ~50% of muscle elasticity has already been lost (Bendall 1951). In the absence of ATP, irreversible bonds between actin and myosin form, leading to muscle shortening and an increase in the isometric tension (Pearson and Young 1989). The degree of shortening that occurs during rigor is critical with respect to tenderness (Harris and Shorthose 1988). The seminal work of Locker and Hagyard (1963) clearly demonstrated that the degree of muscle shortening (relative to pre-rigor length) was highly dependent on the temperature at rigor. In their study, minimal shortening (~10%) was observed at 15−20°C in beef m. sternomandibularis. In later studies from Sweden by Hertzman et al. (1993), Olsson et al. (1994), and Devine et al. (1999), the evidence suggests that the optimal rigor temperature range might be lower, at 10−15°C, in higher quality muscles such as the m. longissimus and m. semimembranosus.
While the functional relationship between the degree of shortening and either objective (e.g. shear force) and/or sensory panel assessments of tenderness/toughness is widely acknowledged (see review by Tornberg 1996), there are exceptions to this dogma (Smulders et al. 1990; Shackelford et al. 1994). Smulders et al. (1990) demonstrated variation in tenderness/toughness that was independent of sarcomere length. In their study, the relationship between sarcomere length and panel tenderness scores of unaged, m. longissimus steaks was almost non-existent for those samples where faster rates of post-mortem glycolysis (i.e. pH <6.3 at 3 h post-mortem) had occurred. The explanation to this hitherto incongruent outcome can be attributed, in part, to the contribution of proteolysis.
Attenuation of rigor
The attenuation of rigor is also known as the tenderisation phase, where the rigor-induced increase in isometric tension is reduced to some degree post-mortem through the actions of enzymatic processes. These cause a weakening of the myofibrillar matrix through the degradation of key structural proteins, which in turn, manifests as an improvement in tenderness. These degradative changes are exploited for commercial benefit via the practice known as ageing, which involves storing meat at low temperatures for periods of several days to weeks.
In his review of the proteolytic systems involved in post-mortem muscle tenderisation, Ouali (1992) identified three main endogenous protease systems: (i) calcium-dependent calpain system (μ-calpain, m-calpain and their inhibitor calpastatin); (ii) lysosomal cathepsins (cathepsin B, D, H and L); and (iii) ubiquitin-proteasome complex (also known as the multicatalytic proteinase). Debate has ensued regarding the relative contributions of these proteinases, particularly the most extensively studied calpains and cathepsins (Koohmaraie 1992, 1996; Roncales et al. 1995; Ouali et al. 2006). However, based on the in vitro data, μ-calpain appears to be responsible for the majority of the post-mortem tenderisation (Roncales et al. 1995; Koohmaraie 1996; Dransfield 1999; Koohmaraie and Geesink 2006).
There has been debate over when proteolysis commences post-mortem. Evidence shows that it begins quite early after stunning (Troy et al. 1986; Koohmaraie et al. 1987). Whether the initial proteolytic activity results in significant structural weakening is a matter of conjecture (Dransfield et al. 1992). Dransfield et al. (1992) argued that, under normal rigor conditions, calpain-mediated tenderisation commences approximately midway through the development of rigor (pH 6.1–6.3).
The prevailing conditions of pH and temperature post-mortem also govern the rate of calpain activation and inactivation (Dransfield 1994; Simmons et al. 1996). In rapidly glycolysing muscle, the rate of proteolytic activity as measured directly (Simmons et al. 1996) and indirectly via electrophoresis (O’Halloran et al. 1997a, 1997b) was accelerated. Dransfield (1994) predicted that calpain activities would be six times greater following rapid glycolysis (i.e. pH 5.5 at 2 h post-mortem) than at more standard rates of glycolysis (i.e. pH 5.5 at 20 h) under standard cooling conditions. Part of this can be explained by the decrease in inhibitory activity of calpastatin during the early post-mortem period when the pH falls below 6.4 (Dransfield 1995; O’Halloran et al. 1997b). Consequently, during rapid glycolysis, the majority of the tenderisation can occur within the first 24 h post-mortem, with very little response to further ageing (Dransfield 1995).
An important caveat here with respect to proteolytic activation during rapid glycolysis is that this only applies when the temperature decline is commensurate with rate of pH decline (Dransfield 1994). This unfortunately is not easily achieved under standard carcass-chilling regimes in commercial abattoirs. Consequently, the corollary of rapid glycolysis is, quite often, higher rigor temperatures. This is undesirable on two fronts. First, it can increase the risk of heat shortening, and second, and perhaps more importantly, it accelerates the inactivation of calpains through autolysis (Dransfield 1994, 1995). In some cases, the rate of inactivation can exceed the rate of calpain activation, thus leading to minimal tenderisation (Dransfield 1995). Consequently, the combination of high temperature and rapid glycolysis can facilitate increases in toughness with very limited ageing potential.
The study by Simmons et al. (1996) nicely illustrates the autolytic effect of temperature on proteolytic activity and the impact on ageing response. They showed that the different pre-rigor temperatures (15, 25 and 35°C) resulted in a 16%, 49% and 74% reduction in calpain activity, respectively, at rigor (i.e. pH 5.5). Higher rigor temperatures also resulted in increased shortening as measured by sarcomere length. Despite this, meat held at 35°C was more tender at rigor than meat held at either 15 or 25°C, indicating that rapid proteolysis helped to negate some of the loss in tenderness through increased shortening. However, any advantage was quickly lost with further ageing, as the reduction in shear force was substantially greater at lower rigor temperatures (45–50%) than at 35°C (20%). This reinforces the assertion of Dransfield (1994, 1995) that high rigor temperatures limit the ageing capacity of meat. Further evidence of this is provided in the more recent studies of Devine et al. (1999) and Devine et al. (2002).
Based on the above evidence, the fast declines in both glycolysis and temperature appear to be optimal with respect to minimising the degree of shortening and maximising the rate and extent of proteolytic tenderisation.
Glycolysis
Glycolysis in muscle is largely responsible for rephosphorylation of ADP during a bout of exercise when oxygen becomes limiting and cannot support mitochondrial respiration, or during post-mortem metabolism. Glycolysis or ‘sweet (sugar) dissolution (loosening)’ is a series of 10 biochemical reactions responsible for metabolising a six-carbon molecule into two three-carbon molecules and, in the process, generates ATP. Often, glycolysis is separated into two phases. The first phase, or preparatory phase, involves five reactions that metabolise the six carbon hexoses into one common product, glyceraldehyde 3-phosphate. The second set of five reactions yield ATP, yet retaining the three-carbon molecules, albeit in the form of pyruvate.
The first rate-limiting enzyme of glycolysis is phosphofructokinase (PFK), which irreversibly catalyses the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate. PFK is often cited as the most profound control point of glycolysis in living tissue, and it may facilitate termination of muscle metabolism post-mortem. To that end, PFK responds positively to AMP, fructose 1,6-phosphate, ADP, phosphate and K+. Conversely, the action of PFK is dramatically attenuated by the presence of ATP and citrate. The latter is a product of fatty acid oxidation. Using his classic reconstituted glycolytic system, Scopes (1974) showed that even at a pH as low as 5.35, PFK remained strongly active, arguing that PFK, although possibly modulating the rate of pH decline, clearly was not responsible for dictating the cessation of glycolysis. Schwägele and Honikel (1988) further supported this notion by showing that PFK activity changed little in pig muscle, ranging in pH from 5.3 to 6.8 at 45 min post-mortem. Even so, there is doubt about whether these enzyme assays were conducted at a neutral pH or at a pH reflecting the environment from which they were extracted. Regardless, PFK remains as one of the most likely candidates in modulating post-mortem metabolism.
Pyruvate kinase (PK), one of the more distal enzymes in the glycolytic pathway, irreversibly catalyses the conversion of phosphoenolpyruvate to pyruvate. It exists in at least three different isoforms and is negatively controlled by ATP, and by acetyl-CoA and fatty acids, both substrates of the TCA cycle. Schwägele et al. (1996) reported that the activity of PK from pale, soft and exudative (PSE) meat was greater at lower pH values. Using isoelectric focusing they proposed that an additional isoform might exist in pigs, making the muscle more prone to a quality aberration. However, such an isoform has not been identified and may simply be an artefact of fast-glycolysing muscle.
Creation of lactate is often considered part of glycolysis. However, lactate is the ultimate product of anaerobic metabolism and may be one of the most misunderstood biochemical events in meat science and among the meat animal community (Scheffler and Gerrard 2007; Scheffler et al. 2011), as well as in exercise physiology (reviewed by Robergs et al. 2004). As outlined previously, the general perception of post-mortem metabolism is that glucose is converted to ‘lactic acid’ and the latter causes muscle pH to decline. However, it is important to understand that lactate formation during anaerobic metabolism serves two purposes. First, this reaction regenerates NAD+, a co-enzyme required by a more proximal glycolytic reaction using the enzyme glyceraldehyde phosphate dehydrogenase (Lehninger et al. 1993). If NADH is not oxidised, glycolysis stops prematurely, or proceeds at an extremely reduced rate. Second, during the reduction of pyruvate to lactate, one hydrogen from NADH and one hydrogen from solution are removed from the cytoplasm. If glycolysis were to proceed without the formation of lactate, the concentration of H+ would increase more rapidly, overwhelming the buffering capacity of the muscle (i.e. Pi, amino acids and various proteins) and decreasing cytosolic pH quicker, thereby compromising the power surrounding contraction, which ultimately results in fatigue (Fitts 1994; Westerblad et al. 2002). Lactate formation essentially prolongs glycolysis by retarding the increase in free hydrogen accumulation in the cytosol (Robergs 2001). In support, the pKa, or the negative log of the dissociation constant of lactate, is ~3.86; therefore, lactic acid is always dissociated and exists as a salt rather than an acid in the muscle tissue. So at any given time during post-mortem metabolism, most of lactate exists in an unprotonated state. Furthermore, recall that the equilibrium constant favours the development of lactate, meaning that any time pyruvate entry into the mitochondria is slightly slowed, lactate is preferentially and immediately formed. These data show the utility of lactate formation for extending anaerobic muscle metabolism and suggest that lactate accumulation is a good indicator of the extent and rate of glycolysis; however, they identify a major pitfall of using lactate directly to predict muscle pH decline.
Some of the most informative studies conducted in the area were reported by Robert K. Scopes while at the Meat Research Institute in Bristol. In particular, he showed that glycolysis was regulated by ATPase activity. Either directly or indirectly, the activities of the aforementioned enzymes are virtually controlled by cellular levels of ADP. When ATP is plentiful, minimal glycolysis is necessary. As such, glycolysis occurring post-mortem is greatly impacted by the disappearance of ATP (Scopes 1974).
Of particular significance to muscle cells are: myofibrillar ATPase (mATPase), sarcoplasmic reticulum Ca2+-ATPase (SR-ATPase), plasmalemma Na+,K+-ATPase, plasmalemma Ca2+-ATPase, and mitochondrial ATPase. Activites of mATPase, SR-ATPase and mitochondrial ATPase are similar immediately post-mortem (Greaser et al. 1969). However, given the abundance of mATPase in skeletal muscle, ATP consumption by the myofibrillar component likely drives post-mortem metabolism. One of the best examples of a rapid glycolysis in muscle post-mortem is that associated with halothane-positive pigs, which often results in PSE pork. In this regard and given the above discussion, the mATPase activity of muscle in PSE-generating pork muscle should be quite high. Contrary to this thesis, however, Greaser et al. (1969) reported mATPase activity is actually greater in myofibrils from normal than halothane-positive pigs. Honikel and Kim (1986) corroborated this finding by showing that sarcomeres of isolated myofibrils from PSE pork were unable to shorten, whereas those of normal pork remained largely functional. These data argue strongly against mATPase driving glycolysis in muscle from halothane-positives pigs. Alternatively, it is well known that the functional difference between halothane-positive and normal pigs resides largely in the ability of the muscles to maintain sarcoplasmic calcium levels. Specifically, halothane-positive pigs possess a mutation in the ryanodine receptor protein, which in muscle functions as a calcium release channel embedded in the sarcoplasmic reticulum (Mickelson et al. 1989). Although difficult to measure, calcium levels in muscle of mutated animals are indeed elevated over those in muscle possessing a normal calcium channel (Lopez et al. 1986; Iaizzo et al. 1988). As a result, ATP consumption by the SR-ATPase directly would remain quite high in the muscle of mutated animals. In response to this aggravated calcium homeostasis, mATPase would be elevated due to rising levels of cellular calcium, and this loss of ATP, or increase in ADP, would increase flux through glycolysis. In fact, Strasburg and Chiang (2009) and others (Allison et al. 2003) have argued that the bulk of variation observed in turkey and pork quality, respectively, is manifested in polymorphisms within the ryanodine receptor gene, which is intuitive given the size and complexity of the tetrameric protein and associated genes (Rossi and Sorrentino 2002). Regardless of the exact ATPase responsible, there is little question that glycolysis responds to declining ATP levels and, by design, must have access to ADP to rephosphorylate.
Continuing the line of thought that energy status (ATP) modulates glycolytic flux, existence of cellular AMP reflects a compromised energy status and, therefore, is a logical activator of glycolysis (Lehninger et al. 1993). Although AMP can directly alter enzyme activity through allosteric means, the primary sensor of cellular AMP in muscle cells is AMP kinase (AMPK), especially in living tissue. Under conditions where the energy expenditure is pronounced, AMP binds to AMPK and becomes activated through various phosphorylation events involving upstream kinases. This, in turn, increases energy-producing biochemical pathways and down-regulates those pathways consuming energy (Winder and Thomson 2007), such as glucose uptake, glycolysis and oxidative phosphorylation, and fatty acid and glycogen syntheses pathways, respectively. Given the profound ability that AMPK activation has on energy metabolism in living muscle, some authors have logically argued that AMPK activation may drive post-mortem glycolysis. Shen and Du (2005) first showed that muscle of mice lacking a functional AMPK failed to experience a normal pH decline post-mortem compared with wild-type mice. They further showed that muscle of mice treated with 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR), a chemical that activates AMPK, had a greater reduction in pH than normal mice. Subsequently, they reported an association between muscle with increased AMPK activation and muscle ultimately developing PSE pork (Shen et al. 2006a). Making the connection to animal handling, Shen et al. (2006b) then reported that pigs transported greater distances had greater AMPK activity and poorer meat-pork quality characteristics. Although changes in AMPK activation before death were not ruled out as a driver of post-mortem metabolism, Shen et al. (2008) culminated their efforts to show AMPK was a driver of meat quality development by showing that administration of compound C, an AMPK inhibitor, to animals immediately before exsanguination was capable of severely retarding muscle pH decline. These data strongly argue that activated AMPK, possibly through modulation of phosphofructokinase 1 and 2 activities—the latter catalyses the production of fructose 2,6-bisphosphate and increases PFK1 activity—may impact post-mortem metabolism and subsequent meat quality development. Others supported this notion by showing that global phosphorylation patterns, possibly through AMPK-mediated events or otherwise, occur post-mortem and may alter the transformation of muscle to meat (Huang et al. 2011; Lametsch et al. 2011).
Alternatively and as pointed out above, it is possible that energy charge of the muscle before slaughter remains the culprit of adverse meat quality development. Although hardly a valid argument, this is more in line with theories initially proposed to underlie handling-related quality issues (Warriss 1990). In the case of the AMPK-mediated aberrations in meat-quality development, a gain-in-function mutation in the gamma 3 subunit has been identified in Rendement Napole pigs (RN) (Milan et al. 2000). These pigs possess muscle chronically attempting to generate extra energy (ATP) because the AMP binding portion of the enzyme is altered in such a manner that it ‘thinks’ it is bound to AMP. Even though, we (Copenhafer et al. 2006) and others (Monin and Sellier 1985; Fernandez et al. 1992; Milan et al. 2000) have shown that RN pigs possess higher glycogen levels than their normal counterparts, these pigs have greater PCr immediately post-stunning (Copenhafer et al. 2006). As discussed, altering the phosphagen system has clear implications on glycolysis, and this, with other as-yet unknown energy substrates, may alter the beginning energy status of muscle at the point of death and thereby alter post-mortem metabolism rather than the fact they have greater activated AMPK at slaughter.
Given that RN pigs produce ‘acid meat’ and have a lower ultimate pH (Monin and Sellier 1985), presumably from augmented, carbohydrate-based metabolism (Przybylski et al. 1994), it is odd that muscle cross-sections stain more intensely for oxidative enzymes (Lebret et al. 1999). This raises the question of how mitochondria contribute to post-mortem metabolism, an issue that has often been ignored in meat science. Energy is clearly stored in the electrochemical gradient that exists across the inner mitochondria membrane, and is captured through the rephosphorylation of ADP by F1F0-ATP synthase in the presence of oxygen. Curiously, however, this synthase reverses itself and functions as an ATPase when oxygen is limiting, in an ill-fated attempt to retain the proton motive forces within the mitochondrion (Scott and Nicholls 1980). The additional consumption of ATP in this manner has been proposed as ‘mitochondrial treason’ and may change the way post-mortem is classically understood, especially in muscle considered more oxidative in nature or the inherent differences between muscles varying in fibre-type composition (Hudson 2012).
Pöso and Puolanne (2005) suggested that transient oxidative metabolism may only account for a small (1–5%) fraction of ATP produced post-mortem by oxygen bound to residual haemoglobin in muscle. This follows on the evidence from Hochachka (2003), who showed that myoglobin is capable of buffering oxygen levels in the muscle during non-steady-state levels of exercise. Indeed, some level of oxidative metabolism can occur during periods of hypoxia or reduced oxygen saturation levels (Marcinek et al. 2003). Therefore, unless oxygen saturation of myoglobin abruptly goes to zero at exsanguination, mitochondrial function may occur during the earliest periods of post-mortem metabolism, perhaps buffering energy depletion and modifying subsequently the transformation of muscle to meat. Alternatively, mitochondria may exacerbate the process by augmenting ATP consumption. Regardless, additional work in this area is needed to understand the full impact of the mitochondria and the cessation of oxidative metabolism on post-mortem metabolism and meat quality development.
Glycogenolysis
Glycogen structure
The structure and metabolism of glycogen were recently reviewed by Roach et al. (2012). Glycogen exists as a branched-chain polymer of glucose. The molecule is believed to comprise ~10 000 glycosyl units linked by α-1,4 (93%) and α-1,6 (7%) glycosidic linkages (Connett and Sahlin 1996). The basic structure of glycogen has been studied extensively, and the Whelan model (Gunja-Smith et al. 1970) is generally accepted (Goldsmith et al. 1982; Melendez-Hevia et al. 1993). The biogenesis of glycogen commences with a self-glycosylating protein primer glycogenin (hexosyltransferase) (see reviews by Alonso et al. 1995; Roach et al. 2012). Based on the Whelan model (Gunja-Smith et al. 1970), the different glycosidic linkages give rise to two different types of branched chains: B-chains, which are branched, and the non-branched A-chains. The degree of branching occurs by a uniform factor of two. Therefore, every B-chain has two branches on it. The molecule is arranged in concentric tiers where the number of chains in any single tier is double that of the previous tier. The mathematical modelling studies of Melendez-Hevia et al. (1993) indicate that the structure of glycogen is functionally optimal as it maximises the storage of glucose within a small volume and the number glycosyl units that are readily phosphorylated.
Histochemical studies (Fridén et al. 1989) reveal that glycogen particles are distributed over five subcellular locations: the subsarcolemmal, intermyofibrillar, para-Z-disk, N2 line (located in the I band), and H zone spaces. Those authors proposed a functional association with these storage depots and were able to demonstrate that the stores near the Z disks and N2 lines were preferentially depleted during exercise. Fridén et al. (1989) also observed the existence of different-sized populations of glycogen particles. Further investigation has revealed that glycogen is present in two discrete forms, which are identifiable based on their solubility in perchloric acid (PCA) and their size (Jansson 1981; Alonso et al. 1995). The smaller (up to 400 kDa) form, known as proglycogen (PG), is not soluble in PCA because of its higher protein/carbohydrate ratio. It is estimated that 10% of proglycogen is protein in the form of glycogenin. By contrast, the more ‘mature’ macroglycogen (MG), by virtue of its size (~103 kDa) and therefore considerably smaller protein/carbohydrate ratio, is soluble in PCA.
The relative proportions of PG and MG vary depending on the total glycogen content (Adamo and Graham 1998; Derave et al. 2000; Bröjer et al. 2002). However, the methodology used to determine the concentrations of PG and MG has been challenged, as it may lead to overestimation of the PG fraction (James et al. 2008). Notwithstanding this, the proportion of total glycogen as MG is recorded as 40% in horses (Bröjer et al. 2002), 24–25% (estimated) in pigs (Young et al. 2009), and 46–57% in sheep (Ferguson et al. 2008).
The physiological roles of these two forms and indeed their metabolic regulation are not fully understood (Alonso et al. 1995; Graham et al. 2001). Contention remains about whether they are discrete entities or whether PG merely represents a continuum of smaller glycogen particles (Alonso et al. 1995; Roach et al. 2012). There is evidence that catabolism of PG and MG may be differentially regulated (Huang et al. 1997; Asp et al. 1999; Derave et al. 2000; Shearer et al. 2001; Graham et al. 2001). In human studies, the results strongly indicate that PG is preferentially depleted over MG during the initial stages of physical activity in muscles with normal to high glycogen concentrations (Graham et al. 2001; Shearer et al. 2001). At lower glycogen concentrations, the two pools contribute equally to glycogenolysis (Shearer et al. 2001). In pigs, the results have been equivocal (Rosenvold et al. 2003, 2010).
Glycogen concentration in ruminant muscle
The muscle glycogen concentration at rest in normal healthy sheep and cattle ranges from 75 to 120 μmol/g (Monin 1981; Lambert et al. 1998; Pethick et al. 1999; Immonen et al. 2000; Gardner 2001; Ferguson et al. 2007). Glycogen levels will vary between muscles, a reflection of their metabolic profile, specifically, fibre type. For example, Monin (1981) reported that ovine muscles with a high proportion of type IIa fibres had higher glycogen concentrations (90–105 μmol/g) than muscles predominantly comprising either type I or IIb fibres (75–80 μmol/g). However, the differences were not as well defined between bovine muscles. Nevertheless, Monin (1981) did observe that glycogen concentration increased in muscles with a higher percentage of type II fibres, which was corroborated by Lacourt and Tarrant (1985). Furthermore, studies in cattle (Pethick et al. 1999; Gardner 2001) suggest, based on the histochemical classification of bovine muscles (Totland and Kryvi 1991), that muscles high in IIa fibres (fast oxidative/glycolytic) have higher glycogen contents than predominantly IIb muscles (fast glycolytic).
Glycogen concentration is, of course, central to the extent of post-mortem pH decline. In healthy animals not unduly stressed before slaughter, this should not be limiting factor, as the concentration is generally higher that that required to attain normal ultimate pH (5.5–5.6). From studies investigating the relationship between pre-slaughter glycogen levels or glycolytic potential – defined by Monin and Sellier (1985) as [lactate] + 2([glycogen] + [glucose-6-phosphate] + [glucose]) – and ultimate pH, it is evident that ultimate pH is only affected once the pre-slaughter glycogen level falls below the critical threshold of 45–55 μmol/g (Howard 1963; Monin 1981; Warriss 1990; Wulf et al. 2002).
Regulation of glycogenolysis
Although the initial substrate ‘powering’ glycolysis is often thought to be glucose, glycogenolysis, or the breakdown of glycogen is actually the sequential liberation of glucose 1-phosphate residues from glycogen by the enzyme glycogen phosphorylase (GP) (Lehninger et al. 1993). This reaction is one of the rate-limiting steps of anaerobic metabolism post-mortem as oxygen is depleted from the tissues. In living tissues, glucose originates in the blood and is immediately phosphorylated to glucose 6-phosphate (G6P) by hexokinase. Once phosphorylated, it is shuttled to storage as part of a larger glycogen molecule, or it enters glycolysis. Free glucose arising from glycogenolysis is not likely to be phosphorylated post-mortem, because G6P is a potent inhibitor of hexokinase. The bottom line is that entry of a glucose molecule into glycolysis requires energy (1 ATP), whereas glycogen residues are already in a form (G6P) to begin metabolism. Although seemingly trivial in nature, it is critical to understanding the origin and significance of various protons emanating from glycolysis and ATP hydrolysis post-mortem. This fact alone causes some disparity in how energy metabolism in exercising muscle differs from that occurring in post-mortem muscle. After all, generation of free hydrogens is responsible for changes in muscle pH post-mortem, and whether glycolysis begins with glucose or G6P can dramatically affect calculations defining the source net hydrogen ion production (Hamm 1977; Robergs 2001). Regardless, once a glucose residue is liberated from glycogen, it is rapidly converted to G6P by an enzyme that repositions the phosphate on the sugar.
Two enzymes operate in parallel during glycogenolysis. As discussed, GP is responsible for the transfer of a glycosyl unit to inorganic phosphate to form glucose-1-phosphate (Connett and Sahlin 1996). It targets the α-1,4 glycosidic linkages on the A-chain branches only, and continues until it reaches a point where four residues remain before the α-1,6 branch point. The bi-functional enzyme amylo-1,6-glucosidase, or debranching enzyme as it is commonly known, is then required to catalyse two successive reactions that result in the transfer of a block of three residues to a nearby non-reducing end (i.e. α-1,4 chain) and the release of the final residue as free glucose (Lehninger et al. 1993). GP is primarily responsible during the initial phase of glycogenolysis, since 40–50% of the glycosyl units exist as α-1,4 linkages in the outer branches (Connett and Sahlin 1996). Consequently, debranching enzyme is not required until the limit dextrin has been reached on each A-chain.
Glycogen phosphorylase exists as two inter-convertible forms and it is regulated by both substrate and allosteric control mechanisms. Consequently, it is therefore recognised as a key rate-limiting enzyme for glycogenolysis (Stanley and Connett 1991; Connett and Sahlin 1996). A cyclic process of covalent modification regulates the inter-conversion between the active form of GP (GPa) and the less active form (GPb) (Meinke and Edstrom 1991). While GPb is less active, it is still allosterically capable of activation by the presence of AMP in times of great energy demand. Conversion to GPa is governed by phosphorylase kinase, which in turn is regulated by calcium (Ca2+, pH, and by β-adrenergic stimulation via cyclic AMP (cAMP); Hargreaves and Richter 1988; Meinke and Edstrom 1991; Connett and Sahlin 1996). The biochemical cascade of events initiated by β-adrenergic stimulation commences with the epinephrine-mediated increase in cAMP, which results in activation of cAMP-dependent protein kinase. This enzyme phosphorylates phosphorylase kinase, which catalyses the conversion of GPb to GPa. The activation of phosphorylase kinase by cAMP and Ca2+ therefore couples activation of glycogenolysis to the adrenergic stress response (i.e. fight or flight response) and muscle contraction, respectively. GP is also allosterically regulated where AMP and IMP are potent activators, while ADP, ATP and glucose-6-phosphate all inhibit GP activity (Connett and Sahlin 1996).
The phosphorylation of GP (i.e. conversion to active form) does not automatically signify higher rates of glycogenolysis. Chasiotis (1988) showed that following epinephrine infusion, the percentage of GP in its active form increased from 22% to 80%. However, despite this, there was minimal effect on glycogenolytic rate. Chasiotis (1988) attributed the low glycogenolytic rate to substrate inhibition of GP by the normally low inorganic phosphate levels in resting muscle. This is overcome at the commencement of muscle contraction where the phosphate concentration rises rapidly through ATP hydrolysis and rephosphorylation of PCr.
In addition to phosphate, evidence from human and rodent studies suggests that glycogen concentration may also regulate GP activity and therefore glycogenolysis (Richter and Galbo 1986; Hespel and Richter 1990, 1992; Hargreaves et al. 1995, 1997; Vandenberghe et al. 1999; Shearer et al. 2001). Paradoxically, the reported Km of GP for glycogen is reported as 1–2 mm (Newsholme and Leech 1983). Therefore, the enzyme should be fully saturated with its substrate given that the normal resting concentrations of glycogen in mammalian muscle ranges from 80 to 100 mm (Connett and Sahlin 1996). However, the relevance of the in vitro Km estimates to in vivo conditions has been questioned (Hargreaves and Richter 1988; Connett and Sahlin 1996).
Further contention about whether glycogen concentration regulates glycogenolysis has been fuelled by equivocal results. Whereas Richter and Galbo (1986), Hespel and Richter (1990, 1992), Hargreaves et al. (1995), Hargreaves et al. (1997), Vandenberghe et al. (1999) and Shearer et al. (2001) all found a positive effect, others (Ren et al. 1990; Spriet et al. 1990; Bangsbo et al. 1992) could not establish an effect. Some of the disparity appears linked to methodological issues, in particular, the intensity of the exercise and the variation in glycogen concentration. According to Vandenberghe et al. (1999), a positive association was more apparent during prolonged exercise than short-term intense activity, although this has not always been the case (e.g. Shearer et al. 2001).
Of significance, however, in the studies by Vandenberghe et al. (1999) and Shearer et al. (2001) was the finding that the active form of GP was elevated in the presence of high glycogen concentrations. This agrees with earlier reports for rodent muscle by Richter and Galbo (1986) and Hespel and Richter (1992). If the reported Km for GP and glycogen is erroneous, the coupling between GP transformation and glycogen concentration could therefore account for the higher glycogenolytic rates. However, this begs the question: what is the mechanistic basis for the coupling in the first instance? Shearer et al. (2001) hypothesised that the substrate-mediated increase in the transformation of GP might be linked to the association and dissociation of GP and other relevant enzymes with the glycogen particle. It is generally accepted that the glycolytic enzymes exist as bound complexes either within the cytosol or with cellular structures (see reviews by Brooks and Storey 1991; Roach et al. 2012). Furthermore, the state of these complexes is not fixed, and this is believed to be implicit in the cellular regulation of glycolytic flux. Using this as a basis, Shearer et al. (2001) put forward the view that the increased glycogenolytic rate may be because more GP is bound with glycogen when glycogen levels are high.
The question of whether the association between glycogen concentration and glycogenolysis was relevant to post-mortem biochemistry in ovine muscle was examined by Daly et al. (2006) and Ferguson et al. (2008). Only Daly et al. (2006) found glycogen concentration to influence the magnitude of the pH response to post-slaughter electrical stimulation. However, there was agreement between the studies regarding the positive association between glycogen concentration and rate of pH decline adjusted to a constant temperature of 38°C (refer Bendall 1978). A significant aspect of both studies was that the muscle glycogen concentration in both studies was relatively low (40–55 μmol/g). Therefore, this does not align with the view by Shearer et al. (2001) that accelerated glycogenolysis is evident primarily at higher glycogen concentrations. This again raises an issue that is paramount to the study of post-mortem metabolism—‘what is the energy status of the tissue prior to slaughter?’—as altered metabolism post-mortem is profoundly a function of those physiological changes that occur in the tissues preceding slaughter.
Clearly, further examination of the association between pre-slaughter muscle glycogen concentration and post-mortem glycolysis is warranted. However, the data and hypotheses of (Copenhafer et al. 2006; Scheffler and Gerrard 2007; Park et al. 2009; Scheffler et al. 2011), including earlier observations of Sellier and Monin (1994), strongly suggest that factors other than glycogen concentration underpin the variation in the rate and extent of post-mortem pH decline.
Factors influencing post-mortem glycolytic rate in ruminant muscle
As stated earlier, the time-dependent changes in pH are typically used to estimate glycolytic rate in post-mortem muscle. In general, depending on the rate of chilling, normal ultimate pH (i.e. 5.5–5.6) in ruminant muscle is attained within 24–48 h (Tarrant and Mothersill 1977; Lister et al. 1981). Glycolytic rate can vary substantially between contemporary animals (O’Halloran et al. 1997a; Butchers et al. 1998; McGeehin et al. 2001) and between muscles within the carcass (Tarrant and Mothersill 1977). The intrinsic and extrinsic factors inherent to this variance are discussed below. It is worth highlighting that, in general, this area has not received critical attention, particularly in ruminants. The opposite is true with respect to the knowledge pertaining to pig muscle, largely because of their inherently faster glycolytic rates and because this can be further accelerated in certain genotypes (e.g. differences in halothane gene expression).
Animal and ante-mortem factors
Muscle fibre type
Muscles vary with respect to their fibre-type composition. These variations in fibre type are reflected by differences between muscles in the physical appearance, mechanical properties and metabolic rates. Not surprisingly, divergent patterns in the post-mortem rates of glycolysis are also evident. Muscles with a high proportion of oxidative type I fibres display faster rates of pH decline than predominantly type IIb fibre muscles (Devine et al. 1984; Aalhus and Price 1991). This seems incongruous, as muscles with a higher proportion of type IIb fibres, and therefore higher activities of glycolytic enzymes (Monin 1981; Talmant et al. 1986), might be expected to have faster rates of pH decline. Aalhus and Price (1991) postulated that the increased buffering capacity in type IIb muscles might explain the apparent anomaly. The data of Talmant et al. (1986) certainly support the fact that slow-twitch, type I muscles have a lower buffering capacity than type II muscles. In their study based on 18 bovine muscles, the buffering capacity ranged from 40 to 55 μmol lactate/g.pH unit.
Importantly, glycogen concentration varies between the fibre types, and this, in turn, can influence the extent of post-mortem glycolysis and therefore ultimate pH. Muscles with predominantly type I fibres have lower glycogen concentrations than those with type II or fast twitch muscles (Monin 1981; Talmant et al. 1986).
Breed/genotype differences
The effects of genotype and environmental factors on the variance in meat quality traits were recently reviewed by Warner et al. (2010). Genetic differences are certainly evident for important traits such as tenderness in cattle (Burrow et al. 2001; Johnston et al. 2003) and sheep (Mortimer et al. 2009; Warner et al. 2010). However, it was not clear whether this could be accounted for by genetic differences in glycolytic rate, because the trait was rarely measured.
Studies contrasting Bos taurus and Bos indicus beef quality (Wheeler et al. 1990a, 1990b; Shackelford et al. 1991) suggest that the rate of pH decline may be slightly slower in Bos indicus muscle. However, it is not clear whether there were differences in the rates of temperature decline, which may have confounded the intrinsic rates of pH decline. This is an important point, as breed differences in maturity pattern will give rise to variations in carcass weight and fatness and, therefore, to variability in carcass cooling rates. Consequently, this may influence the rate of glycolysis, so care needs to be exercised when interpreting data on pH decline made on cooling carcasses. However, it is possible to correct mathematically for the temperature-sensitive variation in pH decline (Bendall 1978), and this will facilitate more meaningful comparisons of pH decline when measurements are made on cooling carcasses.
In a lamb study contrasting five genotypes based on Poll Dorset, Border Leicester and Merino breeds, Hopkins et al. (2007) did not observe differences in post-mortem glycolytic rate (m. longissimus).
With respect to genotype, two genes associated with muscle hyperplasia in cattle (myostatin gene) and muscle hypertrophy in sheep (callipyge gene) may also indirectly influence post-mortem glycolytic rate. Concomitant with the increase in muscle mass, the proportions of type IIb fibres and type IIa fibres increased and decreased, respectively, in double-muscled cattle and callipyge lambs (Holmes and Ashmore 1972, Carpenter et al. 1996; Greenwood and Dunshea 2009). Based on the observations of Devine et al. (1984) and Aalhus and Price (1991), slower rates of pH decline might be expected in these muscles. However, in practice, this may be offset by the increased muscle mass, which will retard muscle temperature decline.
Sex
Due to a lack of comparative studies, little can be concluded about differences in glycolytic rate between the sexes. In one study in lambs, McGeehin et al. (2001) observed faster rates in females than males. Differences in maturity and therefore fat cover may have been a contributing factor.
Nutrition and production system
Nutritionally mediated differences in carcass weight and fatness will influence cooling rate and, potentially, glycolytic rate (Jacob and Hopkins 2014). Grain-fed cattle show elevated core body temperature relative to grass-fed cattle (Jacob et al. 2014), and this can contribute to accelerated glycolytic rate post-slaughter. Furthermore, cattle on grain-based diets show a lower pH early post-slaughter and a higher rigor temperature than grass-fed cattle, even after adjustment for carcass weight and fatness (Warner et al. 2014). In another example, pronounced differences in glycogen concentration can be achieved through varying either the level of feeding or the energy density of the diet (Pethick et al. 1999). As discussed above, glycogen concentration has been shown to influence glycogenolytic rate.
The daily level of physical activity, which can vary between extensive and intensive production systems, may also indirectly affect post-mortem glycolysis, once again through changes in the fibre-type profile. Vestergaard et al. (2000) investigated the effect of finishing system on muscle fibre characteristics in young bulls. In contrast to extensively reared bulls (loose housing plus pasture), the bulls that were intensively housed (tethered in stalls) had lower proportions of type I and IIa fibres and a higher proportion of type IIb fibres. A similar trend, but only for type I fibres, was observed by Moody et al. (1980) in their study comparing pasture and feedlot lambs. Vestergaard et al. (2000) attributed the transition in muscle fibre profile to differences in activity level. This assertion is supported by the general conclusion from human and animal studies that, in response to endurance exercise, there is an increase in muscle oxidative capacity, which is manifest by an increase in type I and IIa fibres (Aalhus and Price 1991; Henriksson 1992; Essen-Gustavsson 1996). The exercise study in lambs by Aalhus and Price (1991) is relevant in the context of this review, as the post-mortem pH declines were measured. Despite the increase in the percentage of type I fibres with exercise, the rate of post-mortem pH decline was comparable to that measured in non-exercised controls. Thus, small changes in fibre-type distribution might not always correlate with altered rates of post-mortem glycolysis.
Pre-slaughter stress
The impact of pre-slaughter stress on muscle glycogen loss and the deleterious consequences for meat quality have been extensively studied in meat animals (see reviews by Tarrant 1989; Lister 1989; Ferguson and Warner 2008). Stress-mediated reductions in glycogen concentration below the critical threshold of 45–55 μmol/g (Howard 1963; Monin 1981; Warriss 1990) will give rise to elevated ultimate pH (pHu). Meat with pHu >5.9 is typically referred to as DFD. It is characterised by a darker colour, increased water-holding capacity and, depending on the pHu, increased toughness (especially at pHu 5.9–6.2; see Purchas and Aungsupakorn 1993).
Glycogen loss during pre-slaughter handling of animals is mediated by the exposure to several stressors such as: fasting, dehydration, novel/unfamiliar environments, transport, increased human contact, changes to social structure (i.e. through separation and mixing), and sudden climatic changes. The magnitude of glycogen loss will depend on the intensity and duration of the various stressors and the susceptibility of the animal to stress (Ferguson et al. 2001). Tarrant (1989), in his review, reported glycogenolytic rates in cattle to vary between 0.05 μmol/g.h (fasting heifers) and 11 μmol/g.h (mixed penning of bulls). The intensity of physical activity is critical, as physical activity per se may not always result in glycogen depletion. For example, Lambert et al. (1998) demonstrated that fast-walking cattle at a speed of 8 km/h over 5 km did not affect glycogen concentration in m. longissimus.
Glycogenolysis will also vary between muscles and fibre types (Tarrant 1989). Muscles along the back and in the hind limbs appear most prone to glycogen depletion in cattle (Tarrant and Sherington 1980). The association between exercise intensity, fibre-type and glycogen mobilisation was shown in an elegant study by Richter et al. (1982). During high-frequency stimulation of perfused rat muscle, the effect of epinephrine on glycogenolysis was most pronounced in slow-twitch fibres, whereas there was virtually no effect in the fast-twitch fibres. By contrast, the opposite was observed when the muscle was exposed to low-frequency stimulation. Lacourt and Tarrant (1985) also showed that type I fibres were more responsive to adrenaline injection in cattle. However, in response to the combination of physical activity and sympatho-adrenal activation associated with mixed penning of bulls, glycogen loss was higher in type II fibres.
Although the association between pre-slaughter stress and muscle glycogen depletion has been extensively studied in ruminants, the same cannot be said for the association between stress pre-slaughter and post-mortem glycolytic rate. This in contrast to the large body of evidence published for pigs (e.g. Klont and Lambooy 1995; Warriss et al. 1995; Channon et al. 2000; Støier et al. 2001). The general finding across these studies was that stress just before stunning resulted in lower initial muscle pH, higher initial muscle temperature, and a faster rate of pH decline in the first hour after death but similar rates beyond that.
The results from several ovine studies where exercise stress was applied just before slaughter are equivocal. Simmons et al. (1997) exercised sheep at 7 km/h for 30 min over a 90-min period and reported that the rate of pH decline at a constant temperature (15°C) was slower in the exercised group then the non-exercised group (0.036 v. 0.073 pH units/h). By contrast, Ferguson (2003) observed no difference in the rate of pH decline between the exercised (running at ~8 km/h for 15 min immediately before slaughter) and non-exercised treatment groups. Pre-slaughter exercise stress also resulted in significant glycogen depletion and elevated muscle temperatures at slaughter. In stark contrast, Bond and Warner (2007) clearly showed that lambs that were exercised for 10 min pre-slaughter had much faster pH declines, particularly in the early post-mortem period.
The application of moderate exercise just before slaughter could be challenged for its relevance to commercial practice. Clearly, best practice pre-slaughter management aims to minimise the intensity and duration of stressors that typically occur during the critical pre-slaughter period. Unfortunately, compliance with best practice does not always occur, and anecdotally, animals are subjected to unnecessary bouts of physical activity before slaughter in some abattoirs. As evidenced in the above studies, increased activity, depending on the intensity and duration, leads to changes in muscle metabolite concentration (e.g. PCr, glycogen), temperature and pH at slaughter. Furthermore, exercise stress has also been shown to affect sarcoplasmic reticulum function, specifically Ca2+ transport, in several animal (Byrd et al. 1989a, 1989b; Luckin et al. 1991; Favero et al. 1993; Ortenblad et al. 2000) and human (Gollnick et al. 1991; Booth et al. 1997) studies. In particular, exercise attenuates Ca2+ uptake and SR Ca2+-ATPase activity (Byrd et al. 1989a, 1989b; Luckin et al. 1991; Gollnick et al. 1991; Booth et al. 1997) or SR Ca2+ release (Favero et al. 1993; Westerblad et al. 1998, Ortenblad et al. 2000).
Kuchenmeister et al. (2001) reported that pre-slaughter stress in pigs also influenced Ca2+ uptake. In sheep, pre-slaughter exercise elicited an 18% reduction in Ca2+ uptake, but this was not found to be significantly different to the uptake rates in the non-exercised controls. It was concluded that pre-slaughter exercise had minimal effect on SR functionality.
Overall, these results suggest that sarcoplasmic reticulum function might be altered by pre-slaughter stress, and consequently, may contribute to variations in post-mortem glycolytic rate. Furthermore, the stress-induced changes in metabolite concentrations, pH and sarcoplasmic reticulum function may also be implicit in the reduced glycolytic response to electrical stimulation (Chrystall et al. 1982; Warner et al. 2000).
Post-mortem factors
Method of stunning
The process of stunning an animal initiates significant changes in energy metabolism in muscle. This can be attributed to neuromuscular activation, and because the plasma concentrations of adrenaline and noradrenaline concentrations rise rapidly during stunning (van der Wal et al. 1999). The choice of stunning method varies between livestock species. In pigs, carbon dioxide and electric stunning are predominantly used, whereas captive bolt and electric stunning are both used to stun cattle and sheep.
In studies comparing different stunning methods, significant differences in the metabolic response have been reported. In porcine muscle, electric stunning resulted in a lower initial pH (Gregory 1995; Bertram et al. 2002) and faster rate of pH decline (Channon et al. 2002) compared with carbon dioxide stunning. Petersen and Blackmore (1982) compared captive bolt and electric stunning in lambs and found that while the rate of pH decline was not affected, electric stunning resulted in a lower initial pH, and this was maintained during rigor.
Another issue relevant to stunning is the degree of involuntary muscle contraction that occurs in animals following stunning (i.e. clonic phase). Further reductions in initial pH values at slaughter can occur depending on the level of muscle activity (Bendall 1973).
Temperature decline
The temperature dependence of post-mortem muscle glycolysis has been studied by several workers (Marsh 1954; Cassens and Newbold 1967a, 1967b; Newbold and Scopes 1967; Bendall 1973; Jeacocke 1977; Tarrant and Mothersill 1977). In the studies where the muscles were excised soon after death and incubated at different temperatures (Marsh 1954; Cassens and Newbold 1967a, 1967b), typically over the range of 1−37°C, the 10°C temperature coefficients (Q10) increased from 1.25 (5−17°C) to 1.7–1.9 (15−37°C). At temperatures ≥37°C, the temperature coefficients rise dramatically. Marsh (1954) reported that the Q10 for the temperature range 37−43°C was nearly double (6.8) that calculated over the range 33.5−37°C (3.7).
It is important to recognise that temperature decline within any given muscle will vary depending on its anatomical location (i.e. deep v. superficial muscles), the weight and fatness of the carcass, and the temperature and air-speed conditions during chilling. Consequently, glycolytic rate varies enormously not only between muscles, but also within a muscle (Tarrant and Mothersill 1977; Sammel et al. 2002). To highlight the within-muscle variation Tarrant and Mothersill (1977) demonstrated that the rate of glycolysis in four beef muscles (m. semimembranosus, m. adducter, m. semitendinosus and m. biceps femoris) was, on average, 64% faster when the measurement was taken at a depth of 8 cm within the muscle compared with 5 cm. The significance of this result should be kept in mind when interpreting pH decline rates based on in situ pH measurements in cooling muscle.
Muscle temperature at slaughter and the subsequent rate of cooling clearly has a profound effect on post-mortem glycolysis.
Electrical stimulation
Application of electrical stimulation to carcasses was designed to accelerate post-mortem glycolysis and therefore minimise the risk of cold shortening during rapid chilling. Excellent reviews on development, scientific basis and methods of electrical stimulation are provided by Bendall (1980) and Chrystall and Devine (1983).
In summary, electrical stimulation results in a biphasic acceleration in muscle pH decline as illustrated in Fig. 1. Initially, during stimulation there is a sharp decrease in pH (ΔpH ~0.4–0.5 pH units). During this phase, the rate of glycolysis is ~100–150 times greater than the rate of normal rigor development (Chrystall and Devine 1983). In the second phase, the rate of pH decline subsequent to stimulation is generally faster (1.5–2 times) than that observed in non-stimulated muscle (Chrystall and Devine 1978; Horgan and Kuypers 1985). Although there has been debate as to whether the latter effect was merely an artefact of higher muscle temperatures (Bendall 1980), the results of Daly (1997) and Chrystall and Devine (1978) strongly suggest that temperature is unlikely to be the sole contributor. Daly (1997) reported a 50–75% increase in post-stimulation pH decline compared with non-stimulated muscle when both were held at constant temperature (35°C).
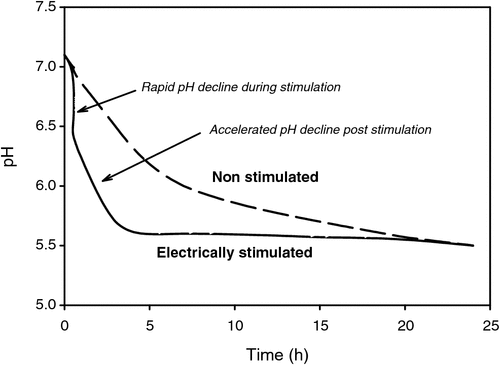
|
The magnitude of change in the ΔpH and/or the post-stimulation rate of pH decline is contingent on several factors including voltage (Chrystall and Devine 1978; Bendall 1980; Horgan and Kuypers 1985; Aalhus et al. 1994), frequency (Chrystall and Devine 1978; Bouton et al. 1980), current and wave form (Chrystall and Devine 1978), and duration of stimulation (Chrystall and Devine 1978; Butchers et al. 1998; Hwang and Thompson 2001). Moreover, intrinsic muscle properties such as the pre-stimulation pH (Chrystall and Devine 1978; Daly et al. 2006; Ferguson et al. 2007) and muscle fibre type (Devine et al. 1984) also influence the glycolytic response to electrical stimulation. In these studies, a larger ΔpH was evident in those muscles with a higher pre-stimulation pH and/or high proportion of glycolytic type IIb fibres. Surprisingly, however, the post-stimulation rate of decline is typically slower in muscles high in type IIb fibres than in those with predominantly type I fibres (Devine et al. 1984).
The mechanisms associated with the post-stimulation increase in pH decline have not been elucidated. Bendall (1980) hypothesised that electrical stimulation affected the sarcoplasmic reticulum capacity to retain Ca2+, which led to an increase in sarcoplasmic reticulum pump activity. However, equivocal results have been found with respect to the immediate changes in SR Ca2+-ATPase activity following electrical stimulation (Tume 1979; Horgan and Kuypers 1985; Ferguson 2003). Tume (1979) and Ferguson (2003) observed a reduction in SR Ca2+-ATPase activity following stimulation in sheep muscle, which contrasts the outcomes of Horgan and Kuypers (1985) using purified sarcoplasmic reticulum from rabbit muscle. Tume (1979) concluded that the reduction in SR Ca2+-ATPase activity following stimulation was permanent and attributed it to a conformational change in the ATPase, resulting in reduced affinity for ATP and inorganic phosphate.
Horgan and Kuypers (1985) speculated that another ATPase, probably actomyosin Ca2+-ATPase, was implicated. However, the prerequisite for increased activity of actomyosin Ca2+-ATPase is, of course, Ca2+. An increase in cytosolic Ca2+ and, perhaps, elevated Ca2+ sensitivity of the regulatory proteins such as troponin would be required to activate this ATPase. Whether stimulation accelerates the normal increase in cytosolic Ca2+ typically observed in post-mortem muscle is not clear yet. However, Daly (1997) proposed that the stimulation-mediated increase in ADP might trigger increased leakage of Ca2+ from the SR.
Conclusions
Post-mortem energy metabolism plays a crucial role in the transformation of living tissue, muscle, into a high-quality food source, meat. The rate and extent of post-mortem glycolysis has a profound effect on the ultimate quality of meat. Those biochemical reactions involved in this energy metabolism are largely well known and characterised in living muscle. Despite this, there is still a widespread misconception about the role of lactate in the development of acidosis following glycolysis. Although predicated on anaerobic metabolism, there are gaps in our understanding of how muscle transitions to this type of metabolism, including the role of mitochondrial function on ATP utilisation early post-mortem. Moreover, the effects of different pre- and post-slaughter factors on the kinetics of those enzymes (e.g. AMPK) involved in carbohydrate metabolism in post-mortem muscle require more investigation. The knowledge gained from further study is likely to yield new directions and potential strategies for optimising the pre- and post-slaughter management of animals and their carcasses, respectively, so that meat quality is maximised.
References
Aalhus JL, Price MA (1991) Endurance-exercised growing sheep: I. Postmortem and histological changes in skeletal muscles. Meat Science 29, 43–56.| Endurance-exercised growing sheep: I. Postmortem and histological changes in skeletal muscles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlOitw%3D%3D&md5=a148ee91ecf95c55e66102412ae00e61CAS | 22060971PubMed |
Aalhus JL, Jones SDM, Lutz S, Best DR, Robertson WM (1994) The efficacy of high and low-voltage electrical-stimulation under different chilling regimes. Canadian Journal of Animal Science 74, 433–442.
| The efficacy of high and low-voltage electrical-stimulation under different chilling regimes.Crossref | GoogleScholarGoogle Scholar |
Adamo KB, Graham TE (1998) Comparison of traditional measurements with macroglycogen and proglycogen analysis of muscle glycogen. Journal of Applied Physiology (Bethesda, Md.) 84, 908–913.
Allison CP, Bates RO, Booren AM, Johnson RC, Doumit ME (2003) Pork quality variation is not explained by glycolytic enzyme capacity. Meat Science 63, 17–22.
| Pork quality variation is not explained by glycolytic enzyme capacity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVGlsg%3D%3D&md5=789e80ed204720ed43f9ccf280b01df0CAS | 22061979PubMed |
Alonso MD, Lomako J, Lomako WM, Whelan WJ (1995) A new look at the biogenesis of glycogen. The FASEB Journal 9, 1126–1137.
Asp S, Daugaard JR, Rohde T, Adamo K, Graham T (1999) Muscle glycogen accumulation after a marathon: roles of fiber type and pro- and macroglycogen. Journal of Applied Physiology (Bethesda, Md.) 86, 474–478.
Bangsbo J, Graham TE, Kiens B, Saltin B (1992) Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. The Journal of Physiology 451, 205–227.
Bate-Smith EC, Bendall JR (1947) Rigor mortis and adenotriphosphate. The Journal of Physiology 106, 107–112.
Bendall JR (1951) The shortening of rabbit muscle during rigor mortis: Relation to the breakdown of adenosine triphosphate and creatine phosphate and to muscular contraction. The Journal of Physiology 114, 71–88.
Bendall JR (1973) Postmortem changes in muscle. In ‘The Structure and Function of Muscle, Vol. 2’. (Ed. GH Bourne) pp. 244–309. (Academic Press: New York)
Bendall JR (1978) Variability in rates of pH fall and of lactate production in the muscles of cooling beef carcasses. Meat Science 2, 91–104.
| Variability in rates of pH fall and of lactate production in the muscles of cooling beef carcasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXkslCgsLc%3D&md5=d4cf116e660d05f3653456c6715ab115CAS | 22054970PubMed |
Bendall JR (1980) The electrical stimulation of carcasses of meat animals. In ‘Developments in meat science’. Vol. 1, pp. 37–59. (Applied Science Publishers: London)
Bertram HC, Stodkilde-Jorgensen H, Karlsson AH, Andersen HJ (2002) Post mortem energy metabolism and meat quality of porcine M. longissimus dorsi as influenced by stunning method—a P-31 NMR spectroscopic study. Meat Science 62, 113–119.
| Post mortem energy metabolism and meat quality of porcine M. longissimus dorsi as influenced by stunning method—a P-31 NMR spectroscopic study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksValt7o%3D&md5=525a840a1b1b78f774c2f283e8196f5eCAS | 22061199PubMed |
Bond JJ, Warner RD (2007) Ion distribution and protein proteolysis affect water holding capacity of Longissimus thoracis et lumborum in meat of lamb subjected to antemortem exercise. Meat Science 75, 406–414.
| Ion distribution and protein proteolysis affect water holding capacity of Longissimus thoracis et lumborum in meat of lamb subjected to antemortem exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVSrsA%3D%3D&md5=b774394570801f7c29247962edbbe07cCAS | 22063796PubMed |
Booth J, McKenna MJ, Ruell PA, Gwinn TH, Davis GM, Thompson MW, Harmer AR, Hunter SK, Sutton JR (1997) Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. Journal of Applied Physiology (Bethesda, Md.) 83, 511–521.
Bouton PE, Weste RR, Shaw FD (1980) Electrical stimulation of calf carcasses: response of various muscles to different waveforms. Journal of Food Science 45, 148–149.
| Electrical stimulation of calf carcasses: response of various muscles to different waveforms.Crossref | GoogleScholarGoogle Scholar |
Briskey EJ, Kastenschmidt LL, Forrest JC, Beecher GR, Judge MD, Cassens RG, Hoekstra WG (1966) Biochemical aspects of post mortem changes in porcine muscle. Journal of Agricultural and Food Chemistry 14, 201–207.
| Biochemical aspects of post mortem changes in porcine muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XktFGntb8%3D&md5=a54df2846d702340c5095f78cb1bb2c2CAS |
Bröjer JT, Stampfli HR, Graham TE (2002) Analysis of proglycogen and macroglycogen content in muscle biopsy specimens obtained from horses. American Journal of Veterinary Research 63, 570–575.
| Analysis of proglycogen and macroglycogen content in muscle biopsy specimens obtained from horses.Crossref | GoogleScholarGoogle Scholar | 11939321PubMed |
Brooks SP, Storey KB (1991) The effect of enzyme-enzyme complexes on the overall glycolytic rate in vivo. Biochemistry International 25, 477–489.
Burrow HM, Moore SS, Johnston DJ, Barendse W, Bindon BM (2001) Quantitative and molecular genetic influences on properties of beef: a review. Australian Journal of Experimental Agriculture 41, 893–919.
| Quantitative and molecular genetic influences on properties of beef: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotlGhtbY%3D&md5=2e84e6b2dc13af00092a1a1c94262ebaCAS |
Butchers ADM, Ferguson DM, Devine CE, Thompson JM (1998) Interaction between pre-slaughter handling and low voltage electrical simulation and effect on beef quality. In ‘Proceedings 44th International Congress of Meat Science and Technology’. pp. 1050–1051. Barcelona, Spain. (ICoMST)
Byrd SK, Bode AK, Klug GA (1989a) Effects of exercise of varying duration on sarcoplasmic reticulum function. Journal of Applied Physiology (Bethesda, Md.) 66, 1383–1389.
Byrd SK, McCutcheon LJ, Hodgson DR, Gollnick PD (1989b) Altered sarcoplasmic reticulum function after high-intensity exercise. Journal of Applied Physiology (Bethesda, Md.) 67, 2072–2077.
Carpenter E, Rice OD, Cockett NE, Snowder GD (1996) Histology and composition of muscles from normal and callipyge lambs. Journal of Animal Science 74, 388–393.
Cassens RG, Newbold RP (1967a) Effect of temperature on the time course of rigor mortis in ox muscle. Journal of Food Science 32, 269–272.
| Effect of temperature on the time course of rigor mortis in ox muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXkslWks7s%3D&md5=e1ae480f1ee58d7b4ef22db77db74838CAS |
Cassens RG, Newbold RP (1967b) Temperature dependence of pH changes in ox muscle post-mortem. Journal of Food Science 32, 13–14.
| Temperature dependence of pH changes in ox muscle post-mortem.Crossref | GoogleScholarGoogle Scholar |
Channon HA, Payne AM, Warner RD (2000) Halothane genotype, pre-slaughter handling and stunning method all influence pork quality. Meat Science 56, 291–299.
| Halothane genotype, pre-slaughter handling and stunning method all influence pork quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVKntg%3D%3D&md5=f211ced8ded52220201560dad79cb056CAS | 22062081PubMed |
Channon HA, Payne AM, Warner RD (2002) Comparison of CO2 stunning with manual electrical stunning (50 Hz) of pigs on carcass and meat quality. Meat Science 60, 63–68.
| Comparison of CO2 stunning with manual electrical stunning (50 Hz) of pigs on carcass and meat quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFCksQ%3D%3D&md5=e2795a825891ac855b7c806f56d4d993CAS | 22063106PubMed |
Chasiotis D (1988) Role of cyclic AMP and inorganic phosphate in the regulation of muscle glycogenolysis during exercise. Medicine and Science in Sports and Exercise 20, 545–550.
| Role of cyclic AMP and inorganic phosphate in the regulation of muscle glycogenolysis during exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtFait7s%3D&md5=883441973915cdf3ab70ac7aca731480CAS | 2853269PubMed |
Cheetham ME, Boobis LH, Brooks S, Williams C (1986) Human muscle metabolism during sprint running. Journal of Applied Physiology 61, 54–60.
Chrystall BB, Devine CE (1978) Electrical stimulation, muscle tension and glycolysis in bovine sternomandibularis. Meat Science 2, 49–58.
| Electrical stimulation, muscle tension and glycolysis in bovine sternomandibularis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhsFSrsLY%3D&md5=3b9a1a98946e849275c97253765c31caCAS | 22054838PubMed |
Chrystall BB, Devine CE (1983) Electrical stimulation: its early development in New Zealand. In ‘Advances in meat science’. Vol. 1, 73–119. (Eds A. M. Pearson and T. R. Dutson): (AVI Publishers: NewYork)
Chrystall BB, Devine CE, Snodgrass M, Ellery S (1982) Tenderness in exercise stressed lambs. New Zealand Journal of Agricultural Research 25, 331–336.
| Tenderness in exercise stressed lambs.Crossref | GoogleScholarGoogle Scholar |
Conley KE, Kemper WF, Crowther GJ (2001) Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. The Journal of Experimental Biology 204, 3189–3194.
Connett RJ, Sahlin K (1996) Control of glycolysis and glycogen metabolism. In ‘Handbook of physiology. 12. Exercise: Regulation and integration of multiple systems’. pp. 870–911 (Eds LB Rowell, JT Shepherd) (Oxford University Press: New York)
Copenhafer TL, Richert BT, Schinckel AP, Grant AL, Gerrard DE (2006) Augmented postmortem glycolysis does not occur early postmortem in AMPK73-mutated porcine muscle of halothane positive pigs. Meat Science 73, 590–599.
| Augmented postmortem glycolysis does not occur early postmortem in AMPK73-mutated porcine muscle of halothane positive pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvVCqt78%3D&md5=0997430c4862290eea75bd17fbe2bf43CAS | 22062557PubMed |
Crowther GJ, Carey MF, Kemper WF, Conley KE (2002) Control of glycolysis in contracting skeletal muscle. I. Turning it on. American Journal of Physiology. Endocrinology and Metabolism 282, E67–E73.
Daly CC (1997) Energy metabolism in post mortem muscle. In ‘Proceedings of 43rd International Congress of Meat Science and Technology’. Auckland, New Zealand (ICoMST)
Daly BL, Gardner GE, Ferguson DM, Thompson JM (2006) The effect of time off feed prior to slaughter on muscle glycogen metabolism and rate of pH decline in three different muscles of stimulated and non-stimulated sheep carcasses. Australian Journal of Agricultural Research 57, 1229–1235.
| The effect of time off feed prior to slaughter on muscle glycogen metabolism and rate of pH decline in three different muscles of stimulated and non-stimulated sheep carcasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFeqsr7M&md5=78d7e290ba25ed0f23be96aea41ed49bCAS |
Derave W, Gao S, Richter EA (2000) Pro- and macroglycogenolysis in contracting rat skeletal muscle. Acta Physiologica Scandinavica 169, 291–296.
| Pro- and macroglycogenolysis in contracting rat skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1yntLc%3D&md5=5ed126f5e286f20a294cd905c08e7691CAS | 10951120PubMed |
Devine CE, Ellery S, Averill S (1984) Response of different types of ox muscle to electrical stimulation. Meat Science 10, 35–51.
| Response of different types of ox muscle to electrical stimulation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFChsA%3D%3D&md5=e1ebe971581f7c985db0b3ac8c7b9dbfCAS | 22055994PubMed |
Devine CE, Wahlgren NM, Tornberg E (1999) Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum. Meat Science 51, 61–72.
| Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWqtA%3D%3D&md5=0fa670a2c0be3f3e48dbef00926751beCAS | 22061537PubMed |
Devine CE, Payne SR, Peachey BM, Lowe TE, Ingram JR, Cook CJ (2002) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| High and low rigor temperature effects on sheep meat tenderness and ageing.Crossref | GoogleScholarGoogle Scholar | 22063237PubMed |
Dransfield E (1994) Modeling postmortem tenderization. 5. Inactivation of calpains. Meat Science 37, 391–409.
| Modeling postmortem tenderization. 5. Inactivation of calpains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt12htrg%3D&md5=0f7a088f9aab1db34f9490fe94eb77d9CAS |
Dransfield E (1995) Control of meat texture at an industrial scale. In ‘Expression of tissue proteinases and regulation of protein degradation as related to meat quality’. pp. 463–484 (Eds A Ouali, DI Demeyer, FJM Smulders) (ECCEAMST: Utrecht, the Netherlands)
Dransfield E (1999) Meat tenderness - the μ-calpain hypothesis. In ‘45th International Congress of Meat Science and Technology’. (Yokohama, Japan) (ICoMST)
Dransfield E, Wakefield DK, Parkman ID (1992) Modeling postmortem tenderization. 1. Texture of electrically stimulated and non-stimulated beef. Meat Science 31, 57–73.
| Modeling postmortem tenderization. 1. Texture of electrically stimulated and non-stimulated beef.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbnt1yrsg%3D%3D&md5=6d1d9bd0fd3f53e70431a97f96c8e6bfCAS | 22059510PubMed |
England EM, Scheffler TL, Kasten SC, Matarneh SK, Gerrad DE (2013) Exploring the unknowns involved in the transformation of muscle to meat. Meat Science 95, 837–843.
| Exploring the unknowns involved in the transformation of muscle to meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnslCisLw%3D&md5=6df79d4dae2fa2e63be356049ee6edd6CAS | 23673227PubMed |
Essen-Gustavsson B (1996) Skeletal muscle adaptation with use and disuse. Comparative aspects between species. In ‘42nd International Congress of Meat Science and Technology’. Lillehammer, Norway. (ICoMST)
Favero TG, Pessah IN, Klug GA (1993) Prolonged exercise reduces Ca2+ release in rat skeletal muscle sarcoplasmic reticulum. Pflugers Archiv 422, 472–475.
| Prolonged exercise reduces Ca2+ release in rat skeletal muscle sarcoplasmic reticulum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3jsVaitg%3D%3D&md5=f991594b5ee751db275745e748200859CAS | 7682687PubMed |
Ferguson DM (2003) Regulation of post-mortem glycolysis in ruminant muscle. PhD thesis. University of New England, Armidale, NSW.
Ferguson DM, Warner RD (2008) Have we underestimated the impact of pre-slaughter stress on meat quality in ruminants? Meat Science 80, 12–19.
| Have we underestimated the impact of pre-slaughter stress on meat quality in ruminants?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFGhtw%3D%3D&md5=323d757bd1f8b7d692a3836e56312925CAS | 22063165PubMed |
Ferguson DM, Bruce HL, Thompson JM, Egan AF, Perry D, Shorthose WR (2001) Factors affecting beef palatability—farmgate to chilled carcass. Australian Journal of Experimental Agriculture 41, 879–891.
| Factors affecting beef palatability—farmgate to chilled carcass.Crossref | GoogleScholarGoogle Scholar |
Ferguson DM, Shaw FD, Stark JL (2007) Effect of reduced lairage duration on beef quality. Australian Journal of Experimental Agriculture 47, 770–773.
| Effect of reduced lairage duration on beef quality.Crossref | GoogleScholarGoogle Scholar |
Ferguson DM, Daly BL, Gardner GE, Tume RK (2008) Effect of glycogen concentration and form on the response to electrical stimulation and rate of post-mortem glycolysis in ovine muscle. Meat Science 78, 202–210.
| Effect of glycogen concentration and form on the response to electrical stimulation and rate of post-mortem glycolysis in ovine muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlCrsbbL&md5=929e5a4f31918aee42feee85823c3b45CAS | 22062271PubMed |
Fernandez X, Tornberg E, Naveau J, Talmant A, Monin G (1992) Bimodal distribution of the muscle glycolytic potential in French and Swedish populations of Hampshire crossbred pigs. Journal of the Science of Food and Agriculture 59, 307–311.
| Bimodal distribution of the muscle glycolytic potential in French and Swedish populations of Hampshire crossbred pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xmtlamtr4%3D&md5=9d4c004480f790242eb80d905e002127CAS |
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiological Reviews 74, 49–94.
Fridén J, Seger J, Ekblom B (1989) Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis. Acta Physiologica Scandinavica 135, 381–391.
| Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis.Crossref | GoogleScholarGoogle Scholar | 2467521PubMed |
Gardner GE (2001) Nutritional regulation of glycogen metabolism in cattle and sheep. PhD thesis. Murdoch University, Perth, W. Aust.
Goldsmith E, Sprang S, Fletterick R (1982) Structure of maltoheptaose by difference Fourier methods and a model for glycogen. Journal of Molecular Biology 156, 411–427.
| Structure of maltoheptaose by difference Fourier methods and a model for glycogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XktlGjsrw%3D&md5=2cfa3671648d4d7f98cc3f083e05280fCAS | 7086906PubMed |
Gollnick PD, Korge P, Karpakka J, Saltin B (1991) Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiologica Scandinavica 142, 135–136.
| Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXksVKksb0%3D&md5=68a69250623eebf722bc04ee8dc9516cCAS | 1831584PubMed |
Graham TE, Adamo KB, Shearer J, Marchand I, Saltin B (2001) Pro- and macroglycogenolysis: relationship with exercise intensity and duration. Journal of Applied Physiology (Bethesda, Md.) 90, 873–879.
Greaser ML, Cassens RG, Briskey EJ, Hoekstra WG (1969) Post-mortem changes in subcellular fractions from normal and pale soft exudative porcine muscle. 1. Calcium accumulation and adenosine triphosphatase activities. Journal of Food Science 34, 120–124.
| Post-mortem changes in subcellular fractions from normal and pale soft exudative porcine muscle. 1. Calcium accumulation and adenosine triphosphatase activities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXkt1SjtLg%3D&md5=ffed94024c9fd905eb08985c32da8a73CAS |
Greenwood PL, Dunshea FR (2009) ‘Biology and regulation of carcass composition .’ (Woodhead Publishing Ltd: Cambridge, UK)
Gregory NG (1995) Recent developments in the gas stunning of pigs. In ‘Meat 95. Australian Meat Industry Research Conference’. 9B1–9B4. Gold Coast. (CSIRO Division of Food Science and Technology: Brisbane)
Gunja-Smith Z, Marshall JJ, Mercier C, Smith EE, Whelan WJ (1970) A revision of the Meyer-Bernfeld model of glycogen and amylopectin. FEBS Letters 12, 101–104.
| A revision of the Meyer-Bernfeld model of glycogen and amylopectin.Crossref | GoogleScholarGoogle Scholar | 11945551PubMed |
Hamm R (1977) Postmortem breakdown of ATP and glycogen in ground muscle: A review. Meat Science 1, 15–39.
| Postmortem breakdown of ATP and glycogen in ground muscle: A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXks1Klsb4%3D&md5=5ee66213826428291297a9ad0579a29dCAS | 22054426PubMed |
Hargreaves M, Richter EA (1988) Regulation of skeletal muscle glycogenolysis during exercise. Canadian Journal of Sport Sciences 13, 197–203.
Hargreaves M, McConell G, Proietto J (1995) Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. Journal of Applied Physiology (Bethesda, Md.) 78, 288–292.
Hargreaves M, Finn JP, Withers RT, Halbert JA, Scroop GC, Mackay M, Snow RJ, Carey MF (1997) Effect of muscle glycogen availability on maximal exercise performance. European Journal of Applied Physiology and Occupational Physiology 75, 188–192.
| Effect of muscle glycogen availability on maximal exercise performance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXislyntLw%3D&md5=223da869ab7bc1893abdec3edc37fb9cCAS | 9118987PubMed |
Harris PV, Shorthose WR (1988) Meat texture. In ‘Development in meat science—4’. (Ed. RA Lawrie). (Elsevier Science Publications Ltd: London)
Hellsten Y, Richter EA, Kiens B, Bangsbo J (1999) AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. Journal of Physiology 520, 909–920.
| AMP deamination and purine exchange in human skeletal muscle during and after intense exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXns1elsrg%3D&md5=7a6f3e021c8951dd69df6a7bd83fd4e6CAS | 10545153PubMed |
Henriksson J (1992) Effects of physical training on the metabolism of skeletal muscle. Diabetes Care 15, 1701–1711.
| Effects of physical training on the metabolism of skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7gsFOqsg%3D%3D&md5=1196eb36e2d71f825b8df702eb712409CAS | 1468304PubMed |
Hertzman C, Olsson U, Tornberg E (1993) The influence of high-temperature, type of muscle and electrical-stimulation on the course of rigor, aging and tenderness of beef muscles. Meat Science 35, 119–141.
| The influence of high-temperature, type of muscle and electrical-stimulation on the course of rigor, aging and tenderness of beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlKjsQ%3D%3D&md5=6f3ca62e08b17e48f991ff29bf19a844CAS | 22060841PubMed |
Hespel P, Richter EA (1990) Glucose uptake and transport in contracting, perfused rat muscle with different pre-contraction glycogen concentrations. The Journal of Physiology 427, 347–359.
Hespel P, Richter EA (1992) Mechanism linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochemical Journal 284, 777–780.
Hochachka PW (2003) Intracellular convection, homeostasis and metabolic regulation. The Journal of Experimental Biology 206, 2001–2009.
| Intracellular convection, homeostasis and metabolic regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlslaltLk%3D&md5=133233f23bad5ba127009359a9cdf3dcCAS | 12756282PubMed |
Hochachka PW, McClelland GB (1997) Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. The Journal of Experimental Biology 200, 381–386.
Holmes JHG, Ashmore CP (1972) A histochemical study of development of muscle fibre type and size in normal and double muscled cattle. Growth 36, 351–372.
Honikel KO, Kim CJ (1986) Causes of the development of PSE pork. Fleischwirtschaft 66, 349–353.
Hopkins DL, Thompson JM (2001) Inhibition of protease activity 2. Degradation of myofibrillar proteins, myofibril examination and determination of free calcium levels. Meat Science 59, 199–209.
| Inhibition of protease activity 2. Degradation of myofibrillar proteins, myofibril examination and determination of free calcium levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlt1Sht78%3D&md5=5e4179107a02bc800d0ca10ce94c5bddCAS | 22062679PubMed |
Hopkins DL, Stanley DF, Martin LC, Toohey ES, Gilmour AR (2007) Genotype and age effects on sheep meat production 3. Meat quality. Australian Journal of Experimental Agriculture 47, 1155–1164.
| Genotype and age effects on sheep meat production 3. Meat quality.Crossref | GoogleScholarGoogle Scholar |
Horgan DJ, Kuypers R (1985) Post-mortem glycolysis in rabbit longissimus dorsi muscles following electrical stimulation. Meat Science 12, 225–241.
| Post-mortem glycolysis in rabbit longissimus dorsi muscles following electrical stimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhvVeqs7w%3D&md5=93aadd82b55774e62ef98394be58d26eCAS |
Howard A (1963) The relation between physiological stress and meat quality. In ‘Carcass composition and appraisal of meat animals’. pp. 11–21 (Ed. DE Tribe) (CSIRO: Melbourne)
Huang M, Lee C, Lin R, Chen R (1997) The exchange between proglycogen and macroglycogen and the metabolic role of the protein-rich glycogen in rat skeletal muscle. The Journal of Clinical Investigation 99, 501–505.
| The exchange between proglycogen and macroglycogen and the metabolic role of the protein-rich glycogen in rat skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtVKktrs%3D&md5=ed68d94f97f0b4ece084cce7791d6749CAS | 9022084PubMed |
Huang HG, Larsen MR, Karlsson AH, Pomponio L, Costa LN, Lametsch R (2011) Gel-based phosphoproteomics analysis of sarcoplasmic proteins in postmortem porcine muscle with pH decline rate and time differences. Proteomics 11, 4063–4076.
| Gel-based phosphoproteomics analysis of sarcoplasmic proteins in postmortem porcine muscle with pH decline rate and time differences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12rtrzE&md5=1eb51addd6b133e3cb9bc2d37a9dd0d9CAS |
Hudson NJ (2012) Mitochondrial treason: a driver of pH decline rate in post-mortem muscle? Animal Production Science 52, 1107–1110.
| Mitochondrial treason: a driver of pH decline rate in post-mortem muscle?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Wju7zK&md5=f687e4da05352dc41a264a4e411a9e9aCAS |
Huffman KL, Miller MF, Hoover LC, Wu CK, Brittin HC, Ramsey CB (1996) Effect of beef tenderness on consumer satisfaction with steaks consumed in the home and restaurant. Journal of Animal Science 74, 91–97.
Hwang IH, Thompson JM (2001) The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle. Meat Science 58, 135–144.
| The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurg%3D&md5=270f7e9f2075e49cd0428af203c46a10CAS | 22062108PubMed |
Iaizzo PA, Klein W, Lehmannhorn F (1988) Fura-2 detected myoplasmic calcium and its correlation with contracture force in skeletal muscle from normal and malignant hyperthermia susceptible pigs. European Journal of Physiology 411, 648–653.
| Fura-2 detected myoplasmic calcium and its correlation with contracture force in skeletal muscle from normal and malignant hyperthermia susceptible pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXktlKisL4%3D&md5=098dbc5a15840ebe453f6fca6057d155CAS | 3412868PubMed |
Immonen K, Kauffman RG, Schaefer DM, Puolanne E (2000) Glycogen concentrations in bovine longissimus dorsi muscle. Meat Science 54, 163–167.
| Glycogen concentrations in bovine longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjvFCqsQ%3D%3D&md5=c3b3fb5b04106e0d94bb699c860d7a17CAS | 22060612PubMed |
Jacob RH, Hopkins DL (2014) Techniques to reduce the temperature of beef muscle early in the post-mortem period – a review. Animal Production Science 54, 482–493.
| Techniques to reduce the temperature of beef muscle early in the post-mortem period – a review.Crossref | GoogleScholarGoogle Scholar |
Jacob RH, Surridge VSM, Beatty DT, Gardner GE, Warner RD (2014) Grain feeding increases core body temperature of beef cattle. Animal Production Science 54, 444–449.
| Grain feeding increases core body temperature of beef cattle.Crossref | GoogleScholarGoogle Scholar |
James AP, Bames PD, Palmer TN, Fournier PA (2008) Proglycogen and macroglycogen: artifacts of glycogen extraction? Metabolism: Clinical and Experimental 57, 535–543.
| Proglycogen and macroglycogen: artifacts of glycogen extraction?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtVens7Y%3D&md5=1326d0f4f981c7dba6667a179277efd1CAS |
Jansson E (1981) Acid soluble and insoluble glycogen in human skeletal muscle. Acta Physiologica Scandinavica 113, 337–340.
| Acid soluble and insoluble glycogen in human skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xjt1Kg&md5=f2d5aec3d8b11a772852ca811e8a4ec3CAS | 6285675PubMed |
Jeacocke RE (1977) The temperature dependence of anaerobic glycolysis in beef muscle held in a linear temperature gradient. Journal of the Science of Food and Agriculture 28, 551–556.
| The temperature dependence of anaerobic glycolysis in beef muscle held in a linear temperature gradient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXltlantrw%3D&md5=bd54f2cc1ff1f34d767d55e1f8e4b430CAS |
Jeacocke RE (1993) The concentrations of free magnesium and free calcium-ions both increase in skeletal-muscle fibers entering rigor-mortis. Meat Science 35, 27–45.
| The concentrations of free magnesium and free calcium-ions both increase in skeletal-muscle fibers entering rigor-mortis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmtVajur8%3D&md5=4d432b44fdbb0b6d206a47032721fa64CAS | 22060835PubMed |
Johnston DJ, Reverter A, Ferguson DM, Thompson JM, Burrow HM (2003) Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 3. Meat quality traits. Australian Journal of Agricultural Research 54, 135–147.
| Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 3. Meat quality traits.Crossref | GoogleScholarGoogle Scholar |
Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J (2004) Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism 286, E245–E251.
| Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsFarsbY%3D&md5=cd2c292f88e2ea966957362b043a72ebCAS | 14559724PubMed |
Kastenschmidt L, Hoekstra WG, Briskey EJ (1968) Glycolytic intermediates and co-factors in fast- and slow-glycolyzing muscle of pig. Journal of Food Science 33, 151–158.
| Glycolytic intermediates and co-factors in fast- and slow-glycolyzing muscle of pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXktFOgt70%3D&md5=61e21b669585fcaa17f2ca4be0405d38CAS |
Kentbraun JA, Miller RG, Weiner MW (1993) Phases of metabolism during progressive exercise to fatigue in human skeletal muscle. Journal of Applied Physiology 75, 573–580.
Klont RE, Lambooy E (1995) Effects of preslaughter muscle exercise on muscle metabolism and meat quality studied in anesthetized pigs of different halothane genotypes. Journal of Animal Science 73, 108–117.
Koohmaraie M (1992) The role of Ca(2+)-dependent proteases (calpains) in post mortem proteolysis and meat tenderness. Biochimie 74, 239–245.
| The role of Ca(2+)-dependent proteases (calpains) in post mortem proteolysis and meat tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVKrsrc%3D&md5=3b9de3cf6df683d04b5b2df9c8022bdeCAS | 1610937PubMed |
Koohmaraie M (1996) Biochemical factors regulating the toughening and tenderization processes of Meat. Meat Science 43, 193–201.
| Biochemical factors regulating the toughening and tenderization processes of Meat.Crossref | GoogleScholarGoogle Scholar |
Koohmaraie M, Geesink GH (2006) Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science 74, 34–43.
| Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1Grsbk%3D&md5=1e6bab8be15345f5680d47f98eb09e37CAS | 22062714PubMed |
Koohmaraie M, Seideman SC, Schollmeyer JE, Dutson TR, Crouse JD (1987) Effect of postmortem storage on Ca++-dependent proteases, their inhibitor and myofibril fragmentation. Meat Science 19, 187–196.
| Effect of postmortem storage on Ca++-dependent proteases, their inhibitor and myofibril fragmentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXlsFejt7s%3D&md5=4f8736daabd1788fe2e6941affc92efcCAS | 22055942PubMed |
Korzeniewski B (2006) AMP deamination delays muscle acidification during heavy exercise and hypoxia. The Journal of Biological Chemistry 281, 3057–3066.
| AMP deamination delays muscle acidification during heavy exercise and hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFSnurs%3D&md5=bcc0947002aebbbe6aa405eac7cd81b8CAS | 16314416PubMed |
Krause U, Wegener G (1996) Control of adenine nucleotide metabolism and glycolysis in vertebrate skeletal muscle during exercise. Experientia 52, 396–403.
| Control of adenine nucleotide metabolism and glycolysis in vertebrate skeletal muscle during exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjsVKnsb0%3D&md5=f3fb23a51451d6c0bc64d136eb861262CAS | 8641374PubMed |
Kuchenmeister U, Langhammer M, Renne U, Nurnberg G, Ender K (2001) Effect of exercise on sarcoplasmic reticulum Ca2+ transport in muscle of mouse lines long-term selected for different performance traits. Archiv Fur Tierzucht 44, 441–450.
Kushmerick MJ, Conley KE (2002) Energetics of muscle contraction: the whole is less than the sum of its parts. Biochemical Society Transactions 30, 227–231.
| Energetics of muscle contraction: the whole is less than the sum of its parts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjvVKgsro%3D&md5=583c1836770b51f4c58c23ad170cde22CAS | 12023856PubMed |
Lacourt A, Tarrant PV (1985) Glycogen depletion patterns in myofibres of cattle during stress. Meat Science 15, 85–100.
| Glycogen depletion patterns in myofibres of cattle during stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xjt1ersw%3D%3D&md5=db6a1260234c99fc55d1ec73f5855489CAS | 22056127PubMed |
Lambert MG, Knight TW, Cosgrove GP, Anderson CB, Death AF, Fisher AD (1998) Exercise effects on muscle glycogen concentration in beef cattle. In ‘Proceedings of the New Zealand Society of Animal Production’. pp. 243–244. Hamilton, New Zealand. (New Zealand Society of Animal Production)
Lametsch R, Larsen MR, Essen-Gustavsson B, Jensen-Waern M, Lundstrom K, Lindahl G (2011) Postmortem changes in pork muscle protein phosphorylation in relation to the RN genotype. Journal of Agricultural and Food Chemistry 59, 11608–11615.
| Postmortem changes in pork muscle protein phosphorylation in relation to the RN genotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlSqsb3N&md5=93558b5c4c9e96ef49af5e5419d722f1CAS | 21958152PubMed |
Lawrie RA (1998) ‘Meat science .’ (Woodhead Publishing Ltd: Cambridge, UK)
Lebret B, Le Roy P, Monin G, Lefaucheur L, Caritez JC, Talmant A, Elsen JM, Sellier P (1999) Influence of the three RN genotypes on chemical composition, enzyme activities, and myofiber characteristics of porcine skeletal muscle. Journal of Animal Science 77, 1482–1489.
Lehninger AL, Nelson DL, Cox MM (1993) ‘Principles of biochemistry.’ (Worth Publishers New York)
Lister D (1989) Muscle metabolism and animal physiology in the dark cutting condition. In ‘Dark-cutting in cattle and sheep. Proceedings of an Australian workshop’. (Eds SU Fabiansson, WR Shorthose, RD Warner) pp. 19–25. (Australian Meat & Live-stock Research & Development Corporation. Sydney)
Lister D, Gregory NG, Warriss PD (1981) ‘Stress in meat animals.’ (Applied Science Publishers: London)
Locker RH, Hagyard CJ (1963) A cold shortening effect in beef muscles. Journal of the Science of Food and Agriculture 14, 787–793.
| A cold shortening effect in beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXlsFOiug%3D%3D&md5=ebcef65dee72d68932c06451127cd7e1CAS |
Lopez JR, Alamo LA, Allen P, Sreter F (1986) Intracellular free calcium concentration in muscle fibers of swine susceptible to malignant hyperthermia. Acta Anaesthesiologica Italica 37, 563–567.
Lowenstein JM (1990) The purine nucleotide cycle revisted. International Journal of Sports Medicine 11, S37–S46.
| The purine nucleotide cycle revisted.Crossref | GoogleScholarGoogle Scholar | 2193892PubMed |
Luckin KA, Favero TG, Klug GA (1991) Prolonged exercise induces structural changes in SR Ca(2+)-ATPase of rat muscle. Biochemical Medicine and Metabolic Biology 46, 391–405.
| Prolonged exercise induces structural changes in SR Ca(2+)-ATPase of rat muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xls1aksg%3D%3D&md5=1a6d3644228e647824ff2648054d7d54CAS | 1838929PubMed |
Marcinek DJ, Ciesielski WA, Conley KE, Schenkman KA (2003) Oxygen regulation and limitation to cellular respiration in mouse skeletal muscle in vivo. American Journal of Physiology. Heart and Circulatory Physiology 285, H1900–H1908.
Marsh BB (1954) Rigor mortis in beef. Journal of the Science of Food and Agriculture 5, 70–75.
| Rigor mortis in beef.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2cXjtlKqsQ%3D%3D&md5=20c13aeeb6fdd9ec8cb0cb2d4e1acd6dCAS |
McGeehin B, Sheridan JJ, Butler F (2001) Factors affecting the pH decline in lamb after slaughter. Meat Science 58, 79–84.
| Factors affecting the pH decline in lamb after slaughter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntlyjug%3D%3D&md5=444b44ddae57620ab17dc3504433fe49CAS | 22061923PubMed |
Meinke MH, Edstrom RD (1991) Muscle glycogenolysis. Regulation of the cyclic interconversion of phosphorylase a and phosphorylase b. The Journal of Biological Chemistry 266, 2259–2266.
Melendez-Hevia E, Waddell TG, Shelton ED (1993) Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochemical Journal 295, 477–483.
Meyer RA, Foley JM (1996) Cellular processes integrating the metabolic response to exercise. In ‘Handbook of physiology. Section 12: Exercise: regulation and integration of multiple systems’. (Eds LR Rowell, JT Shepherd) pp. 841–869. (Oxford University Press: New York)
Mickelson JR, Louis CF (1993) Calcium (Ca2+) regulation in porcine skeleatl muscle - Review. In ‘Pork quality: Genetic and metabolic factors’. pp. 160–184. (Eds E Puolanne, DI Demeyer) (CAB International: Wallingford, UK)
Mickelson JR, Gallant EM, Rempel WE, Johnson KM, Litterer LA, Jacobson BA, Louis CF (1989) Effects of the halothane sensitivity gene on sarcoplasmic reticulum function. The American Journal of Physiology 257, C787–C794.
Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Le Roy P, Chardon P, Andersson L (2000) A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 288, 1248–1251.
| A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjs1Wlu7w%3D&md5=d65a237634e16a53a2d6421658e6aee8CAS | 10818001PubMed |
Monin G (1981) Muscle metabolic type and the DFD condition. In ‘The problem of dark cutting in beef. Current topics in veterinary medicine and animal science’. (Eds DE Hood, PV Tarrant) Vol. 10, pp. 63–81. (Martinus Nijhoff Publishers: The Hague)
Monin G, Sellier P (1985) Pork of low technological quality with a normal rate of muscle pH fall in the intermediate post-mortem period: the case of the Hampshire breed. Meat Science 13, 49–63.
| Pork of low technological quality with a normal rate of muscle pH fall in the intermediate post-mortem period: the case of the Hampshire breed.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFWjuw%3D%3D&md5=ff57fc79945a72070afd515453e3d5b5CAS | 22055445PubMed |
Moody WG, Kemp JD, Mahyuddin M, Johnston DM, Ely DG (1980) Effect of feeding systems, slaughter weight and sex on histological properties of lamb carcasses. Journal of Animal Science 50, 249–256.
Mortimer SI, Pearce KL, Jacobs RH, Hopkins DL, Warner RD, Geesink GH, Edwards JEH, Pethick DW, van der Werf JHJ, Ball AJ (2009) ‘The information nucleus—genetically improving Australian lamb production.’ (Association for the Advancement of Animal Breeding and Genetics)
Newbold RP, Scopes RK (1967) Post-mortem glycolysis in ox skeletal muscle. Effect of temperature on the concentrations of glycolytic intermediates and cofactors. Biochemical Journal 105, 127–136.
Newsholme EA, Leech AR (1983) ‘Biochemistry for the medical sciences.’ (Wiley: New York)
O’Halloran GR, Troy DJ, Buckley DJ (1997a) The relationship between early post-mortem pH and the tenderisation of beef muscles. Meat Science 45, 239–251.
| The relationship between early post-mortem pH and the tenderisation of beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXitlKlsL8%3D&md5=11a530c7c10f5e04db7ad5a5abb9ee41CAS | 22061306PubMed |
O’Halloran GR, Troy DJ, Buckley DJ, Reville WJ (1997b) The role of endogenous proteases in the tenderisation of fast glycolysing muscle. Meat Science 47, 187–210.
| The role of endogenous proteases in the tenderisation of fast glycolysing muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXksFKgsQ%3D%3D&md5=5a21d7909412611a11c672d4ccf72bf3CAS | 22062733PubMed |
Olsson U, Hertzman C, Tornberg E (1994) The influence of low-temperature, type of muscle and electrical-stimulation on the course of rigor-mortis, aging and tenderness of beef muscles. Meat Science 37, 115–131.
| The influence of low-temperature, type of muscle and electrical-stimulation on the course of rigor-mortis, aging and tenderness of beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbnt1ynuw%3D%3D&md5=b7a5d925f251261bd2ae820a3baf4722CAS | 22059417PubMed |
Ortenblad N, Sjogaard G, Madsen K (2000) Impaired sarcoplasmic reticulum Ca(2+) release rate after fatiguing stimulation in rat skeletal muscle. Journal of Applied Physiology (Bethesda, Md.) 89, 210–217.
Ouali A (1992) Proteolytic and physicochemical mechanisms involved in meat texture development. Biochimie 74, 251–265.
| Proteolytic and physicochemical mechanisms involved in meat texture development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVKrs78%3D&md5=6a2a0220f9ed21f5e397bf0feb76cf18CAS | 1535227PubMed |
Ouali A, Herrera-Mendez CH, Coulis G, Becila S, Boudjellal A, Aubry L, Sentandreu MA (2006) Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Science 74, 44–58.
| Revisiting the conversion of muscle into meat and the underlying mechanisms.Crossref | GoogleScholarGoogle Scholar | 22062715PubMed |
Park S, Scheffler TL, Gunawan AM, Shi H, Zeng C, Hannon KM, Grant AL, Gerrard DE (2009) Chronic elevated calcium blocks AMPK-induced GLUT-4 expression in skeletal muscle. American Journal of Physiology. Cell Physiology 296, C106–C115.
| Chronic elevated calcium blocks AMPK-induced GLUT-4 expression in skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFCitg%3D%3D&md5=91989fd0db2c28020d28bb496a7364d2CAS | 18971392PubMed |
Pearson AM, Young RB (1989) ‘Muscle and meat biochemistry.’ (Academic Press Inc.: San Diego, CA, USA)
Petersen GV, Blackmore DK (1982) The effect of different slaughter methods on the post-mortem glycolysis of muscle in lambs. New Zealand Veterinary Journal 30, 195–198.
| The effect of different slaughter methods on the post-mortem glycolysis of muscle in lambs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2Mznt1Oltw%3D%3D&md5=1ec46e37ffbb90fa3a92c143d11954b8CAS | 16030845PubMed |
Pethick DW, Cummins L, Gardner GE, Knee BW, McDowell M, McIntyre BW, Tudor G, Walker PJ, Warner RD (1999) The regulation by nutrition of glycogen in the muscle of ruminants. Recent Advances in Animal Nutrition in Australia. 12, 145–151.
Pethick D, Pleasants A, Gee A, Hopkins D, Ross IR (2006) Eating quality of commercial meat cuts from Australian lambs and sheep. In ‘Proceedings of the New Zealand Society of Animal Production’. pp. 363–367. (New Zealand Society of Animal Production)
Pöso AR, Puolanne E (2005) Carbohydrate metabolism in meat animals. Meat Science 70, 423–434.
| Carbohydrate metabolism in meat animals.Crossref | GoogleScholarGoogle Scholar | 22063742PubMed |
Przybylski W, Vernin P, Monin G (1994) Relationship between glycolytic potential and ultimate pH in bovine, porcine and ovine muscles. Journal of Muscle Foods 5, 245–255.
| Relationship between glycolytic potential and ultimate pH in bovine, porcine and ovine muscles.Crossref | GoogleScholarGoogle Scholar |
Purchas RW, Aungsupakorn R (1993) Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers. Meat Science 34, 163–178.
| Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVOqsL0%3D&md5=d32768ebd3b63090e0b29ed548ec9569CAS | 22060661PubMed |
Ren JM, Hultman E (1989) Regulation of glycogenolysis in human skeletal muscle. Journal of Applied Physiology (Bethesda, Md.) 67, 2243–2248.
Ren JM, Broberg S, Sahlin K, Hultman E (1990) Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiologica Scandinavica 139, 467–474.
| Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlt12rtLs%3D&md5=cf5b925364cde0d814107806156f6721CAS | 2239350PubMed |
Richter EA, Galbo H (1986) High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. Journal of Applied Physiology (Bethesda, Md.) 61, 827–831.
Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H (1982) Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. The American Journal of Physiology 242, E25–E32.
Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS (2012) Glycogen and its metabolism: some new developments and old themes. Biochemical Journal 441, 763–787.
| Glycogen and its metabolism: some new developments and old themes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFKktg%3D%3D&md5=477ba837052011df03b876bf9c3d5678CAS | 22248338PubMed |
Robergs RA (2001) Exercise-induced metabolic acidosis: where do the protons come from? Sportscience 5, R502–R516.
Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 287, R502–R516.
| Biochemistry of exercise-induced metabolic acidosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvVaqt7g%3D&md5=898484d8fa91a2117f20b4be1937c3c8CAS | 15308499PubMed |
Roncales P, Geesink GH, van Laack RLJM, Jaime I, Beltran JA, Barnier VMH, Smulders FJM (1995) Meat tenderisation: enzymatic mechanisms. In ‘Expression of tissue proteinases and regulation of protein degradation as related to meat quality’. pp. 311–332. (Eds A Ouali, DI Demeyer, FJM Smulders) (ECCEAMST: Utrecht, the Netherlands)
Rosenvold K, Essen-Gustavsson B, Andersen HJ (2003) Dietary manipulation of pro- and macroglycogen in porcine skeletal muscle. Journal of Animal Science 81, 130–134.
Rosenvold K, Borup U, Therkildsen M (2010) Stepwise chilling: Tender pork without compromising water-holding capacity. Journal of Animal Science 88, 1830–1841.
| Stepwise chilling: Tender pork without compromising water-holding capacity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsVWrs78%3D&md5=2dff5a0c107546f38a4631b51511eb18CAS | 20118418PubMed |
Rossi D, Sorrentino V (2002) Molecular genetics of ryanodine receptors Ca2+- release channels. Cell Calcium 32, 307–319.
| Molecular genetics of ryanodine receptors Ca2+- release channels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtFKntbo%3D&md5=4345a9c00565459f9f3f01f8e4209192CAS | 12543091PubMed |
Sahlin K (1978) Intracellular pH and energy metabolism in skeletal muscle of man with specific reference to exercise. Acta Physiologica Scandinavica 455, 7–56.
Sahlin K (1985) NADH in human skeletal muscle during sort-term intense exercise. European Journal of Physiology 403, 193–196.
| NADH in human skeletal muscle during sort-term intense exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFSlsbk%3D&md5=9ddd1f2c09fa0bbf74f3986e90a53b2fCAS | 3982970PubMed |
Sammel LM, Hunt MC, Kropf DH, Hachmeister KA, Kastner CL, Johnson DE (2002) Influence of chemical characteristics of beef inside and outside semimembranosus on color traits. Journal of Food Science 67, 1323–1330.
| Influence of chemical characteristics of beef inside and outside semimembranosus on color traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvVyqsb4%3D&md5=0704891bf821bf0a99289d18ec1a063fCAS |
Scheffler TL, Gerrard DE (2007) Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Science 77, 7–16.
| Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvVOgtr0%3D&md5=162fdaf99d4b5b55f39699a837224fb3CAS | 22061391PubMed |
Scheffler TL, Park S, Gerrard DE (2011) Lessons to learn about postmortem metabolism using the AMPK gamma 3(R200Q) mutation in the pig. Meat Science 89, 244–250.
| Lessons to learn about postmortem metabolism using the AMPK gamma 3(R200Q) mutation in the pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptlagtLc%3D&md5=57ae2d8d2a4f4e4471a4ad0ab1c7eaf9CAS | 21632185PubMed |
Schwägele F, Honikel KO (1988) Studies in postmortem metabolism of PSE-prone pork muscles. In ‘Proceedings of the 34th International Congress of Meat Science and Technology’. pp. 26–28.
Schwägele F, Haschke C, Honikel KO, Krauss G (1996) Enzymological investigations on the causes for the PSE-syndrome. 1. Comparative studies on pyruvate kinase from PSE- and normal pig muscles. Meat Science 44, 27–40.
| Enzymological investigations on the causes for the PSE-syndrome. 1. Comparative studies on pyruvate kinase from PSE- and normal pig muscles.Crossref | GoogleScholarGoogle Scholar | 22060753PubMed |
Scopes RK (1974) Studies with a reconstituted muscle glycolytic system. Biochemical Journal 142, 79–86.
Scott ID, Nicholls DG (1980) Energy transduction in intact synaptosomes—influence of plasma membrane depolarisation in the respiration and membrane potential of internal mitochondria determine in situ. Biochemical Journal 186, 21–33.
Sellier P, Monin G (1994) Genetics of pig meat quality: A review. Journal of Muscle Foods 5, 187–219.
| Genetics of pig meat quality: A review.Crossref | GoogleScholarGoogle Scholar |
Shackelford SD, Koohmaraie M, Miller MF, Crouse JD, Reagan JO (1991) An evaluation of tenderness of the longissimus muscle of Angus by Hereford versus Brahman crossbred heifers. Journal of Animal Science 69, 171–177.
Shackelford SD, Koohmaraie M, Savell JW (1994) Evaluation of longissimus-dorsi muscle pH at 3 hours postmortem as a predictor of beef tenderness. Meat Science 37, 195–204.
| Evaluation of longissimus-dorsi muscle pH at 3 hours postmortem as a predictor of beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbnt1yltA%3D%3D&md5=1c601bc0133cbfaa76caf57f401ca475CAS | 22059494PubMed |
Shearer J, Marchand I, Tarnopolsky MA, Dyck DJ, Graham TE (2001) Pro- and macroglycogenolysis during repeated exercise: roles of glycogen content and phosphorylase activation. Journal of Applied Physiology (Bethesda, Md.) 90, 880–888.
Shen QW, Du M (2005) Role of AMP-activated protein kinase in the glycolysis of postmortem muscle. Journal of the Science of Food and Agriculture 85, 2401–2406.
| Role of AMP-activated protein kinase in the glycolysis of postmortem muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKjsbjM&md5=eccf4f33c257482b84a9e3ff32717a78CAS |
Shen QW, Means WJ, Thompson SA, Underwood KR, Zhu MJ, McCormick RJ, Ford SP, Du M (2006a) Pre-slaughter transport, AMP-activated protein kinase, glycolysis, and quality of pork loin. Meat Science 74, 388–395.
| Pre-slaughter transport, AMP-activated protein kinase, glycolysis, and quality of pork loin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntlWrt74%3D&md5=d20e35aa5c05581277f37256885ef6fbCAS | 22062850PubMed |
Shen QW, Means WJ, Underwood KR, Thompson SA, Zhu MJ, McCormick RJ, Ford SP, Ellis M, Du M (2006b) Early post-mortem AMP-activated protein kinase (AMPK) activation leads to phosphofructokinase-2 and -1 (PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle. Journal of Agricultural and Food Chemistry 54, 5583–5589.
| Early post-mortem AMP-activated protein kinase (AMPK) activation leads to phosphofructokinase-2 and -1 (PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtF2qsrY%3D&md5=7607532a38b9e90529bf1ea3f9ce299fCAS | 16848549PubMed |
Shen QWW, Gerrard DE, Du M (2008) Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem. Meat Science 78, 323–330.
| Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlCrtr7M&md5=d1fe4d8b210629909b521a5ee3757d50CAS |
Simmons NJ, Singh K, Dobbie PM, Devine CE (1996) The effect of pre-rigor holding temperature on calpain and calpastatin activity and meat tenderness. In ‘42nd International Congress of Meat Science and Technology’. Lillehammer, Norway. (ICoMST)
Simmons NJ, Young OA, Dobbie PM, Singh K, Thompson BC, Speck PA (1997) Post-mortem calpain-system kinetics in lamb: Effects of clenbuterol and preslaughter exercise. Meat Science 47, 135–146.
| Post-mortem calpain-system kinetics in lamb: Effects of clenbuterol and preslaughter exercise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXns1yitbk%3D&md5=68489722692ef35ff5ed23868c904eefCAS | 22062624PubMed |
Smulders FJM, Marsh BB, Swartz DR, Russell RL, Hoenecke ME (1990) Beef tenderness and sarcomere-length. Meat Science 28, 349–363.
| Beef tenderness and sarcomere-length.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFagsQ%3D%3D&md5=7e4dc6cb5c35de1a3948efe9fc27fddeCAS |
Spriet LL, Berardinucci L, Marsh DR, Campbell CB, Graham TE (1990) Glycogen content has no effect on skeletal muscle glycogenolysis during short-term tetanic stimulation. Journal of Applied Physiology (Bethesda, Md.) 68, 1883–1888.
Stanley WC, Connett RJ (1991) Regulation of muscle carbohydrate metabolism during exercise. The FASEB Journal 5, 2155–2159.
Støier S, Aaslyng MD, Olsen EV, Henckel P (2001) The effect of stress during lairage and stunning on muscle metabolism and drip loss in Danish pork. Meat Science 59, 127–131.
| The effect of stress during lairage and stunning on muscle metabolism and drip loss in Danish pork.Crossref | GoogleScholarGoogle Scholar | 22062670PubMed |
Strasburg GM, Chiang W (2009) Pale, soft, exudative turkey—The role of ryanodine receptor variation in meat quality. Poultry Science 88, 1497–1505.
| Pale, soft, exudative turkey—The role of ryanodine receptor variation in meat quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Omtbc%3D&md5=a6fd5a58ad40502ec88d5b5212f4240dCAS | 19531723PubMed |
Talmant A, Monin G, Briand M, Dadet M, Briand Y (1986) Activities of metabolic and contractile enzymes in 18 bovine muscles. Meat Science 18, 23–40.
| Activities of metabolic and contractile enzymes in 18 bovine muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXjtFGisg%3D%3D&md5=7456e283964d2bd8b8f135e9b61f72edCAS | 22055463PubMed |
Tarrant PV (1989) Animal behavior and environment in the dark-cutting condition in beef—a review. Irish Journal of Food Science and Technology 13, 1–21.
Tarrant PV, Mothersill C (1977) Glycolysis and associated changes in beef carcasses. Journal of the Science of Food and Agriculture 28, 739–749.
| Glycolysis and associated changes in beef carcasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXls1Kitbg%3D&md5=03dc1ca58578fb9c995d87844c8b3af0CAS |
Tarrant PV, Sherington J (1980) An investigation of ultimate pH in the muscles of commercial beef carcasses. Meat Science 4, 287–297.
| An investigation of ultimate pH in the muscles of commercial beef carcasses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFarsQ%3D%3D&md5=0e0d39270ae3e48932adc82bf3c3de89CAS | 22055770PubMed |
Tornberg E (1996) Biophysical aspects of meat tenderness. Meat Science 43, 175–191.
| Biophysical aspects of meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Totland GK, Kryvi H (1991) Distribution patterns of muscle fibre types in major muscles of the bull (Bos taurus). Anatomy and Embryology 184, 441–450.
| Distribution patterns of muscle fibre types in major muscles of the bull (Bos taurus).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FnsVGktA%3D%3D&md5=4234351e6edf7cd147a67716958eba4cCAS | 1835822PubMed |
Troy DJ, Tarrant VP, Harrington MG (1986) Electrophoretic analysis of changes in beef myofibrillar proteins during the early postmortem period. Biochemical Society Transactions 14, 436–438.
Tullson PC, Terjung RL (1991) Adenine-nucleotide synthesis in exercising and endurance-trained skeletal muscle. The American Journal of Physiology 261, C342–C347.
Tullson PC, Rundell KW, Sabina RL, Terjung RL (1996) Creatine analogue beta-guanidinopropionic acid alters skeletal muscle AMP deaminase activity. American Journal of Physiology. Cell Physiology 270, C76–C85.
Tume RK (1979) Post-mortem electrical stimulation of muscle and its effects on sarcoplasmic reticulum adenosine triphosphatase. Australian Journal of Biological Sciences 32, 163–176.
van der Wal PG, Engel B, Reimert HGM (1999) The effect of stress, applied immediately before stunning, on pork quality. Meat Science 53, 101–106.
| The effect of stress, applied immediately before stunning, on pork quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFClsg%3D%3D&md5=39a84174d3d96ee672654b11d9667d2dCAS | 22063086PubMed |
Vandenberghe K, Richter EA, Hespel P (1999) Regulation of glycogen breakdown by glycogen level in contracting rat muscle. Acta Physiologica Scandinavica 165, 307–314.
| Regulation of glycogen breakdown by glycogen level in contracting rat muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVejtbY%3D&md5=d2e65a3554264d072260152abc1246b2CAS | 10192181PubMed |
Vestergaard M, Oksbjerg N, Henckel P (2000) Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls. Meat Science 54, 177–185.
| Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlCgtw%3D%3D&md5=d0d841f609750d8e3769308d7e87327eCAS | 22060614PubMed |
Warner RD, Bond JJ, Kerr MG (2000) Meat quality traits in lamb m.longissimus thoracis et lumborum: the effect of pre-slaughter stress and electrical stimulation. In ‘Proceedings 46th International Congress of Meat Science and Technology’. Buenes Aries, Argentina. (ICoMST)
Warner RD, Greenwood PL, Pethick DW, Ferguson DM (2010) Genetic and environmental effects on meat quality. Meat Science 86, 171–183.
| Genetic and environmental effects on meat quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXos1Cntr4%3D&md5=3d78893cb6b54556286f3f5646a745c9CAS | 20561754PubMed |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Warriss PD (1990) The handling of cattle pre-slaughter and its effects on carcass and meat quality. Applied Animal Behaviour Science 28, 171–186.
| The handling of cattle pre-slaughter and its effects on carcass and meat quality.Crossref | GoogleScholarGoogle Scholar |
Warriss PD, Brown SN, Nute GR, Knowles TG, Edwards JE, Perry AM, Johnson SP (1995) Potential interactions between the effects of preslaughter stress and postmortem electrical-stimulation of the carcasses on meat quality in pigs. Meat Science 41, 55–68.
| Potential interactions between the effects of preslaughter stress and postmortem electrical-stimulation of the carcasses on meat quality in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbntlagug%3D%3D&md5=a759d59b112cb5b7462b1ae4dc32570eCAS | 22060113PubMed |
Watson R, Gee A, Polkinghorne R, Porter M (2008) Consumer assessment of eating quality - development of protocols for Meat Standards Australia (MSA) testing. Australian Journal of Experimental Agriculture 48, 1360–1367.
| Consumer assessment of eating quality - development of protocols for Meat Standards Australia (MSA) testing.Crossref | GoogleScholarGoogle Scholar |
Westerblad H, Allen DG, Bruton JD, Andrade FH, Lannergren J (1998) Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiologica Scandinavica 162, 253–260.
| Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitlGqt7Y%3D&md5=3afc9dd2da520b9cb041ea8e33482673CAS | 9578370PubMed |
Westerblad H, Allen DG, Lannergren J (2002) Muscle fatigue: lactic acid or inorganic phosphate the major cause? News in Physiological Sciences 17, 17–21.
Wheeler TL, Savell JW, Cross HR, Lunt DK, Smith SB (1990a) Effect of postmortem treatments on the tenderness of meat from Hereford, Brahman and Brahman-cross beef-cattle. Journal of Animal Science 68, 3677–3686.
Wheeler TL, Savell JW, Cross HR, Lunt DK, Smith SB (1990b) Mechanisms associated with the variation in tenderness of meat from Brahman and Hereford cattle. Journal of Animal Science 68, 4206–4220.
Winder WW, Thomson DM (2007) Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochemistry and Biophysics 47, 332–347.
| Cellular energy sensing and signaling by AMP-activated protein kinase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnt1Clur8%3D&md5=5ec26ea6164a9a11060366311e3809c9CAS | 17652779PubMed |
Wulf DM, Emnett RS, Leheska JM, Moeller SJ (2002) Relationships among glycolytic potential, dark cutting (dark, firm, and dry) beef, and cooked beef palatability. Journal of Animal Science 80, 1895–1903.
Young JF, Bertram HC, Oksbjerg N (2009) Rest before slaughter ameliorates pre-slaughter stress-induced increased drip loss but not stress-induced increase in the toughness of pork. Meat Science 83, 634–641.
| Rest before slaughter ameliorates pre-slaughter stress-induced increased drip loss but not stress-induced increase in the toughness of pork.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFGns73E&md5=577576fc848da33a3afdd4799480bb43CAS | 20416646PubMed |


