Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner
K. J. Copping A , A. Hoare B , M. Callaghan C , I. C. McMillen D , R. J. Rodgers A and V. E. A. Perry E FA Robinson Institute, University of Adelaide, Frome Road, SA 5001, Australia.
B South East Vets, Mount Gambier, SA 5290, Australia.
C Ridley Agriproducts, Toowong, Qld 4066, Australia.
D The Chancellery, University of Newcastle, Callaghan, NSW 2308, Australia.
E School of Veterinary and Medical Science, University of Nottingham, Sutton Bonington, LE12 5RD, UK.
F Corresponding author. Email: viv.perry@nottingham.ac.uk
Animal Production Science 54(9) 1333-1337 https://doi.org/10.1071/AN14278
Submitted: 13 March 2014 Accepted: 23 May 2014 Published: 17 July 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Protein restriction in early bovine gestation affects post-natal reproduction and production traits in progeny. This experiment evaluated the effects of dietary protein restriction during the peri-conception period and first trimester in yearling heifers on conceptus growth and development; this period of dietary intervention being earlier than any previous bovine fetal programming studies. Three-hundred and sixty primiparous 12-month-old Santa Gertrudis heifers were individually fed high [14% crude protein (CP)] or low (7% CP) diets for 60 days before conception. At 23 days post-conception (dpc), each high (HPERI) or low (LPERI) group was again split into high (HPOST) or low (LPOST) protein groups yielding four treatment groups in a 2 × 2 factorial design. From the end of the first trimester of gestation (98dpc), the pregnant heifers were individually fed a 12% CP diet until parturition. Forty-six fetuses were excised at 98dpc. Sixty-four heifers went on to calve. Conceptus development was assessed via transrectal ultrasound from 36dpc, fetal necropsy at 98dpc and live calf measures at term. At 36dpc, HPERI diet increased fetal crown–rump length (CRL) (P < 0.05) and at the 60dpc scan, biparietal diameter (BPD) tended to be increased by HPOST diet (P < 0.1) though the greater effect upon BPD was still the HPERI diet (P < 0.05). At 60dpc, BPD in the male fetus was affected by the peri-conception diet (P < 0.05), while in females, BPD was not different among nutritional groups. These ultrasound measures of fetal growth were validated by measures of the excised fetus at 98dpc. Fetal weight was heavier (P < 0.01) in those whose mothers were fed the HPOST diet than their LPOST counterparts. Males fetuses were heavier than female fetuses (P < 0.001). Fetal CRL was increased by HPERI diet (P < 0.05) and tended to be increased by HPOST diet (P < 0.1). Fetal BPD tended to be increased by HPERI diet (P < 0.1). In males, BPD tended to be increased in those fetuses whose mothers were fed HPERI (P < 0.1). For females, maternal nutrition during PERI or POST did not affect BPD at 98dpc (P > 0.1). At term, no dietary effect on birthweight was observed (P > 0.1) and males were not heavier than females (P > 0.1). These results suggest that maternal protein intake during the peri-conception (–60 to 23dpc) and first trimester (24–98dpc) may influence early conceptus growth and development in the bovine. The long-term effects on offspring metabolism and post-natal development of this dietary intervention are yet to be determined.
Additional keywords: beef, embryo, fetus, nutrition, oocyte.
Introduction
Protein is the major limiting nutrient in range beef cattle in Australia. It has previously been established that protein restriction in early gestation may affect post-natal reproduction and production traits in progeny (Sullivan et al. 2009, 2010; Micke et al. 2010a, 2011; Mossa et al. 2013). Further, studies have also shown effects of maternal diet upon fetal growth to 123 days post-conception (dpc) after dietary intervention 30–123dpc (Long et al. 2009) although this effect was no longer apparent at birth (Long et al. 2012).
Critical windows exist during oocyte, embryo and fetal development where decreased maternal dietary protein may reset early cell lineages (Wu et al. 2006). Maternal nutrition affects both the number of oocytes that ovulate and their quality (Ashworth et al. 2009), which has then been associated with differences in embryo survival (Borowczyk et al. 2006). The gender of an embryo has also been shown to affect the susceptibility of the embryo to altered nutrition before mating (Vinsky et al. 2006). Gender-specific differences in embryo DNA methylation pattern (Dobbs et al. 2013), expression of key developmental genes (Kwong et al. 2006), fetal and placental perfusion (Prior et al. 2013) post-natal carcass traits (Micke et al. 2011) have all been reported. Intriguingly, neonatal and fetal death in cattle is increased if the fetus is male, as with most mammalian species.
The aim of this study is to further evaluate the critical windows during development where dietary protein may alter fetal development. By examining the effects of protein diets similar to those experienced in range conditions in supplemented and non-supplemented breeders during the period from oocyte development in the ovary to the end of the first trimester we aim to establish the effects of such restriction.
This paper focuses on the effects of protein restriction in early life upon conceptus growth and development. We hypothesise that low maternal protein during the peri-conception period and the first trimester will reduce fetal growth in a gender dependent manner.
Materials and methods
This project was approved by the University of South Australia IMVS Animal Ethics Committee (Approval number 18/11) on 11 March 2011.
Experimental animals
Three-hundred and sixty primiparous 12-month-old Santa Gertrudis (Bos taurus × Bos indicus) heifers were selected based on weight and age from SK Kidman herds at Glengyle and Morney Plains, south-western Queensland. All heifers were vaccinated against viral and bacterial diseases on two occasions 4 weeks apart before transport. The heifers were transported to Sedan, South Australia to purpose-built, shaded feedlot pens, acclimatised to diet, and trained to individual stall feeding.
At 12 months of age, 60 days before artificial insemination (AI), the heifers were randomly assigned to two equal groups stratified by weight. Each heifer was individually fed a high [14% crude protein (CP)] or low (7% CP) protein diet consisting of pellet diet fed in stalls and straw (5% CP) ad libitum in pens. The digestible energy content of the diet was as similar as possible in the ruminant and supplemented with a vitamin and mineral commercial preparation (Table 1).

|
Heifers underwent an 8-day progesterone-based oestrous synchronisation program. On Day 0, heifers were AI sequentially with frozen semen from one low birthweight Santa Gertrudis bull, selected on the basis of estimated breeding value.
At 23dpc each high (HPERI) or low (LPERI) group was again split into high (HPOST) or low (LPOST) protein groups yielding four treatment groups [high high (HH), high low (HL), low high (LH), low low LL)] in a 2 × 2 factorial design. Detailed composition of the heifer rations is given in Table 1.
Pregnancy was positively diagnosed in 124 heifers (HH = 33; HL = 37; LH = 30; LL = 28) at 36dpc via transrectal ultrasound and non-pregnant heifers were removed from the trial. At 60dpc fetuses were sexed via ultrasonography enabling equal numbers of sex fetuses to be excised at slaughter from each of the four treatment groups. From the end of the first trimester of gestation (98dpc), the remaining pregnant heifers were individually fed at the NRC recommended ration for weight containing 12% CP until parturition.
Fetal calf measurements
Fetuses were measured using transrectal ultrasound (Sonosite M-Turbo; Sonosite Inc., Bothell, Washington, DC, USA) at 36, 60 and 95dpc. Fetal sex was determined at 60dpc by rectal ultrasound. Measurements of the conceptus were taken at the time of ultrasonsography (Micke et al. 2010b). All fetal body measurements were in centimetres. Crown–rump length (CRL) was measured from a lateral view of the fetus from the tip of nose to the base of the tail. Biparietal diameter (BPD) was measured from a dorso-ventral view of the cranium perpendicular to the sagittal crest at the widest span between the most lateral parts of the parietal bone.
Fetal excision 98dpc
A subset of heifers (HH = 12; HL = 15; LH = 10; LL = 9) were killed in a commercial abattoir at 98dpc and used for human consumption. Tissues for examination were immediately collected from the uterus and fetus after removal from the kill floor. The fetus was excised from the uterus, cord blood collected, weighed, measured and then dissected. Sex of fetus was recorded during dissection. Measures of BPD and CRL were obtained from fetuses using sliding calipers. The remaining heifers were taken to term.
Calf measurements
At calving, heifers (n = 64; 19 female, 45 male) were monitored individually and assistance provided where necessary. Calves were collected within 15 min of birth, before sucking, and sex, birthweight and CRL recorded.
Statistical analyses
Multifactorial ANOVA using STATA SE version 11 (Stata Corporation, College Station, TX, USA) was used to interpret the main effects of peri- and post-conception diet, sex and their interaction terms on ultrasound measures of embryo and fetal body dimensions from 36dpc, fetal measures at 98dpc and live calf measures at term. Individual calf was considered the experimental unit, with gestation length as a co-variate for birth measures only. Males and females were analysed together and independently. A probability of 5% (P < 0.05) was accepted as the level of significance and trends reported as P < 0.1. Data presented as mean ± standard errors of the mean.
Results
Fetal measures in utero
At 36dpc high protein (14% CP) preconception diets increased fetal crown–rump length (CRL) compared with low protein (7% CP) (P < 0.05) (Fig. 1) and by 60dpc BPD tended to be increased by diet post-conception (P < 0.1) though the greater effect upon BPD was still peri-conception diet (P < 0.05) (Fig. 1). In males, BPD was increased in those fetuses whose mothers were fed HPERI (P < 0.05). For females, maternal nutrition during PERI or POST did not affect BPD at 60dpc (P > 0.1).
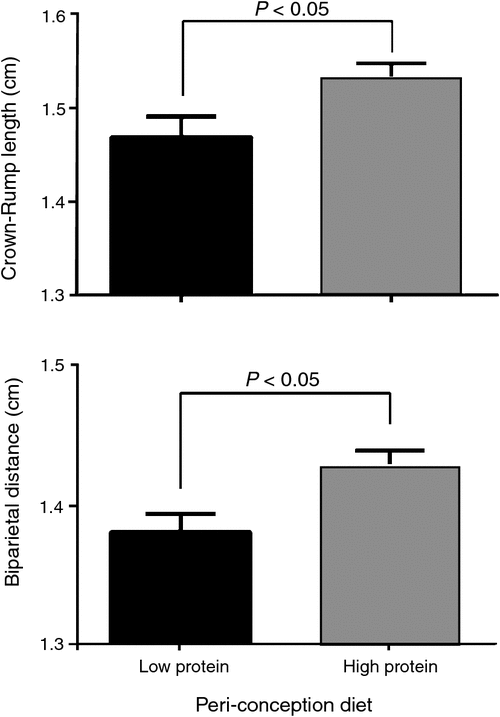
|
Weight gain during peri- and post-conception
Initial weight of the heifers at acclimatisation was 350 kg. The average daily gain of heifers was 0.4 kg/day during the high and 0.2 kg/day during the low protein treatment periods (Fig. 2).
Fetal size at 98dpc
In measures of the excised fetus at 98dpc, fetal weight was heavier (P < 0.01) in those whose mothers were fed HPOST diet (HH + LH = 326.3 ± 6.7 g) compared to their low counterparts (HL + LL = 301.2 ± 7.6 g). Male fetuses were heavier than female fetuses (P < 0.001). Fetal CRL at 98dpc was increased by HPERI diet compared to LPERI (P = < 0.05) and by this stage of development also tended to be increased by HPOST diet compared to LPOST (P < 0.1). Fetal BPD tended to be increased by HPERI diet (P < 0.1). In males, BPD tended to be increased in those fetuses whose mothers were fed the HPERI diet compared to LPERI (P < 0.1). For females, maternal nutrition during PERI or POST did not affect BPD at 98dpc (P > 0.1).
Calf size at birth
There was no effect of dietary treatment on birthweight, CRL or head measures (all P > 0.1). Gestation length did however affect birthweight (P < 0.05). Male calves were not larger or heavier at birth than female calves (P > 0.1).
Discussion
The present study demonstrates a significant difference in bovine conceptus size as early as 36 days post-conception in response to maternal dietary protein restriction during peri-conception and first trimester. Further, this effect was gender specific there being a greater effect of early dietary intervention on measures of male fetal growth at least until the end of the first trimester.
Nutritional intervention
The 360 heifers were individually fed for a total of 14 months (including acclimatisation) and inseminated via fixed time AI over 1.5 days. Thus, the nutritional regimens were instigated at exactly the same time points in gestation and were controlled with heifers being fed individually from 60 days before AI and throughout gestation until term. This is similar to previous fetal programming studies reported by (Sullivan et al. 2009; Micke et al. 2010a) but not others (Cafe et al. 2006; Greenwood and Cafe 2007; Hickson et al. 2008; Long et al. 2009, 2012). Furthermore, in these latter three studies, realimentation of the restricted heifers was applied so that all calved at similar weight. In this study the high protein diet group had average daily gain of 0.4 kg/day and low protein 0.2 kg/day over the treatment period. After this time they continued to be individually fed throughout gestation at positive weight gain [unlike studies with negative gain in the low treatment (Cafe et al. 2006)]; there being also no attempt, to attain similar liveweight at parturition. This difference in (1) nutritional window of dietary intervention and (2) severity and period of restriction applied, may explain the apparently conflicting results in fetal development reported from bovine fetal programming studies.
Sexual dimorphism
This period of dietary intervention is earlier than any previous studies investigating bovine fetal growth trajectory in utero. The sexual dimorphism observed, such that the effect peri-conception protein restriction effect was greater in the male, supports our previous findings in beef cattle (Micke et al. 2011, 2014). This suggests that the male embryo is more susceptible to early gestational intervention than the female, as seen in the in vitro embryo (Bermejo-Alvarez et al. 2011), and that this effect continues until at least 98dpc. The reason for this gender difference may be that the pattern of DNA methylation during embryo development in the two sexes is different over time making susceptibility to epigenetic change both gender and time dependent (Dobbs et al. 2013). We note however, that the expected gender effect on birthweight where the male is larger and heavier than the female was not observed in this study.
Birthweight
The finding that maternal peri-conception and first trimester nutrition did not affect birthweight at term may be an important dystocia management consideration when considering timing of supplementation to heifers. This is in agreement with previous reports following first trimester intervention (Micke et al. 2010b) and between 30 and 123dpc (Long et al. 2009). If restriction is applied during the second trimester (Micke et al. 2010b), however, or second and third trimester (Cafe et al. 2006) birthweight has reportedly been affected.
The lack of effect on gross morphology at birth, however, does not necessarily reflect the impact of early gestational intervention upon long-term growth, development and health in the offspring (Wu et al. 2006) as we have previously reported. In sheep, peri-conceptional undernutrition does not necessarily result in altered birthweight but has been associated with altered fetal growth trajectory and altered post-natal growth, metabolic and endocrine regulation (Jaquiery et al. 2011). The post-natal effects of similar periods of peri-conceptional undernutrition in the bovine are less well understood. Further studies of the post-natal development of the progeny are under way.
Acknowledgements
We are indebted to S. Kidman and Son for the provision of cattle and monetary support for this project and similarly to Ridley Agriproducts for the provision of feedstuffs and monetary support. This work was also funded by an ARClinkage grant. We are grateful for the excellent technical assistance provided by Ray Cranney, Mark Irrgang (Dec.), Wendy Bonner, Peter Johnston, Maddie Jonas and Tony Mott.
References
Ashworth CJ, Toma LM, Hunter MG (2009) Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philosophical Transactions of the Royal Society. B. Biological Sciences 364, 3351–3361.| Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability.Crossref | GoogleScholarGoogle Scholar |
Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A (2011) Transcriptional sexual dimorphism in elongating bovine embryos: implications for XCI and sex determination genes. Reproduction 141, 801–808.
| Transcriptional sexual dimorphism in elongating bovine embryos: implications for XCI and sex determination genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVCktLg%3D&md5=36407a2283c983eb9ec65b65d328d1bfCAS | 21411694PubMed |
Borowczyk E, Caton JS, Redmer DA, Bilski JJ, Weigl RM, Vonnahme KA, Borowicz P, Kirsch JD, Kraft KC, Reynolds LP, Grazul-Bilska AT (2006) Effects of plane of nutrition on in vitro fertilization and early embryonic development in sheep. Journal of Animal Science 84, 1593–1599.
Cafe LM, Hennessy DW, Hearnshaw H, Morris ST, Greenwood PL (2006) Influences of nutrition during pregnancy and lactation on birthweights and growth to weaning of calves sired by Piedmontese and Wagyu bulls. Australian Journal of Experimental Agriculture 46, 245–255.
| Influences of nutrition during pregnancy and lactation on birthweights and growth to weaning of calves sired by Piedmontese and Wagyu bulls.Crossref | GoogleScholarGoogle Scholar |
Dobbs KB, Rodriguez M, Sudano MJ, Ortega MS, Hansen PJ (2013) Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS ONE 8, e66230
| Dynamics of DNA methylation during early development of the preimplantation bovine embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVemsLvJ&md5=e42342e875dc9e5da2081697a33a2ce3CAS | 23799080PubMed |
Greenwood PL, Cafe LM (2007) Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production. Animal 1, 1283–1296.
| Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vpt1Wmtg%3D%3D&md5=de08c8a09c331d64c4fa0dc36a7b3aa2CAS | 22444884PubMed |
Hickson RE, Kenyon PR, Lopez-Villalobos N, Morris ST (2008) Effects of liveweight gain during pregnancy of 15-month-old Angus heifers on dystocia and birth weight, body dimensions, estimated milk intake and weaning weight of the calves. New Zealand Journal of Agricultural Research 51, 171–180.
| Effects of liveweight gain during pregnancy of 15-month-old Angus heifers on dystocia and birth weight, body dimensions, estimated milk intake and weaning weight of the calves.Crossref | GoogleScholarGoogle Scholar |
Jaquiery AL, Oliver MH, Bloomfield FH, Harding JE (2011) Periconceptional events perturb postnatal growth regulation in sheep. Pediatric Research 70, 261–266.
| Periconceptional events perturb postnatal growth regulation in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVSgu7vK&md5=3ca2ed7f34a619251bbe03826d1086c2CAS | 21587096PubMed |
Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond CW, Anthony F, Fleming TP (2006) Imprinted gene expression in the rat embryo–fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 132, 265–277.
| Imprinted gene expression in the rat embryo–fetal axis is altered in response to periconceptional maternal low protein diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Wjsrc%3D&md5=16e4c5c29ba794d2c2dd3481186ded95CAS | 16885535PubMed |
Long NM, Vonnahme KA, Hess BW, Nathanielsz PW, Ford SP (2009) Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. Journal of Animal Science 87, 1950–1959.
| Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1eju74%3D&md5=6cee9a873c51447792957be96d5cdf97CAS | 19213703PubMed |
Long NM, Tousley CB, Underwood KR, Paisley SI, Means WJ, Hess BW, Du M, Ford SP (2012) Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle. Journal of Animal Science 90, 197–206.
| Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvFOitQ%3D%3D&md5=cc993dd29743ec05648566c87d035fc1CAS | 21908644PubMed |
Micke GC, Sullivan TM, Gatford KL, Owens JA, Perry VEA (2010a) Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Animal Reproduction Science 121, 208–217.
| Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsrrE&md5=ab90c8b02e423ac86ba7f0cacc629726CAS | 20591585PubMed |
Micke GC, Sullivan TM, Magalhaes RJS, Rolls PJ, Norman ST, Perry VEA (2010b) Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Animal Reproduction Science 117, 1–10.
| Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlyjt7vO&md5=dfafe58885006d584349d497bf7fe45cCAS | 19394770PubMed |
Micke GC, Sullivan TM, McMillen IC, Gentili S, Perry VEA (2011) Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R. Molecular and Cellular Endocrinology 332, 234–241.
| Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1WqtLfL&md5=64f4e762b7692185caefc9a5fdbaf334CAS | 21056085PubMed |
Micke G, Hernandez J, Sullivan TM, Kennaway D, Perry VEA (2014) Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones, growth and appetite of their progeny. Theriogenology
Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JLH, Hildebrandt TB, Lonergan P, Ireland JJ, Evans ACO (2013) Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biology of Reproduction 88, 1–9.
Prior T, Wild M, Mullins E, Bennett P, Kumar S (2013) Sex specific differences in fetal middle cerebral artery and umbilical venous doppler. PLoS ONE 8, e56933
| Sex specific differences in fetal middle cerebral artery and umbilical venous doppler.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsF2itLY%3D&md5=e7490165d89ba54e482e77e4789d5519CAS | 23437275PubMed |
Sullivan TM, Micke GC, Perry VE (2009) Influences of diet during gestation on potential postpartum reproductive performance and milk production of beef heifers. Theriogenology 72, 1202–1214.
| Influences of diet during gestation on potential postpartum reproductive performance and milk production of beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCgtr3O&md5=c33acc133404a7a050b2722d6041b601CAS | 19796799PubMed |
Sullivan TM, Micke GC, Greer RM, Perry VEA (2010) Dietary manipulation of Bos indicus × heifers during gestation affects the prepubertal reproductive development of their bull calves. Animal Reproduction Science 118, 131–139.
Vinsky MD, Novak S, Dixon WT, Dyck MK, Foxcroft GR (2006) Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development. Reproduction, Fertility and Development 18, 347–355.
| Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD287ms1Slug%3D%3D&md5=3960f6b1db23ad639ed04605bfbb6b89CAS |
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Intrauterine growth retardation: implications for the animal sciences. Journal of Animal Science 84, 2316–2337.
| Intrauterine growth retardation: implications for the animal sciences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFGktLs%3D&md5=eb547f3a5d86acb2ff72a1c14ecd16cdCAS | 16908634PubMed |



