Ergovaline, an endophytic alkaloid. 2. Intake and impact on animal production, with reference to New Zealand
A. M. Nicol A and J. L. Klotz B CA AMN Consulting, 1248 Old West Coast Road, RD 1, Christchurch 7671, New Zealand.
B USDA-ARS, Forage-Animal Production Research Unit, Lexington, KY 40546, USA.
C Corresponding author. Email: james.klotz@ars.usda.gov
Animal Production Science 56(11) 1775-1786 https://doi.org/10.1071/AN14963
Submitted: 26 November 2014 Accepted: 28 June 2016 Published: 19 August 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
On the basis of published reports, the daily intake of the alkaloid ergovaline from the consumption of endophyte-containing ryegrass in New Zealand ranges from 0.008 to 0.287 mg ergovaline/kg LW0.75.day. Most of the reports are based on the use of standard endophyte-containing ryegrass and, thus, it is difficult to disassociate the impact of ergovaline consumption from that of lolitrem B. However, physiological effects of ergovaline consumption, such as reduced circulating prolactin concentration, vasoconstriction and elevated core temperature, have been detected at fairly low ergovaline intake, whereas decreased feed intake, liveweight gain and milk production have not generally been observed in animals at an intake below 0.07 mg ergovaline/kg LW0.75.day. Intakes above this value represent only 17% of published values. There are insufficient data to suggest a threshold ergovaline intake associated with heat stress with animal-welfare implications. The relationship between published ergovaline intake and the corresponding ergovaline concentration in pasture is poor (R2 = 0.48), but on average an intake of 0.07 ergovaline/kg LW0.75.day is associated with an ergovaline concentration in ryegrass of 0.70 mg/kg DM. About 16–18% of published ergovaline concentrations in ryegrass pasture exceed this value. The ergovaline concentration in ryegrass is greater in the basal parts of the plant than in the leaf and during the late summer–autumn than in spring. Animals grazing in the lower sward horizons (horizontal grazing plane) are more at risk of high ergovaline intake, although the reduction in grazing intake induced by grazing at low pasture height aids in limiting ergovaline intake. As pasture growth rates decline in late summer, supplementary feed may be used to maintain stocking rate and, if such feeds have zero ergovaline concentration, they serve to dilute the mean dietary ergovaline intake. Ergovaline-containing ryegrass pastures are widely used in New Zealand. It appears that farmers consider the risks of depressed animal production on these pastures to be less than the benefits ergovaline bestows through its deterrent effect of specific insect attack and thus greater survival and pasture persistence.
Additional keywords: ergot alkaloids, livestock, perennial ryegrass, threshold.
Introduction
The endophyte Neotyphodium lolii has a symbiotic relationship with the dominant grass species (Lolium perenne) in New Zealand pastures (di Menna et al. 2012). The advantage of this symbiosis to ryegrass is the protection against insect attack and thus greater pasture persistence (Popay and Hume 2011) provided by the endophytic alkaloids, chiefly, lolitrem B, peramine, ergovaline and epoxy-janthitrems. The disadvantage of endophyte intake by grazing animals is potential physiological disruption and decreased productivity. A high intake of lolitrem B is associated with ‘ryegrass staggers’ and this syndrome has been well reviewed (Fletcher and Easton 2007; di Menna et al. 2012; Thom et al. 2012). Strains of Neotyphodium lolii in which ergovaline rather than lolitrem B is the dominant alkaloid have been discovered and ryegrass cultivars containing these strains are commercially available (di Menna et al. 2012).
A companion review (Klotz and Nicol 2016) identified a wide range of physiological effects that can be induced by ergovaline consumption. The aims of the present review are to document published research data on the intake of ergovaline by New Zealand livestock, so as to relate these intakes to physiologically and economically important effects, and assess the probability of detrimentally high ergovaline intake occurring in New Zealand.
Ergovaline intake
The results of most of the New Zealand research that compares animals grazing endophyte-containing pasture with those on endophyte-free or AR1 (does not produce lolitrems or ergot alkaloids) pasture are summarised in Table 1. Only experiments where experimental details allow estimates of ergovaline intake are included. Treatments have been ranked on daily ergovaline intake (mg/kg LW0.75.day). These comparisons, with the exception of the work of Layton et al. (2004) and that of Cosgrove et al. (1996) using 187BB endophyte, have involved standard endophyte and thus include possible interfering effects of lolitrem B. The importance of such a confounding factor is discussed later.
The data summarised in Table 1 indicate that the effects of ergovaline consumption appear to be dose-related. Reduced serum prolactin concentration and increased respiration rate have been observed at relatively low ergovaline intakes of <0.05 mg/kg LW0.75.day. However, these signs have not been associated with increased core temperature until ergovaline intake exceeds >0.07 mg/kg LW0.75.day. Economically important symptoms of reduced DM intake, milk production or liveweight gain are seldom associated with a daily ergovaline intake below 0.07 mg/kg LW0.75.day. There are insufficient published data to suggest a threshold ergovaline intake for overt heat stress. Ryegrass staggers have been observed over a wide range of ergovaline intakes, but are related to the concurrent lolitrem B intake (see section Data interpretation).
A schematic of the suggested ranking of these responses (Fig. 1) in an increasing order of importance is as follows:
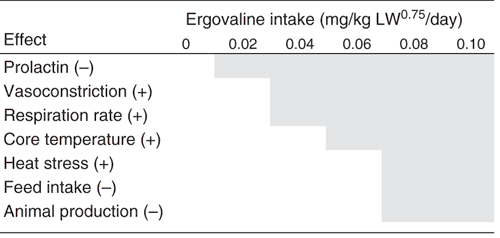
|
(1) Decreased circulating prolactin concentration
Ergot alkaloids, such as ergovaline, are D2 dopamine-receptor agonists that result in a suppression of plasma prolactin concentration (Larson et al. 1995). The USA fescue toxicosis literature has demonstrated that circulating prolactin concentration is a very sensitive, but variable, indicator of ergopeptide (ergovaline) modulation of the D2 dopamine receptor (Oliver 1997). Several reports (Table 1) have shown a depression in circulating prolactin concentration as a response to ergovaline intake from endophyte-infected ryegrass. However, using dairy cattle, Bluett et al. (2003a) provided evidence that serum prolactin concentration may not be useful as an indicator of an ergovaline effect on animal production because it has not been well correlated with effects on liveweight gains in animals (Aldrich et al. 1993). In essence, a suppressed prolactin concentration is a good indicator of ergovaline intake but not of the level of intake, or magnitude of other responses.
(2) Vasoconstriction
Vasoconstriction is often measured in research settings, but is not a response typically observed at the producer level. Also, it can contribute to several other responses, such as heat stress. The interaction of ergovaline with α2-adrenoreceptors has been shown to cause bronchoconstriction in sheep (Nolan et al. 1986) and calves (Gustin et al. 1989). Serotonergic-2 receptors have also been shown to be involved in bronchoconstriction in calves (Linden et al. 1993). Vasoconstriction of the peripheral vasculature by ergovaline interacting with adrenergic and serotonergic receptors results in a decreased ability to dissipate body heat and can contribute to increased core temperature (Spiers et al. 1995). Foote et al. (2013) indicated that ergovaline-induced vasoconstriction at the rumen epithelium may affect nutrient absorption, and hypothesised that this may affect intake as well as liveweight gain. It is debatable whether it is the decrease in prolactin concentration or a measureable vasoconstrictive response that occurs first in response to ergovaline exposure. It is thought that recovery differs between these two responses. There is a rapid recovery of prolactin concentrations after removal from an ergovaline source; whereas vascular recovery takes a considerably longer period of time (Aiken et al. 2013). Because they are more sensitive responses to ergovaline exposure, vasoconstriction and reduced prolactin effects alone are not good indicators of animal production or welfare issues.
(3) Increased respiration rate
An increase in respiration rate is part of a normal homeostatic mechanism to increase evaporative heat loss (Ganong 1981). In the case of ergovaline intake, the increase in respiration rate is presumably in response to decreased evaporative heat loss from the skin surface due to ergovaline-induced vasoconstriction. Unless an increase in respiration rate is prolonged (not mitigated by lower night-time ambient temperature) or leads to overt heat stress, it is not in itself a detrimental, i.e. welfare-concerning, physiological response. This response to ergovaline exposure is also complicated by the observance of respiratory distress in animals exposed to lolitrem B and exhibiting symptoms of ryegrass staggers (Oliver 1997).
(4) Increased core body temperature
Animals have diurnal fluctuations in core temperature within their normal daily life of ~0.5–0.7°C (Breen and Barrell 2002; Refinetti and Piccione 2005). The increases in core temperature, reported as a response of ergovaline intake, of 0.2–0.4°C, are within this normal circadian range. It can be argued that there may be advantages for animals consuming ergovaline to allow their ‘set-point’ of core temperature to increase. The higher core temperature would raise the temperature differential between pleural blood and inspired air, which together with an increased respiration rate potentially enhance evaporative heat loss from the lungs as a compensation for reduced peripheral heat loss due to the ergovaline-induced vasoconstriction.
(5) Decreased feed intake, decreased production (e.g. liveweight gain, milk production)
If ergovaline intake is of sufficient magnitude and duration (see later section), the effects of ergovaline consumption can be of economic significance to livestock producers. However, of the 35 studies summarised in Table 1, only 6 (17%) identified significant decreases in animal production. The effects of ergovaline intake on milksolids production ranged from 7–11% more on endophyte-free pasture over the trial period (Clark et al. 1996) to 8–12% less from December to January, but which accounted for only 4% and 0% decrease over the complete season (Thom et al. 2013). A larger effect of a 23% reduction in milksolids production over a period from October to April recorded by Keogh et al. (1999) on a standard endophyte-containing pasture was confounded by pasture source. Effects of ergovaline intake on liveweight gain, extrapolated from the results of Layton et al. (2004), showed a less than 15% reduction at an ergovaline intake below ~0.075 (hoggets) and 0.15 mg/kg LW0.75.day (heifers). Such ergovaline intakes are well above the average reported (Table 1).
(6) Heat stress
Prolonged and a high incidence of heat stress can be an animal-welfare issue and represents the most important potentially detrimental effect of high ergovaline intake. Symptoms of heat stress (e.g. panting, excess salivation) can be induced during exposure to high ambient temperature (32−36°C) and relative humidity (69% RH) in sheep that have had a moderate intake of ergovaline (Fletcher et al. 1994; Bluett et al. 2001). When evaluating the effects of ergovaline in endophyte-infected tall fescue, the effects of fescue toxicosis are exacerbated at higher ambient temperatures. Aldrich et al. (1993) demonstrated that at 22°C, cattle fed a diet containing ergovaline-free tall fescue seed ate more than those fed a diet containing ergovaline. When the ambient temperature was increased to 32°C, intake in both groups decreased but the reduction was greater in cattle consuming ergovaline.
Incidences of heat stress in cattle have been recorded in Northland (Easton et al. 2000) where ergovaline intake has been implicated as a cause. Bryant et al. (2007) reported a small decline in herd test daily milksolids production of (10 g/day.unit temperature humidity index, THI) when the average 3-day THI exceeded 68–73. In the paper by Bryant et al. (2007), THI was calculated as 0.8T + [RH/100 × (T – 14.4)] + 46.4, where T = daily maximum ambient temperature and RH = relative humidity.
However, separating the effects of ergovaline from the overall effects of heat stress has been difficult and there is little formal evidence to confirm that ergovaline intake significantly increases the incidence or severity of heat stress. No work has established the relationship between ergovaline intake and the upper critical temperature or threshold THI of cattle or sheep and this is an area that may require more study.
Heat stress may also be modulated by regional differences in the New Zealand environment. In the Canterbury region of the South Island, no evidence of reduced milk production was associated with hot weather (Laird and Barrell 2010) and, presumably, many of these cows were grazing novel endophyte-containing ryegrass pasture. Thom et al. (2013) concluded that production of milksolids was not affected by wild-type endophyte in Waikato region of the North Island and, on the basis of the same farmlet data, Bluett et al. (2003b) noted that the number of heat-stress days was similar for cows on both standard endophyte-containing and AR1-containing (no lolitrem B or ergovaline) pasture.
Data interpretation
One of the greatest issues in interpreting data in Table 1 is the concomitant presence of both ergovaline and lolitrem B in standard endophyte and in NEA2 novel perennial ryegrass endophyte. This makes it unrealistic to apportion separately the effects of the two alkaloids or to determine the primary suspect of the pair, particularly if there is possible synergy between them (Bluett et al. 2001). Of the New Zealand data, only the work of Layton et al. (2004) and Cosgrove et al. (1996), using 187BB-containing (a high ergovaline- low lolitrem-producing endophyte) pasture removed the potential for a major lolitrem B effect and minimised confounding. However, their data, when compared with those from standard endophyte pastures, are not suggestive of important synergistic or antagonistic relationships between ergovaline and lolitrem B. The general consensus, as illustrated by the data of Thom et al. (2013), is that the incidence of ryegrass staggers is more closely correlated with the lolitrem B concentration than the ergovaline concentration in standard endophyte-containing pastures. Hovermale and Craig (2001) analysed 459 ryegrass samples to report that when an average concentration of 2 mg lolitrem B/kg DM is reached in the plant, the average concentration of ergovaline is 0.175 mg/kg DM. At these concentrations the clinical effects of ryegrass staggers will prevent any effects of ergovaline from being observed and will likely result in a decreased intake of ergovaline as a result of reduced feed intake due to lolitrem B.
The question as to which is the dominant alkaloid, in terms of limits to pastoral livestock production, also exists for pasture with ‘novel’ endophytes that have different concentrations and ratios of lolitrem B to ergovaline from those of standard endophytes. The qualitative data presented in Table 2 (Stewart et al. 2014) suggest that ergovaline may be the dominant alkaloid in ryegrass containing the NEA2 endophyte, but quantitative data on the ergovaline profiles of novel endophytes in both the grazed and residual portions of the pasture are needed. The novel endophytes are owned, and their use licenced, by New Zealand proprietary ryegrass seed-producing companies.
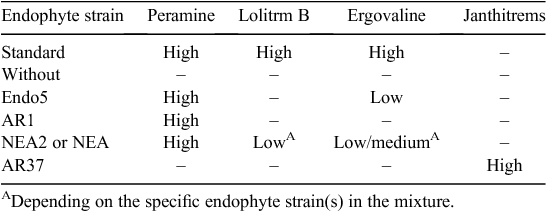
|
Given that there are examples of reduced feed intake and lowered animal production associated with ergovaline intake from grazed perennial ryegrass pasture in New Zealand, the question now becomes as follows: ‘What is the likelihood that a detrimentally high ergovaline intake will occur?’.
Predicting ergovaline intake
Ergovaline intake is the product of DM intake and the ergovaline concentration of the DM consumed. Clearly, the higher the DM intake at a common ergovaline content, the higher the animal’s ergovaline intake. For example, a dairy cow at maintenance will have an ergovaline intake of only 25% of that of a cow producing 2.0 kg milksolids/day. Furthermore, the same high-producing cow consuming pasture with an ergovaline concentration of 0.25 mg/kg DM will have an ergovaline intake similar to that of a similar-weight cow at maintenance consuming pasture with an ergovaline concentration of 1.0 mg/kg DM. For these reasons, liveweight and level of animal performance need to be recorded in trials investigating the impact of ergovaline-containing pasture, so that ergovaline intake can be estimated.
Ergovaline in perennial ryegrass
A major factor that can influence animal intake of ergovaline is the concentration of ergovaline in the plant. The main factors affecting ergovaline concentration within perennial ryegrass are anatomical variation within the plant and seasonal variation and cultivar.
Anatomical variation
Ergovaline concentration is highest in the parts of the plants critical for the host–plant survival, i.e. the basal region. (Lane et al. 1997). Concentrations of ergovaline decrease from basal to apical regions of the vegetative plant, with the crown or lowest 5 mm of basal stem having the highest (0.5–5 mg/kg DM) and leaf having the lowest (0.0–0.5 mg/kg DM) concentrations (Lane et al. 1997). The distribution of the endophyte mycelium, although correlated, showed only a weak correlation with alkaloid accumulation in the tissues and appears to be only a minor factor in determining alkaloid concentrations. This variation in ergovaline within a tiller will have an obvious effect on consumption by the animal, bioavailability within the animal and sampling by research personnel. The potential impacts of these aspects are discussed in subsequent sections of the present review. The concentration of ergovaline can also be affected by the host-plant genotype (Easton et al. 2002), the reproductive status of the plant (Lane et al. 1997) and tissue position and age (Spiering et al. 2005). The implication of this within-plant variation in ergovaline concentration is that when grazing is concentrated in the higher pasture horizons, thus being mainly leaf, ergovaline intake will be low compared with that when grazing includes or is exclusively focussed on the lower horizons.
Seasonal variation
In New Zealand, ergovaline concentrations in perennial ryegrass show a seasonal pattern and are highest in summer (January, February, March; ~0.8–2.0 mg/kg DM) and lowest in winter (~0.1–0.4) (Fletcher et al. 2000; Thom et al. 2013). A typical seasonal pattern is illustrated in Fig. 2 and is based on samples cut to ground level. Similar seasonality in ergovaline concentration in perennial ryegrass has been observed in several Australian locations (Reed et al. 2011). This seasonal pattern is most highly correlated with soil temperature (Fletcher et al. 2000) or a THI index (Thom et al. 2013).
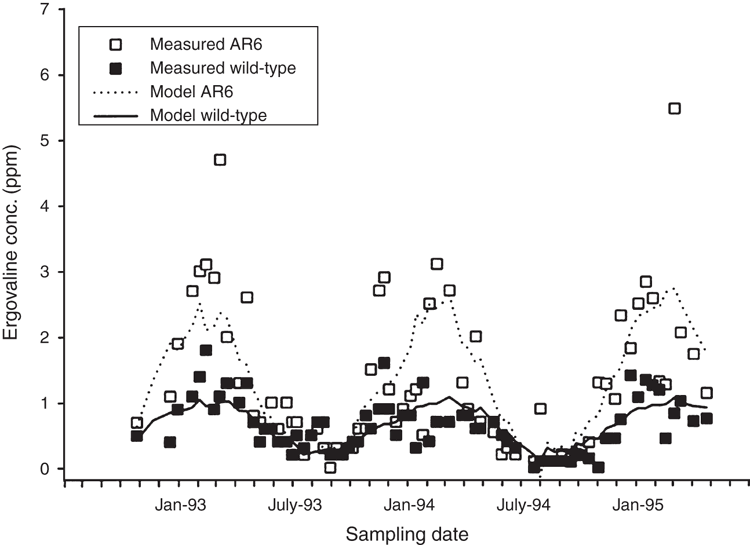
|
The implications of this seasonal pattern of ergovaline concentration are as follows:
-
For at least 6 months of the year, ergovaline concentration of standard endophyte in perennial ryegrass is unlikely to be greater than 0.5 mg/kg DM. Concentrations higher than this have been detected for some novel endophytes such as AR6 and have not been documented for others (e.g. NEA2).
-
During the summer months, most animal-production systems, with the exception of lamb finishing, have passed their peak annual production, so DM intake will be declining, thus decreasing potential ergovaline intake. Furthermore, because pasture production is lower in summer than spring, some animal-production systems incorporate supplementary feeding to help maintain animal production through the summer, which effectively reduces ergovaline intake (see Mitigation section).
-
During the higher summer temperatures when ergovaline concentrations are peaking, grazing animals are at the greatest risk of heat stress (see Heat stress section).
Linking ergovaline concentration in pasture to ergovaline intake by the animal
Daily ergovaline intake should be used as the predictor of the ‘risk’ from ergovaline consumption, but often only ergovaline concentration in pasture is available to assess this risk associated with grazing a given pasture. The data in Table 1 were used to determine the relationship between ergovaline intake (mg/kg LW0.75.day; Y) and ergovaline concentration in the pasture (mg/kg DM; X). The relationship was found to be Y = 0.086X, R2 = 0.42. The ergovaline concentration in pasture is a poor predictor of daily ergovaline intake, which is not surprising given the discussion above. However, this relationship suggests that as a crude working value, an ergovaline concentration of 0.1 mg/kg DM equates to an ergovaline intake of 0.01 mg/kg LW0.75.day. An analysis of 48 published values of ergovaline concentrations in Table 1 shows that 68% of the values are less than 0.7 mg ergovaline/kg DM (Fig. 3). This is not dissimilar to the figure of 70% less than 1.0 mg ergovaline/kg DM from a survey of ryegrass pastures in the Northland region (Easton et al. 1996). On this basis, a high proportion of New Zealand pastures should (on average) not contain sufficient ergovaline to provide an ergovaline intake high enough to depress animal productivity or cause animal-welfare concerns.

|
Defining an ergovaline intake threshold: prudence or folly?
Often the difference between a compound being labelled as a medicine or a poison is the dose. It is tempting, although risky, if based on the limited data, to suggest a critical threshold of ergovaline intake above which detrimental effects on animal production and animal welfare are likely to be observed. The cumulative incidence of observations in Table 1 showing a significant depression, or no significant effect on milk production or liveweight gain, is illustrated in Fig. 3, which suggests a crossover at 0.07 mg/kg LW0.75.day. In Table 1, the incidence of a significant decrease in animal production with an ergovaline intake below 0.07 mg/kg LW0.75.day is less than 1 in 6 and, above this intake, the incidence of a significant or non-significant effect is close to 1 in 1. Clark et al. (1996) suggested a value of 10 mg/kg LW.day (equivalent to 0.1 mg ergovaline/kg LW0.75.day) for dairy cows. These values are higher than the suggested threshold ergovaline intake from tall fescue in the USA (0.035–0.045 mg/kg LW0.75.day (Bohnert and Merrill 2006). Some suggested explanations for this difference are discussed in other sections.
Others have sought to define the dietary minimum concentration of ergovaline that an animal can tolerate before exhibiting negative effects or an ergovaline threshold (e.g. Tor-Agbidye et al. 2001). A general value would be advantageous to producers who could use the information as a tool to aid in management decisions. Hovermale and Craig (2001) suggested a threshold range of ergovaline of 0.3–0.4 mg/kg DM that was associated with clinical signs in cattle grazing tall fescue in USA. Stamm et al. (1994) also reported a threshold value of 0.475 mg/kg DM ergovaline, again in the USA. A suggested working figure for the dietary maximum safe concentration on the basis of the New Zealand pasture reviewed here is an ergovaline concentration of 0.7 mg/kg DM, which is considerably higher than the USA thresholds.
Interpretation of experimental data on ergovaline
There are many reasons to be cautious about the above interpretation of experimental data on the impact of ergovaline on animal production and, thus, the thresholds for intake and pasture concentration of ergovaline. These include method of pasture sampling, grazing intensity, pasture quality and composition, length of exposure and experimental design, each of which will be considered here.
Method of pasture sampling
Pasture samples collected for ergovaline concentration may be cut to ground level, to a specific height above ground level, or taken from the grazed horizons. Although sampling to ground level provides an indication of maximum possible ergovaline exposure, given the general increase in ergovaline concentration from leaf blade tip to pseudostem (see section Anatomical variation), such samples are likely to overestimate ergovaline intake, as grazing is seldom to ground level. Pasture samples for measurement of ergovaline concentration should probably be specific to grazed horizons and grazing height should be used to enable such sampling. This, of course, increases the resource requirement and the costs of estimating ergovaline intake and requires researchers to predetermine grazing-height limits and manage the animals to achieve these targets.
Often details of pasture sampling methods and details of pre- and post-grazing pasture height are not well documented, all of which makes estimation of ergovaline intake difficult. Standard protocols need to be established. Differences in pasture-sampling protocols may contribute to the differences in suggested threshold ergovaline concentrations between New Zealand and the USA.
Grazing intensity
At a greater grazing intensity, sheep and cattle graze to a lower post-grazing pasture height (Cosgrove and Edwards 2007), resulting in a higher mean ergovaline concentration in the herbage consumed. Whether this results in a higher ergovaline intake depends not only on the increase in endophyte concentration in lower plant parts but also on the counterbalancing effect of reduced grazing intake associated with the lower post-grazing pasture height. This interaction was explored by Bluett et al. (2001) who incorporated ‘leader’ and ‘follower’ groups of lambs grazing standard endophyte-containing pastures. However, in this work the decrease in grazing intake of the followers was small (4%) and not enough to significantly change the ergovaline intake of the two groups of lambs.
Some trials (e.g. Fletcher and Sutherland (2009)) have been designed so that animals graze into the lower horizons (<6 cm) with high endophyte content as a ‘worst-case scenario’, to test for potential animal effects of endophytes. Results from such experiments will not translate well to situations of less intensive grazing where higher levels of animal performance are targeted.
Pasture quality and composition
Layton et al. (2004) suggested that an explanation of why increasing ergovaline intake caused much less of a decrease in liveweight gain in their heifers than in their hoggets was the higher quality of the basal pasture diet of the heifers. They hypothesised that the faster rate of gastrointestinal-tract passage of the higher-quality cattle pasture may reduce absorption of ergot alkaloids. If this is true, a higher-quality basal pasture (ryegrass) in New Zealand, compared with USA (tall fescue), may contribute to a higher suggested threshold for ergovaline intake in New Zealand.
In theory, ryegrass pastures that contain a significant proportion of clover or herbs, such as plantain or chicory or some weeds, will dilute ergovaline intake, if the companion plants are not likely to contain ergovaline. Cosgrove et al. (1996) found a lower mean ryegrass-stagger score in cattle grazing standard endophyte-containing pastures when the pasture contained clover, although the details of the clover content of the pasture were not provided. However, Clark et al. (1996) found that the nutritional benefits of white clover for increasing milksolids was much reduced by the presence of a standard endophyte. Similar data do not exist for ergovaline alone. Pastures with high clover content are preferred to promote high levels of animal performance, so it will be of value to establish more clearly any interaction between clover content of the pasture and ergovaline intake. Most short-term trials designed to compare ryegrass cultivars and their interaction with endophytes have been sown without clover, and, thus, do not shed light on this interaction.
Period of exposure and experimental design
Animals grazing ergovaline-containing pastures in New Zealand may be exposed to a high ergovaline intake only in late summer and autumn (see section Seasonal variation) when ergovaline concentration in the pasture is high, or if grazing to a low post-grazing height (see above). The length of such exposure may be 4–6 weeks in the former (high ergovaline concentration) and a day or two in the latter (grazing to low pasture height) before the pasture grazed has a low ergovaline concentration. There is good evidence for compensatory growth following exposure to ergovaline intake that depresses intake and liveweight gain (Emile et al. 2000) and Thom et al. (2013) showed that the reduction in milk yield of cows grazing standard endophyte-containing pastures was much less when measured over the whole lactation than that during the actual period of exposure to pasture with high endophyte concentration. This suggests that if experiments do not conclude at the end of the ergovaline-exposure period, effects on animal performance are likely to be undetected or diminished in magnitude.
Changes in digesta load may enhance the negative effect (Emile et al. 2000) or reduce (Koontz et al. 2013) the apparent impact of ergovaline intake on liveweight gain. To avoid either of these possible confounding effects on estimates of liveweight gain, experimental animals should be reweighed after 2–3 days on a common ergovaline-free feed type at the conclusion of a trial, for an assessment of the impact of ergovaline consumption on liveweight gain.
Low ergovaline intake, low ergovaline concentration in pasture, inappropriate pasture sampling methods, short exposure to ergovaline intake, and full recovery and gut-fill effects of the animals may well contribute to the many comments in the literature on the lack of large effects of exposure to endophyte-containing pasture. Below are some examples of these comments, which are mostly based on observations using standard endophyte.
Under the environmental conditions experienced in the Manawatu, endophyte alkaloids have minimal direct effect on cattle performance. (Cosgrove et al. 1996, p. 43)
These results amount to an irregular picture, with (standard) endophyte sometimes having no measurable effect on cattle and sometimes depressing milk production and weight gain. (Easton et al. 2000, p. 362)
We conclude that (standard) endophyte in both pasture and pasture silage can have a transitory effect on milk and protein yield during the autumn. (Clark et al. 1996, p. 292)
Only 6 out of 16 short term trials showed a depressing effect of +E and some of these when endophyte levels were low. (Thom et al. 1999, p. 41)
The effect of wild type (standard) on cattle health and productivity in summer and autumn can be significant and economically important but they are not readily predictable. (Easton et al. 2000, p. 362)
The effects of ergovaline intake on cattle performance were small. (Layton et al. 2004, p. 193)
There are many factors (other than variable ergovaline concentrations across the tiller and throughout the year) that might or will explain the variable results described in the literature and cause values for threshold intake to change. These include the following:
(1) Animal genetics
Selection experiments have demonstrated genetic diversity in animal sensitivity to lolitrem B (Morris et al. 2007) and to sporidesmin (Morris et al. 2004), so it would be surprising if similar genetic diversity did not exist for tolerance to ergovaline.
(2) Physiological status of the animal
The physiological status of the animal not only affects ergovaline intake (Table 3), but may change the sensitivity of the animal to any particular dose.
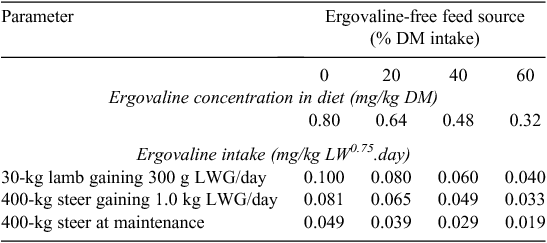
|
(3) Interaction with other secondary plant compounds
A synergistic relationship between ergovaline and lolitrem B intake has been suggested (Bluett et al. 2001) and exposure to other compounds such as isoflavones or phyto-oestrogens may influence the effects of ergovaline.
(4) Environment (temperature–humidity)
The evidence that the effects of ergovaline consumption are exacerbated at higher ambient temperatures and humidity (see section Heat stress) suggests that threshold intakes are likely to be lower in the north of the North Island than elsewhere in New Zealand. Or, alternatively, upper critical temperature or THI are likely to be lower for animals with a high ergovaline intake.
No good data exist to quantify these criteria and their significance in the hotter, more humid regions of New Zealand. But to crudely assess the possible impact of climate, the following analysis was performed. The New Zealand mean monthly maxima and minima ambient temperatures and relative humidity records over the period from 1850 to 2014 (NIWA 2014) were trawled. They identified February in Kaitaia, Northland, as the site and month at which the mean monthly THI (calculated as per Bryant et al. (2007)) was greatest. From an analysis of the daily data for this site in February over the period 2009–2014, the impact of a reduction in threshold THI below that of 72 (a commonly accepted threshold THI for dairy cows; Laird and Barrell 2010) was established.
This analysis showed that if the threshold THI associated with a high ergovaline intake was to drop from 72 to 70, the number of ‘heat-stress days’ in February would increase by between 1 and 5 days (or an average of 15%). This assessment was made using the maximum daily ambient temperature. On no days during these years did a THI based on the daily minimum ambient temperature exceed 68. Bryant et al. (2007) showed a small decrease in daily milksolids production (10 g/day.unit THI), where critical THI was exceeded for three consecutive days. Furthermore, Igono et al. (1992) demonstrated in dairy cows that a cool period of below 21°C for at least 3 h will minimise any decline in milk yield. The above analysis of climate records indicated that the environmental impact of ergovaline on grazing animals is likely to be minimal, even in the hottest regions of New Zealand.
(5) Health status
As with many challenges to normal function, previous illness or other health issues may compromise the animal’s ability to deal with normally low concentrations of ergovaline, but we know of no evidence to substantiate this.
Mitigation of potential high ergovaline intake
In situations where environmental conditions (hot and humid) and pasture ergovaline concentrations (>0.7 mg/kg DM) could potentially lead to detectable detrimental effects on animal production and welfare, there are several mitigating options available. These include dietary dilution, reducing the level of animal performance, and self-regulation of feed intake.
Dietary dilution
Provision and consumption of an alternative feed source to a ‘risky’ pasture can reduce ergovaline intake (Table 3). Over 20% of the total DM intake needs to be ergovaline-free to substantially reduce the average ergovaline concentration of the diet consumed. When supplementary feeds of high nutritional value are offered, substitution of pasture by supplement intake can help reduce ergovaline intake (Clark and Woodward 2007).
Level of animal performance
Further reference to Table 3 demonstrates that animals with a high target level of animal performance are at a greater risk of experiencing a high ergovaline intake at any given dietary ergovaline concentration. For example, a moderate dietary ergovaline concentration of 0.64 mg/kg DM is likely to lead to a marginally high ergovaline intake of 0.08 mg/kg LW0.75.day for a 30-kg lamb growing at 300 g/day. In contrast, a relatively high dietary ergovaline concentration of 0.80 mg/kg DM is calculated to lead to a low ergovaline intake of 0.049 mg/kg LW0.75.day for a 400-kg steer at maintenance. Where practical, ‘risky’ pastures should be grazed by animals with a low target level of animal performance.
Self-regulation of endophyte intake
There are two mechanisms by which animals may self-regulate their ergovaline intake. Sheep have been shown to have a lower preference for standard endophyte-containing pasture than for endophyte-free pasture when presented with the two sources in separate plots (Edwards et al. 1993; Cosgrove et al. 2002) and Layton et al. (2004) found that both sheep and cattle rejected ground ryegrass seed with a very high ergovaline concentration. Preference tests with pastures containing varying concentrations of ergovaline-only swards have not been performed.
When seeds of varying endophyte status are sown together during pasture establishment, it is unlikely that grazing animals will be able to discriminate sufficiently between individual plants or tillers to materially change the endophyte status of their intake. Any proportional change in endophyte status is more likely to result from differential survival of plants following insect attack and a corresponding change in pasture cultivar composition.
As animals graze closer to the ground, daily DM intake declines as a result of decreased bite size and intake rate (Cosgrove and Edwards 2007). This decrease in intake may, at least partially, offset an increase in ergovaline concentration in the lower plant horizons. Edwards et al. (1993) observed a lesser decline in pasture height and mass over time with hoggets on standard endophyte-containing ryegrass than on endophyte-free ryegrass pasture. Such self-limiting of grazing intake with ergovaline-type endophytes has not been studied. It can be argued that self-limitation of DM intake at low pasture height with endophyte-containing pastures may contribute to greater persistence of such pastures.
Conclusions
In the scientific literature, experimental conditions are created that deliberately favour high ergovaline intakes so as to evaluate its effect. A progression of effects has been described that is caused by ergovaline consumption. At high ergovaline intakes, the effects can be severe (reduced feed intake, reduced milk production, increased susceptibility to heat stress), but can be, and often are, managed to minimise their occurrence. The early onset signs of ergovaline intake (reduced plasma prolactin concentration, vasoconstriction and increase in core body temperature) are of low economic significance and are less likely to be observed at a farm production level than in a scientific experimental setting, but producers should be aware of the underlying physiological changes. The key issue for New Zealand producers is keeping ergovaline intake low enough not to induce negative production responses or welfare issues, but having concentrations in pasture sufficient to impart the positive benefits on pasture persistence that are provided by ergovaline. The effects of ergovaline on animal physiology are becoming increasingly well defined. However, these should be weighed against the importance of maintaining sufficient presence of ergovaline in the pasture for reduced insect herbivory and enhanced drought resistance. This desirable balance should be the basis for the production of ergovaline endophyte types ideally suited for the New Zealand environment.
A review of the New Zealand data shows that in only a small proportion (15%) of formal comparisons has feed intake or animal production been depressed (up to ~15%) on endophyte-containing pasture compared with ergovaline-free pasture. These have been associated with an ergovaline intake of >0.07 mg/kg LW0.75.day. Furthermore, less than 20% of pasture samples show ergovaline concentrations (>0.7 mg/kg DM) high enough to risk depressing animal performance. The incidence of high pasture ergovaline concentrations is greater in summer and in the basal horizons (<5 cm) of the pasture and is less when high levels of animal production are required, e.g. when grazing is restricted to the upper pasture horizons. Threshold upper critical temperature and THI will be lower for animals consuming ergovaline, but the extent of these changes is not known. However, under these conditions, various mitigation strategies can be invoked to reduce ergovaline intake.
Ergovaline-containing perennial ryegrass pastures are used widely in New Zealand, as indicated by the selection of ergovaline-containing cultivars for the reference standards of the national forage variety trials in New Zealand (NFVT 2013). Given the low incidence of significant effects of ergovaline intake on animal productivity, the restricted period of the year during which ergovaline concentration in ryegrass pasture is sufficiently high to result in high ergovaline intake, and the mitigation strategies used in farm systems, it is unlikely that the use of ergovaline-containing cultivars is a major issue for New Zealand farmers. Future developments in endophyte research may modify this conclusion.
References
Aiken GE, Klotz JL, Johnson JM, Strickland JR, Schrick FN (2013) Postgraze assessment of toxicosis symptoms for steers grazed on toxic endophyte-infected tall fescue pasture. Journal of Animal Science 91, 5878–5884.| Postgraze assessment of toxicosis symptoms for steers grazed on toxic endophyte-infected tall fescue pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFOrur3I&md5=4dfda04f5f4b8adaecd283be4c053b63CAS | 24126272PubMed |
Aiken GE, Sutherland BL, Fletcher LR (2011) Haemodynamics of lambs grazing perennial ryegrass (Lolium perenne L.) either infected with AR6 novel, wild-type endophyte, or endophyte-free. New Zealand Veterinary Journal 59, 179–184.
Aldrich CG, Paterson JA, Tate JL, Kerley MS (1993) The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. Journal of Animal Science 71, 164–170.
Bluett SJ, Hodgson J, Kemp PD, Barry TN (2001) Performance of lambs and the incidence of staggers and heat stress on two perennial ryegrass (Lolium perenne) cultivars using a leader-follower rotational grazing management system. Journal of Agricultural Science, Cambridge. 136, 99–110.
| Performance of lambs and the incidence of staggers and heat stress on two perennial ryegrass (Lolium perenne) cultivars using a leader-follower rotational grazing management system.Crossref | GoogleScholarGoogle Scholar |
Bluett SJ, Kolver ES, Auldist MJ, Thom ER, Davis SR, Farr VC, Tapper BA (2003a) Perennial ryegrass endophyte effects on plasma prolactin concentration in dairy cows. New Zealand Journal of Agricultural Research 46, 9–14.
| Perennial ryegrass endophyte effects on plasma prolactin concentration in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Bluett SJ, Thom ER, Clark DA, MacDonald KA, Minnee EMK (2003b) Milk solids production from cows grazing perennial ryegrass containing AR1 or wild type endophyte. Proceedings of the New Zealand Grassland Association 65, 83–90.
Bluett SJ, Thom ER, Clark DA, Macdonald KA, Minnee EMK (2005a) Effects of perennial ryegrass infected with either AR1 or wild type endophyte on dairy production in the Waikato. New Zealand Journal of Agricultural Research 48, 197–212.
| Effects of perennial ryegrass infected with either AR1 or wild type endophyte on dairy production in the Waikato.Crossref | GoogleScholarGoogle Scholar |
Bluett SJ, Thom ER, Clark DA, Waugh CD (2005b) Effects of a novel ryegrass endophyte on pasture production, dairy cow milk production and calf liveweight gain. Australian Journal of Experimental Agriculture 45, 11–19.
| Effects of a novel ryegrass endophyte on pasture production, dairy cow milk production and calf liveweight gain.Crossref | GoogleScholarGoogle Scholar |
Bohnert D, Merrill M (2006) Management strategies for use of high-alkaloid grass seed straw. In ‘Proceedings of the 41st annual Pacific Northwest nutrition conference’, Vancouver, British Columbia, Canada. (Ed. M von Keyserlingk) pp. 113–125. (CR Press Inc.: Portland, OR)
Breen GP, Barrell GK (2002) Effects of pregnancy on the fever response of sheep to a Gram-negative pyrogen. Proceedings of the New Zealand Society of Animal Production 62, 337–339.
Bryant JR, López-Villalobos N, Price JE, Holmes CW, Johnson DL (2007) Quantifying the effect of thermal environment on production traits in three breeds of dairy cattle in New Zealand. New Zealand Journal of Agricultural Research 50, 327–338.
| Quantifying the effect of thermal environment on production traits in three breeds of dairy cattle in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Clark DA, Woodward SL (2007) ‘Supplementation of dairy cows, beef cattle, and sheep grazing pasture.’ In ‘Pasture and supplements for grazing animals’. Occasional publication no. 14. (Eds PV Rattray, IM Brookes, AM Nicol) pp. 117–131. (New Zealand Society of Animal Production)
Clark DA, Thom ER, Waugh CD (1996) Milk production from pastures and pasture silage with different levels of endophyte infection. Proceedings of the New Zealand Society of Animal Production 56, 292–296.
Clark DA, Thom ER, Waugh CD, Burggraff VT (1999) Milk production from perennial ryegrass pastures containing different levels of endophyte. Proceedings of the New Zealand Society of Animal Production 59, 258–262.
Cosgrove GP, Edwards GR (2007) Control of grazing intake. In ‘Pasture and supplements for grazing animals’. No. 14. (Eds PV Rattray, IM Brookes, AM Nicol) pp. 61–80. (New Zealand Society of Animal Production)
Cosgrove GP, Anderson CB, Berquist TRN (1996) Fungal endophyte effects on intake, health, and liveweight gain of grazing cattle. Proceedings of the New Zealand Society of Animal Production 57, 43–48.
Cosgrove GP, Anderson CB, Phillot M, Nyfeler D, Hume DE, Parsons AJ, Lane GA (2002) The effect of endophyte alkaloids on diet selection by sheep. Proceedings of the New Zealand Society of Animal Production 62, 167–170.
di Menna ME, Finch SC, Popay AJ, Smith BL (2012) A review of the Neotyphodium lolii/Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. New Zealand Veterinary Journal 60, 315–328.
| A review of the Neotyphodium lolii/Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bgsVWjuw%3D%3D&md5=b73d9bf777e579085bd51e86604dc29fCAS | 22913513PubMed |
Easton HS, Lane GA, Tapper BA, Keogh RG, Cooper BM, Blackwell M, Anderson M, Fletcher LR (1996) Ryegrass endophyte-related heat stress in cattle. Proceedings of the New Zealand Grassland Association 57, 5
Easton HS, Couchman JN, Keogh RG, Tapper BA (2000) Ryegrass endophyte and cattle in northern New Zealand. In ‘Fourth international Neotyphodium/grass interaction symposium’. (Eds HV Paul, PD Dapprich) pp. 357–363. (Soest, Germany)
Easton HS, Latch GC, Tapper BA, Ball OJ (2002) Ryegrass host genetic control of concentrations of endophyte-derived alkaloids. Crop Science 42, 51–57.
| Ryegrass host genetic control of concentrations of endophyte-derived alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsVantb8%3D&md5=202d7e91cfc7c81c1bd937e68c290fb3CAS | 11756253PubMed |
Edwards GR, Lucas RJ, Johnson MR (1993) Grazing preference for pasture species by sheep is affected by endophyte and nitrogen fertility. Proceedings of the New Zealand Grassland Association 55, 137–141.
Emile JC, Bony S, Ghesquiere M (2000) Influence of consumption of endophyte-infested tall fescue hay on performance of heifers and lambs. Journal of Animal Science 78, 358–364.
Fletcher LR (1999) ‘Non-toxic’ endophytes in ryegrass and their effect on livestock health and production. In ‘Ryegrass endophyte: an essential New Zealand symbiosis’. Grassland research and practice series no. 7. (Eds DR Woodfield, C Matthew) pp. 133–139.
Fletcher LR (2013) ‘Safety of Trojan NEA2 for grazing sheep.’ AgResearch 7. (AgResearch: New Zealand)
Fletcher LR, Easton HS (2007) ‘The importance of endophyte in agricultural systems: changing plant and animal productivity. New Zealand Grassland Association: endophyte symposium.’ pp. 11–18.
Fletcher LR, Sutherland BL (2009) Sheep responses to grazing ryegrass with AR37 endophyte. Proceedings of the New Zealand Grassland Association 71, 127–132.
Fletcher LR, Markham LJ, White SR (1994) Endophytes and heat tolerance in lambs grazing perennial ryegrass. Proceedings of the New Zealand Grassland Association 56, 265–270.
Fletcher LR, Lane GA, Baird DB, Davies E (2000) Seasonal variation of alkaloid concentrations in two perennial ryegrass-endophyte associations. In ‘Fourth international Neotyphodium/grass interactions symposium’. (Eds HV Paul, PD Dapprich) pp. 535–541. (Soest, Germany)
Foote AP, Kristensen NB, Klotz JL, Kim DH, Koontz AF, McLeod KR, Bush LP, Schrick FN, Harmon DL (2013) Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. Journal of Animal Science 91, 5366–5378.
| Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKkt7vK&md5=f14644ab61948b34a76ee31f5653bba6CAS |
Ganong WF (1981) ‘Review of medical physiology.’ (Lange Medical Publications: Los Altos, CA)
Gustin P, Dhem AR, Lekeux P, Lomba F, Landser FJ, Van de Woestijne KP (1989) Regulation of bronchomotor tone in conscious calves. Journal of Veterinary Pharmacology and Therapeutics 12, 58–64.
| Regulation of bronchomotor tone in conscious calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M7pvFOhug%3D%3D&md5=6b02b386a27461f71c828749c7dc250dCAS | 2704063PubMed |
Hovermale JT, Craig AM (2001) Correlation of ergovaline and lolitrem B levels in endophyte-infected perennial ryegrass (Lolium perenne). Journal of Veterinary Diagnostic Investigation 13, 323–327.
| Correlation of ergovaline and lolitrem B levels in endophyte-infected perennial ryegrass (Lolium perenne).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2Fis1Gjsg%3D%3D&md5=ec2c2f61059f74004668a5f8e05baaa4CAS | 11478604PubMed |
Igono MO, Bjotvedt G, Sanford-Crane HT (1992) Environmental profile and critical temperature effects on milk production of Holstein cows in desert climate. International Journal of Biometeorology 36, 77–87.
| Environmental profile and critical temperature effects on milk production of Holstein cows in desert climate.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zktVWluw%3D%3D&md5=d1c2dc8a1386cc7f0cc0274a709d955aCAS | 1634283PubMed |
Keogh RG, Blackwell MB, Shepherd P (1999) Performance of dairy cows grazing pasture with or without ergovaline and lolitrem B in Northland. Proceedings of the New Zealand Society of Animal Production 59, 254–257.
Klotz JL, Nicol AM (2016) Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism. Animal Production Science 56, 1761–1774.
| Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism.Crossref | GoogleScholarGoogle Scholar |
Koontz AF, Kim DH, Foote AP, Bush LP, Klotz JL, McLeod KR, Harmon DL (2013) Alteration of fasting heat production during fescue toxicosis in Holstein steers. Journal of Animal Science 91, 3881–3888.
| Alteration of fasting heat production during fescue toxicosis in Holstein steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlOjsbfK&md5=63571c2562033abd954cf1c6b96abd08CAS | 23908162PubMed |
Laird FC, Barrell GK (2010) Lack of effect of hot summer conditions in Canterbury on milk production of dairy cows. Proceedings of the New Zealand Society of Animal Production 70, 19–22.
Lane GA, Ball OJP, Davies E, Davidson C (1997) Ergovaline distribution in perennial ryegrass naturally infected with endophyte. In ‘Neotyphodium/grass interactions’. (Eds CW Bacon, NS Hill) pp. 65–67. (Plenum Press: New York)
Larson BT, Samford MD, Camden JM, Piper EL, Kerley MS, Paterson JA, Turner JT (1995) Ergovaline binding and activation of D2 dopamine receptors in GH4ZR7 cells. Journal of Animal Science 73, 1396–1400.
Layton DL, Fletcher LR, Litherland AJ, Scannell MG, Sprosen J, Hoogendoorn CJ, Lambert MG (2004) Efects of ergot alkaloids on liveweight gain and urine lysergol level in ewe hoggets and cattle. Proceedings of the New Zealand Society of Animal Production 64, 192–196.
Linden AS, Desmecht DJ, Amory H, Rollin FA, Michaux CL, Lekeux P (1993) Pulmonary response to intravenous administration of 5-hydroxytryptamine after type-2 receptor blockade in healthy calves. American Journal of Veterinary Research 54, 168–173.
Morris CA, Towers NR, Hohenboken WD, Maqbool N, Smith BL, Phua SH (2004) Inheritance of resistance to facial eczema: a review of research findings from sheep and cattle in New Zealand. New Zealand Veterinary Journal 52, 205–215.
| Inheritance of resistance to facial eczema: a review of research findings from sheep and cattle in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptFKjsbc%3D&md5=e81cf5d424e53015915be16451f2cd88CAS | 15768115PubMed |
Morris CA, Amyes NC, Orchard RL (2007) Responses to selection in ryegrass staggers lines of sheep. Proceedings of the New Zealand Society of Animal Production 67, 204–208.
Nicol AM, Brookes IM (2007) The metabolisable energy requirements of grazing livestock. In ‘Pasture and supplements for grazing animals’. Occasional publication no. 14. (Eds PV Rattray, IM Brookes, AM Nicol) pp. 151–172. (New Zealand Society of Animal Production)
NIWA (2014) New Zealand national climate database. Available at climo.niwa.co.nz [Verified 20 November 2014]
Nolan A, Livingston A, Waterman A (1986) The effects of alpha 2 adrenoceptor agonists on airway pressure in anaesthetized sheep. Journal of Veterinary Pharmacology and Therapeutics 9, 157–163.
| The effects of alpha 2 adrenoceptor agonists on airway pressure in anaesthetized sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XktlGmt7w%3D&md5=988c927be13eb60a4da55cd4a1bd22acCAS | 2873256PubMed |
NFVT (2013) ‘National forage variety trials: trial protocol 2013.’ (New Zealand Plant Breeding Research Association: Christchurch, New Zealand)
Oliver JW (1997) Physiological manifestations of endophyte toxicosis in ruminant and laboratory species. In ‘Neotyphodium/grass interactions’. (Eds CW Bacon, NS Hill) pp. 311–346. (Springer: New York)
Popay AJ, Hume DE (2011) Endophytes improve ryegrass persistence by controlling insects. Pasture persistence.
Reed KFM, Nie ZN, Walker LV, Kearney G (2011) Fluctuations in the concentration of ergovaline and lolitrem B produced by the wild-type endophyte (Neotyphodium lolii) in perennial ryegrass (Lolium perenne) pasture. Animal Production Science 51, 1098–1108.
| Fluctuations in the concentration of ergovaline and lolitrem B produced by the wild-type endophyte (Neotyphodium lolii) in perennial ryegrass (Lolium perenne) pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFertb3L&md5=d0d8d577db1c2e375cb3c90b4cbf27c1CAS |
Refinetti R, Piccione G (2005) Intra- and inter-individual variability in the circadian rhythm of body temperature of rats, squirrels, dogs and horses. Journal of Thermal Biology 30, 139–146.
| Intra- and inter-individual variability in the circadian rhythm of body temperature of rats, squirrels, dogs and horses.Crossref | GoogleScholarGoogle Scholar |
Spiering MJ, Lane GA, Christensen MJ, Schmid J (2005) Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants. Phytochemistry 66, 195–202.
| Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVektw%3D%3D&md5=437f3b0d15fb87bb88ae44dcc7ea1e49CAS | 15652576PubMed |
Spiers DE, Zhang Q, Eichen PA, Rottinghaus GE, Garner GB, Ellersieck MR (1995) Temperature-dependent responses of rats to ergovaline derived from endophyte-infected tall fescue. Journal of Animal Science 73, 1954–1961.
Stamm MM, DelCurto T, Horney MR, Brandyberry SD, Barton RK (1994) Influence of alkaloid concentration of tall fescue straw on the nutrition, physiology, and subsequent performance of beef steers. Journal of Animal Science 72, 1068–1075.
Stewart, A, Kerr, G, Lissaman, W, Rowarth, J (2014) Chapter 8. Endophyte in ryegrass and fescue. In ‘Pasture and forage plants for New Zealand’. Grassland research and practice series no. 8. pp. 66–77. (New Zealand Grassland Association)
Thom ER, Clark DA, Waugh CD (1999) Endophyte and dairy production in New Zealand: experience at the Dairy Research Corporation. Grassland research and practice series. Vol. 7. (New Zealand Grasslands Association)
Thom ER, Popay AJ, Hume DE, Fletcher LR (2012) Evaluating the performance of endophytes in farm systems to improve farmer outcomes: a review. Crop and Pasture Science 63, 927–943.
| Evaluating the performance of endophytes in farm systems to improve farmer outcomes: a review.Crossref | GoogleScholarGoogle Scholar |
Thom ER, Waugh CD, Minnee EM, Waghorn GC (2013) Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production. New Zealand Veterinary Journal 61, 87–97.
| Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bgsVWnsA%3D%3D&md5=9f5e28c4175c61b4ae346638d81a4fcfCAS | 22913546PubMed |
Tor-Agbidye J, Blythe LL, Craig AM (2001) Correlation of endophyte toxins (ergovaline and lolitrem B) with clinical disease: fescue foot and perennial ryegrass staggers. Veterinary and Human Toxicology 43, 140–146.
1 Mention of trade name, proprietary product, or specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable.



