Reducing the carbon footprint of Australian milk production by mitigation of enteric methane emissions
Peter J. Moate A D , Matthew H. Deighton A , S. Richard O. Williams A , Jennie E. Pryce B , Ben J. Hayes B , Joe L. Jacobs A , Richard J. Eckard A C , Murray C. Hannah A and William J. Wales AA Department of Economic Development, Jobs, Transport and Resources, 1301 Hazeldean Road, Ellinbank, Vic. 3821, Australia.
B Department of Economic Development, Jobs, Transport and Resources, AgriBio, 5 Ring Road, Bundoora, Vic. 3086, Australia.
C Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
D Corresponding author. Email: peter.moate@ecodev.vic.gov.au
Animal Production Science 56(7) 1017-1034 https://doi.org/10.1071/AN15222
Submitted: 30 April 2015 Accepted: 21 June 2015 Published: 15 September 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
This review examines research aimed at reducing enteric methane emissions from the Australian dairy industry. Calorimeter measurements of 220 forage-fed cows indicate an average methane yield of 21.1 g methane (CH4)/kg dry matter intake. Adoption of this empirical methane yield, rather than the equation currently used in the Australian greenhouse gas inventory, would reduce the methane emissions attributed to the Australian dairy industry by ~10%. Research also indicates that dietary lipid supplements and feeding high amounts of wheat substantially reduce methane emissions. It is estimated that, in 1980, the Australian dairy industry produced ~185 000 t of enteric methane and total enteric methane intensity was ~33.6 g CH4/kg milk. In 2010, the estimated production of enteric methane was 182 000 t, but total enteric methane intensity had declined ~40% to 19.9 g CH4/kg milk. This remarkable decline in methane intensity and the resultant improvement in the carbon footprint of Australian milk production was mainly achieved by increased per-cow milk yield, brought about by the on-farm adoption of research findings related to the feeding and breeding of dairy cows. Options currently available to further reduce the carbon footprint of Australian milk production include the feeding of lipid-rich supplements such as cottonseed, brewers grains, cold-pressed canola, hominy meal and grape marc, as well as feeding of higher rates of wheat. Future technologies for further reducing methane emissions include genetic selection of cows for improved feed conversion to milk or low methane intensity, vaccines to reduce ruminal methanogens and chemical inhibitors of methanogenesis.
Additional keywords: abatement, climate change, dairy.
Introduction
In 2013–2014, there were 1.69 million dairy cows in the Australian dairy herd within 6300 registered dairy farms producing ~9.5 billion litres of milk (Dairy Australia 2014). The Holstein and Holstein × Friesian breeds account for ~70% of the national herd, with other breeds including Jersey, Holstein × Jersey, Brown Swiss, Ayrshire, Australian Red and Illawara. Dairying is an established land use across the temperate regions of southern Australia and the subtropical regions of northern New South Wales and southern Queensland. The majority of milk by volume is produced in the southern mainland state of Victoria (66%), where ~68% of registered farms are located. Australian feeding systems have evolved to suit different regions, climate conditions and feed availability and range from pasture-based grazing with minimal grain supplementation, through to zero-grazing feedlot systems. The feeding systems used in the temperate rain-fed and irrigated regions, including Victoria, Tasmania, southern New South Wales, South Australia and Western Australia, represent more than 93% of the industry and are characterised by grazed pasture (typically perennial ryegrass, Lolium perenne) and supplementary feeding an average of 1.7 t grain/cow.year (Dharma et al. 2012; Dairy Australia 2014). Dairy products are the single largest commodity by value exported from south-eastern Australia and their production is responsible for ~12% of Australia’s agricultural greenhouse gas (GHG) emissions (methane and nitrous oxide; DCCEE 2012a).
The average carbon footprint of Australian milk production has been calculated to be 1.11 kg carbon dioxide equivalent (CO2eq) per kg fat- and protein-corrected milk (Dairy Australia 2012). Enteric methane emissions are the greatest contributor to the carbon footprint, comprising 57% of total on-farm GHG emissions associated with milk production (Dairy Australia 2012). Therefore, mitigation of enteric methane emissions is key to reducing the carbon footprint of Australian milk production.
The Australian Carbon Farming Initiative (CFI) was an incentive policy mechanism for farmers to generate additional income through the sale of carbon offsets generated through reductions in methane and nitrous oxide emissions, or increased carbon storage in soils and trees (DCCEE 2012b). However, the legislation concerning carbon tax and future emissions trading scheme was repealed in July 2014, effectively removing the main purchaser of agricultural offsets under the CFI. The current Australian government has proposed a scheme of direct action, whereby government becomes the purchaser of all emission reductions, through the establishment of an Emission Reduction Fund (ERF; ComLaw 2014).
Australia has invested significantly into research to mitigate methane and nitrous oxide emissions and sequester carbon in soils and trees, initially through the Climate Change Research Program (Department of Agriculture 2013) and then the Carbon Farming Futures Fund – Filling the Research Gap Program (Department of Agriculture 2014). The aim of these programs has been to develop cost-effective solutions to mitigate agricultural GHG and provide cost-effective options for farmers to participate in the ERF. Under the Australian ERF, several offset methodologies have been developed for dairy farmers to increase carbon storage in pasture soils, reduce enteric methane through feeding dietary lipids, establish environmental plantings and capture methane emissions from effluent ponds (CER 2014). However, many of these methods are not currently cost-effective for dairy farmers relative to opportunities to simply increase production efficiency (e.g. Alcock and Hegarty 2011; Bell et al. 2013; Browne et al. 2015). No projects have yet been registered against these methodologies by dairy farmers and therefore, no carbon-offset credits have been issued by the Australian government to the dairy industry.
The major funders of enteric methane mitigation research within the Australian dairy industry have been Dairy Australia, state departments, universities and the federal Department of Agriculture, with major activities being undertaken by Victorian Department of Economic Development, Jobs, Transport and Resources and The University of Melbourne, through the Primary Industries Climate Challenges Centre, the Commonwealth Scientific and Industrial Research Organisation, the University of Sydney, the University of Western Australia and Royal Melbourne Institute of Technology.
Several comprehensive reviews have been published that describe options to mitigate GHG from livestock production (e.g. Eckard et al. 2010; Martin et al. 2010; Buddle et al. 2011; Cottle et al. 2011; Doreau et al. 2011; Grainger and Beauchemin 2011; Henry et al. 2012; Meale et al. 2012; Clark 2013; Hristov et al. 2013; Montes et al. 2013; Knapp et al. 2014; Kumar et al. 2014; Pacheco et al. 2014). These reviews on methane mitigation have been global in scope and have focussed on methane-mitigation research conducted during the past 10 years. The present review focuses on more recent Australian dairy industry methane-mitigation research and includes a revised method for calculating methane emissions from the Australian dairy industry, which is recommended as a replacement of the current Australian GHG inventory methodology.
The majority of dairy cows in the temperate regions of Australia graze on perennial ryegrass-dominant pastures for the entire year (Dairy Australia 2014). In contrast, North American and European dairy cow diets are usually based on total mixed rations composed of lucerne hay, maize silage, maize grain and soybeans (Mowrey and Spain 1999; VandeHaar and St-Pierre 2006). However, the dairy industries in New Zealand, United Kingdom and the Republic of Ireland also are based on grazed perennial ryegrass-dominant pastures. Thus, the research findings concerning methane emissions from dairy cows fed pasture-based diets in these countries are likely to have relevance to the Australian dairy industry, and vice versa.
The major aims of this review are to summarise the most recent methane-mitigation research for the Australian dairy industry, including the following; to identify those strategies that can currently be used to reduce methane emissions, and those technologies that have potential to be used as mitigation strategies in the future; to estimate the methane yield when dairy cows consume diets typically available in Australia; to propose an improvement in the way the Australian GHG inventory calculates enteric methane emissions from the Australian dairy herd; to estimate the total enteric methane intensity of Australian milk and to suggest future research opportunities for reducing methane emissions from the Australian dairy herd.
Metric of emissions
International markets increasingly require certification of the carbon footprint of imported dairy products. To achieve this, the Australian dairy industry participates in a carbon footprint project, using the methodology of the International Dairy Federation (International Dairy Federation 2010). There is currently no differentiation of the carbon footprint of products produced by individual dairy companies in Australia but carbon footprint analysis could be used as a point of product differentiation in the future. By its nature, a carbon footprint analysis derived from the CO2eq is an emission-intensity metric because it is based on emissions per unit of product produced, rather than an absolute measure of total emissions. Use of the emission-intensity metric allows for further industry growth, in keeping with international food demand, but with proportionally less environmental impact per unit of product. There are three generally accepted metrics used to quantify enteric methane emissions from dairy cows, including (1) methane emission, i.e. methane emitted per cow per day (g/day) or per unit of farm land (g/ha), (2) methane yield, i.e. methane emitted per unit of feed eaten (g/kg dry matter intake, DMI), and (3) methane intensity, i.e. methane emitted per unit of product (g/kg milk or g/kg milk solids).
Although the definition of methane intensity seems straightforward, methane intensity has been used as a metric by several authors to describe quite different things. The majority of authors have used ‘methane intensity’ to describe a short-term measure of the efficiency of methane production with respect to milk production. For example, many authors when describing short-term feeding experiments, have measured the total amount of enteric methane produced by a specific group of lactating cows in a particular period of an experiment, and then divided this amount by the total amount of milk produced by these same cows during the same period (e.g. Wims et al. 2010; Moate et al. 2011). We propose that this measure of methane (CH4) intensity, which has units of g CH4/kg milk or milk solids (MS), should be defined as ‘partial enteric methane intensity’ (PEMI) because it does not take into account the enteric methane that is necessarily produced by growing heifers, non-lactating cows and bulls. Such factors as level of milk production and stage of lactation can greatly affect the magnitude of PEMI, and for this reason, PEMI should be used only to compare emission intensity within an experiment or a range of related experiments involving cows of similar milk yield and stage of lactation. If we consider the total lifetime enteric-methane emissions of a cow, and divide this amount by the total lifetime milk production of the cow, we again have a metric of methane intensity, with units of g CH4/kg milk, but this metric is more appropriate to calculate the enteric methane intensity of an individual cow. We propose that this should be called ‘individual enteric methane intensity’ (IEMI), an ideal metric for the selection of low methane-emitting dairy cows over their lifetime. By an extension of this reasoning, it is apparent that we can also compare the methane intensity of different herds or indeed national herds by calculating the total annual production of enteric methane by all of the animals in the herd, including methane produced by the non-lactating growing heifers, the non-lactating cows, and the bulls as well as the lactating cows, and dividing this amount by the total annual production of milk from all of the lactating cows in the herd. This metric also has units of g CH4/kg milk, and we propose that this metric should be called ‘total enteric methane intensity’ (TEMI).
Mitigation can be defined as a reduction in any one of these metrics. Currently, national inventories account only for total emissions, while customers are increasingly interested in emission intensity, most commonly expressed as a product’s carbon footprint. Research efforts have been concerned with all of these metrics, with a reduction in one metric sometimes associated with a reduction in another. Under the policies of the Australian government, the aim has been to reduce total emissions. However, there is growing consensus in Australia of the need to increase dairy production to supply the increasing international demand for dairy products. Not surprisingly, there is recognition by many scientists that enteric-methane mitigation may, therefore, need to focus on emission intensity (Henry et al. 2012; Knapp et al. 2014). This also recognises that if dairy production is to be increased, options to reduce total per cow or per hectare emissions may be limited (Henry et al. 2012; Hristov et al. 2013; Knapp et al. 2014).
The current constraints to mitigation mainly concern the lack of effective, practical and profitable offset methodologies. Farmers are unlikely to accept changes that are detrimental to profitability, or that affect normal farm operation. If suitable technologies are identified, then the reduction of emissions would need to be quantified to provide appropriate financial reward, unless the mitigant(s) increase farm profitability. The mitigation challenge is greater in pastoral dairying, where the focus is on low-cost, pasture-based milk production, compared with dairying that involves feeding of mixed rations that provides a means to feed components that can reduce enteric-methane formation (e.g. plant lipids, tannins or methanogen-specific inhibitors). Lowering emission intensity fits well with farmer objectives, namely, to maximise profit through efficient production, increased cow fertility and longevity (e.g. Lovett et al. 2006; Beukes et al. 2010; Waghorn and Hegarty 2011). Therefore, to ensure that mitigation of enteric methane does not lead to increased GHG emissions in other sectors, life-cycle analyses with appropriately defined boundaries and assumptions are essential to avoid unforseen increases in overall emissions (e.g. Williams et al. 2014a; Zehetmeier et al. 2014a, 2014b).
Measurement of methane emissions
Any attempt to mitigate emissions first requires the accurate measurement of enteric methane emissions from dairy cows. There have been several recent reviews that have specifically addressed the various methods for measuring enteric methane emissions from ruminants (e.g. Lassey 2007; Storm et al. 2012), and it is the intent of this review to provide only a summary of the methods used in Australia.
Calorimetric chambers are often acknowledged as the gold standard for measurement of methane emissions from individual animals (Grainger et al. 2007; Williams et al. 2013). This is due to the accepted accuracy of calorimetric chambers; however, their establishment and running costs and complexity of their operation generally limits their availability and, consequently, throughput is limited to small numbers of animals. Despite the acknowledged accuracy of calorimetric chambers, substantial errors in methane measurement can occur if appropriate calibration procedures are not routinely followed (Gardiner et al. 2015).
As well as measuring methane emissions by means of chambers, the sulfur hexafluoride (SF6) tracer-gas technique has also been used extensively for many years by researchers in Australia. Unlike the calorimetric chamber method, the SF6 tracer technique can be used on large numbers of animals simultaneously (e.g. McNaughton et al. 2005; O’Neill et al. 2012) and, importantly, for Australian dairy research, can be used to determine the methane emissions from grazing or non-grazing dairy cows (e.g. Grainger et al. 2007, 2008a).
Methane emissions from cattle can also be measured by the GreenFeed system (C-lock Inc., Rapid City, SD, USA) patented by Zimmerman (2011). There have been no Australian studies on dairy cows using the GreenFeed system. However, Velazco et al. (2014) compared the methane emissions from Angus steers fed diets supplemented with either nitrate or urea. They reported that nitrate-fed steers consumed more meals per day, resulting in a shorter time interval between consuming a meal and having methane measured by the GreenFeed system. Velazco et al. (2014) concluded that there was a need for caution in extrapolating ‘short-term emission measures’, as are obtained by the GreenFeed system, to daily methane-emission rates. This need for caution when employing the GreenFeed system was also recently echoed by Hammond et al. (2015). Hammond et al. (2015) conducted two experiments in which Holstein heifers were fed various diets, and one experiment in which growing heifers rotationally grazed swards of either ryegrass, clover or flowers. They found that methane emissions as measured by the GreenFeed system were not concordant with emissions measured by the respiration chamber, and only in moderate agreement with measurements made by the SF6 technique. Hammond et al. (2015) concluded that ‘under our conditions of use the GreenFeed system was unable to detect significant treatment and individual animal differences in methane emissions that were identified using both respiration chambers and SF6 techniques, in part due to limited numbers and timing of measurements obtained’. Thus, before the GreenFeed system should be used in Australia in applied research, additional research is required to determine appropriate operating protocols and its accuracy must be validated against measurements made by the chamber technique.
The open-path FTIR tracer method was developed for use on free-ranging cattle in Victoria (Griffith et al. 2006); however, there are no peer reviewed papers reporting the use of open path or micrometeorological methods to determine methane emissions from dairy cattle in Australia.
Methane emissions from cows fed pasture
Almost all Australian experiments in which methane emissions have been measured, involved diets containing pasture or some other forage supplemented with some amount of cereal grain. However, there have been three Australian experiments in which cows have been fed measured amounts of harvested pasture (without supplement) and their mean methane emissions ranged from 369 to 458 g CH4/cow.day, methane yields ranged between 21.9 and 24.6 g CH4/kg DMI and PEMI ranged between 15.1 and 18.3 g CH4/kg milk (Williams et al. 2013; Deighton et al. 2014b; Moate et al. 2014a). These methane-emission metrics are similar to those reported from pasture-fed dairy cows in other countries. In New Zealand, Waghorn et al. (2008) fed cows harvested perennial ryegrass-dominant pasture, and measured methane emissions in the range 273–352 g CH4/cow.day, methane yields in the range 17.7–21.2 g CH4/kg DMI and PEMI of 11.5–16.9 g CH4/kg milk. In Ireland, O’Neill et al. (2011) measured methane emissions of cows fed harvested perennial ryegrass as 251 g CH4/cow.day with a methane yield of 18.1 g CH4/kg DMI and a PEMI of 12.8 g CH4/kg milk.
Dietary strategies to mitigate methane emissions
Lipid-containing plant by-products
There is now strong evidence that addition of plant lipids to the diet of dairy cows can reduce their enteric methane emissions (Grainger and Beauchemin 2011; Moate et al. 2011). In Australia, there are several industries that produce plant by-products that, relative to pasture, contain a high concentration of lipids and are, therefore, potentially useful as feed supplements for ruminants. These by-products are typically fibrous residues resulting from the extraction of higher-value constituents used in the production of primary products (e.g. brewers grains from beer production; cold-pressed canola from oil production; cottonseed from cotton production; grape marc from wine making, and hominy meal from maize milling). Given that these by-products have little economic value compared with the primary product resulting from their production, the GHG emissions associated with their production can mostly be attributed to the primary product (Williams et al. 2014a).
Grainger et al. (2008a, 2010b) showed that supplementary feeding of whole cottonseed to dairy cows could cause a substantial decrease in methane emissions without adversely affecting milk production. They speculated that it was the concentration of lipid in cottonseed that was responsible for the methane mitigation. Since then, there have been several reviews that have identified dietary supplementation with plant lipids as one of the most effective ways to reduce methane emissions from ruminants (Beauchemin et al. 2009; Martin et al. 2010). More recently, Moate et al. (2011) compared brewers grains, cold-pressed canola and hominy meal for their methane-mitigation potential and found that all three by-products could substantially reduce enteric methane emissions from dairy cows.
Using data from 17 cattle experiments published in the international peer-reviewed scientific literature, Moate et al. (2011) quantified the effect of dietary lipid concentration on methane emissions from dairy cows. Methane yield (g/kg DMI) was linearly related to the lipid concentration in the diet (Eqn 1) and this equation has been used in the development of an offset methodology (ComLaw 2013) by the Australian Federal Government.
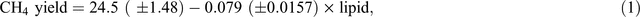
where CH4 yield is the enteric methane yield (g CH4/kg DMI) and lipid is the concentration of lipid in the diet (g lipid/kg DMI). From Eqn 1, it can be calculated that, for a typical dairy diet containing 30 g lipid/kg DMI, each additional 1% increase in dietary fat concentration will decrease methane emissions by ~3.5%.
Since the work of Moate et al. (2011), other Australian research has examined the effects on methane emissions when dairy cows were fed other by-products rich in plant lipids. Grape marc is one such by-product. The feeding of red grape marc to dairy cows reduced methane emissions and methane yield by ~20% (Moate et al. 2014b). Recently, the feeding of grape marcs from red and white grapes were found to be equally effective at inhibiting enteric methane emissions from dairy cows (Moate et al. 2014c). As well as lipids, grape marc contains tannins, lignin, tartaric acid, p-coumaric acid, resveratrol and copper which all have potential to inhibit enteric methanogenesis (Moate et al. 2014b).
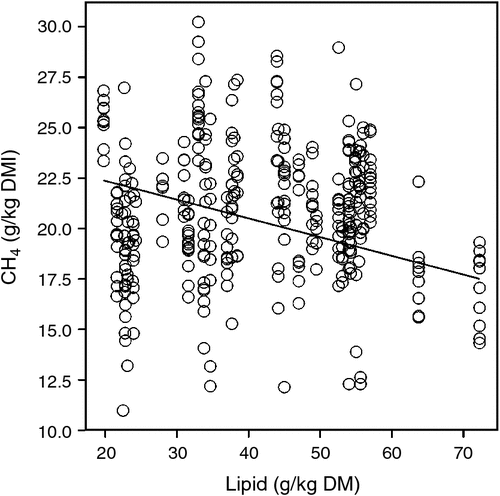
A compilation of data from the most recent Australian feeding studies, including seven published experiments (Moate et al. 2011, 2013, 2014a, 2014b, 2014c; Deighton et al. 2014b; Williams et al. 2014b) and five other unpublished experiments from these authors, provides additional quantification of the lipid effect and, for the first time, quantification of the effect of wheat in the diet, on methane emissions from dairy cows. This dataset included measurements from 362 lactating dairy cows from six experiments in which methane was measured by open-circuit calorimeters and six experiments in which methane was measured by the SF6 technique (Williams et al. 2011a; Deighton et al. 2014b). In these experiments, cows were in various stages of lactation and consumed diets containing a diverse range of dietary ingredients. These factors are likely to have contributed to the variation in methane yield shown in Fig. 1. A meta-analysis was performed on the data from these experiments using a linear mixed model with nested random effects for experiment and cow within experiment. Orthogonal linear and quadratic polynomial terms in wheat and lipid diet percentage were used to develop the fixed-effect regression terms. A parsimonious model consisting of only statistically significant terms (P < 0.01) was refitted to the data using original (non-centred and non-orthogonalised) covariates, for presentation. Data analysis was conducted using Genstat 17 software. The concentrations of lipid in these diets ranged from 20 to 70 g/kg DM and methane yield declined linearly by 0.093 ± 0.0174 g CH4/kg DMI per gram dietary lipid concentration (Fig. 1). This response in methane yield is not significantly (P > 0.05) different from the response shown in Eqn 1 above. In Australia, dietary-lipid supplements such as brewers grains, cold-pressed canola meal, cottonseed, hominy meal and grape marc will continue to have an important role in supporting milk production, with the ancillary benefit of reducing methane emissions. Although there has been considerable speculation as to the mechanisms by which dietary lipids suppress methane emissions (Beauchemin et al. 2009; Martin et al. 2010), definitive evidence identifying the most important mechanisms is still lacking and represents a clear knowledge gap.
|
Cereal- and protein-based concentrates
Most international research on the effect of concentrate feeding has been carried out with mixtures of maize grain and soybean meal (Yan et al. 2010; Hristov et al. 2013). However, it is known that diets containing a high proportion of starch-containing grains decrease methane yield compared with forage-based diets (Johnson and Johnson 1995; Beauchemin and McGinn 2005). In general, most international research has shown that methane yield starts to decline when maize grain or soybean concentrates make up more than 60% of the diet (Lovett et al. 2003; Sauvant et al. 2011). In the temperate regions of south-eastern Australia, grazed pasture generally constitutes the majority of feed consumed by dairy cows, although the consumption of concentrates has been increasing steadily. By 2011, dairy cows in Australia consumed, on average, 1.7 t DM of concentrates per year (Dharma et al. 2012). Most of this concentrate was wheat grain, yet there are few reports on the effect of dietary wheat on methane emissions from dairy cows (Moate et al. 2012).
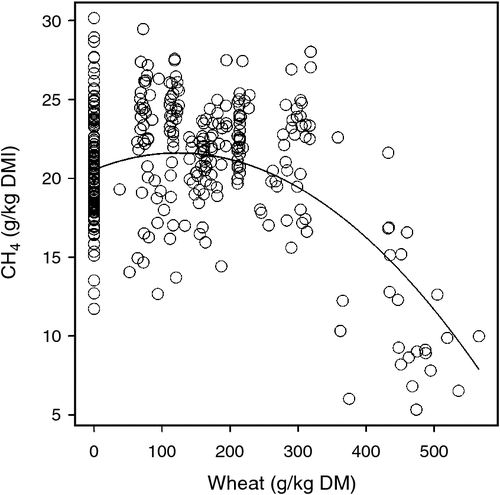
The meta-analysis described above also showed that, as well as a lipid effect, methane yield (g CH4/kg DMI) varied quadratically with respect to the concentration of wheat grain in diets (Fig. 2, Eqn 2).
|
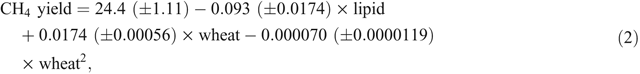
where CH4 yield is the enteric methane yield (g CH4/kg DMI), lipid is the concentration of lipid in the diet (g lipid/kg DMI) and wheat is the concentration of wheat in the diet (g wheat/kg DMI).
Plant secondary compounds
Inclusion of condensed tannins from bark of black wattle (Acacia mearnsi) in the diet of dairy cows has been shown to substantially reduce methane emissions (Grainger et al. 2009). However, milk yield and DMI were also reduced. Tannins are also present in many forage species, such as birdsfoot trefoil (Lotus corniculatus), chicory (Cichorium intybus) and sulla (Hedysarum coronarium). When dairy cows in New Zealand grazed birdsfoot trefoil, their methane emissions were reduced (Woodward et al. 2004). Similarly, when dairy cows grazed sulla, methane emissions were reduced without compromising milk production (Woodward et al. 2002). There is also some evidence that the feeding of chicory to sheep may be associated with reduced methane emissions (Sun et al. 2012).
Forage brassicas have shown mixed results as a methane mitigating feed. Although most brassicas fed to sheep have resulted in similar methane yields, an exception is the significantly lower methane yield (~20%) when swedes or forage rape were fed, compared with ryegrass, turnips and kale (Sun et al. 2012). The mechanism of this reduction is unknown and further research is warranted to investigate the methane mitigation effects when these forage brassicas are fed to dairy cows.
Methane inhibitors
Monensin, a polyether antibiotic, has shown promise as a methane mitigant in dairy cattle where grains form a substantial proportion of the diet (e.g. Odongo et al. 2007). However, monensin has not shown any consistent methane-mitigating effect in the pasture-based systems of Australia and New Zealand (Moate et al. 1997; van Vugt et al. 2005; Waghorn et al. 2008; Grainger et al. 2010a).
Nitrates, when fed as a dietary supplement, have been shown to reduce methane emissions from dairy cows consuming diets containing low concentrations of nitrogen (van Zijderveld et al. 2011). This mitigation option may not be attractive to Australian dairy farmers because dietary nitrate supplementation may decrease DMI (Lund et al. 2014) and, therefore, potentially decrease milk production. Dietary nitrate supplementation will also increase the risk of nitrate poisoning of dairy cows, especially when pastures already contain a high concentration of crude protein (Callaghan et al. 2014).
Methanogen-specific inhibitors are a potentially effective mitigation technology because they may exploit the evolutionary distinctiveness of methanogenic micoorganisms. Methanogens are the most prevalent archaea species found in the rumen (Janssen and Kirs 2008). Archaea are evolutionarily distinct from other rumen organisms (bacteria, protozoa, fungi and viruses) and, as all methanogens share a similar methanogenesis pathway (Hedderich and Whitman 2013), inhibitors of this pathway may specifically target methanogens without directly affecting other rumen microorganisms. Halogenated compounds were identified as inhibitors of ruminal methane formation over 40 years ago (Johnson et al. 1972; Clapperton 1974). Considerable research has been conducted with halogenated compounds (e.g. bromochloromethane, bromoethanesulfonate and chloroform) and, in some cases, administration of halogenated compounds to ruminants has caused substantial decreases in enteric methane emissions (Trei et al. 1972; Abecia et al. 2012). Artificially synthesised halogenated chemicals are unlikely to be used in Australia because of their potential toxicity, the risk of residues in milk, their ozone-depleting properties and because there are strict laws governing their importation and use. However, some plants such as the red sea weed, Asparagopsis taxiformis, contain high concentrations of bromoform and other halogen compounds (Burreson et al. 1976) and, in a recent in vitro experiment, Asparagopsis taxiformis was shown to reduce methane production by 99% (Machado et al. 2014). Thus, the supplementation of ruminant diets with red sea weed may offer a natural means of methane mitigation.
A novel inhibitor 3-nitrooxypropanol (NOP) has been shown to have anti-methanogenic properties. NOP acts against archaea in the rumen by interfering with the last enzymatic step in the formation of methane (Duval and Kindermann 2012). Recent research with dairy cows in Canada and the USA has shown that administration of NOP was associated with reduced methane production (Haisan et al. 2014; Hristov et al. 2015). The efficacy of NOP as a dietary additive for pasture-fed cows has not yet been determined; however, before any such inhibitor could be used on Australian farms, research focussing on toxicology and residues would be required.
Emerging technologies to measure methane emissions and facilitate mitigation
In the context of this review, emerging technologies are those technologies that, by themselves, may not mitigate enteric methane emissions, but facilitate the accurate measurement of methane emissions (e.g. improved SF6 methodology; development of intra-ruminal sensors), prediction of methane emissions (e.g. modelling), or the development of other technologies that can be used to mitigate enteric methane emissions (e.g. ruminomics).
Improved methodology for the SF6 tracer technique
Several researchers have shown that methane yields measured using the original SF6 technique (Zimmerman 1993; Johnson et al. 1994) were not concordant with methane yields measured in calorimetric chambers (e.g. Pinares-Patiño et al. 2008; Grainger et al. 2010b; Muñoz et al. 2012) or that the variance in methane yield measured by the original SF6 technique was generally greater than the variance of methane yield measured by the chamber technique (Vlaming et al. 2008; Hammond et al. 2009, 2015).
In 2011, the Global Research Alliance on Agricultural Greenhouse Gases sponsored a workshop in New Zealand on the SF6 technique, resulting in publication of guidelines describing how the original SF6 technique should be implemented (Berndt et al. 2014). Since this workshop, there has been considerable research undertaken in Ireland and Australia to improve the accuracy and reliability of the SF6 technique (e.g. Williams et al. 2011a; Deighton et al. 2013a, 2014a, 2014b; Moate et al. 2014c, 2015). This research has identified the source of measurement errors within the SF6 tracer technique and has led to the development and validation of a modified method (Deighton et al. 2014b). Implementation of the SF6 technique using these modifications enabled measurement of CH4 emissions to be highly concordant with measurements made using calorimetric chambers (Deighton et al. 2013b). Another recent experiment has demonstrated the high degree of accuracy of the modified SF6 technique (Deighton et al. 2014b). In this experiment, lactating dairy cows were fed on freshly harvested perennial ryegrass and mean CH4 yield (g/kg DMI) was 21.9 ± 1.65 when measured by calorimetric chamber and 22.3 ± 1.44 when measured by the SF6 technique. The between-cow coefficient of variation was 7.5% when CH4 yield was measured in chambers and 6.5% when measured by the SF6 technique. Recently, Moate et al. (2015) showed that Michaelis–Menten kinetics accurately predict the rate of SF6 release from permeation tubes used to estimate methane emissions from ruminants. The important implications of this latest research are that when Michaelis–Menten kinetics are used to predict SF6 release rate from permeation tubes, this will increase the accuracy of the estimation of methane emissions from ruminants and extend the period during which methane emissions can be accurately measured for up to 1 year after deployment of the tubes.
Intra-ruminal gas sensor
An intra-ruminal device with the capability of measuring the concentrations of methane and carbon dioxide dissolved in rumen fluid has recently been developed (CSIRO 2014). The rumen is a particularly hostile environment for electronic instruments because hydrogen sulfide can diffuse across the membrane that encloses the device and cause corrosion of electrical circuits. Recently, researchers have shown that silver nano-particles incorporated into membranes can substantially reduce the permeation of hydrogen sulfide through membranes (Nour et al. 2014). For an intra-ruminal device to be able to dynamically measure the concentrations of gases in the rumen, the gases must be able to quickly permeate through the membrane. Recently, Nour et al. (2013) and Berean et al. (2014) reported that membrane permeability of methane, hydrogen and carbon dioxide could be substantially altered by the use of composite membranes and by optimising the temperature at which the membranes were made. This fundamental research will be crucial for the further development of robust intra-ruminal gas-sensing devices, and also for gas-sensing devices that could be attached to in-vitro fermentation apparatus to enable real time measurements of the composition of fermentation gases. Nevertheless, additional research will be needed to determine whether, or how, the in situ measurement of ruminal gas composition can be used to determine methane emissions from individual animals.
In vitro measurement of fermentation gases
Globally, the most common screening process used to identify potential methane mitigants involves in vitro fermentation procedures. In Australia, several research groups are currently using in vitro fermentation procedures. A series of in vitro experiments have been conducted to investigate the effect of plant essential oils (Chaves et al. 2012), endophyte toxins (Meale et al. 2013) and organic acids (Reis et al. 2014) on methane production. However, none of these substrates inhibited methane production. In vitro procedures have been used to screen Australian native plants for their methane-mitigation potential. The most promising forage plant identified is emu bush (Eremophila glabra; Li et al. 2014). Researchers have also screened a wide range of feed additives for their methane-mitigation potential and have found reduced methane production from nine feed additives, eight essential oils and two plant extracts (Durmic et al. 2014). Tropical marine algae (sea-weed) have been screened for their methane-mitigation potential. The brown algae (Cystoseira trinodis and Dictyota bartayresii), and the red algae (Asparagopsis taxiformis) were identified as having particularly potent methane-inhibiting effects in vitro (Dubois et al. 2013; Machado et al. 2014).
Proxy measures of methane emissions
The measurement of enteric methane emissions from ruminants is difficult, labour intensive and expensive and, for these reasons, there has been considerable international research to develop proxy measures for enteric methane emissions. In Europe, research has focussed on the possibility of using the concentrations of specific fatty acids in milk as predictors of methane emissions from dairy cows (Chilliard et al. 2009; Dijkstra et al. 2011). Furthermore, the fatty acid composition of milk fat can influence the mid-infrared spectra of milk and researchers in Belgium have related the mid-infrared spectrum of milk from individual cows to their methane emissions (Dehareng et al. 2012; Vanlierde et al. 2013). In Australia, Williams et al. (2014c) found only weak relationships between methane emissions and the concentrations of specific fatty acids in milk fat. Further research is required to examine potential relationships between the yields of specific milk fatty acids and enteric methane emissions.
Rumen metagenome profiling, proteomics and high-energy forages
The rumen microbiome of individual cattle is the collection of microbial species living in the rumen, including bacteria, protozoa, archaea and virus. The composition of an animal’s microbiome is of great interest, as it could influence methane emissions. In the past, it was difficult to assess rumen microbial composition as many of the rumen microbial species are resistant to culture. High-throughput, massively parallel sequencing overcomes this problem as DNA extracted from rumen samples can be sequenced directly without an intervening culture step. If individual rumen samples are sequenced, the sequences can be used to infer relative composition of each microbiome profile. This requires a rumen metagenome reference sequence – a representation of DNA from all of the species likely to occur in the rumen. While Brulc et al. (2009) and Hess et al. (2011) have assembled rumen metagenome reference sequences, many species remain unknown, given the large number of species in the rumen.
The repeatability (stability) of an animal’s rumen metagenome over time remains to be determined and has important consequences for the validity of rumen metagenome profiling as a research tool. However, Ross et al. (2012) collected rumen fluid samples from three locations within the rumen of three cows. DNA from the samples was sequenced using massively parallel sequencing. When the reads were aligned to the rumen metagenome references, the rumen metagenome profiles were repeatable (P < 0.00001) by cow regardless of location of sampling within the rumen. There is preliminary evidence that rumen metagenome profiles can be used to predict methane yield of dairy cows (Ross et al. 2013b). Ross et al. (2013a) extracted DNA from rumen fluid from 39 cows either fed diets with a large effect on methane (grapemarc, fat and tannins), or a control diet, and compared these with measured methane emissions from these cows. Rumen metagenome profiles for each cow were associated with methane yield using the best linear unbiased prediction method, which allowed information from the relative abundance of many microbes to be used simultaneously. The correlation between predicted methane yield and actual methane yield was 0.47. This is encouraging, as it suggests that rumen metagenome profiles could be used either to select cattle with lower methane yields, or as a proxy phenotype for methane yield, for example, to develop genomic breeding values for methane yield, as described above.
The accuracy of using rumen microbial profiles to predict methane yield, while useful, could be improved. If the complete DNA sequence of rumen microbes was known, profiles could be generated that more accurately reflected the rumen microbial composition of individual cattle. The Hungate 1000 project (Leahy et al. 2013) aims to completely sequence large numbers of rumen microorganisms, including bacteria and archaea. The accuracy of predicting methane yield from rumen microbial profiling would also be increased with a larger set of cattle measured for methane, and with rumen microbial profiles. Such efforts are underway in Australia in the Pangenome project (P. Vercoe, pers. comm.) and, internationally, in the Ruminomics project. Extracting RNA (i.e. gene expression) information from rumen samples may also be useful as it has recently been demonstrated that the magnitude of methane emissions in sheep was associated with the level of gene expression of methanogenesis pathway genes (Shi et al. 2014).
While most rumen metagenome studies have been focussed on bacteria and archaea, a recent paper demonstrated that the rumen is also host to viruses, in the form of bacteriophages and archaeophages (Ross et al. 2013c). While the importance of phage abundance and species on methane yield is unknown, this is certainly an interesting area for future investigation. A question of great interest is to what extent does the host ruminant control the abundance and composition of the rumen microbial community. Possible mechanisms for this control would be gene expression in rumen papillae, and salivary proteins (given the huge amount of saliva ingested each day). An atlas of the bovine saliva proteome (the proteins expressed in saliva) revealed that at least 447 proteins are expressed in bovine saliva (Ang et al. 2011). More recent work has demonstrated considerable variability between cattle in the relative abundance of these proteins in saliva samples. However, no link to methane yield could be demonstrated (in a small number of cattle). A vaccine that delivered an antibody to methanogens through saliva has been suggested as a means to a reduce methane yield (Wedlock et al. 2013). In the future, transgenic approaches could possibly be used to modify saliva protein expression.
Genomic selection
Selection for higher milk production in the Australian dairy cattle population has already resulted in a reduction in methane emissions per kilogram milk, i.e. reduced PEMI (Hayes et al. 2013; Fig. 3).
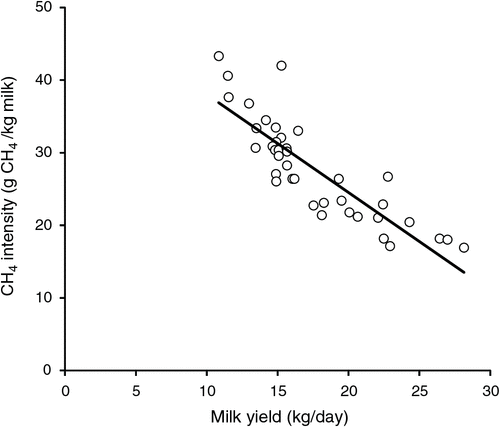
|
Genomic selection, combined with the direct measurement of methane emissions and feed conversion efficiency, could result in reductions in methane emission intensity of milk production. Genomic selection identifies many of the causative mutations affecting an animal’s phenotype through tagging with many thousands of genetic markers, known as single nucleotide polymorphisms. Provided sufficient phenotypes are available within a genotyped subset of dairy cows that are representative of the national herd, genomic prediction equations can be derived and applied to cows that have single nucleotype polymorphism genotypes, but for whom phenotypes for traits affecting methane production are unknown (Pryce et al. 2014). Genomic selection is now used routinely in many countries for genetic evaluation of traits that already have an estimated breeding value derived from a combination of pedigree and phenotype information (Spelman et al. 2013). The advantage of genomic selection for these traits is that the rate of genetic gain is accelerated by 40–50% (Spelman et al. 2013).
Genomic selection offers powerful new opportunities to select for traits that are difficult and/or expensive to measure, such as phenotypes associated with methane production. An example of the implementation of genomic selection within the Australian Holstein–Friesian population is selection for improved feed conversion efficiency (Williams et al. 2011b). The trait used by Williams et al. (2011b) was residual feed intake (RFI), which is the difference between actual and predicted feed intake. Negative values are indicative of cows that eat less than predicted for their level of milk production. Developing a genomic prediction tool for this trait has been the focus of a large collaboration between researchers in Australia and New Zealand, initially involving measurement of feed-intake phenotypes of ~1000 Holstein–Friesian heifers of ~6 months of age in each country (Williams et al. 2011b). The accuracy of trait prediction using this population was ~0.4 (Pryce et al. 2012), which is equivalent to a reliability of 0.16. While considerably lower than achieved for production traits (Spelman et al. 2013), this is similar to deterministic predictions of accuracy based on population size and the heritability of the trait (Pryce et al. 2014). Accuracy of trait prediction is limited by the size of the reference population. One possible solution is to collaborate with other researchers to establish an even larger reference population. For example, de Haas et al. (2012) combined DMI phenotypes from Dutch and UK cows with Australian heifer phenotypes and found that the accuracy of genomic prediction was 5.5% higher when a multi-country reference population was used, than with single-trait models. Since then, there has been further international collaboration through the global Dry Matter Initiative (gDMI) to build an even larger reference population (Berry et al. 2014). Initial results on genomic prediction using this reference population look promising.
So as to use genomic selection for reduced methane emissions, there are two options; to copy the gDMI model and build a reference population of methane phenotypes, where countries share the in vivo phenotype measurement and genotyping work, or to select for traits that are correlated with methane intensity, such as feed efficiency.
RFI is showing promise as a selection criterion for reducing methane emissions. Selection for RFI has been reported to lead to reductions in methane emissions in Australian beef cattle by ~13.5 g CH4/kg RFI (Hegarty et al. 2007). Recently, using methane-emission data from calorimetry chambers collected over a 3-day period from 32 cows that were part of a study on RFI, the potential for abatement in Holstein dairy cows was found to be ~17.5 g CH4/kg RFI (J. E. Pryce, unpubl. data). In another Australian study, Bell et al. (2013) suggested that in addition to feed efficiency, the greatest lifetime reduction in emissions per cow and per unit product (i.e. milk production) may be through selecting animals for longevity because of a reduction in the number of replacement animals required and the increased milk yield per lactation of multiparous cows. It is expected that, in the future, a combination of these two strategies will make a substantial contribution to reducing methane emissions from the Australian dairy herd.
Modelling enteric methane production
There are two main classes of model that can be used to predict enteric methane emissions from dairy cows. These are dynamic mechanistic models and static empirical statistical models. Within these classes of models, there are both whole farm-system models and rumen or ruminant-animal models.
Dynamic mechanistic models can describe the dynamic changes that occur in the rumen as ingested feed is fermented (Volpe et al. 2005). Thus, by definition, dynamic mechanistic models involve a set of differential equations that describe changes in a set of state variables over time. State variables are variables such as the amount of a particular substrate, metabolite or agent of change in the rumen. Examples of state variables are the amount of fermentable carbohydrate present in the rumen at any time, the amount of soluble protein present in the rumen at any time, and the number of bacteria in the rumen. Typically, dynamic mechanistic models may have between 12 and 50 state variables and there may be complex interactions between these variables. Globally, there are currently four major dynamic mechanistic models that have the capacity to dynamically model fermentation within the rumen of dairy cows and predict methane production. These are Molly (Baldwin 1995), Anje/COWPOL (Dijkstra et al. 1992; Benchaar et al. 1998; Mills et al. 2001), Karoline (Danfaer et al. 2006) and DairyMod (Johnson et al. 2012). These models are suitable for research purposes but, due to their complexity and their requirement for extensive parameterisation reflecting dietary components, they are unlikely to be suitable for day-to-day use predicting the methane emissions from cows grazing a wide range of constantly varying diets, as occurs in the pasture-based dairying systems of Australia. Thus, they are unlikely to be sufficiently accurate to generate the national inventory of GHG emissions from the Australian dairy herd. Molly, Anje/COWPOL and Karoline are not in common use in Australia, but DairyMod has been extensively used to evaluate a range of methane and nitrous oxide mitigation options in the context of Australian whole-farm systems (Browne et al. 2013, 2015).
Static empirical models have the following three key strengths: (1) simplicity, they generally involve a small number of variables (e.g. DMI, dietary lipid%, dietary lignin%), (2) they can be implemented in a simple spreadsheet, and (3) they are transparent and can be easily tested on a variety of datasets. The national GHG inventory of Australia currently uses a static empirical model to estimate emissions of methane from ruminant livestock. The equation used is based on the equation of Blaxter and Clapperton (1965), as corrected by Wilkerson and Casper (1995), and is as follows:
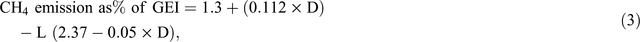
where CH4 emission (CH4% of gross energy intake, GEI) is expressed as a % of GEI (MJ), D is the apparent digestibility of dietary energy at maintenance (expressed as a %) and L is the energy intake expressed as a multiple of that required for maintenance (%).
The Dairy Greenhouse Gas Abatement Strategy model, which captures the Australian inventory methods in a simple farm tool, has been used to predict enteric methane emissions from 60 Tasmanian dairy farms (Christie et al. 2011). The principal disadvantage of all of these empirical static models is that they do not rely on an understanding of the biology of methanogenesis in the rumen, and, hence, they are of limited use in the development of new strategies to mitigate enteric methane emissions from ruminants. Static empirical models are also limited in predicting beyond the data used for their development.
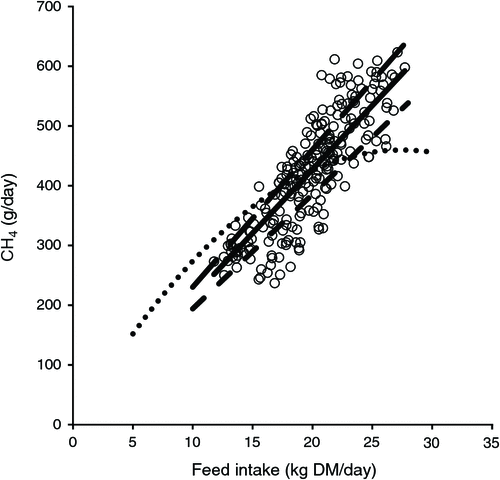
So as to be able to quantify the magnitude to which various interventions mitigate methane emissions, it is first necessary to quantify methane emissions in the absence of mitigation interventions. Figure 4 depicts daily DMI and daily methane production data from 220 cows from eight experiments using Holstein–Friesian cows (Grainger et al. 2008b; 2010a; Moate et al. 2013; Williams et al. 2013; Deighton et al. 2014b; and three unpublished experiments, using the same methodology). The cows were at different stages of lactation and were fed a wide variety of diets containing in excess of 70% forage (pasture, pasture hay, pasture silage, or lucerne hay) and between 0% and 30% cereal grain (barley or wheat), with none of these diets containing any known methane mitigants. These diets represent diets that have been used on Australian dairy farms during the past 40 years (Kellaway and Harrington 2004). The methane emissions from individual cows were measured in open-circuit calorimeters, with the chambers and method of operation previously described in detail (Williams et al. 2013). These methane-yield data were analysed using a mixed model with a single fixed effect consisting of just the mean and with random effects for experiment, treatment within experiment, period within experiment, cow within experiment and a residual term (period within cow within experiment). This model (Eqn 4) is essentially the same as a random coefficients regression-through-the-origin model for methane (CH4 g/day) versus DMI (kg/day).
|
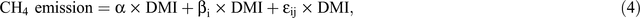
where α is the mean slope of the relationship between methane and DMI, and βi is a random coefficient with mean 0, that depends on factors such as experiment, treatment, period and cow, and εij is a residual error coefficient for each datum, with residual error plausibly proportional to DMI (or CH4). The primary objective of the analysis was to estimate the mean slope, α, with an appropriate standard error. Rearranging Eqn 4 gives Eqn 5 that is, a linear mixed model for methane yield, as follows:

Each random term was tested using a chi-square change-in-deviance test on exclusion of the term from the model, and the most parsimonious model was selected on the basis of Akaike information criterion (AIC). Distributional assumptions of normality and constant variance were checked graphically using plots of residual versus fitted value (in the case of lowest stratum residuals) histograms and normal quantile plots, and residual plots by experiment and treatment class (for both lowest stratum residuals and for residuals combining all random effects). Data analysis was conducted using Genstat 17 software (VSN International 2014).
The resulting linear relationship between methane emissions (g CH4/cow.day) and DMI (kg/cow.day) is shown in Fig. 4 and is described by Eqn 6, as follows:

The coefficient of 21.1 g CH4/kg DMI is similar to the coefficient of 21.6 used in the New Zealand GHG inventory (Ministry for the Environment 2014). Furthermore, this coefficient for dairy cows is less than the 23.1 g CH4/kg DMI (Fig. 4) reported by Dijkstra et al. (2011) for dairy cows in The Netherlands, but more than the coefficient of 19.4 for diets based on a mixture of maize silage, maize grain and soybeans, as is typically used in the USA (Hristov et al. 2013).
When lactating dairy cows are fed different amounts of a diet dominated by forage, we have found the response in methane emissions to be linear (Fig. 4). This is in contrast to the diminishing methane yield predicted by the equation which is used to predict methane emissions for dairy and beef cattle in the current Australian GHG inventory (Department of the Environment 2014), based on Blaxter and Clapperton (1965), the non-linear Mitscherslich (Mits3) model of Mills et al. (2003), which is the current model recommended by the USDA (Powers et al. 2014), and the recent model of Knapp et al. (2014). We propose that for the Australian GHG inventory, Eqn 6 is more appropriate than the current method. There are six reason for using Eqn 6 instead of Eqn 3. These are as follows:
-
The choice of dependent and independent variables in Eqn 3 is problematic. The dependent variable, CH4 emissions, is expressed as a percentage of the GEI (MJ). GEI is determined as the product of DMI and the concentration of gross energy in the feed (MJ/kg DM). The concentration of gross energy in a feed is normally determined by using a bomb calorimeter, and it is an exacting process prone to error. Also the apparent digestibility of dietary energy at maintenance (D) for most Australian feeds has rarely, if ever, been measured in dairy cows. For any given animal, the determination of D and the level of energy intake relative to maintenance (L) is quite difficult to determine and subject to error. Therefore, this calculation is based on variables that are themselves very difficult to accurately measure and about which there is limited published data for Australian feeds.
-
The second issue with Eqn 3 is that it is primarily based on data that came from studies on sheep (Blaxter and Clapperton 1965). Equation 3 predicts that, for a feed of a given D value, the methane production as a % of GEI will decline with increasing L. There is some recent evidence that this may be the case for sheep (Pacheco et al. 2014), but the data reported here (Fig. 4) and in the scientific literature now provide incontrovertible evidence that, for cattle fed forage-dominant diets, methane emissions (g CH4/day) are linearly related to DMI (Kriss 1930; Ellis et al. 2010; Hristov et al. 2013).
-
The third issue with Eqn 3 is that it is based on feeds available in the UK during the early 1960s. Not only are these qualitatively different from current Australian cattle diets, but some of the diets in the database of Blaxter and Clapperton (1965) included high proportions of flaked maize and sugar-beet pulp, which may be expected to have ruminal fermentation characteristics different from those of forage-based diets typically fed to dairy cows in Australia.
-
The fourth problem with the Blaxter and Clapperton (1965) equation is that it introduces an error into the estimation of methane emissions, by relating methane production to energy intake. This is particularly problematic in diets containing a high concentration of lipid. The Blaxter and Clapperton (1965) equation predicts that cows consuming a lipid-rich diet would produce more methane than cows consuming a diet with a low lipid concentration. However, the reverse is observed (Beauchemin et al. 2009; Moate et al. 2011).
-
The fifth reason why the Blaxter and Clapperton (1965) equation should be replaced is that it was developed at a time before statistical programs had been developed that could take into account multiple covariates and colinearity between independent variables or perform appropriate meta-analyses. From the vantage point of 2015, we cannot be confident that appropriate statistical modelling was performed on the data of Blaxter and Clapperton (1965).
-
Last, more than 50 years have passed since the data of Blaxter and Clapperton (1965) were obtained from experiments conducted during the late 1950s and early 1960s. There have been substantial changes in the genetic merit of dairy cows during the past 50 years. Therefore, it would be prudent that mathematical models to predict methane emissions from the Australian dairy sector should be based on data from modern lactating dairy cows representative of the current Australian dairy herd.
If Eqn 6 was to be employed to estimate enteric methane emissions from dairy cows, then the estimate of enteric methane emissions from the Australian dairy industry would be ~10% less than the current inventory estimate.
Methane emissions and intensity of Australian milk production
First-world consumers are increasingly concerned with the environmental impact of their lifestyles (Fraj and Martinez 2006; Young et al. 2010). An important determinant of the carbon footprint of dairy products is how much total enteric methane is associated with each kilogram of milk produced (TEMI; g CH4/kg milk). Thus, practical steps to mitigate methane emissions associated with dairy production must necessarily be concerned with reducing methane intensity. In this regard, the question facing researchers and consumers is ‘What is the TEMI of Australian milk production?’.
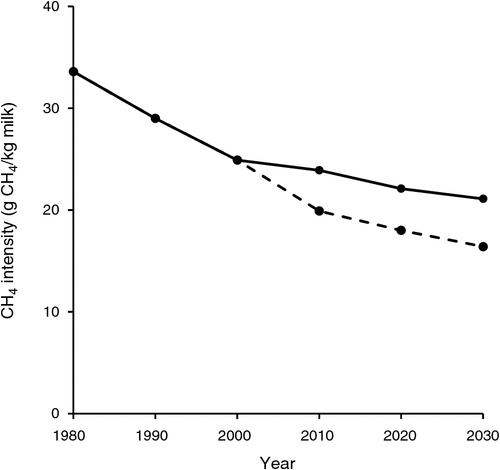
Calculation of methane intensity is complicated by the fact that methane is produced by dairy cows during the growth phase, before commencement of lactation at about 2 years of age, when they are not lactating and when they are lactating. Nevertheless, using the calculator described by Heard et al. (2011), it is possible to estimate the total annual DMI of all animals (calves, growing heifers, milking cows and dry cows) on an Australian dairy farm. Several assumptions are required to make this calculation. These include the average bodyweight of cows, the percentage annual replacement rate of cows, and the estimated metabolisable energy (ME) concentration (11.0 MJ/kg DM) of pasture consumed, the estimated ME concentration (12.0 MJ/kg DM) of concentrate consumed, the lipid concentration of the diet and the impact that the proportion of wheat in the diet has on the methane yield. A conservative approach to calculating enteric methane emissions from these animals involves use of Eqn 6. Alternatively, the impact that wheat consumption has on methane emissions can be estimated by using Eqn 2. We have applied Eqn 6 for Years 1980, 1990, 2000, 2010, 2020 and 2030 and Eqn 2 for Years 2010, 2020 and 2030. When making these calculations, we used data on total annual milk production and the total number of dairy cows in Australia (Dairy Australia 2014). In modelling the effect of wheat consumption on methane emissions, it has been assumed that half of the Australian dairy herd consume 25% of their ME intake as wheat and half consume 50% of their ME intake as wheat, overall equating to 38% of ME intake as wheat. This approximates the average proportion of wheat currently in the diet of dairy cows in Australia. Thus we assumed that average wheat intakes in 2010, 2020 and 2030 were, respectively, 1.7, 2.4 and 2.5 t wheat DM per cow per year. Table 1 shows the data used to estimate methane emissions and TEMI from 1980 to 2030.
In 1980, Australia’s annual milk production was ~5.4 billion kg, and this increased to ~8.9 billion kg by 2010, a 64% increase. We estimate that in 1980, the Australian dairy industry produced ~185 000 t of enteric methane annually, and by 2010, enteric methane production remained almost the same at ~182 000 t (3.8 M t CO2e). Thus, the important issue highlighted by the data in Table 1 and Fig. 5 is that, as milk production per cow has increased, TEMI has decreased. Indeed, between 1980 and 2010, TEMI has decreased by ~40%, and by 2030, the modelling indicates that with wheat feeding, TEMI may be less than half of that in 1980. The data presented in Table 1 and Fig. 5 also highlight the fact that currently and into the near future, wheat feeding can be expected to reduce TEMI by ~20%. This substantial reduction in estimated TEMI relies on our estimated methane response to wheat feeding, as is shown in Fig. 2. In view of the potential magnitude of the dietary wheat effect, additional research is needed to better quantify the methane response to wheat feeding, such as the following: to determine whether the methane mitigation response observed in short-term experiments persists into the longer term; to identify potential additive or synergistic effects if diets contain wheat as well as other methane mitigants such as lipid supplements; to compare the methane mitigation responses when wheat of various qualities (e.g. Australian general-purpose or feed wheat) are fed to cows; and to elucidate the chemical and physical attributes of wheat that are associated with methane mitigation.
|
It has been suggested that as arable land becomes scarce, diets containing a high proportion of cereal grains should not be fed to ruminants on ethical grounds since cereals could be fed directly to humans or to monogastric animals (Doreau et al. 2014). However, in 2010, Australian dairy cows consumed less than 10% of the 27.4 million tonnes of wheat grown in Australia (Index mundi 2014). Most of the wheat consumed by cattle was classified as either Australian general-purpose or feed wheat. These wheat classes generally comprise grain that has failed to meet minimum standards for milling, due to foreign materials, weather damage or sprouting (Queensland Government 2009). Furthermore, in terms of producing food for human consumption, it is more efficient to feed grain to cows and produce milk than to use grain to produce chicken or pig meats (Wilkinson 2011). Thus, it seems likely that wheat will continue to constitute a significant proportion of the diet of Australian dairy cows in the future.
Future research opportunities
During the past 30 years, the on-farm application of research in dairy cow nutrition, rumen microbiology and dairy cow genetics has led to substantial increases in milk production from individual cows. In 1980, Australian dairy cows produced on average 2889 kg milk per year and, in 2010, average production had increased 64% to 5654 kg milk per year. Analysis of data collated in the present review has shown that dairy cows in Australia produce 21.1 g methane for each kg of DM consumed. Using this coefficient, the average TEMI from Australian dairy cows was estimated to have been 33.6 g methane/kg milk (9.8 t CO2e/t MS) in 1980, but this had decreased to 19.9 g methane/kg milk (6.0 t CO2e/t MS) in 2010. This reduction in TEMI intensity was brought about by improvements in nutrition and genetics, leading to increases in milk production, that have occurred in the intervening years.
Currently, there are only a few practical and cost-effective strategies that can be used on Australian farms to achieve further reductions in total methane emissions. Those currently available include the feeding of lipid-rich feed supplements, such as brewers grains, cold-pressed canola, cottonseed, hominy meal, grape marc, and the feeding of wheat. Other promising strategies are in early stages of development but offer the possibility of long-term mitigation. These include genetic selection of cows that are efficient at feed conversion to milk, genetic selection of low-methane-emitting cows, vaccines to reduce ruminal methanogens and intra-ruminal administration of specific chemical inhibitors of methanogens. However, well-resourced research on methane mitigation in Australia has been undertaken for less than 15 years and it is likely that further research will be required for significant, sustainable and cost-effective solutions to be developed.
The present review has identified that the following areas should have high priority in future methane-mitigation research:
-
The quantification of methane mitigation resulting from feeding wheat
-
The elucidation of the mechanisms by which the feeding of fatty feed supplements and the feeding of wheat reduce the methane emissions of ruminants
-
The elucidation of how rumen microbiology influences enteric methane production
-
The development of low-cost methods for measuring enteric methane production
-
Large-scale screening of dairy cows to identify low methane-emitting animals
-
Research to enhance the productivity of dairy cows so as to further reduce their methane intensity
Conclusions
The current Australian GHG inventory overestimates the contribution made by enteric methane emissions from the dairy sector due to the use of a now outdated calculation based on the research of Blaxter and Clapperton (1965). We conclude that empirical evidence demonstrates that the methane yield of forage-fed dairy cows in Australia is ~10% less than the current inventory with an average of 21.1 g CH4/kg DMI.
We report that the current TEMI of Australian national milk production is ~19.9 g CH4/kg milk (6.0 t CO2e/t MS).
Implementation of research to improve Australian milk production has led to a substantial (40%) reduction in the enteric methane emission associated with Australian milk production, from 33.6 in 1980 to 19.9 g CH4/kg milk in 2010. Current research has demonstrated that the TEMI of Australian milk production can be further reduced by the inclusion of lipid-rich by-products in the diet of dairy cows. The methane emissions of cows consuming forage diets containing 30 g lipid/kg DMI can be reduced by ~3.5% for every 1% increase in dietary fat concentration. Recent Australian research has also indicated that inclusion of feed wheat in the diet of dairy cows also results in a substantial reduction in methane yield; however, further research is required to quantify this effect and its complementarity with other dietary mitigation options. A further reduction in the methane intensity of Australian milk production is anticipated in the near future, given the increasing inclusion of wheat in the diet of Australian dairy cows.
Further substantial reductions in methane emissions will probably not occur by the application of a single technology, but by the application of an integrated suite of technologies. Against these advances, continuing growth in consumer demand for dairy products, especially in Asia, is likely to stimulate dairy production in Australia, and in the absence of further mitigation research, potentially cause a concomitant increase in production of GHG, and in particular, methane, in the absence of further mitigation research. Future research should, therefore, focus on both the medium- and long-term options to profitably reduce the emission footprint of dairy production systems. In the short term, the most prospective focus for mitigation research would be on dietary manipulation and animal management. However, a long-term research focus on rumen manipulation and plant and animal breeding is imperative to underpin the future sustainability and environmental footprint credentials of the Australian dairy industry.
Acknowledgements
The authors acknowledge the support of the Department of Economic Development, Jobs, Transport and Resources Victoria, the Australian Department of Agriculture, Meat and Livestock Australia, and Dairy Australia. Parts of this review were presented as a keynote address to the 6th Australasian Dairy Science Symposium held at Hamilton, New Zealand in November 2014 (Moate et al. 2014d).
References
Abecia L, Toral PG, Martín-García AI, Martínez G, Tomkins NW, Molina-Alcaide E, Newbold CJ, Yáñez-Ruiz DR (2012) Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. Journal of Dairy Science 95, 2027–2036.| Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xksleitrw%3D&md5=8d6884b6cd22cf46be335d176e3f3e66CAS | 22459848PubMed |
Alcock DJ, Hegarty RS (2011) Potential effects of animal management and genetic improvement on enteric methane emissions, emissions intensity and productivity of sheep enterprises at Cowra, Australia. Animal Feed Science and Technology 166–167, 749–760.
| Potential effects of animal management and genetic improvement on enteric methane emissions, emissions intensity and productivity of sheep enterprises at Cowra, Australia.Crossref | GoogleScholarGoogle Scholar |
Ang CS, Binos S, Knight MI, Moate PJ, Cocks BG, McDonagh MB (2011) Global survey of the bovine salivary proteome: integrating multidimensional prefractionation, targeted, and glycocapture strategies. Journal of Proteome Research 10, 5059–5069.
| Global survey of the bovine salivary proteome: integrating multidimensional prefractionation, targeted, and glycocapture strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1amsb3I&md5=476ab93d0c8c732f551d3c2b5ef94e9eCAS | 21902196PubMed |
Baldwin RL (1995) ‘Modeling ruminant digestion and metabolism.’ (Chapman & Hall: London)
Beauchemin KA, McGinn SM (2005) Methane emissions from feedlot cattle fed barley or corn diets. Journal of Animal Science 83, 653–661.
Beauchemin KA, McAllister TA, McGinn SM (2009) Dietary mitigation of enteric methane from cattle. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 4, 1–18.
| Dietary mitigation of enteric methane from cattle.Crossref | GoogleScholarGoogle Scholar |
Bell MJ, Eckard RJ, Haile-Mariam M, Pryce JE (2013) The effect of changing cow production and fitness traits on net income and greenhouse gas emissions from Australian dairy systems. Journal of Dairy Science 96, 7918–7931.
| The effect of changing cow production and fitness traits on net income and greenhouse gas emissions from Australian dairy systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1CqsbfI&md5=62e159900fd1c83a8433ec0224dfb7aeCAS | 24140333PubMed |
Benchaar C, Rivest J, Chiquette J (1998) Prediction of methane production form dairy cows using existing mechanistic and regression equations. Journal of Animal Science 76, 617–627.
Berean K, Ou JZ, Nour M, Latham K, McSweeney C, Paull D, Halim A, Kentish S, Doherty CM, Hill AJ, Kalantar-Zadeh K (2014) The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Separation and Purification Technology 122, 96–104.
| The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1KhsL4%3D&md5=f62f46eec3140e7b8d1a07cd1704ce66CAS |
Berndt A, Boland TM, Deighton MH, Gere JI, Grainger C, Hegarty RS, Iwaasa AD, Koolaard JP, Lassey KR, Luo D, Martin RJ, Martin C, Moate PJ, Molano G, Pinares-Patiño C, Ribaux BE, Swainson NM, Waghorn GC, Williams SRO (2014) Guidelines for use of sulphur hexafluoride (SF6) tracer technique to measure enteric methane emissions from ruminants. (Ed. MG Lambert) (Ministry of Primary Industries: Wellington, New Zealand)
Berry DP, Coffey MP, Pryce JE, de Haas Y, Løvendahl P, Krattenmacher N, Crowley JJ, Wang Z, Spurlock D, Weigel K, Macdonald K, Veerkamp RF (2014) International genetic evaluations for feed intake in dairy cattle through the collation of data from multiple sources. Journal of Dairy Science 97, 3894–3905.
| International genetic evaluations for feed intake in dairy cattle through the collation of data from multiple sources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtFagsr0%3D&md5=9ab84a2752bf2373a567f71be7875c1dCAS | 24731627PubMed |
Beukes PC, Gregorini P, Romera AJ, Levy G, Waghorn GC (2010) Improving production efficiency as a strategy to mitigate greenhouse gas emissions on pastoral dairy farms in New Zealand. Agriculture, Ecosystems & Environment 136, 358–365.
| Improving production efficiency as a strategy to mitigate greenhouse gas emissions on pastoral dairy farms in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFenurY%3D&md5=f8e854b876d86ed6e9d1a4839fe00c9fCAS |
Blaxter KL, Clapperton JL (1965) Prediction of the amount of methane produced by ruminants. British Journal of Nutrition 19, 511–522.
| Prediction of the amount of methane produced by ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XitFKktg%3D%3D&md5=2a0b9f2ba383587ab81bcf6489c6b257CAS | 5852118PubMed |
Browne N, Kingwell R, Behrendt R, Eckard R (2013) The relative profitability of dairy, sheep, beef and grain farm enterprises in southeast Australia under selected rainfall and price scenarios. Agricultural Systems 117, 35–44.
| The relative profitability of dairy, sheep, beef and grain farm enterprises in southeast Australia under selected rainfall and price scenarios.Crossref | GoogleScholarGoogle Scholar |
Browne NA, Behrendt R, Kingwell RS, Eckard RJ (2015) Does producing more product over a lifetime reduce greenhouse gas emissions and increase profitability in dairy and wool enterprises? Animal Production Science 55, 49–55.
| Does producing more product over a lifetime reduce greenhouse gas emissions and increase profitability in dairy and wool enterprises?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVKqsrrL&md5=1c47d1c1758e5ffc25a498a491cd96a4CAS |
Brulc JM, Antonopoulos DA, Berg Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, Edwards RE, Frank ED, Emerson JB, Wacklin P, Coutinho PM, Henrissat B, Nelson KE, White BA (2009) Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proceedings of the National Academy of Sciences of the United States of America 106, 1948–1953.
| Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitVKitro%3D&md5=4de112df74d0a50b17d3df1b993e45ffCAS | 19181843PubMed |
Buddle BM, Denis M, Arrwood GT, Alterman E, Janssen PH, Ronimus RS, Pinares-Patino CS, Muetzel S, Wedlock DN (2011) Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Veterinary Journal (London, England) 188, 11–17.
| Strategies to reduce methane emissions from farmed ruminants grazing on pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1Sgt7k%3D&md5=18fdc4698a72394fec8ee2533fb136a5CAS |
Burreson BJ, Moore RE, Roller (1976) Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta). Journal of Agricultural and Food Chemistry 24, 856–861.
| Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksVGju7k%3D&md5=f31b8f0a4b53ee41f9c330984a7a3547CAS |
Callaghan MJ, Tomkins NW, Benu I, Parker AJ (2014) How feasible is it to replace urea with nitrates to mitigate greenhouse gas emissions from extensively managed beef cattle? Animal Production Science 54, 1300–1304.
CER (2014) ‘Carbon Farming Initiative, methdology determinations.’ Available at http://www.cleanenergyregulator.gov.au/Carbon-Farming-Initiative/methodology-determinations/Pages/default.aspx [Verified 26 March 2015]
Chaves AV, Baah J, Wang Y, McAllister TA, Benchaar C (2012) Effects of cinnamon leaf, oregano and sweet orange essential oils on fermentation and aerobic stability of barley silage. Journal of the Science of Food and Agriculture 92, 906–915.
| Effects of cinnamon leaf, oregano and sweet orange essential oils on fermentation and aerobic stability of barley silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSns7w%3D&md5=22d5f8b34521d28ded7b5a5d0ea2a4efCAS | 22413147PubMed |
Chilliard Y, Martin C, Rouel J, Doreau M (2009) Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output. Journal of Dairy Science 92, 5199–5211.
| Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtF2qtrfE&md5=c592b6f996b067b93da8786c687ab00fCAS | 19762838PubMed |
Christie KM, Rawnsley RP, Eckard RJ (2011) A whole farm systems analysis of greenhouse gas emissions of 60 Tasmanian dairy farms. Animal Feed Science and Technology 166–167, 653–662.
| A whole farm systems analysis of greenhouse gas emissions of 60 Tasmanian dairy farms.Crossref | GoogleScholarGoogle Scholar |
Clapperton JL (1974) The effect of trichloroacetamide, chloroform and linseed oil given into the rumen of sheep on some of the end-products of rumen digestion. British Journal of Nutrition 32, 155–161.
| The effect of trichloroacetamide, chloroform and linseed oil given into the rumen of sheep on some of the end-products of rumen digestion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXltVeltL8%3D&md5=dd429ed76576bdf3af971cd68e354a4cCAS | 4408011PubMed |
Clark H (2013) Nutritional and host effects on methanogenesis in the grazing ruminant. Animal 7, 41–48.
| Nutritional and host effects on methanogenesis in the grazing ruminant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFeisbo%3D&md5=8d87951ea48d6ca06720c9784c4eb661CAS | 23127524PubMed |
ComLaw (2013) ‘Carbon credits (Carbon Farming Initiative) (reducing greenhouse gas emissions by feeding dietary additives to milking cows) methodology determination 2013 – F2013L01554.’ Available at http://www.comlaw.gov.au/Details/F2013L01554 [Verified 26 March 2015]
ComLaw (2014) Carbon Farming Initiative Amendment Act 2014. An ‘Act to amend the Carbon Credits (Carbon Farming Initiative) Act 2011, and for other purposes’. ComLaw Authoritative Act C2014A00119, p. 161. Available at http://www.comlaw.gov.au/Details/C2014A00119 [Verified 3 March 2015]
Cottle DJ, Nolan JV, Wiedemann SG (2011) Ruminant enteric methane mitigation: a review. Animal Production Science 51, 491–514.
| Ruminant enteric methane mitigation: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntVGisLY%3D&md5=f55dc07f1a9764dc6d98213fd08001e5CAS |
CSIRO (2014) ‘System, method and device for measuring a gas in the stomach of a mammal. Patent application W020213/003892.’ Available at http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013003892&recNum=76&docAn=AU2012000784&queryString=FP:(sensor)&maxRec=440955 [Verified 26 March 2015]
Dairy Australia (2012) Summary of the final report: farming-carbon footprint of the Australian dairy industry. Dairy Australia Ltd, Melbourne. Available at http://www.dairyaustralia.com.au/Home/Standard-Items/~/media/Documents/Animal%20management/Environment/Climate%20footprint%20LCA/DA%20LCA%20-%20Australian%20dairy%20carbon%20footprint%20-%20Farm%20Report.pdf [Verified 26 March 2015]
Dairy Australia (2014) Australian dairy industry. In ‘Focus’. (Dairy Australia Ltd: Melbourne). Available at http://www.dairyaustralia.com.au/~/media/Documents/Stats%20and%20markets/In%20Focus/Australian%20Dairy%20Industry%20In%20Focus%202014.pdf [Verified 26 March 2015]
Danfaer A, Huhtanen P, Uden P, Sveinbjornsson J, Volden H (2006) The Nordic dairy cow model, Karoline-description. In ‘Nutrient digestion and utilization in farm animals. Modelling approaches’. (Eds E Kebreab, J Dijkstra, A Bannink, WJJ Garrits, J France) pp. 407–415. (CAB International: Wallingfords, UK)
DCCEE (2012a) Australian National Greenhouse Accounts, National Inventory Report 2010. Australian Government Department of Climate Change and Energy Efficiency, Canberra. Available at http://www.environment.gov.au/climate-change/greenhouse-gas-measurement/publications/national-inventory-report-2010 [Verified 26 March 2015]
DCCEE (2012b) ‘An overview of the Carbon Farming Initiative.’ (Australian Government Department of Climate Change and Energy Efficiency: Canberra, ACT 2601, Australia) Available at http://www.environment.gov.au/climate-change/emissions-reduction-fund/cfi/about [Verified 26 March 2015]
de Haas Y, Calus MPL, Veerkamp RF, Wall E, Coffey MP, Daetwyler HD, Hayes BJ, Pryce JE (2012) Improved accuracy of genomic prediction for dry matter intake of dairy cattle from combined European and Australian data sets. Journal of Dairy Science 95, 6103–6112.
| Improved accuracy of genomic prediction for dry matter intake of dairy cattle from combined European and Australian data sets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVCnsL%2FL&md5=85dd8dacb6f746ff94743f8fd60000d6CAS | 22863091PubMed |
Dehareng F, Delfosse C, Froidmont E, Soyeurt H, Martin C, Gengler N, Vanlierde A, Dardenne P (2012) Potential use of milk mid-infrared spectra to predict individual methane emission of dairy cows. Animal 6, 1694–1701.
| Potential use of milk mid-infrared spectra to predict individual methane emission of dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Gis7zP&md5=3b054d28549d3edd1d4519cbc9170320CAS | 23031566PubMed |
Deighton MH, O’Loughlin BM, Williams SRO, Moate PJ, Kennedy E, Boland TM, Eckard RJ (2013a) Declining sulphur hexafluoride permeability of fluoropolymer membranes causes overestimation of calculated ruminant methane emissions using the tracer technique. Animal Feed Science and Technology 183, 86–95.
| Declining sulphur hexafluoride permeability of fluoropolymer membranes causes overestimation of calculated ruminant methane emissions using the tracer technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovVWnsb0%3D&md5=23dc8c267581733b301000bdb1777edbCAS |
Deighton MH, Williams SRO, Eckard RJ, Boland TM, Moate PJ (2013b) High concordance of CH4 emissions is possible between the SF6 tracer and respiration chamber techniques. In ‘Advances in animal biosciences: proceedings of the 5th greenhouse gases and animal agriculture conference (GGAA 2013)’, Dublin, Ireland. Vol. 4. Part 2. p. 411. (Cambridge University Press: Cambridge, UK)
Deighton MH, Williams SRO, Lassey KR, Hannah MC, Boland TM, Eckard RJ, Moate PJ (2014a) Temperature, but not submersion or orientation, influences the rate of sulphur hexafluoride release from permeation tubes used for estimation of ruminant methane emissions. Animal Feed Science and Technology 194, 71–80.
| Temperature, but not submersion or orientation, influences the rate of sulphur hexafluoride release from permeation tubes used for estimation of ruminant methane emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVSht7fO&md5=65e41a5318691064f35ae3f249a9b2ccCAS |
Deighton MH, Williams SRO, Hannah MC, Eckard RJ, Boland TM, Wales WJ, Moate PJ (2014b) A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Animal Feed Science and Technology 197, 47–63.
| A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVSqsbvO&md5=87f7b0e86e5b782831341929c05182eeCAS |
Department of Agriculture (2013) ‘Australian agriculture: reducing emissions and adapting to a changing climate: key findings of the climate change research program.’ (Australian Government Department of Agriculture: Canberra) Available at http://www.agriculture.gov.au/Style%20Library/Images/DAFF/__data/assets/pdffile/0006/2359815/reducing-emissons-adapting-changing-climate.pdf [Verified 26 March 2015]
Department of Agriculture (2014) ‘Filling the Research Gap (FtRG) program.’ (Australian Government Department of Agriculture: Canberra) Available at http://www.daff.gov.au/climatechange/carbonfarmingfutures/ftrg [Verified 26 March 2015]
Department of the Environment (2014) National inventory report 2012, vol. 1. The Australian Government submission to the United Nations Framework Convention on Climate Change, Australian National Greenhouse Accounts. Australian Government Department of the Environment, Canberra. Available at http://www.environment.gov.au/system/files/resources/6b894230-f15f-4a69-a50c-5577fecc8bc2/files/national-inventory-report-2012-vol1.pdf [Verified 26 March 2015]
Dharma S, Shafron W, Oliver M (2012) ‘Australian dairy farm technology and management practices 2010–11.’ (Australian Government Department of Agriculture, Fisheries amd Forestry – Australian Bureau of Agricultural and Resource Economics and Sciences (ABARES): Canberra). Available at http://data.daff.gov.au/data/warehouse/9aab/9aabf/2012/adftm9aabf006/AustDairyFarmTechManagPrac_v1.0.0.pdf [Verified 26 March 2015]
Dijkstra J, Neal HD, Beever D, France J (1992) Simulation of nutrient digestion, absorption and outflow in the rumen: model description. The Journal of Nutrition 122, 2239–2255.
Dijkstra J, van Zijderveld SM, Apajalahti JA, Bannink A, Gerrits WJJ, Newbold JR, Perdok HB, Berends H (2011) Relationships between methane production and milk fatty acid profiles in dairy cattle. Animal Feed Science and Technology 166–167, 590–595.
| Relationships between methane production and milk fatty acid profiles in dairy cattle.Crossref | GoogleScholarGoogle Scholar |
Doreau M, Martin C, Eugène M, Popova M, Morgavi DP (2011) Leviers d’action pour réduire la production de méthane entérique par les ruminants. Productions Animales 24, 461–474.
Doreau M, Bamière L, Pellerin S, Lherm M, Benoit M (2014) Mitigation of enteric methane for French cattle: potential extent and cost of selected actions. Animal Production Science 54, 1417–1422.
Dubois B, Tomkins NW, Kinley RD, Bai M, Seymour S, Paul NA, de Nys R (2013) Effect of tropical algae additives on rumen in vitro gas production and fermentation characteristics. American Journal of Plant Sciences 4, 34–43.
| Effect of tropical algae additives on rumen in vitro gas production and fermentation characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjsFart74%3D&md5=6eb2904999cd3dbacd3d06b901b43ff5CAS |
Durmic Z, Moate PJ, Eckard R, Revell DK, Williams R, Vercoe PE (2014) In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. Journal of the Science of Food and Agriculture 94, 1191–1196.
| In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1ertr%2FN&md5=e524efa402104e15a4e562178d81f2f6CAS | 24105682PubMed |
Duval S, Kindermann M (2012) ‘Use of nitrooxy organic molecules in feed for reducing enteric methane emissions in ruminants, and/or to improve ruminant performance.’ International patent application WO 2012/084629 A1. (World Intellectual Property Organization)
Eckard RJ, Grainger C, de Klein CAM (2010) Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livestock Science 130, 47–56.
| Options for the abatement of methane and nitrous oxide from ruminant production: a review.Crossref | GoogleScholarGoogle Scholar |
Ellis JL, Bannink A, France J, Kebreab E, Dijkstra J (2010) Evaluation of enteric methane prediction equations for dairy cows used in whole farm models. Global Change Biology 16, 3246–3256.
| Evaluation of enteric methane prediction equations for dairy cows used in whole farm models.Crossref | GoogleScholarGoogle Scholar |
Fraj E, Martinez E (2006) Environmental values and lifestyles as determining factors of ecological consumer behaviour: an empirical analysis. Journal of Consumer Marketing 23, 133–144.
| Environmental values and lifestyles as determining factors of ecological consumer behaviour: an empirical analysis.Crossref | GoogleScholarGoogle Scholar |
Gardiner TD, Coleman MD, Innocenti F, Tompkins J, Connor A, Garnsworthy PC, Moorby JM, Reynolds CK, Waterhouse A, Wills D (2015) Determination of the absolute accuraccy of UK chamber facilities used in measuring methane emissions from livestock. Measurement 66, 272–279.
| Determination of the absolute accuraccy of UK chamber facilities used in measuring methane emissions from livestock.Crossref | GoogleScholarGoogle Scholar |
Grainger C, Beauchemin KA (2011) Can enteric methane emissions from ruminants be lowered without lowering their production? Animal Feed Science and Technology 166–167, 308–320.
| Can enteric methane emissions from ruminants be lowered without lowering their production?Crossref | GoogleScholarGoogle Scholar |
Grainger C, Clarke T, McGinn SM, Auldist MJ, Beauchemin KA, Hannah MC, Waghorn GC, Clark H, Eckard RJ (2007) Methane emissions from dairy cows measured using the sulphur hexafluoride (SF6) tracer and chamber techniques. Journal of Dairy Science 90, 2755–2766.
| Methane emissions from dairy cows measured using the sulphur hexafluoride (SF6) tracer and chamber techniques.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvFOitr0%3D&md5=31e9af0171520957adc3a531ed7b340bCAS | 17517715PubMed |
Grainger C, Clarke T, Beauchemin KA, McGinn SM, Eckard RJ (2008a) Supplementation with whole cottonseed reduces methane emissions and can profitably increase milk production of dairy cows offered a forage and cereal grain diet. Australian Journal of Experimental Agriculture 48, 73–76.
| Supplementation with whole cottonseed reduces methane emissions and can profitably increase milk production of dairy cows offered a forage and cereal grain diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVKm&md5=ebcc410d4122aeaa09bea0f285de180cCAS |
Grainger C, Auldist MJ, Clarke T, Beauchemin KA, McGinn SM, Hannah MC, Eckard TJ, Lowe LB (2008b) Use of monensin controlled-release capsules to reduce methane emissions and improve milk production of dairy cows offered pasture supplemented with grain. Journal of Dairy Science 91, 1159–1165.
| Use of monensin controlled-release capsules to reduce methane emissions and improve milk production of dairy cows offered pasture supplemented with grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXivFemtb8%3D&md5=4f1e454f49c2e52137e628ad5aba972cCAS | 18292272PubMed |
Grainger C, Clarke T, Auldist MJ, Beauchemin KA, McGinn SM, Waghorn GC, Eckard RJ (2009) Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Canadian Journal of Animal Science 21, 19–38.
Grainger C, Williams R, Eckard RJ, Hannah MC (2010a) A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain. Journal of Dairy Science 93, 5300–5308.
| A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsl2qsA%3D%3D&md5=2876474c7da48572f49621f5d02f3798CAS | 20965346PubMed |
Grainger C, Williams R, Clarke T, Wright ADG, Eckard RJ (2010b) Supplementation with whole cottonseed causes long term reduction of methane emissions from lactating dairy cows offered a forage and cereal grain diet. Journal of Dairy Science 93, 2612–2619.
| Supplementation with whole cottonseed causes long term reduction of methane emissions from lactating dairy cows offered a forage and cereal grain diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVahs7nP&md5=5415cbda700e89865db71b88574ca355CAS | 20494170PubMed |
Griffith DWT, Bryant GW, Tonini M, Kettlewell G, Denmead OT, Leuning R, Chen D, Eckard R (2006) Novel FTIR and laser spectroscopy methods for measuring trace gas emissions from agriculture and forests. In ‘Proceedings for the workshop on agricultural air quality: state of the science’, 5–8 June 2006. (Eds VP Aneja, WH Schlesinger, R Knighton, G Jennings, D Niyogi, W Gilliam, CS Duke) pp. 210–213. (Bolger Center: Potomac, MD)
Haisan J, Sun Y, Guan LL, Beauchemin KA, Iwaasa A, Duval S, Barreda DR, Oba M (2014) The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. Journal of Dairy Science 97, 3110–3119.
| The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktlWlsLk%3D&md5=1d8f848557dff5ed98f997bfda030380CAS | 24630651PubMed |
Hammond KJ, Muetzel S, Waghorn GC, Pinares-Patiño CS, Burke JL, Hoskin SO (2009) The variation in methane emissions from sheep and cattle is not explained by the chemical composition of ryegrass. Proceedings of the New Zealand Society of Animal Production 69, 174–178.
Hammond KJ, Humphries DJ, Crompton LA, Green C, Reynolds CK (2015) Methane emissions from cattle: Estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Animal Feed Science and Technology 203, 41–52.
| Methane emissions from cattle: Estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjvFeqtbc%3D&md5=2ff752f36051f5f6e3eb09c56779f738CAS |
Hayes BJ, Lewin HA, Goddard ME (2013) The future of livestock breeding: genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends in Genetics 29, 206–214.
| The future of livestock breeding: genomic selection for efficiency, reduced emissions intensity, and adaptation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKqurbM&md5=0dab15ed952491d003a7a6e185b53d8dCAS | 23261029PubMed |
Heard JW, Doyle PT, Francis SA, Staines MVH, Wales WJ (2011) Calculating dry matter consumption of dairy herds in Australia: the need to fully account for energy requirements and issues with estimating energy supply. Animal Production Science 51, 605–614.
| Calculating dry matter consumption of dairy herds in Australia: the need to fully account for energy requirements and issues with estimating energy supply.Crossref | GoogleScholarGoogle Scholar |
Hedderich R, Whitman WB (2013) Physiology and biochemistry of the methane-producing archaea. In ‘The prokaryotes’. 4th edn. (Eds E Rosenberg, EF DeLong, S Lory, E Stackebrandt, F Thompson) pp. 635–662. (Springer: Berlin)
Hegarty RS, Goopy JP, Herd RM, McCorkell B (2007) Cattle selected for lower residual feed intake have reduced daily methane production. Journal of Animal Science 85, 1479–1486.
| Cattle selected for lower residual feed intake have reduced daily methane production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1ant7c%3D&md5=ea9cf9bfd2c6b1e9568da47bb96e6892CAS | 17296777PubMed |
Henry B, Charmley E, Eckard R, Gaughan JB, Hegarty R (2012) Livestock production in a changing climate: adaptation and mitigation research in Australia. Crop and Pasture Science 63, 191–202.
| Livestock production in a changing climate: adaptation and mitigation research in Australia.Crossref | GoogleScholarGoogle Scholar |
Hess M, Sczyrba A, Egan R, Kim T-W, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM (2011) Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331, 463–467.
| Metagenomic discovery of biomass-degrading genes and genomes from cow rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsF2mtA%3D%3D&md5=9c3c03bb0ba6515153f16cc119bc5abfCAS | 21273488PubMed |
Hristov AN, Oh J, Firkins JL, Dijkstra J, Kebreab E, Waghorn G, Makkar HPS, Adesogan AT, Yang W, Lee C, Gerber PJ, Henderson B, Tricarico JM (2013) Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. Journal of Animal Science 91, 5045–5069.
| Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKktrrL&md5=232196d68807bd4a5064908acacdfea8CAS | 24045497PubMed |
Hristov AN, Oh J, Giallongo F, Frederick TW, Harper MT, Weeks HL, Branco AF, Moate PJ, Deighton MH, Williams SRO, Kindermann M, Duval S (2015) An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proceedings of the National Academy of Science of the Unites States of America 112, 10 663–10 668.
| An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1GrsL3P&md5=23585cf409f54b82d65b521f25e20cb9CAS |
Index mundi (2014) ‘Australian wheat production by year.’ Available at http://www.indexmundi.com/agriculture/?country=au&commodity=wheat&graph=production [Verified 26 March 2015]
International Dairy Federation (2010) ‘A common carbon footprint methodology for the dairy sector.’ Bulletin of the International Dairy Federation 445/2010. (International Dairy Federation: Brussels, Belgium) Available at http://idf-lca-guide.org/Files/media/Documents/445-2010-A-common-carbon-footprint-approach-for-dairy.pdf [Verified 26 March 2015]
Janssen PH, Kirs M (2008) Structure of the archaeal community of the rumen. Applied and Environmental Microbiology 74, 3619–3625.
| Structure of the archaeal community of the rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFWlsrk%3D&md5=44cb75aecd44dde6f9efa9fb4d66e17aCAS | 18424540PubMed |
Johnson KA, Johnson DE (1995) Methane emissions from cattle. Journal of Animal Science 73, 2483–2492.
Johnson DE, Wood AS, Stone JB, Moran ET (1972) Some effects of methane inhibition in ruminants (steers). Canadian Journal of Animal Science 52, 703–712.
| Some effects of methane inhibition in ruminants (steers).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXkt1SitQ%3D%3D&md5=73830555c771a6d8e270a38ae1141e8cCAS |
Johnson K, Huyler M, Westberg H, Lamb B, Zimmerman P (1994) Measurement of methane emissions from ruminant livestock using a SF6 tracer technique. Environmental Science & Technology 28, 359–362.
| Measurement of methane emissions from ruminant livestock using a SF6 tracer technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmtFahtA%3D%3D&md5=0064e1a5426af33063e026a8886af305CAS |
Johnson IR, France J, Thornley JHM, Bell MJ, Eckard RJ (2012) A generic model of growth, energy metabolism, and body composition for cattle and sheep. Journal of Animal Science 90, 4741–4751.
| A generic model of growth, energy metabolism, and body composition for cattle and sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXisV2qurc%3D&md5=b0bb0294a5fc04583c331f4d884595f6CAS | 22871927PubMed |
Kellaway R, Harrington T (2004) ‘Feeding concentrates: supplements for dairy cows.’ (Land links Press: Melbourne)
Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM (2014) Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. Journal of Dairy Science 97, 3231–3261.
| Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtlCnt74%3D&md5=a988b8ca75867d19f75b1203819a4b32CAS | 24746124PubMed |
Kriss M (1930) Quantitative relations of the dry matter of the food consumed, the heat production, the gaseous outgo, and the insensible loss in body weight of cattle. Journal of Agricultural Research 40, 283–295.
Kumar S, Choudhury PK, Carro MD, Griffith GW, Dagar SS, Puniya M, Calabro S, Ravella SR, Dhewa T, Upadhyay RC, Sirohi SK, Kundu SS, Wanapat M, Puniya AK (2014) Mini-review: new aspects and strategies for methane mitigation from ruminants. Applied Microbiology and Biotechnology 98, 31–44.
| Mini-review: new aspects and strategies for methane mitigation from ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslyqur3J&md5=dac1f381e2a99408d336cd3d64f13c63CAS | 24247990PubMed |
Lassey KR (2007) Livestock methane emission: from the individual grazing animal through national inventories to the global methane cycle. Agricultural and Forest Meteorology 142, 120–132.
| Livestock methane emission: from the individual grazing animal through national inventories to the global methane cycle.Crossref | GoogleScholarGoogle Scholar |
Leahy SC, Kelly WJ, Ronimus RS, Wedlock N, Altermann E, Attwood GT (2013) Genome sequencing of rumen bacteria and archaea and its application to methane mitigation strategies. Animal 7, 235–243.
| Genome sequencing of rumen bacteria and archaea and its application to methane mitigation strategies.Crossref | GoogleScholarGoogle Scholar | 23739466PubMed |
Li XX, Durmic Z, Liu SM, McSweeney CS, Vercoe PE (2014) Eremophila glabra reduces methane production and methanogen populations when fermented in a rusitec. Anaerobe 29, 100–107.
| Eremophila glabra reduces methane production and methanogen populations when fermented in a rusitec.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVKis7nM&md5=aa5eceb9d4dad2d5e524222dec50a9c0CAS |
Lovett D, Lovell S, Stack L, Callan J, Finlay M, Conolly J, O’Mara FP (2003) Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers. Livestock Production Science 84, 135–146.
| Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers.Crossref | GoogleScholarGoogle Scholar |
Lovett DK, Shalloo L, Dillon P, O’Mara FP (2006) A systems approach to quantify greenhouse gas fluxes from dairy production as affected by management regimen. Agricultural Systems 88, 156–179.
| A systems approach to quantify greenhouse gas fluxes from dairy production as affected by management regimen.Crossref | GoogleScholarGoogle Scholar |
Lund P, Dahl R, Yang HJ, Hellwing ALF, Cao BB, Weisbjerg MR (2014) The acute effect of addition of nitrate on in vitro and in vivo methane emission in dairy cows. Animal Production Science 54, 1432–1435.
Machado L, Magnusson M, Paul NA, de Nys R, Tomkins N (2014) Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One 9, e85289
| Effects of marine and freshwater macroalgae on in vitro total gas and methane production.Crossref | GoogleScholarGoogle Scholar | 24465524PubMed |
Martin C, Morgavi DP, Doreau M (2010) Methane mitigation in ruminants: from microbe to the farm scale. Animal 4, 351–365.
| Methane mitigation in ruminants: from microbe to the farm scale.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslWgs7k%3D&md5=ec86c02a2eac08f6c5be320152290e71CAS | 22443940PubMed |
McNaughton LR, Berry DP, Clark H, Pinares-Patiño CS, Harcourt S, Spelman RJ (2005) Factors affecting methane production in Friesian × Jersey dairy cattle. Proceedings of the New Zealand Society of Animal Production 65, 352–355.
Meale SJ, McAllister TA, Beauchemin KA, Harstad OM, Chaves AV (2012) Strategies to reduce greenhouse gases from ruminant livestock. Acta Agriculturae Scandinavica. Section A. Animal Science 62, 199–211.
| Strategies to reduce greenhouse gases from ruminant livestock.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVCrurs%3D&md5=e8bdb4eef95b6d70bb7c9dccf78b72e1CAS |
Meale SJ, Chaves AV, Hannah MC, Williams SRO, Hume DE, Mace WJ, Moate PJ (2013) Comparison of wild type, AR1 and AR37 endophyte infected perennial ryegrass on in vitro methanogenesis. Animal Feed Science and Technology 180, 10–17.
| Comparison of wild type, AR1 and AR37 endophyte infected perennial ryegrass on in vitro methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Mills JAN, Dijkstra J, Bannink A, Cammell SB, Kebreab E, France J (2001) A mechanistic model of whole-tract and methanogenesis in the lactating dairy cow: model development, evaluation, and application. Journal of Animal Science 79, 1584–1597.
Mills JAN, Kebreab E, Yates CM, Crompton LA, Cammell SB, Dhanoa MS, Agnew RE, France J (2003) Alternative approaches to predicting methane emissions from dairy cows. Journal of Animal Science 81, 3141–3150.
Ministry for the Environment (2014) ‘New Zealand’s greenhouse gas inventory 1990–2012.’ (New Zealand Ministry for the Environment: Wellington, New Zealand) Available at http://www.mfe.govt.nz/sites/default/files/media/Climate%20Change/ghg-inventory-1990-2012.pdf [Verified 26 March 2015]
Moate PJ, Clarke T, Davis LH, Laby RH (1997) Rumen gases and bloat in grazing dairy cows. The Journal of Agricultural Science 129, 459–469.
| Rumen gases and bloat in grazing dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXot1OmtA%3D%3D&md5=c9fb934838a72c7d86783705f74309b8CAS |
Moate PJ, Williams SRO, Grainger G, Hannah MC, Ponnampalam EN, Eckard RJ (2011) Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows. Journal of Animal Feed Science and Technology 166–167, 254–264.
| Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows.Crossref | GoogleScholarGoogle Scholar |
Moate PJ, Williams SRO, Deighton MH, Wales WJ (2012) A comparison between wheat or maize grain fed as a high proportion of the diet on milk production and methane emissions from dairy cows. In ‘Proceedings of the 5th Australasian dairy science symposium’, 13–15 November, Melbourne, Australia. (Ed. J Jacobs) pp. 452–453. (The Australasian Dairy Science Symposium Committee: Melbourne)
Moate PJ, Williams SRO, Torok VA, Hannah MC, Eckard RJ, Auldist MJ, Ribaux BE, Jacobs JL, Wales WJ (2013) Effects of feeding algal meal high in docosahexanoic acid on feed intake milk production and methane emissions in dairy cows. Journal of Dairy Science 96, 3177–3188.
| Effects of feeding algal meal high in docosahexanoic acid on feed intake milk production and methane emissions in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktFaksb8%3D&md5=21a1a908e2ecf8ba151909af8b58a990CAS | 23498011PubMed |
Moate PJ, Williams SRO, Deighton MH, Wales WJ, Jacobs JL (2014a) Supplementary feeding of wheat to cows fed harvested pasture increases milk production and reduces methane yield. In ‘Proceedings of the 6th Australasian dairy science symposium’, 19–21 November, Hamilton, New Zealand. (Ed. J Roche) pp. 176–178. (The Australasian Dairy Science Symposium Committee: Hamilton, New Zealand)
Moate PJ, Williams SRO, Torok VA, Hannah MC, Ribaux BE, Tavendale MH, Eckard RJ, Jacobs JL, Auldist MJ, Wales WJ (2014b) Grape marc reduces methane emissions when fed to dairy cows. Journal of Dairy Science 97, 5073–5087.
| Grape marc reduces methane emissions when fed to dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVCjur3E&md5=a39281ae168116e8c364f9bc9f48e006CAS | 24952778PubMed |
Moate PJ, Williams SRO, Deighton MH, Wales WJ, Jacobs JL (2014c) Effects of white and red grape marcs on milk production and methane emissions from dairy cows. In ‘Proceedings of the 6th Australasian dairy science symposium’, 19–21 November, Hamilton, New Zealand. (Ed. J Roche) pp. 174–175. (The Australasian Dairy Science Symposium Committee: Hamilton, New Zealand)
Moate PJ, Williams SRO, Deighton MH, Pryce JE, Hayes BJ, Jacobs JL, Eckard RJ, Hannah MC, Wales WJ (2014d) Mitigation of enteric methane emissions from the Australian dairy industry. In ‘Proceedings of the 6th Australasian dairy science symposium’, 19–21 November, Hamilton, New Zealand. (Ed. J Roche) pp. 121–140. (The Australasian Dairy Science Symposium Committee: Hamilton, New Zealand)
Moate PJ, Deighton MH, Ribaux BE, Hannah MC, Wales WJ, Williams SRO (2015) Michaelis–Menten kinetics predict the rate of SF6 release from permeation tubes used to estimate methane emissions from ruminants. Animal Feed Science and Technology 200, 47–56.
| Michaelis–Menten kinetics predict the rate of SF6 release from permeation tubes used to estimate methane emissions from ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1Oqt7w%3D&md5=647b8ec3585647d002351f17d1a93526CAS |
Montes F, Meinen R, Dell C, Rotz A, Hristov AN, Oh J, Waghorn G, Gerber PJ, Henderson B, Makkar HPS, Dijkstra J (2013) Mitigation of methane and nitrous oxide emissions from animal operations II. A review of manure management mitigation options. Journal of Animal Science 91, 5070–5094.
| Mitigation of methane and nitrous oxide emissions from animal operations II. A review of manure management mitigation options.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKktrvN&md5=f54f33946a939b2b6e2788acc77013ccCAS | 24045493PubMed |
Mowrey A, Spain JN (1999) Results of a nationwide survey to determine feedstuffs fed to lactating dairy cows. Journal of Dairy Science 82, 445–451.
| Results of a nationwide survey to determine feedstuffs fed to lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhvVWhtrY%3D&md5=a63c814d60a68567ea9e4b8a84fefe23CAS | 10068966PubMed |
Muñoz C, Yan T, Will DA, Murray S, Gordon AW (2012) Comparison of the sulphur hexafluoride tracer and respiration chamber techniques for estimating methane emissions and correction for rectum methane output from dairy cows. Journal of Dairy Science 95, 3139–3148.
| Comparison of the sulphur hexafluoride tracer and respiration chamber techniques for estimating methane emissions and correction for rectum methane output from dairy cows.Crossref | GoogleScholarGoogle Scholar | 22612950PubMed |
Nour M, Berean K, Balendhran S, Ou JZ, Du Plessis J, Mc Sweeney C, Bhaskaran M, Sriram S, Kalantar-zadeh K (2013) CNT/PDMS composite membranes for H2 and CH4 gas separation. International Journal of Hydrogen Energy 38, 10494–10501.
| CNT/PDMS composite membranes for H2 and CH4 gas separation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVyktrbI&md5=8c9e9a7d9405d669f20db2bb260b01f9CAS |
Nour M, Berean K, Chrimes A, Zoolfakar AS, Latham K, McSweeney C, Field MR, Sriram S, Kalantar-zadeh K, Ou JZ (2014) Silver nanoparticle/PDMS nanocomposite catalytic membranes for H2S gas removal. Journal of Membrane Science 470, 346–355.
| Silver nanoparticle/PDMS nanocomposite catalytic membranes for H2S gas removal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlWmtL%2FF&md5=9f5a80a0e38b548214390a0cfb1f5954CAS |
O’Neill BF, Deighton MH, O’Loughlin BM, Mulligan FJ, Boland TM, O’Donovan M, Lewis E (2011) Effects of a perennial ryegrass diet or total mixed ration diet offered to spring-calving Holstein-Friesian dairy cows on methane emissions, dry matter intake, and milk production. Journal of Dairy Science 94, 1941–1951.
| Effects of a perennial ryegrass diet or total mixed ration diet offered to spring-calving Holstein-Friesian dairy cows on methane emissions, dry matter intake, and milk production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFChtbg%3D&md5=b3868bb95e45b1003223edbe3f91638fCAS | 21426985PubMed |
O’Neill BF, Deighton MH, O’Loughlin BM, Galvin N, O’Donovan M, Lewis E (2012) The effects of supplementing grazing dairy cows with partial mixed ration on enteric methane emissions and milk production during mid to late lactation. Journal of Dairy Science 95, 6582–6590.
| The effects of supplementing grazing dairy cows with partial mixed ration on enteric methane emissions and milk production during mid to late lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFCksrrK&md5=b486379e0690a4df4bbb1097f0c13106CAS | 22959938PubMed |
Odongo NE, Bagg R, Vessie G, Dick P, Or-Rashid MM, Hook SE, Gray JT, Kebreab E, France J, McBride BW (2007) Long-term effects of feeding monensin on methane production in lactating dairy cows. Journal of Dairy Science 90, 1781–1788.
| Long-term effects of feeding monensin on methane production in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjs1ahs78%3D&md5=31324755cd8a3d6c6ac2e23e0f94ccafCAS | 17369219PubMed |
Pacheco D, Waghorn G, Janssen PH (2014) Decreasing methane emissions from ruminants grazing forages: a fit with productive and financial realities. Animal Production Science 54, 1141–1154.
Pinares-Patiño CS, Holmes CW, Lassey KR, Ulyatt MJ (2008) Measurement of methane emission from sheep by the sulphur hexafluoride tracer technique and by the calorimetric chamber: failure and success. Animal 2, 141–148.
| Measurement of methane emission from sheep by the sulphur hexafluoride tracer technique and by the calorimetric chamber: failure and success.Crossref | GoogleScholarGoogle Scholar | 22444973PubMed |
Powers W, Auvermann B, Cole A, Gooch C, Grant R, Hatfield J, Hunt P, Johnson K, Leytem A, Liao W, Powell JM (2014) Chapter 5: quantifying greenhouse gas sources and sinks in animal production systems. In ‘Quantifying greenhouse gas fluxes in agriculture and forestry: methods for entity‐scale inventory’. Technical Bulletin Number 1939, July 2014. (Eds M Eve, D Pape, M Flugge, R Steele, D Man, M Riley‐Gilbert, S Biggar) pp. 1–160. (Office of the Chief Economist, US Department of Agriculture: Washington, DC) Available at http://www.usda.gov/oce/climate_change/Quantifying_GHG/Chapter5S.pdf [Verified 26 March 2015]
Pryce JE, Arias J, Bowman PJ, Davis SR, Macdonald KA, Waghorn GC, Wales WJ, Williams YJ, Spelman RJ, Hayes BJ (2012) Accuracy of genomic predictions of residual feed intake and 250 day bodyweight in growing heifers using 625 000 SNP markers. Journal of Dairy Science 95, 2108–2119.
| Accuracy of genomic predictions of residual feed intake and 250 day bodyweight in growing heifers using 625 000 SNP markers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkslejtLg%3D&md5=df294ca2769b5499bf5b52066d14c53dCAS | 22459856PubMed |
Pryce JE, Wales WJ, De Haas Y, Veerkamp RF, Hayes BJ (2014) Genomic selection for feed efficiency in dairy cattle. Animal 8, 1–10.
| Genomic selection for feed efficiency in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c%2FltVehsQ%3D%3D&md5=cf2dad62b3efd96c28db89db1c001f19CAS | 24128704PubMed |
Queensland Government (2009) ‘Wheat quality and markets in Queensland.’ (Queensland Government Department of Primary Industries and Fisheries: Brisbane, Qld) Available at http://www.nvtonline.com.au/wp-content/uploads/2013/03/Crop-Guide-QLD-Wheat-Quality-Markets-in-QLD.pdf [Verified 26 March 2015]
Reis LG, Chaves AV, Williams SRO, Moate PJ (2014) Comparison of enantiomers of organic acids for their effects on methane production in vitro. Animal Production Science 54, 1345–1349.
Ross EM, Moate PJ, Bath CR, Davidson S, Sawbridge TI, Guthridge KM, Cocks BG, Hayes BJ (2012) High throughput whole rumen metagenome profiling using untargeted massively parallel sequencing. BMC Genetics 13, 53
| High throughput whole rumen metagenome profiling using untargeted massively parallel sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslWjt77E&md5=525c69761bb84853ff16d6f39174c260CAS | 22747657PubMed |
Ross EM, Moate PJ, Marett L, Cocks BG, Hayes BJ (2013a) Investigating the effect of two methane-mitigating diets on the rumen microbiome using massively parallel sequencing. Journal of Dairy Science 96, 6030–6046.
| Investigating the effect of two methane-mitigating diets on the rumen microbiome using massively parallel sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFaksb3O&md5=62ca31ef3232b14287aa45c3d867aab8CAS | 23871375PubMed |
Ross EM, Moate PJ, Marett L, Cocks BG, Hayes BJ (2013b) Metagenomic predictions: from microbiome to complex health and environmental phenotypes in humans and cattle. PLoS One 8, e73056
| Metagenomic predictions: from microbiome to complex health and environmental phenotypes in humans and cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVCrsrnE&md5=68e06f22f65594eafcb063e62c637e0dCAS | 24023808PubMed |
Ross EM, Petrovski S, Moate PJ, Hayes BJ (2013c) Metagenomics of rumen bacteriophage from thirteen lactating dairy cows. BMC Microbiology 13, 242
| Metagenomics of rumen bacteriophage from thirteen lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 24180266PubMed |
Sauvant D, Giger-Reverdin S, Serment A, Broudiscou L (2011) Influences des régimes et e leur fermentation dans le rumen sur la production de méthane par les ruminants. INRA Production Animaux 24, 433–446.
Shi W, Moon CD, Leahy SC, Kang D, Froula J, Kittelmann S, Fan C, Deutsch S, Gagic D, Seedorf H, Kelly WJ, Atua R, Sang C, Soni P, Li D, Pinares-Patiño CS, McEwan JC, Janssen PH, Chen F, Visel A, Wang Z, Attwood GT, Rubin EM (2014) Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Research 24, 1517–1525.
| Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFaksLnJ&md5=e8d4dea7e69d170c1dc6a9041fdd6591CAS | 24907284PubMed |
Spelman R, Hayes BJ, Berry DP (2013) Use of molecular technologies for the advancement of animal breeding: genomic selection in dairy cattle populations in Australia, Ireland and New Zealand. Animal Production Science 53, 869–875.
Storm IMLD, Hellwing ALF, Nielsen NI, Madsen J (2012) Methods for measuring and estimating methane emission from ruminants. Animals 2, 160–183.
| Methods for measuring and estimating methane emission from ruminants.Crossref | GoogleScholarGoogle Scholar |
Sun XZ, Waghorn GC, Hoskin SO, Harrison SJ, Muetzel S, Pacheco D (2012) Methane emissions from sheep fed fresh brassicas (Brassica spp.) compared to perennial ryegrass (Lolium perenne). Animal Feed Science and Technology 176, 107–116.
| Methane emissions from sheep fed fresh brassicas (Brassica spp.) compared to perennial ryegrass (Lolium perenne).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVykt7zE&md5=b908b1ae99302041ab450a1ee3c0c8d9CAS |
Trei JE, Scott GC, Parish RC (1972) Influence of methane inhibition on energetic efficiency of lambs. Journal of Animal Science 34, 510–515.
van Vugt SJ, Waghorn GC, Clark DA, Woodward SL (2005) Impact of monensin on methane production and performance of cows fed forage diets. Proceedings of the New Zealand Society of Animal Production 65, 362–366.
van Zijderveld SM, Gerrits WJJ, Dijkstra J, Newbold JR, Hulshof RBA, Perdok HB (2011) Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. Journal of Dairy Science 94, 4028–4038.
| Persistency of methane mitigation by dietary nitrate supplementation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsVylur4%3D&md5=084e8f16cfadbcd6b4d0d69aceee4a28CAS | 21787938PubMed |
VandeHaar MJ, St-Pierre N (2006) Major advances in nutrition: relevance to the sustainability of the dairy industry. Journal of Dairy Science 89, 1280–1291.
| Major advances in nutrition: relevance to the sustainability of the dairy industry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjt1Sjsb4%3D&md5=fa87b0dd58b46b57c3a928059d054aaeCAS | 16537960PubMed |
Vanlierde A, Dehareng F, Froidmont E, Dardenne P, Kandel PB, Gengler N, Deighton MH, Buckley F, Lewis E, McParland S, Berry D, Soyeurt H (2013) Prediction of individual enteric methane emission of dairy cows from milk mid-infrared spectra. In ‘Advances in animal biosciences: proceedings of the 5th greenhouse gases and animal agriculture conference (GGAA 2013)’, Dublin, Ireland. Vol. 4. Part 2. p. 433. (Cambridge University Press: Cambridge, UK)
Velazco JI, Cottle DJ, Hegarty RS (2014) Methane emissions and feeding behaviour of feedlot cattle supplemented with nitrate or urea. Animal Production Science 54, 1737–1740.
| Methane emissions and feeding behaviour of feedlot cattle supplemented with nitrate or urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVWjtrbJ&md5=8df245dd6f25be9d9ccdb5254d07f32aCAS |
Vlaming JB, Lopez-Villalobos N, Brookes IM, Hoskin SO, Clark H (2008) Within- and between-animal variance in methane emissions in non-lactating dairy cows. Australian Journal of Experimental Agriculture 48, 124–127.
| Within- and between-animal variance in methane emissions in non-lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovV2r&md5=035aa3dca45df0a0601781fbc3fb571eCAS |
Volpe V, Boston RC, Susmel P, Moate PJ, France J, Dijkstra J (2005) Chapter 7. The use of WinSAAM for modelling complex ruminal metabolism. In ‘Mathematical modeling in nutrition and toxicology’. (Eds JL Hargrove, CD Berdanier) pp. 111–139. (Mathematical Biology Press: Athens, GA)
VSN International (2014) ‘Genstat for Windows.’ 17th edn. (VSN International: Hemel Hempstead, UK) Available at http://www.genstat.co.uk [Verified 1 May 2015]
Waghorn GC, Hegarty RH (2011) Lowering ruminant methane emissions through improved feed conversion efficiency. Animal Feed Science and Technology 166–167, 291–301.
| Lowering ruminant methane emissions through improved feed conversion efficiency.Crossref | GoogleScholarGoogle Scholar |
Waghorn GC, Clark H, Taufa V, Cavanagh A (2008) Monensin controlled release capsules for mitigating methane in dairy cows fed pasture. Australian Journal of Experimental Agriculture 48, 65–68.
| Monensin controlled release capsules for mitigating methane in dairy cows fed pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFSn&md5=ae70b030418375e174dc85682d3a98beCAS |
Wedlock DN, Janssen PH, Leahy SC, Shu D, Buddle BM (2013) Progress in the development of vaccines against rumen methanogens. Animal 7, 244–252.
| Progress in the development of vaccines against rumen methanogens.Crossref | GoogleScholarGoogle Scholar | 23739467PubMed |
Wilkerson VA, Casper DP (1995) The prediction of methane production of Holstein cows by several equations. Journal of Dairy Science 78, 2402–2414.
| The prediction of methane production of Holstein cows by several equations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtFOrtA%3D%3D&md5=ac345018e88bf971f330f478c16be111CAS | 8747332PubMed |
Wilkinson JM (2011) Re-defining efficiency of feed use by livestock. Animal 5, 1014–1022.
| Re-defining efficiency of feed use by livestock.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vovV2juw%3D%3D&md5=2453a784824a74bc9c59ff84b1e62846CAS | 22440097PubMed |
Williams SRO, Moate PJ, Hannah MC, Ribaux BE, Wales WJ, Eckard RJ (2011a) Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: a critical evaluation of the SF6 procedure. Animal Feed Science and Technology 170, 265–276.
| Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: a critical evaluation of the SF6 procedure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVOrurfJ&md5=85f85eb1fe748fee8404f1fb82ef05f2CAS |
Williams YJ, Pryce JE, Grainger C, Wales WJ, Linden N, Porker M, Hayes BJ (2011b) Variation in residual feed intake in Holstein-Friesian dairy heifers in southern Australia. Journal of Dairy Science 94, 4715–4725.
| Variation in residual feed intake in Holstein-Friesian dairy heifers in southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtV2gsb%2FE&md5=20f69a7039a4d92a41a4384d45518b6dCAS | 21854946PubMed |
Williams SRO, Clarke T, Hannah MC, Marrett LC, Moate PJ, Auldist MJ, Wales WJ (2013) Energy partitioning in herbage-fed dairy cows offered supplementary grain during an extended lactation. Journal of Dairy Science 96, 484–494.
| Energy partitioning in herbage-fed dairy cows offered supplementary grain during an extended lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvV2ktbfL&md5=104899176baf949ec2f83d7957d31aa8CAS |
Williams SRO, Fisher PD, Berrisford T, Moate PJ, Reynard K (2014a) Reducing methane on-farm by feeding diets high in fat may not always reduce life cycle greenhouse gas emissions. The International Journal of Life Cycle Assessment 19, 69–78.
| Reducing methane on-farm by feeding diets high in fat may not always reduce life cycle greenhouse gas emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXps1Squw%3D%3D&md5=e3f2d383d89ec6ce54c63c35402b785bCAS |
Williams SRO, Deighton MH, Jacobs JL, Wales WJ, Moate PJ (2014b) Almond hulls and citrus pulp can be used as an alternative source of fibre for dairy cows but neither has any methane mitigation potential. In ‘Proceedings of the 6th Australasian dairy science symposium’, 19–21 November, Hamilton, New Zealand. (Ed. J Roche) pp. 273–275. (The Australasian Dairy Science Symposium Committee: Hamilton, New Zealand)
Williams SRO, Moate PJ, Deighton MH, Hannah MC, Wales WJ (2014c) Methane emissions of dairy cows cannot be predicted by the concentrations of C8:0 and total C18 fatty acids in milk. Animal Production Science 54, 1757–1761.
| Methane emissions of dairy cows cannot be predicted by the concentrations of C8:0 and total C18 fatty acids in milk.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVWjtrfI&md5=5cf97ab5d9857756bff27e4d15e7d564CAS |
Wims CM, Deighton MH, Lewis E, O’Loughlin B, Delaby L, Boland TM, O’Donovan M (2010) Effect of pregrazing herbage mass on methane production, dry matter intake, and milk production of grazing dairy cows during the mid-season period. Journal of Dairy Science 93, 4976–4985.
| Effect of pregrazing herbage mass on methane production, dry matter intake, and milk production of grazing dairy cows during the mid-season period.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFKmsb%2FN&md5=636203d59bad529279315f5c8aa68fbfCAS | 20855032PubMed |
Woodward SL, Waghorn GC, Lassey KR, Laboyrie PG (2002) Does feeding sulla (Hedysarum coronarium) reduce methane emissions from dairy cows? Proceedings of the New Zealand Society of Animal Production 62, 227–230.
Woodward SL, Waghorn GC, Laboyrie PG (2004) Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proceedings of the New Zealand Society of Animal Production 64, 160–164.
Yan T, Mayne CS, Gordon FG, Porter MG, Agnew RE, Patterson DC, Ferris CP, Kilpatrick DJ (2010) mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating cows. Journal of Dairy Science 93, 2630–2638.
| mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVahs7nJ&md5=d85c8daebef15c16d20a4f99876e7372CAS | 20494172PubMed |
Young W, Hwang K, McDonald S, Oates CJ (2010) Sustainable consumption: green consumer behaviour when purchacing products. Sustainable Development 18, 20–31.
Zehetmeier M, Gandorfer M, Hoffmann H, Müller UK, De Boer IJM, Heißenhuber A (2014a) The impact of uncertainties on predicted greenhouse gas emissions of dairy cow production systems. Journal of Cleaner Production 73, 116–124.
| The impact of uncertainties on predicted greenhouse gas emissions of dairy cow production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslerur3J&md5=e74d5182392d660289a4fcc1b9eb3ac8CAS |
Zehetmeier M, Hoffmann H, Sauer J, Hofmann G, Dorfner G, O’Brien D (2014b) A dominance analysis of greenhouse gas emissions, beef output and land use of German dairy farms. Agricultural Systems 129, 55–67.
| A dominance analysis of greenhouse gas emissions, beef output and land use of German dairy farms.Crossref | GoogleScholarGoogle Scholar |
Zimmerman PR (1993) System for measuring metabolic gas emissions from animals. Patent no. 5 265 618. United States Patent and Trademark Office, Alexandria, VA.
Zimmerman PR (2011) ‘Method and system for monitoring and reducing ruminant methane production.’ Patent no. 7 966 971 B2. United States Patent and Trademark Office, Alexandria, VA.



