Resilience in farm animals: biology, management, breeding and implications for animal welfare
Ian G. Colditz A B and Brad C. Hine AA CSIRO Agriculture, Locked Bag 1, Post Office, Armidale, NSW 2350, Australia.
B Corresponding author. Email: ian.colditz@csiro.au
Animal Production Science 56(12) 1961-1983 https://doi.org/10.1071/AN15297
Submitted: 11 June 2015 Accepted: 27 September 2015 Published: 18 February 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
A capacity for the animal to recover quickly from the impact of physical and social stressors and disease challenges is likely to improve evolutionary fitness of wild species and welfare and performance of farm animals. Salience and valence of stimuli sensed through neurosensors, chemosensors and immunosensors are perceived and integrated centrally to generate emotions and engage physiological, behavioural, immune, cognitive and morphological responses that defend against noxious challenges. These responses can be refined through experience to provide anticipatory and learned reactions at lower cost than innate less-specific reactions. Influences of behaviour type, coping style, and affective state and the relationships between immune responsiveness, disease resistance and resilience are reviewed. We define resilience as the capacity of animals to cope with short-term perturbations in their environment and return rapidly to their pre-challenge status. It is manifested in response to episodic, sporadic or situation-specific attributes of the environment and can be optimised via facultative learning by the individual. It is a comparative measure of differences between individuals in the outcomes that follow exposure to potentially adverse situations. In contrast, robustness is the capacity to maintain productivity in a wide range of environments without compromising reproduction, health and wellbeing. Robustness is manifested in response to persistent or cyclical attributes of the environment and is effected via activity of innate regulatory pathways. We suggest that for farm animals, husbandry practices that incorporate physical and social stressors and interactions with humans such as weaning, change of housing, and introduction to the milking parlour can be used to characterise resilience phenotypes. In these settings, resilience is likely to be more readily identified through the rate of return of variables to pre-challenge or normal status rather than through measuring the activity of diverse stress response and adaptation mechanisms. Our strategy for phenotyping resilience of sheep and cattle during weaning is described. Opportunities are examined to increase resilience through genetic selection and through improved management practices that provide emotional and cognitive enrichment and stress inoculation.
Additional keywords: affective state, allostasis, animal behaviour, animal temperament, animal welfare, disease resistance, genetic selection, homeostasis, resilience, robustness, stress inoculation.
‘Well,’ said Pooh, ‘what I like best,’ and then he had to stop and think. Because although Eating Honey was a very good thing to do, there was a moment just before you began to eat it which was better than when you were, but he didn’t know what it was called. A.A. Milne, Winnie-the-Pooh
1. Introduction
During its development and during later life, each animal is exposed to a diversity of stimuli arising from its internal and external environments. It is desirable for the animal’s welfare, and for its commercial productivity and environmental fitness that it has a capacity to cope with these challenges and to bounce back rapidly when insults to its integrity occur. Although factors influencing the development of phenotype have been a focus of research for over a century (Strandberg 2009), the past decade has seen a substantial increase in interest in resilience and robustness of animals to environmental effects (Klopcic et al. 2009; Hermesch and Dominik 2014). Resilience to the effects of parasitic disease in farm animals was first recognised 80 years ago by Clunies Ross (1932), yet, in recent years, a broader concept of resilience has emerged in animal and human sciences that encompasses not only the response of the individual to disease challenges but also the individual’s response to environmental and social stressors (Russo et al. 2012; Wu et al. 2013; Hermesch and Dominik 2014). Therefore, in Section 2 of this review, we examine the biological processes whereby animals respond to signals from their internal and external environments, the influence of personality, emotion and cognitive functions of the animal on these responses, how responses to stimuli are regulated and what happens when signals are noxious or overtax the capacity of the animal to cope. Two patterns of environmental challenges are identified. The first are episodic, sporadic or situation-specific challenges that evoke acute stress responses and accommodation via facultative learning. The second are persistent or cyclical challenges that the animal adjusts to via innate regulatory pathways. From this biological background, we examine in Section 3, the current concepts of resilience and robustness, and suggest a broader definition of resilience that encompasses the animal’s capacity to cope with environmental, social and disease challenges. We propose that resilience can be described as the capacity of the animal to be minimally affected by a disturbance or to rapidly return to the physiological, behavioural, cognitive, health, affective and production states that pertained before exposure to a disturbance. In the remainder of the review, we examine approaches for managing and breeding animals to improve resilience.
2. Sensing, reacting to and coping with the environment
The interactions of an animal with its environment are central to the concept of resilience and it is the biology of these responses that are explored below. Readers with little interest in the biological concepts underlying resilience could proceed directly to Section 2.8 for a summary of this section.
2.1. Sensing environmental stimuli
Sensing the environment is the first step by which an animal becomes aware of potential threats to its integrity. More broadly, the animal interacts with its environment so it can gather resources and can express functions and activities that contribute to its life history. To engage in these actions, the animal requires information about the environment it occupies, including its internal environment. Information from these environments is gained via three main media: (1) sensors in the peripheral nervous system, which transmit information to the central nervous system via axons; (2) receptors throughout the body, which respond to chemical and hormonal stimuli and communicate with the host cell bearing the sensor, with neighbouring cells, with distant organs and with the central nervous system via autocrine, paracrine and endocrine messages that are distributed throughout the body by interstitial fluid, lymph, cerebrospinal fluid and blood; and (3) the immune system. These can be abbreviated to neurosensors, chemosensors and immunosensors.
In a broad ranging classification of neurosensors, Sherrington (1900) recognised five sense modalities: teloreception (vision and hearing), proprioception (limb position), exterioception (touch, pain and temperature), chemoreception (taste and smell) and interoception (visceral sense). Stimulation of sensors in the sense organs (e.g. eye, inner ear) or on axons in peripheral tissues (e.g. stretch receptors in the rumen wall) invokes action potentials, which convey signals via afferent nerves to the central nervous system where sensations are perceived. New sensibilities of these sensors, such as a primary taste sensibility for fat in humans (Keast and Constanzo 2015), continue to be found. Among these senses, the interoceptive sense is least well characterised. Although it was recognised by Sherrington in 1900, the neuroanatomical pathways for interoception have only been identified in recent years, and it is now considered that interoception provides a sense of the physiological condition of the entire body rather than being limited to sensing visceral organs (Craig 2002). This interoceptive sense has also been called the homeostatic sense and may provide a sense of somatic self which, at least in humans, provides through self-awareness a sense of ‘how one feels’ (Craig 2002, 2003).
Immunosensors detect the presence of foreign (non-self) molecules through germ line-encoded receptors of the innate immune system and through receptors that are generated throughout life by receptor gene hypermutation in the T and B lymphocytes of the adaptive immune system. The immune system shares with other cells and organs of the body the capacity to communicate via autocrine, paracrine and endocrine pathways and is intimately linked with the autonomic nervous system (Czura and Tracey 2005; Wrona 2006). Chemosensors provide the means for cellular and organ exchange and utilisation of resources and one medium for central regulation of these functions. Importantly, the nutrient economy of the cell is not managed autonomously by the cell but is regulated via extracellular signals received predominantly via chemosensors (Fox et al. 2005).
2.2. Processing environmental stimuli
Sensors need stimulation for the development and ongoing expression of normal functions. Stimulation is particularly important during critical periods in the ontogeny of the sense functions (Wiesel 1982). The concept of critical periods was first developed to describe acquisition of behaviours and social skills (Scott 1962) and is applied now throughout developmental biology and epigenetics in the study of environmental influences on phenotype. For instance, a critical period is seen in the immune system where stimulation during early postnatal life develops immune capabilities and influences response characteristics expressed in later life (Kelly and Coutts 2000) that influence metabolic profiles and disease susceptibility (Tilg and Kaser 2011). The function of sense organs is adapted to an optimal range of stimulus intensity and either too low or too high a rate of stimulation of some types of sensors can have adverse effects for the animal (Wiesel 1982).
Animals respond both to quantitative and qualitative attributes of stimuli. Quantitative attributes include frequency and intensity of stimuli whereas qualitative attributes include salience and valence. The ways that tissues respond at a local level and the way the animal responds at a central level are influenced by the state of the system and by the stream of stimuli it is receiving. The stream of stimuli impinging on the animal creates a context within which an individual stimulus is nested and this context influences the ability of the animal to discriminate a single stimulus from background (the stimulus salience). Thus, context can influence the ability of the animal to attend to and respond to a stimulus. The aversiveness or pleasantness of a stimulus (its valence) is also influenced by context. For instance, the speed with which temperature changes within a constant range influences the extent of emotional and physiological responses in cattle (Desire et al. 2002). A detailed account of how stimulation of thermosensors in skin at a single temperature can be perceived as either pleasant or painful depending of the context of the stimulus is provided by Craig (2002). The state of the system, known as its affective state, can also influence the valence of a stimulus (Mendl et al. 2010), as demonstrated in sheep (Doyle et al. 2010) and as described in more detail in Section 2.5.3.
Individual sensors respond to a limited range of the stimulus spectrum and thus impose an initial filter on the environmental information relayed to the animal. Additional filtration at the sensor level occurs due to sensor response characteristics such as hysteresis, refractory period, co-operativity and blockade by antagonists (Gether and Kobilka 1998; Dumont et al. 2002). Following stimulus sampling and filtration by the sensor, messages from neurosensors and some chemosensors and immunosensors are processed by the central nervous system. Cells in peripheral tissues also process signals from chemosensors and immunosensors. The salience and valence of a stimulus are in part attributes or qualia that arise from the animal’s perception of the stimulus rather than qualities intrinsic to the stimulus or the filtered sample relayed by the sensor. Perception of a stimulus is largely a function of the central nervous system that involves integrating multiple sources of information. Tissue and immune system perception of stimulus salience and valence are also important concepts but will not be pursued here.
2.3. Regulation of responses to environmental stimuli
The need for the animal to maintain a stable internal environment in the face of environmental fluctuations was first recognised by Claude Bernard (Cooper 2008). Walter Cannon subsequently called this process of physiological regulation homeostasis (Cannon 1929). The involvement of behavioural anticipations and behavioural reactions in maintaining the internal environment was first recognised by Pavlov (1904; Smith 2008) and Richter (Woods and Ramsay 2007). With the progressive development of engineering systems during World Wars I and II to improve the targeting of weapons by use of negative feedback to correct errors, it was proposed that animals also use negative feedback to control physiological responses (Rosenblueth et al. 1943). In subsequent decades, negative feedback to control a physiological variable at a fixed set point became the default model of homeostatic regulation (Carpenter 2004). In fact, much more complex mechanisms of physiological regulation occur, and the inaccuracy of a set point model for describing regulation via negative feedback has long been recognised (Carpenter 2004; Cooper 2008). Rather, it is considered that physiological variables are regulated within a range, with excursions above and below the range controlled by various effector mechanisms which often act independently. Importantly, there is no error signal generated by comparison between the current value of the regulated variable and a notional target value or set point (Ramsay and Woods 2014). This model of regulation leads to the concept that the physiological variable is regulated towards a settling range or balancing range rather than a fixed point. Using thermoregulation as an example, a detailed account of the balancing point or balancing range model of regulation is provided by Ramsay and Woods (2014). These authors note that measuring a regulated variable often tells us little about the activity status of the various effector mechanisms that influence the variable. This observation can be generalised to the notion that proximate mechanisms require a different level of enquiry than the ultimate biological functions they act on (Bateson and Gluckman 2011). Thus, physiological processes can be viewed as defending critical functions of the animal, such as nutrient storage in body mass, through multiple effector mechanisms.
2.4. Adaptation to environmental change
The changing nature of environmental conditions requires continual adaptation (acclimatisation) by the animal during the course of its life. Koolhaas et al. (2011) suggest that physiological processes are adapted to a range of values for each environmental variable and that the capacity of the physiological response to adapt to environmental fluctuations is greater towards the centre of this range and diminishes at its periphery. Changing environmental conditions can shift the regulatory range. This is exemplified by the shift in the thermoneutral range of sheep adapted to cold (Webster et al. 1969) and in cattle adapted to heat (Kadzere et al. 2002). When animals are exposed to an increasing heat load their adaptive capacity is reduced and the cost of maintaining normal functionality is increased (Kadzere et al. 2002; Roberts 2007). This is seen for instance in the increased maintenance costs (Collier et al. 2009) and lower milk production in heat-stressed cows than in pair-fed control cows (Rhoads et al. 2007). At a cellular level, microRNA play an important role in reprogramming gene expression during such adaptation (Leung and Sharp 2010). Fig. 1 illustrates the increasing recruitment of adaptive responses in sheep as they attempt to control body temperature during exposure to increasing ambient temperatures.
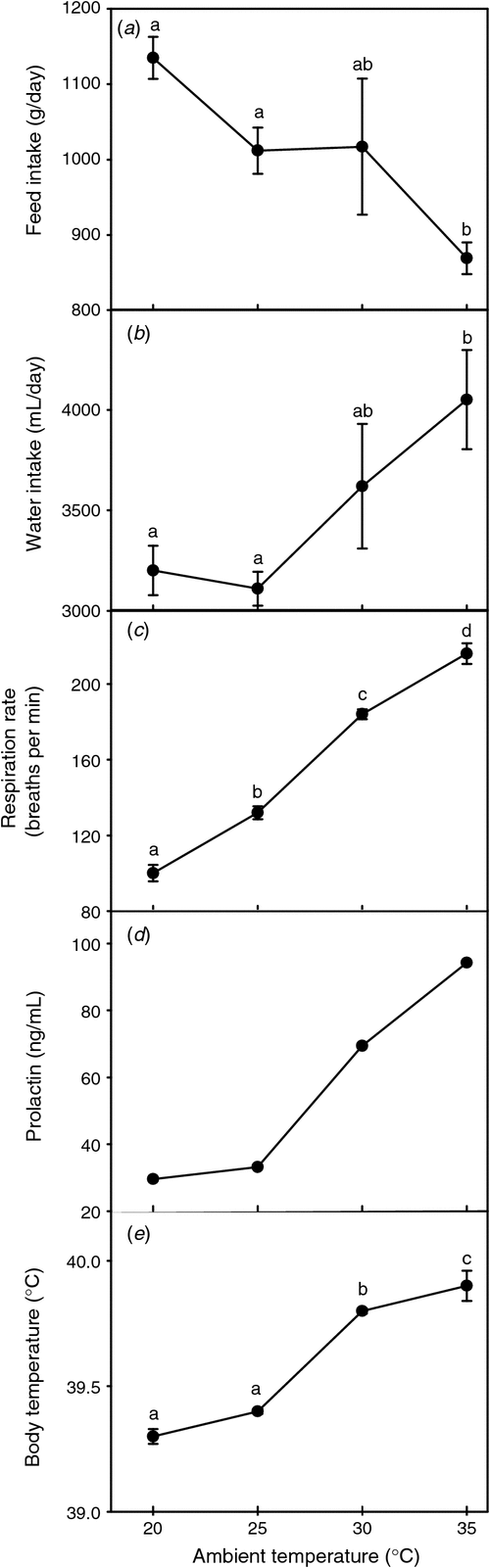
|
The physiological demands of life events such as pregnancy, lactation, migration, and hibernation are coordinated by changes in hormonal activities that alter nutrient utilisation by tissues (Bauman and Currie 1980; Bell 1995; Bell and Bauman 1997). These homeorhetic changes anticipate the demands of changing tissue activities that are linked to cyclical or persistent environmental stimuli such as seasonal changes and the reproductive cycle.
2.5. Sources of variation between individuals in responses to environmental stimuli
2.5.1. Behavioural types
The individual’s response to environmental stimuli is influenced by numerous factors including ontogeny, experience, genetics, age, sex, physiological status, emotional state, cognitive function and season (von Holst 1998; Cockram 2004; Veissier and Miele 2014). Furthermore, individual variation can occur at each level of the response: sensor stimulation, perception, reaction, and outcome (Moberg 2000). Within this underlying variation, an individual animal can show a consistent type of behavioural and physiological response in different challenge situations and on different occasions when exposed to a single type of challenge. When the behavioural response is consistent across time and across situations it is described as a behavioural type, temperament or personality (Sih et al. 2004; MacKay and Haskell 2015). A combination of genetic, physiological and cognitive attributes creates a so called behavioural architecture that supports associations between behaviour type and metabolic rate, cerebral lateralisation and response to stressors (Wolf and Weissing 2012). The position of an individual on several behavioural axes is recognised as temperament traits. Prominent axes for characterising temperament traits are frequently described with terms equating with bold–fearful, exploratory–neophobic, active–inactive, social–asocial, and aggressive–non-aggressive (Réale et al. 2007). Alternatively, temperament is sometimes described as the position on a single factor vector, for example degree of fearfulness, rather than a position on a spectrum between two behavioural antonyms (Mehta and Gosling 2008). Temperament influences several types of responses to environmental stimuli (Stamps and Groothuis 2010) and hence is likely to be one of the factors influencing resilience to potentially adverse challenges.
2.5.2. Coping styles
Correlated behavioural and physiological responses to stressful stimuli have been termed coping styles (von Holst 1998; Koolhaas et al. 1999). Two major coping styles to stressor challenges are proactive and reactive coping. The proactive style is associated with higher behavioural activity, elevated reactivity of the sympathetic autonomic nervous system (SAM) and higher sensitivity of the dopaminergic reward system whereas the reactive coping style displays lower behavioural activity (withdrawal, freezing, less aggression), and high hypothalamic-pituitary-adrenal (HPA) axis and parasympathetic reactivity in stressful situations (Coppens et al. 2010). Importantly, coping style does not equate with success in coping with stressors: the term describes the strategy or processes an animal employs when reacting to stressors rather than the outcome of the coping response (von Holst 1998). The distribution of individuals along the coping style axis can differ between populations and this distribution may have been influenced by selection of farm animals for certain production traits due to genetic correlations between the selected traits and the physiological and behavioural processes underpinning coping styles. For instance, in recent decades, genetic selection in pigs (Knap 2005) and meat sheep (Rowe and Banks 2015) has resulted in reduced carcass fat that in pigs has been accompanied by a reduction in urinary cortisol concentrations (Foury et al. 2009) that might also reflect a change in coping style in the selected population. Furthermore, recent studies indicate that catecholamines like adrenaline produced by the SAM during stress responses stimulate proliferation and virulence of common pathogens of farm animals (Freestone et al. 2008). Thus, coping style, and as we shall see later resilience phenotype, could be closely linked to disease susceptibility in farm animals, whereas the impact of selection for production traits on the SAM/HPA balance noted above could be contributing to increased disease susceptibility in high-production genotypes (Rauw et al. 1998).
Use of environmental resources can differ between behavioural types, as demonstrated by the influence of boldness/shyness on grazing behaviours in sheep (Michelena et al. 2009). Some differences in susceptibility to stress-related disease and some differences in reactivity to immunological stimuli have been noted between coping styles. For instance, sheep with a proactive coping style had a more protracted increase in body temperature, a greater reduction in feed intake, and a smaller increase in cortisol in response to challenge with endotoxin, which is a potent stimulator of the innate immune system, than sheep with a reactive coping style (Lee et al. 2014). Pigs that were classified retrospectively as proactive on the basis of aggression in a test of social confrontation together with resistance in the backtest had greater in vivo and in vitro activity of cell-mediated adaptive immunity and lower antibody responses in the humoral arm of adaptive immunity (Hessing et al. 1995). Interestingly, cell-mediated adaptive immunity was suppressed by weaning stress in the proactive but not the reactive pigs. In a separate study, pigs classified by the backtest as proactive also had lower antibody responses (adaptive immunity) to ovalbumin, keyhole limpet hemocyanin and tetanus toxoid than reactive coping-style pigs (Schrama et al. 1997). It is noteworthy that behavioural tests used in the identification of coping styles are also used to define temperament in farm animals.
2.5.3. Influences of affective states
The operational state of the central nervous system influences the way an animal responds to environmental stimuli. The operational state, more commonly termed the affective state, acts as both gate keeper and modulator of stimuli as they pass from afferent sensory inputs to the efferent outputs that activate the response modalities of the physiological, immune, behavioural, and cognitive systems and tissue morphology (Fig. 2). Just as the passage of light is influenced by the molecular structure and the interface angle as it traverses a prism, processing of stimuli through the central nervous system is influenced by two principal aspects of affective state, its valence and its arousal. As well as modulating outputs, valence and arousal can themselves be influenced by afferent inputs (Mendl et al. 2010). Two important proximate outputs of stimulus modulation by the operational state of the central nervous systems are the affects termed emotions and moods of the animal (Mendl et al. 2010). As well as inputs from the external environment, endogenous stimuli from the animal’s internal environment provide important inputs which influence affective state. For instance, interoceptive inputs (Section 2.1) can generate positive and negative valence and the affect of homeostatic wellness (Craig 2003; Wiens 2005), and certain sensory nerve fibres provide inputs that can generate strong negative valence and the affect of pain (Shackman et al. 2011).
Despite longstanding interest in the expression of emotions in animals (Darwin 1965), acceptance that animals do indeed experience emotions has been slow to develop (Veissier and Miele 2014). Opinions differ on the nature of emotions, their proximate mechanisms and the ultimate functions they serve. An evolutionary role for emotions in enhancing fitness through effects on the animal’s success in acquiring rewards and avoiding punishments has been proposed (Mendl et al. 2010). Emotions can be considered to be transient changes in affect, which can accumulate to create longer-lasting affective states such as moods (Boissy et al. 2007; Mendl et al. 2010; Boissy and Lee 2014).
A valuable insight into the nature of emotions is provided by Barrett (2012) who suggests that the named emotions such as fear, joy, and anger in humans are socially constructed concepts that are learnt as the child matures. Barrett illustrates this proposition in the following manner. Plants exist in the physical world. When viewed by humans, plants can acquire functions such as becoming a weed or becoming a flower that are not intrinsic to their biological activities as plants. The function emerges from the interaction between the physical object (the plant) and the conceptual constructs of the human agent. In like manner she suggests that in humans, the changes in the physiology, behaviour, voice and neural activity that occur during emotional reactions acquire functions through application of the learned and socially shared concepts that we know as acculturated individuals by names like fear, joy and anger. The functions of these named emotions are a property of secondary consciousness (awareness of awareness), which is a human trait that appears to not be shared by farm animals (Hobson 2009). Barrett’s viewpoint has been strongly influenced by her team’s own research and a meta-analysis of numerous studies, which failed to find unique neurophysiological signatures for the individually named emotions (Lindquist et al. 2012). Further exploration of this concept is beyond the scope of the current review, except to say that if Barrett’s proposition pertains, then as animal scientists we may be forcing the observable reactions of the animal to certain environmental challenges into categories (e.g. fear, joy, aggression) that are not functionally discriminated by the animal itself. But this is not to say that Winnie the Pooh did not enjoy the anticipation of eating honey for want of a name to describe the emotion. As we shall see in Section 3, constructing the concepts of resilience and robustness carries this same risk of imposing values-based functions on characteristics of animals where biological functions might not exist.
A conceptual framework for assessing discrete emotions through behavioural testing in farm animals was introduced by Desire et al. (2002). These authors proposed using test paradigms controlling the suddenness, novelty, intrinsic pleasantness, predictability and controllability of stimuli, and then monitoring subsequent neurophysiological, motor and motivation reactions of the animal from which emotional reactions could then be inferred. In addition, test paradigms to assess the impact of valence and arousal dimensions of affective states on cognitive functions of farm animals have been developed (Mendl et al. 2009, 2010). From studies employing these test paradigms in farm animals, and from a very large body of research in laboratory animals, several principles have emerged:
-
Negative affective states can diminish cognitive functions, expression of production traits and health outcomes (Boissy et al. 2007; Doyle et al. 2011).
-
Predictability and controllability of exposure to a stressor can diminish the perceived negative valence of the stressor and subsequent magnitude and negative valence of responses (Greiveldinger et al. 2007, 2009).
-
Anticipation can increase the hedonic value of a reward (Boissy et al. 2007).
-
A negative affective state (frustration) can arise from failure to receive an anticipated hedonic reward or failure to fulfil a motivated behavioural drive (Greiveldinger et al. 2011).
-
The negative value of a diminished reward is perceived to be greater than the positive value of an enhanced reward (Boissy et al. 2007).
Sustained adverse environmental conditions such as heat or cold, and low body mass during periods of limited feed availability or when body reserves are being used to support lactation impose substantial stress on farm animals (Bell and Bauman 1997; Collier et al. 2009; Sordillo and Mavangira 2014). Studies using operant conditioning show that animals will work hard to alleviate heat stress and undernutrition and suggest that these stresses create negative affective states (Roberts 2007; Roche et al. 2009; Stockman et al. 2014). The contribution of negative affective states to the impact of these common production stressors on the animal is an issue deserving more attention. Fig. 3 illustrates the behavioural demand of sheep to alleviate heat stress in an operant conditioning model. In conclusion, affective states influence the perception of stimuli as positive or negative, and also influence health, productivity, and the likelihood of the animal recovering quickly or developing adverse responses to environmental perturbations.
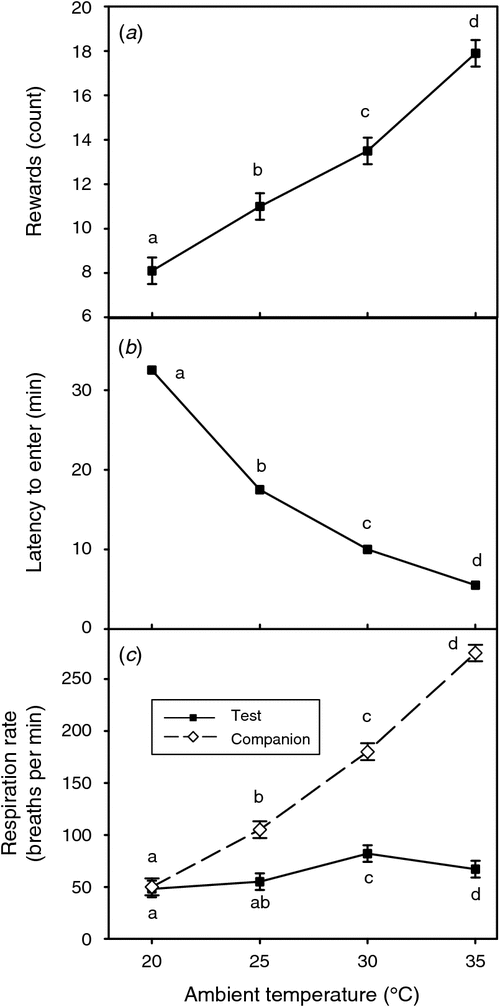
|
2.5.4. Social effects on individual responses
In social species, interactions between individuals and social isolation can be a source of stress and can influence access of the individual to environmental and social resources (Langbein and Puppe 2004; Greenwood et al. 2014). Position of the individual within the social structure of the group can influence physiological and immune responses and disease outcomes (Hessing et al. 1994; Tuchscherer et al. 1998; von Holst 1998). Social conflict has been used in tests of coping style in farm animals (Koolhaas et al. 1999; Lee et al. 2014) and in animal models of human resilience (Russo et al. 2012; Wu et al. 2013). Social conflict provides strong afferent inputs to affective state to influence the response modality of the individual to the stressor (Wu et al. 2013).
2.5.5. Immune response types
A dimensional model is also sometimes used to describe adaptive immune responses to antigenic stimuli. The dimensions are described as Type I and Type II immunity on one axis and the degree of immune responsiveness on the second axis. When faced with a pathogen challenge the body usually mounts an effective and efficient immune response. Some pathogens have devised means by which they enter cells of the body, classified as intracellular pathogens, whereas others remain in the environment external to cells, classified as extracellular pathogens. Elimination of intracellular pathogens generally requires that infected cells be destroyed by phagocytic and cytotoxic immune cells, the action of which can be collectively described as ‘cell-mediated immune responses’. In contrast, extracellular pathogens and soluble antigens are more effectively controlled by ‘antibody-mediated immune responses’. Antibodies act by binding to pathogens and soluble antigens in the extracellular environment, preventing them from damaging or entering cells and tagging them for destruction by immune cells. T-helper (TH) cells, a type of T lymphocyte, play an important role in orchestrating adaptive immune responses by promoting host immune responses tailored to the encountered pathogen. Distinct populations of TH cells, based on their function and the cytokines they secrete, were first described in mice (Mosmann et al. 1986). TH1 cells secrete Type I cytokines, including interferon-γ and interleukin-12, which largely promote cell-mediated immune responses whereas TH2 cells produce Type II cytokines, including IL-4 and IL-5, which largely promote antibody-mediated immune responses. The existence of a similar TH1/TH2 paradigm has been investigated in several farm animal species including chickens (Vandaveer et al. 2001; Erf 2004), pigs (Schmied et al. 2012); (Raymond and Wilkie 2004) and cattle (Estes et al. 1998). Although polarised cell-mediated and antibody-mediated responses to intracellular and extracellular pathogens respectively are observed in these production animals, evidence suggests that the TH1/TH2 paradigm established in mice is an oversimplification of a more complex immunoregulatory framework operating in these species (Brown et al. 1998). Nonetheless, the Type I/Type II paradigm provides a useful framework for assessing immune responses in livestock, with strength of the immune response being the second and perhaps more important dimension to assess. Immune responsiveness influences the outcome of challenges to the animal’s integrity by disease causing organisms and, as discussed in Section 3, is therefore likely to participate in the mechanisms conferring resilience.
2.6. Effects from underload and overload by environmental stimuli
Claude Bernard considered that maintenance of the internal environment was necessary for the efficient functioning of the animal (Cooper 2008). Through studies on the consequences of prolonged exposure to diverse noxious stimuli, Hans Selye proposed that animals exhibit a general adaptation syndrome that is a prelude to diseases of (mal)adaptation, which occur when resistance to the noxious stimuli breaks down (Selye 1936, 1946). The consequences of failure to adapt to a burden of noxious insults impair functioning of the animal. An enormous body of research and conceptualisation since Selye’s early work has attempted to categorise the processes and quantify the costs of progression from maintenance of normal function through stages of dysregulation to final collapse. Following Selye, the early focus of stress research on physical stressors like exercise, tissue damage and toxins addressed physiological reactions and failed to recognise the biological significance of the emotional component of the animal’s response to stressors. This balance was changed by the work of Mason who showed that in a stress challenge paradigm containing psychological and physical components, the greater part of the physiological response was attributable to the psychological component of the stressor (Mason 1971; Veissier and Miele 2014).
For a naïve animal exposed to a novel stressor such as a swim test in rats, responses of the SAM system and HPA axis differ from those seen in experienced rats (Koolhaas et al. 2011). Energy mobilisation in experienced rats more closely matches energy needs during the swim test. Importantly, response variables return to normal more rapidly in experienced rats. Behavioural changes also occur, with Norwegian rats for whom water is a natural habitat, voluntarily entering water once they become experienced with the test. This example provided by Koolhaas et al. (2011) illustrates the progression from non-specific to more specific responses with experience of a stressor. The same phenomenon is seen within the immune system in which innate responses to foreign agents in naïve animals recruit a wide range of non-specific host defence reactions including fever and production of acute phase proteins and pro-inflammatory cytokines, whereas on repeated exposure the response of the adaptive immune system is better focussed on the molecular characteristics of the foreign agent and entrains fewer of the non-specific defence components of the innate immune system (Colditz 2008a). Energy mobilisation and changes in priorities for nutrient utilisation are prominent features of the non-specific defence response (Elsasser et al. 2000; Colditz 2002). Thus, defence of the animal’s integrity by physiological and immune functions share a common strategy of progressing from costly non-specific responses on initial exposure to less costly responses of higher specificity and lower cost on re-exposure to the stressor (Colditz 2008a).
No clear boundary has been identified to denote where responses to stimuli pass from normal maintenance of physiological equilibria into the domain of stress responses. Following Mason, some authors identify a negative emotional state as indicating that a (dis)stress response has occurred (Rushen 1986; Cockram 2004). Several physiological mediators expressed during activation of innate immune responses such as pro-inflammatory cytokines, micro RNA, heat shock proteins and acute phase proteins can also be upregulated by physical and psychological stressors that have no immunological component (Colditz 2008a; Leung and Sharp 2010). Behavioural repertoires change as animals experience an increasing stressor load, with increasing expression of abnormal behaviours in distressed animals (Cockram 2004; Dwyer and Bornett 2004). The commonality of non-specific reactions to novel noxious stimuli perceived by neurosensors, chemosensors and immunosensors is noteworthy. We can characterise these sensors as the afferent arms of host defence. Activation of these sensors by novel stimuli recruits a broad range of responses that generally act with low stimulus specificity to protect the host. Refinement of responses through experience and learning increases the specificity of the responses for eliminating or diminishing the impact of the noxious stressors. Stimulus specificity of host defence responses can be provided by specialised behaviours, anticipatory and adapted physiological responses, by so called affinity maturation of receptors of the adaptive immune system and by cognition. A more nuanced version of Barrett’s model of emotions acquiring functionality in humans might hold that the refinement of the emotional response from an initial shared pool of neurophysiological, physiological and behavioural reactions towards the named functions learned as conceptual constructs improves their utility for dealing with the context of the emotion. These refinements to the effectiveness of host defence through experience can be termed facultative learning in the sense that they are not obligate refinements. Furthermore, the refinements are not necessarily expressed in like manner or to the same degree between individuals. A question arising from this characterisation of host defence is whether a negative affective state occurring during exposure of naïve animals to a novel stressor is an innate protective reaction acting as an advantageous component of non-specific host defence, or whether (as often viewed from the perspective of animal welfare) a negative affective state is a negative outcome whenever it occurs. From an evolutionary perspective, some negative affective states are considered to improve avoidance of threats to the animal (Mendl et al. 2010).
A problem when considering stress responses is to account for maladaptations where system functions settle at suboptimal or deleterious operational states in the absence of any substantial genetic lesion that might predispose the animal to the disease state. The concept of allostasis was introduced in 1988 in part to account for such lifestyle diseases and dysfunctional physiological states that develop under a sustained burden of stressors (Sterling and Eyer 1988; Sterling 2004). Although the validity of the claimed differences between allostasis and homeostasis has been energetically debated (McEwen 1998; McEwen and Wingfield 2003; Woods and Ramsay 2007; Booth 2008; Cooper 2008; Romero et al. 2009; Koolhaas et al. 2011; Sterling 2012; Ramsay and Woods 2014), the discussion has drawn attention to important aspects of physiological regulation and to changes that occur during adaptation (acclimatisation) of the individual to environmental conditions and during chronic stress. The reader is referred to the above references for a history and comparative analysis of the concepts of homeostasis and allostasis.
2.7. A summary of physiological regulation of responses to environmental stimuli
If we step outside the constraints of definitions of regulatory models such as homeostasis, homeorhesis and allostasis, the key points about physiological regulation can be summarised as follows:
-
The valence and salience of stimuli (including stressors) are influenced by the context of the stimulus, and the affective state and residual adaptive capacity of the animal.
-
Physiological variables are regulated towards a balancing point within a balancing range that is reflected in the typical reference intervals for variables used in clinical pathology, for example (Lepherd et al. 2009).
-
Balance is achieved by operation of multiple effectors that defend key functionalities of the animal. For example, during thermoregulation to defend core body temperature, autonomic effectors independently modify metabolism, blood flow to skin, evaporation, and piloerection, whereas behavioural effectors influence respiration, body posture, shade seeking, and so on (Ramsay and Woods 2014).
-
Regulating the internal environment comes at a cost to the animal; however, activation of physiological and behavioural effectors in anticipation of a stimulus or stressor challenge generally reduces this cost.
-
Increments in the stimulus or stressor load cause decrements in the residual capacity to adapt (Koolhaas et al. 2011).
-
Costs increase with increments of the stressor load and/or decrements of residual adaptive capacity.
-
Disturbance of behavioural repertoires, expression of abnormal behaviours and prevalence of negative affective states increase with stressor load.
-
Regulatory behaviours and physiological responses can be influenced by ontogeny and by recent experience.
-
Learned and predictive physiological, behavioural and immune responses generally reduce costs
-
Animals adapt (acclimatise) to a range of environmental conditions. Adaptation leads to a change in the environmental sensitivity of effectors, for example, thermogenesis in sheep.
-
Responses can vary due to characteristics of the individual including ontogeny, behavioural type, social status, affective state, physiological state, gender, age, season, prior experience and cognitive ability.
2.8. Two response patterns
Two patterns emerge from the above description of response and adaptation to environmental stimuli. In the first class, environmental stimuli tend to be sporadic, episodic or situation-specific. Examples include challenges to the animal’s integrity created by infection, social conflict and physical trauma. The stimuli can also be situation-specific such as periodic supplementary feeding or husbandry procedures. Innate responses to these stimuli such as innate immune responses and behavioural characteristics dictated by the animal’s temperament can be optimised through learned coupling of environmental cues with behavioural, physiological and immune responses. Through coupling with environmental cues, the learned responses become predictive and anticipatory and prepare the animal for the impending load it will be exposed to.
The second class of stimuli are provided by cyclical or persistent characteristics of the environment such as circadian and seasonal changes in temperature, light, and pasture availability. Internal stimuli with these characteristics include stimuli associated with reproductive cycles. Environmental cues provided by these stimuli activate innate regulatory pathways such as the hormone pathways directing homeorhetic responses. Adaptation (acclimatisation) to these persistent environmental characteristics occurs principally through adjustment within the adaptive range (allostasis) rather than through facultative learning of new metabolic response patterns. Consistent with the view, we are unaware of evidence that the efficiency of homeorhetic changes is improved by experience within the lifetime of the individual.
In Section 3 we illuminate the distinction between these two classes of stimuli through the analogy of weather and climate.
The affective state of the animal and learned responses in the behavioural, immune and physiological systems influence whether an environmental challenge is perceived as noxious or benign and whether the outcome to the challenge is beneficial, such as rapid recovery from an insult, or detrimental, such as a protracted stress responses. In the next section we examine resilience and robustness to environmental stimuli and propose an approach for identifying animals with a phenotype that confers resilience to the potentially adverse impacts of the first class of environmental stimuli that are commonly experienced within the farm animal production environment.
3. Resilience and robustness
From the perspective of evolutionary biology, traits of an animal have merit in terms of their influence on the flow of genetic and non-genetic (e.g. cultural) information to subsequent generations and their contribution to environmental outcomes such as niche construction and ecological services. For animals within the care and responsibility of humans, it is economic, ethical and aesthetic values rather than measures of evolutionary and environmental influence that are brought to the consideration of the merits of traits and the circumstances under which the animal lives. For farm animals, resilience and robustness are examples of two such conceptual constructs developed from human values. Animal welfare, which bridges aspects of resilience and robustness is an example of a third such values-based conceptual construct (Sørensen et al. 2001). Differentiation between the concepts of resilience and robustness is merited if different values are brought to the concepts or if some differing biological processes underpin their management through environmental manipulation or genetic selection.
3.1. Historical concepts of resilience
Clunies Ross (1932) appears to have been the first to recognise the distinction between resistance to infection, in terms of parasite numbers, and resistance to the impact of infection, in terms of host disease. Although Clunies Ross did not use the term, later studies described the latter phenomenon as disease resilience (Albers et al. 1987; Bisset and Morris 1996; Bishop 2012; Doeschl-Wilson and Lough 2014), which is typically defined in terms such as a capacity to maintain high productivity in the face of ongoing infection (Bisset and Morris 1996; Bishop 2012). More recently, interest in the impact of non-infectious stressors on animal performance has used the term resilience to describe these broader aspects of an animal’s response to environmental challenges (Hermesch and Dominik 2014). In human studies, resilience is described in negative terms as the absence of persistent adverse behavioural, physiological, affective and cognitive outcomes following periods of extreme physical trauma, psychological stress or life threatening situations that in non-resilient individuals are linked to conditions such as post-traumatic stress disorder, depression and other psychiatric conditions (Russo et al. 2012; Wu et al. 2013).
3.2. Models of resilience in humans
Several animal models of the conceptual construct of human resilience have been developed that provide valuable insights for research into resilience in farm animals. Two aspects of resilience are identified in these models: (1) insensitivity or low sensitivity to stimuli found to be noxious by some conspecifics, and (2) adaptive responses involving neurophysiological and behavioural changes (Russo et al. 2012). Animal models use a range of physical (e.g. forced swim test, electric shocks) and psychosocial (e.g. social defeat) stressors and define resilience with negative terms such as subsequent absence of social avoidance, stress-induced hyperthermia, anhedonia (e.g. reduced interest in food rewards or sex), or metabolic syndrome (Russo et al. 2012). Interestingly, even within highly inbred strains of mice drawn from a single cohort, animals exhibiting maladaptive responses (that is, animals lacking resilience) typically comprise a substantial proportion (e.g. 30%) of the study population. This observation provides a hint that even within a highly genetically homogeneous group, a social ecology might be in operation drawing individuals into distinctive resilience phenotypes. Adverse outcomes are increased by lack of ability to control exposure to the stressor including unpredictability of the stressor, and by exposure to some early life stressors. Conversely, resilience is enhanced by ability to predict and control exposure to stressors, by development of behaviours that reduce exposure to stressors, and by graded exposure to some stressors early in life. The latter phenomenon has been termed stress inoculation (Levine 1962). Several genetic factors influencing neuroendocrine and neurotransmitter pathways have also been identified and are discussed below.
3.3. General environmental resilience
In view of the concordance between mechanistic studies in mouse models of human resilience and the biological response pathways to environmental challenges in farm animals reviewed in Section 2, we propose a characterisation of resilience in farm animals that focuses on the outcome of exposure to infectious, social and other environmental stressors. In this synthesis, resilience can be described as the capacity of the animal to be minimally affected by a disturbance or to rapidly return to the physiological, behavioural, cognitive, health, affective and production states that pertained before exposure to a disturbance. This broader characterisation of resilience can be considered to describe general environmental resilience to distinguish it from the narrower concept of disease resilience previously used by others. This formulation encapsulates low sensitivity or lack of sensitivity to disturbances that other conspecifics can find stressful or detrimental, and values a rapid recovery when situations with negative consequences on morphological, physiological, behavioural, cognitive, health, affective or productive functions do occur. The environmental challenges linked with resilience tend to be episodic, sporadic or situation-specific challenges rather than persistent characteristics of the environment. In common with its characterisation in humans (Rutter 2012), resilience is a comparative measure of differences between individuals in the outcomes that follow exposure to potentially adverse situations. In this formulation, biological processes underpinning resilience relate to perception of stimuli and the efficacy of the short-term stress and adaptive responses that ensue in minimising the impact of the stressor. As indicated by studies on responses to environmental stressors in farm animals (reviewed in Section 2) and by studies of resilience in humans, innate mechanisms conferring resilience are likely to be found within sensor sensitivity to environmental stimuli, central processing functions and the response architecture provided by temperament, affective state, cognition, immune competence, behaviour and morphology of the animal. Facultative learning within these response modalities provides a means by which the resilience of the animal can improve with experience. Clearly, much work is needed in this area.
3.4. Resilience or robustness?
An important question arises as to the relationship between resilience and robustness. The recognition that several functional and conformation traits including health, reproduction and leg strength have deteriorated during selection for production traits in farm animals (Rauw et al. 1998) has led to a strong call to increase robustness through genetic selection to improve functional traits (Knap 2005; Star et al. 2008). The definition provided by Knap (2005) is commonly used to characterise robustness in farm animals. The robust farm animal has the ability to express its production potential in a wide range of environments without compromising its reproduction, health and wellbeing. The environmental effects that influence expression of functional and production traits tend to accumulate within the animal over longer time frames than the disturbances that can be minimised by resilience of the animal. Furthermore, these environmental effects tend to be cyclical or persistent. An analogy for the time frame in operation here is the distinction between environmental fluctuations described as weather from those described as climate. Indeed the somewhat arbitrary time boundary between climate and weather may provide a useful boundary for identifying the temporal difference between resilience and robustness. Animals cope with short-term, episodic fluctuations in environmental conditions through acute physiological stress responses coupled with innate and learnt behaviours. When perturbations persist over longer time frames whereby the environmental conditions are better described as characteristic of the environment than as temporary perturbations, homeorhetic physiological responses enable acclimatisation and adaptation (Collier et al. 2009). Others describe this adaptive process as allostasis. Nonetheless, these characterisations of the temporal boundary between resilience and robustness are somewhat arbitrary and tautological. The difference between environmental factors influencing resilience and robustness are also termed as microenvironmental and macroenvironmental effects respectively (Strandberg 2009).
Two aspects of robustness are commonly pursued in animal breeding. The first quantifies robustness through consistency of trait expression despite variations in genotype and environment (Strandberg 2009). A particular focus of this aspect in animal breeding is the ability of the animal to achieve its genetic potential for production traits in environments that have greater resource constraints or a larger load of adverse environmental conditions than the environment in which the genetic merit of parents was assessed. This consistency of production performance across environments is usually identified through estimation of genotype by environment interactions and reaction norms (Bryant et al. 2005; Knap 2005). The second aspect addresses the biological mechanism through which trade-offs within the animal between production traits and functional traits occur and is generally approached within the framework of resource allocation theory (Beilharz et al. 1993). Consideration of the genetic models and mechanisms related to resilience and robustness is beyond the scope of this review.
The impact of human values on the concept of robustness can be seen in the approach of researchers to lactational anoestrus in high-producing dairy cows. A negative genetic correlation between milk production and reproductive performance has seen a decline in fertility as milk yield increases (Pryce et al. 2009). From evolutionary and physiological perspectives, lactational anoestrus can be viewed as allocating resources to the current lactation in order to improve survival of the current offspring and to protect the cow from demands of the future pregnancy and lactation while body nutrient reserves are under pressure from the current lactation (Martin et al. 2008), creating a so called temporal trade-off (Phocas et al. 2014). Attempts to breed ‘robust’ cows by decoupling ovulatory activity from protective physiological regulators is driven by human values such as a management preference for an annual production cycle that matches feed availability for cows grazing at pasture with the lactation cycle (Pryce et al. 2009). We do not disagree with this general objective but note ethical implications such as responsibility to better manage nutrition and health (Knap 2005) and the other consequences of decoupling such as reduced residual adaptive capacity of the cow resulting from the increased metabolic load associated with higher milk production (Chagas et al. 2007).
3.5. Relationship between general environmental resilience and disease resilience
A second question raised by the concept of general environmental resilience is its relationship to disease resilience. Infectious agents are a very significant source of environmental challenge to animals. From studies on resistance of animals to the effects of infection, two concepts have been developed: disease tolerance and disease resilience. In farm animals, disease tolerance provides a phenotypic measure of the net impact of a given level of infection on performance whereas disease resilience is a phenotypic measure of productivity during infection (Bishop 2012; Doeschl-Wilson et al. 2012). The substantial biological significance of this minor semantic difference is explored in detail by Bishop (2012) and Doeschl-Wilson et al. (2012). The biological mechanisms contributing to these phenotypic outcomes can differ between pathogens and are not considered further here. For further details of the immune mechanisms contributing to disease tolerance the reader is referred to reviews by Glass (2012) and Ayres and Schneider (2012). A schematic relation of the terms disease resistance, disease resilience and disease tolerance to general environmental resilience is presented in Fig. 4.
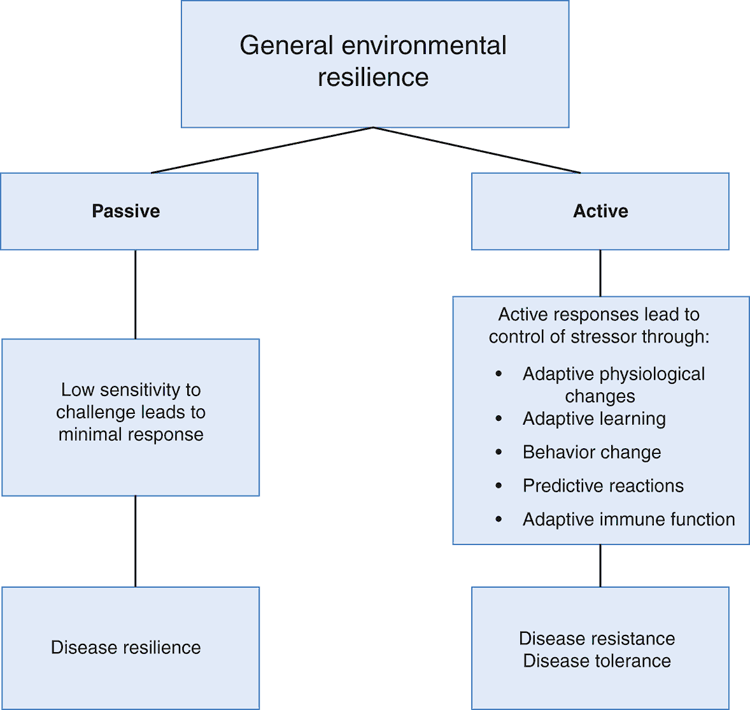
|
4. Managing animals to enhance resilience
During life on a farm, during transport and at slaughter, farm animals are exposed to many potential stressors, which can negatively impact their physiological, behavioural, and affective states resulting in reduced production, poor health and bad welfare. A very large body of work has focussed on identifying management and environmental factors that lead to these negative outcomes in order to reduce or eliminate their effects. The preceding discussion suggests that as well as improving resilience by providing comfortable, non-threatening environments, it may be possible to enhance resilience through provision of cognitive and emotional enrichment to the animal (Wechsler and Lea 2007; Spinka and Wemelsfelder 2011; Boissy and Lee 2014). The recent progress in developing tests to assess cognition and affective states is stimulating the development of a conceptual framework for understanding how we can improve welfare and resilience through cognitive and emotional enrichment (Boissy et al. 2007; Boissy and Lee 2014). Boissy and Lee (2014) suggest three ways that cognitive and emotional enrichment can be achieved: (1) signalling a reward in advance, (2) providing a reward that is greater than expected, and (3) providing the animal with the ability to control fulfilment of ‘wants’ and ‘likes’. However, experience of French shepherds suggests that too great a predictability of the timing of feed rewards can distract goats from engaging in normal grazing activities and reduce overall feed intake while they await the reward (Villalba et al. 2015). Thus, complexity in the reward structure is needed to avoid frustration from unfulfilled expectations (Greiveldinger et al. 2011). In contrast to using rewards to modify cognition and affect, exposing an animal to situations that provide stress inoculation and enable it to learn to control its environment has the potential to increase the animal’s agency (Wechsler and Lea 2007; Spinka and Wemelsfelder 2011). Additional strategies for cognitive and emotional enrichment and for increasing animal agency are likely to be found and lead to new management methods for increasing resilience.
5. Genetic selection to enhance resilience
5.1. Defining the phenotype
A starting point for genetic manipulation of resilience is the description and measurement of desirable phenotypes. The substantial evidence discussed above suggests that a resilience syndrome can be exhibited across diverse stressor situations and thus the resilience phenotype exhibited to one stressor challenge is likely to be correlated with resilience to other stressful environmental challenges that the animal may be exposed to. This proposition will need validating as measures of resilient phenotypes are further developed for farm animals. From the definition of resilience as reduced sensitivity to potential disturbances or rapid recovery from their impact, it follows that the desirable phenotype might be most easily identified by measuring the rate of recovery to baseline and normality of behavioural, physiological, affective, immune, cognitive or production traits following the disturbance rather than measuring the magnitude or direction of excursion of these variables while the animal attempts to cope with the stressor.
The physiological variables that change and the types of abnormal behaviours expressed can vary with the nature of the stressor and with the coping style of the animal (von Holst 1998), thus too strong a focus on measuring particular physiological response variables (for instance reactivity in the HPA axis during the stress response) or particular behaviours might characterise a phenotype that is specific to the stressor paradigm or coping style of the animal rather than be representative of the resilience syndrome. During mobilisation of defences in response to stressors, the normal dynamic range and circadian patterns of physiological and behavioural activities of the animals are reduced as defences are being forced to protect the animal. We suggest that a diagnostic signature for resilience will be more easily identified by measuring summary characteristics of response variables rather than by attempting to identify correlative relationships between the numerous labile variables that change in diverse ways in differing challenge scenarios. Thus, we advocate a predominantly outcomes-based approach to identify resilient phenotypes rather than a mechanisms-based approach.
The principle variables connoting normality are likely to differ between species and their choice will also be influenced by ease of measurement. We suggest that phenotyping be done during exposure of animals to a stressful event that is intrinsic to their management environment, and which includes a novelty component, a social component and if possible also includes a change in the pattern of exposure of animals to humans, rather than be undertaken during exposure to an experimentally imposed stressful situation. Examples from the repertoire of management-induced stressors that could provide suitable situations for phenotyping resilience include change of housing, change of group structure, weaning, and transition to the milking parlour. We suggest that a subset of the following variables that are cognate with the chosen stressors is likely to be useful for defining resilient phenotypes.
-
Core body temperature. Return to normality as defined by the time of day for and difference between maximum and minimum (i.e. normality of circadian pattern and dynamic range).
-
Heart rate and heart rate variability (von Borell et al. 2007). The desirable outcome is normal heart rate and normal heart rate variability rather than the deviated heart rate and reduced heart rate variability seen during the change in autonomic tone that occurs during response to stressors.
-
Normality of circadian ethogram and expression of behavioural complexity (MacIntosh et al. 2011).
-
Feed intake.
-
Growth rate, or principle production variable of the species.
-
Immune responsiveness. Capacity to exhibit a strong immune response without excessive bias towards a Type I or Type II response to vaccines delivered during the period of exposure to the stressor.
-
Normality of demeanour.
-
Speed of acquisition of predictive behaviours that are cognate with positive environmental cues such as feeding or entry to the milking parlour (milk let-down).
-
Normality of vocalisations.
The last three measures are included to provide information on affective state and cognitive abilities. A growing body of empirical evidence acquired from studies employing the methodology of qualitative behavioural assessment indicates that aspects of the animal’s demeanour are indicative of affective state (Wemelsfelder and Lawrence 2001; Wemelsfelder et al. 2001; Rousing and Wemelsfelder 2006; Brscic et al. 2009; Stockman et al. 2011, 2012; Napolitano et al. 2012; Rutherford et al. 2012). Wechsler and Lea (2007) have suggested that speed with which the animal learns to respond to new environmental cues is an overlooked indicator of cognitive ability and adaptation by learning in farm animals. In view of the detrimental impact of negative affective states on cognitive functions (Mendl et al. 2009), measuring the speed of learning to respond to cues associated with rewards (e.g. feeding, milking) is a composite measure of learning ability and resilience of learning ability to the negative impact of the stressor. Briefer (2012) suggests that there is considerable potential to use vocalisations as measures of affective states, which seems especially pertinent in view of the increasing use of animal-borne sensor technologies, including acoustic sensors, to monitor animals in real time and to automate phenotyping (Hocquette et al. 2012; Greenwood and Bell 2014).
The dynamics of adverse health outcomes can be highly situation-specific such that occurrence and distribution within a group can be strongly influenced by factors other than underlying resilience (Bishop 2012). We suggest therefore that function of the immune system is better assessed through measuring responsiveness to a standardised controlled challenge from vaccination with industry relevant vaccines rather than relying on the occurrence of disease challenges during the assessment period.
Some temperament traits are likely to be correlated with resilience and are likely to be a valuable component of a resilience breeding goal. It is thought that domestication has increased the threshold of behavioural responses to aversive environmental stimuli rather than led to loss from the behavioural repertoire (Price 1999; Canario et al. 2013), with increases in the thresholds for expression of fearfulness and fear of humans being particularly important (Mignon-Grasteau et al. 2005). With the inclusion of a novel environment (e.g. change of housing, change of feed type), a social stressor (e.g. mixing, weaning) and presence of humans in the situation under which resilience is phenotyped, there should be an opportunity for the influence of temperament traits on resilience to be expressed. Additional direct measurements of temperament undertaken outside the period of resilience phenotyping could also be valuable, although the extent of desirable associations between temperament and resilience still need to be ascertained.
When performance traits such as immune responsiveness, production and learning ability are measured during the response to a stressor, the measured trait is influenced by a combination of the underlying genetic potential for performance of the trait and by sensitivity of the trait to the impact of the stressor. Using the example of disease resilience to gastro-intestinal parasites, Doeschl-Wilson and Lough (2014) have noted that for accurate estimation of resilience of the performance trait to the stressor, performance should also be measured in the absence of the stressor as well as in the presence of the stressor so that the decrement in performance due to the effect of the stressor can be estimated. This limitation may be more pertinent to persistent stressors such as infections that are not cleared by host defence reactions than to transient stressors.
Measurement of the resilience syndrome would be improved by phenotyping resilience in a broad range of acute environmental challenge situations so that reaction norms can be used to assess the situational sensitivity of resilience (Knap 2005; Dingemanse et al. 2010) rather than using a single situation as we propose here.
5.2. The role of immune responsiveness in general environmental resilience
When exposed to a pathogen challenge, both active and passive responses make important contributions to the animal’s general environmental resilience, defined in terms of their capacity to be minimally affected by the disease challenge and rapidly return to a normal health status.
Although the Type I and Type II arms of the adaptive immune system work together to protect the host, negative genetic and phenotypic correlations between an animal’s ability to mount antibody-mediated and cell-mediated immune responses have been reported in dairy cattle (Hernandez et al. 2006; Thompson-Crispi et al. 2012b). The strength of this negative association may differ between species, but this observation is important to consider when breeding for resistance to a specific disease. Breeding animals resistant to a disease largely controlled by cell-mediated immune responses in the host may inadvertently increase their susceptibility to diseases largely controlled by antibody-mediated immune responses and vice versa. In support of this concept, an inverse relationship between antibody production and phagocytic cell function has been reported in Biozzi mice selected for high and low antibody production (Hale and Howard 1981) and in cattle selected for resistance or susceptibility to Brucella abortus (Price et al. 1990). Although the importance of breeding strategies targeting resistance to specific diseases of economic importance, such as internal parasites in sheep (LeJambre et al. 1971) and mastitis in dairy cattle (Heringstad et al. 2000) is acknowledged, we suggest that breeding strategies should also incorporate selection for general disease resistance as manifested by an animal’s ability to mount both cell-mediated and antibody-mediated immune responses to diverse antigens (Wilkie and Mallard 1999).
When deciding in selective breeding strategies whether to target disease resistance, disease tolerance or disease resilience as the basis for improving animal health, many factors need to be considered. For example, disease resistance and disease tolerance are generally negatively correlated, therefore individuals identified as susceptible to disease tend to be more tolerant, and conversely, individuals with resistant genotypes tend to be less tolerant (Raberg et al. 2007). It is also known that the host mechanisms and genes underlying disease resistance, disease tolerance and disease resilience differ, and have varied impacts on the evolving pathogen (Simms and Triplett 1994). Such factors highlight the importance of considering the preferred final outcomes for both the host and pathogen when establishing selection strategies to improve animal health. When developing strategies to improve the environmental resilience of livestock in Australian production systems through improved animal health, the approach taken by our team has been to target general disease resistance following the strategy first proposed by Wilkie and Mallard (1999). Selection for general disease resistance through selection for enhanced immune responsiveness was targeted because in many cases of infectious disease it is critical to eliminate the causal agent in order to prevent mortality and unintended pathogen transmission to the environment and hence to other hosts. Furthermore, animals identified as having enhanced general disease resistance are likely to be resistant to a wide range of pathological agents.
Many genes contribute to the general immune responsiveness of the animal (Kelley et al. 2005), which can be assessed as a quantitative trait by measuring responses to test antigens (Wilkie and Mallard 1999). This was first demonstrated among livestock species in Yorkshire pigs, where measures of innate and adaptive immunity (both cell-mediated and antibody-mediated) were combined to generate estimated breeding values for general immune responsiveness and to rank boars and gilts as high, intermediate and low immune responder (IR) phenotypes for use in future breeding programs (Mallard et al. 1992). This strategy aimed to simultaneously improve the ability of animals to mount both antibody- and cell-mediated adaptive responses, and as a consequence, enhance general disease resistance. Pigs classified as high IR using this strategy were found to have superior antibody responses to test antigens and several commercial vaccines (Wilkie and Mallard 1999), a lower frequency of non-responders when vaccinated with inactivated influenza vaccine (Wilke et al. 1998) and higher antibody avidity for target antigen (Appleyard et al. 1992), relative to their intermediate and low IR counterparts. Although such findings provided overwhelming evidence that selection using this strategy successfully enhanced the general immune responsiveness of selected pigs, when challenged with Mycoplasma hyorhinis, high IR pigs displayed more severe arthritis than low IR pigs, suggesting that high IR phenotype pigs may be more prone to generating inflammatory responses (Magnusson et al. 1998). It is noteworthy however, that in the same study, high IR pigs were found to have less severe peritonitis, less severe pleuritis and an enhanced ability to produce serum antibody against M. hyorhinis.
More recently, research efforts have been focussed on developing protocols to assess general immune responsiveness in dairy cattle (Mallard et al. 2015), beef cattle and sheep (Hine et al. 2015). Although testing protocols developed to assess general immune responsiveness in these species have not incorporated measures of innate immunity to date, it is well recognised that strong adaptive immune responses are underpinned by strong innate immune responses. Associations between the immune responsiveness phenotypes of individual dairy cows and their incidence of disease have been investigated on large-scale commercial farms in North America (Thompson-Crispi et al. 2012a). Results showed that the incidence of several common diseases of dairy cattle, including mastitis, displaced abomasums and retained fetal membranes were observed more frequently in average and/or low IR cows as compared with high IR cows in the same herd. This strategy, to assess general immune responsiveness, has been used to investigate the influence of hybrid vigour on general immune responsiveness in purebred and crossbreed dairy cattle (Begley et al. 2009; Cartwright et al. 2012), the influence of age and pregnancy status on general immune responsiveness in dairy heifers (Hine et al. 2011), leukocyte populations in high and low IR dairy heifers (Hine et al. 2012) and the influence of geographical location on immune response profiles of Canadian dairy cattle (Thompson-Crispi and Mallard 2012). We conclude that a high level of general immune responsiveness in both Type I and Type II arms of the adaptive immune system has the capacity to improve the resilience of animals to diverse disease challenges.
5.3. Candidate genes for resilience
Research in mice and humans has identified several gene polymorphisms in the neuroendocrine and neurotransmitter pathways involved in stress, reward and learning responses to stressors that differ between resilient and susceptible phenotypes. Better characterisation of resilient phenotypes in farm animals should provide the opportunity to look for similar gene differences in these species. Genes and pathways of interest include: neuropeptide Y gene; CRH receptor 1 gene and FK binding protein 5 gene (HPA axis); catechol-O-methyltransferase gene (noradrenergic and dopaminergic pathways); dopamine transported gene and dopamine receptor genes (dopaminergic pathway); promoter region of serotonin transported gene and serotonin receptor genes (serotinergic pathway); brain-derived neurotrophic factor gene; and the fatty acid amide hydrolase gene (endocannabinoid pathway) (Wu et al. 2013; Dincheva et al. 2015). The gene associated with porcine stress syndrome provides an extreme example of the potential for single gene effects to influence stress responsiveness and resilience (O’Brien et al. 1993).
5.4. Group performance
The influence of social factors on the response of the individual to stressors suggests that some aspects of resilience will be influenced by group characteristics. Thus, genetic selection for resilience may benefit from selection on performance of the group rather than the individual (Muir 2005), especially where social stressors are likely to be a major cause of environmental stress to farm animals (Muir 1996; Bergsma et al. 2008; Bolhuis et al. 2009). Genetic models to disentangle direct and social effects have been developed by Bijma et al. (2007). The potential for social ecological factors within a group to evoke a non-resilient phenotype in some individuals irrespective of their genotype also needs consideration.
5.5. Our approach to phenotyping sheep and cattle for resilience
Our team is currently assessing resilience using weaning in sheep and beef cattle and introduction to the milking parlour in dairy heifers as the management-induced stressor situations during which animals are phenotyped (Hine et al. 2015). In the majority of Australian production systems, breeding groups of sheep and beef cattle are run at pasture, with a low level of interaction with humans. Progeny are typically handled once or twice before weaning. During these handling events the routine husbandry procedures of castration, earmarking and primary vaccinations against common diseases of livestock are performed. At weaning, beef calves are usually held in an open air stock handling facility for 7–10 days, fed high quality hay and introduced to small quantities of pelleted rations. During this time they are moved through the forcing yards and stock race several times to familiarise them with human interactions and restraint, and to receive secondary or boost vaccinations where required. A comparable practice is increasingly being used for weaning lambs. The procedure termed yard weaning leads to greater ease in handling the livestock. Progeny are usually returned to pasture after weaning. In the beef industry, a portion of the progeny is often transferred to a feedlot several months after weaning to be finished for slaughter. Yard weaned calves have improved health outcomes and faster growth rates during feedlot finishing than calves weaned without the yard weaning procedure (Fell et al. 1998, 1999; Colditz et al. 2006; Walker et al. 2007).
Our resilience phenotyping procedure is being performed on genotyped progeny from genetic benchmarking programs in the sheep, beef and dairy industries. A typical procedure involves immunologically challenging animals at the commencement of yard weaning (lambs and beef calves), or introduction to the milking parlour (dairy heifers), and subsequently measuring both cell-mediated and antibody-mediated immune responses to vaccine components in order to assess the general immune responsiveness of individual animals while exposed to a management-induced stressor. Other phenotypic measures made on individual animals during the testing procedure include temperament assessment using flight speed testing (Burrow et al. 1988) and or crush/restraint score (Grandin 1993), liveweight change over the yard weaning or induction period and stress responsiveness to the management-induced stressor. Stress responsiveness is assessed by measuring changes in serum haptoglobin levels induced by the management-induced stressor each animal is exposed to during the testing procedure (Slocombe and Colditz 2005). Current work is focussed on investigating associations between these various resilience component traits, important production traits and animal health and welfare outcomes to guide the development of selection tools for farmers aiming to improve the environmental resilience of animals in their production system.
5.6. Will selection for resilience improve robustness?
Persistent environmental challenges from poor design of facilities, poor stock management and climatic extremes pose a substantial burden on the welfare and productivity of animals. The capacity of the animal to adapt to less extreme aspects of infrastructure, husbandry and climate is also likely to have been reduced through diversion of nutrients to production traits, in accord with resource allocation theory. The extent to which adaptability to short-term environmental challenges confers long-term benefits on performance of functional and production traits remains to be seen; however, it seems likely that the mechanisms enabling adaptation to short-term perturbations (resilience) might differ from the mechanisms enabling adaptation to persistent environmental (robustness). Some potential similarities and differences between resilience and robustness are outlined in Table 1.

|
6. Resilience and animal welfare
The concept of animal welfare developed by the World Organisation for Animal Health (2014) is commonly used to describe welfare. It states: ‘Animal welfare means how an animal is coping with the conditions in which it lives. An animal is in a good state of welfare if (as indicated by scientific evidence) it is healthy, comfortable, well nourished, safe, able to express innate behaviour, and if it is not suffering from unpleasant states such as pain, fear, and distress. Good animal welfare requires disease prevention and veterinary treatment, appropriate shelter, management, nutrition, humane handling and humane slaughter/killing. Animal welfare refers to the state of the animal; the treatment that an animal receives is covered by other terms such as animal care, animal husbandry, and humane treatment’.
Short-term challenges to the animal can reduce its welfare. Managing the animal so that its experience of the environment improves its ability to adapt, and breeding for resilience traits should help lead to better welfare outcomes. Nonetheless, as noted by others, selection for resilience, adaptability and robustness in farm animals should not be viewed as a substitute for good housing, good management and fitting the genotype of the animal to the production environment (Star et al. 2008; Fraser et al. 2013; Ferguson 2014). Rather, selection for resilience can be seen as a continuation of the genetic change associated with the process of domestication that has been occurring over several millennia (Price 1999; Mignon-Grasteau et al. 2005; Canario et al. 2013).
A challenging concept arising from work on physiological regulation and affective states in animals and from work on resilience in rodent models and humans is the importance of what can be termed negative experiences in maintaining the dynamic responsiveness of the animal and in shaping its capacity to cope with challenges (Korte et al. 2007; Russo et al. 2012; Wu et al. 2013; Bastian et al. 2014). This leads to a formulation of good welfare that includes nurturing the capacity of the animal to cope with challenges that are intrinsic to its life history and that cannot be eliminated by other aspects of good housing, good breeding, good husbandry, good disease control and good climatic environment. Thus, a proximate cost to the animal from controlled exposure to situations that invoke short-term negative effects could be necessary for the ultimate benefit of good whole-of-life welfare outcomes. The positive value of negative experiences is encapsulated in the concept of stress inoculation (Levine 1962).
7. Conclusions
Interactions with the environment expose the animal to beneficial and threatening situations. The animal’s perception of environmental stimuli is influenced by historical events, its contemporary status, and the behavioural, cognitive and immune attributes of the animal. The animal can learn from experience to anticipate stimuli and minimise their impact by predictive adaptive behaviours and predictive physiological responses. Stimuli lacking predictability and controllability generally have more adverse effects on the animal than predictable and controllable stimuli. Thus, the animal can learn to modify its exposure to the environment and its reactions to the environment so that the future occurrence of negative experiences is minimised, although when the animal is in a negative affective state when exposed to a noxious stimulus its capacity to learn from that event is compromised. Temperament and behavioural traits of the animal can also influence its perception of the valence of stimuli and influence subsequent reactions. Correlated behavioural and physiological reactions to environmental challenges have been termed coping styles. Two prominent styles are proactive and reactive coping. These styles describe the strategy of the animal for dealing with a challenge rather than the success of the outcome of its response to that challenge.
The concept of resilience differs between species (e.g. humans versus non-human animals) and between disciplines. We characterise resilience as the capacity of the animal to return rapidly to its pre-challenge state following short-term exposure to a challenging situation. Resilience is a comparative measure of differences between animals in the impact of a challenge. Resilience can arise due to lower sensitivity or better adaptability to the challenge and be improved through learnt responses. It is likely that resilience of an individual is a trait expressed consistently across diverse challenge situations, although the mechanisms by which animals achieve resilience may differ between individuals.
The concept of robustness of animals addresses both consistency of phenotype across environments and competition for resources between functional and production traits. Resilience tends to be expressed in response to environmental challenges lasting a matter of days whereas robustness is a consequence of longer lasting environmental conditions. Thus, resilience relies particularly on the reaction of the animal to stressors, whereas robustness is influenced by the capacity of the animal to adapt (acclimatise) to persistent characteristics of its environment. In animals selected for elite performance, the functional and production traits may be operating near their limits of adaptive capacity resulting in reduced residual capacity for arbitrage between production, defence and function. Some differences between resilience and robustness are open to somewhat arbitrary definition. A provisional characterisation of differences between resilience and robustness is presented in Table 1.
We suggest that for use in farm animal production, the resilient phenotype can be characterised by the rate of return of key variables to the pre-challenge or normal state that is achieved through low sensitivity or an enhanced ability to overcome the challenge, rather than by measuring the dynamic changes in variables within stress response pathways during reaction to the challenge. To minimise the need for additional animal handling, challenge situations could be based on routine management events in the life of the animal such as weaning, change of social grouping, transition to the milking parlour or change of housing. It is desirable for the challenge situation to include elements of novelty, social stress and interaction with humans.
Ignoring stress response mechanisms in identifying the resilient phenotype entails a risk and a benefit. Focussing on resilience outcomes rather than resilience mechanisms carries a risk that negatively correlated mechanisms compete in achievement of the resilience outcome and that individuals achieve resilience by employing one of two or more discrete strategies rather than bits of each. Coping style provides an analogy here. Thus, selection based solely on the resilience outcome without considering underlying mechanisms could be impeded if different individuals expressing the resilience outcome are employing different negatively correlated mechanisms to achieve that outcome. A benefit of focusing on outcomes is that progress might be made without first unravelling the underlying mechanisms. Of course, this is a perennial dilemma in animal breeding.
The realisation that stress responses increase disease susceptibility not only through suppression of host defence functions but also through stimulation of pathogens by host-derived catecholamine mediators of stress responses suggests that improving resilience of farm animals could provide multiple benefits for their health.
Advances in research on affective states and cognition in farm animals suggests that resilience might be improved with little management cost or effort by providing animals with environments and activities that provide cognitive and emotional enrichment. In addition, controlled exposure to negative experience might provide stress inoculation that makes animals more resilient to challenges that are intrinsic to their life history and production environment. An increased focus on breeding and managing animals for improved resilience should contribute to ongoing improvements in animal welfare.
Acknowledgements
The financial support of Australian taxpayers and Meat and Livestock Australia is gratefully acknowledged.
References
Albers GAA, Gray GD, Piper LR, Barker JSF, Le Jambre LF, Barger IA (1987) The genetics of resistance and resilience to Haemonchus contortus in young Merino sheep. International Journal for Parasitology 17, 1355–1363.| The genetics of resistance and resilience to Haemonchus contortus in young Merino sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7hsVejsw%3D%3D&md5=5b536b7d1e6269c9fcabdaf13464b559CAS |
Appleyard G, Wilkie BN, Kennedy BW, Mallard BA (1992) Antibody avidity in Yorkshire pigs of high and low immune response groups. Veterinary Immunology and Immunopathology 31, 229–240.
| Antibody avidity in Yorkshire pigs of high and low immune response groups.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK383ntFCnsA%3D%3D&md5=9ac6e5ec2a4757f9c26b07968a0dae45CAS | 1589953PubMed |
Ayres JS, Schneider DS (2012) Tolerance of infections. Annual Review of Immunology 30, 271–294.
| Tolerance of infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmsVKmtbg%3D&md5=d7988cf5dde2a8db7063904de1edc8b0CAS | 22224770PubMed |
Barrett LF (2012) Emotions are real. Emotion (Washington, D.C.) 12, 413–429.
| Emotions are real.Crossref | GoogleScholarGoogle Scholar |
Bastian B, Jetten J, Hornsey MJ, Leknes S (2014) The positive consequences of pain: a biopsychosocial approach. Personality and Social Psychology Review 18, 256–279.
| The positive consequences of pain: a biopsychosocial approach.Crossref | GoogleScholarGoogle Scholar | 24727972PubMed |
Bateson P, Gluckman P (2011) ‘Plasticity, robustness, development and evolution.’ (Cambridge University Press: Cambridge)
Bauman DE, Currie WB (1980) Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. Journal of Dairy Science 63, 1514–1529.
| Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXmtFygu7s%3D&md5=a2f6270fa31b2ba452df274bff6e889fCAS | 7000867PubMed |
Begley N, Buckley F, Pierce KM, Fahey AG, Mallard BA (2009) Differences in udder health and immune response traits of Holstein-Friesians, Norwegian Reds, and their crosses in second lactation. Journal of Dairy Science 92, 749–757.
| Differences in udder health and immune response traits of Holstein-Friesians, Norwegian Reds, and their crosses in second lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1KisLc%3D&md5=d6cf922831d794c4f2e2d22744763608CAS | 19164687PubMed |
Beilharz RG, Luxford BG, Wilkinson JL (1993) Quantitative genetics and evolution: is our understanding of genetics sufficient to explain evolution? Journal of Animal Breeding and Genetics 110, 161–170.
| Quantitative genetics and evolution: is our understanding of genetics sufficient to explain evolution?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3mtF2htg%3D%3D&md5=a9be671e750209b0fa64d1eed1cb2d09CAS | 21395715PubMed |
Bell AW (1995) Regulation of organic nutrient metabolism during transition form late pregnancy to early lactation. Journal of Animal Science 73, 2804–2819.
Bell AW, Bauman DE (1997) Adaptations of glucose metabolism during pregnancy and lactation. Journal of Mammary Gland Biology and Neoplasia 2, 265–278.
| Adaptations of glucose metabolism during pregnancy and lactation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3czosF2htg%3D%3D&md5=cd2830cedc75cc3dda0436bfa779f8c3CAS | 10882310PubMed |
Bergsma R, Kanis E, Knol EF, Bijma P (2008) The contribution of social effects to heritable variation in finishing traits of domestic pigs (Sus scrofa). Genetics 178, 1559–1570.
| The contribution of social effects to heritable variation in finishing traits of domestic pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3isVyiug%3D%3D&md5=7566506aef6a7ec94b6ccd75c00ed599CAS | 18245326PubMed |
Bijma P, Muir WM, Van Arendonk JA (2007) Multilevel selection 1: quantitative genetics of inheritance and response to selection. Genetics 175, 277–288.
| Multilevel selection 1: quantitative genetics of inheritance and response to selection.Crossref | GoogleScholarGoogle Scholar | 17110494PubMed |
Bishop SC (2012) A consideration of resistance and tolerance for ruminant nematode infections. Frontiers in Genetics 3, 168
| A consideration of resistance and tolerance for ruminant nematode infections.Crossref | GoogleScholarGoogle Scholar | 23248638PubMed |
Bisset SA, Morris CA (1996) Feasibility and implications of breeding sheep for resilience to nematode challenge. International Journal for Parasitology 26, 857–868.
| Feasibility and implications of breeding sheep for resilience to nematode challenge.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FotFyqsA%3D%3D&md5=8911c8c18b2d414e5bd2a1ab48ecf691CAS | 8923135PubMed |
Boissy A, Lee C (2014) How assessing relationships between emotions and cognition can improve farm animal welfare. Revue Scientifique et Technique (International Office of Epizootics) 33, 103–110.
Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Winckler C, Forkman B, Dimitrov I, Langbein J (2007) Assessment of positive emotions in animals to improve their welfare. Physiology & Behavior 92, 375–397.
| Assessment of positive emotions in animals to improve their welfare.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2htr7K&md5=fbeceb771c4101fce5cc216632949df3CAS |
Bolhuis JE, Ellen ED, van Reenen CG, De Groot J, Ten Napel J, Koopmanschap RE, Reilingh GDV, Uitdehaag KA, Kemp B, Rodenburg TB (2009) Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks. Physiology & Behavior 97, 470–475.
| Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFeqsLg%3D&md5=8615fb890257d1fbaf6de1d99d7c51bcCAS |
Booth DA (2008) Physiological regulation through learnt control of appetites by contingencies among signals from external and internal environments. Appetite 51, 433–441.
| Physiological regulation through learnt control of appetites by contingencies among signals from external and internal environments.Crossref | GoogleScholarGoogle Scholar | 18640162PubMed |
Briefer EF (2012) Vocal expression of emotions in mammals: mechanisms of production and evidence. Journal of Zoology 288, 1–20.
| Vocal expression of emotions in mammals: mechanisms of production and evidence.Crossref | GoogleScholarGoogle Scholar |
Brown WC, Rice-Ficht AC, Estes DM (1998) Bovine type 1 and type 2 responses. Veterinary Immunology and Immunopathology 63, 45–55.
| Bovine type 1 and type 2 responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtVKqtr4%3D&md5=864e40a8d8eceb04fc832635850aea8aCAS | 9656440PubMed |
Brscic M, Wemelsfelder F, Tessitore E, Gottardol F, Cozzi G, van Reenen CG (2009) Welfare assessment: correlations and integration between a Qualitative Behavioural Assessment and a clinical/health protocol applied in veal calves farms. Italian Journal of Animal Science 8, 601–603.
| Welfare assessment: correlations and integration between a Qualitative Behavioural Assessment and a clinical/health protocol applied in veal calves farms.Crossref | GoogleScholarGoogle Scholar |
Bryant J, Lopez-Villalobos N, Holmes C, Pryce J (2005) Simulation modelling of dairy cattle performance based on knowledge of genotype, environment and genotype by environment interactions: current status. Agricultural Systems 86, 121–143.
| Simulation modelling of dairy cattle performance based on knowledge of genotype, environment and genotype by environment interactions: current status.Crossref | GoogleScholarGoogle Scholar |
Burrow HM, Seifert GW, Corbet NJ (1988) A new technique for measuring temperament in cattle. Proceedings of the Australian Society for Animal Production 17, 154–157.
Canario L, Mignon-Grasteau S, Dupont-Nivet M, Phocas F (2013) Genetics of behavioural adaptation of livestock to farming conditions. Animal 7, 357–377.
| Genetics of behavioural adaptation of livestock to farming conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3s7htVSqsg%3D%3D&md5=57546d654be8a1d10198fb9a679ea765CAS | 23127553PubMed |
Cannon WB (1929) Organization for physiological regulation. Physiological Reviews 9, 399–431.
Carpenter RHS (2004) Homeostasis: a plea for a unified approach. Advances in Physiology Education 28, 180–187.
| Homeostasis: a plea for a unified approach.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2crntFOquw%3D%3D&md5=6fdb19f4abbe85fccdf3201cb0843cddCAS |
Cartwright SL, Schaeffer LR, Burnside EB, Mallard BA (2012) Adaptive immune response, survival, and somatic cell score between postpartum Holstein and Norwegian Red × Holstein first-calf heifers. Journal of Animal Science 90, 2970–2978.
| Adaptive immune response, survival, and somatic cell score between postpartum Holstein and Norwegian Red × Holstein first-calf heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVKku7vI&md5=21e35494342e6fe9d04bf7c94347851bCAS | 22585796PubMed |
Chagas LM, Bass JJ, Blache D, Burke CR, Kay JK, Lindsay DR, Lucy MC, Martin GB, Meier S, Rhodes FM (2007) Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. Journal of Dairy Science 90, 4022–4032.
| Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsleqtb0%3D&md5=cb89e99e4e2ab231651a269fb06d483cCAS | 17699018PubMed |
Clunies Ross I (1932) Observations on the resistance of sheep to infestation by the stomach worm (Haemonchus contortus). Journal. Council for Scientific and Industrial Research (Australia) 5, 73–80.
Cockram MS (2004) A review of behavioural and physiological responses of sheep to stressors to identify potential behavioural signs of distress. Animal Welfare (South Mimms, England) 13, 283–291.
Colditz IG (2002) Effects of the immune system on metabolism: implications for production and disease resistance in livestock. Livestock Production Science 75, 257–268.
| Effects of the immune system on metabolism: implications for production and disease resistance in livestock.Crossref | GoogleScholarGoogle Scholar |
Colditz IG (2008a) The costs of immune responses. In ‘Resource allocation theory applied to farm animal production’. (Ed. WM Rauw) pp. 192–209. (CABI Publishing: Wallingford, UK)
Colditz IG (2008b) Six costs of immunity to gastrointestinal nematode infections. Parasite Immunology 30, 63–70.
Colditz IG, Watson DL, Kilgour R, Ferguson DM, Prideaux C, Ruby J, Kirkland PD, Sullivan K (2006) Impact of animal health and welfare research within the CRC for Cattle and Beef Quality on Australian beef production. Australian Journal of Experimental Agriculture 46, 233–244.
| Impact of animal health and welfare research within the CRC for Cattle and Beef Quality on Australian beef production.Crossref | GoogleScholarGoogle Scholar |
Collier RJ, Limesand SW, Rhoads ML, Rhoads RP, Baumgard LH, Rauw WM (2009) Homeorhesis during heat stress. In ‘Resource allocation theory applied to farm animal production’. (Ed. WM Rauw) pp. 72–88. (CABI Publishing: Wallingford, UK)
Cooper SJ (2008) From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite 51, 419–427.
| From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis.Crossref | GoogleScholarGoogle Scholar | 18634840PubMed |
Coppens CM, de Boer SF, Koolhaas JM (2010) Coping styles and behavioural flexibility: towards underlying mechanisms. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365, 4021–4028.
| Coping styles and behavioural flexibility: towards underlying mechanisms.Crossref | GoogleScholarGoogle Scholar | 21078654PubMed |
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience 3, 655–666.
| How do you feel? Interoception: the sense of the physiological condition of the body.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlslGrt7s%3D&md5=aa775cdd2e68ee4eb4bdca6d1a9d669fCAS | 12154366PubMed |
Craig AD (2003) Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology 13, 500–505.
| Interoception: the sense of the physiological condition of the body.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntVKhsLY%3D&md5=a702c7df1d52d2825fb1f5584e46764cCAS | 12965300PubMed |
Czura CJ, Tracey KJ (2005) Autonomic neural regulation of immunity. Journal of Internal Medicine 257, 156–166.
| Autonomic neural regulation of immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXit1ejurk%3D&md5=5c382e8968cd6ea31c1b6af005184301CAS | 15656874PubMed |
Darwin C (1965) ‘The expression of the emotions in man and animals.’ (University of Chicago Press: Chicago, IL)
Desire L, Boissy A, Veissier I (2002) Emotions in farm animals: a new approach to animal welfare in applied ethology. Behavioural Processes 60, 165–180.
Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM (2015) FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications 6, 6395
| FAAH genetic variation enhances fronto-amygdala function in mouse and human.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtF2itb7F&md5=9db4b2695818942a23238febe5a6696dCAS | 25731744PubMed |
Dingemanse NJ, Kazem AJ, Reale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends in Ecology & Evolution 25, 81–89.
| Behavioural reaction norms: animal personality meets individual plasticity.Crossref | GoogleScholarGoogle Scholar |
Doeschl-Wilson A, Lough G (2014) Inferring genetic resilience of animals to infectious pathogens – opportunities and pitfalls. In ‘Breeding Focus 2014 improving resilience’. (Eds S Hermesch, S Dominik) pp. 19–30. (Animal Genetics and Breeding Unit: Armidale)
Doeschl-Wilson AB, Villanueva B, Kyriazakis I (2012) The first step toward genetic selection for host tolerance to infectious pathogens: obtaining the tolerance phenotype through group estimates. Frontiers in Genetics 3, 265
| The first step toward genetic selection for host tolerance to infectious pathogens: obtaining the tolerance phenotype through group estimates.Crossref | GoogleScholarGoogle Scholar | 23412990PubMed |
Doyle RE, Fisher AD, Hinch GN, Boissy A, Lee C (2010) Release from restraint generates a positive judgement bias in sheep. Applied Animal Behaviour Science 122, 28–34.
| Release from restraint generates a positive judgement bias in sheep.Crossref | GoogleScholarGoogle Scholar |
Doyle RE, Lee C, Deiss V, Fisher AD, Hinch GN, Boissy A (2011) Measuring judgement bias and emotional reactivity in sheep following long-term exposure to unpredictable and aversive events. Physiology & Behavior 102, 503–510.
| Measuring judgement bias and emotional reactivity in sheep following long-term exposure to unpredictable and aversive events.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvFeiurs%3D&md5=ac7c2ec53e76042b177b015032f42e35CAS |
Dumont JE, Dremier S, Pirson I, Maenhaut C (2002) Cross signaling, cell specificity, and physiology. American Journal of Physiology. Cell Physiology 283, C2–C28.
| Cross signaling, cell specificity, and physiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVCkurY%3D&md5=8c5b8cca37e6f07d38a94fa05568fe7bCAS | 12055068PubMed |
Dwyer CM, Bornett HLI (2004) Chronic stress in sheep: assessment tools and their use in different management conditions. Animal Welfare (South Mimms, England) 13, 293–304.
Elsasser TH, Klasing KC, Filipov N, Thompson F (2000) The metabolic consequences of stress: targets for stress and priorities of nutrient use. In ‘The biology of animal stress’. (Eds GP Moberg, JA Mench) pp. 77–110. (CABI Publishing: Wallingford, UK)
Erf GF (2004) Cell-mediated immunity in poultry. Poultry Science 83, 580–590.
| Cell-mediated immunity in poultry.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3htFWlsQ%3D%3D&md5=c5bd7759d2f4a52db0f3735e425d341aCAS | 15109055PubMed |
Estes DM, Tuo W, Brown WC, Goin J (1998) Effects of type I/type II interferons and transforming growth factor-beta on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology 95, 604–611.
| Effects of type I/type II interferons and transforming growth factor-beta on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFakuw%3D%3D&md5=3ef24107908e03aa90f697b4fdc047b4CAS | 9893052PubMed |
Fell LR, Walker KH, Reddacliff LA, Davies L, Vallance HJ, House JR, Wilson SC (1998) Effects of yard weaning and pre-feedlot vaccination on feedlot performance of Bos taurus steers. Animal Production Australia 22, 173–176.
Fell LR, Colditz IG, Walker KH, Watson DL (1999) Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Australian Journal of Experimental Agriculture 39, 795–802.
| Associations between temperament, performance and immune function in cattle entering a commercial feedlot.Crossref | GoogleScholarGoogle Scholar |
Ferguson DM (2014) Key features of ‘environmental fit’ that promote good animal welfare in different husbandry systems. Revue Scientifique et Technique (International Office of Epizootics) 33, 161–169.
Foury A, Tribout T, Bazin C, Billon Y, Bouffaud M, Gogue JM, Bidanel JP, Mormede P (2009) Estimation of genetic trends from 1977 to 2000 for stress-responsive systems in French Large White and Landrace pig populations using frozen semen. Animal 3, 1681–1687.
| Estimation of genetic trends from 1977 to 2000 for stress-responsive systems in French Large White and Landrace pig populations using frozen semen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1Sjt77K&md5=b267b58691eea76dc2c70fc92f082e2dCAS | 22443552PubMed |
Fox CJ, Hammerman PS, Thompson CB (2005) Fuel feeds function: energy metabolism and the T-cell response. Nature Reviews. Immunology 5, 844–852.
| Fuel feeds function: energy metabolism and the T-cell response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKls7jO&md5=7b8fd8ff49737c5aadb817cc7247978fCAS | 16239903PubMed |
Fraser D, Duncan IJ, Edwards SA, Grandin T, Gregory NG, Guyonnet V, Hemsworth PH, Huertas SM, Huzzey JM, Mellor DJ (2013) General principles for the welfare of animals in production systems: the underlying science and its application. Veterinary Journal (London, England) 198, 19–27.
| General principles for the welfare of animals in production systems: the underlying science and its application.Crossref | GoogleScholarGoogle Scholar |
Freestone PP, Sandrini SM, Haigh RD, Lyte M (2008) Microbial endocrinology: how stress influences susceptibility to infection. Trends in Microbiology 16, 55–64.
| Microbial endocrinology: how stress influences susceptibility to infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhslOlsLY%3D&md5=dae92ff8eccbcf1ce6142f2e496722d5CAS | 18191570PubMed |
Gether U, Kobilka BK (1998) G protein-coupled receptors II. Mechanism of agonist activation. The Journal of Biological Chemistry 273, 17979–17982.
| G protein-coupled receptors II. Mechanism of agonist activation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvVChsr8%3D&md5=8c5f5319b56f9b887e82adf3e3f26087CAS | 9660746PubMed |
Glass EJ (2012) The molecular pathways underlying host resistance and tolerance to pathogens. Frontiers in Genetics 3, 263
| The molecular pathways underlying host resistance and tolerance to pathogens.Crossref | GoogleScholarGoogle Scholar | 23403960PubMed |
Grandin T (1993) Behavioral agitation during handling of cattle is persistent over time. Applied Animal Behaviour Science 36, 1–9.
| Behavioral agitation during handling of cattle is persistent over time.Crossref | GoogleScholarGoogle Scholar |
Greenwood PL, Bell AW (2014) Consequences of nutrition during gestation, and the challenge to better understand and enhance livestock productivity and efficiency in pastoral ecosystems. Animal Production Science 54, 1109–1118.
Greenwood EC, Plush KJ, van Wettere WHEJ, Hughes PE (2014) Hierarchy formation in newly mixed, group housed sows and management strategies aimed at reducing its impact. Applied Animal Behaviour Science 160, 1–11.
| Hierarchy formation in newly mixed, group housed sows and management strategies aimed at reducing its impact.Crossref | GoogleScholarGoogle Scholar |
Greiveldinger L, Veissier I, Boissy A (2007) Emotional experience in sheep: predictability of a sudden event lowers subsequent emotional responses. Physiology & Behavior 92, 675–683.
| Emotional experience in sheep: predictability of a sudden event lowers subsequent emotional responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1GltrzK&md5=12dc189483a0f7fe0407563a195accf2CAS |
Greiveldinger L, Veissier I, Boissy A (2009) Behavioural and physiological responses of lambs to controllable vs. uncontrollable aversive events. Psychoneuroendocrinology 34, 805–814.
| Behavioural and physiological responses of lambs to controllable vs. uncontrollable aversive events.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzhtFaqtA%3D%3D&md5=985486196bf5f5a6e57d3e9f29587135CAS | 19084342PubMed |
Greiveldinger L, Veissier I, Boissy A (2011) The ability of lambs to form expectations and the emotional consequences of a discrepancy from their expectations. Psychoneuroendocrinology 36, 806–815.
| The ability of lambs to form expectations and the emotional consequences of a discrepancy from their expectations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MrlsF2gtQ%3D%3D&md5=20e73da40c5fe04ac043e8cd87cc0226CAS | 21232873PubMed |
Hale CHRI, Howard JG (1981) Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice. Parasite Immunology 3, 45–55.
| Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7nsVeitQ%3D%3D&md5=d30b10936fa33c3e30ce819ad96d0a9aCAS |
Heringstad Br, Klemetsdal G, Ruane J (2000) Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries. Livestock Production Science 64, 95–106.
| Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries.Crossref | GoogleScholarGoogle Scholar |
Hermesch S, Dominik S (2014) ‘Breeding Focus 2014 − improving resilience.’ (Eds S Hermesch, S Dominik) (Animal Genetics and Breeding Unit, University of New England: Armidale)
Hernandez A, Quinton M, Miglior F, Mallard BA (2006) ‘Genetic parameters of dairy cattle immune response traits.’ (International Committee for World Congress on Genetics Applied to Livestock Production: Belo Horizonte, MG, Brazil)
Hessing MJC, Scheepens CJM, Schouten WGP, Tielen MJM, Wiepkema PR (1994) Social rank and disease susceptibility in pigs. Veterinary Immunology and Immunopathology 43, 373–387.
| Social rank and disease susceptibility in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M7lvFCjuw%3D%3D&md5=166045a9a4e65cbb72605ae3310b0e44CAS |
Hessing MJC, Coenen GJ, Vaiman M, Renard C (1995) Individual differences in cell-mediated and humoral immunity in pigs. Veterinary Immunology and Immunopathology 45, 97–113.
| Individual differences in cell-mediated and humoral immunity in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzivVamtA%3D%3D&md5=25f4822899b4728b18e5e5f5681e26a8CAS |
Hine BC, Cartwright SL, Mallard BA (2011) Effect of age and pregnancy status on adaptive immune responses of Canadian Holstein replacement heifers. Journal of Dairy Science 94, 981–991.
| Effect of age and pregnancy status on adaptive immune responses of Canadian Holstein replacement heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXls1Ggt7Y%3D&md5=0a5f60b7f205b9645250de692f1ad6a6CAS | 21257066PubMed |
Hine BC, Cartwright SL, Mallard BA (2012) Analysis of leukocyte populations in Canadian Holsteins classified as high or low immune responders for antibody- or cell-mediated immune response. Canadian Journal of Veterinary Research-Revue Canadienne de Recherche Veterinaire 76, 149–156.
Hine BC, Mallard BA, Ingham AB, Colditz IG (2015) Immune competence in livestock. In ‘Breeding Focus 2014 improving resilience’. (Eds S Hermesch, S Dominik) pp. 49–64. (Animal Genetics and Breeding Unit, University of New England: Armidale)
Hobson JA (2009) REM sleep and dreaming: towards a theory of protoconsciousness. Nature Reviews. Neuroscience 10, 803–813.
Hocquette JF, Capel C, David V, Guemene D, Bidanel J, Ponsart C, Gastinel PÉ, Bail PL, Monget P, Mormede P (2012) Objectives and applications of phenotyping network set-up for livestock. Animal Science Journal 83, 517–528.
| Objectives and applications of phenotyping network set-up for livestock.Crossref | GoogleScholarGoogle Scholar | 22776789PubMed |
Kadzere CT, Murphy MR, Silanikove N, Maltz E (2002) Heat stress in lactating dairy cows: a review. Livestock Production Science 77, 59–91.
| Heat stress in lactating dairy cows: a review.Crossref | GoogleScholarGoogle Scholar |
Keast SJ, Constanzo A (2015) Is fat the sixth taste primary? Evidence and implications. Flavour 4, 5
| Is fat the sixth taste primary? Evidence and implications.Crossref | GoogleScholarGoogle Scholar |
Kelley J, de Bono B, Trowsdale J (2005) IRIS: a database surveying known human immune system genes. Genomics 85, 503–511.
| IRIS: a database surveying known human immune system genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisVWltb0%3D&md5=1fffffd5f10fdbcb7131f6e430f25290CAS | 15780753PubMed |
Kelly D, Coutts AGP (2000) Early nutrition and the development of immune function in the neonate. The Proceedings of the Nutrition Society 59, 177–185.
| Early nutrition and the development of immune function in the neonate.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7lt1Clsw%3D%3D&md5=088ad363af706087bcfe761d8c3ead9bCAS | 10946785PubMed |
Klopcic M, Reents R, Philipsson J, Kulpers A (2009) ‘Breeding for robustness in cattle.’ (Wageningen Academic Publishers: Wageningen)
Knap PW (2005) Breeding robust pigs. Australian Journal of Experimental Agriculture 45, 763–773.
| Breeding robust pigs.Crossref | GoogleScholarGoogle Scholar |
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews 23, 925–935.
| Coping styles in animals: current status in behavior and stress-physiology.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2Fks1amsA%3D%3D&md5=6b1840bd74ed071f949f921f1c117a62CAS | 10580307PubMed |
Koolhaas JM, Bartolomucci A, Buwalda B, De Boer SF, Fluegge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P (2011) Stress revisited: a critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews 35, 1291–1301.
| Stress revisited: a critical evaluation of the stress concept.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3mtVyqug%3D%3D&md5=477fe9bb88f0ec13872b68aba1328a69CAS | 21316391PubMed |
Korte SM, Olivier B, Koolhass JM (2007) A new concept of animal welfare based on allostasis. Physiology & Behavior 92, 422–428.
| A new concept of animal welfare based on allostasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2htr%2FN&md5=76fdc3b6a492373e08b9a90c1f1f9a6fCAS |
Langbein J, Puppe B (2004) Analysing dominance relationships by sociometric methods – plea for a more standardised and precise approach in farm animals. Applied Animal Behaviour Science 87, 293–315.
| Analysing dominance relationships by sociometric methods – plea for a more standardised and precise approach in farm animals.Crossref | GoogleScholarGoogle Scholar |
Lee TK, Lee C, Bischof R, Lambert GW, Clarke IJ, Henry BA (2014) Stress-induced behavioral and metabolic adaptations lead to an obesity-prone phenotype in ewes with elevated cortisol responses. Psychoneuroendocrinology 47, 166–177.
| Stress-induced behavioral and metabolic adaptations lead to an obesity-prone phenotype in ewes with elevated cortisol responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFSls7jF&md5=5918f5be8dacc5b498e3a9bc7a365de8CAS | 25001966PubMed |
LeJambre LF, Ractliffe LH, Uhazy LS, Whitlock JH (1971) Fecal egg output of lambs in relationship to Haemonchus contortus burden. International Journal for Parasitology 1, 157–160.
| Fecal egg output of lambs in relationship to Haemonchus contortus burden.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3s%2FpsFyisw%3D%3D&md5=3b9774f40072c9c29ab99b41be64fbacCAS | 5170631PubMed |
Lepherd ML, Canfield PJ, Hunt GB, Bosward KL (2009) Haematological, biochemical and selected acute phase protein reference intervals for weaned female Merino lambs. Australian Veterinary Journal 87, 5–11.
| Haematological, biochemical and selected acute phase protein reference intervals for weaned female Merino lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtF2gur8%3D&md5=71cc98fba5f78401dc170b09ecfaadebCAS | 19178470PubMed |
Leung AK, Sharp PA (2010) MicroRNA functions in stress responses. Molecular Cell 40, 205–215.
| MicroRNA functions in stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlCjtr7I&md5=b57c1763c08e7341f32d16304f08842cCAS | 20965416PubMed |
Levine S (1962) Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science 135, 795–796.
| Plasma-free corticosteroid response to electric shock in rats stimulated in infancy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF38%2Fms1WjtA%3D%3D&md5=e2aead80a09f01987a81b5b7c6c51d95CAS | 14464660PubMed |
Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences 35, 121–143.
| The brain basis of emotion: a meta-analytic review.Crossref | GoogleScholarGoogle Scholar | 22617651PubMed |
MacIntosh AJJ, Alados CL, Huffman MA (2011) Fractal analysis of behaviour in a wild primate: behavioural complexity in health and disease. Journal of the Royal Society, Interface 8, 1497–1509.
| Fractal analysis of behaviour in a wild primate: behavioural complexity in health and disease.Crossref | GoogleScholarGoogle Scholar |
MacKay JR, Haskell MJ (2015) Consistent individual behavioral variation: the difference between temperament, personality and behavioral syndromes. Animals (Basel) 5, 455–478.
| Consistent individual behavioral variation: the difference between temperament, personality and behavioral syndromes.Crossref | GoogleScholarGoogle Scholar | 26479368PubMed |
Magnusson U, Wilkie B, Mallard B, Rosendal S, Kennedy B (1998) Mycoplasma hyorhinis infection of pigs selectively bred for high and low immune response. Veterinary Immunology and Immunopathology 61, 83–96.
| Mycoplasma hyorhinis infection of pigs selectively bred for high and low immune response.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3ntlGgsg%3D%3D&md5=50063a9d1d8c609898c695dd56f52abcCAS | 9613474PubMed |
Mallard BA, Wilkie BN, Kennedy BW, Quinton M (1992) Use of estimated breeding values in a selection index to breed Yorkshire pigs for high and low immune and innate resistance factors. Animal Biotechnology 3, 257–280.
| Use of estimated breeding values in a selection index to breed Yorkshire pigs for high and low immune and innate resistance factors.Crossref | GoogleScholarGoogle Scholar |
Mallard BA, Emam M, Paibomesai M, Thompson-Crispi K, Wagter-Lesperance L (2015) Genetic selection of cattle for improved immunity and health. The Japanese Journal of Veterinary Research 63, S37–S44.
Martin GB, Blache D, Williams IH (2008) Allocation of resources to reproduction. In ‘Resource allocation theory applied to farm animal production’. (Ed. W Rauw) pp. 169–191. (CABI Publishing: Wallingford, UK)
Mason JW (1971) A re-evaluation of the concept of non-specificity in stress theory. Journal of Psychiatric Research 8, 323–333.
| A re-evaluation of the concept of non-specificity in stress theory.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE38%2Fms1ymsA%3D%3D&md5=30f37cfc6b09b5f7773f256a2b7795d9CAS | 4331538PubMed |
McEwen BS (1998) Stress, adaptation, and disease: allostasis and allostatic load. Annals of the New York Academy of Sciences 840, 33–44.
| Stress, adaptation, and disease: allostasis and allostatic load.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3pt1ykuw%3D%3D&md5=b594429e1d3b880a526d0d4bc22528a3CAS | 9629234PubMed |
McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Hormones and Behavior 43, 2–15.
| The concept of allostasis in biology and biomedicine.Crossref | GoogleScholarGoogle Scholar | 12614627PubMed |
Mehta PH, Gosling SD (2008) Bridging human and animal research: a comparative approach to studies of personality and health. Brain, Behavior, and Immunity 22, 651–661.
| Bridging human and animal research: a comparative approach to studies of personality and health.Crossref | GoogleScholarGoogle Scholar | 18343094PubMed |
Mendl M, Burman OH, Parker RM, Paul ES (2009) Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Applied Animal Behaviour Science 118, 161–181.
| Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms.Crossref | GoogleScholarGoogle Scholar |
Mendl M, Burman OH, Paul ES (2010) An integrative and functional framework for the study of animal emotion and mood. Proceedings. Biological Sciences 277, 2895–2904.
| An integrative and functional framework for the study of animal emotion and mood.Crossref | GoogleScholarGoogle Scholar |
Michelena P, Sibbald AM, Erhard HW, McLeod JE (2009) Effects of group size and personality on social foraging: the distribution of sheep across patches. Behavioral Ecology 20, 145–152.
| Effects of group size and personality on social foraging: the distribution of sheep across patches.Crossref | GoogleScholarGoogle Scholar |
Mignon-Grasteau S, Boissy A, Bouix J, Faure JM, Fisher AD, Hinch GN, Jensen P, Le Neindre P, Mormede P, Prunet P (2005) Genetics of adaptation and domestication in livestock. Livestock Production Science 93, 3–14.
| Genetics of adaptation and domestication in livestock.Crossref | GoogleScholarGoogle Scholar |
Moberg GP (2000) Biological response to stress: implications for animal welfare. In ‘The biology of animal stress’. (Eds GP Moberg, JA Mench) pp. 1–22. (CABI Publishing: Wallingford)
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of Immunology (Baltimore, MD.: 1950) 136, 2348–2357.
Muir WM (1996) Group selection for adaptation to multiple-hen cages: selection program and direct responses. Poultry Science 75, 447–458.
| Group selection for adaptation to multiple-hen cages: selection program and direct responses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283nt1CqtQ%3D%3D&md5=de93822d893854f2ed3f438da1a9dd7eCAS | 8786932PubMed |
Muir WM (2005) Incorporation of competitive effects in forest tree or animal breeding programs. Genetics 170, 1247–1259.
| Incorporation of competitive effects in forest tree or animal breeding programs.Crossref | GoogleScholarGoogle Scholar | 15911590PubMed |
Napolitano F, De Rosa G, Grasso F, Wemelsfelder F (2012) Qualitative behaviour assessment of dairy buffaloes (Bubalus bubalis). Applied Animal Behaviour Science 141, 91–100.
| Qualitative behaviour assessment of dairy buffaloes (Bubalus bubalis).Crossref | GoogleScholarGoogle Scholar |
O’Brien PJ, Shen H, Cory CR, Zhang X (1993) Use of a DNA-based test for the mutation associated with porcine stress syndrome (malignant hyperthermia) in 10,000 breeding swine. Journal of the American Veterinary Medical Association 203, 842–851.
Pavlov IP (1904) Physiology of digestion. In ‘Nobel lectures: physiology or medicine, 1901–1921’. (Ed. Nobel Foundation, 1967) pp. 141–155. (Elsevier: New York, NY)
Phocas F, Bobe J, Bodin L, Charley B, Dourmad JY, Friggens NC, Hocquette JF, Le Bail PY, Le Bihan-Duval E, Mormede P, Quere P, Schelcher F (2014) Des animaux plus robustes: un enjeu majeur pour le developpement durable des productions animales necessitant l’essor du phenotypage fin et a haut debit. INRA Production Animale 27, 181–194.
Price EO (1999) Behavioral development in animals undergoing domestication. Applied Animal Behaviour Science 65, 245–271.
| Behavioral development in animals undergoing domestication.Crossref | GoogleScholarGoogle Scholar |
Price RE, Templeton JW, Smith R, Adams LG (1990) Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infection and Immunity 58, 879–886.
Pryce JE, Harris BL, Bryant JR, Montgomerie WA, Klopcic M, Reents R, Philipsson J, Kuipers A (2009) Do robust dairy cows already exist? In ‘Breeding for robustness in cattle’. (Eds M Klopcic, R Reents, J Philipsson, A Kuipers) pp. 99–109. (Wageningen Academic Publishers: Wageningen)
Raberg L, Sim D, Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814.
| Disentangling genetic variation for resistance and tolerance to infectious diseases in animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1elsrfK&md5=442cee15115eee388fb99dfb3565f8faCAS | 17975068PubMed |
Ramsay DS, Woods SC (2014) Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychological Review 121, 225–247.
| Clarifying the roles of homeostasis and allostasis in physiological regulation.Crossref | GoogleScholarGoogle Scholar | 24730599PubMed |
Rauw WM, Kanis E, Noordhuizen-Stassen EN, Grommers FJ (1998) Undesirable side effects of selection for high production efficiency in farm animals, a review. Livestock Production Science 56, 15–33.
| Undesirable side effects of selection for high production efficiency in farm animals, a review.Crossref | GoogleScholarGoogle Scholar |
Raymond CR, Wilkie BN (2004) Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells. Vaccine 22, 1016–1023.
| Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOkur4%3D&md5=b57f2a2d0fbe224845b8a8dc5d067619CAS | 15161079PubMed |
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biological Reviews of the Cambridge Philosophical Society 82, 291–318.
| Integrating animal temperament within ecology and evolution.Crossref | GoogleScholarGoogle Scholar | 17437562PubMed |
Rhoads ML, Rhoads RP, Sanders SR, Carroll SH, Weber WJ, Crooker BA, Collier RJ, VanBaale MJ, Baumgard LH (2007) Effects of heat stress on production, lipid metabolism and somatotropin variables in lactating cows. Journal of Dairy Science 90, 230
Roberts N (2007) Links between behavioural and physiological parameters during adaptation to stress. PhD Thesis, University of New England, Armidale.
Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP (2009) Invited review: body condition score and its association with dairy cow productivity, health, and welfare. Journal of Dairy Science 92, 5769–5801.
| Invited review: body condition score and its association with dairy cow productivity, health, and welfare.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFWisbbN&md5=bb8f50e79243179b7e715c4354fa3cfaCAS | 19923585PubMed |
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model – a new model integrating homeostasis, allostasis, and stress. Hormones and Behavior 55, 375–389.
| The reactive scope model – a new model integrating homeostasis, allostasis, and stress.Crossref | GoogleScholarGoogle Scholar | 19470371PubMed |
Rosenblueth A, Wiener N, Bigelow J (1943) Behavior, purpose and teleology. Philosophy of Science 10, 18–24.
| Behavior, purpose and teleology.Crossref | GoogleScholarGoogle Scholar |
Rousing T, Wemelsfelder F (2006) Qualitative assessment of social behaviour of dairy cows housed in loose housing systems. Applied Animal Behaviour Science 101, 40–53.
| Qualitative assessment of social behaviour of dairy cows housed in loose housing systems.Crossref | GoogleScholarGoogle Scholar |
Rowe J, Banks R (2015) Sheep industry productivity – the role of genomics and digital data. Farm Policy Journal 12, 21–31.
Rushen J (1986) Some problems with the physiological concept of ‘stress’. Australian Veterinary Journal 63, 359–361.
| Some problems with the physiological concept of ‘stress’.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s7ls1eqsg%3D%3D&md5=db103bf105a87ba27f352386a4a1c4a7CAS | 3548689PubMed |
Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012) Neurobiology of resilience. Nature Neuroscience 15, 1475–1484.
| Neurobiology of resilience.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsV2rsbrP&md5=0a0609b4287d821c9340f84ec39bf7b1CAS | 23064380PubMed |
Rutherford KM, Donald RD, Lawrence AB, Wemelsfelder F (2012) Qualitative behavioural assessment of emotionality in pigs. Applied Animal Behaviour Science 139, 218–224.
| Qualitative behavioural assessment of emotionality in pigs.Crossref | GoogleScholarGoogle Scholar | 22915833PubMed |
Rutter M (2012) Resilience: causal pathways and social ecology. In ‘The social ecology of resilience’. (Ed. M Ungar) pp. 33–42. (Springer: New York, NY)
Schmied J, Hamilton K, Rupa P, Oh SY, Wilkie B (2012) Immune response phenotype induced by controlled immunization of neonatal pigs varies in type 1: type 2 bias. Veterinary Immunology and Immunopathology 149, 11–19.
| Immune response phenotype induced by controlled immunization of neonatal pigs varies in type 1: type 2 bias.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFWitr8%3D&md5=cba47032d89cdc9df5e9af2833a7b9d8CAS | 22698390PubMed |
Schrama JW, Schouten JM, Swinkels JW, Gentry JL, de Vries Reilingh G, Parmentier HK (1997) Effect of hemoglobin status on humoral immune response of weanling pigs differing in coping styles. Journal of Animal Science 75, 2588–2596.
Scott JP (1962) Critical periods in behavioral development. Science 138, 949–958.
Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138, 32
| A syndrome produced by diverse nocuous agents.Crossref | GoogleScholarGoogle Scholar |
Selye H (1946) The general adaptation syndrome and the diseases of adaptation. The Journal of Clinical Endocrinology 6, 117–230.
| The general adaptation syndrome and the diseases of adaptation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaH28XhvFGmug%3D%3D&md5=18c0f75dcbaacb336e9830254d7ea71fCAS |
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience 12, 154–167.
| The integration of negative affect, pain and cognitive control in the cingulate cortex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitVOnsL8%3D&md5=09ae53a671fed656841ee5a9019bf1f2CAS | 21331082PubMed |
Sherrington CS (1900) Cutaneous sensations. In ‘Text-book of physiology’. (Ed. EA Schäfer) pp. 920–1001. (Pentland: Edinburgh, UK)
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution 19, 372–378.
| Behavioral syndromes: an ecological and evolutionary overview.Crossref | GoogleScholarGoogle Scholar |
Simms EL, Triplett J (1994) Costs and benefits of plant responses to disease: resistance and tolerance. Evolution 48, 1973–1985.
| Costs and benefits of plant responses to disease: resistance and tolerance.Crossref | GoogleScholarGoogle Scholar |
Slocombe LL, Colditz IG (2005) Evaluating the stress of production in cattle using haptoglobin. In ‘Proceedings of the 5th International Colloquium on Acute Phase Proteins, Dublin, Ireland’. (Scientific Committee and Enterprise Ireland, Biotechnology Directorate: Dublin, Ireland) 30.
Smith GP (2008) Unacknowledged contributions of Pavlov and Barcroft to Cannon’s theory of homeostasis. Appetite 51, 428–432.
| Unacknowledged contributions of Pavlov and Barcroft to Cannon’s theory of homeostasis.Crossref | GoogleScholarGoogle Scholar | 18675307PubMed |
Sordillo LM, Mavangira V (2014) The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Animal Production Science 54, 1204–1214.
Sørensen JT, Sandøe P, Halberg N (2001) Animal welfare as one among several values to be considered at farm level: the idea of an ethical account for livestock farming. Acta Agriculturae Scandinavica, Section A – Animal Science 51, 11–16.
| Animal welfare as one among several values to be considered at farm level: the idea of an ethical account for livestock farming.Crossref | GoogleScholarGoogle Scholar |
Spinka MARE, Wemelsfelder F (2011) Environmental challenge and animal agency. In ‘Animal welfare’. (Eds MC Appleby, JA Mench, IAS Olsson, BO Hughes) pp. 27–43. (CABI International: Wallingford, UK)
Stamps J, Groothuis TG (2010) The development of animal personality: relevance, concepts and perspectives. Biological Reviews of the Cambridge Philosophical Society 85, 301–325.
| The development of animal personality: relevance, concepts and perspectives.Crossref | GoogleScholarGoogle Scholar | 19961473PubMed |
Star L, Ellen ED, Uitdehaag K, Brom FWA (2008) A plea to implement robustness into a breeding goal: poultry as an example. Journal of Agricultural & Environmental Ethics 21, 109–125.
| A plea to implement robustness into a breeding goal: poultry as an example.Crossref | GoogleScholarGoogle Scholar |
Sterling P (2004) Principles of allostasis: optimal design, predictive regulation, pathophysiology and rational therapeutics. In ‘Allostasis, homeostasis, and the costs of adaptation’. (Ed. J Schulkin) pp. 17–64. (Cambridge University Press: Cambridge)
Sterling P (2012) Allostasis: a model of predictive regulation. Physiology & Behavior 106, 5–15.
| Allostasis: a model of predictive regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFSntLg%3D&md5=7fec00926c54aa5147a5d11b80b8e812CAS |
Sterling P, Eyer J (1988) Allostasis: a new paradigm to explain arousal pathology. In ‘Handbook of life stress, cognition and health’. (Eds S Fisher, J Reason) pp. 629–649. (J. Wiley and Sons: New York)
Stockman CA, Collins T, Barnes AL, Miller D, Wickham SL, Beatty DT, Blache D, Wemelsfelder F, Fleming PA (2011) Qualitative behavioural assessment and quantitative physiological measurement of cattle naive and habituated to road transport. Animal Production Science 51, 240–249.
| Qualitative behavioural assessment and quantitative physiological measurement of cattle naive and habituated to road transport.Crossref | GoogleScholarGoogle Scholar |
Stockman CA, McGilchrist P, Collins T, Barnes AL, Miller D, Wickham SL, Greenwood PL, Cafe LM, Blache D, Wemelsfelder F (2012) Qualitative Behavioural Assessment of Angus steers during pre-slaughter handling and relationship with temperament and physiological responses. Applied Animal Behaviour Science 142, 125–133.
| Qualitative Behavioural Assessment of Angus steers during pre-slaughter handling and relationship with temperament and physiological responses.Crossref | GoogleScholarGoogle Scholar |
Stockman CA, Collins T, Barnes AL, Miller D, Wickham SL, Verbeek E, Matthews L, Ferguson D, Wemelsfelder F, Fleming PA (2014) Qualitative behavioural assessment of the motivation for feed in sheep in response to altered body condition score. Animal Production Science 54, 922–929.
Strandberg E (2009) The role of environmental sensitivity and plasticity in breeding for robustness: lessons from evolutionary genetics. In ‘Breeding for robustness in cattle’. (Eds M Klopcic, R Reents, J Philipsson, A Kuipers) pp. 17–33. EAAP Publication. (Wageningen Academic Publishers: Wageningen, The Netherlands)
Thompson-Crispi KA, Mallard BA (2012) Type 1 and type 2 immune response profiles of commercial dairy cows in 4 regions across Canada. Canadian Journal of Veterinary Research 76, 120
Thompson-Crispi KA, Hine B, Quinton M, Miglior F, Mallard BA (2012a) Short communication: Association of disease incidence and adaptive immune response in Holstein dairy cows. Journal of Dairy Science 95, 3888–3893.
| Short communication: Association of disease incidence and adaptive immune response in Holstein dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XoslGmsLc%3D&md5=550bdd07ae3533a2c69705f9d9a48113CAS | 22720943PubMed |
Thompson-Crispi KA, Sewalem A, Miglior F, Mallard BA (2012b) Genetic parameters of adaptive immune response traits in Canadian Holsteins. Journal of Dairy Science 95, 401–409.
| Genetic parameters of adaptive immune response traits in Canadian Holsteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1OlsrnF&md5=7e1625a2970dd0dce432e2354097141fCAS | 22192219PubMed |
Tilg H, Kaser A (2011) Gut microbiome, obesity, and metabolic dysfunction. The Journal of Clinical Investigation 121, 2126–2132.
| Gut microbiome, obesity, and metabolic dysfunction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVaisL4%3D&md5=8c109f76d82eb794fbfc5e04bb8fc8aaCAS | 21633181PubMed |
Tuchscherer M, Puppe B, Tuchscherer A, Kanitz E (1998) Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Physiology & Behavior 64, 353–360.
| Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlvVygtbs%3D&md5=f76fd33f4fbe3b84e178b00ed5fc134cCAS |
Vandaveer SS, Erf GF, Durdik JM (2001) Avian T helper one/two immune response balance can be shifted toward inflammation by antigen delivery to scavenger receptors. Poultry Science 80, 172–181.
| Avian T helper one/two immune response balance can be shifted toward inflammation by antigen delivery to scavenger receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXitVKgt7o%3D&md5=0b8489afcdee92982f7c4adfe5280366CAS | 11233005PubMed |
Veissier I, Miele M (2014) Animal welfare: towards transdisciplinarity – the European experience. Animal Production Science 54, 1119–1129.
Villalba JJ, Provenza FD, Catanese F, Distel RA (2015) Understanding and manipulating diet choice in grazing animals. Animal Production Science 55, 261–271.
| Understanding and manipulating diet choice in grazing animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXisF2nu7o%3D&md5=2f46dae370ec80abc996727758cac39bCAS |
von Borell E, Langbein J, Despres G, Hansen S, Leterrier C, Marchant-Forde J, Marchant-Forde R, Minero M, Mohr E, Prunier A (2007) Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals – a review. Physiology & Behavior 92, 293–316.
| Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2hsbfF&md5=50a22e18648d99438b55d3ecd5970c77CAS |
von Holst D (1998) The concept of stress and its relevance for animal behavior. Advances in the Study of Behavior 27, 1–131.
| The concept of stress and its relevance for animal behavior.Crossref | GoogleScholarGoogle Scholar |
Walker KH, Fell LR, Reddacliff LA, Kilgour RJ, House JR, Wilson SC, Nicholls PJ (2007) Effects of yard weaning and training on the behavioural adaptation of cattle to a feedlot. Livestock Science 106, 210–217.
| Effects of yard weaning and training on the behavioural adaptation of cattle to a feedlot.Crossref | GoogleScholarGoogle Scholar |
Webster AJF, Hicks AM, Hays FL (1969) Cold climate and cold temperature induced changes in the heat production and thermal insulation of sheep. Canadian Journal of Physiology and Pharmacology 47, 553–562.
| Cold climate and cold temperature induced changes in the heat production and thermal insulation of sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M3ktlClsA%3D%3D&md5=be0119bc1dcc2abe70f167a0a9992e1eCAS |
Wechsler B, Lea SE (2007) Adaptation by learning: its significance for farm animal husbandry. Applied Animal Behaviour Science 108, 197–214.
| Adaptation by learning: its significance for farm animal husbandry.Crossref | GoogleScholarGoogle Scholar |
Wemelsfelder F, Lawrence AB (2001) Qualitative assessment of animal behaviour as an on-farm welfare-monitoring tool. Acta Agriculturae Scandinavica, Section A – Animal Science 51, 21–25.
Wemelsfelder F, Hunter TE, Mendl MT, Lawrence AB (2001) Assessing the whole animal: a free choice profiling approach. Animal Behaviour 62, 209–220.
| Assessing the whole animal: a free choice profiling approach.Crossref | GoogleScholarGoogle Scholar |
Wiens S (2005) Interoception in emotional experience. Current Opinion in Neurology 18, 442–447.
| Interoception in emotional experience.Crossref | GoogleScholarGoogle Scholar | 16003122PubMed |
Wiesel TN (1982) The postnatal development of the visual cortex and the influence of environment. Bioscience Reports 2, 351–377.
| The postnatal development of the visual cortex and the influence of environment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL383lslKkuw%3D%3D&md5=9cd8197a1751744340121a6fc2b7b84aCAS | 7049262PubMed |
Wilke BN, Mallard BA, Quinton M, Gibson J (1998) Multi-trait selection for immune response: a possible alternative strategy for enhanced livestock health and productivity. In ‘Progress in pig science’. (Ed. J Wiseman) pp. 29–38. (Nottingham University Press: Nottingham, UK)
Wilkie B, Mallard B (1999) Selection for high immune response: an alternative approach to animal health maintenance? Veterinary Immunology and Immunopathology 72, 231–235.
| Selection for high immune response: an alternative approach to animal health maintenance?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnsl2ltbg%3D&md5=bf571b5104f62b234473c412271da822CAS | 10614513PubMed |
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends in Ecology & Evolution 27, 452–461.
| Animal personalities: consequences for ecology and evolution.Crossref | GoogleScholarGoogle Scholar |
Woods SC, Ramsay DS (2007) Homeostasis: beyond Curt Richter. Appetite 49, 388–398.
| Homeostasis: beyond Curt Richter.Crossref | GoogleScholarGoogle Scholar | 17524521PubMed |
World Organisation for Animal Health (2014) Terrestrial Animal Health Code. Chapter 7.1. Available at http://www.oie.int/en/international-standard-setting/terrestrial-code/access-online/ [Verified 28 November 2015]
Wrona D (2006) Neural−immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. Journal of Neuroimmunology 172, 38–58.
| Neural−immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVWgsrs%3D&md5=964f2585d9db906c4a9a944825328e28CAS | 16375977PubMed |
Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathe AA (2013) Understanding resilience. Frontiers in Behavioral Neuroscience 7, 1–10.
| Understanding resilience.Crossref | GoogleScholarGoogle Scholar |



