RNA interference-based technology: what role in animal agriculture?
B. J. Bradford A C , C. A. Cooper B , M. L. Tizard B , T. J. Doran B and T. M. Hinton BA Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506, USA.
B CSIRO Biosecurity Flagship, Geelong, Vic. 3219, Australia.
C Corresponding author. Email: bbradfor@ksu.edu
Animal Production Science 57(1) 1-15 https://doi.org/10.1071/AN15437
Submitted: 8 August 2015 Accepted: 18 January 2016 Published: 23 May 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Animal agriculture faces a broad array of challenges, ranging from disease threats to adverse environmental conditions, while attempting to increase productivity using fewer resources. RNA interference (RNAi) is a biological phenomenon with the potential to provide novel solutions to some of these challenges. Discovered just 20 years ago, the mechanisms underlying RNAi are now well described in plants and animals. Intracellular double-stranded RNA triggers a conserved response that leads to cleavage and degradation of complementary mRNA strands, thereby preventing production of the corresponding protein product. RNAi can be naturally induced by expression of endogenous microRNA, which are critical in the regulation of protein synthesis, providing a mechanism for rapid adaptation of physiological function. This endogenous pathway can be co-opted for targeted RNAi either through delivery of exogenous small interfering RNA (siRNA) into target cells or by transgenic expression of short hairpin RNA (shRNA). Potentially valuable RNAi targets for livestock include endogenous genes such as developmental regulators, transcripts involved in adaptations to new physiological states, immune response mediators, and also exogenous genes such as those encoded by viruses. RNAi approaches have shown promise in cell culture and rodent models as well as some livestock studies, but technical and market barriers still need to be addressed before commercial applications of RNAi in animal agriculture can be realised. Key challenges for exogenous delivery of siRNA include appropriate formulation for physical delivery, internal transport and eventual cellular uptake of the siRNA; additionally, rigorous safety and residue studies in target species will be necessary for siRNA delivery nanoparticles currently under evaluation. However, genomic incorporation of shRNA can overcome these issues, but optimal promoters to drive shRNA expression are needed, and genetic engineering may attract more resistance from consumers than the use of exogenous siRNA. Despite these hurdles, the convergence of greater understanding of RNAi mechanisms, detailed descriptions of regulatory processes in animal development and disease, and breakthroughs in synthetic chemistry and genome engineering has created exciting possibilities for using RNAi to enhance the sustainability of animal agriculture.
Additional keywords: gene silencing, livestock, pharmacology, RNAi.
Introduction
Animal science occupies a unique position at the interface of basic biology, medicine, and agriculture. Indeed, research and commercial application of technologies ranging from quantitative and genomic genetic selection programs, artificial insemination, embryo transfer, and various uses of endocrine and pharmaceutical products have contributed to the dramatically increased resource efficiency of animal agriculture over the past 5–7 decades (Capper 2011). Although potential risks must be evaluated for each novel technology, applications of RNA interference (RNAi) hold substantial promise for addressing both existing and potential threats to the sustainability of global animal agriculture. Our aim in this review is to introduce a broader group of animal scientists to the fundamental mechanics and broad utility of RNAi, and also to introduce to those in the RNAi field the many potential applications of this technology in animal agriculture.
What is RNA interference?
RNAi is a key intracellular mechanism regulating the function of cells, and in turn, organisms. This process generated substantial excitement upon its discovery in the late 1990s (Fire et al. 1998), resulting in a Nobel Prize for its co-discovers in 2006 (the shortest period ever from discovery to award). RNAi is believed to have emerged as an anti-viral defence system whereby double-stranded RNA used as viral genetic code is recognised and cleaved, destroying the message (Obbard et al. 2009). However, this toolkit was adapted over evolutionary time to contribute to the regulation of eukaryotic protein synthesis. Both plant and animal genomes encode a variety of short RNA strands, typically referred to as micro RNA (miRNA), that are not destined for translation but as a secondary level of transcriptional control (Iwakawa and Tomari 2015). Pri-miRNA are produced in the nucleus, generally as hairpin structures present in mRNA introns. The microprocessor complex comprised of two proteins, the RNase III enzyme Drosha and the double-stranded RNA binding protein Pasha, excise the pre-miRNA hairpin from the mRNA. Pre-miRNA are transported into the cytoplasm by a nucleocytoplasmic transport factor exportin 5. Once in the cytoplasm another RNase III enzyme, dicer, removes the loop structure from the pre-miRNA hairpin producing a short double-stranded mature miRNA. The miRNA then associates with multiprotein RNA induced silencing complex (RISC), which comprises argonaute 2, dicer, and the human immunodeficiency virus transactivating response RNA-binding protein. The guide strand is integrated into the RISC. The remaining strand, the passenger strand, is degraded as a RISC complex substrate. The RISC with a bound miRNA can then recognise and bind complementary mRNA, leading to formation of double-stranded RNA. At this point, the RISC cleaves the target mRNA at the position facing nucleotides 10 and 11 of the miRNA. If the sequence is not fully complementary, RISC can still silence target genes by recruiting additional effector proteins, which induce translational repression and/or mRNA decay in a manner independent of endonucleolytic cleavage (Chapman and Carrington 2007). Short binding sequences complementary to individual miRNA are often found on an array of different mRNA targets, allowing for transcription of a single miRNA to suppress translation of a whole class of proteins. This process therefore provides a rapid, responsive method for a cell to downregulate entire enzyme or signalling/response pathways (Inui et al. 2010; Dumortier et al. 2013).
Small interfering RNA (siRNA), when discussed in the context of higher animals, generally refers to synthetic molecules that mimic the effects of miRNA, although the potential sequences targeted by siRNA are not limited to those targeted by genomic miRNA. Synthetic siRNA is typically comprised of double-stranded RNA (dsRNA) molecules 20–25 base pairs in length with 3ʹ overhangs on each strand (Elbashir et al. 2001). Another class of molecules that can induce RNAi is shRNA, which are single RNA strands that are self-complementary, resulting in the formation of hairpin-shaped structures like those formed by miRNA (Paddison et al. 2002).
Unfortunately, siRNA binding to target sequences is not so specific that off-target mRNA knockdown is impossible. Partial complementation of either the ‘guide strand’, (which corresponds to the antisense strand) or the passenger strand (corresponding to the sense strand) can result in off-target mRNA cleavage (Matveeva 2013). Furthermore, imperfect pairing can also lead to translational repression even if the mRNA is not degraded (Saxena et al. 2003). However, perfect complementarity and specificity does not guarantee efficacy; the stability of the siRNA-mRNA complex and the structure of the mRNA must be appropriate to allow for degradation of the transcript. Despite these challenges, observed off-target silencing is often subtle compared with suppression of the intended target, and a large body of work has now detailed the characteristics of functional and specific siRNA sequences (Rettig and Behlke 2012; Matveeva 2013). Despite the need to test for off-target effects, RNAi nevertheless provides the unique opportunity to target essentially any gene product in an animal with reasonable specificity. As such, RNAi has become a standard tool in functional genomics studies, with genome-wide siRNA and shRNA libraries readily available for several animal genomes.
The enzyme complex responsible for mediating RNA-induced silencing forms in the cytosol of the cell (Kim et al. 2014). Therefore, triggering this process requires the siRNA to find its way into target cells, which is no small challenge for in vivo application of RNAi (Rivera and Yuan 2012). Two distinct approaches have been taken to address this problem, detailed below.
Transgenic expression of RNAi-inducing molecules
The most common approach to delivery of RNAi-inducing molecules to date has been via genomic integration of shRNA constructs (Fig. 1). For detailed reviews of transgene insertion techniques, see Gama Sosa et al. (2010) and Maksimenko et al. (2013). Traditional methods of transgene insertion include the following approaches:
-
DNA microinjection. The transgene containing the shRNA is injected into the nucleus of a fertilised egg where it is randomly inserted into the host genome. The fertilised egg is then transferred to a foster mother, and the offspring will carry the transgene if integration was successful (Gordon and Ruddle 1981).
-
Embryonic stem cell-mediated gene transfer. Embryonic stem cells derived from early stage mouse embryos can be cultured indefinitely. The targeting vector consisting of the endogenous gene carrying the shRNA required is introduced into these cells and inserted into the genome by homologous recombination. The altered embryonic stem cells are then injected into early mouse embryos, where they mix with the endogenous cells of the embryo resulting in a chimeric animal. If the altered cells contribute to the germ cells of the mouse, progeny in a subsequent mating will inherit the shRNA expressing gene (Smithies et al. 1985).
-
Somatic cell nuclear transfer. Somatic cells are maintained in culture. A targeting vector consisting of an endogenous gene carrying the shRNA required is introduced into these somatic cells and inserted into the genome by homologous recombination. The somatic cells are screened to find cells that have correctly integrated the shRNA. Oocytes are enucleated and nuclei of shRNA-integrated cells are transferred into the enucleated oocytes, and then fused with an electrical pulse. Once fused, the cells behave like typical in vitro fertilised embryos, and are grown in culture until the blastocyst stage, when they are transferred into recipient animals.
-
Retrovirus-mediated gene transfer. Retroviruses have the ability to infect host cells and integrate into the genome. Therefore, they are commonly used as vectors for transgenic applications, creating a chimeric embryo with the DNA inserted randomly (Nagano et al. 2001).
-
Transposon-mediated gene transfer. Transposons are mobile genetic elements that can facilitate the transposition of DNA from plasmid vectors into chromosomes. During transposition, the transposase recognises transposon-specific inverted terminal repeat sequences flanking the shRNA transgene, and moves the shRNA transgene into a random chromosomal site (Macdonald et al. 2012).
-
Sperm-mediated gene transfer. In this method the transgene containing the shRNA is attached or inserted into the sperm head and then used to fertilise eggs (Moreira et al. 2007). DNA can be introduce to the sperm head in several ways including transfection (Collares et al. 2011), attachment by antibodies (Chang et al. 2002) and disruption by repeated freeze–thaw cycles or exposure to detergents (Perry et al. 2001).
Random insertion of the shRNA construct into the genome can lead to problems including overloading of the RNAi machinery (Grimm et al. 2006), complete elimination of a required gene product, or epigenetic silencing of the construct. Newer precision genome engineering tools such as zinc finger nucleases, clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas-based RNA-guided DNA endonucleases and transcription activator-like effector nucleases (TALEN) can be used to avoid some of these problems (Gaj et al. 2013; Sander and Joung 2014). These technologies act as ‘molecular scissors’ creating specific double-stranded breaks at targeted locations in the genome. The transgene with flanking homologous sequences is then inserted by homologous recombination, utilising the cell’s endogenous DNA repair mechanism to repair the break. Combining precision genome engineering and careful selection of the promoter for the construct can, in theory, address the issues with traditional transgenic methods and can also potentially target shRNA expression to specific cell types or developmental stages. These approaches will be explored in the reminder of the text.
Administration of exogenously produced RNA molecules
Exogenous siRNA have become a routinely used tool in cell culture research over the past decade. Additionally, because of the potential of RNAi for treatment of human diseases, substantial work has been carried out in rodent models to determine optimal approaches to in vivo administration of siRNA molecules. Early studies employed hydrodynamic intravenous administration of dsRNA to essentially flood RNA degradation processes and induce gene silencing systemically, with particularly dramatic effects in the liver (Kawakami and Hashida 2007). However, the rapid administration of large volumes into the tail vein of mice may lead to tissue damage and stress responses, and this approach is unlikely to be feasible in large animals anyway. Most current work employs nanoparticles to encapsulate siRNA (Hong and Nam 2014) or relies on chemical modification of the siRNA to slow its degradation (Engels 2013). Upon delivery to target cells, siRNA still must enter the cell – typically through endosomes – and make its way to the cytoplasm. In addition to enhancing the stability of siRNA, current work on nanoparticle design for siRNA delivery focuses on incorporation of targeting moieties that trigger uptake by specific cell types and cleavage sites allowing for efficient dissociation of the nanoparticle in endocytic vesicles.
The relative difficulty of gene silencing with exogenous siRNA varies greatly across organs. The liver, kidneys, spleen, and phagocytic cells can be affected by many delivery formulations, with little or no targeting required (Shi et al. 2011). Although many other organs are difficult to target with systemic siRNA administration, there has been some success in targeting muscle tissue (Kinouchi et al. 2008) and adipocytes (Won et al. 2014). Additional target organs, including the lungs (Bitko et al. 2005), uterus (Brooks et al. 2015), mammary gland (Brock et al. 2014), and muscle (Koganti et al. 2015) can be altered by direct delivery of siRNA.
Advantages of exogenous RNAi versus the transgenic RNAi approach
Exogenous delivery has several advantages over the transgenic approach. One of these is the short-term effect of delivering the treatment; this is beneficial for transitioning between physiological states, regulation of genes specifically during development for improved physiological traits and for protection from outbreaks of infectious disease. Exogenously delivered siRNA may also be advantageous over transgenic expression to combat infectious diseases, as many viruses can mutate rapidly, potentially negating the advantage of the transgenic line. Newly emerging viruses can also be addressed more rapidly with synthetic siRNA, as once the new viral sequence is known, development of siRNA is relatively straightforward. Exogenous RNAi also avoids the detrimental effects of overexpression of transgenic shRNA, unanticipated negative consequences from prolonged downregulation of genes and does not require manipulation of the genome, making it accessible for human therapeutics and bypassing the perception issues with genetically modified organisms. Exogenous RNAi can also be rapidly adapted for multiple species, where the genome sequence is available. Similarly, exogenous RNAi can be applied on a much larger scale suitable for agricultural applications through food supplies or as plant insecticide delivery for pest control.
Potential applications of RNAi in animal agriculture
In plant agriculture, RNAi has already entered the commercial domain. Transgenic potatoes and apples utilising RNAi have been approved in the USA (Waltz 2015), and many more crops employing this technology are being developed (Koch and Kogel 2014). Although there are additional challenges for animal agriculture to utilise these tools, the advances in plant agriculture point to the potential of RNAi.
Previous reviews on potential uses of RNAi in animal agriculture were published more than 5 years ago (Wise et al. 2008; Long et al. 2010), and significant advances in our general understanding of RNAi, as well as new examples of in vivo RNAi applications in livestock, have occurred during that time. We will outline a variety of opportunities for RNAi in animal agriculture while highlighting examples of successful RNAi approaches in livestock to date.
Altering developmental programs
In many agricultural species, research on fetal and early postnatal programming has revealed that even subtle differences in the environment during development can have long-term impacts on the animal that influence productivity, efficiency, and product characteristics (Reynolds et al. 2010). These findings demonstrate the broad potential impacts of altering gene translation during development.
Altering muscle development. Finding ways to increase overall muscle mass has long been a topic of study in animal agriculture research. One of the most studied genes relating to control of muscle mass is myostatin, which negatively regulates skeletal muscle mass by reducing protein synthesis. Long before the identification of the myostatin gene, farmers have been selecting animals with naturally occurring myostatin mutations. Both the Belgian Blue and Piedmontese cattle breeds were first established in the 1800s based on observed muscle hypertrophy phenotypes, which more than 100 years later were found to be due to mutations within the myostatin gene (Kambadur et al. 1997). However, during calving, these breeds have a high incidence of dystocia and Caesarean sections due to the muscle hypertrophy of their offspring.
New genome engineering tools such as TALEN and CRISPR allow scientists to make precise changes to the genome. There are multiple groups that have used these tools to produce myostatin knockout animals, including cattle (Proudfoot et al. 2015), goats (Ni et al. 2014), and fish (Dong et al. 2011). These knockout animals are expected to have similar muscle hypertrophy phenotypes to those breeds with naturally occurring myostatin mutations. However, precision engineering of myostatin mutations in mammals leaves the reproductive issues unresolved. Previous research shows that translation of the myostatin gene is partially regulated by endogenous microRNA, indicating that the myostatin gene is a good candidate for RNAi knockdown (Miretti et al. 2013). Multiple groups have now successfully produced myostatin knockdown animals, by delivery of siRNA molecules in mice (Adachi et al. 2010) and through transgenic integration of shRNA constructs in cattle (Tessanne et al. 2012) and sheep (Hu et al. 2013). These RNAi-based approaches offer some advantages over the precision engineered knockout animals as RNAi knocks down gene expression, but does not completely knock it out, which could lead to a more moderate muscle hypertrophy phenotype. Overall, RNAi-based approaches allow for greater customisation than gene deletion; however, the shRNA constructs used to produce myostatin knockdown in large animals to date have utilised constitutive promoters to drive abundant transcription of small RNA (Ma et al. 2014).
As our understanding of RNAi improves, systems to tailor the knockdown of myostatin expression – in a tissue- or time-specific manner – could allow for enhanced muscle development without negative reproductive outcomes. For example, precision genome engineering could be used to place a shRNA construct in a known ‘safe harbour’ in the genome, which would not disrupt transcription of any endogenous genes. These systems will likely have promoters that primarily function in postnatal muscle tissue, such as FBXO40 (Ye et al. 2007) or the muscle creatine kinase promoter (Trask and Billadello 1990). The muscle creatine kinase promoter has been successfully used to drive muscle-specific transgene expression in mice (Johnson et al. 1989; Brüning et al. 1998; Wang et al. 2008; Li et al. 2013). Additionally, the muscle creatine kinase promoter was used to drive production of artificial microRNA targeted at activin receptor type IIB, another regulator of muscle fibre size, in goat myoblasts in vitro, resulting in up to 57% protein silencing and enhanced proliferation and differentiation of myoblasts (Patel et al. 2014). Although tissue-specific RNAi systems for myostatin knockdown have not yet been used in livestock in vivo, successful knockdown of the known milk allergen β-lactoglobulin specifically in the mammary gland of cattle has been achieved (Jabed et al. 2012), which demonstrates the promise of such approaches.
Exogenous siRNA could also be useful for modulating development early in life. Using a chemically modified oligonucleotide intended to induce translational repression of the target gene (known as a morpholino; Morcos et al. 2008) Brooks et al. (2015) demonstrated the dramatic influence of peroxisome proliferator activator receptor gamma on elongation of the ovine conceptus between Days 7 and 14 after fertilisation. These investigators used osmotic minipumps to deliver the morpholinos into the uterus over 7 days. Although the goal of this particular study was not to influence postnatal characteristics of the offspring, it nevertheless shows the potential for targeted gene knockdown at critical prenatal stages to alter developmental physiology. Less invasive strategies for RNAi manipulation of the reproductive tract have also been investigated (Yang et al. 2013).
Altering sex ratios. Another major area of interest is the generation of single sex populations. In the poultry layer industry, for example, modulation of sex determination to generate only female chickens would greatly increase productivity. It would also provide an alternative to culling of non-laying male chicks, which currently presents a major welfare concern to the egg industry. Female-only hatches may also lead to increased uptake of in ovo delivered vaccines in the layer industry, improving control of diseases that impact egg production. Conversely, the generation of only male chicks would be of great benefit to the broiler industry as male chickens grow faster than females.
The most attractive way of stably manipulating sex is by modifying the activity of key sex-determining genes during embryonic development. In 2009 the Z-linked gene, DMRT1, was identified as a major factor required for testis development in the chicken embryo (Smith et al. 2009). Retrovirus-delivered RNAi targeting DMRT1 induced the gonads of genetic males (ZZ) to develop partial or complete ovaries, suggesting that it may be possible to produce genetically male chickens with female attributes. Similarly, aromatase expression is a hallmark of female development in chickens (Lambeth et al. 2013). By using RNAi to knockdown aromatase in the broiler industry, it may be possible to produce only male chickens.
In silkworms (Bombyx mori), male-only rearing has long been desired because males show better fitness, lesser food consumption, and greater silk yield (Nagaraju 2002). Using TALEN to specifically knock out the female Bmdsx gene led to 100% of the resulting females being sterile and having developmental defects, pointing to a potential use for RNAi in silk production (Xu et al. 2014).
Supporting transitions between physiological states
Across livestock industries, many of the most difficult health challenges occur as animals adapt to a new stage of life or environment. In all species (wild and domestic), transitioning to a new physiological state induces stress that can impair health and productivity. Examples include neonatal morbidity and mortality (Mellor and Stafford 2004), post-weaning stress in cattle (Duff and Galyean 2007) and pigs (Pluske et al. 1997), pregnancy toxaemia in sheep (Mavrogianni and Brozos 2008), osteoporosis in laying hens (Webster 2004), transition disorders in early lactation dairy cattle (Vergara et al. 2014), and heat stress across species (Renaudeau et al. 2012). Much effort has gone into using genetics, nutrition, environmental management, and traditional pharmaceuticals to prevent these costly problems, yet many of these issues remain a thorn in the side of livestock producers.
As a tool that can precisely alter animal function, RNAi offers exciting possibilities for aiding the adaptation to a new physiological state. Furthermore, because the onset of these health challenges are predictable and they diminish once a new steady-state is achieved, the short window of efficacy for a single exogenous siRNA treatment is of limited concern. As an example of the potential use of siRNA as an adaptation aide, we will consider the case of the dairy cow transitioning into lactation.
Metabolic and infectious diseases affect more than 50% of all dairy cattle in the first 2 weeks of lactation (Vergara et al. 2014), manifested in a variety of clinical symptoms including ketosis, displaced abomasum, milk fever, mastitis, and metritis. Such problems not only harm the welfare of animals, but also contribute to many cows being removed from the lactating herd during early lactation, limiting the profitability and sustainability of the dairy industry. With the exception of milk fever, the incidence of these problems has not decreased in the past 25 years (USDA 2007). The reason for the high incidence of some of these disorders in early lactation remain unresolved despite decades of research. Arguably, this slow progress is largely due to the lack of efficacious tools to interrupt specific signals that may contribute to disease states during the transition to lactation.
The centrality of the liver in many of the transition cow problems provides a particularly good opportunity to employ RNAi tools, because intravenous delivery of siRNA nanoparticles consistently results in liver uptake of siRNA, often along with delivery to the spleen and kidneys (Jiang et al. 2011). Hypothesised roles for oxidative stress response pathways in postpartum liver dysfunction (Gessner et al. 2013), as an example, could be evaluated by targeting one or more pathway members through RNA silencing. If cows respond to exogenous siRNA in a manner similar to mice, it may be possible to suppress target protein expression by >90% for at least 2 weeks after a single intravenous treatment with a siRNA nanoparticle (Love et al. 2010). Therefore, siRNA delivery may help to prevent maladaptive responses and subsequent diseases during the transition to lactation. Even if not successful at preventing disease, such a strategy could at least rule out proposed mechanisms underlying these disorders.
Combating infectious disease
Infectious diseases remain a substantial problem in animal agriculture, with different but overlapping issues in intensive and extensive production systems (Perry et al. 2013). Despite progress in research technologies, diagnostic capabilities, and manufacturing methods, there remain many infectious diseases for which no effective vaccines exist. Furthermore, vaccination can drive the emergence of resistant and more infectious viruses, as in the case of Mareks disease virus of poultry (Witter 1997; Nair 2005). Many of the existing viral vaccines are ineffective against the prevailing strains in the field, and new vaccines have to be generated from field strains with each new outbreak, which can take months. Global availability, field compliance, effectiveness, and safety are also significant concerns.
Despite the need for new and improved vaccines, the development of vaccines is becoming more complex and expensive. There is also significant concern by the public about the use of antibiotics and other pharmaceutical products in agricultural animals, which has significant impact on control of many diseases. Ongoing work is exploring three distinct uses of RNAi to overcome these challenges to combat infectious diseases in livestock.
Directly targeting pathogens/disease agents. RNAi has an advantage over classical vaccines and small drug molecules in that it is highly selective for the pathogen and can be made to large scale very quickly (Dykxhoorn and Lieberman 2005). Entire genomes of emerging viruses can be sequenced within a day with the advent of high throughput sequencing. Once this is known, siRNA can be rapidly designed using published algorithms and can be tested in vitro and in vivo within days. By comparison, it can take months to screen the vast number of candidate small drug molecules against a new virus or to develop and test inactivated vaccines.
RNAi has been shown to inhibit a wide range of agriculturally significant pathogens both in vitro and in vivo, including foot and mouth disease virus (FMDV; Jiao et al. 2013; Gismondi et al. 2014), African swine fever virus (Keita et al. 2010), classical swine fever virus (Porntrakulpipat et al. 2010), influenza A (Stoppani et al. 2015), highly pathogenic avian influenza (Stewart et al. 2011), chicken anaemia virus (Hinton and Doran 2008), bovine viral diarrhoea virus (Lambeth et al. 2007), and infectious bursal disease virus (Wang et al. 2010).
When directly targeting a pathogen, the RNAi target site needs to be in a highly conserved region of the genome (usually within a gene essential for replication) and must take into account the three-dimensional structure of the viral genome. In the case of FMDV, the conformational folding of the RNA genome has been shown to inhibit the activity of a range of siRNA sequences (Gismondi et al. 2014). This has also been demonstrated in HIV (Westerhout et al. 2005). Studies have also shown that to ensure complete coverage of the population and to avoid the emergence of siRNA-resistant mutants, multiple siRNA sequences should be employed (Kahana et al. 2004; ter Brake et al. 2006).
Four examples of transgenic animals expressing shRNA targeting disease agents have been published. The first example was the development of a goat that expressed shRNA targeting the prion protein that causes transmissible spongiform encephalopathies (Golding et al. 2006). The transgenic fetus was collected on day 81 of gestation and had a >90% reduction in prion protein abundance in the brain compared with a non-transgenic fetus at a similar stage of development. Using a similar approach to produce a viable calf expressing an anti-prion shRNA, Wongsrikeao et al. (2011) reported that the prion mRNA and protein levels in nervous tissue were 24% and 86% lower, respectively, compared with the Control animals. More recently, porcine reproductive and respiratory syndrome shRNA pigs were produced. These animals survived 11 days longer than the Control pigs when challenged with porcine reproductive and respiratory syndrome, with the siRNA working early in infection, similar to the vaccinated Control group (Li et al. 2014). In the same year, transgenic mice expressing two anti-FMDV shRNA, targeting the viral polymerase protein 3D and the non-structural protein 2B regions, showed 19–27% higher survival rates than wild type mice (Chang et al. 2014). In each of these examples, moderate inhibition of the disease or knockdown of the gene target was found. Although these approaches are in the early stage of development, RNAi combined with transgenic technology offers the possibility to genetically engineer livestock to promote resistance to viral infections and prion diseases.
There are multiple examples of exogenously delivered siRNA inhibiting viral infections, including important human pathogens such as influenza virus, respiratory syncytial virus, and hepatitis viruses B and C in animal models, and siRNA therapeutics targeting respiratory syncytial virus and Ebola virus have been evaluated in clinical trials (Kanasty et al. 2013). Few examples, however, are published for agriculturally important diseases. Inhibition of influenza virus infection has been demonstrated in both mice and chicken embryos (Tompkins et al. 2004; Hinton et al. 2014), and adenovirus constructs expressing shRNA were shown to successfully inhibit FMDV in guinea-pigs (Xu et al. 2012). Exogenously delivered RNAi has also been shown to be functional in aquatic species. In lower vertebrates, RNAi can be delivered as long dsRNA, as in the example where L. vannamei shrimp were protected from White spot syndrome virus (Robalino et al. 2005). Another study demonstrated protection from yellow head virus by injection of a dsRNA (Saksmerprome et al. 2009). Penaeus japonicus (the kuruma prawn) was also protected from White spot syndrome virus after siRNA injection (Xu et al. 2007). Exogenously delivered siRNA can be a useful therapeutic tool and should be considered as an alternative strategy in the control of livestock diseases.
Targeting disease vectors. An alternative strategy for disease control with RNAi is to target the animal or insect vector that transmits the disease. The sterile insect technique (SIT) is a common method to biologically control pest insects that heavily damage agriculture and forestry or transmit deadly diseases to animals and humans (Whyard et al. 2015). In America SIT has successfully eradicated the screw worm (blow) fly that caused significant losses to the livestock industries, due to infestation of maggots in the wounds of cattle and other livestock. Similarly, in Zanzibar SIT eradicated the tsetse fly, which was responsible for cyclical transmission of the disease trypanosomosis and annual losses estimated at US$2 million due to loss of calves, reduced meat and milk production and disease control costs. Traditional SIT works by releasing overwhelming numbers of males steralised by radiation. The sterile males compete with the wild males for female insects, thus reducing the next generation’s population. Whyard et al. (2015) demonstrated that feeding sex-specific dsRNA to larvae of the dengue vector Aedes aegypti halted sperm production in adult males, producing fit substantially sterile males. Therefore, sterilising males by administering RNAi through food sources or by transgenics could provide an environmentally friendly, sustainable and humane approach to controlling insect pests (Whyard et al. 2015).
Another strategy is to target genes that can decrease colonisation and transmission of the insect vector. Multiple tick genera such as Dermacentor, Ixodes, Margaropus, and Rhipicephalus from the hard bodied tick (Ixodidae) family and Argas, Ornithodoros, and Otobius from the soft tick (Argasidae) family are responsible for the transmission of numerous important human and agricultural pathogens (Anderson and Magnarelli 2008). These include the bacterial pathogens Borrelia, Anaplasma, Coxiella, Francisella, Rickettsia, and Babesia, as well as viruses such as the tick-borne encephalitis virus (de la Fuente and Kocan 2006). The pathogens that cause babesiosis (B. bovis and B. bigemina) and anaplasmosis (A. marginale) are transmitted by infestation with the cattle tick, Rhipicephalus microplus. This tick also has a significant economic impact on cattle production in tropical and subtropical regions of the world by reducing weight gain and milk production. Functional analyses conducted by RNAi have been performed on multiple pathogen–tick interactions to elucidate the roles of specific genes in the pathogen lifecycle within the vector (de la Fuente and Kocan 2006; de la Fuente et al. 2007). This approach enables rapid identification of potential pathogen colonisation or transmission-blocking tick RNAi candidates (de la Fuente et al. 2007). In B. burgdorferi colonisation of the deer tick (Ixodes scapularis), the repression of TROSPA expression via RNAi reduced the bacterial adherence to the tick gut in vivo, thereby preventing efficient colonisation of the tick vector and decreasing pathogen transmission to the host (Pal et al. 2004). A functional screen of salivary proteins in I. scapularis during infection with Anaplasma phagocytophilum identified significant upregulation of the gene salp16. Subsequent RNAi silencing of this salivary protein inhibited initial infection of the tick salivary gland, eliminating the ability to transmit the pathogen (Sukumaran et al. 2006).
Similarly, new tick-specific antigens have been identified by RNAi screens, raising the possibility of vaccination strategies against tick-borne pathogens as a cost-effective, environmentally friendly alternative to chemical control (de la Fuente et al. 2005, 2007). RNAi inhibition of subolesin resulted in sterile wood ticks (Dermacentor variabilis) with under-developed salivary glands (de la Fuente et al. 2006b). Subsequently, mice vaccinated with subolesin were shown to have a 3-fold reduction in the number of I. scapularis nymphs infected with A. phagocytophilum. Subolesin-based vaccines may therefore disrupt the transmission of A. phagocytophilum by affecting pathogen infection and/or development in tick salivary glands (de la Fuente et al. 2006a).
Altering animal responses to disease. Many pathogens, particularly viruses, continually mutate, making vaccination-based control strategies difficult. New strategies to combat these diseases require an expanded knowledge of host–virus interactions as a crucial first step. Whole genome siRNA screens of infected cells have recently begun to elucidate host genes that are important in infection. In one such screening study, 21 121 pools of four chemically synthesised siRNA targeting nearly every gene in the human genome were individually transfected into a human cell line and the effect on viral replication observed (Lee et al. 2014). In the case of vesicular stomatitis virus that infects cattle, horses and pigs, the genome-wide siRNA screen identified 23 genes that reduced vesicular stomatitis virus replication when silenced, without affecting cell viability (Lee et al. 2014). Silencing one or more of these 23 host genes, which are apparently important for viral replication, may lead to a new therapeutic treatment for vesicular stomatitis virus. Similarly, FMDV is known to use host αvβ1, αvβ3, αvβ6, and αvβ8 integrins as cellular receptors to initiate infection through interactions with viral proteins. Development of transgenic suckling mice that artificially expressed αv integrin-specific miRNAs resulted in significantly higher survival rates following FMDV infection compared with their non-transgenic littermates (Du et al. 2014). Therefore, suppression of the host-cell αv integrin gene inhibited FMDV infection. Despite the promise of these approaches, it must be noted that life-long knockdown of host factors (e.g. through genomic introduction of shRNA) may have unanticipated negative consequences for the animal, as their function is sometimes unknown and may not be sufficiently redundant to avoid problems (Meliopoulos et al. 2012).
Another approach is to alter the immune response to trigger a more appropriate response to infection, which may mean driving a more aggressive response to some pathogens or limiting runaway inflammation in other pathologies (Goldsmith et al. 2011). Such strategies can take many forms, depending on the cell types and signalling cascades involved. One innovative approach was used to combat induced colitis in a mouse model (Peer et al. 2008). In this study, antibody-conjugated nanoparticles were used to target anti-cyclin D1 siRNA specifically to leukocytes. Intravenous administration of these nanoparticles dramatically improved systemic measures of health and intestinal tissue morphology, presumably by dampening the T helper 1 cytokine responses in the intestine (Peer et al. 2008). Similarly, knockdown of a proapoptotic gene from the Bcl-2 family, Bcl2-interacting mediator of cell death, increased sepsis survival to 90% compared with 50% survival in Control septic mice (Schwulst et al. 2008). In contrast to these inflammatory pathologies, hepatitis B virus interferes with pattern recognition receptor signalling, which prevents an innate immune response and allows a persistent infection to develop. To address this condition, Han and colleagues (2011) designed siRNA that targeted hepatitis B virus genes, but also triggered pattern recognition receptors to engage the innate immune system. This approach significantly slowed viral replication in vivo (Han et al. 2011). Therefore, RNAi can be used to either suppress or amplify immune system activation.
RNAi approaches can also be used to amplify vaccine response. In one example, siRNA silencing of suppressor of cytokine signalling 1 in dendritic cells before exposure to HIV proteins led to increased antibody production as well as expansion of antigen-specific T-cell populations in mice (Song et al. 2006). In vivo studies using IL-10-silencing siRNA, co-delivered with plasmid DNA encoding for the hepatitis-B surface antigen, exhibited efficient ‘switching’ towards a T helper 1 immune response and increased the cytotoxic T-cell response (Singh et al. 2008).
Whether targeting host, pathogen, or vector, the range of RNAi targets to combat infectious disease is broad and continually expanding. RNAi therapeutics, then, have the potential to improve animal welfare and the sustainability of animal agriculture in both extensive and intensive management systems. Still, there are several technical and market challenges that must be addressed before this potential can be realised.
Barriers to adoption
Despite the exciting laboratory results there are still many hurdles to overcome before RNAi therapeutics can be adopted in the field. Some are based on public perception, particularly with respect to genetically engineered animals that express RNAi molecules. Most, however, are due to the incomplete scientific understanding about how to deliver the technology. The two main methods for adoption – genome engineering and delivery of exogenous RNAi molecules – face separate hurdles.
Technology
Genome engineering. The ability to directly manipulate the DNA of agricultural animals has progressed significantly in the past several years due to the latest targeted gene editing tools including zinc fingers nucleases, TALEN and CRISPR, which enable site-specific insertion or deletion of genes and RNAi expression cassettes (Gaj et al. 2013; Sander and Joung 2014). Nevertheless, adoption of this technology to engineer animals expressing RNAi molecules is still in the early stages. Many barriers are still to be overcome in both the genome manipulation technology and the understanding of the RNAi mechanism (Gaj et al. 2013; Sander and Joung 2014).
Site-specific genome editing based on CRISPR and TALEN still requires extensive knowledge about the genome of the animal. Although most agricultural species genomes have been sequenced, identification of the role of the genes and non-coding sequences is challenging. RNAi expression cassettes are commonly inserted in the non-coding regions of the genome; however, recent evidence suggests that much of that DNA and transcribed RNA is actually important for cell function (Guttman et al. 2009; Wapinski and Chang 2011; ENCODE Project Consortium 2012; Picardi et al. 2014). Therefore, careful placement of the transgene is required. Insertion into non-specific sites is also a drawback, highlighting the need to engineer improved specificity (Gabriel et al. 2011; Mali et al. 2013).
Initial development of genetically engineered mice expressing shRNA resulted in severe medical problems due to overexpression of the RNAi molecule, which led to dysfunction in the processing of the required endogenous miRNA (Grimm et al. 2006). This has also been observed in transgenic pigs expressing anti-classical swine fever virus shRNA (Dai et al. 2014). This can be overcome by decreasing the strength of the promoter, incorporating the shRNA into an endogenous miRNA intron or using tissue-specific or transducible promoters (Furth et al. 1994; Dickins et al. 2007).
Several siRNA have also been shown to activate an immune response through toll-like receptor activation (Sledz et al. 2003; Judge et al. 2005; Stewart et al. 2011). Some toll-like receptor-activating sequences have been identified; however, rigorous testing of the shRNA sequence is required before use in transgenes. Another drawback of transgenic RNAi animals is the off target effects or suppression of genes that are not the primary target (Jackson et al. 2003; Persengiev et al. 2004). The majority of the off-target gene silencing of siRNA is due to partial sequence homology, especially within the 3ʹ untranslated region, of mRNA other than the intended target mRNA (Saxena et al. 2003; Scacheri et al. 2004). Both of these challenges also apply to synthetic RNAi delivery.
siRNA delivery. Significant research is ongoing in the use of exogenously delivered RNAi for combating a plethora of human diseases. However, due to the current cost of synthetic RNAi molecules, particularly siRNA, very little research into in vivo siRNA therapeutics for agricultural animals has been performed. The cost of oligonucleotides is expected to continue dropping, and the potential for large-scale synthesis for commercially viable siRNA would likely overcome current cost issues.
Beyond this concern, the same barriers that exist for human therapeutics will need to be overcome for animal agricultural applications. These challenges include degradation by serum nucleases, the inability to cross negatively charged lipid membranes, and clearance by the kidney.
To overcome these significant hurdles, potential siRNA delivery vehicles for human therapeutic applications are being extensively investigated. Amongst these are polymer-based nanoparticles, lipid-based nanoparticles, aggregation of the siRNA itself, and viral and bacterial carriers of RNAi molecules (Lee et al. 2013; Dong et al. 2014; Hong and Nam 2014). Many examples have shown sufficient promise that they are reaching clinical trials; however, these nanoparticles also have significant drawbacks (Rettig and Behlke 2012). These include targeting the appropriate tissue, clearance through the kidney, serum opsonisation and efficient release of the siRNA once in the cell. Nevertheless, the first phase III clinical trial of a systemic siRNA therapy, for the treatment of transthyretin-mediated amyloidosis, is now underway (https://clinicaltrials.gov/ct2/show/NCT01960348, verified 5 May 2016). This milestone provides hope that the technical challenges for RNAi therapies are surmountable and human clinical applications may finally be on the horizon.
Additional difficulties will be faced for agricultural applications. One challenge is that many of the nanoparticles under investigation require intravenous injection, which may not be feasible for large-scale agricultural applications, such as the poultry industry, or some extensive management systems in the beef industry. In such scenarios, nasal delivery via nebulisation or delivery to the gut via feed is likely to be the preferred method. Minimal research into these delivery methods is currently ongoing, with some promising results for local effects in the lung or gastrointestinal tissue, but little evidence of systemic efficacy via these routes (Rettig and Behlke 2012). Investigation into subcutaneous injection has shown some promise with simple conjugates such as the GalNAc-siRNA (Tesz et al. 2011). However, nanoparticle selection for livestock will have to include consideration of not only target animal safety but also safety of the end product, which may limit the options available for animal agriculture.
Consumer and regulatory barriers
Consumer and regulatory barriers around transgenic expression of RNAi molecules. From a commercialisation standpoint, there are numerous market barriers preventing the use of RNAi-based technologies in animal agriculture. Transgenic expression of RNAi-inducing molecules in agricultural species would face the high regulatory hurdles that all transgenic animals face, which few have passed. In the United States, transgenic animals are regulated by the Center for Veterinary Medicine of the Food and Drug Administration. Under this paradigm, transgenes are treated as drugs and require investigational new drug applications when being developed for commercial use. The review process consists of seven phases (FDA 2015). The first five phases cover transgene characterisation, including assessing the makeup of the transgene, its site of integration in the animal, expression in the animal, its transmission and durability across generations, and overall phenotypic assessment of the animal. Phenotypic assessment requires submission of clinical chemistry, haematology, histopathology, and post-mortem results for multiple transgenic animals from multiple generations to determine if there are any off-target effects.
After the genotypic and phenotypic characterisation is the food/feed safety and environmental safety assessments. The food/feed safety portion involves proving that any food product for human consumption or feed product for consumption by other animals that is derived from the transgenic animal is as safe as the conventional food/feed. This portion of the assessment can be particularly onerous, as there is often little existing data on the food/feed safety of conventionally produced items, and there is little clarity on the types of studies and desired endpoints considered acceptable to meet this requirement. The environmental safety assessment is used to evaluate the risk posed if the transgenic animal were to escape into the wild. Depending on the species, this part of the assessment can be very straightforward (for most domestic livestock species for which there are no wild populations), but this assessment can be very complicated for aquatic or avian species that have wild counterparts, like farmed trout or ducks.
After the environmental assessment, the application reaches the final phase, which is claims validation. If the purpose of the transgene is to confer disease resistance, for example, data must be provided to support that claim. Claims are often framed to include a set of conditions under which the claim is made; for example, a claim may state that transgenic animals, when maintained under appropriate husbandry conditions including adequate nutrition, appropriate vaccinations, and regular veterinary care, are resistant to the target disease.
To date, only two transgenic animal lines have made it through the United States regulatory process. The first animals approved were goats that produce recombinant therapeutic proteins in their milk, and are not approved for human consumption (Kling 2009). In November 2015, the AquAdvantage salmon became the first transgenic animal to be approved for human consumption (Ledford 2015). However, this step was approximately 20 years in the making, and all regulatory assessments, the draft Environmental Assessment, a preliminary Finding of No Significant Impact, and the public comment period were all completed by early 2013, more than 2 years before the final approval by the Food and Drug Administration. Currently, the regulatory burden for transgenic animals is extremely high, and for transgenic RNAi expression systems to be applied in agricultural animals, the regulatory process will likely need to become more streamlined.
In Australia, genetically engineered foods are regulated under the Australia New Zealand Food Standards Code (Australian Government 2014). The standard is an enforceable regulation with two provisions – mandatory pre-market approval (including a food safety assessment) and mandatory labelling requirements. Each food safety assessment is carried out on a case by case basis where the genetically engineered food is compared with a similar, commonly eaten conventional form. Assessment involves a molecular, toxicological, nutritional and compositional characterisation. If the genetic modification causes an unexpected effect in the food, such as increasing its allergenicity or toxicity, or decreases nutritional value, it will not be approved. At this point in time, animal feeding studies are not required, as it is believed they are unlikely to contribute any further useful information. So far, no safety concerns with any genetically engineered foods have been identified, similar to other national regulators. However, only plant-based products have been approved and tested, and regulations may change when Australia begins to consider genetically engineered animal products.
Consumer acceptance of RNAi transgenes in animal products is difficult to predict. There is certainly a small and vocal portion of the population that will not purchase these products and will actively campaign against them. However, most consumers in several countries buy and consume transgenic plant material; the question is whether they will view transgenic animals differently following regulatory approval. Consumers may be more or less likely to accept products produced from transgenic animals depending on the application, with a particular focus on whether from the point of view of the consumer it is the producer, the animal, or the consumer that is receiving the most benefit. Although animals with improved growth and efficiency alone are less likely to appeal to the general public, animals that are resistant to zoonotic diseases or have improved welfare are likely to be viewed more favourably (Heiman and Zilberman 2011).
Regulatory issues around exogenous delivery of RNAi molecules. The regulatory framework for exogenously delivered siRNA in animals has yet to be established, and we are not aware of any published work on consumer perceptions on the use of exogenous RNAi strategies. However, given that several genetically engineered crop varieties employing RNAi machinery have now been approved, the public health issues to consider around potential siRNA residues have been detailed (Petrick et al. 2013). One difference from transgenic approaches to RNAi delivery is that delivery of exogenous siRNA is unlikely to require evaluation of multiple generations of animals, as the time scale for siRNA effects are more like those of traditional pharmaceuticals. In fact, it is likely that any nanoparticles or chemical modifications necessary to deliver siRNA to target organs will raise greater concern than the siRNA itself, and nanoparticle toxicology as well as degradation and clearance kinetics will need to be determined to assess whether withdrawal times might be necessary following such treatments. These details will depend heavily on the class of nanoparticles that emerges as the best fit for livestock applications of RNAi.
|
Conclusions
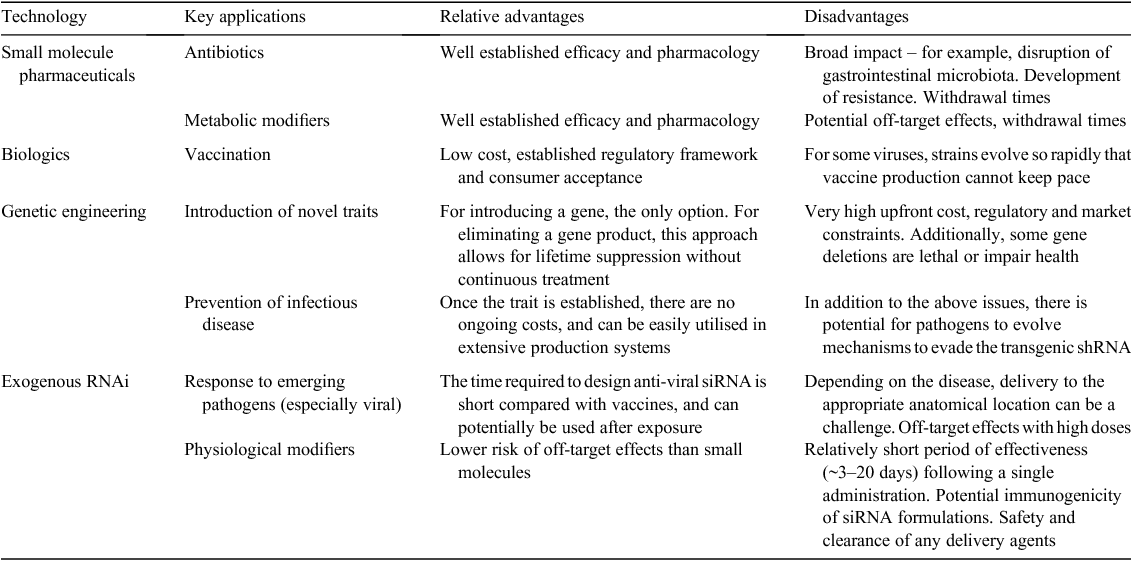
Efforts to improve the health and productivity of livestock through genetics, nutrition, and management improvements over the past 100 years have resulted in dramatic progress in the quality and sustainability of animal-derived products. Nevertheless, continuing challenges such as shifting market demands, climate change, problematic transitions between physiological states, and emerging infectious diseases require continued efforts to meet the growing demand for these products without additional inputs. RNAi offers exciting novel approaches to these challenges (Table 1). The ability of dsRNA molecules to precisely induce the silencing of a single mRNA target, coupled with technologies to deliver the dsRNA to target cells, offers hope of developing RNAi therapeutics with few side effects. Genetic engineering can be used to hard-wire the RNAi process, or exogenous RNAi approaches can be used, sidestepping consumer resistance to genetic engineering in food animals. However, numerous hurdles remain for RNAi in animal agriculture, including the lack of efficient techniques to deliver exogenous siRNA to certain organs, the current cost of both dsRNA and delivery nanoparticles, regulatory uncertainty, and questions about how consumers will perceive this technology.
Acknowledgements
This review was partially supported by a Senior Scholar award to BJB from the Australian-American Fulbright Commission.
References
Adachi T, Kawakami E, Ishimaru N, Ochiya T, Hayashi Y, Ohuchi H, Tanihara M, Tanaka E, Noji S (2010) Delivery of small interfering RNA with a synthetic collagen poly(Pro-Hyp-Gly) for gene silencing in vitro and in vivo. Development, Growth & Differentiation 52, 693–699.| Delivery of small interfering RNA with a synthetic collagen poly(Pro-Hyp-Gly) for gene silencing in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVWjs7fN&md5=568c1c3691ec22b03b8641d70afc9749CAS |
Anderson JF, Magnarelli LA (2008) Biology of ticks. Infectious Disease Clinics of North America 22, 195–215.
| Biology of ticks.Crossref | GoogleScholarGoogle Scholar | 18452797PubMed |
Australian Government (2014) Australia New Zealand food standards code – Standard 1.5.2 – Food produced using gene technology. Available at www.comlaw.gov.au/Details/F2014C01175 [Verified 3 August 2015]
Bitko V, Musiyenko A, Shulyayeva O, Barik S (2005) Inhibition of respiratory viruses by nasally administered siRNA. Nature Medicine 11, 50–55.
| Inhibition of respiratory viruses by nasally administered siRNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXovF2q&md5=79c8c508eb1e76c341263736aa7893d9CAS | 15619632PubMed |
Brock A, Krause S, Li H, Kowalski M, Goldberg MS, Collins JJ, Ingber DE (2014) Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Science Translational Medicine 6, 217ra2
| Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice.Crossref | GoogleScholarGoogle Scholar | 24382894PubMed |
Brooks KE, Burns GW, Spencer TE (2015) Peroxisome proliferator activator receptor gamma (PPARG) regulates conceptus elongation in sheep. Biology of Reproduction 92, 42
| Peroxisome proliferator activator receptor gamma (PPARG) regulates conceptus elongation in sheep.Crossref | GoogleScholarGoogle Scholar | 25519185PubMed |
Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Molecular Cell 2, 559–569.
| A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance.Crossref | GoogleScholarGoogle Scholar | 9844629PubMed |
Capper JL (2011) Replacing rose-tinted spectacles with a high-powered microscope: the historical versus modern carbon footprint of animal agriculture. Animal frontiers 1, 26–32.
| Replacing rose-tinted spectacles with a high-powered microscope: the historical versus modern carbon footprint of animal agriculture.Crossref | GoogleScholarGoogle Scholar |
Chang K, Qian J, Jiang M, Liu YH, Wu MC, Chen CD, Lai CK, Lo HL, Hsiao CT, Brown L, Bolen J, Huang HI, Ho PY, Shih PY, Yao CW, Lin WJ, Chen CH, Wu FY, Lin YJ, Wang K (2002) Effective generation of transgenic pigs and mice by linker based sperm-mediated gene transfer. BMC Biotechnology 2, 5
| Effective generation of transgenic pigs and mice by linker based sperm-mediated gene transfer.Crossref | GoogleScholarGoogle Scholar | 11964188PubMed |
Chang Y, Dou Y, Bao H, Luo X, Liu X, Mu K, Liu Z, Liu X, Cai X (2014) Multiple microRNAs targeted to internal ribosome entry site against foot-and-mouth disease virus infection in vitro and in vivo. Virology Journal 11, 1
| Multiple microRNAs targeted to internal ribosome entry site against foot-and-mouth disease virus infection in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 24393133PubMed |
Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nature Reviews. Genetics 8, 884–896.
| Specialization and evolution of endogenous small RNA pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFOksL7K&md5=137e110701c1610b7fa0d16407935c7bCAS | 17943195PubMed |
Collares T, Campos VF, de Leon PMM, Cavalcanti PV, Amaral MG, Dellagostin OA, Deschamps JC, Seixas FK (2011) Transgene transmission in chickens by sperm-mediated gene transfer after seminal plasma removal and exogenous DNA treated with dimethylsulfoxide or N, N-dimethylacetamide. Journal of Biosciences 36, 613–620.
| Transgene transmission in chickens by sperm-mediated gene transfer after seminal plasma removal and exogenous DNA treated with dimethylsulfoxide or N, N-dimethylacetamide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Giu7vO&md5=0f9eb84c760c5c600515d7af73be85a0CAS | 21857108PubMed |
Dai Z, Wu R, Zhao YC, Wang KK, Huang YY, Yang X, Xie ZC, Tu CC, Ouyang HS, Wang TD, Pang DX (2014) Early lethality of shRNA-transgenic pigs due to saturation of microRNA pathways. Journal of Zhejiang University. Science. B 15, 466–473.
| Early lethality of shRNA-transgenic pigs due to saturation of microRNA pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXotFSrurg%3D&md5=6d41064b61155742e9a80d7f5aef55b0CAS | 24793764PubMed |
de la Fuente J, Kocan KM (2006) Strategies for development of vaccines for control of ixodid tick species. Parasite Immunology 28, 275–283.
| Strategies for development of vaccines for control of ixodid tick species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1ynuro%3D&md5=519fdbcc5e2cdd2399d3bd4a4d1cb55fCAS | 16842264PubMed |
de la Fuente J, Almazán C, Blouin E, Naranjo V, Kocan K (2005) RNA interference screening in ticks for identification of protective antigens. Parasitology Research 96, 137–141.
| RNA interference screening in ticks for identification of protective antigens.Crossref | GoogleScholarGoogle Scholar | 15824899PubMed |
de la Fuente J, Almazán C, Blouin E, Naranjo V, Kocan K (2006a) Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin. Parasitology Research 100, 85–91.
| Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin.Crossref | GoogleScholarGoogle Scholar | 16816958PubMed |
de la Fuente J, Almazán C, Naranjo V, Blouin EF, Meyer JM, Kocan KM (2006b) Autocidal control of ticks by silencing of a single gene by RNA interference. Biochemical and Biophysical Research Communications 344, 332–338.
| Autocidal control of ticks by silencing of a single gene by RNA interference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvFWntrs%3D&md5=a56beadb0673eac8ca6a0695fbae49a8CAS | 16630571PubMed |
de la Fuente J, Kocan KM, Almazán C, Blouin EF (2007) RNA interference for the study and genetic manipulation of ticks. Trends in Parasitology 23, 427–433.
| RNA interference for the study and genetic manipulation of ticks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsFGmsLg%3D&md5=99d661187690143ec12e4fc173683984CAS | 17656154PubMed |
Dickins RA, McJunkin K, Hernando E, Premsrirut PK, Krizhanovsky V, Burgess DJ, Kim SY, Cordon-Cardo C, Zender L, Hannon GJ, Lowe SW (2007) Tissue-specific and reversible RNA interference in transgenic mice. Nature Genetics 39, 914–921.
| Tissue-specific and reversible RNA interference in transgenic mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvFKls7w%3D&md5=bb4c1530b54ef57b2165953fd14ec1f1CAS | 17572676PubMed |
Dong Z, Ge J, Li K, Xu Z, Liang D, Li J, Li J, Jia W, Li Y, Dong X, Cao S, Wang X, Pan J, Zhao Q (2011) Heritable targeted inactivation of myostatin gene in yellow catfish (Pelteobagrus fulvidraco) using engineered zinc finger nucleases. PLoS One 6, e28897
| Heritable targeted inactivation of myostatin gene in yellow catfish (Pelteobagrus fulvidraco) using engineered zinc finger nucleases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xis1Snsg%3D%3D&md5=4b26338db77b00c4039817c10ffdb326CAS | 22194943PubMed |
Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, Alabi CA, Sahay G, Olejnik KT, Wang W, Schroeder A, Lytton-Jean AK, Siegwart DJ, Akinc A, Barnes C, Barros SA, Carioto M, Fitzgerald K, Hettinger J, Kumar V, Novobrantseva TI, Qin J, Querbes W, Koteliansky V, Langer R, Anderson DG (2014) Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America 111, 3955–3960.
| Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtlyhsL4%3D&md5=60b44ba660a38334e2a8781e15472cb9CAS | 24516150PubMed |
Du J, Guo X, Gao S, Luo J, Gong X, Hao C, Yang B, Lin T, Shao J, Cong G, Chang H (2014) Induction of protection against foot-and-mouth disease virus in cell culture and transgenic suckling mice by miRNA targeting integrin αv receptor. Journal of Biotechnology 187, 154–161.
| Induction of protection against foot-and-mouth disease virus in cell culture and transgenic suckling mice by miRNA targeting integrin αv receptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVOmsLzL&md5=b2e3d80b7ced885cc08ccd6267372466CAS | 25016204PubMed |
Duff GC, Galyean ML (2007) Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. Journal of Animal Science 85, 823–840.
| Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXit1Wktbs%3D&md5=47318506abbd8440920e2ed36c6926edCAS | 17085724PubMed |
Dumortier O, Hinault C, Van Obberghen E (2013) MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metabolism 18, 312–324.
| MicroRNAs and metabolism crosstalk in energy homeostasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtV2rsL%2FI&md5=5fdd088b6793683b3ad0c0c7befba74dCAS | 23850315PubMed |
Dykxhoorn DM, Lieberman J (2005) The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annual Review of Medicine 56, 401–423.
| The silent revolution: RNA interference as basic biology, research tool, and therapeutic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisVSrtro%3D&md5=e1e4b2fee7922d0dbd837cd47d34ecdbCAS | 15660519PubMed |
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498.
| Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1ejt7Y%3D&md5=173a3747f1a488cb4d8912a83e556ca3CAS | 11373684PubMed |
ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74.
Engels JW (2013) Gene silencing by chemically modified siRNAs. New Biotechnology 30, 302–307.
| Gene silencing by chemically modified siRNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVGitrw%3D&md5=0d43733fe6dcd890246e01595e865dc9CAS | 22820489PubMed |
FDA (2015) Guidance for Industry: regulation of genetically engineered animals containing heritable recombinant DNA constructs [Online]. Available at http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm113903.pdf [Verified 3 August 2015]
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811.
| Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtlCju74%3D&md5=c39dcd3936ce512fb26405aef182c2adCAS | 9486653PubMed |
Furth PA, St Onge L, Böger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L (1994) Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proceedings of the National Academy of Sciences of the United States of America 91, 9302–9306.
| Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmvFeqt7c%3D&md5=ebad0cd1dae4c4daffe712b260a16b1cCAS | 7937760PubMed |
Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman G, Holmes MC, Gregory PD, Glimm H, Schmidt M, Naldini L, von Kalle C (2011) An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nature Biotechnology 29, 816–823.
| An unbiased genome-wide analysis of zinc-finger nuclease specificity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvVOmur0%3D&md5=6ade2f5cf2d5430b785019fcb8b67d4fCAS | 21822255PubMed |
Gaj T, Gersbach CA, Barbas CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology 31, 397–405.
| ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsVyiu7c%3D&md5=cfe3fffce532f86f6d06a25e2d923959CAS | 23664777PubMed |
Gama Sosa MA, De Gasperi R, Elder GA (2010) Animal transgenesis: an overview. Brain Structure & Function 214, 91–109.
| Animal transgenesis: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktVCnsbs%3D&md5=dd071a707c667bc38f223c570adc9457CAS |
Gessner DK, Schlegel G, Keller J, Schwarz FJ, Ringseis R, Eder K (2013) Expression of target genes of nuclear factor E2-related factor 2 in the liver of dairy cows in the transition period and at different stages of lactation. Journal of Dairy Science 96, 1038–1043.
| Expression of target genes of nuclear factor E2-related factor 2 in the liver of dairy cows in the transition period and at different stages of lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVCrsbbM&md5=d7bc0dead22799f5cfb035a43913940cCAS | 23245956PubMed |
Gismondi MI, Ortiz XP, Currá AP, Asurmendi S, Taboga O (2014) Artificial microRNAs as antiviral strategy to FMDV: structural implications of target selection. Journal of Virological Methods 199, 1–10.
| Artificial microRNAs as antiviral strategy to FMDV: structural implications of target selection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjsVWlt7w%3D&md5=6618ec05232a5d2e6e00a9765bf96c4cCAS | 24406623PubMed |
Golding MC, Long CR, Carmell MA, Hannon GJ, Westhusin ME (2006) Suppression of prion protein in livestock by RNA interference. Proceedings of the National Academy of Sciences of the United States of America 103, 5285–5290.
| Suppression of prion protein in livestock by RNA interference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjslaqtbo%3D&md5=1e24c108926a3774fce0e30424a5fa7fCAS | 16567624PubMed |
Goldsmith M, Mizrahy S, Peer D (2011) Grand challenges in modulating the immune response with RNAi nanomedicines. Nanomedicine (London) 6, 1771–1785.
| Grand challenges in modulating the immune response with RNAi nanomedicines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFCms7fO&md5=e6db9887662648bf9507c8ebdb3fbb91CAS |
Gordon JW, Ruddle FH (1981) Integration and stable germ line transmission of genes injected into mouse pronuclei. Science 214, 1244–1246.
| Integration and stable germ line transmission of genes injected into mouse pronuclei.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XivFersA%3D%3D&md5=feb1c26d64f5190ff3d43246069beb22CAS | 6272397PubMed |
Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441, 537–541.
| Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvVyku7g%3D&md5=386f6fcfae653d015f67a1f3153d8d77CAS | 16724069PubMed |
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227.
| Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehsbo%3D&md5=c62e1f85070df18fbe6a9580092fd2d5CAS | 19182780PubMed |
Han Q, Zhang C, Zhang J, Tian Z (2011) Reversal of hepatitis B virus‐induced immune tolerance by an immunostimulatory 3p‐HBx‐siRNAs in a retinoic acid inducible gene I–dependent manner. Hepatology (Baltimore, Md.) 54, 1179–1189.
| Reversal of hepatitis B virus‐induced immune tolerance by an immunostimulatory 3p‐HBx‐siRNAs in a retinoic acid inducible gene I–dependent manner.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1amtbzN&md5=b9bef95d5ca65adccfc9b587f0af936dCAS |
Heiman A, Zilberman D (2011) The effects of framing on consumers’ choice of GM foods. AgBioForum 14, 171–179.
Hinton TM, Doran TJ (2008) Inhibition of chicken anaemia virus replication using multiple short-hairpin RNAs. Antiviral Research 80, 143–149.
| Inhibition of chicken anaemia virus replication using multiple short-hairpin RNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ensLnM&md5=04b149b2262453243ea00a40bceb3a63CAS | 18603312PubMed |
Hinton TM, Challagulla A, Stewart CR, Guerrero-Sanchez C, Grusche FA, Shi S, Bean AG, Monaghan P, Gunatillake PA, Thang SH, Tizard ML (2014) Inhibition of influenza virus in vivo by siRNA delivered using ABA triblock copolymer synthesized by reversible addition-fragmentation chain-transfer polymerization. Nanomedicine (London) 9, 1141–1154.
| Inhibition of influenza virus in vivo by siRNA delivered using ABA triblock copolymer synthesized by reversible addition-fragmentation chain-transfer polymerization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlKmsb3O&md5=4ede31537ce403e12e84bcb8c0d3dfd5CAS |
Hong CA, Nam YS (2014) Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics 4, 1211–1232.
| Functional nanostructures for effective delivery of small interfering RNA therapeutics.Crossref | GoogleScholarGoogle Scholar | 25285170PubMed |
Hu S, Ni W, Sai W, Zi H, Qiao J, Wang P, Sheng J, Chen C (2013) Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep. PLoS One 8, e58521
| Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltFyqt7c%3D&md5=bd938ebf901102823903a3f3533a6675CAS | 23526994PubMed |
Inui M, Martello G, Piccolo S (2010) MicroRNA control of signal transduction. Nature Reviews. Molecular Cell Biology 11, 252–263.
| MicroRNA control of signal transduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivFKnsbg%3D&md5=f75cfaa1d9086e04864cc9132cc08a9dCAS | 20216554PubMed |
Iwakawa HO, Tomari Y (2015) The functions of microRNAs: mRNA decay and translational repression. Trends in Cell Biology 25, 651–665.
| The functions of microRNAs: mRNA decay and translational repression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1Sht7rE&md5=5153354c16992a3391c468776f7195a3CAS | 26437588PubMed |
Jabed A, Wagner S, McCracken J, Wells DN, Laible G (2012) Targeted microRNA expression in dairy cattle directs production of β-lactoglobulin-free, high-casein milk. Proceedings of the National Academy of Sciences of the United States of America 109, 16811–16816.
| Targeted microRNA expression in dairy cattle directs production of β-lactoglobulin-free, high-casein milk.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Wks7fE&md5=0a584a152d26b8b47d703a94796fb2faCAS | 23027958PubMed |
Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS (2003) Expression profiling reveals off-target gene regulation by RNAi. Nature Biotechnology 21, 635–637.
| Expression profiling reveals off-target gene regulation by RNAi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFSlu7c%3D&md5=8df93642ddfc406c52bc55e3cfda5832CAS | 12754523PubMed |
Jiang N, Zhang X, Zheng X, Chen D, Zhang Y, Siu LKS, Xin HB, Li R, Zhao H, Riordan N, Ichim TE, Quan D, Jevnikar AM, Chen G, Min W (2011) Targeted gene silencing of TLR4 using liposomal nanoparticles for preventing liver ischemia reperfusion injury. American Journal of Transplantation 11, 1835–1844.
| Targeted gene silencing of TLR4 using liposomal nanoparticles for preventing liver ischemia reperfusion injury.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12ktLrN&md5=4703935e2c0c1748522889bb7ccb88a7CAS | 21794086PubMed |
Jiao Y, Gong X, Du J, Liu M, Guo X, Chen L, Miao W, Jin T, Chang H, Zeng Y, Zheng Z (2013) Transgenically mediated shRNAs targeting conserved regions of foot-and-mouth disease virus provide heritable resistance in porcine cell lines and suckling mice. Veterinary Research 44, 47
| Transgenically mediated shRNAs targeting conserved regions of foot-and-mouth disease virus provide heritable resistance in porcine cell lines and suckling mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1ersLbI&md5=daf0e6be1e56d6688b08c4d0cc5eae97CAS | 23822604PubMed |
Johnson JE, Wold BJ, Hauschka SD (1989) Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Molecular and Cellular Biology 9, 3393–3399.
| Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXlt1yiur8%3D&md5=f49b1efc3815ad39dbbe3c884de1e404CAS | 2796990PubMed |
Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnology 23, 457–462.
| Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivFOhtLk%3D&md5=022e49a7ae2e90885c79c23c98d8a2b7CAS | 15778705PubMed |
Kahana R, Kuznetzova L, Rogel A, Shemesh M, Hai D, Yadin H, Stram Y (2004) Inhibition of foot-and-mouth disease virus replication by small interfering RNA. The Journal of General Virology 85, 3213–3217.
| Inhibition of foot-and-mouth disease virus replication by small interfering RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpsVSms7g%3D&md5=9d72c1c46dbb7ea7a057f1071da9a6a7CAS | 15483234PubMed |
Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Research 7, 910–916.
Kanasty R, Dorkin JR, Vegas A, Anderson D (2013) Delivery materials for siRNA therapeutics. Nature Materials 12, 967–977.
| Delivery materials for siRNA therapeutics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Grt7rM&md5=2a1107998d5245e2b6e7e294d1ccc244CAS | 24150415PubMed |
Kawakami S, Hashida M (2007) Targeted delivery systems of small interfering RNA by systemic administration. Drug Metabolism and Pharmacokinetics 22, 142–151.
| Targeted delivery systems of small interfering RNA by systemic administration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpslCmtL0%3D&md5=6894ac99511646d4b251e3b670829e42CAS | 17603214PubMed |
Keita D, Heath L, Albina E (2010) Control of African swine fever virus replication by small interfering RNA targeting the A151R and VP72 genes. Antiviral Therapy 15, 727–736.
| Control of African swine fever virus replication by small interfering RNA targeting the A151R and VP72 genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGms7rO&md5=b813cdade9bc12dad10ff642402a73ceCAS | 20710054PubMed |
Kim YJ, Maizel A, Chen X (2014) Traffic into silence: endomembranes and post-transcriptional RNA silencing. The EMBO Journal 33, 968–980.
| Traffic into silence: endomembranes and post-transcriptional RNA silencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtV2iu7%2FI&md5=9633ba512f98e7f51243775474f53f2eCAS | 24668229PubMed |
Kinouchi N, Ohsawa Y, Ishimaru N, Ohuchi H, Sunada Y, Hayashi Y, Tanimoto Y, Moriyama K, Noji S (2008) Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass. Gene Therapy 15, 1126–1130.
| Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVygtLc%3D&md5=be98366e149dd6f141cdacfb654f88e3CAS | 18323791PubMed |
Kling J (2009) First US approval for a transgenic animal drug. Nature Biotechnology 27, 302–304.
| First US approval for a transgenic animal drug.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktFaksLo%3D&md5=e8eee08ac9ac60f5bdb8b79e6697e706CAS | 19352350PubMed |
Koch A, Kogel KH (2014) New wind in the sails: improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnology Journal 12, 821–831.
| New wind in the sails: improving the agronomic value of crop plants through RNAi-mediated gene silencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVSrtL%2FO&md5=3e85fca28cd54b5b749ff716acca97e9CAS | 25040343PubMed |
Koganti SRK, Zhu Z, Subbotina E, Gao Z, Sierra A, Proenza M, Yang L, Alekseev A, Hodgson-Zingman D, Zingman L (2015) Disruption of KATP channel expression in skeletal muscle by targeted oligonucleotide delivery promotes activity-linked thermogenesis. Molecular Therapy 23, 707–716.
| Disruption of KATP channel expression in skeletal muscle by targeted oligonucleotide delivery promotes activity-linked thermogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXktVCmsL8%3D&md5=da016bcfd68c035e89505d3ec9ddc7d8CAS |
Lambeth LS, Moore RJ, Muralitharan MS, Doran TJ (2007) Suppression of bovine viral diarrhea virus replication by small interfering RNA and short hairpin RNA-mediated RNA interference. Veterinary Microbiology 119, 132–143.
| Suppression of bovine viral diarrhea virus replication by small interfering RNA and short hairpin RNA-mediated RNA interference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1emsA%3D%3D&md5=72c1bebf3c799e12f6faa38f8e662cdcCAS | 17052865PubMed |
Lambeth LS, Cummins DM, Doran TJ, Sinclair AH, Smith CA (2013) Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLoS One 8, e68362
| Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFahsLzN&md5=ee3dc11109a07377bd845ecffd7b0521CAS | 23840850PubMed |
Ledford H (2015) Salmon approval heralds rethink of transgenic animals. Nature 527, 417–418.
Lee SJ, Son S, Yhee JY, Choi K, Kwon IC, Kim SH, Kim K (2013) Structural modification of siRNA for efficient gene silencing. Biotechnology Advances 31, 491–503.
| Structural modification of siRNA for efficient gene silencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslCnt73O&md5=ae32167b72efbc8eba69f3fd73b976d8CAS | 22985697PubMed |
Lee ASY, Burdeinick-Kerr R, Whelan SPJ (2014) A genome-wide small interfering RNA screen identifies host factors required for vesicular stomatitis virus infection. Journal of Virology 88, 8355–8360.
| A genome-wide small interfering RNA screen identifies host factors required for vesicular stomatitis virus infection.Crossref | GoogleScholarGoogle Scholar |
Li T, Xu D, Zuo B, Lei M, Xiong Y, Chen H, Zhou Y, Wu X (2013) Ectopic overexpression of porcine DGAT1 increases intramuscular fat content in mouse skeletal muscle. Transgenic Research 22, 187–194.
| Ectopic overexpression of porcine DGAT1 increases intramuscular fat content in mouse skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 22826105PubMed |
Li L, Li Q, Bao Y, Li J, Chen Z, Yu X, Zhao Y, Tian K, Li N (2014) RNAi-based inhibition of porcine reproductive and respiratory syndrome virus replication in transgenic pigs. Journal of Biotechnology 171, 17–24.
| RNAi-based inhibition of porcine reproductive and respiratory syndrome virus replication in transgenic pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Grsr4%3D&md5=dd19a2dff72d341460df202901278aabCAS | 24333125PubMed |
Long CR, Tessanne KJ, Golding MC (2010) Applications of RNA interference-based gene silencing in animal agriculture. Reproduction, Fertility and Development 22, 47–58.
| Applications of RNA interference-based gene silencing in animal agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlagurk%3D&md5=784aead5a861087b38b7016bf64944d8CAS |
Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DWY, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG (2010) Lipid-like materials for low-dose, in vivo gene silencing. Proceedings of the National Academy of Sciences of the United States of America 107, 1864–1869.
| Lipid-like materials for low-dose, in vivo gene silencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFSnsr0%3D&md5=f3cdf68fb24cd15ecab2157bb9f548c8CAS | 20080679PubMed |
Ma H, Wu Y, Dang Y, Choi JG, Zhang J, Wu H (2014) Pol III promoters to express small RNAs: delineation of transcription initiation. Molecular Therapy. Nucleic Acids 3, e161
| Pol III promoters to express small RNAs: delineation of transcription initiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFagurzP&md5=bb77bb4a93ca06f7c4d34406f84af9f7CAS | 24803291PubMed |
Macdonald J, Taylor L, Sherman A, Kawakami K, Takahashi Y, Sang HM, McGrew MJ (2012) Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proceedings of the National Academy of Sciences of the United States of America 109, E1466–E1472.
| Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovF2ns7c%3D&md5=c8c1a17c0a8fd977207ce1f403f5e716CAS | 22586100PubMed |
Maksimenko OG, Deykin AV, Khodarovich YM, Georgiev PG (2013) Use of transgenic animals in biotechnology: prospects and problems. Acta Naturae 5, 33–46.
Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM (2013) CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nature Biotechnology 31, 833–838.
| CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1SjsL7I&md5=fd8b5256d858225c59c471a4417761b0CAS | 23907171PubMed |
Matveeva O (2013) What parameters to consider and which software tools to use for target selection and molecular design of small interfering RNAs. In ‘siRNA design’. (Ed. DJ Taxman) pp. 1–16. (Humana Press: New York, NY)
Mavrogianni VS, Brozos C (2008) Reflections on the causes and the diagnosis of peri-parturient losses of ewes. Small Ruminant Research 76, 77–82.
| Reflections on the causes and the diagnosis of peri-parturient losses of ewes.Crossref | GoogleScholarGoogle Scholar |
Meliopoulos VA, Andersen LE, Birrer KF, Simpson KJ, Lowenthal JW, Bean AG, Stambas J, Stewart CR, Tompkins SM, van Beusechem VW, Fraser I, Mhlanga M, Barichievy S, Smith Q, Leake D, Karpilow J, Buck A, Jona G, Tripp RA (2012) Host gene targets for novel influenza therapies elucidated by high-throughput RNA interference screens. The FASEB Journal 26, 1372–1386.
| Host gene targets for novel influenza therapies elucidated by high-throughput RNA interference screens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsVChsLo%3D&md5=5d761c8f8c83d32ad590c8f3b70ce22eCAS | 22247330PubMed |
Mellor DJ, Stafford KJ (2004) Animal welfare implications of neonatal mortality and morbidity in farm animals. Veterinary Journal (London, England) 168, 118–133.
| Animal welfare implications of neonatal mortality and morbidity in farm animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2czpvFWntQ%3D%3D&md5=838374ba13f57b04411b8e4349087b70CAS |
Miretti S, Martignani E, Accornero P, Baratta M (2013) Functional effect of mir-27b on myostatin expression: a relationship in Piedmontese cattle with double-muscled phenotype. BMC Genomics 14, 194
| Functional effect of mir-27b on myostatin expression: a relationship in Piedmontese cattle with double-muscled phenotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXot1yit7Y%3D&md5=69fa4f7e23982e82dd97e85916fdd720CAS | 23510267PubMed |
Morcos P, Li Y, Jiang S (2008) Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques 45, 613–623.
| Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFahsr7I&md5=7af26646b65b449227039dc0bb059cb4CAS | 19238792PubMed |
Moreira PN, Pozueta J, Pérez-Crespo M, Valdivieso F, Gutiérrez-Adán A, Montoliu L (2007) Improving the generation of genomic-type transgenic mice by ICSI. Transgenic Research 16, 163–168.
| Improving the generation of genomic-type transgenic mice by ICSI.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjt1Sqt7Y%3D&md5=5a61c520011aa6bd381725e0cb7e8afdCAS | 17372844PubMed |
Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL (2001) Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proceedings of the National Academy of Sciences of the United States of America 98, 13090–13095.
| Transgenic mice produced by retroviral transduction of male germ-line stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosFygsLs%3D&md5=305f956af003cace99170cca6bfbbb38CAS | 11606778PubMed |
Nagaraju J (2002) Application of genetic principles for improving silk production. Current Science 83, 409–414.
Nair V (2005) Evolution of Marek’s disease – A paradigm for incessant race between the pathogen and the host. Veterinary Journal (London, England) 170, 175–183.
| Evolution of Marek’s disease – A paradigm for incessant race between the pathogen and the host.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpslymurw%3D&md5=d6e4ad7c47efc5d55353a9c27ae4ba22CAS |
Ni W, Qiao J, Hu S, Zhao X, Regouski M, Yang M, Polejaeva IA, Chen C (2014) Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS One 9, e106718
| Efficient gene knockout in goats using CRISPR/Cas9 system.Crossref | GoogleScholarGoogle Scholar | 25188313PubMed |
Obbard DJ, Gordon KHJ, Buck AH, Jiggins FM (2009) The evolution of RNAi as a defence against viruses and transposable elements. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364, 99–115.
| The evolution of RNAi as a defence against viruses and transposable elements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtVOqsbs%3D&md5=094fb4bad809018405582a2f3f5756ebCAS | 18926973PubMed |
Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes & Development 16, 948–958.
| Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjt1Gktbs%3D&md5=d62f0eda2af93bff3d3a6d3882b275e7CAS |
Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, deSilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E (2004) TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119, 457–468.
| TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVCjt7fN&md5=aab5f71b1baee43d8110a94c205f5c30CAS | 15537536PubMed |
Patel AK, Shah RK, Patel UA, Tripathi AK, Joshi CG (2014) Goat activin receptor type IIB knockdown by muscle specific promoter driven artificial microRNAs. Journal of Biotechnology 187, 87–97.
| Goat activin receptor type IIB knockdown by muscle specific promoter driven artificial microRNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtleju7bN&md5=8995a20c61c2c030a185b517fea42ba8CAS | 25107506PubMed |
Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M (2008) Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630.
| Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Knt7g%3D&md5=a4d974c72d26cf427c92e5aa85d62645CAS | 18239128PubMed |
Perry AC, Rothman A, Jose I, Feinstein P, Mombaerts P, Cooke HJ, Wakayama T (2001) Efficient metaphase II transgenesis with different transgene archetypes. Nature Biotechnology 19, 1071–1073.
| Efficient metaphase II transgenesis with different transgene archetypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXot1eqt7Y%3D&md5=57d5fbd65b3ce0c58a47caa975bac57fCAS | 11689854PubMed |
Perry BD, Grace D, Sones K (2013) Current drivers and future directions of global livestock disease dynamics. Proceedings of the National Academy of Sciences of the United States of America 110, 20871–20877.
| Current drivers and future directions of global livestock disease dynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnsFyktQ%3D%3D&md5=88a53b5f063a9817d33d2d15560ff59cCAS | 21576468PubMed |
Persengiev SP, Zhu X, Green MR (2004) Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA (New York, N.Y.) 10, 12–18.
| Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsFWguw%3D%3D&md5=e3015578141e0f57f31e49d90010c227CAS |
Petrick JS, Brower-Toland B, Jackson AL, Kier LD (2013) Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regulatory Toxicology and Pharmacology 66, 167–176.
| Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosFOntro%3D&md5=90ff7a47853b73966c77086b3a90828eCAS | 23557984PubMed |
Picardi E, D’Erchia AM, Gallo A, Montalvo A, Pesole G (2014) Uncovering RNA editing sites in long non-coding RNAs. Frontiers in Bioengineering and Biotechnology 2, 64
| Uncovering RNA editing sites in long non-coding RNAs.Crossref | GoogleScholarGoogle Scholar | 25538940PubMed |
Pluske JR, Hampson DJ, Williams IH (1997) Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science 51, 215–236.
| Factors influencing the structure and function of the small intestine in the weaned pig: a review.Crossref | GoogleScholarGoogle Scholar |
Porntrakulpipat S, Supankong S, Chatchawanchonteera A, Pakdee P (2010) RNA interference targeting nucleocapsid protein (C) inhibits classical swine fever virus replication in SK-6 cells. Veterinary Microbiology 142, 41–44.
| RNA interference targeting nucleocapsid protein (C) inhibits classical swine fever virus replication in SK-6 cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjs1aks7k%3D&md5=2d5724e73b5e0c0d045eeab85b7d33f8CAS | 19850420PubMed |
Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, Lillico SG, Mileham AJ, McLaren DG, Whitelaw CB, Fahrenkrug SC (2015) Genome edited sheep and cattle. Transgenic Research 24, 147–153.
| Genome edited sheep and cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFSgsLrF&md5=e8ec0a8f61aa0fb4c04b8ba5ff6009a6CAS | 25204701PubMed |
Renaudeau D, Collin A, Yahav S, de Basilio V, Gourdine JL, Collier RJ (2012) Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6, 707–728.
| Adaptation to hot climate and strategies to alleviate heat stress in livestock production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38ngsFejtw%3D%3D&md5=2c39d852eef38b35bbb7ec689db67582CAS | 22558920PubMed |
Rettig GR, Behlke MA (2012) Progress toward in vivo use of siRNAs-II. Molecular Therapy 20, 483–512.
| Progress toward in vivo use of siRNAs-II.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gjs7nP&md5=0090c1d625ccc63220b90c1f5c0f977cCAS | 22186795PubMed |
Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Hammer CJ, Maddock Carlin KR, Grazul-Bilska AT, Redmer DA (2010) Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. Journal of Animal Science 88, E61–E72.
| Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3psl2ksw%3D%3D&md5=5594e3f1d85499c0e5a18c2427bfffa8CAS | 20023136PubMed |
Rivera S, Yuan F (2012) Critical issues in delivery of RNAi therapeutics in vivo. Current Pharmaceutical Biotechnology 13, 1279–1291.
| Critical issues in delivery of RNAi therapeutics in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1CgsrfL&md5=276eea512f7d4871ef0e0c5d88e027a2CAS | 22201583PubMed |
Robalino J, Bartlett T, Shepard E, Prior S, Jaramillo G, Scura E, Chapman RW, Gross PS, Browdy CL, Warr GW (2005) Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? Journal of Virology 79, 13561–13571.
| Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKjtr%2FN&md5=d45f1d8f8a2c55088d7b3699bfe21159CAS | 16227276PubMed |
Saksmerprome V, Charoonnart P, Gangnonngiw W, Withyachumnarnkul B (2009) A novel and inexpensive application of RNAi technology to protect shrimp from viral disease. Journal of Virological Methods 162, 213–217.
| A novel and inexpensive application of RNAi technology to protect shrimp from viral disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Ggur7P&md5=dbaecb1e1f5f456e20d1f6cd3ece4609CAS | 19712700PubMed |
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology 32, 347–355.
| CRISPR-Cas systems for editing, regulating and targeting genomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtlyrsLo%3D&md5=00cec15000003566e100f46d9beb8a90CAS | 24584096PubMed |
Saxena S, Jónsson ZO, Dutta A (2003) Small RNAs with imperfect match to endogenous mRNA repress translation: implications for off-target activity of small inhibitory RNA in mammalian cells. The Journal of Biological Chemistry 278, 44312–44319.
| Small RNAs with imperfect match to endogenous mRNA repress translation: implications for off-target activity of small inhibitory RNA in mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXoslWmtLY%3D&md5=7a971018f678ef2e1968a055cb074ecbCAS | 12952966PubMed |
Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M, Collins FS (2004) Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America 101, 1892–1897.
| Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhs1Sktbw%3D&md5=9176c9dc4bff2521b28a8d1714448e71CAS | 14769924PubMed |
Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS (2008) BIM siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock (Augusta, Ga.) 30, 127–134.
Shi B, Keough E, Matter A, Leander K, Young S, Carlini E, Sachs AB, Tao W, Abrams M, Howell B, Sepp-Lorenzino L (2011) Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. The Journal of Histochemistry and Cytochemistry 59, 727–740.
| Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvVyhsro%3D&md5=4b2f43dddd133fc72fab105d1c7a79e1CAS | 21804077PubMed |
Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K (2008) Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Molecular Therapy 16, 2011–2021.
| Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVSjsbvL&md5=0b4d4c6312224431b6039526c0725be7CAS | 18813280PubMed |
Sledz C, Holko M, de Veer M, Silverman R, Williams B (2003) Activation of the interferon system by short-interfering RNAs. Nature Cell Biology 5, 834–839.
| Activation of the interferon system by short-interfering RNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvVWlur4%3D&md5=4d36ab7d11423ba9a97073d437b2bf69CAS | 12942087PubMed |
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH (2009) The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271.
| The avian Z-linked gene DMRT1 is required for male sex determination in the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGiurvN&md5=264aba0c24074dbb6c6404b2fefbbb1fCAS | 19710650PubMed |
Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS (1985) Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317, 230–234.
| Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXmtVyjtr8%3D&md5=16b6834fdd2be1bda9645f130e64b0d4CAS | 2995814PubMed |
Song X, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, Chen S (2006) An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Medicine 3, e11
| An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells.Crossref | GoogleScholarGoogle Scholar | 16381597PubMed |
Stewart CR, Karpala AJ, Lowther S, Lowenthal JW, Bean AG (2011) Immunostimulatory motifs enhance antiviral siRNAs targeting highly pathogenic avian influenza H5N1. PLoS One 6, e21552
| Immunostimulatory motifs enhance antiviral siRNAs targeting highly pathogenic avian influenza H5N1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFyntLc%3D&md5=89a03ac36511584b58b6d94e324a1b3eCAS | 21747939PubMed |
Stoppani E, Bassi I, Dotti S, Lizier M, Ferrari M, Lucchini F (2015) Expression of a single siRNA against a conserved region of NP gene strongly inhibits in vitro replication of different Influenza A virus strains of avian and swine origin. Antiviral Research 120, 16–22.
| Expression of a single siRNA against a conserved region of NP gene strongly inhibits in vitro replication of different Influenza A virus strains of avian and swine origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXos1elt78%3D&md5=7efae7b30eb24504f2f6f1aeaa32fd86CAS | 25986248PubMed |
Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, Fish D, Telford SR, Kantor FS, Fikrig E (2006) An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. The Journal of Experimental Medicine 203, 1507–1517.
| An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtVantro%3D&md5=bb23ab10e453618c8df21b542bdf95baCAS | 16717118PubMed |
ter Brake O, Konstantinova P, Ceylan M, Berkhout B (2006) Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Molecular Therapy 14, 883–892.
| Silencing of HIV-1 with RNA interference: a multiple shRNA approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Cis7zJ&md5=61b1d2e71f1ea27e8640e066bc034abcCAS | 16959541PubMed |
Tessanne K, Golding MC, Long CR, Peoples MD, Hannon G, Westhusin ME (2012) Production of transgenic calves expressing an shRNA targeting myostatin. Molecular Reproduction and Development 79, 176–185.
| Production of transgenic calves expressing an shRNA targeting myostatin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFGku7jM&md5=77cf29adc1291a38b8577af205419c9bCAS | 22139943PubMed |
Tesz GJ, Aouadi M, Prot M, Nicoloro SM, Boutet E, Amano SU, Goller A, Wang M, Guo CA, Salomon WE, Virbasius JV, Baum RA, O’Connor MJ, Soto E, Ostroff GR, Czech MP (2011) Glucan particles for selective delivery of siRNA to phagocytic cells in mice. The Biochemical Journal 436, 351–362.
| Glucan particles for selective delivery of siRNA to phagocytic cells in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFGitrg%3D&md5=67be9d08386ad37ded520cf449b35489CAS | 21418037PubMed |
Tompkins SM, Lo C-Y, Tumpey TM, Epstein SL (2004) Protection against lethal influenza virus challenge by RNA interference in vivo. Proceedings of the National Academy of Sciences of the United States of America 101, 8682–8686.
| Protection against lethal influenza virus challenge by RNA interference in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFKksLk%3D&md5=67c02001c6fa12ece587c21a4ae40c7dCAS | 15173583PubMed |
Trask RV, Billadello JJ (1990) Tissue-specific distribution and developmental regulation of M and B creatine kinase mRNAs. Biochimica et Biophysica Acta 1049, 182–188.
| Tissue-specific distribution and developmental regulation of M and B creatine kinase mRNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkslGnu7Y%3D&md5=83c8c0d12801db2c16f5a81a377b3554CAS | 2364108PubMed |
USDA (2007) NAHMS Dairy 2007 Part I: reference of dairy cattle health and management practices in the United States, 2007. United States Department of Agriculture, Washington, DC.
Vergara CF, Döpfer D, Cook NB, Nordlund KV, McArt JAA, Nydam DV, Oetzel GR (2014) Risk factors for postpartum problems in dairy cows: explanatory and predictive modeling. Journal of Dairy Science 97, 4127–4140.
| Risk factors for postpartum problems in dairy cows: explanatory and predictive modeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnsVWrsrY%3D&md5=9cf41187619a4a1e50282830ac273c2eCAS | 24792805PubMed |
Waltz E (2015) Nonbrowning GM apple cleared for market [Online]. In Trade Secrets blog. Available at http://blogs.nature.com/tradesecrets/2015/03/30/nonbrowning-gm-apple-cleared-for-market [Verified 3 August 2015]
Wang B, Li J, Fu FH, Chen C, Zhu X, Zhou L, Jiang X, Xiao X (2008) Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Therapy 15, 1489–1499.
| Construction and analysis of compact muscle-specific promoters for AAV vectors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlais7rJ&md5=874452dd03a865985e7964fecc33768cCAS | 18563184PubMed |
Wang Y, Sun H, Shen P, Zhang X, Xia X, Xia B (2010) Effective inhibition of replication of infectious bursal disease virus by miRNAs delivered by vectors and targeting the VP2 gene. Journal of Virological Methods 165, 127–132.
| Effective inhibition of replication of infectious bursal disease virus by miRNAs delivered by vectors and targeting the VP2 gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVaqur4%3D&md5=0b6d3db4b9339b8a541b6fad8f0a89ddCAS | 19189848PubMed |
Wapinski O, Chang HY (2011) Long noncoding RNAs and human disease. Trends in Cell Biology 21, 354–361.
| Long noncoding RNAs and human disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntlGltLw%3D&md5=ee3bf2ee448b6eadda569c9ba655221dCAS | 21550244PubMed |
Webster AB (2004) Welfare implications of avian osteoporosis. Poultry Science 83, 184–192.
| Welfare implications of avian osteoporosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c%2FovFygsQ%3D%3D&md5=5c21a0eed3ebbf67a2758a390e3acd98CAS | 14979568PubMed |
Westerhout EM, Ooms M, Vink M, Das AT, Berkhout B (2005) HIV-1 can escape from RNA interference by evolving an alternative structure in its RNA genome. Nucleic Acids Research 33, 796–804.
| HIV-1 can escape from RNA interference by evolving an alternative structure in its RNA genome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtV2qt7g%3D&md5=d91df2acb73af8645cde317a0a72924bCAS | 15687388PubMed |
Whyard S, Erdelyan CN, Partridge AL, Singh AD, Beebe NW, Capina R (2015) Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasites & Vectors 8, 96
| Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs.Crossref | GoogleScholarGoogle Scholar |
Wise TG, Schafer DS, Lowenthal JW, Doran TJ (2008) The use of RNAi and transgenics to develop viral disease resistant livestock. Developments in Biologicals 132, 377–382.
| The use of RNAi and transgenics to develop viral disease resistant livestock.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmt1Crug%3D%3D&md5=664dccbe394b1d8f983d898967f9fc9eCAS | 18817330PubMed |
Witter RL (1997) Increased virulence of Marek’s disease virus field isolates. Avian Diseases 41, 149–163.
| Increased virulence of Marek’s disease virus field isolates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3kt1ehuw%3D%3D&md5=0f9a078fbba07f7f74c605c0b4222abfCAS | 9087332PubMed |
Won YW, Adhikary PP, Lim KS, Kim HJ, Kim JK, Kim YH (2014) Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nature Materials 13, 1157–1164.
| Oligopeptide complex for targeted non-viral gene delivery to adipocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1yht7vJ&md5=851e5c68195978b4a73961cb220e0c11CAS | 25282508PubMed |
Wongsrikeao P, Sutou S, Kunishi M, Dong YJ, Bai X, Otoi T (2011) Combination of the somatic cell nuclear transfer method and RNAi technology for the production of a prion gene-knockdown calf using plasmid vectors harboring the U6 or tRNA promoter. Prion 5, 39–46.
| Combination of the somatic cell nuclear transfer method and RNAi technology for the production of a prion gene-knockdown calf using plasmid vectors harboring the U6 or tRNA promoter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFWhurg%3D&md5=a4febcc569dc91a31f2e41a614094421CAS | 21084838PubMed |
Xu J, Han F, Zhang X (2007) Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA. Antiviral Research 73, 126–131.
| Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVCitb4%3D&md5=46c5c65663f45f6f24458154dd34f061CAS | 17011052PubMed |
Xu Y-F, Shen H-Y, Zhao M-Q, Chen L-J, Li Y-G, Liao M, Jia J-T, Lv Y-R, Yi L, Chen J-D (2012) Adenovirus-vectored shRNAs targeted to the highly conserved regions of VP1 and 2B in tandem inhibits replication of foot-and-mouth disease virus both in vitro and in vivo. Journal of Virological Methods 181, 51–58.
| Adenovirus-vectored shRNAs targeted to the highly conserved regions of VP1 and 2B in tandem inhibits replication of foot-and-mouth disease virus both in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtF2htLg%3D&md5=e8f0638ab6a16dcfa2971d8325f631d1CAS | 22327142PubMed |
Xu J, Wang Y, Li Z, Ling L, Zeng B, James AA, Tan A, Huang Y (2014) Transcription activator‐like effector nuclease (TALEN)‐mediated female‐specific sterility in the silkworm, Bombyx mori. Insect Molecular Biology 23, 800–807.
| Transcription activator‐like effector nuclease (TALEN)‐mediated female‐specific sterility in the silkworm, Bombyx mori.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVGjtbrI&md5=2717113b4159f0345c0903e1f268b4e9CAS | 25125145PubMed |
Yang S, Chen Y, Ahmadie R, Ho EA (2013) Advancements in the field of intravaginal siRNA delivery. Journal of Controlled Release 167, 29–39.
| Advancements in the field of intravaginal siRNA delivery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktlSjsrY%3D&md5=f65c484ecc54c89a0e089b4a8831340eCAS | 23298612PubMed |
Ye J, Zhang Y, Xu J, Zhang Q, Zhu D (2007) FBXO40, a gene encoding a novel muscle-specific F-box protein, is upregulated in denervation-related muscle atrophy. Gene 404, 53–60.
| FBXO40, a gene encoding a novel muscle-specific F-box protein, is upregulated in denervation-related muscle atrophy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFyku7nL&md5=23ced1f718c560431af82df41cd3b577CAS | 17928169PubMed |



