Comparative performance of broiler chickens offered nutritionally equivalent diets based on six diverse, ‘tannin-free’ sorghum varieties with quantified concentrations of phenolic compounds, kafirin, and phytate
Ha H. Truong A B , Karlie A. Neilson C , Bernard V. McInerney C , Ali Khoddami D , Thomas H. Roberts D , David J. Cadogan E , Sonia Yun Liu A and Peter H. Selle A FA Poultry Research Foundation within the Faculty of Veterinary Science, The University of Sydney, 425 Werombi Road, Camden, NSW 2570, Australia.
B Poultry CRC, University of New England, Armidale, NSW 2351, Australia.
C Australian Proteome Analysis Facility, Macquarie University, Sydney, NSW 2109, Australia.
D Faculty of Agriculture and Environment, The University of Sydney, NSW 2006, Australia.
E Feedworks Pty Ltd, Romsey, Vic. 3434, Australia.
F Corresponding author. Email: peter.selle@sydney.edu.au
Animal Production Science 57(5) 828-838 https://doi.org/10.1071/AN16073
Submitted: 8 February 2016 Accepted: 9 February 2016 Published: 1 June 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Starch is the main source of energy in sorghum-based diets but starch/energy utilisation by broiler chickens offered these diets may be substandard. Both in vitro and in vivo data indicate that the digestibility of sorghum starch is inferior to that of other feed grains, especially maize. Three ‘starch-extrinsic’ factors in grain sorghum, namely ‘non-tannin’ phenolic compounds, kafirin and phytate may negatively influence starch/energy utilisation in sorghum-based broiler diets. To test this hypothesis, concentrations of polyphenols, free, bound and conjugated phenolic acids, kafirin and phytate were quantified in six diverse ‘tannin-free’ (Type I) grain sorghum varieties. These sorghums were incorporated into nutritionally equivalent diets at 620 g/kg and offered to male broiler chickens from 7 to 28 days post-hatch. Growth performance, nutrient utilisation (AME, ME : GE ratios, N retention, AMEn) and starch and protein (N) digestibility coefficients and disappearance rates in four small intestinal segments were determined. Numerous relationships that were either significant (P < 0.05), or approached significance (P < 0.10), were detected that indicated various ‘non-tannin’ phenolic compounds, kafirin and phytate in sorghums negatively influenced nutrient utilisation parameters in broiler chickens. ME : GE ratios are sensitive indicators of efficiency of energy utilisation and were most negatively influenced by flavan-4-ols (r = –0.919; P < 0.015), which are polyphenolic compounds. Moreover, flavan-4-ols in tandem with conjugated vanillic acid negatively influenced (r = –0.993; P < 0.005) ME : GE ratios on the basis of a valid multiple linear regression. Similarly, conjugated vanillic and bound ferulic acids in tandem negatively influenced AME (r = –0.990; P < 0.005). N retention was most negatively influenced by kafirin (r = –0.887; P < 0.025). Thus, it appears that both phenolic compounds and kafirin may have deleterious effects on nutrient utilisation of sorghum-based broiler diets and recommendations are made that should enhance the quality of sorghum as a feedstuff for chicken-meat production based on these findings.
Additional keywords: condensed tannin, conjugated and bound phenolic acids, ferulic acid, free, polyphenols.
Introduction
Most energy in broiler diets is derived from starch; however, the utilisation of starch/energy in sorghum-based broiler diets may be substandard. For example, the amount of energy required to generate 1 kg of liveweight gain in broilers offered a sorghum-based diet was shown to exceed that for a wheat-based broiler diet (20.9 vs 19.8 MJ/AME.kg gain; P < 0.05) as reported by Black et al. (2005). On the basis of both in vitro and in vivo data the digestibility of sorghum starch is inferior in comparison to the starch component of maize. The in vitro potential starch digestibility of 14 maize samples was 95.0 in comparison to only 70.4 g/100 g dry starch in 11 sorghum samples (Giuberti et al. 2012). In a review of 11 studies, the mean ileal starch digestibility coefficient of 0.950 (range: 0.873–0.993) in broilers offered maize-based broiler diets exceeded the mean value of 0.883 (range: 0.846–0.921) in seven sorghum assays (Truong et al. 2016). Condensed tannin would be a contributing factor to this disparity if it were present in grain sorghum (Nyachoti et al. 1997); however, sorghum crops now grown in Australia almost certainly do not contain condensed tannin (Khoddami et al. 2015). The present study follows two similar studies by Khoddami et al. (2015) and Truong et al. (2015a). In the first study sorghum-casein diets containing 809 g/kg of six red sorghum varieties were offered to broilers from 7 to 23 days post-hatch. Among other findings, concentrations of conjugated phenolic acids were negatively correlated with ME : GE ratios (r = –0.832; P < 0.05) or the efficiency of energy utilisation. In the second study, conventional diets based on two of the six sorghum varieties were compared in broiler chickens from 7 to 28 days post-hatch. One sorghum was clearly inferior in terms of weight gain, feed conversion efficiency and parameters of nutrient utilisation and this appeared to be associated with a higher kafirin concentration (61.5 vs 50.7 g/kg). One contention is that three factors in grain sorghum, or colloquially the ‘Bermuda Triangle’, are negatively influencing starch/energy utilisation in sorghum-based broiler diets (Liu et al. 2015). The three apices of this triangle are ‘non-tannin’ phenolic compounds, kafirin, the dominant protein fraction in sorghum and phytate, a ubiquitous constituent in plant-sourced feedstuffs. Based on the Clorox bleach test (Waniska et al. 1992), the six sorghum varieties used in this study possessed non-pigmented testas and thus did not contain condensed tannin. However, the established anti-nutritive properties of condensed tannin may extend to the balance of phenolic compounds, which are abundant in sorghum, and the anti-nutritive properties of phenolic compounds are not solely the province of condensed tannin as proposed by Khoddami et al. (2015). It is widely held that the close proximity of kafirin protein bodies to starch granules in sorghum endosperm interferes with starch utilisation (Taylor 2005). The presence of phytate in broiler diets has anti-nutritive effects and although the negative impacts of phytate on the utilisation of protein and amino acids are established, these impacts may extend to starch and glucose utilisation (Selle and Ravindran 2007; Selle et al. 2012). To investigate this proposition, concentrations of polyphenolic compounds, free, conjugated and bound phenolic acids, kafirin, and phytate in six diverse grain sorghum varieties were quantified. Six nutritionally equivalent diets containing 620 g/kg sorghum were formulated and offered to broiler chicks in a 7 to 28 days post-hatch bioassay in order to examine relationships between the nominated components of sorghum and broiler performance parameters.
Materials and methods
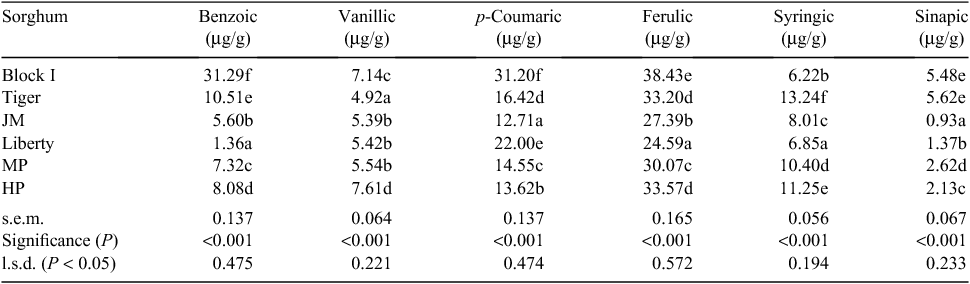
Grains of six diverse sorghum varieties (Block I, HP, Liberty, Tiger, MP, JM) grown in New South Wales and Queensland and harvested in 2012, 2013 or 2014 (Table 1) were analysed for phenolic compounds, kafirin and phytate. Kafirin was quantified by procedures adapted from those of Wallace et al. (1990) and Hamaker et al. (1995), which has been described in detail (Truong et al. 2015a). Total phosphorus (P) levels were determined by inductively coupled plasma mass spectrometry and phytate-P or phytate concentrations by high performance liquid chromatography procedures. Kafirin and phytate concentrations and other relevant characteristics including AusScan NIR profiles of the six sorghum varieties are shown in Table 2. Concentrations of polyphenols, free, conjugated, bound and total phenolic acids are recorded in Tables 3–7 inclusive. The complex methodologies used in their quantification have been documented by Khoddami et al. (2015).

|

|
On the basis of the above data, six broiler diets containing 620 g/kg sorghum were formulated to be nutritionally equivalent with an energy density of 12.95 MJ/kg and similar profiles for key amino acids (Table 8). The diets were steam-pelleted at a conditioning temperature of 84°C and crumbled after sorghum grain was ground through a 3.2-mm hammer-mill screen. Feather-sexed, male broiler chicks (Ross 308) were housed in an environmentally controlled facility, initially fed a proprietary starter ration, weighed at Day 7 and distributed among 48 cages so that mean bodyweights in each cage and their variations were almost identical. The six dietary treatments were offered to eight replicates (six birds per cage) from 7 to 28 days post-hatch. Bodyweights were determined on Days 7 and 28 and feed intakes recorded to calculate feed conversion ratio (FCR) with adjustments made from the weight of dead or culled birds, which were monitored on a daily basis. Total excreta were collected from 23 to 26 days post-hatch from each cage to determine apparent metabolisable energy (AME), ME : GE ratios, nitrogen (N) retention and N-corrected apparent metabolisable energy (AMEn). AME values (MJ/kg) on a dry matter basis and were calculated using the following formula:

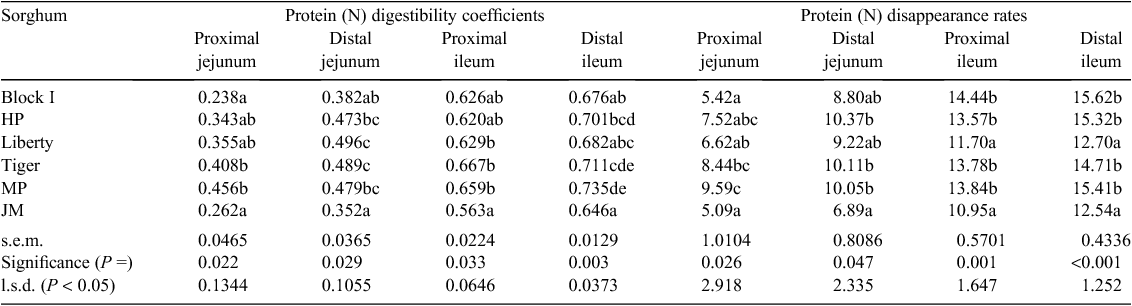
|
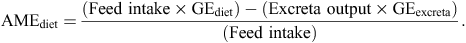
ME : GE ratios were calculated by dividing AME values by the gross energy (GE) of the relevant diets. N retention was calculated using the following formula:
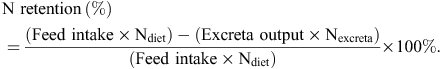
N-corrected AME values were calculated by correcting to zero N retention by applying the factor of 36.54 kJ/g N retained in the body (Hill and Anderson 1958). Acid insoluble ash was used as the inert dietary marker and acid insoluble ash concentrations were determined by the method of Siriwan et al. (1993). N content of the diets and excreta were obtained using an FP-428 determinator (Leco Corporation, St Joseph, MI, USA) and starch content were determined by a procedure based on dimethyl sulfoxide, α-amylase and amyloglucosidase, as described by Mahasukhonthachat et al. (2010). On Day 28, digesta was collected in its entirety from the proximal and distal halves of the jejunum and ileum to determine apparent digestibility coefficients in four small intestinal segments: proximal jejunum (PJ), distal jejunum (DJ), proximal ileum (PI) and distal ileum (DI). The four small intestinal segments were defined by the end of the duodenal loop, Meckel’s diverticulum, the ileal-caecal junction and their midpoints. Apparent digestibility coefficients of starch and protein (N) were calculated from the following equation:
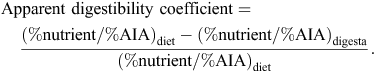
Feed intakes over the final 2 days of the 7 to 28-day feeding period were recorded. Starch and protein (N) disappearance rates (g/bird.day) were deduced from feed intakes over the final 2 days from the following equation:
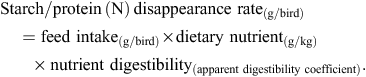
Ratios of starch to protein disappearance rates in the four small intestinal segments were calculated. Experimental data were analysed using the IBM SPSS Statistics 20 program (IBM Corporation, Somers, NY, USA). Statistical procedures included one-way univariate ANOVA using general linear models procedures, Pearson correlations, linear and multiple regressions. A probability level of less than 5% was considered to be statistically significant. There were numerous significant relationships between sorghum characteristics and bird performance parameters but many of these were complicated by significant correlations between sorghum characteristics. Therefore, focus was placed on the most significant linear relationship with the highest correlation coefficient (r =) between a given factor in sorghum and selected bird performance parameters. Where this sorghum factor was not correlated with others, valid multiple linear regressions were detected where possible. In a valid model, the two or more unrelated sorghum factors have significant impacts on a combined and individual basis. The feeding study complied with specific guidelines approved by the Animal Ethics Committee of Sydney University.
Results
Kafirin concentrations of the six sorghums averaged 51.9 g/kg with a range from 41.4 to 67.1 g/kg. Phytate concentrations averaged 8.02 g/kg with a range from 4.93 to 9.79 g/kg (Table 2). Concentrations of phenolic compounds are shown in Tables 3–7. These include eight categories of polyphenols (Table 3), six free (Table 4), six conjugated (Table 5) and six bound phenolic acids (Table 6). Total free, conjugated and bound phenolic acid and the overall sum of phenolic acids are shown in Table 7.
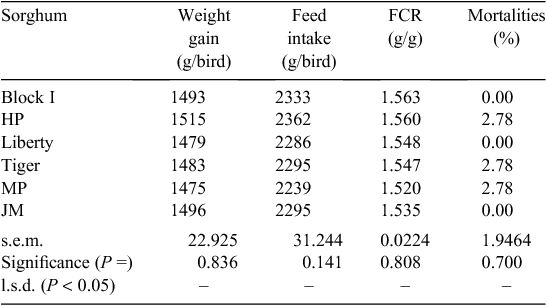
There were no significant differences between the dietary treatments in weight gain (P > 0.80), feed intake (P > 0.10) and FCR (P > 0.80) from 7 to 28 days post-hatch as shown in Table 9. The overall weight gain of 1490 g/bird and feed intake of 2302 g/bird exceeded Ross 308 objectives by 6.7% and 12.2%, respectively, but FCR were inferior by 4.5% (1.545 vs 1.479). The overall mortality rate of 1.4% was not related (P > 0.65) to dietary treatments.
|
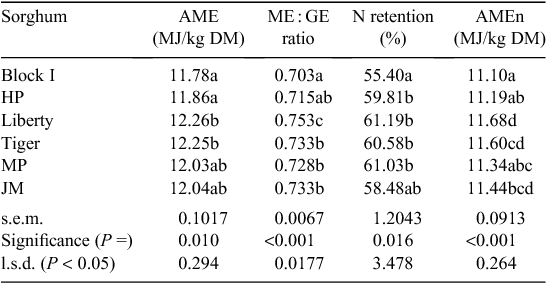
The effects of grain variety in six sorghum-based diets on parameters of nutrient utilisation are shown in Table 10 where there significant treatment differences for ME : GE ratios, AMEn (P < 0.001), AME and N retention (P < 0.02). Among the sorghum-based diets, Liberty (white) was superior and Block I (red) inferior across all parameters. Advantages held by Liberty relative to Block I were 0.48 MJ (12.26 vs 11.78 MJ/kg; P = 0.002) in AME, 7.11% (0.753 vs 0.703; P < 0.001) in ME : GE ratios, 10.5% (61.19 vs 55.40%; P = 0.002) in N retention and 0.58 MJ (11.68 vs 11.10 MJ/kg; P < 0.001) in AMEn. The probability values in parentheses are on the basis of pair-wise comparisons between the two dietary treatments.
|
The effects of sorghum variety on starch digestibility coefficients and accumulative starch disappearance rates in four small intestinal segments of broiler chickens at 28 days post-hatch are shown in Table 11. There were no significant differences between dietary treatments for starch digestibility coefficients where average digestibilities progressively increased along the small intestine from 0.684 in PJ, to 0.753 in DJ, 0.838 in PI and to 0.871 in DI. Significant differences in starch disappearance rates were observed in PI (P < 0.005) and DI (P < 0.001). Starch disappearance rates ranged from 34.60 to 40.91 g/bird.day (P < 0.001) in PI and from 36.52 to 42.59 g/bird.day (P = 0.004) in DI where Liberty was the superior and JM the inferior sorghum variety.
The effects of sorghum variety on protein (N) digestibility coefficients and disappearance rates included significant treatment effects (P = 0.033–0.003) for protein digestibility in all four small intestinal segments (Table 12). Average protein digestibility coefficients were 0.344 in PJ, 0.445 in DJ, 0.627 in PI and 0.692 in DI. The lowest digestibility coefficients were recorded for sorghum variety JM in the three caudal small intestinal segments. There were also significant treatment effects (P = 0.026 – <0.001) in four segments for accumulative protein disappearance rates. Average protein disappearance rates were 7.11 in PJ, 9.24 in DJ, 13.05 in PI and 14.38 g/bird.day in DI.
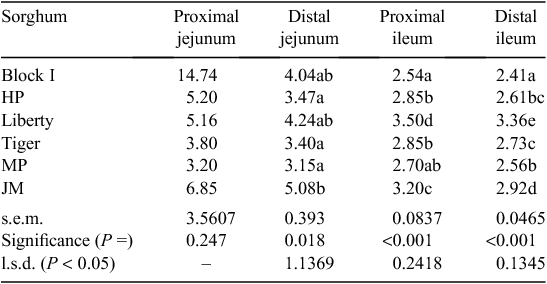
The effects of sorghum variety on starch : protein (N) disappearance rate ratios in four small intestinal segments of broilers at 28 days post-hatch included significant effects in DJ (P < 0.02), PI and DI (P < 0.001) as shown in Table 13. In the final two segments Liberty had the highest starch : protein ratios of 3.50 and 3.36, respectively, and Block I the lowest ratios of 2.54 and 2.41, respectively.
|
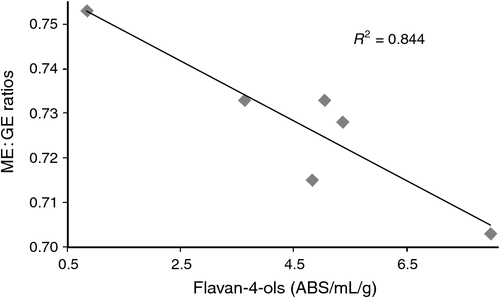
Linear regressions between concentrations of phenolic compounds, kafirin and phytate in six grain sorghums with parameters of nutrient utilisation and distal ileal digestive dynamics of broilers offered sorghum-based diets appear in Table 14. Conjugated vanillic acid was negatively correlated with AME (r = –0.879; P < 0.025) but it was not significantly correlated with bound ferulic acid. In combination, these two phenolic acids were negatively correlated (r2 = 0.980; P > 0.005) and the multiple linear regression equation is tabulated. The polyphenolic flavan-4-ols were the most significantly related (r = –0.919; P < 0.015) with ME : GE ratios but were not correlated with conjugated vanillic acid. Collectively, both phenolic compounds were negatively related (r2 = 0.986; P > 0.0025) to ME : GE ratios and the multiple linear regression equation is tabulated. Kafirin was dominant in respect of its negative relationship (r = –0.887; P < 0.025) with N retention in this respect. There were no significant correlations with AMEn, although the negative relationship with flavan-4-ols (r = –0.795; P < 0.06) approached significance. In respect of distal ileal starch : protein disappearance rate ratios, flavan-4-ols (r = –0.960; P < 0.005) held the most significant negative correlation.
Discussion
Among our results, the lack of significant differences in growth performance across the six sorghum-based diets was somewhat surprising. This observation may stem from the absence of any significant differences in starch digestibility coefficients in the four small intestinal segments. However, the overall distal ileal starch digestibility coefficient of 0.871 for sorghum-based diets in the present study is both substandard and consistent with previously published data (Truong et al. 2016). After correcting FCR for weight gain (25 g ≡ 0.01), the overall efficiency of feed conversion of 1.508 for gain-corrected FCR remained inferior to the 1.479 Ross 308 performance objective. It is also noteworthy that the diets were formulated to an energy density of 12.95 MJ/kg; however, the average AME recorded in broilers was 12.04 MJ/kg. Arguably, this shortfall vividly illustrates the substandard energy utilisation of broiler chickens offered sorghum-based diets.
It is appreciated that correlations do not establish causation; nevertheless, there were numerous correlations between concentrations of phenolic compounds, kafirin and phytate in six grain sorghums with bird performance parameters that either approached (P < 0.10) or were (P < 0.05) significant. An interpretation is complicated by the fact that several of these sorghum characteristics were themselves significantly correlated. However, the significant negative regression between conjugated vanillic and bound ferulic acids in tandem with AME (P < 0.025) suggests that phenolic acids in grain sorghum negatively impact on AME as an indicator of energy utilisation. ME : GE ratios may be a better indicator of energy utilisation; taken individually, flavan-4-ols, conjugated ferulic and benzoic acids, kafirin and apigeninidin were negatively correlated with ME : GE ratios to significant extents. Flavan-4-ols was the most significantly correlated factor (r = –0.919; P < 0.015) with ME : GE ratios as shown in Fig. 1. In addition, the combined negative impact of flavan-4-ols and conjugated vanillic acid on ME : GE ratios was highly significant (P < 0.0025). This outcome suggests that (non-tannin) polyphenols and phenolic acids in sorghum negatively impact on energy utilisation. On an individual basis, several sorghum characteristics including kafirin, total phenolics, apigeninidin, 7-methoxy-apigeninidin, conjugated benzoic and vanillic acids were negatively correlated with N retention to significant extents. However, kafirin was dominant (r = –0.887; P < 0.025) in this respect. It was not possible to find any valid multiple linear regressions for this parameter; however, this outcome suggests that kafirin, in addition to phenolic compounds, can compromise nutrient utilisation in broilers offered sorghum-based diets.
|
There were significant, negative correlations between flavan-4-ols, conjugated ferulic acid, phytate, total phenolic compounds, bound ferulic acid and kafirin, with distal ileal starch : protein disappearance rate ratios on an individual basis. Valid multiple linear regressions could not be detected. These ratios are indicators of starch : protein digestive dynamics of sorghum-based diets and it appears that total phenolics, kafirin and phytate may all influence the bilateral bioavailability of starch and protein.
Several significant negative relationships between polyphenols, notably flavan-4-ols, and phenolic acids, where ferulic acid is dominant, with broiler performance parameters were observed. Thus, it appears that phenolic compounds, other than condensed tannin (absent in these Type I sorghums), can negatively influence starch/energy utilisation in broilers offered sorghum-based diets. The complex interactions between starch and phenolic compounds were recently reviewed by Zhu (2015) and in their extensive review, Tomasik and Schilling (1998) stated that phenolics readily form starch complexes and probably have a greater propensity to bind with amylose than amylopectin. However, the researchers did allow that the structure and stability of phenol-starch complexes are not clearly understood. It appears that phenolic compounds may interact with starch through hydrogen bonds, covalent bonds or chelation via their carboxyl and hydroxyl groups (Yu et al. 2001). Interactions between polyphenols and starch molecules were reported by Barros et al. (2012) where phenolic extracts from both tannin and non-tannin sorghums interacted with starch, including hydrophobic and hydrogen bonding with amylose. Phenolic extracts from sorghums were shown to increase the generation of resistant starch under in vitro conditions in the Barros et al. (2012) study. Interestingly, negative relationships between phenolic intakes and blood glucose responses have been observed in humans (Thompson et al. 1983) and phenolics have been shown to inhibit Na+-dependent intestinal glucose uptakes in rats (Welsch et al. 1989).
In the present study, ferulic acid was the dominant phenolic acid in soluble, conjugated and insoluble, bound forms. Conjugated ferulic acid was negatively correlated with ME : GE ratios (r = –0.919; P < 0.015) and bound ferulic acid in combination with conjugated vanillic acid was negatively correlated with AME (r = –0.990; P < 0.005). Khoddami et al. (2015) reported negative correlations between conjugated ferulic acid with AME (r = –0.808; P = 0.052), ME : GE ratios (r = –0.831; P = 0.042) and AMEn (r = –0.769; P = 0.074) in a recent study involving six red sorghum cultivars. Thus, the implication is that phenolic acids have a deleterious impact on energy utilisation in broilers offered sorghum-based diets. Instructively, Kandil et al. (2012) found that phenolic acids in feed grains play an important role in the resistance of starch to hydrolysis under in vitro conditions. Phenolic acids are capable of cross-linking with cell wall macromolecules via ester and ether linkages through reactions involving their carboxyl and phenolic groups (Yu et al. 2001). Ferulic acid is the dominant phenolic acid in sorghum but it is also found in barley, maize, triticale and wheat (Kandil et al. 2012). Interestingly, ferulic acid has been shown to influence starch pasting profiles as determined by rapid visco-analysis of maize and sorghum, which suggests that phenolic acids have the capacity to interact with starch (Beta and Corke 2004). Also, Hung et al. (2013) reported that ferulic acid has the capacity to complex with debranched starch and that these starch-ferulic acid complexes were associated with increases in resistance of starch to digestion.
Liberty, the white sorghum variety, contained lower concentrations of polyphenols than five red varieties (Table 3). This was to be expected as the colouration of red sorghums is due to polyphenolic pigments including anthocyanins and anthocyanidins (Taylor 2005). However, Liberty also contained lower concentrations of bound phenolic acids by 35.1% (229 vs mean of 353 μg/g) and total phenolic acids by 34.1% (303 vs mean of 460 μg/g) in comparison to the five red sorghum varieties (Table 7). In a comparison by Liu et al. (2015), another sample of white sorghum (Liberty) contained lower concentrations of bound phenolic acids by 48.3% (282 vs 545 μg/g) and total phenolic acids by 41.3% (374 vs 637 μg/g) in comparison to a red sorghum (Buster). Thus, it appears that white sorghums may routinely contain lesser concentrations of both polyphenols and phenolic acids than red sorghums and, anecdotally, white sorghums as a feed grain for pigs and poultry are considered to be superior to red varieties under Australian conditions.
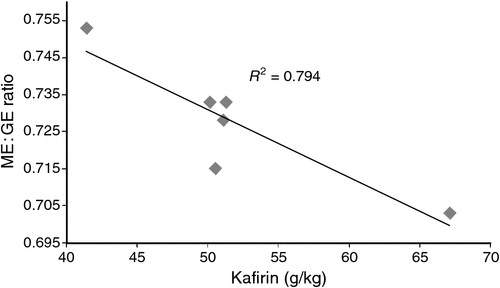
The significant negative relationships between absolute kafirin concentrations on an individual basis with N retention and ME : GE ratios (Fig. 2) imply that kafirin compromises the efficiency of nutrient utilisation in sorghum-based diets. Salinas et al. (2006) claimed that kafirin, as a percentage of protein in 12 sorghum hybrids, was negatively correlated with AME and TMEn to significant extents in caecetomised roosters. Moreover, Truong et al. (2015a) found, in a comparison of broiler diets based on two sorghums with kafirin concentrations of 61.5 and 50.7 g/kg, that the sorghum with the lower kafirin concentration was associated with substantial and significant advantages in AME (13.61 vs 12.55 MJ/kg), ME : GE ratios (0.806 vs 0.769) and AMEn (12.38 vs 11.35 MJ/kg).
|
It seems reasonable to conclude that kafirin compromises starch/energy utilisation in sorghum-based diets, which is consistent with opinions expressed in Taylor (2005), Wong et al. (2010), de Mesa-Stonestreet et al. (2010) and other research groups. Kafirin protein bodies and starch granules are both embedded in the glutelin protein matrix of sorghum endosperm (Selle et al. 2010). This close proximity facilitates any physical or chemical starch-protein interactions. It has been argued that kafirin physically impedes the swelling of starch granules and their gelatinisation (Chandrashekar and Kirleis 1998). Chemical interactions between starch and protein in sorghum and other feed grains are considered to be important (Rooney and Pflugfelder 1986); however, as discussed by Truong et al. (2016), these starch-protein interactions have not been precisely defined. One possible interaction could involve disulfide cross-linking between the β- and γ-kafirin fractions located in the periphery of protein bodies and starch granule-associated proteins, which may be amplified by steam-pelleting of broiler diets (Selle et al. 2013). Although based on indirect evidence, it has been argued that the kafirin proportion of sorghum protein is escalating in sorghum crops grown in Australia as an inadvertent consequence of breeding programs (Selle 2011). Kafirin is not a readily digestible protein (Selle et al. 2010) but arguably nutritionists can accommodate for this because kafirin only comprises some 15% of total protein in sorghum-based diets. Thus, in the context of protein, the prospect of ‘high-kafirin’ sorghums may not be overly adverse. However, if kafirin additionally compromises energy utilisation then the prospect of ‘high-kafirin’ sorghums constitutes a potentially tangible problem for chicken-meat production.
The six sorghums contained an average level of 2.26 g/kg phytate-P or 8.02 g/kg phytate, which are very similar to values recorded in an earlier local survey (Selle et al. 2003). Phytate was negatively correlated with starch disappearance rates in the distal jejunum (r = –0.845; P < 0.04) and proximal ileum (r = –0.890; P < 0.02) on an individual basis (data not shown). This is consistent with a recent report (Truong et al. 2015b) in which phytase supplementation of maize-based broiler diets significantly increased starch disappearance rates in the proximal jejunum (58.0 vs 43.4 g/bird.day) and proximal ileum (80.8 vs 71.4 g/bird.day). This positive ‘starch/energy effect’ of phytase may stem from enhanced glucose absorption to a greater extent than improved starch digestion. Interestingly both phytate and phenolic compounds have been shown to depress starch digestibility in vitro (Thompson and Yoon 1984) and both components may share analogous anti-nutritive properties (Selle et al. 2010). Thus, it appears that phytate may depress energy utilisation in sorghum but to a lesser extent than phenolics and kafirin. Responses to exogenous phytases in sorghum-based diets are not always robust (Selle et al. 2013); this may be because exogenous phytases does not counteract the deleterious impacts of kafirin and phenolic compounds in this context.
Conclusions
This study provides evidence that ‘non-tannin’ phenolic compounds, kafirin and, to a lesser extent, phytate collectively exert a negative influence on starch/energy utilisation in broiler chickens offered sorghum-based diets. More detailed considerations of the likely responsible underlying mechanisms may be found in two reviews (Selle et al. 2013; Liu et al. 2015). One recommendation arising out of this study is that white sorghum varieties with low-protein contents would be advantageous. White sorghums inherently contain lower concentrations of polyphenols and, quite possibly, phenolic acids. Low-protein cultivars axiomatically contain less kafirin; moreover, data generated by Taylor et al. (1984) indicates that the proportion of kafirin increases at the expense of glutelin as protein contents in sorghum increase. Exogenous phytases are routinely included in broiler diets but in the context of lower phenolic and kafirin concentrations, phytases may generate more robust responses. Over the longer term, this study also suggests that sorghum breeding programs should be re-directed in order to reduce kafirin proportions of protein in grain sorghum, which should benefit both starch and protein utilisation in broiler chickens offered sorghum-based diets in the future.
Acknowledgements
The authors acknowledge the support of RIRDC Chicken-meat for funding the sorghum starch project and the Poultry CRC for providing Ms Ha Truong with a PhD scholarship. Also we thank Ms Denise Thomas and Ms Fei Chi at the Australian Proteome Analysis Facility (APAF), Macquarie University for their contribution to amino acid analyses and the quantification of the kafirin. The research at APAF was facilitated using infrastructure provided by the Australian Government under the National Collaborative Research Infrastructure Strategy (NCRIS).
References
Barros F, Awika JM, Rooney LW (2012) Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. Journal of Agricultural and Food Chemistry 60, 11 609–11 617.| Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1WltrjO&md5=5403419372f42fec28e8df08fcbc959eCAS |
Beta T, Corke H (2004) Effect of ferulic acid and catechin on sorghum and maize starch pasting properties. Cereal Chemistry 81, 418–422.
| Effect of ferulic acid and catechin on sorghum and maize starch pasting properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvFelt7o%3D&md5=7ed0a9785c98b94df6695ba728992316CAS |
Black JL, Hughes RJ, Nielsen SG, Tredrea AM, MacAlpine R, Van Barneveld RJ (2005) The energy value of cereal grains, particularly wheat and sorghum, for poultry. Proceedings of the Australian Poultry Science Symposium 17, 21–29.
Chandrashekar A, Kirleis A (1998) Influence of protein on starch gelatinization in sorghum. Cereal Chemistry 65, 457–462.
de Mesa-Stonestreet NJ, Alavi S, Bean SR (2010) Sorghum proteins: the concentration, isolation, modification, and food applications of kafirins. Journal of Food Science 75, 90–104.
Giuberti G, Gallo A, Cerioli C, Masoero F (2012) In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition. Animal Feed Science and Technology 174, 163–173.
| In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvF2ju7w%3D&md5=a8d42d856d56793a7317f170460293bdCAS |
Hamaker BR, Mohamed AA, Habben JE, Huang CP, Larkins BA (1995) Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than conventional method. Cereal Chemistry 72, 583–588.
Hill FW, Anderson DL (1958) Comparison of metabolisable energy and productive energy deerminations with growing chicks. The Journal of Nutrition 64, 587–603.
Hung PV, Phat NH, Phi NTL (2013) Physicochemical properties and antioxidant capacity of debranched starch-ferulic acid complexes. Stärke 65, 382–389.
Kandil A, Li J, Vasanthan T, Bressler DC (2012) Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. Journal of Agricultural and Food Chemistry 60, 8444–8449.
| Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVaktrzL&md5=5f83ace6824283756b34650a3cc1119dCAS | 22793673PubMed |
Khoddami A, Truong HH, Liu SY, Roberts TH, Selle PH (2015) Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broilers. Animal Feed Science and Technology
| Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broilers.Crossref | GoogleScholarGoogle Scholar |
Liu SY, Fox G, Khoddami A, Neilsen KA, Truong HH, Moss AF, Selle PH (2015) Grain sorghum: a conundrum for chicken-meat production. Agriculture 5, 1224–1251.
| Grain sorghum: a conundrum for chicken-meat production.Crossref | GoogleScholarGoogle Scholar |
Mahasukhonthachat K, Sopade PA, Gidley MJ (2010) Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. Journal of Cereal Science 51, 392–401.
| Kinetics of starch digestion and functional properties of twin-screw extruded sorghum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVeksrg%3D&md5=c0420e99504f876534a4a43ff11c3653CAS |
Nyachoti CM, Atkinson JL, Leeson S (1997) Sorghum tannins: a review. World’s Poultry Science Journal 53, 5–21.
| Sorghum tannins: a review.Crossref | GoogleScholarGoogle Scholar |
Rooney LW, Pflugfelder RL (1986) Factors affecting starch digestibility with special emphasis on sorghum and corn. Journal of Animal Science 63, 1607–1623.
Salinas I, Pro A, Salinas Y, Sosa E, Becerril CM, Cuca M, Cervantes M, Gallegos J (2006) Compositional variation amongst sorghum hybrids: effect of kafirin concentration on metabolizable energy. Journal of Cereal Science 44, 342–346.
| Compositional variation amongst sorghum hybrids: effect of kafirin concentration on metabolizable energy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtF2qu7%2FM&md5=67085bd131060fce8f620da6cd788118CAS |
Selle PH (2011) The protein quality of sorghum grain. Proceedings of the Australian Poultry Science Symposium 22, 147–156.
Selle PH, Ravindran V (2007) Microbial phytase in poultry nutrition. Animal Feed Science and Technology 135, 1–41.
| Microbial phytase in poultry nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkt1artb8%3D&md5=10c873cc2a2fa70b4c8c35c36f7ce39aCAS |
Selle PH, Walker AR, Bryden WL (2003) Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Australian Journal of Experimental Agriculture 43, 475–479.
| Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlslOnsbY%3D&md5=a74bd3ec461d75175387cae678b82441CAS |
Selle PH, Cadogan DJ, Li X, Bryden WL (2010) Implications of sorghum in broiler chicken nutrition. Animal Feed Science and Technology 156, 57–74.
| Implications of sorghum in broiler chicken nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVCksr4%3D&md5=80de3bfba630d56db0d6e23cc497213aCAS |
Selle PH, Cowieson AJ, Cowieson NP, Ravindran V (2012) Protein-phytate interactions in pig and poultry nutrition; a reappraisal. Nutrition Research Reviews 25, 1–17.
| Protein-phytate interactions in pig and poultry nutrition; a reappraisal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvVOisb0%3D&md5=0d23dafc48ae2e0b3549e3f327eecb1eCAS | 22309781PubMed |
Selle PH, Liu SY, Cowieson AJ (2013) Sorghum: an enigmatic grain for chicken-meat production. In ‘Sorghum: production, growth habits and health benefits’. (Ed. PC Parra) pp. 1–44. (Nova Publishers Inc.: Hauppauge, NY)
Siriwan P, Bryden WL, Mollah Y, Annison EF (1993) Measurement of endogenous amino-acid losses in poultry. British Poultry Science 34, 939–949.
| Measurement of endogenous amino-acid losses in poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFOgsLg%3D&md5=9cf977713d25c4cadedec9959d9ad230CAS | 8156432PubMed |
Taylor JRN (2005) Non-starch polysaccharides, proteins and starch: form function and feed – highlight on sorghum. Proceedings of the Australian Poultry Science Symposium 17, 10–16.
Taylor JRN, Schüssler L, van der Walt WH (1984) Fractionation of proteins from low-tannin sorghum grain. Journal of Agricultural and Food Chemistry 32, 149–154.
| Fractionation of proteins from low-tannin sorghum grain.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c7mtlSjtQ%3D%3D&md5=073418d78cde38667f788919652ebb68CAS |
Thompson LU, Yoon JH (1984) Starch digestibility as affected by polyphenols and phytic acid. Journal of Food Science 49, 1228–1229.
| Starch digestibility as affected by polyphenols and phytic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlvFSjsbo%3D&md5=f6ef04cc67bf363063a94bcb1a7dc92aCAS |
Thompson LU, Yoon JH, Jenkins DJA, Wolever TMS, Jenkins AL (1983) Relationships between polyphenol intake and blood glucose response in normal and diabetic individuals. The American Journal of Clinical Nutrition 39, 745–751.
Tomasik P, Schilling CH (1998) Complexes of starch with organic guests. Advances in Carbohydrate Chemistry and Biochemistry 53, 345–426.
| Complexes of starch with organic guests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkvFCg&md5=bb6439b63b20fef7f2c2b6b8954fbbdaCAS |
Truong HH, Neilson KA, McInerney BV, Khoddami A, Roberts TH, Liu SY, Selle PH (2015a) Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or reground mash following steam-pelleting at 65 or 97°C conditioning temperatures. Animal Nutrition 1, 220–228.
| Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or reground mash following steam-pelleting at 65 or 97°C conditioning temperatures.Crossref | GoogleScholarGoogle Scholar |
Truong HH, Bold RM, Liu SY, Selle PH (2015b) Standard phytase inclusion in maize-based broiler diets enhances digestibility coefficients of starch, amino acids and sodium in four small intestinal segments and digestive dynamics of starch and protein. Animal Feed Science and Technology 209, 240–248.
| Standard phytase inclusion in maize-based broiler diets enhances digestibility coefficients of starch, amino acids and sodium in four small intestinal segments and digestive dynamics of starch and protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFeitLrJ&md5=b7798a72548136930b704fba49f5a138CAS |
Truong HH, Liu SY, Selle PH (2016) Starch utilisation in chicken-meat production: the foremost factors. Animal Production Science 56, 797–814.
Wallace JC, Lopes MA, Palva E, Larkins BA (1990) New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize. Plant Physiology 92, 191–196.
| New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXpslWhsA%3D%3D&md5=48f8114f128f953a07da61bb9a0bfec0CAS | 16667246PubMed |
Waniska RD, Hugo LF, Rooney LW (1992) Practical methods to determine the presence of tannins in sorghum. Journal of Applied Poultry Research 1, 122–128.
| Practical methods to determine the presence of tannins in sorghum.Crossref | GoogleScholarGoogle Scholar |
Welsch CA, Lachance PA, Wasserman BP (1989) Dietary phenolic compounds: inhibition of Na+-dependent d-glucose uptake in rat intestinal brush border membrane vesicles. The Journal of Nutrition 119, 1698–1704.
Wong JH, Marx DB, Wilson JD, Buchanan BB, Lemaux PG, Pedersen JF (2010) Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Science 179, 598–611.
| Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKht7jK&md5=2cbb2f708586ae0895512b992284a11cCAS |
Yu J, Vasanthan T, Temelli F (2001) Analysis of phenolic acids in barley by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry 49, 4352–4358.
| Analysis of phenolic acids in barley by high-performance liquid chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtVWktLo%3D&md5=11a308512661364e31724d5e491f1f65CAS | 11559137PubMed |
Zhu F (2015) Interactions between starch and phenolic compound. Trends in Food Science & Technology 43, 129–143.
| Interactions between starch and phenolic compound.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjs1ajsL0%3D&md5=5bb08110c768bb1f77cf81b94e689870CAS |