Feeding wet distillers grains plus solubles contributes to sarcoplasmic reticulum membrane instability
M. D. Chao A B , K. I. Domenech-Perez A , L. S. Senaratne-Lenagala A and C. R. Calkins A CA Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE 68583-0908, USA.
B Present address: College of Agriculture, California State University, Chico, CA 95929, USA.
C Corresponding author. Email: Ccalkins1@unl.edu
Animal Production Science 58(12) 2215-2223 https://doi.org/10.1071/AN16784
Submitted: 1 December 2016 Accepted: 22 June 2017 Published: 8 August 2017
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Feeding wet distillers grains plus solubles (WDGS) increases polyunsaturated fatty acid (PUFA) levels in beef. It was hypothesised that WDGS in feedlot diets increases PUFA concentration in the sarcoplasmic reticulum (SR) membrane, thereby altering membrane integrity, resulting in more rapid intracellular calcium leakage and improved tenderness. The objective of this study was to evaluate this hypothesis. Ninety-six crossbred steers were fed either a corn-based diet with 0% WDGS or 50% WDGS. Fifteen strip loins per treatment were collected, fabricated into steaks, aged and placed under retail display conditions. Steaks were used to measure tenderness, proteolysis, free calcium concentrations, lipid oxidation, sarcomere length and SR membrane fatty acid, phospholipid lipid, neutral lipid and total lipid profiles. Compared with steaks from steers fed 0% WDGS, steaks from steers fed 50% WDGS were more tender (P < 0.05) and had greater (P < 0.05) free calcium concentrations early post-mortem. Feeding 50% WDGS also tended to increase (P < 0.10) total PUFA concentrations, decrease (P < 0.10) total phospholipid concentration and increase (P < 0.10) total neutral lipid concentration for SR membrane. Steaks from steers fed 0% WDGS had greater (P < 0.05) lipid oxidation (TBARS values) than steaks from steers fed 50% WDGS after extended aging. Although differences in tenderness between the two treatments were detected, there were no corresponding differences (P > 0.10) in sarcomere length or proteolysis. This study showed that feeding WDGS may increase tenderness, possibly by increasing free calcium in muscle early post-mortem. However, the true mechanism that contributes to these differences is still unclear.
Additional keywords: beef, fatty acids, phospholipids.
Introduction
Tenderness has repeatedly been cited as the most important element for both eating quality and consumer purchasing decisions (Miller et al. 2001; Platter et al. 2005) and is a high priority for research in the meat industry (Boleman et al. 1998; McKenna et al. 2002; Garcia et al. 2008). The mechanism of meat tenderisation is a well understood subject. However, how animal diets affect the basic mechanism of meat tenderisation requires further exploration.
When compared with corn, distillers grains are not only less expensive, but also contain up to three times the levels of protein, fibre, and fat (Klopfenstein et al. 2008). Hence, distillers grains are used widely in feedlot diets at levels varying from 10% to 80% on a DM basis. Although many beef quality studies (Roeber et al. 2005; Koger et al. 2010; Mello et al. 2012) on feeding distillers grains reported no differences in tenderness between control and treatments, a recent study (Senaratne 2012) revealed an intriguing phenomenon. Beef from steers fed 30% wet distillers grains plus solubles (WDGS) was more tender than beef from steers not fed WDGS, and this is not the first time that feeding distillers grains is reported to improve tenderness (Depenbusch et al. 2009; Segers et al. 2011).
The hypothesis was that polyunsaturated fatty acid (PUFA) content of the sarcoplasmic reticulum (SR) membrane would increase as a consequence of feeding WDGS, which may predispose the SR membrane to release calcium earlier post-mortem as a result of rapid membrane oxidation, causing an early activation of calcium dependent proteases (the calpain system) and thereby enhancing tenderness. The objective of this study was to evaluate this hypothesis through examination of tenderness, lipid oxidation, sarcomere length, and proteolysis during aging and retail display as well as changes in SR membrane fatty acid, phospholipid, neutral lipid and total lipid profiles of beef with varying degrees of oxidation capacity. The mechanism of this increased tenderisation was the subject of this research.
Materials and methods
All animal use protocols were approved by the University of Nebraska-Lincoln’s Animal Care and Use Committee.
Animals
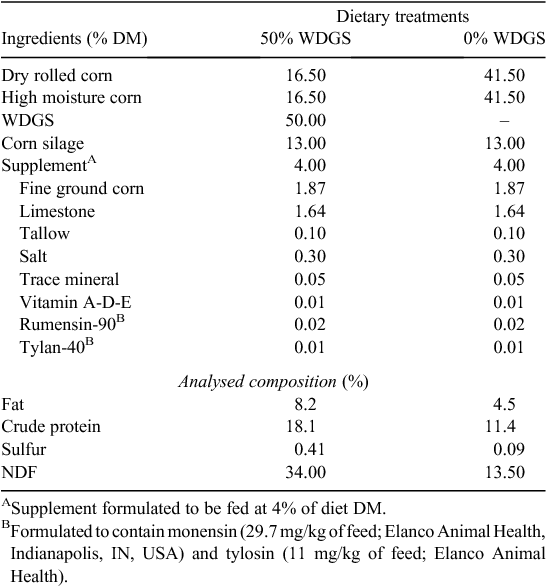
A total of 96 Continental × British steers were used in a single factor randomised complete block design study with two treatments with each pen used as the experimental unit. Steers were fed for 147 days on either a corn-based diet with 0% WDGS or 50% WDGS (DM basis; Table 1). As inclusion of WDGS increased from 0% to 50%, the percentages of dry rolled corn and high moisture corn (1 : 1) decreased accordingly to formulate the diets with equal amounts of corn silage (13% DM basis) and supplement (4% DM basis). All WDGS were produced from a single ethanol plant (KAAPA Ethanol, Minden, NE, USA), and all diets contained Rumensin90 (monensin, 29.7 mg/kg of feed; Elanco Animal Health, Indianapolis, IN, USA) and Tylan40 (tylosin, 11 mg/kg of feed; Elanco Animal Health). On Day 1 of the feeding period, steers were implanted with Revalor-XS (Merck Animal Health, Summit, NJ, USA), and steers were blocked by bodyweight (BW), stratified by BW within each block, and assigned randomly to pens within block. Pens were randomly assigned to one of the two treatments with six pens per treatment and eight steers per pen.
|
Sample collection, fabrication and preparation
All steers were harvested on the same day at a commercial abattoir (Greater Omaha Packing, Omaha, NE, USA). After 48 h of post-mortem chilling, 30 out of 96 carcasses were selected. Carcass selection was based on treatments (n = 15) and quality grade (USDA Choice). The rest of the carcasses that came in after a treatment group was fulfilled or did not meet the quality grade criteria were untagged and mixed with the plant’s normal production. The strip loins (Longissimus lumborum) from all selected carcasses were collected, vacuumed-packaged and transported to Loeffel meat laboratory at the University of Nebraska-Lincoln.
Strip loins from the left sides of the carcasses were fabricated on post-mortem aging Day 2 and 7, and strip loins from the right sides of carcasses were fabricated on post-mortem aging Day 14 and 21. Each strip loin was fabricated into two tenderness samples (2.54 cm) and three laboratory samples (1.27 cm) for each aging period, from the anterior to the posterior end of the loin muscle. The remaining portions of the strip loins were immediately vacuum-packaged in vacuum pouches (3-mm STD barrier, Prime Source, St Louis, MO, USA) on a Multivac packaging machine (Multivac C500, Multivac, Kansas City, MO, USA) and aged to the next designated aging period.
Other than the steaks designated for 0-day retail display, all steaks were overwrapped in Styrofoam trays (Styro-Tech, Denver, CO, USA) with oxygen permeable polyvinyl chloride film (PSM18, Prime Source) and subjected to retail display conditions (2 ± 2°C, and exposed to continuous 1000–1800 lx warm white fluorescence lighting). Objective tenderness, free calcium concentrations, and proteolysis samples were obtained from steaks displayed for 0 and 7 days of each aging period, and lipid oxidation samples were obtained from steaks displayed for 0, 4, and 7 days of each aging period. Sarcomere length samples were obtained from steaks aged for 2 days with 0-day retail display. For SR membrane fatty acids, phospholipid, neutral lipid and total lipid analyses, samples were obtained from steaks aged for 14 days with 0 days of retail display. Samples were vacuum-packaged and frozen at −20°C (tenderness samples) or −80°C (laboratory samples) until analysis. The laboratory samples were pulverised in liquid nitrogen with a blender (model 51BL32, Waring Commercial, Torring, CT, USA) before each assay.
Objective tenderness (Warner–Bratzler shear force – WBSF)
Steaks were removed from the freezer and thawed at 4°C for 24 h before grilling. An insulated type T thermocouple (5SC-TT-T-30–120, OMEGA Engineering, Inc., Stamford, CT, USA) was inserted into the geometric centre of each steak and attached to an OMEGA 450-ATT thermometer (OMEGA Engineering, Inc.) to monitor the internal temperature of the steak. Steaks were grilled on a Hamilton Beach Indoor-Outdoor grill (Model 31605A, Proctor-Silex, Inc., Washington, NC, USA), flipped once when the internal temperature reached 35°C, and removed from the grill when they reached an internal temperature of 71°C. Grilled steaks were cooled at 4°C for 24 h, and six cores, 1.27 cm in diameter, were removed parallel to the muscle fibres using a drill press. Cores were sheared on a texture analyser (TMS-PRO, Food Technology Crop., Sterling, VA, USA) with a Warner–Bratzler blade. The mean peak shear force (kg) of six cores was calculated for each steak.
Free calcium concentration
Free calcium was quantified using the methods described by Parrish et al. (1981) with modifications. Three grams of powdered sample was centrifuged at 196 000g (Beckman L7–65 ultracentrifuge with a SW55Ti rotor; Beckman Coulter, Brea, CA, USA) at 4°C for 30 min. Seven-hundred μL of the supernatant was collected and treated with 0.1 mL of 27.5% trichloroacetic acid. Samples were centrifuged at 6000g (Eppendorf model 5430; Eppendorf, Hamburg, Germany) at room temperature for 10 min. Four-hundred μL of supernatant was transferred to a syringe, and the volume was brought to 4 mL with deionised distilled water (ddH2O). The diluted calcium sample was filtered through 13-mm-diameter Millex-LG 0.20-μm syringe filters (Millipore, Bedford, MA, USA). Calcium concentration of samples was quantified using an inductively-coupled plasma emission spectrometer (iCAP 6500 Radial; Thermo Electron, Cambridge, UK) with calcium concentration standards of 0, 25, 50, 150 mg/L.
Lipid oxidation
Lipid oxidation samples were prepared using the thiobarbituric acid assay (TBARS) described by Ahn et al. (1998). Duplicate 200-μL aliquots of supernatant from each sample was transferred to 96-well plates and read with a microplate spectrophotometer (Model Epoch, Biotek, Winooski, VT, USA) at 540 nm. All 96-well plates had standards to calculate standard curves, and each sample was calculated as mg of malonaldehyde per kg of muscle tissue using the standard curve from each plate.
Sarcomere length
A few specks of muscle powder from each sample were gently spread on a microscope slide. A single drop of 0.25 M sucrose and 0.002 M potassium chloride solution at pH 7.0 was applied to the muscle powder on the microscope slide. The prepared microscope slide was placed on the stage of a Helium-Neon laser (Melles Griot, Carlsbad, CA, USA) stand, and the average length of five sarcomere positions per sample were determined using the light diffraction method described by Cross et al. (1981). Dolazza and Lorenzen (2014) demonstrated that there was no difference in sarcomere length between fresh whole muscle and powdered liquid nitrogen frozen muscle. However, laboratory work time can be shortened significantly by applying the powdering method for sarcomere length measurement.
Proteolysis
Myofibrillar proteins were isolated according the method described by Pietrzak et al. (1997) with modifications. Three g of powdered meat sample were suspended in 10 mL ice-cold rigor buffer (0.1 M KCl, 2 mM MgCl2, 1 mM EGTA, and 10 mM K2HPO4) at pH 7.4 and homogenised using a Polytron homogeniser (model CH-6010; Kinematica, Luzern, Switzerland) at setting 6 for 15 s. The homogenate was filtered thorough doubled-layered cheese cloth to remove connective tissue and fat. One mL of homogenate was transferred and centrifuged at 4000g for 5 min at room temperature. The supernatant was decanted and the pellet was resuspended in 1 mL of ice-cold rigor buffer. The pellet washing step was repeated three times. One mL of extraction buffer (0.1 M TRIS-HCl, 1.25 mM EDTA, 5% SDS) at pH 8 was added to the washed pellet and vortexed thoroughly. Protein concentration was determined using a Pierce bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). All myofibrillar protein samples were diluted to 2 mg/mL with ddH2O.
Degree of proteolysis was measured by troponin-T (TNT) degradation. All of the following procedures were conducted at room temperature. Twenty-five μL of the 2 mg/mL myofibrillar protein samples were mixed with 2x Laemmli sample buffer (65.8 mM TRIS-HCl, 2.1% SDS, 26.3% glycerol, 0.01% bromophenol blue) with 5% β-mercaptoethanol at 1 : 1 ratio. All samples were heated at 95°C for 5 min. Five μL Kaleidoscope Pre-stained Protein Standard and prepared myofibrillar protein samples (5 μg) were loaded on 4 to 20% Mini-PROTEAN TGX™ precast polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA, USA) using a Bio-Rad Mini-PROTEIN 2 Cells system (Bio-Rad Laboratories). The system was run at constant voltage of 200 V for 40 min with a running buffer consisting 25 mM Tris-base, 192 mM glycine and 0.1% SDS (pH 8.3). Proteins in the gels were blotted to polyvinylidene difluoride membranes (0.45 μm, Immobilon-FL transfer membrane; Millipore) using a Bio-Rad Mini-Trans-Blot Electrophoretic transfer cell (Bio-Rad Laboratories) for 60 min at a constant voltage of 100 V with ice-cold transfer buffer (25 mM Tris-base, 192 mM glycine, 20% methanol, 0.1% SDS at pH 9.2). Membranes were blocked for 2 h in Odyssey Blocking Buffer (LI-COR, Lincoln, NE, USA) and incubated for 1 h in primary anti-TNT antibody (JLT-12; Sigma-Aldrich, St Louis, MO, USA) at a dilution of 1 : 10 000 in Odyssey blocking buffer containing 0.2% Tween-20. Membranes were washed three times with Tris Buffered Saline containing 0.2% Tween-20 for 15 min and incubated in IRDye 680 LT Conjugated Goat Anti-Mouse IgG1 secondary antibody (LI-COR) at a dilution of 1 : 10 000 in Odyssey blocking buffer containing 0.2% Tween-20 for 1 h. Membranes were washed three times with TBST and scanned using Odyssey Infrared Imaging system (LI-COR) at 700 nm.
All intact TNT and degraded TNT products were measured by quantifying band intensities (k. pixels) using Odyssey application software version 1.1. Bands ranging from 38 and 35 kD corresponded to intact TNT because their intensities decreased over time, whereas, bands ranging from 30 and 28 kD corresponded to degraded TNT because these bands increased in intensities over time. Percent TNT degraded was measured by band intensities of degraded bands divided by band intensities of all bands in a specific lane.
SR membrane extraction
The SR membrane was extracted from muscle tissue according to the method described by Hemmings (2001) with modifications. Ten g of powdered samples were suspended in 35 mL of ice-cold homogenisation buffer (10 mM NaHCO3, 2 mM sodium azide, 10 mM Tris-Cl, and 1 mM dithiothreitol) at pH 7.5 and homogenised using a Polytron homogeniser (Kinematica) at setting 6 for 15 s. Homogenate was transferred into a 50-mL plastic centrifuge tube and centrifuged at 2000g for 10 min at 4°C. The supernatant was collected and centrifuged at 10 000g (Sorvall RC5B Superspeed Centrifuge; Thermo Scientific, Rockford, IL, USA) for 30 min at 4°C. Four and a half percent KCl (w/v) was added to the supernatant and stirred for 30 min on ice. The supernatant was centrifuged at 100 000g for 60 min at 4°C (Beckman L7–65 Ultracentrifuge with a SW28 rotor; Beckman Coulter). The final supernatant was discarded, and the pellet was resuspended in 1 mL of 10 mM tris buffer and stored at −80°C until use.
SR membrane fatty acids
For SR membrane fatty acids, total lipid was extracted following the procedure of Bligh and Dyer (1959). After extraction, lipids were converted to fatty acid methyl esters according to Morrison and Smith (1964) and Metcalfe et al. (1966). The prepared fatty acid methyl esters were further concentrated by drying at 60°C under constant nitrogen gas purging and mixed with 100 μL of hexane. The fatty acid methyl esters were transferred to 100-μL spring bottom vial inserts and inserted into the GC vials. The samples were analysed using gas chromatography (Hewlett-Packard 6890 FID GC System; Agilent Technologies, Santa Clara, CA, USA) with setups described by Mello et al. (2012).
SR membrane phospholipids
Total lipids were extracted from a second set of SR membrane samples. Thirty μL of 2% methanol and 1% ddH2O in chloroform was added to each lipid sample. For SR membrane phospholipid, neutral lipid and total lipid profile, samples were separated into 10 different lipid groups by one-dimensional thin-layer chromatography (TLC) described by Leray et al. (1987) with modifications. Briefly, Whatman LK5 TLC plates (Whatman, Clifton, NJ, USA) were prewashed by migration up to 1 cm from the top with chloroform/methanol (1/1, v/v) and wetted thoroughly with 2.3% (w/v) boric acid solution. All 30 µL of each extracted lipid sample was deposited on the plate and placed in the chromatography tank containing the following solvent: chloroform/ethanol/ddH2O/triethylamine (30/35/7/35, v/v). After the migration was complete, lipid and phospholipid spots were quantified by the method described by Baron and Coburn (1984) with modifications. Each plate was dampened with a 10% (w/v) cupric sulfate solution in 8% (w/v) phosphoric acid and placed in an oven at 180°C for 10–15 min. The isolated fractions were identified by comparing their Rf values with known lipid standards. The plate was scanned by a desktop scanner (Artisan 730, Epson, Nagano, Japan) and the isolated fractions were quantified using Quantity One 1-D Analysis Software (Bio-Rad). Each phospholipid was measured as a percentage of total phospholipids in one lane.
Statistical analyses
Data for WBSF, free calcium concentration, lipid oxidation, and TNT degradation were analysed as a split-split plot design with dietary treatments as the whole-plot, aging period as the split-plot and retail display time as the split-split plot. Animals within diet was considered the whole-plot error term, and age by diet was considered as the split-plot error term and display day by diet was considered the split-split plot error term. Sarcomere length, SR membrane fatty acid, phospholipid and total lipid profiles were analysed as a completely randomised design. Animal was considered the random effect. Tukey’s test was used for multiple comparisons. Data were analysed using the GLIMMIX procedure of SAS (version 9.2, 2009, Cary, NC, USA). For all analysis, separation of means was conducted using LSMEANS procedure (least significant differences) at P < 0.05.
Results and discussion
SR membrane fatty acids
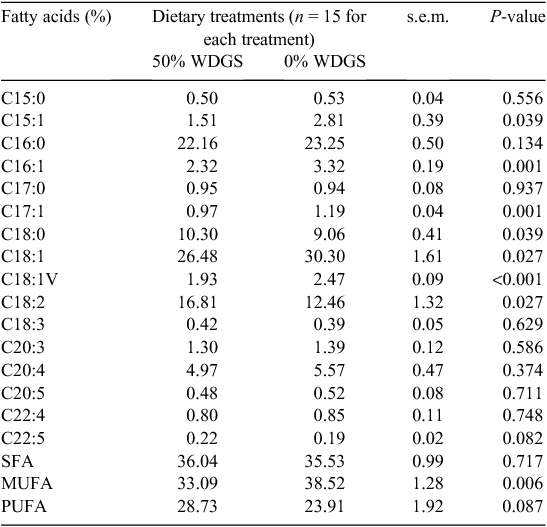
Results for the SR membrane fatty acid profile analyses are presented in Table 2. Feeding 50% WDGS decreased (P < 0.05) concentrations of 15:1, 16:1, 17:1, 18:1, 18:1V and total monounsaturated fatty acid (MUFA), increased (P < 0.05) concentrations of 18 : 0 and 18 : 2 fatty acids and tended to increase (P < 0.10) total PUFA in the SR membrane. Long and very long chain PUFA like 20:3, 20:4, 20:5, 22:4, 22 : 5 were not affected by the dietary treatment. When compared the beef SR membrane fatty acid profiles from this study to the beef muscle tissue fatty acid profiles (Domenech et al. 2014) from the same set of cattle, total PUFA quadrupled in SR membrane compared with the PUFA content of muscle tissue. About half of the total lipids from the SR membrane measured in this study are phospholipids (Table 3). Larick and Turner (1990) and Noci et al. (2005) reported that PUFA, especially 18:2, are predominately associated with the phospholipid fraction. Therefore, the greater content of PUFA in SR membrane than muscle tissue was expected. The increase in 18 : 2 fatty acid and total PUFA of the SR membrane from 50% WDGS steers supported our hypothesis that feeding WDGS may impair SR membrane integrity. Modification of beef SR membrane fatty acid profiles by feeding WDGS likely occurred by the same mechanism that modification of beef muscle fatty acid profiles occurs. Although the majority of the unsaturated fatty acids in the diet were biohydrogenated to saturated fatty acid (SFA) by the rumen microflora, WDGS has double the amount of corn oil (Ham et al. 1994) compared with corn, which led to greater deposition of 18 : 2 and PUFA in the muscle tissue and SR membrane. All individual MUFA and total MUFA decreased in SR membrane for WDGS-fed steers. However, the reason for such a decrease is still unclear. Unlike 18:2, which is entirely derived from the diet, medium and long chain MUFA are products of biohydrogenation from dietary PUFA or SFA to MUFA conversion from delta-9-desaturase (Wood et al. 2008; Smith et al. 2006). Perhaps, the 0% WDGS steers needed to generate more MUFA from SFA because less PUFA was available in the plasma to incorporate in the organelle membrane. The other possibility is that the 50% WDGS diet (high in PUFA) may have suppressed the stearoyl-CoA desaturase gene, which is the gene that encodes for delta-9-desaturase (Chung et al. 2007).
|
|
SR membrane phospholipids
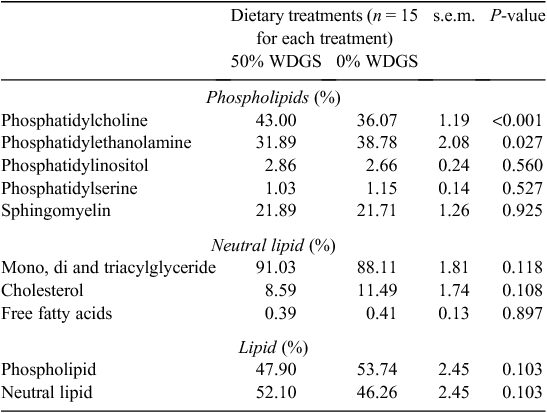
Results for the SR membrane phospholipids, neutral lipid, and total lipid profiles are presented in Table 3. Feeding 50% WDGS tended to decrease (P = 0.10) phospholipid concentration and tended to increase (P = 0.10) total neutral lipid concentrations in the SR membrane. Feeding 50% WDGS also increased (P < 0.01) concentration of phosphatidylcholine (PC), but decreased (P < 0.05) concentration of phosphatidylethanolamine (PE) in the SR membrane phospholipids. Deaver et al. (1986), Brasitus et al. (1985) and Thi-Dinh et al. (1990) found that feeding rats a high unsaturated fat diet decreased the phospholipid concentration in liver, enterocytes and the plasma membrane of adipocytes, respectively. Feed stuffs rich in PUFA, like pasture and WDGS, are known to increase the proportion of PUFA in muscle tissue phospholipids of beef (Dannenberger et al. 2007), fish (Huang et al. 1998) and pork (Nurnberg et al. 1998). Mead et al. (1980) further suggested lipid oxidation can increase the activity of phospholipases to remove esterified fatty acids. Perhaps, the greater PUFA content contributed to oxidation of SR membrane phospholipids, thus resulting in accelerated SR membrane phospholipid degradation and causing differences in phospholipid concentration between the two treatments. Mlekusch et al. (1993) also found that feeding rats a diet high in PUFA increased concentration of PC, but decreased the concentration of PE in rat liver. However, no clear explanation was given for the phenomenon. Beare and Kates (1964) reported that rat muscle tissue PC can incorporate 18 : 2 better than PE can. In the present study, feeding 50% WDGS increased 18 : 2 content of beef SR membrane by 35%. Perhaps, the shifts of phospholipid profiles found in Mlekusch et al. (1993) and this study can be explained by the increase of 18 : 2 content in the diets.
WBSF and free calcium concentration
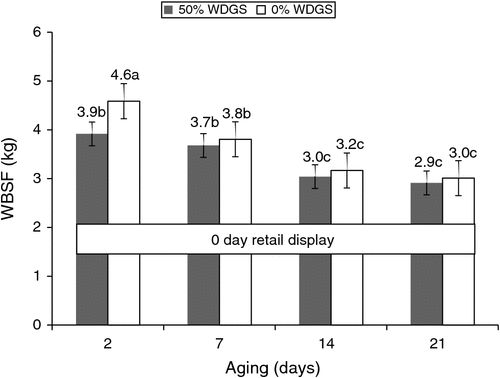
There was a (P < 0.05) three-way interaction among treatment, aging and retail display time on steak WBSF. Compared with steaks from steers fed 0% WDGS, steaks from the 50% WDGS group were more tender (P < 0.01) at 2 days of aging with 0 days of retail display (Fig. 1). There was no difference tenderness concentrations between treatments for any other aging and retail display period (data not presented). Segers et al. (2011) reported that beef from cattle supplemented with 25% dried distillers plus solubles for 100 days was more tender than beef from cattle supplemented with soybean meal at 7 days of aging, and Depenbusch et al. (2009) reported sensory overall tenderness ratings increased linearly as dietary level of distillers grains increased from 0% to 75%. Finally, Senaratne (2012) reported that beef from steers fed 30% WDGS tended (P < 0.10) to be more tender than beef from steers not fed a corn-only diet. However, extended aging beyond 2 days appeared to mitigate the tenderisation effects from the WDGS treatments for this study.
|
There was also a (P < 0.05) three-way interaction among treatments, aging and retail display day on free calcium concentrations. Steaks from steers fed 50% WDGS had greater (P < 0.01) free calcium concentrations at 2 days of aging after 7 days of retail display (Fig. 2), and there was no difference in free calcium concentrations between treatments for any other aging and retail display period (data not presented). Senaratne (2012) was the first to the authors’ knowledge to evaluate diet effect on beef muscle tissue free calcium level, and that study reported beef from steers fed 30% WDGS tended (P < 0.10) to have greater free calcium concentrations than beef from steers fed corn-only. Miller et al. (2011) also found differences in free calcium concentration between tender and tough samples early post-mortem (24 and 48 h). However, the tenderness difference from that study was created through implant and β-agonist rather than diet, and no measurement on SR membrane integrity was performed.
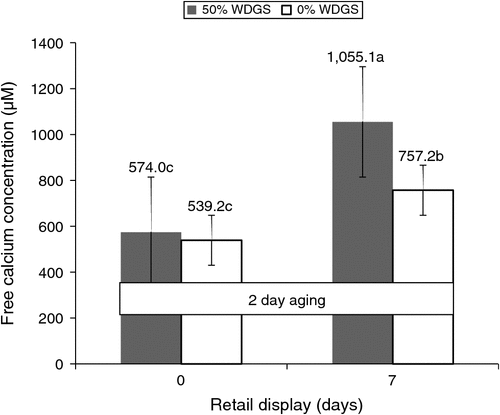
|
Although differences were detected in both WBSF and free calcium concentration assays, the results from this study did not fully support the authors’ hypothesis. The time courses for free calcium concentrations differences (9 days post-mortem) and tenderness differences (2 days post-mortem) between treatments did not match up with the time courses proposed in the authors’ hypothesis.
Lipid oxidation
There was a significant (P < 0.01) interaction between aging and treatments for lipid oxidation. Steaks from steers fed 0% WDGS had greater malonaldehyde concentrations than steaks from steers fed 50% WDGS (P < 0.05) at 21 days of aging (Fig. 3). Lipid oxidation data from this study revealed another intriguing phenomenon that beef from cattle fed 0% WDGS was more oxidised than beef from cattle fed 50% WDGS during retail display. This result contradicted the results from many other studies that reported feeding cattle high concentration of WDGS increases lipid oxidation levels compared with feeding 0% WDGS (Gill 1996; Kinman et al. 2011; Senaratne et al. 2011). However, this is not the first time that feeding distillers grains was reported to decrease lipid oxidation. Song et al. (2013) also found that lipid oxidation values were not different between pigs fed 30% dried distillers grains plus solubles or corn/soybean-based control diets. One explanation for such a phenomenon is that the greater concentration of sulfur in 50% WDGS diet [0.41% vs 0.09% in 0% WDGS diet (Jolly et al. 2014)] may cause an antioxidant effect. The high concentration of sulfur may synthesise sulfur-containing amino acids and thus alleviate the oxidative stress induced by PUFA in WDGS (Song et al. 2013). Sulfur-containing amino acids such as methionine, cystine, taurine, and glutathione have been studied extensively for their antioxidant properties (Parcell 2002; Atmaca 2004; Battin and Brumaghim 2009). Hwang et al. (2000) fed 5% taurine to rats and observed decreased liver lipid oxidation from feeding diets containing 3% oxidised fish oil, which indicated that taurine may protect against lipid oxidation. Sulfur in water and feed exists in the form of sulfate; in ruminants, sulfate-reducing bacteria reduce sulfate to hydrogen sulfide, which is utilised to produce sulfur-containing amino acids (Drewnoski et al. 2014). It is possible that the increase in hydrogen sulfide also increased the production of sulfur-containing amino acids, thus triggering the antioxidant effect to protect PUFA in beef.
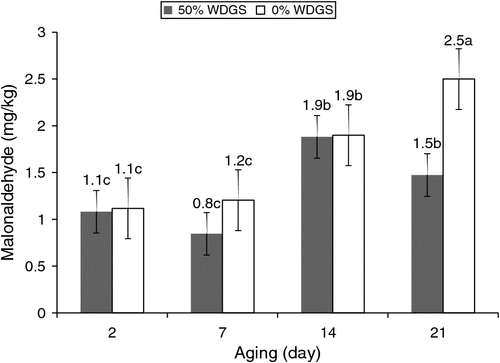
|
Sarcomere length and proteolysis
No differences (P > 0.10) in sarcomere length between treatments were observed. The average sarcomere length of strip loins from 50% WDGS and 0% WDGS steers were 1.84 and 1.82 μm, respectively. Similarly, Oltra et al. (2008) also reported that feeding distillers grains had no effect on sarcomere length. Sarcomere length is established as a marker for pre-rigor free calcium level (Pearson et al. 1973). It is likely there were still plenty of antioxidants available to prevent membrane lipid oxidation early post-mortem, so the pre-rigor time course of calcium release was not be affected by the greater PUFA content in the SR membrane. In fact, Stanley (1991) pointed out that the presence of PUFA in membranes can inhibit hydrocarbon chain packing, thereby better preserve membrane integrity at lower temperature.
An example of a western blot used to quantify TNT degradation is presented in Fig. 4. There were no differences (P > 0.10) in TNT degradation between treatments in any of the aging or display period (data not presented). TNT degradation results indicated that the calpain activity was not different between treatments, but the WBSF results indicated otherwise. Taylor et al. (1995) pointed out that the proteolytic degradation of specific proteins may be attributed to inconsistent relationship between WBSF and proteolysis. It is possible that proteolysis was different for some other proteins such as titin, desmin, vinculin, or nebulin in this study.
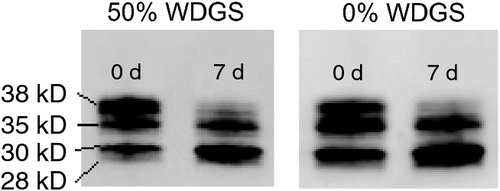
|
Possible mechanisms
Based on the results from this study, three possible mechanisms were speculated for the differences in free calcium concentrations between treatments. First, Cheah (1981) reported that free 18 : 2 fatty acid can stimulate the release of calcium and inhibit calcium uptake from the SR membrane. It is possible that 18 : 2 from the SR membrane released by endogenous phospholipase A2 induced the ryanodine receptor (RyR1) to release calcium. Second, phospholipid PE has the ability to enhance the activity of sarco-endoplasmic reticulum calcium ATPase in SR membrane through specific headgroup interactions (Hunter et al. 1999). It is possible that a reduction in SR membrane PE in WDGS-fed cattle impeded calcium influx, resulting in an increase of sarcoplasmic free calcium concentration. Third, Ji and Takahashi (2006) reported that concentrations of SR membrane phospholipid are negatively correlated with sarcoplasmic calcium concentration during aging of pork and beef, and they hypothesised that calcium ions leaked into the sarcoplasm through channels formed by the degradation of phospholipids. It is possible that the decrease in SR membrane phospholipid concentration in 50% WDGS samples contributes to the increase of free calcium.
In addition to SR membrane lipid oxidation, the contribution of protein oxidation should also be considered. The RyR1 of SR membrane possesses several highly reactive sulfhydryl (SH) groups that are susceptible to oxidation (Sun et al. 2001). Abramson and Salama (1989) reported that oxidised SH groups can stimulate calcium release by forming disulfide bonds, which cause the RyR1 to maintain in the open state. Hidalgo et al. (2000) further reported that oxidised RyR1 becomes active at low (μM) luminal calcium concentrations and was not inhibited by high (mM) sarcoplasmic calcium concentrations. Unfortunately, no measurement of SR membrane protein oxidation was made in this study.
Conclusions
This study reported that feeding WDGS may increase early post-mortem tenderness and free calcium release. The increase in free calcium concentration was likely the result of increased total PUFA and decreased total phospholipids in the SR membrane from early onset of oxidation. Although the true mechanism and time course of membrane oxidation and free calcium release and their effects on beef tenderness are still unclear, results from this study provide conceptual foundation for a new research perspective on meat tenderisation.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank all the past and current graduate and undergraduate students who helped with this project; as well as University of Nebraska Research Council (Lincoln, NE) and The Beef Checkoff (Centennial, CO) for funding this research.
References
Abramson JJ, Salama G (1989) Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. Journal of Bioenergetics and Biomembranes 21, 283–294.| Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlGitbY%3D&md5=77730b1d554edd6a2b5ab59f62c2eb24CAS |
Ahn DU, Olson DG, Jo C, Chen X, Wu C, Lee JI (1998) Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Science 49, 27–39.
| Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFygtrg%3D&md5=830eacd7bfeb896597c988e5ef203239CAS |
Atmaca G (2004) Antioxidant effects of sulfur-containing amino acids. Yonsei Medical Journal 45, 776–788.
| Antioxidant effects of sulfur-containing amino acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVKitbvM&md5=aff207d0c1c3a2068c8c3c4448252914CAS |
Baron CB, Coburn RF (1984) Comparison of two copper reagents for detection of saturated and unsaturated neutral lipids by charring densitometry. Journal of Liquid Chromatography 7, 2793–2801.
| Comparison of two copper reagents for detection of saturated and unsaturated neutral lipids by charring densitometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlvVOgtQ%3D%3D&md5=329a4f26167bec02416fd317673e9d68CAS |
Battin EE, Brumaghim JL (2009) Antioxidant activity of sulfur and selenium, a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochemistry and Biophysics 55, 1–23.
| Antioxidant activity of sulfur and selenium, a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosV2ksL8%3D&md5=385269f05a5739e962f827b8c1c0a279CAS |
Beare JL, Kates M (1964) The deposition of linoleic acid in rats fed corn oil. Canadian Journal of Biochemistry 42, 1477–1486.
| The deposition of linoleic acid in rats fed corn oil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXkvFCltbw%3D&md5=c37ace9881b6d4178c6f5d8e721421faCAS |
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917.
| A rapid method of total lipid extraction and purification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXhtVSgt70%3D&md5=4e60a5c754d3f3b9f9701b07dd6a3f60CAS |
Boleman S, Boleman S, Morgan W, Hale D, Griffin D, Savell J, Ames R, Smith M, Tatum J, Field T (1998) National Beef Quality Audit-1995: survey of producer-related defects and carcass quality and quantity attributes. Journal of Animal Science 76, 96–103.
| National Beef Quality Audit-1995: survey of producer-related defects and carcass quality and quantity attributes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVKjtw%3D%3D&md5=9d04dc551e97124be6fc90991deab3c3CAS |
Brasitus TA, Davidson NO, Schachter D (1985) Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes. BBA-Biomembranes 812, 460–472.
| Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFajtb4%3D&md5=f36108dfe40110a1a4c3ce67e838f14dCAS |
Cheah AM (1981) Effect of long chain unsaturated fatty acids on the calcium transport of sarcoplasmic reticulum. BBA-Biomembranes 648, 113–119.
| Effect of long chain unsaturated fatty acids on the calcium transport of sarcoplasmic reticulum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xit1yh&md5=fd63545f7771497aa7f073db28ab0bafCAS |
Chung KD, Lunt D, Kawachi H, Yano H, Smith S (2007) Lipogenesis and stearoyl-CoA desaturase gene expression and enzyme activity in adipose tissue of short-and long-fed Angus and Wagyu steers fed corn-or hay-based diets. Journal of Animal Science 85, 380–387.
| Lipogenesis and stearoyl-CoA desaturase gene expression and enzyme activity in adipose tissue of short-and long-fed Angus and Wagyu steers fed corn-or hay-based diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1elsrY%3D&md5=7700494125f65b6858154955f1d59b53CAS |
Cross H, West R, Dutson T (1981) Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Science 5, 261–266.
| Comparison of methods for measuring sarcomere length in beef semitendinosus muscle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFClsA%3D%3D&md5=f8f551c8f2ea61d908630f19b59ffccbCAS |
Dannenberger D, Nuernberg G, Scollan N, Ender K, Nuernberg K (2007) Diet alters the fatty acid composition of individual phospholipid classes in beef muscle. Journal of Agricultural and Food Chemistry 55, 452–460.
| Diet alters the fatty acid composition of individual phospholipid classes in beef muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhtlaru7rF&md5=ee673b57d51469335a416046fbfb95f1CAS |
Deaver OE, Wander RC, McCusker R, Berdanier CD (1986) Diet effects on membrane phospholipid fatty acids and mitochondrial function in BHE rats. The Journal of Nutrition 116, 1148–1155.
Depenbusch BE, Coleman CM, Higgins JJ, Drouillard JS (2009) Effects of increasing levels of dried corn distillers grains with solubles on growth performance, carcass characteristics, and meat quality of yearling heifers. Journal of Animal Science 87, 2653–2663.
| Effects of increasing levels of dried corn distillers grains with solubles on growth performance, carcass characteristics, and meat quality of yearling heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptV2gtL8%3D&md5=f01c2c415cba201bf0d407cf4370e2b8CAS |
Dolazza RM, Lorenzen CL (2014) Can samples be powdered to determine sarcomere length? Journal of Animal Science 92, 129–130.
Domenech K, Varnold KA, Semler ME, Chao M, Jones T, Erickson GE, Calkins CR (2014) Effects of feeding de-oiled wet distillers grains plus solubles on beef fatty acid profiles. Nebraska Beef Report MP99, 116–118.
Drewnoski M, Pogge D, Hansen S (2014) High-sulfur in beef cattle diets: a review. Journal of Animal Science 92, 3763–3780.
| High-sulfur in beef cattle diets: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslSjtbbK&md5=8c42aa02d4d44b3762dbde14512dab47CAS |
Garcia L, Nicholson K, Hoffman T, Lawrence T, Hale D, Griffin D, Savell J, VanOverbeke D, Morgan J, Belk K (2008) National Beef Quality Audit–2005: Survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers. Journal of Animal Science 86, 3533–3543.
| National Beef Quality Audit–2005: Survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsV2jsbrI&md5=bafc092a88c249de5983e91de04537a3CAS |
Gill C (1996) Extending the storage life of raw chilled meats. Meat Science 43, 99–109.
| Extending the storage life of raw chilled meats.Crossref | GoogleScholarGoogle Scholar |
Ham G, Stock R, Klopfenstein T, Larson E, Shain D, Huffman R (1994) Wet corn distillers byproducts compared with dried corn distillers grains with solubles as a source of protein and energy for ruminants. Journal of Animal Science 72, 3246–3257.
Hemmings SJ (2001) New methods for the isolation of skeletal muscle sarcolemma and sarcoplasmic reticulum allowing a comparison between the mammalian and amphibian beta(2)-adrenergic receptors and calcium pumps. Cell Biochemistry and Function 19, 133–141.
| New methods for the isolation of skeletal muscle sarcolemma and sarcoplasmic reticulum allowing a comparison between the mammalian and amphibian beta(2)-adrenergic receptors and calcium pumps.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVSrtb4%3D&md5=33cf63000e53ba913601cbd8dd29f3b3CAS |
Hidalgo C, Bull R, Marengo JJ, Perez CF, Donoso P (2000) SH oxidation stimulates calcium release channels (ryanodine receptors) from excitable cells. Biological Research 33, 113–124.
| SH oxidation stimulates calcium release channels (ryanodine receptors) from excitable cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtVamsbg%3D&md5=ff653df2ae3779ac940becb83e2e0290CAS |
Huang CH, Huang MC, Hou PC (1998) Effect of dietary lipids on fatty acid composition and lipid peroxidation in sarcoplasmic reticulum of hybrid tilapia, Oreochromis niloticus x O. aureus. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 120, 331–336.
| Effect of dietary lipids on fatty acid composition and lipid peroxidation in sarcoplasmic reticulum of hybrid tilapia, Oreochromis niloticus x O. aureus.Crossref | GoogleScholarGoogle Scholar |
Hunter GW, Negash S, Squier TC (1999) Phosphatidylethanolamine modulates Ca-ATPase function and dynamics. Biochemistry 38, 1356–1364.
| Phosphatidylethanolamine modulates Ca-ATPase function and dynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhslOnuw%3D%3D&md5=69194a1ad5e5fca4e7e7d523a82edaa2CAS |
Hwang D, Hour J, Cheng H (2000) Effect of taurine on toxicity of oxidized fish oil in rats. Food and Chemical Toxicology 38, 585–591.
| Effect of taurine on toxicity of oxidized fish oil in rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlt1Giu7w%3D&md5=c80194155a89499a796a80f977cfaa57CAS |
Ji JR, Takahashi K (2006) Changes in concentration of sarcoplasmic free calcium during post-mortem ageing of meat. Meat Science 73, 395–403.
| Changes in concentration of sarcoplasmic free calcium during post-mortem ageing of meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksF2jsLc%3D&md5=80da70fe80761b623c099e4ac01c74f7CAS |
Jolly ML, Nuttelman BL, Burken D, Schneider CJ, Klopfenstein TJ, Erickson GE (2014) Effects of increasing inclusion of wet distillers grains plus solubles with and without oil extraction on finishing performance. Nebraska Beef Report MP99, 81–82.
Kinman L, Hilton G, Richards C, Morgan J, Krehbiel C, Hicks R, Dillwith J, VanOverbeke D (2011) Impact of feeding various amounts of wet and dry distillers grains to yearling steers on palatability, fatty acid profile, and retail case life of longissimus muscle. Journal of Animal Science 89, 179–184.
| Impact of feeding various amounts of wet and dry distillers grains to yearling steers on palatability, fatty acid profile, and retail case life of longissimus muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotl2ktA%3D%3D&md5=5f2e23d99365111c9a6f3555b6b6b493CAS |
Klopfenstein TJ, Erickson GE, Bremer VR (2008) Board-invited review: Use of distillers byproducts in the beef cattle feeding industry. Journal of Animal Science 86, 1223–1231.
| Board-invited review: Use of distillers byproducts in the beef cattle feeding industry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltlWruro%3D&md5=9668678fd700d0f03804f3407f79c78aCAS |
Koger TJ, Wulf DM, Weaver AD, Wright CL, Tjardes KE, Mateo KS, Engle TE, Maddock RJ, Smart AJ (2010) Influence of feeding various quantities of wet and dry distillers grains to finishing steers on carcass characteristics, meat quality, retail-case life of ground beef, and fatty acid profile of longissimus muscle. Journal of Animal Science 88, 3399–3408.
| Influence of feeding various quantities of wet and dry distillers grains to finishing steers on carcass characteristics, meat quality, retail-case life of ground beef, and fatty acid profile of longissimus muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtF2rtb3O&md5=5a1f4cc6aaedde5f26204875af225b11CAS |
Larick DK, Turner BE (1990) Flavor characteristics of forage-fed and grain-fed beef as influenced by phospholipid and fatty acid compositional differences. Journal of Food Science 55, 312–317.
| Flavor characteristics of forage-fed and grain-fed beef as influenced by phospholipid and fatty acid compositional differences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXktl2jtbk%3D&md5=15268d6c9c25799c68dda07f2cde0fb1CAS |
Leray C, Pelletier X, Hemmendinger S, Cazenave JP (1987) Thin-layer chromatography of human platelet phospholipids with fatty acid analysis. Journal of Chromatography. A 420, 411–416.
| Thin-layer chromatography of human platelet phospholipids with fatty acid analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXlvFCgurk%3D&md5=32aaef7ffd22bc3c6144f92e7517065eCAS |
McKenna D, Roebert D, Bates P, Schmidt T, Hale D, Griffin D, Savell J, Brooks J, Morgan J, Montgomery T (2002) National Beef Quality Audit-2000: survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers. Journal of Animal Science 80, 1212–1222.
| National Beef Quality Audit-2000: survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVeksb0%3D&md5=f5e8638c06c5e2e9991f80dd905811a8CAS |
Mead JF, Stein RA, Wu GS, Sevanian A, Gan-Elepano M (1980) Peroxidation of lipids in model systems and in biomembranes. In ‘Autoxidation in food and biological systems’. (Eds MG Simic, M Karel) pp. 413–428. (Springer: New York)
Mello AS, Calkins CR, Jenschke BE, Carr TP, Dugan MER, Erickson GE (2012) Beef quality of calf-fed steers finished on varying levels of corn-based wet distillers grains plus solubles. Journal of Animal Science 90, 4625–4633.
| Beef quality of calf-fed steers finished on varying levels of corn-based wet distillers grains plus solubles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXns1SqtQ%3D%3D&md5=fd9e7ac20cc95e179c354ccff49c3421CAS |
Metcalfe L, Schmitz AA, Pelka J (1966) Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Analytical Chemistry 38, 514–515.
| Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XosFKltA%3D%3D&md5=6a95b21233ca7a4bc6f6cd6906654dbdCAS |
Miller MF, Carr MA, Ramsey CB, Crockett CL, Hoover LC (2001) Consumer thresholds for establishing the value of beef tenderness. Journal of Animal Science 79, 3062–3068.
| Consumer thresholds for establishing the value of beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFCmtA%3D%3D&md5=bbcef837330f9c5959c29885b7173fe3CAS |
Miller RK, Smith SB, Carstens GE (2011) New Beef Tenderness Theory – Executive Summary. Prepared for the National Cattlemen’s Beef Association, Centenial, CO.
Mlekusch W, Celedin C, Aloia RC, Moller R (1993) Effect of a high fat diet on phospholipid class distribution and fatty acid composition in rat liver. The International Journal of Biochemistry 25, 1539–1547.
| Effect of a high fat diet on phospholipid class distribution and fatty acid composition in rat liver.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivF2mug%3D%3D&md5=a735b2e58af102e4e69e467ced7b988aCAS |
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. Journal of Lipid Research 5, 600–608.
Noci F, Monahan FJ, French P, Moloney AP (2005) The fatty acid composition of muscle fat and subcutaneous adipose tissue of pasture-fed beef heifers: Influence of the duration of grazing. Journal of Animal Science 83, 1167–1178.
| The fatty acid composition of muscle fat and subcutaneous adipose tissue of pasture-fed beef heifers: Influence of the duration of grazing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslShtLg%3D&md5=47f5231fa453acdfac4eba4ad46b5ae3CAS |
Nurnberg K, Kuchenmeister U, Ender K, Nurnberg G, Hackl W (1998) Influence of dietary n-3 fatty acids on the membrane properties of skeletal muscle in pigs. European Journal of Lipid Science and Technology 100, 353–358.
Oltra O, Farmer L, Moss B, Birnie J (2008) The effect of distiller’s and brewer’s grains on beef acceptability. Proceeding of International Congress of Meat science and Technology 54. Cape Town, South Africa.
Parcell S (2002) Sulfur in human nutrition and applications in medicine. Alternative Medicine Review 7, 22–44.
Parrish FC, Selvig CJ, Culler RD, Zeece MG (1981) CAF activity, calcium concentration, and the 30,000-dalton component of tough and tender bovine longissimus muscle. Journal of Food Science 46, 308–311.
| CAF activity, calcium concentration, and the 30,000-dalton component of tough and tender bovine longissimus muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXot1Cisw%3D%3D&md5=c94c1331ff8ba81f2c8cda1ce4d4e26aCAS |
Pearson AW, Carse WA, Davey CL, Locker RH, Hagyard CJ, Kirton AH (1973) Influence of epinephrine and calcium upon glycolysis, tenderness and shortening of sheep muscle. Journal of Food Science 38, 1124–1127.
| Influence of epinephrine and calcium upon glycolysis, tenderness and shortening of sheep muscle.Crossref | GoogleScholarGoogle Scholar |
Pietrzak M, Greaser ML, Sosnicki AA (1997) Effect of rapid rigor mortis processes on protein functionality in pectoralis major muscle of domestic turkeys. Journal of Animal Science 75, 2106–2116.
| Effect of rapid rigor mortis processes on protein functionality in pectoralis major muscle of domestic turkeys.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXltl2itLk%3D&md5=04785c5ec36c27543aa4ccac135f643fCAS |
Platter WJ, Tatum JD, Belk KE, Koontz SR, Chapman PL, Smith GC (2005) Effects of marbling and shear force on consumers’ willingness to pay for beef strip loin steaks. Journal of Animal Science 83, 890–899.
| Effects of marbling and shear force on consumers’ willingness to pay for beef strip loin steaks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivVCqsLY%3D&md5=fbd5cb47c4f6dda209930f53362940dcCAS |
Roeber D, Gill R, DiCostanzo A (2005) Meat quality responses to feeding distiller’s grains to finishing Holstein steers. Journal of Animal Science 83, 2455–2460.
| Meat quality responses to feeding distiller’s grains to finishing Holstein steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKqsLnO&md5=320421d889c8a07a967b7e34f0374a41CAS |
Segers J, Stewart R, Lents C, Pringle T, Froetschel M, Lowe B, McKeith R, Stelzleni A (2011) Effect of long-term corn by-product feeding on beef quality, strip loin fatty acid profiles, and shelf life. Journal of Animal Science 89, 3792–3802.
| Effect of long-term corn by-product feeding on beef quality, strip loin fatty acid profiles, and shelf life.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqsrjP&md5=7ab5cf5c074afd881ea59e7a7a348b35CAS |
Senaratne LS (2012) Mechanism and control of beef Toughening during retail display in high oxygen modified atmosphere packages. PhD Thesis, University of Nebraska-Lincoln, USA.
Senaratne LS, Calkins C, Vasconcelos J, de Mello AS, Andersen MA, Furman SA, Pokharel S (2011) Shelf life of m. longissimus lumborum from beef fed antioxidants and wet distillers grains. Nebraska Beef Report MP94, 100–102.
Smith SB, Lunt DK, Chung KY, Choi CB, Tume RK, Zembayashi M (2006) Adiposity, fatty acid composition, and delta‐9 desaturase activity during growth in beef cattle. Animal Science Journal 77, 478–486.
| Adiposity, fatty acid composition, and delta‐9 desaturase activity during growth in beef cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1egs7nM&md5=2647061fa8713350882a8e1ebfb023abCAS |
Song R, Chen C, Wang L, Johnston L, Kerr B, Weber T, Shurson G (2013) High sulfur content in corn dried distillers grains with solubles protects against oxidized lipids by increasing sulfur-containing antioxidants in nursery pigs. Journal of Animal Science 91, 2715–2728.
| High sulfur content in corn dried distillers grains with solubles protects against oxidized lipids by increasing sulfur-containing antioxidants in nursery pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpvFCrtr8%3D&md5=e72ec1a0755b2daa1d3fd6452f0900afCAS |
Stanley DW (1991) Biological membrane deterioration and associated quality losses in food tissues. Critical Reviews in Food Science and Nutrition 30, 487–553.
| Biological membrane deterioration and associated quality losses in food tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xitleqsbc%3D&md5=da172cc495d07fb29914cce84b3f9f34CAS |
Sun J, Xu L, Eu JP, Stamler JS, Meissner G (2001) Classes of thiols that influence the activity of the skeletal muscle calcium release channel. The Journal of Biological Chemistry 276, 15625–15630.
| Classes of thiols that influence the activity of the skeletal muscle calcium release channel.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvFalu7w%3D&md5=b00f73e652fc1fba6bcf73483ad3f1d3CAS |
Taylor RG, Geesink G, Thompson V, Koohmaraie M, Goll D (1995) Is Z-disk degradation responsible for post-mortem tenderization? Journal of Animal Science 73, 1351–1367.
| Is Z-disk degradation responsible for post-mortem tenderization?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlsFSjtb0%3D&md5=add3dc4e6e48bdde56cd06b397592469CAS |
Thi-Dinh KK, Demarne Y, Nicolas C, Lhuillery C (1990) Effect of dietary fat on phospholipid class distribution and fatty acid composition in rat fat cell plasma membrane. Lipids 25, 278–283.
| Effect of dietary fat on phospholipid class distribution and fatty acid composition in rat fat cell plasma membrane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXktl2gsrw%3D&md5=4db7d921ff64bb78680b56d8729366f6CAS |
Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM (2008) Fat deposition, fatty acid composition and meat quality: a review. Meat Science 78, 343–358.
| Fat deposition, fatty acid composition and meat quality: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCls7s%3D&md5=5087ec4dbce501875a943f79ddaa74f8CAS |


