Mannitol and galactose as markers of gastrointestinal tract morphology in pigs after gradual or conventional weaning
D. L. Turpin A C , P. L. Langendijk B and J. R. Pluske AA Murdoch University, Murdoch, WA 6150.
B Trouw Nutrition, Boxmeer 5831, The Netherlands.
C Corresponding author. Email: D.Turpin@murdoch.edu.au
Animal Production Science 57(12) 2408-2408 https://doi.org/10.1071/ANv57n12Ab028
Published: 20 November 2017
Intermittent suckling sows and litters suckle, piglets suck (IS), where a sow and her piglets are separated for a period of time each day before weaning, can attenuate weaning-associated villous atrophy in progeny from multiparous sows (Berkeveld et al. 2009). The effect of such a management strategy on progeny from primiparous sows might be different given differences in gastrointestinal (GIT) function at weaning (Cottrell et al. 2017). Sugar absorption tests (SAT) using mannitol (MAN) and galactose (GAL) were used to assess GIT morphology with results validated using standard histological methods. Mannitol and GAL are usually absorbed in vivo across the epithelium via transcellular passive or active pathways, respectively. It was hypothesised that (1) MAN and GAL SAT would detect GIT changes at weaning, and (2) changes would be less profound in IS pigs from primiparous sows due to habituation with creep feed and maternal separation in lactation.
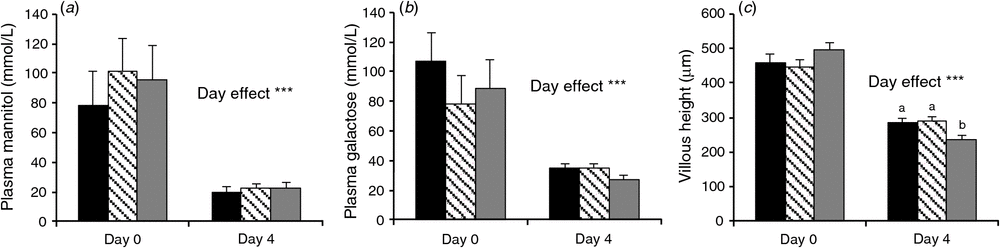
Gilt litters (n = 15), Large White x Landrace, were allocated to one of three weaning regimes: (1) conventional weaning (CW), where piglets had continuous access to the sow until weaning at 26.4 ± 1.34 days (mean ± s.d.), (2) IS, where piglets were separated from the sow for 16 h overnight (0700 to 1500 h) for three nights before weaning (IS16), and (3) IS for 8 h per day (0700 to 1500 h) for 6 days before weaning (IS8). Creep feed was offered ad libitum from 10 days of age. At weaning, litters were mixed within treatment and housed in pens of 9.8 ± 0.41. Two hours (d 0) and 4 days after weaning one piglet per pen was selected, fasted for 3 h and given an oral dose of 20% MAN (2.5 mL/kg bodyweight (BW)) and 20% GAL (2.5 mL/kg BW). A blood sample was taken 20 min later. Pigs were then killed and the jejunum was removed for histological examination. Plasma MAN, GAL and jejunum villous height were compared between treatments using the GLM procedures of SPSS (v22.0, IBM, Armonk, NY, USA). No differences in SAT between treatments were found (P > 0.05), hence data were combined to compare SAT data with GIT histology. Quadratic regressions (y = a +bx+ cx2) were calculated for all relationships.
Plasma MAN and GAL were highly correlated with the jejunum villous height (r = 0.76, P < 0.001 and r = 0.73, P < 0.001 respectively), with all measures decreasing 4 days after weaning compared with the day of weaning (P < 0.05; Fig. 1). These results suggest that MAN and GAL (as single marker probes) are effective measures of changes in small intestine surface area, which supports the first hypothesis. However, given IS8 pigs had the lowest villous height (P < 0.01; Fig. 1C) compared with the other treatment groups 4 days after weaning, there may be some limitations to MAN and GAL SAT when differences between treatments are more subtle. Furthermore, IS did not improve GIT morphological adaptation to weaning in progeny from primiparous dams, with IS8 pigs performing worse than CW pigs with respect to villous height in the immediate post-weaning period. Therefore, our second hypothesis was not supported.
References
Berkeveld M, Langendijk P, Soede NM, Kemp B, Taverne MA, Verheijden JH, Kuijken N, Koets AP (2009) Journal of Animal Science 87, 3156–3166.| Crossref | GoogleScholarGoogle Scholar |
Cottrell J, Craig J, Wijesiriwardana UA, Fothergill L, Ringuet MT, O’Halloran K, Turpin DL, Munoz LM, Collins CL, Furness JB, Dunshea FR, Pluske JR (2017) The FASEB Journal 31, 792.24
Supported by Pork CRC Limited Australia.