Predicting oestrus and ovulation in sows using the vulva, cervical mucus and body temperature
D. Glencorse A B , C. G. Grupen A and R. Bathgate AA Sydney School of Veterinary Science, Faculty of Science, The University of Sydney, NSW 2006.
B Corresponding author. Email: dannielle.glencorse@sunporkfarms.com.au
Animal Production Science 57(12) 2473-2473 https://doi.org/10.1071/ANv57n12Ab098
Published: 20 November 2017
The most common method of oestrus detection in sows relies on observing behavioural changes indicative of sexual receptivity. This technique is time consuming, labour intensive and due to its subjective nature, often provides inconsistent results between observers (Soede et al. 2011). Consequently, it would be beneficial to identify alternative markers for oestrus, and more importantly for the onset of ovulation, by validating more quantifiable and objective measures. The hypothesis follows that fluctuations in vulva size, mucus composition and body temperature will occur within 24 h of ovulation and would be suitable measures to assist with the determination of the onset of oestrus.
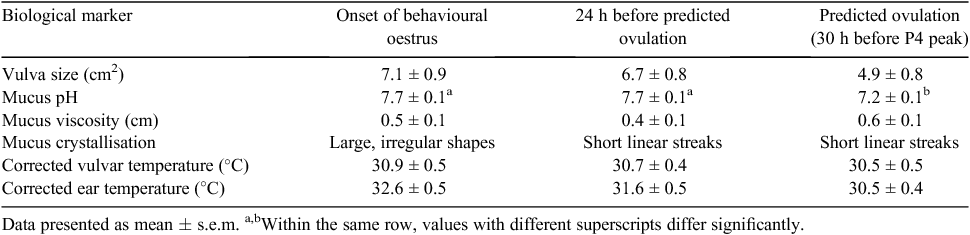
In this pilot study, a range of biological markers were measured on 46 multiparous (mean parity ± s.e.m. 3.22 ± 0.15, range = 2–5) Large White × Landrace sows over the anticipated period of oestrus, based on time post-weaning. Vulva size, vulva and ear temperature and cervical mucus pH, viscosity and crystallisation patterns, were recorded. Measurements were recorded at 12 h intervals from 3 days post-weaning to 2 days after the last observed oestrus behaviour. Vulva size was measured with a ruler and calculated as length × width. Cervical mucus was extracted using a Rocket cervical mucus syringe and split into three aliquots. One aliquot was air-dried on a microscope slide, observed at ×100 magnification and classified into six patterns based on the predominant shapes present (Abusineina 1962). The remaining aliquots were used to detect pH using test strips and viscosity length by measuring the stretch of mucus with a ruler (Rijnders et al. 2007). Internal vulva and ear canal temperatures were obtained using an infrared gun at a distance of 10 cm from the body surface and corrected for ambient temperature. The time of predicted ovulation was defined as 30 h before an increase in faecal progesterone (P4) as determined by ELISA, accounting for the 24 h hormone passage rate (Shaw and Foxcroft 1985).
The measurements were mapped to three time points; the onset of behavioural oestrus determined by the first instance of standing heat in response to back pressure, 24 h before predicted ovulation and the point of predicted ovulation (Table 1). Each marker was analysed using an ANOVA in Genstat 16 (VSN International, Hemel Hempstead, UK). Mucus pH (P < 0.001) decreased at the point of predicted ovulation to facilitate sperm survival during conception. The predominant mucus crystallisation pattern changed from large irregular fern shapes at the onset of behavioural oestrus to shortened, linear patterns 24 h before predicted ovulation (P = 0.013) potentially indicating a decrease in ionic compounds resulting from elevated oestrogen levels. There were no significant differences in vulva size, mucus viscosity, vulvar temperature or ear temperature detected between the time points.
|
These results indicated that cervical mucus properties have potential as an alternative oestrus detection tool in addition to existing monitoring programs. Further investigation is required to determine if using these markers can reduce the time for oestrus detection and to predict insemination timing results in satisfactory fertility.
References
Abusineina M (1962) The Veterinary Record 74, 619–621.Rijnders S, Bolscher J, McDonnell J, Vermeiden J (2007) Journal of Andrology 28, 461–465.
| Crossref | GoogleScholarGoogle Scholar |
Shaw HJ, Foxcroft GR (1985) Journal of Reproduction and Fertility 75, 17–28.
| Crossref | GoogleScholarGoogle Scholar |
Soede NM, Langendijk P, Kemp B (2011) Animal Reproduction Science 124, 251–258.
| Crossref | GoogleScholarGoogle Scholar |


