Relict islands of the temperate rainforest tree Aextoxicon punctatum (Aextoxicaceae) in semi-arid Chile: genetic diversity and biogeographic history
Mariela C. Núñez-Ávila A B C D and Juan J. Armesto A CA CMEB, Laboratorio de Ecología Forestal, Facultad de Ciencias, Universidad de Chile, Santiago, Chile.
B Present address: Instituto de Silvicultura, Universidad Austral de Chile, Valdivia, Chile.
C CASEB, Departamento de Ecología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Casilla 114-D, Santiago, Chile.
D Corresponding author. Email: marielanunez@uach.cl
Australian Journal of Botany 54(8) 733-743 https://doi.org/10.1071/BT06022
Submitted: 8 February 2006 Accepted: 10 July 2006 Published: 29 November 2006
Abstract
Aextoxicon punctatum, the only representative of the family Aextoxicaceae, is a tree species endemic to temperate forests of western South America. This species exhibits a disjunct distribution pattern, with few isolated populations occurring on coastal hilltops of the Chilean semi-arid zone (SAZ), 30–32°S; small populations mixed with sclerophyllous forest in some gorges of the central Chilean Mediterranean coastal range (MCR), 32–39°S; more continuous forests extended along the southern temperate coastal range (TCR), 39–43°S; and fragmented populations eastward in the south temperate central depression and Andean foothills (TAF), 39–41°S. This geographic disjunction is the result of climatic and tectonic changes that took place in southern South America since the late Tertiary, leading to the isolation of forest fragments in the SAZ from the rest of the distribution. According to palynological evidence, populations of TCR and TAF originated from postglacial population expansions from refuges located on the coastal range, north of 40°S. We examined how the present genetic structure and diversity of Aextoxicon populations in Chile reflects this biogeographic history. Random amplified polymorphic DNA (RAPD) markers were used to characterise genetic differences within and among 16 populations of this species throughout its natural range. AMOVA and UPGMA analysis showed high genetic differentiation between the geographically closer SAZ and MCR populations, suggesting a long history of restricted genetic exchange between populations in these two zones. Estimates of Shannon’s genetic diversity and percentage polymorphism were relatively low compared with other southern temperate forest trees (mainly conifers) that were less affected by the glaciations. Positive correlations between genetic and geographic distances were found for TCR but not for TAF populations, suggesting earlier postglacial population expansion southwards along the TCR and more recent eastward migration from coastal locations to TAF during the Holocene.
Introduction
Historical events, such as range fragmentation, range expansion and population bottlenecks, appear to have had a stronger influence on patterns of genetic differentiation within species than has been accounted for in traditional population genetic models (Schaal et al. 1998). This historic legacy is particularly evident in temperate regions, such as southern South America, where Pleistocene glaciations and postglacial climatic fluctuations promoted contraction and expansion of geographic ranges of many plant species (Villagrán 1991, 2001; Heusser et al. 1999).
Aextoxicon punctatum Ruiz & Pav. (Olivillo) is the only member of the phylogenetically isolated family Aextoxicaceae, which is endemic to the narrow strip of temperate forests occurring along the western margin of southern South America. The present geographic range of Olivillo (30–43°S) exceeds the northern limit of austral temperate rainforests (39°S), extending northwards as a chain of remnant forest communities occurring in small populations mixed with sclerophyllous forest in some gorges of the central Chilean Mediterranean coastal range (39–33°S) and further north, on isolated coastal hilltops of the Chilean semi-arid zone between 30 and 32°S. These northern outposts of Olivillo forest occur within a matrix of xerophytic vegetation, but exhibit remarkable floristic affinities with temperate rain forests located 1000 km to the south (Villagrán et al. 2004).
The enigmatic origin and persistence of these isolated forest fragments in semi-arid Chile has puzzled naturalists and botanists since their early description by Philippi in 1884. Hypotheses regarding the age and origin of these remnants have been advanced by Looser (1935), Muñoz and Pisano (1947), Wolffhügel (1949), Schmithüsen (1956), Troncoso et al. (1980), Skottsberg (1984), Hinojosa and Villagrán (1997) and Villagrán et al. (2004). Close relatives of the woody species present today in these relict forests are known from Paleogene fossil floras (early Tertiary) and are descendants of ancient tropical lineages with Australasian relatives, suggesting that they occurred in South America before the breakup of Gondwana (Hinojosa and Villagrán 1997). Fossil trunks compatible with Aextoxicon are known from the Dorotea Hill Formation (51°33′S) in southern Patagonia, corresponding to the late Paleocene (Nishida et al. 1988). Additionally, angiosperm molecular phylogenies consider the family Aextoxicaceae as a sister group of Berberidopsidaceae, a family of Gondwanan distribution (Savolainen et al. 2000). Because of their phylogenetic isolation, it has been suggested that Aextoxicon and Berberidopsis may represent specialised relicts from ancient floras for which the intermediate linking taxa are now extinct (Savolainen et al. 2000). Consequently, isolated patches of Olivillo forest in semi-arid Chile are considered remnants of an ancient forest community, which became gradually segregated from its main range, at higher latitude, as a result of pronounced aridisation in north-central Chile since the Plio-Pleistocene period (Villagrán et al. 2004).
Regarding the more recent Quaternary history of Aextoxicon, Troncoso et al. (1980) and Villagrán et al. (2004) postulated that in central Chile Olivillo forests had a more continuous distribution at low elevations during wetter glacial periods. Strong postglacial aridity, as recorded during the early to mid-Holocene in central Chile (Villagrán and Varela 1990; Villa-Martínez and Villagrán 1997; Jenny et al. 2002; Maldonado and Villagrán 2002), disrupted Olivillo’s geographic range (Villagrán et al. 2004). As a consequence, the Olivillo-dominated community became restricted to humid coastal gorges of the central Chilean Mediterranean coastal range, where it occurs today (Troncoso et al. 1980; Villagrán et al. 2004).
At higher latitudes in southern South America (39–43°S), pollen records show that many Valdivian rainforest species, including A. punctatum, contracted their geographical ranges during the last glacial maximum (18 000–20 000 years before present) when glaciers covered the Andes and temperatures decreased by 6–7°C (Villagrán 1991, 2001). Following postglacial climatic warming (11 000–9500 years before present), pollen records indicate that Valdivian tree species expanded south and eastwards from glacial refugia located on the coastal range, north of 40°S (Villagrán 1991, 2001).
Population genetic studies of Chilean tree species are few and have focused mainly on cold-resistant, temperate forest conifers (e.g. Fitzroya cupressoides I.M.Johnst., Allnutt et al. 1999; Podocarpus saligna D.Don, Allnutt et al. 2001; Araucaria araucana K.Koch, Bekessy et al. 2002; Pilgerodendron uviferum Florin, Allnutt et al. 2003), whose ranges often expanded during glacial periods (Villagrán and Roig 2004). However, we lack information about the patterns of genetic variation of broadly distributed, cold-sensitive angiosperm trees, such as A. punctatum. Patterns of genetic divergence among Olivillo populations can provide clues to the glacial and post-glacial history of Valdivian rainforests and the consequences of climatic cycles for tree species that reduced their ranges during the glacial ages.
The aim of this study was to assess the amount and distribution of genetic variability within and between populations of A. punctatum throughout its present geographic range. We addressed the following questions: (1) Are there genetic differences between isolated Aextoxicon populations on coastal hilltops of the Chilean semi-arid zone and more continuous populations of the Mediterranean and temperate zones of Chile? (2) To what extent can genetic differentiation of Aextoxicon be linked to its Tertiary history and more recent glacial and post-glacial climate change? (3) Can genetic data be used to suggest or confirm possible locations of glacial refugia and patterns of post-glacial migration for Olivillo?
To address these questions, we employed the molecular technique random amplified polymorphism DNA (RAPD; Williams et al. 1990). This method was chosen because it is immediately applicable to a wide range of taxa without previous knowledge of DNA sequences (Dawson et al. 1995). This was necessary because A. punctatum is a phylogenetically isolated species. Despite these advantages, the use of RAPD markers in genetic diversity studies has been questioned (e.g. Szmidt et al. 1996) because of the lack of reproducibility (Nybom and Bartish 2000). However, this limitation has been largely overcome through improved laboratory techniques and band scoring procedures. An additional disadvantage is that heterozygous individuals cannot be readily identified and allele frequencies cannot be determined accurately, thus, leading to an underestimation of the frequency of recessive alleles (Szmidt et al. 1996). Potential biases due to these problems can partly be offset by examination of a large number of polymorphic RAPD bands, increased sample sizes and careful consideration of the most appropriate method for data analysis (Lynch and Milligan 1994).
Materials and methods
Current distribution of Aextoxicon
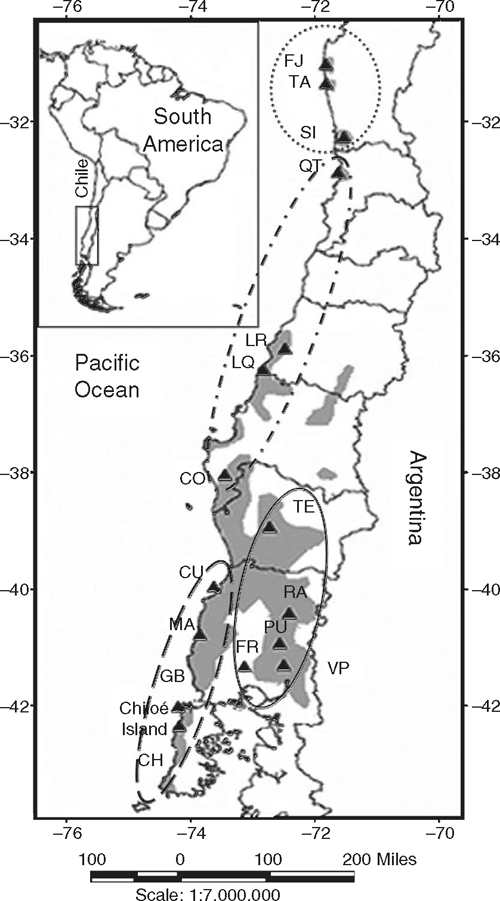
The extensive latitudinal range of Olivillo can be divided a priori into four distinct geographic zones (Fig. 1), on the basis of latitude, as well as topographic, climatic and historical contrasts. (i) Isolated populations in forest patches found on coastal hilltops (470–700 m) of the Chilean semi-arid zone (SAZ, 30–32°S), where annual precipitation is less than 150 mm (Pérez and Villagrán 1994). Dominant vegetation is a xerophytic scrub, except where forest patches occur. Forest patches are restricted to hilltops and subsist because of the constant influence of marine fogs (Kummerow 1966; del-Val et al. 2006). (ii) Small populations, mixed with sclerophyllous shrubs, in deep gorges of the central Chilean Mediterranean coastal range (MCR, 33–39°S), under a Mediterranean climate characterised by winter rains of 400–600 mm per year. Gorges facing the ocean maintain sufficient moisture for the development of Olivillo (Pérez and Villagrán 1994). In addition to historical restriction to coastal gorges due to Holocene aridity, populations have been severely reduced in recent times due to disturbance by fire, logging and replacement by commercial plantations of exotic pines and eucalypts (Armesto et al. 1998). The northernmost fragment in coastal gorges is known as ‘Quebrada del Tigre’ (Fig. 1). (iii) Continuous populations occurring on the forested western slopes of the southern temperate coastal range (TCR), from 39 to 43°S, receiving between 1000 and 3000 mm of rain per year. Olivillo is the dominant tree at elevations ranging from sea level to about 150 m. At higher altitudes in the coastal range, Olivillo forms a mixed canopy with other evergreen trees characteristic of Valdivian temperate rainforests up to approximately 250 m above sea level (Pérez and Villagrán 1994). In TCR we find the last remnants of continuous undisturbed coastal rain forest, characterised by high species diversity and endemism (Smith-Ramírez 2004). (iv) Forest fragments located eastwards in the southern temperate central depression and mainly on Andean foothills (TAF), between 37 and 41°S, generally segregated from the main range of Olivillo on the western slopes of TCR at the same latitudes. As no geographic barrier prevents genetic exchange between populations in the central depression and Andean foothills, we considered them as one unit. Because of land cover change associated with human occupation, Olivillo populations in TAF are highly fragmented. One Olivillo population (not sampled) is known from east of the Andes in Lake Puelo, Argentina.
|
Sampled populations differed in size, human impact and associated woody flora. Nearly all populations had suffered some degree of selective logging, partial burning and/or occasional browsing by livestock, and a few had been severely degraded as a result. Disturbance by fire was more intense in MCR. Only Guabún and Chiloé (Table 1) were forests in better state of conservation.
|
Sampling
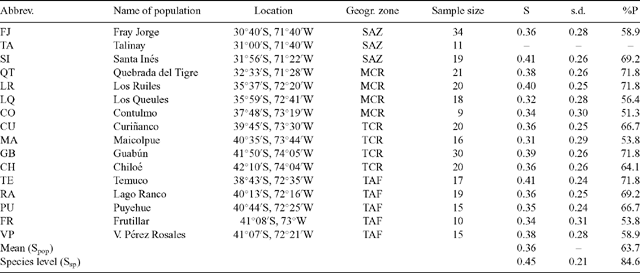
Leaf samples from 16 sites were collected during an extensive field campaign in early 2003, covering the entire range of A. punctatum in Chile and all four geographic zones described above (Fig. 1; Table 1). At each site we collected leaves from 9 to 34 trees, depending on population size (Table 1). Trees sampled were separated by at least 50 m from one another to minimise the chance of sampling genetically identical individuals or close relatives. Young leaves without signs of insect or pathogen damage were collected from trees. Samples were washed, dried inside plastic bags containing silica gel, and stored at 4°C prior to DNA extraction (Chase and Hills 1991).
DNA extraction
From each individual sample, DNA was extracted after grinding the leaves in liquid nitrogen to obtain 50 µL and mixed with a similar volume of Polyvinylpyrrolidone (PVP) and 700 µL of CTAB buffer (3% CTAB in 1 M Tris–HCl, pH 8.0, 1.4 M NaCl, 20 mM EDTA) and RNAse. The reaction was stirred every 10 min at 60°C, for 40 min, and then centrifuged at 12 000 rpm at 20°C, for 20 min. The supernatant was transferred to clean tubes and 700 µL of chloroform: isoamyl alcohol (24 : 1) was added. After the sample was vortexed for 1 min and centrifuged for 20 min at 12 000 rpm, the supernatant was precipitated with 0.6 volumes of 100% (v/v) isopropanol at –20°C overnight. The sample was then centrifuged at 14 000 rpm at 4°C, for 20 min. The DNA pellet resulting from centrifugation was washed in 70% (v/v) EtOH, dried and resuspended in water (50 µL). CHELEX was added to purify the DNA from polysaccharides (5% (w/v) Chelex, 90 mM Tris–HCL, pH 8, 5 µ? EDTA) in four volumes of concentrated DNA. Samples were incubated in a thermoregulated bath at 100°C for 15 min, homogenised and cooled to –20°C in less than 1 min, and centrifuged to 14 000 rpm for 1 min at 20°C. The supernatant was recovered and the DNA concentration of each sample was quantified at 280/260 nm in an UV spectrophotometer in order to dilute it to the 15 ng µL–1 required for the RAPD reactions.
RAPD reactions
The following conditions per 20-µL reaction were optimised to give repeatable results for the markers. RAPD reactions were performed in an Applied Biosystems (model 9700) thermal cycler by using the following components: 3 µL of DNA (15 ng µL–1), 2 µL of buffer PCR (10×), 3 µL of primers (10 µM, Invitrogen technologies), 8.5 µL ultrapurified water (GIBCO technologies), 1.6 µL MgCl2 (25 mM), 1.6 µL dNTPs (2.5 mM) and 0.3 µL Taq polymerase (5 U cµL–1, Fermentas #EP0402). The thermocycle consisted of 5 min of initial denaturation at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 38°C and 90 s at 72°C, with a final extension of 10 min a 72°C. PCR products were visualised on 1.5% agarose gel with ethidium bromide (1 µL each 100 mL) in 1× TAE buffer. Molecular markers (GeneRuler™ 100 bp DNA Ladder, Fermentas #SM0241) were run adjacent to the samples at either end of the agarose gel. Prior to loading the samples onto the agarose gel, 6× loading dye solution (supplied in Fermentas #SM0241) was added to each individual PCR product. The samples were electrophoresed at 90 mV for three hours. RAPD products were visualised and photographed over UV light with a red filter.
A caution has been expressed concerning the reproducibility of results obtained with RAPD markers. Minimisation of the problem of non-reproducible fragments can be achieved by the use of high-quality DNA only, careful optimisation of PCR conditions and assays of the reproducibility of each marker (Newton et al. 1999). These considerations were followed in this study.
During preliminary RAPD studies of Aextoxicon punctatum, 25 primers (Operon technologies, sets A, B, C, D, N, O and X) were screened for their ability to produce scoreable RAPD markers. From these, a subset of 12 primers was screened in two individuals from each of the 16 populations. We selected four primers (X17, C2, X1 and X7) having reproducible variation characterised by well-defined and darkly staining bands. DNA samples from Talinay population (in the SAZ) did not amplify for one primer (X7) and hence this site was excluded from most analyses (see below).
Only RAPD bands that could be unequivocally scored were counted. Generally, only bands in size ranges close to clearly visible monomorphic bands were scored. This greatly reduced the potential number of bands that could have been scored but, importantly, avoided mis-scoring of bands for which comigration could not be ascertained (Allnutt et al. 1999). Band scoring was performed simultaneously on a total of 39 markers, from which 33 were polymorphic, accurately scoreable and reproducible. Repeatability of banding patterns was checked for each primer on several samples. Only those RAPD markers that reproduced consistently across successful PCR were included in the analysis.
Data analyses
RAPD markers were considered to be ‘genetic’ phenotypes, with each PCR product assumed to represent a single locus. Loci were scored for presence (1) and absence (0) and entered into a binary data matrix.
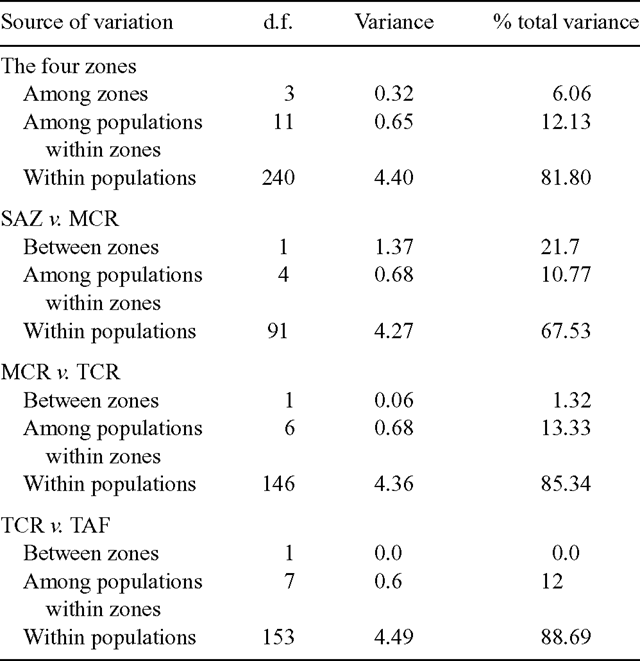
Where allele frequencies were used (for all analyses, except percent of polymorphism), they were calculated from RAPD band frequencies following the methods and corrections employed by Lynch and Milligan (1994). A Euclidean distance matrix was constructed (AMOVA-PREP 1.01; Miller 1998) and used as the input file for an analysis of molecular variance (AMOVA; Excoffier et al. 1992). Variance components were estimated for (i) individuals within populations; (ii) individuals between populations; and (iii) among populations in the four pre-defined zones (see above and Table 1). In order to determine the differences between pairs of zones that made the greatest contribution to the overall difference among the four geographic zones, we made the following planned comparisons between geographically contiguous pairs, namely SAZ v. MCR, MCR v. TCR, and TCR v. TAF.
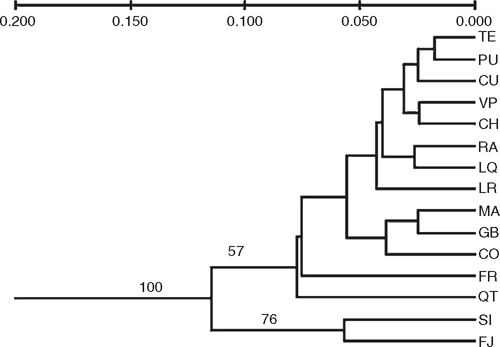
The genetic distance matrix among populations based on Nei’s (1978) unbiased distance estimates was used to construct unrooted phenetic trees with the program TFPGA (Miller 1997), by an average method (UPGMA; Rohlf 1992). Bootstrap values for clusters were calculated after 1000 replicates. Only values greater than 50% are reported (Fig. 2). Additionally, to examine the relationship of Talinay site (located in the SAZ, Fig. 1) with the rest of the populations, we also conducted this analysis excluding loci from X7 primer and including the Talinay population.
|
Shannon’s index (S) provided a relative estimate of the degree of variation within each population (Lewontin 1972). This was calculated separately for each putative locus, and by averaging over all loci for each population the mean value of the index was then produced. This approach ensures that estimates of phenotypic diversity from each putative locus are equally weighted in the calculation of Spop diversity statistic. The pooled species-level value, which considers all individuals sampled in the entire geographic range as belonging to one population, was also determined. The percent of polymorphic RAPD loci (%P) was calculated for each population, as well as the mean value for all populations and a pooled species-level value.
Geographic distances among populations were estimated from GPS coordinates. The correlation between the genetic (Φst) and geographic distance matrices was analysed with Mantel’s randomisation test (Mantel 1967) with the program Mantel-Structure 1.0 (Miller 1999). In this analysis, distances from one matrix were regressed onto distances in the second matrix. Significance of regressions was evaluated by randomising one of the matrices 1000 times while keeping the other constant, and determining the percentage of randomisations resulting in a regression value exceeding the observed ‘r’ value. When the observed regression value exceeded 95% of the randomly generated regressions, the former was considered statistically significant.
Results
Genetic diversity and structure
The magnitude of genetic diversity within each population of Aextoxicon measured by Shannon’s index varied from 0.31 (Maicolpue) to 0.41 (Santa Inés, Los Ruiles and Temuco) (Table 1). The three populations having the greatest genetic diversity are located north of 40°S. The mean genetic diversity for all populations (Spop) was 0.36 and the pooled species-level value (Ssp) was 0.45. The percentages of polymorphic RAPD loci varied from 51.3% (Contulmo) to 71.8% (Quebrada del Tigre, Los Ruiles, Temuco and Guabún). The mean percentage of polymorphic loci for all populations was 63.7% and the pooled species-level value was 84.6% (Table 1).
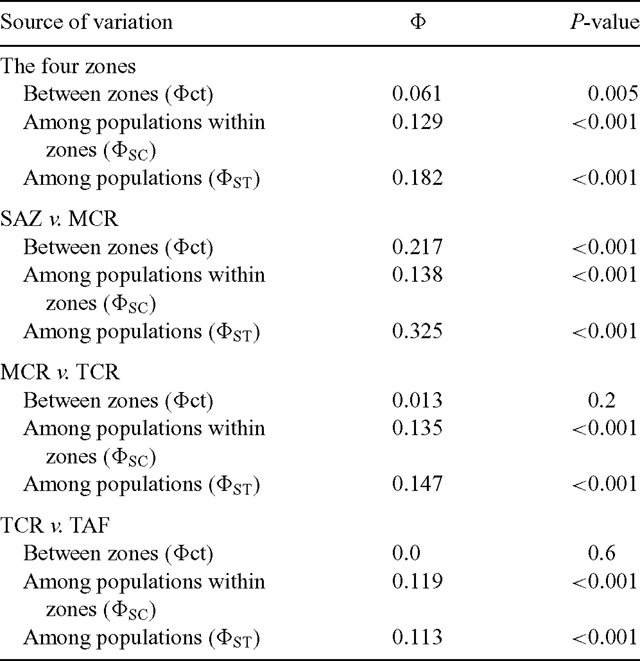
The distribution of genetic variation in Olivillo was examined by AMOVA. Although most of the variation (81.8%) was found within populations (Table 2), a significant proportion of the variance was attributable to differences among populations (12.1%) and among the four pre-defined geographic zones (6.1%) (P < 0.001 and P < 0.005, respectively, tested with 1000 bootstrap replicates; Table 3).
|
|
The Φ values among zones given by AMOVA indicate that, overall, significant genetic differences exist among the four geographic and climatic zones defined in this study (Table 3). However, after planned pairwise comparisons, the highest genetic difference found was between SAZ and MCR (Table 3), which are geographically closer but not continuous. In this case, 21.7% of the genetic diversity was attributable to differences between zones (Table 2). In contrast, planned pairwise comparisons of MCR v. TCR, and TCR v. TAF (Table 3) showed no significant genetic differences between these zones. This result indicates that genetic divergence among the four geographic zones is due primarily to the high genetic differentiation of the isolated relicts in the SAZ from the rest of the populations. The high magnitude of the genetic difference between SAZ and MCR populations stands out (ΦST = 0.325; P < 0.001; Table 3), compared with much lower genetic differentiation between MCR and TCR populations (ΦST = 0.147; P < 0.001; Table 3) and between TCR and TAF populations (ΦST = 0.113; P < 0.001; Table 3).
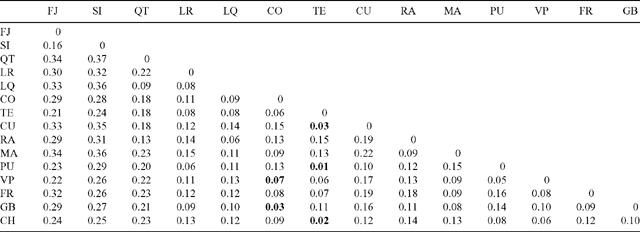
The isolated Olivillo populations of Fray Jorge and Santa Inés located in the SAZ showed the highest ΦST values with respect to all other populations (Table 4). Only five pairwise comparisons among populations were not significant and these populations were localised in the southern temperate zone. The ΦST values, or genetic differences among pairs of populations, tend to decrease at higher latitudes (Table 4).
|
Relationship between geographic and genetic distances
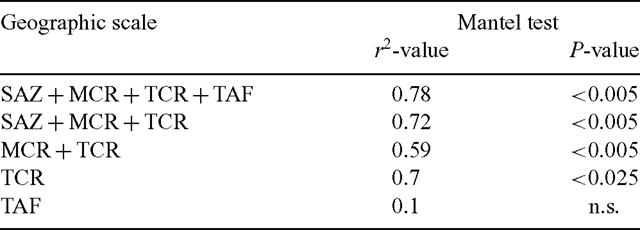
The correlation between genetic and geographic distances among populations was high and significant when considering the entire geographic range of Olivillo in Chile (r2 = 0.78; p < 0.005; Table 5), and also when considering only those populations in the TCR (r2 = 0.7; P < 0.025; Table 5). In contrast, the correlation between geographic and genetic distances for TAF populations only, excluding coastal sites at similar latitudes, was not statistically significant (Table 5).
|
When Nei’s genetic distance (1978) was used to construct an UPGMA dendrogram (Fig. 2), the most pronounced difference was between Fray Jorge and Santa Inés populations in the SAZ and all the other populations. When the Talinay population, located on the summit of coastal hills immediately south of Fray Jorge, was included in the UPGMA dendrogram, the same topology was achieved (data not shown), with Talinay population clustering together with the other northern populations of Fray Jorge and Santa Inés (boostrap value = 0.51). The Olivillo population in Quebrada del Tigre, a coastal gorge in central Chile, also emerged as relatively distinct (Fig. 2), despite the fact that it is just 70 km south of Santa Inés (Fig. 1). Other clusters grouping MCR, TCR and TAF populations in the dendrogram were not significant (bootstrap values <50).
Discussion
The magnitude and geographic distribution of genetic diversity within and between populations of A. punctatum provides a vehicle to examine the legacy of biogeographical history on current patterns of genetic divergence in a Valdivian rainforest tree species. In addition, knowledge of the magnitude and structure of genetic diversity in populations of this tree species at the present margins of its geographic range and in zones degraded by human impact may help guide and inform conservation strategies.
Patterns of genetic diversity and history
Islands of Olivillo located in the SAZ (Fray Jorge, Talinay and Santa Inés) are thought to represent ancient remnants of preglacial subtropical rainforests (Villagrán et al. 2004). These rain forest islands are currently immersed in a xerophytic vegetation matrix, but their remarkable floristic affinity with temperate rain forests situated 1000 km to the south, in southern Chile (Villagrán et al. 2004), suggests that a continuous forest flora may have existed in the past. The floristic elements of this woody flora have close relatives in the Paleogene’s Mixed Paleoflora, which was dominated by Australasian-tropical elements that colonised southern South American before the break up of Gondwana (Hinojosa and Villagrán 1997). Climatic and tectonic events concentrated during the Plio-Pleistocene transition, such as, the onset of west-Antarctic glaciation, the start of the Humboldt Current running northwards along the Chilean coast and the final uplift of the Andes, determined the development of strong aridity in western South America north of 30°S (Hinojosa and Villagrán 1997). This process was the driver of the present isolation of South American temperate forests from other continental forests and of the initial fragmentation of remnant SAZ forest in north-central Chile. The long geographic isolation of fragmented SAZ populations, which dates at least from the onset of aridity in the western margin of South America, is the reason to consider these patches as relict forests. The high level of genetic divergence between relict populations in the SAZ and the complex of populations of central and southern Chile (Fig. 2; Tables 3, 4) documented here lends support to the hypothesis of ancient isolation of these northern patches. Ancient isolation of rain forest patches in the SAZ is also supported by a phylogeographic study of two species and one variety of the rainforest tree genus Drimys, present in southern Chile and Argentina (Jara et al. 2002). By using RAPD markers, these authors also showed high genetic differentiation between the populations of Drimys winteri Forst. from Fray Jorge forest in the Chilean SAZ and all the other populations in mainland Chile (Jara et al. 2002). A long history of geographic discontinuity between populations in the SAZ and the main range of the species would have produced the high genetic differences documented for Olivillo and Drimys.
Despite glacial and post-glacial climate change and recent human impact, gene flow has apparently been strong among the more continuous populations of central and southern Chile, as shown by their low genetic differentiation (Tables 3, 4). The northernmost population of MCR in central Chile, Quebrada del Tigre (QT), documented a unique case. This population was genetically closer to the more distant populations of MCR, TCR and TFA (QT v. LQ, ΦST = 0.09; QT v. TE, ΦST = 0.18; QT v. PU, ΦST = 0.20, respectively; Table 4) than to its nearest neighbour to the north (QT v. SI, ΦST = 0.37; Table 4). This is shown by the dendrogram, where the QT population was not grouped with the geographically closer sites in the SAZ (Fig. 2). This suggests that populations situated south of the SAZ probably maintained gene flow and presumably a more continuous geographic range during glacial periods. Small and highly degraded remnant populations of Aextoxicon (not sampled by us) that occur in gorges of the MCR, between 33° and 34°S (Pérez and Villagrán 1994; C. Villagrán, unpubl. data) are evidence of this formerly continuous range, which linked QT with populations along the MCR and TCR. If some of the original genetic diversity of these coastal remnants of MCR populations has been conserved, these populations are of key importance for conservation and restoration purposes. From our data, we predict that presently highly degraded Aextoxicon populations in coastal gorges of MCR would be genetically similar to Quebrada del Tigre.
Evidence of glacial refugia
Intrapopulation genetic diversity was low and similar in the different populations across the range of Olivillo, showing a weak tendency to decline south of 40°S (Table 1). There were no significant differences in the values of genetic diversity (Shannon’s index and percentage polymorphism) between populations located north and south of 40°S. Although this was not consistent with the presumed location of glacial refuges (Villagrán 1991), Olivillo populations that exhibited the highest genetic diversity in this study (Santa Inés, Los Ruiles and Temuco; Table 1) are all located north of 40°S. However, this latitudinal range also includes populations with low genetic diversity such as Los Queules and Contulmo (Table 1). It is likely that the centres of high genetic diversity that persisted during the glacial cycles, serving as sources of seeds for the recolonisation of austral latitudes and TAF sites, may now be losing genetic richness because of increased fragmentation of native forests within this latitudinal range (Armesto et al. 1996). By the method used in this study, it is impossible to discriminate whether low genetic diversity of these populations is due to recent fragmentation or has a longer-time component.
Correlation between genetic and geographic distances
Significant genetic and geographic structure for populations throughout the geographic range of Aextoxicon (Table 5) is interpreted as the result of vicariant events that restricted gene flow among a subset of populations (Bossart and Prowell 1998). The long genetic and geographic isolation of SAZ populations, as shown in the high value of ΦST and statistically significant pairwise population differences (Table 4) should increase the correlation between genetic and geographic distances. On the other hand, positive and significant correlations between genetic and geographic distances for Aextoxicon populations along the southern TCR (Table 5) were interpreted as following the isolation-by-distance model, where neighbouring populations exchange more migrants than distant ones (Bossart and Prowell 1998). In contrast, TAF populations in the same latitudinal range showed no correlation between genetic and geographic distances. Accordingly, we suggest that post-glacial expansion of Olivillo southwards along the Coastal Range occurred earlier in Holocene than eastward migration of populations into glaciated areas of the TAF (see also Villagrán 1991). Eastward expansion of Olivillo populations from TCR may have occurred from more than one source population, although our data are inconclusive in this respect.
Geographic vicinity of forests in TAF may also account for the low level of genetic differentiation among populations (Table 5). Seeds of Aextoxicon are disseminated by frugivorous birds (M. Salvande, J. Figueroa and J. J. Armesto, unpubl. data), which may enhance genetic exchange, especially among contiguous populations in the southern zone, thus, reducing genetic differences. Nevertheless, due to the extensive latitudinal range of Olivillo (approx. 1200 km) it is unlikely that effective seed dispersal can occur over the entire range of the species, as shown by the high genetic differentiation between SAZ and MCR populations (Table 3). Knowledge of pollination mechanism of Olivillo suggests that the main pollen vector is wind (M. Núñez-Ávila and J. J. Armesto, unpubl. data), which generally reduces subdivision within populations and prevents divergence among populations (Loveless and Hamrick 1984). In wind-pollinated species, background pollen levels are sufficient to prevent differentiation over fairly large geographic ranges (Antonovics 1968). Although we lack measurements of pollen flow in Olivillo, effective pollen dispersal by wind is more likely in TAF populations, thus, reducing genetic differentiation among these populations as shown in this study (Table 5).
Genetic diversity of Aextoxicon and southern rain forest conifers
Aextoxicon punctatum is a cold-sensitive Valdivian rain forest species, as documented by its evergreen habit, broad-leaved foliage and its distribution predominantly on coastal and low elevation sites (Fig. 1; Smith-Ramírez et al. 2005). Because the geographic distribution of Olivillo is currently quite extensive (1200 km), extending the northern limit of temperate rainforests of South America into the MCR and SAZ, and because it is an obligate outbreeder, a higher value of genetic diversity could have been expected for this species (Loveless and Hamrick 1984).
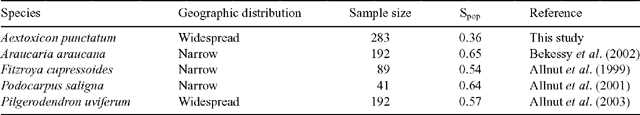
However, average genetic diversity estimated from RAPD markers for A. punctatum populations, using Shannon’s index (Spop = 0.36) was lower than RAPD–based Shannon’s indices calculated for conifer tree species from South American temperate forests (Table 6). These conifers are characterised today by restricted geographic ranges in southern forests (Villagrán and Roig 2004), but according to fossil pollen data (Villagrán 2001) and fossil wood remains (Villagrán and Roig 2004), they expanded their ranges in the Chilean Lake District during glacial periods, occupying their presently narrower ranges only in the warmer Holocene. Genetic data for the native Chilean conifers Fitzroya cupressoides (Allnutt et al. 1999), Podocarpus saligna (Allnutt et al. 2001) and Araucaria araucana (Bekessy et al. 2002) showed higher genetic diversity (Spop = 0.54, 0.64 and 0.65, respectively). The southernmost conifer in the world, Pilgerodendron uvíferum, which has an extended but fragmented distribution from 41 to 55°S, has a Shannon’s genetic diversity index of 0.57 (Allnutt et al. 2003). We propose that the higher genetic diversity observed in these conifer species today (Allnutt et al. 1999, 2001, 2003; Bekessy et al. 2002) can be a legacy of their extensive and more continuous geographic ranges during the repeated and prolonged glacial periods.
|
Low genetic diversity of Aextoxicon at the species level is therefore, interpreted as a consequence of the impact of glacial cycles on its geographic range. In contrast to the temperate conifers, Olivillo would have experienced repeated contractions of its southern geographic range for long periods during glacial ages. This range contraction, associated with glacial cycles, could have resulted in progressive losses of genetic variability, as this has study revealed.
Future directions
Climatic and tectonic events that affected southern South America since the late Tertiary have undoubtedly influenced the population structure of tree species (Allnutt et al. 1999, 2001, 2003; Etisham-Ul-Haq et al. 2001; Martínez and Núñez-Ávila 2001; Bekessy et al. 2002; Bull-Hereñu et al. 2005). Each species has responded differently to historical phenomena depending upon its own physiological, ecological and reproductive attributes. Further studies should contrast the pattern of genetic variation documented for Aextoxicon with those of other species that belong to this relict forest community. The following species possess disjunct geographic ranges separated by nearly 1000 km between Olivillo forest fragments in SAZ and southern rain forests: Azara microphylla Hook.f., Griselinia scandens Taub., Nertera granadensis Druce., Mitraria coccinea Cav., Sarmienta repens Ruiz & Pav., and several species of ferns, mosses and liverworts (Villagrán et al. 2004). If ancient geographic isolation has caused marked genetic differentiation of Olivillo populations in the SAZ, despite its generation time of more than 200 years (A. G. Gutierrez and J. J. Armesto, unpubl. data), plants with shorter generation times should exhibit higher levels of genetic divergence.
Conservation of the small remnants of Olivillo forest on coastal hilltops of SAZ is of singular importance because of their high genetic differentiation from all other populations. Particularly important is the small and isolated Santa Inés population in the SAZ, with the highest genetic diversity among all populations studied here. These relict islands are the present-day refuges of an ancient forest community that was once continuous throughout the coastal range of south-central Chile. The persistence of these isolated relict patches depends on the continued input of fog water on ocean-facing hilltops (del-Val et al. 2006). Climatic variability, on millennial scales, including changes in precipitation and fog inputs, could trigger expansions or contractions of these relict rainforest patches. We recommend the collection and propagation of germplasm from genetically divergent and highly threatened Olivillo populations in the Chilean SAZ and MCR, with the purpose of assuring its availability for future restoration projects.
Acknowledgments
We dedicate this paper to our colleague Carolina Villagrán, who introduced us to the enigmatic history of Olivillo forests and stimulated us to pursue this study. We thank many friends and colleagues for helping with field sampling. Pete Hollingsworth, Adrian Newton, Richard Ennos, Sylvain Fulgeiron, Mark Hershkovitz and Elie Poulin offered technical advice and comments on earlier versions of this manuscript. CONAF and landowners are thanked for allowing access to research sites. This work was supported by graduate fellowships from CMEB (P99-103F-ICM) and from Universidad de Chile (PG/28/02), and by grants from FONDAP-FONDECYT 1501-0001 (CASEB, Pontificia Universidad Católica de Chile) and BIOCORES Project funded by EC under INCO IV program (contract ICA 4-CT-2001-10095). This is a contribution to the research program of Senda Darwin Biological Station, Chile.
Allnutt TR,
Newton AC,
Lara A,
Premoli A,
Armesto JJ,
Vergara R, Gardner M
(1999) Genetic variation in Fitzroya cupressoides (alerce), a threatened South American conifer. Molecular Ecology 8, 975–987.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Allnutt TR,
Courtis JR,
Gardner M, Newton AC
(2001) Genetic variation and wild Chilean and cultivated British populations of Podocarpus salignus D.Don (Podocarpaceae). Edinburgh Journal of Botany 58, 459–473.

Allnutt TR,
Newton AC,
Premoli AC, Lara A
(2003) Genetic variation in the threatened South American conifer Pilgerodendron uviferum (Cupressaceae), detected using RAPD markers. Biological Conservation 114, 245–253.
| Crossref | GoogleScholarGoogle Scholar |

Antonovics J
(1968) Evolution in closely adjacent populations. VI. Manifold effects of gene flow. Heredity 23, 507–524.

Armesto JJ,
Rozzi R,
Smith-Ramirez C, Arroyo MTK
(1998) Conservation targets in South American temperate forests. Science 282, 1271–1272.
| Crossref | GoogleScholarGoogle Scholar |

Bekessy SA,
Allnutt TR,
Premoli AC,
Lara A,
Ennos RA,
Burgman MA,
Cortes M, Newton AC
(2002) Genetic variation in the vulnerable and endemic monkey puzzle tree, detected using RAPDs. Heredity 88, 243–249.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bossart JL, Prowell DP
(1998) Genetic estimates of population structure and gene flow: limitations, lessons and new directions. Trends in Ecology and Evolution 13, 202–205.
| Crossref | GoogleScholarGoogle Scholar |

Bull-Hereñu K,
Martínez EA, Squeo FA
(2005) Structure and genetic diversity in Colliguaja odorífera Mol. (Euphorbiaceae), a shrub subjected to Pleisto-Holocenic natural perturbations in a Mediterranean South American region. Journal of Biogeography 32, 1129–1138.
| Crossref | GoogleScholarGoogle Scholar |

Chase HW, Hills HH
(1991) Silica gel: an ideal material for field preservation of leaf samples for DNA studies. Taxon 40, 215–220.
| Crossref | GoogleScholarGoogle Scholar |

Dawson IK,
Simons AJ,
Waugh R, Powell W
(1995) Diversity and genetic differentiation among subpopulations of Gliricidia sepium revealed by PCR-based assays. Heredity 74, 10–18.
| PubMed |

Etisham-Ul-Haq M,
Allnutt TR,
Smith-Ramírez C,
Gardner MF,
Armesto JJ, Newton AC
(2001) Patterns of genetic variation in and ex situ populations of the threatened Chilean vine Berberidopsis corallina, detected using RAPD markers. Annals of Botany 87, 813–821.
| Crossref | GoogleScholarGoogle Scholar |

Excoffier L,
Smouse PE, Quattro JM
(1992) Analysis of molecular variance inferred from metric distances among DNA haplogroups: applications to human mitochondrial DNA restriction data. Genetics 131, 479–491.
| PubMed |

Heusser CJ,
Heusser LE, Lowell TV
(1999) Paleoecology of the southern Chilean Lake District Isla Grande de Chiloé during middle–late Llanquihue glaciation and deglaciation. Geografiska Annaler 81, 231–284.
| Crossref | GoogleScholarGoogle Scholar |

Hinojosa LF, Villagrán C
(1997) Historia de los bosques del sur de Sudamerica I: Antecedentes paleobotánicos, geológicos y climáticos del terciario del cono sur de América. Revista Chilena de Historia Natural 70, 225–239.

Jara P,
Squeo FA, Hershkovitz M
(2002) Divergencia genética en Drimys (canelo) en Chile, mediante análisis con RAPD. Biological Research 35, R-54.

Jenny B,
Valero-Garcés BL,
Villa-Martínez R,
Urrutia R,
Geyh M, Veit H
(2002) Early to Mid-Holecene aridity in central Chile and the southern westerlies: The Laguna Aculeo record (34°S). Quaternary Research 58, 160–170.
| Crossref | GoogleScholarGoogle Scholar |

Kummerow J
(1966) Aporte al conocimiento de las condiciones climáticas del bosque de Fray Jorge. Boletín Técnico de la Facultad de Agronomía, Universidad de Chile 24, 21–28.

Lewontin RC
(1972) The apportionment of human diversity. Evolutionary Biology 6, 381–398.

Looser G
(1935) Argumentos botánicos a favor de un cambio de clima en Chile central en tiempos geológicos recientes. Revista Universitaria 20, 843–857.

Loveless MD, Hamrick JL
(1984) Ecological determinants of genetic structure in plants populations. Annual Review of Ecology and Systematics 15, 65–95.
| Crossref | GoogleScholarGoogle Scholar |

Lynch M, Milligan BG
(1994) Analysis of population genetic structure with RAPD markers. Molecular Ecology 3, 91–99.
| PubMed |

Maldonado A, Villagrán C
(2002) Paleoenvironmental changes in the semi-arid coast of Chile (32°S) during the last 6200 cal years inferred from a swamp forest pollen records. Quaternary Research 58, 130–138.
| Crossref | GoogleScholarGoogle Scholar |

Mantel N
(1967) The detection of disease clustering and a generalized regression approach. Cancer Research 27, 209–220.
| PubMed |

Martínez EA, Núñez-Ávila M
(2001) Uso de marcadores moleculares para evaluar el estado híbrido de Colliguaja salicifolia. Biological Research 34, R36.

Muñoz C, Pisano E
(1947) Estudio de la vegetación y flora de los parques nacionales de Fray Jorge y Talinay. Agricultura Técnica 7, 71–190.

Nei M
(1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590.

Newton AC,
Allnut T,
Gillies AC,
Lowe A, Ennos RA
(1999) Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends in Ecology and Evolution 14, 140–145.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nybom H, Bartish VI
(2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology, Evolution and Systematics 3, 93–114.
| Crossref | GoogleScholarGoogle Scholar |

Pérez C, Villagrán C
(1994) Influencias del clima en el cambio florístico, vegetacional y edáfico de lo bosques de ’olivillo‘ (Aextoxicon punctatum R. et Pav.) de la Cordillera de Costa de Chile: implicancias biogeográficas. Revista Chilena de Historia Natural 67, 77–90.

Philippi F
(1884) A visit to the northernmost forest of Chile. Journal of Botany 22, 202–211.

Savolainen V,
Chase MW,
Hoot SB,
Morton CM,
Soltis DE,
Bayer C,
Fay MF,
De Bruijn AY,
Sullivan S, Qiu YL
(2000) Phylogenetics of flowering plants based on combined analysis of plastid atpB and rbcL gene sequences. Systematic Biology 49, 306–362.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schaal BA,
Hayworth DA,
Olsen KM,
Rauscher JT, Smith WA
(1998) Phylogeographic studies in plants: problems and prospects. Molecular Ecology 7, 465–474.
| Crossref | GoogleScholarGoogle Scholar |

Schmithüsen J
(1956) Die räumliche Ordnung der chilenischen Vegetation. Bonner Geographische Abhandlungen 17, 1–86.

Skottsberg C
(1984) Apuntes sobre la historia y vegetación de Fray Jorge (Coquimbo, Chile). Acta Horti Gotoburgensis 18, 91–184.

Smith-Ramírez C
(2004) The Chilean coastal range: a vanishing center of biodiversity and endemism in South American temperate forests. Biodiversity and Conservation 13, 373–393.
| Crossref | GoogleScholarGoogle Scholar |

Szmidt AE,
Wang XR, Lu MZ
(1996) Empirical assessment of allozyme and RAPD variation in Pinus sylvestris (L.) using haploid tissue analysis. Heredity 76, 412–420.

Troncoso AC,
Villagrán C, Muñoz M
(1980) Una nueva hipótesis acerca del origen y edad del bosque de Fray Jorge (Coquimbo, Chile). Boletín del Museo Nacional de Historia Natural de Chile 37, 117–152.

del-Val E,
Armesto JJ,
Barbosa O,
Christie DA,
Gutiérrez AG,
Jones CG,
Marquet PA, Weathers KC
(2006) Rain forest islands in the Chilean semi-arid region: fog-dependency, ecosystem persistence and tree regeneration. Ecosystems 9, 598–608.
| Crossref | GoogleScholarGoogle Scholar |

Villa-Martínez R, Villagrán C
(1997) Historia de la vegetación de los bosques pantanosos de la costa de Chile central durante el Holoceno medio y tardío. Revista Chilena de Historia Natural 7, 391–401.

Villagrán C
(1991) Historia de los bosques templados del sur de Chile durante el tardiglacial y postglacial. Revista Chilena de Historia Natural 64, 447–460.

Villagrán C
(2001) Un modelo de la historia de la vegetación de la Cordillera de la costa de Chile central-sur: la hipótesis glacial de Darwin. Revista Chilena de Historia Natural 74, 793–803.

Villagrán C, Varela J
(1990) Palynological evidence for increased aridity on the central Chilean coast during the Holocene. Quaternary Research 34, 198–207.
| Crossref | GoogleScholarGoogle Scholar |

Williams JGK,
Kubelik AR,
Livak KJ,
Rafalski JA, Tingey SV
(1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18, 6531–6536.
| PubMed |

Wolffhügel K
(1949) Rätsel der Notohylaea. Revista Sudamericana de Botánica 8, 45–58.



