Spatial and temporal variation in damage and dieback in a threatened subantarctic cushion species
J. Whinam A C , J. A. Abdul-Rahman B , M. Visoiu A , M.-B. F. di Folco B and J. B. Kirkpatrick BA Resource Management and Conservation Division, Department of Primary Industries, Parks, Water & Environment, 134 Macquarie Street, Hobart, Tas. 7000, Australia.
B School of Geography and Environmental Studies, University of Tasmania, Private Bag78, GPO, Hobart, Tas. 7001, Australia.
C Corresponding author. Email: Jennie.Whinam@dpipwe.tas.gov.au
Australian Journal of Botany 62(1) 10-21 https://doi.org/10.1071/BT13207
Submitted: 21 August 2013 Accepted: 15 February 2014 Published: 14 April 2014
Abstract
A decline was observed in the subantarctic Macquarie Island endemic cushion, Azorella macquariensis, during the summer of 2008–2009, resulting in the listing of the species as critically endangered in 2010. Photographs of A. macquariensis in the period 2009–2013 were used to (1) identify types of damage, (2) determine the likely causes of three distinct types of damage, (3) establish whether dieback was spreading from affected to unaffected sites and (4) find out whether dieback was associated with the expansion of Agrostis magellanica. Grey damage occurred on the most wind-exposed parts of cushions and on the most wind-exposed sites. Speck damage occurred in the opposite situations and was consistent in its location, attributes and timing with rabbit grazing. Yellow dieback was sporadic in its occurrence. Its symptoms were consistent with those of a pathogen. Yellow damage expanded between spring 2009 and autumn 2010, with neither grey nor speck damage increasing. Yellow damage was associated with a marked decline in live cushion cover in plots between 2010 and 2013. The cushion was not eliminated from any plots, despite increased cover of A. magellanica in plots with dead cushions. Only one site not affected by yellow damage in 2010 had become affected by 2013. Given these results, and given that yellow damage has been observed in the past, 2008–2010 may have been an infrequent extreme outbreak of a pathogen and/or a response of a pathogen to ongoing climatic change.
Additional keywords: cushion plant, pathogen, wind damage.
Introduction
In an era of global climatic change and accelerated mixing of species from different biotic realms (Lebouvier et al. 2011), biologists have grown to expect the occasional rapid decline of a native species. In the first decade of the 21st century, there were several rapid declines of native species on subantarctic Macquarie Island associated with fluctuations in rabbit populations (Copson and Whinam 1998, 2001; Scott and Kirkpatrick 2008, 2013; Bergstrom et al. 2009; Shaw et al. 2011). During the 2008–2009 austral summer, a dieback in the Macquarie Island endemic cushion plant, Azorella macquariensis Orchard (henceforth Azorella), was reported (Threatened Species Section 2009; Threatened Species Scientific Committee 2010). Necrosis was observed across the entire range of the species, severely affecting up to 90% of cushions in some areas (Threatened Species Section 2009). By the winter of 2009, cushions on the northern plateau appeared to be worst-affected and only patches of cushions on isolated rock stacks and in wet valley bottoms were found to be unaffected (Threatened Species Section 2009). The species was listed as endangered under the Threatened Species Protection Act (Tas.) 1995 in late 2009, and as critically endangered under the Environmental Protection and Biodiversity Conservation Act (Cth) 1999 in August 2010 (Threatened Species Section 2009; Threatened Species Scientific Committee 2010).
Damage in cushions has been associated with climatic extremes, particularly strong winds (Taylor 1955a, 1955b; Kirkpatrick and Harwood 1980; Kirkpatrick et al. 2002), shading by other plants (Hauri and Schröter 1914; le Roux et al. 2005), mechanical damage from burrowing species (Selkirk et al. 1990; Phiri et al. 2009), herbivory (Chapuis et al. 1994; Rundel and Palma 2000), human trampling (Scott and Kirkpatrick 1994; Whinam and Chilcott 1999, 2003) and human exploitation for fuel and medicine (Núñez and Grosjean 2003; Kleier and Rundel 2004). There is no published work that shows that disease is a cause of the rapid spread of necrosis in and between individuals (TSS 2009), although diseases do damage cushion plants (e.g. Marr 1997). We make a distinction between dieback, as an observed process of expansion of morbidity or mortality, and damage, which is necrosis or foliage removal observed at a particular time, which may, or may not, be a symptom of a process of dieback at the time of observation, as damage might be evident where a process of dieback has ceased or where recolonisation is taking place.
Azorella cushions on Macquarie Island have been suggested to be damaged by wind, rabbits, mice, birds and pathogens. Partial mortality of cushions has been posited to be a result of exposure to wind, with total death being suggested to occur when wind is extremely strong (Taylor 1955a, 1955b; Ashton and Gill 1965). Rabbits have been observed to scratch and dig into cushions (Taylor 1955b). Cushions die within the nesting areas of giant petrels (Taylor 1955b; Selkirk et al. 1990). Ashton and Gill (1965) observed that 11.4% of the area of Azorella cushions on the plateau was dead, hypothesising that necrosis radiated from the windward peak of the cushions, leaving a ring of live foliage. Selkirk (2012), presenting data from 1980, recorded a very low cover of dead cushions. Azorella exhibits seasonal leaf browning (winter senescence), which occurs between late autumn and mid-spring (Taylor 1955b).
During the austral summers of 1986–1987 and 1989–1990, several cushions on Macquarie Island were observed to have a form of damage that was brown, with a ring-shaped zone of yellow leaves on its margin, and that appeared as if it radiated within and among individual cushions, suggesting a fungus or another pathogen (P. Selkirk, pers. comm. 2010). The same yellow margin was thought to be associated with the patchy onset of winter browning by Taylor (1955b), and was widely observed where the cushions appeared to be dying in 2008–2009.
In response to the rapid cushion decline in 2008–2009, a need for spatial and temporal mapping of the cushion dieback to help determine possible causes, and the degree of threat to the species, was apparent (Kirkpatrick 2009). Our mapping occurred as part of a larger research program to identify the causes and consequences of the phenomenon (D. Bergstrom, pers. comm. 2009).
Our aim was to use temporal and spatial observations to suggest possible causes of damage and the implications of any dieback for the future of the species. In the first stage of our investigation, we determined whether there was variation in the symptoms of damage on the cushions. In the second stage, we determined whether spatial variation in each of three types of damage was consistent with patterns that could be expected from possible causes of damage. In the third stage, we determined whether any of the three types of damage was associated with dieback between spring 2009 and autumn 2010. Finally, we determined whether dieback was occurring between 2010 and 2013, whether it locally eliminated Azorella, and whether it promoted the expansion of Agrostis magellanica Lam. (henceforth Agrostis) into places previously occupied by Azorella.
Materials and methods
Site selection
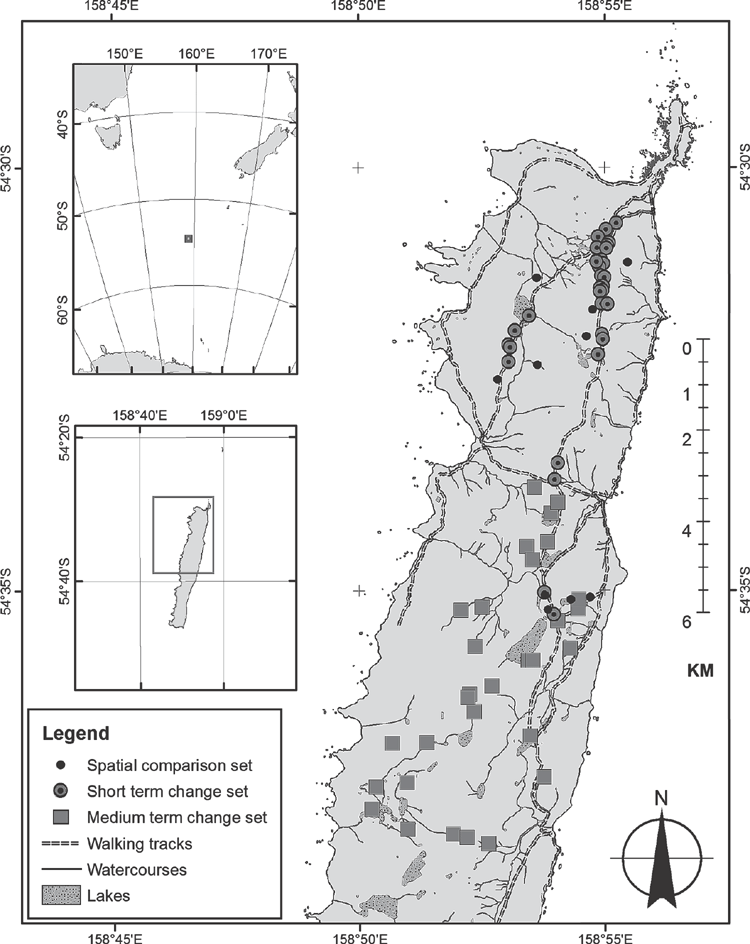
We collected three datasets with the purposes of spatial comparison, observation of short-term change and observation of medium-term change. The spatial-comparison dataset tested whether there was variation between sites in damage in the 2009–2010 summer. The sites were measured only once. Ten sites on the northern half of the plateau with Azorella (Fig. 1) were preselected from the stratified random study sites of Bricher et al. (2013).
For the set of observations of short-term change (spring 2009–autumn 2010), 31 Azorella sites on the northern half of the island, within 10–50 m of walking tracks, were randomly selected for monitoring using ESRI ArcMap (ESRI, Redlands, CA, USA) (Fig. 1). The sites were selected close to tracks to allow easy access for repeat visits, and far enough away from tracks to avoid disturbance by human traffic. Sites were rejected if they lacked cushion plants. The nearest cushion plant was placed in the centre of a repeat photograph quadrat, of which there was one per site.
For the set of observations of medium-term change (summer 2010–summer 2013), 30 sites with Azorella were subjectively selected in the northern third of the island (Fig. 1). Sixteen sites, on a range of aspects, elevations and soil types, had yellow discoloration of cushions. Fourteen sites, selected to be in the same range of environments, had no yellowing of any cushions. The change observations were repeated for the same plots so as to detect dieback.
Our spatial-comparison and medium term-change observations took place in mid-summer when winter browning of cushions was not evident anywhere on the island. Our short term-change observations picked up some winter browning in the earliest and latest observations. This was distinguished from yellow damage in spring by the greening of the foliage in later observations and in autumn by the lack of a yellow line.
Data collection for spatial comparison
At each of the 10 sites used for spatial comparison, one 30–80-cm maximum-diameter cushion in each of good health, medium health and poor health was selected. Cushions with green leaves forming the majority of the surface cover and with nil to minor damage were classed as in good health. Cushions with a surface cover comprising a mixture of green and yellow and/or brown leaves, and with minor to moderate damage were classed as in medium health. Cushions with brown and/or dead leaves forming the majority of the surface cover were classed as in poor health.
The nearest cushion in the direct line of every cardinal and intermediate directions from the three selected cushions was selected. Each of the 27 cushions per site was photographed vertically with its surrounds. A compass was located within the frame of each photograph. The elevation and geographic position of each of the 27 cushions at each site was mapped using a tape, compass and clinometer.
Data collection for short-term change
The 31 1 × 1-m quadrats were repeat photographed at intervals of ~3 weeks between 30 October 2009 and 24 March 2010. A tripod was used to position the camera ~2 m above the ground.
Data collection for medium-term change
The sites consisted of a marked 2 × 2-m space. This space was photographed in 1 × 1-m quadrat segments in January–February 2010, 2011, 2012 and 2013, although some sites were not relocated in 2011 and 2013. Elevation (m), slope (0–2°, >2°), aspect (south-westerly, north-easterly) and vegetation type (fjaeldmark or herbfield) were noted.
Observations on root systems
Twelve plants of Azorella with yellow damage were excavated in 2009 and observations made of the state of their root systems. In 2013, 10 plants that had previously had yellow damage and had surviving green shoots were excavated and observations made on the origin and characteristics of the live shoots. Several apparently healthy seedlings in adjacent rocky areas were excavated and examined at the same time.
Laboratory analysis in spatial-comparison study
The photographs were examined to determine whether there were different types of damage on the cushions. The percentage of each of the cushions that consisted of each of three types of damage (Fig. 2) was estimated to the nearest per cent by using a grid overlay as a guide. Cushion size was classed into large (>70-cm maximum diameter), medium (31–70-cm maximum diameter) and small (<30-cm maximum diameter).
Dominant wind direction (north, north-east, east, south-east, south, south-west, west, north-west) was recorded from the photographs on the basis of the orientations of distortions to vegetation surrounding the target cushion, following Noguchi (1979), Robertson (1987) and Wooldridge et al. (1996). We assumed that asymmetry in mosses, herbs and grasses was equivalent to asymmetry in tree crowns. The direction, or lack thereof, of concentration of each type of damage on each cushion was recorded. The sector with the highest proportion of damaged cushion surface of the particular type was selected. Thus, if the slope of the cushion facing south-west had the most grey damage, south-west was recorded.
By using the photographs, cushions were subjectively classed by one observer (JAR) as sheltered from the direct force of the wind, partially protected or exposed (>50% of windward side unprotected from the direct force of the wind). Protection from wind was deduced by identifying obstacles that could reduce the impact of the wind against the cushion surface, such as rocks, tall vegetation to the windward or plants growing epiphytically on the windward part of the cushions.
The photographs were used to determine ground cover, which was the nature of the surrounding surface material. Cushions growing on an approximately equal combination of moss-bed, organic material and particles <2 mm in diameter were classed as having a substrate of moss-bed and soil. Cushions observed to be growing mainly on particles <2 mm in diameter were classed as having a substrate of soil. Cushions observed to be growing on an approximately equal combination of particles <2 mm and >2 mm in diameter were classed as having a substrate of soil and gravel. Cushions observed to be growing only on particles >2 mm were classed as having a substrate of gravel.
Areas in the photographs in the immediate vicinity of each cushion with vegetation dominated by bryophytes, cushions and lichens and with a ground cover consisting mainly of exposed gravel and aeolian sediments were classified as fjaeldmark. Areas with vegetation dominated by herbs, cushions and sedges and with a ground cover consisting mainly of vegetation were classified as herbfield.
Laboratory analysis of data from short and medium term-change sites
Areas of cushion damage were marked in layers overlaid onto the images from each visit to the 31 1 × 1 short term-change sites, identifying the three damage types. The covers of live and dead Azorella and live Agrostis were measured on the 2 × 2 m medium term-change plot photographs, and the presence of yellow damage was noted. A wind-exposure variable was calculated from the aspect readings (south-west = 1, south and west = 2, north-west and south-east = 3, north and east = 4, north-east = 5). The strongest winds on the island are south-westerly.
Statistical methods for the spatial-comparison data
All analyses for all datasets were undertaken in Minitab16 (Minitab Inc. 2010) or R (version 2.15.1, R Core Team 2012). Chi-square was used to determine the significance of the co-occurrence of counts of each type of damage with classes of each of cushion size, exposure, ground cover and vegetation type. Chi-square was also used to determine whether the relationships between these predictor variables deviated from random.
Beta regression models (R betareg package, Cribari-Neto and Zeileis 2010) were used to determine the influence of ground cover, cushion size, vegetation type and exposure on percentage damage for each of the damage types. Both maximum likelihood and Akaike information criterion (AIC) were used to determine the combination of variables best related to percentage Azorella damage. The β regression model was selected for its appropriateness of use for proportion response variables that display both asymmetry and heteroscedasticity. The model was fitted via maximum likelihood using a parameterisation with a mean and precision parameter (phi) (Cribari-Neto and Zeileis, 2010). In instances of 0, the following conversion was applied: y × (n – 1) + 0.5)/n, where n is the sample size (Smithson and Verkuilen 2006). The variables were assessed using partial Wald tests.
The size-class distributions of the three types of damage were graphed. The strong wind directions as indicated by vegetation distortion and the direction of concentration of each type of damage were graphed. The relationships between percentage damage of each type and elevation were analysed for each site by using Pearson’s product moment correlation coefficient.
Statistical methods for the temporal-change data
One-way ANOVA was used to determine whether elevation varied between the medium term-change plots with yellow damage in 2010 and those without such damage. For each of slope, aspect and vegetation type, chi-square was used for the same purpose.
The changes in the live cover of each of Azorella and Agrostis between 2010–2011, 2011–2012 and 2012–2013 were calculated for the 2 × 2 m medium term-change plots and standard errors were calculated. Analysis of covariance with quasibinomial errors was applied in two generalised linear models, as follows: live Azorella cover in 2013 = live Azorella cover in 2010, yellowing in 2010, live Azorella cover in 2010 × yellowing in 2010; Agrostis cover in 2013 = Agrostis cover in 2010, yellowing in 2010, Agrostis cover in 2010 × yellowing in 2010. Pearson’s product moment correlation coefficient was used to determine the relationship between the wind-exposure index and percentage damage to cushions for each of 2010, 2011, 2012 and 2013, and the relationship between change in live Azorella cover between 2010 and 2013 and the change in Agrostis cover between 2010 and 2013.
Results
Damage type
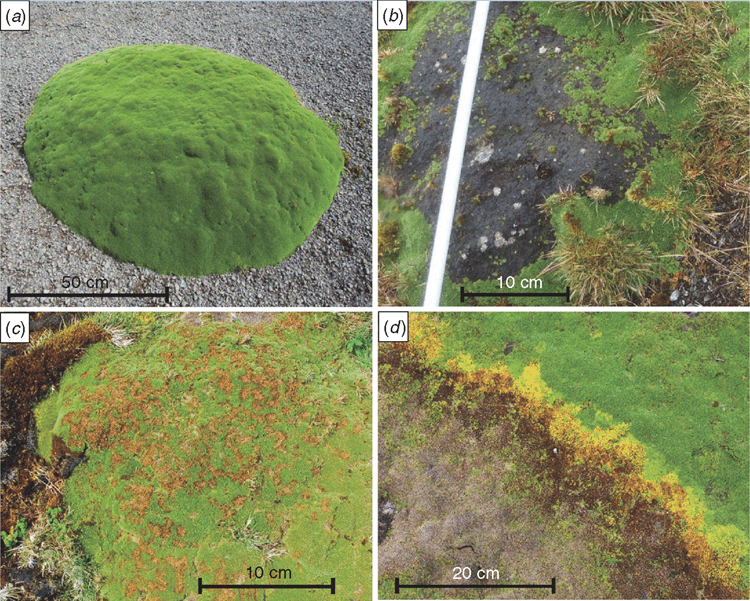
The three types of damage that were discerned were distinguished by colour, surface characteristics and size. In grey damage, the surface of the cushion was smooth and grey to black (Fig. 2b). In speck damage, there were small reddish-brown patches or streaks of leaves, with their tips removed and their stems exposed (Fig. 2c). In yellow damage, intact leaves had yellow discoloration, and were adjacent to leaves with brown discoloration (Fig. 2d).
Spatial variation in damage type
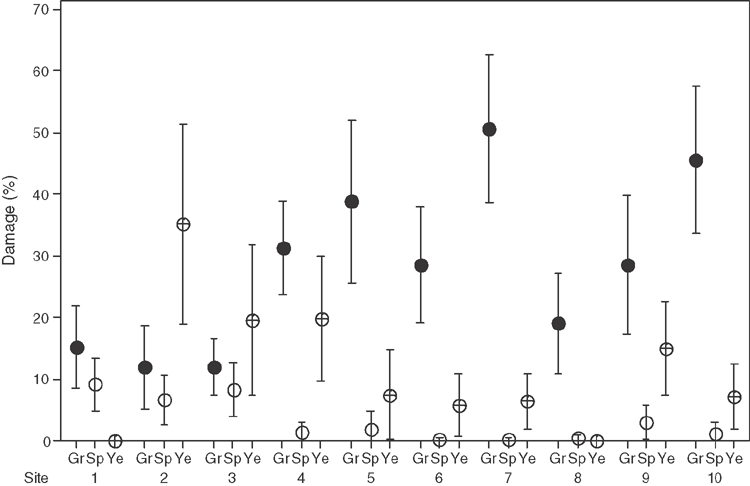
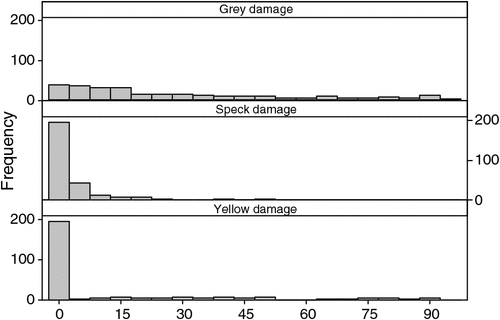
Grey damage was dominant at eight of the spatial-comparison sites, yellow damage was dominant at two and speck damage was dominant in none, while being absent from several (Fig. 3). Grey damage varied enormously in its cover (Fig. 4). Most cushions had no yellow damage, but those cushions that did have it varied widely in its percentage cover (Fig. 4). Speck damage was rare and usually did not cover much of the cushion (Fig. 4).
|
|
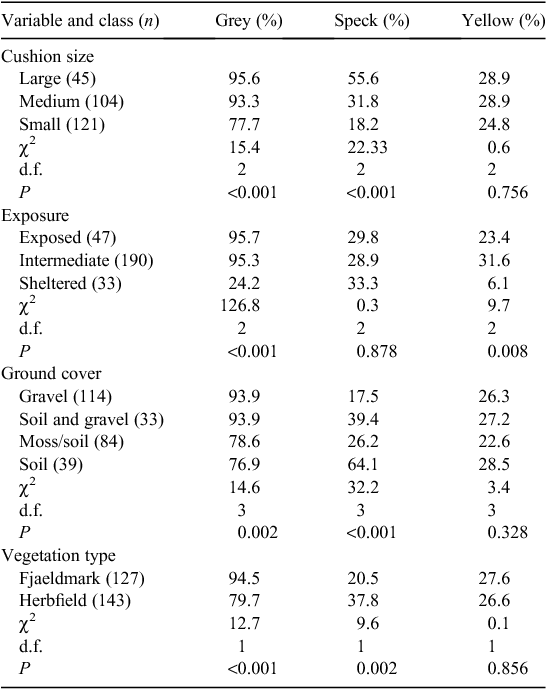
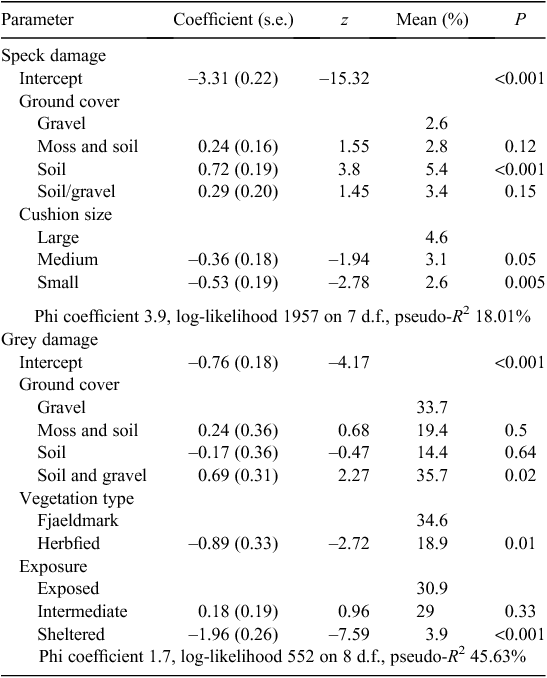
The four predictor variables were partly coincident in their variation, with P < 0.001 (χ2) for all pairs. The frequency of yellow damage was low on the most sheltered cushions (Table 1). The other available predictor variables did not explain yellow-damage frequency (Table 1) and no model for yellow-damage cover could be derived (Table 2).
|
|
Ground cover, vegetation type and exposure were the variables retained in the final model for percentage grey damage (Table 2), which was high on gravelly soils, in fjaeldmark and on the most exposed cushions (Table 1). There was significant variation in the frequency of grey damage in relation to these variables and cushion size, grey damage being most frequent on larger cushions (Table 1).
Cushions size and ground cover were the components in the model for percentage speck damage, with values being highest on soil and larger cushions (Table 2). The same two predictors were significant for frequency, as was vegetation type, with speck damage being greater in herbfield than fjaeldmark (Table 1).
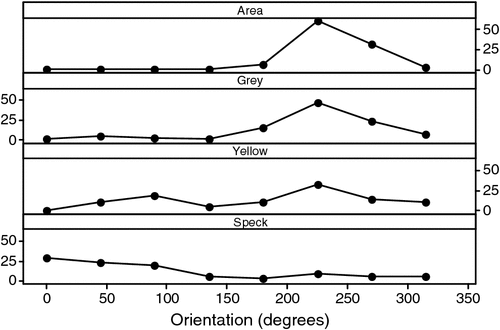
Grey damage was concentrated in the south-west of the cushions (Fig. 5), the direction from which the prevailing winds emanate (Fig. 5), whereas speck damage was concentrated to the north and east (Fig. 5) and yellow damage, although tending to the south-west, was variable in its direction of concentration (Fig. 5).
|
Grey damage increased with elevation at one site (r25 = 0.575, P = 0.002), while decreasing with elevation at others (r25 = –0.565, P = 0.002; r25 = –0.669, P = <0.001; r25 = –0.678, P = <0.001). Speck damage decreased with elevation at one site (r25 = –0.397, P = 0.040). Yellow damage increased with elevation at the same site (r25 = 0.449, P = 0.019), but decreased with elevation at another (r25 = –0.419, P = 0.030).
Short-term change
Grey damage was observed on 28 of the 31 cushions when first photographed. This type of damage exhibited minimal temporal variation, although, in some cases, being partially replaced by green foliage between visits. Speck damage occurred in 26 of the 31 sites. Mild speck damage often disappeared between visits, whereas severe damage persisted. Overall, speck damage remained constant. Yellow damage occurred during observations in 5 of the 31 sites. At all of these sites, it expanded markedly in a clear dieback process, sometimes apparently spreading from one cushion to another (Fig. 6).
|
Medium-term change
Elevation did not differ between the plots with and without yellow damage in 2010 (F1,28 = 0.59, P = 0.856). There was also no significant difference for aspect (χ2 = 0.11, d.f. = 1, P = 0.772), slope (χ2 = 0.01, d.f. = 1, P = 0.919) or vegetation type (χ2 = 0.15, d.f. = 1, P = 0.696).
By 2013, yellow-coloured cushion foliage had disappeared from all plots in which it occurred in 2010. In all cases, this disappearance was associated with a transition from chlorosis to extensive necrosis rather than recovery to healthy tissue. In 1 case of 14, yellow foliage became apparent in a plot in 2012 and 2013 that had green (healthy) cushions in 2010.
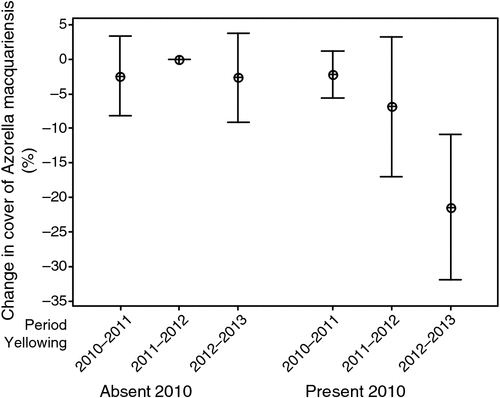
Yellow damage, Azorella live cover in 2010 and their interaction were all good predictors of Azorella live cover in 2013 in the generalised linear model (Table 3). Azorella live cover in 2013 less its live cover in 2010 was –4.65% for plots without yellow damage in 2010 and –21.73% for plots with some yellow damage at this time. Azorella had a live cover of >10% of cushion area in all plots in 2013. On the plots with the most dieback, Azorella was present as live branchlets in or between dead cushions. Heterogeneity in change in healthy cushion cover increased through time in the 2010 yellow-damage plots (Fig. 7).
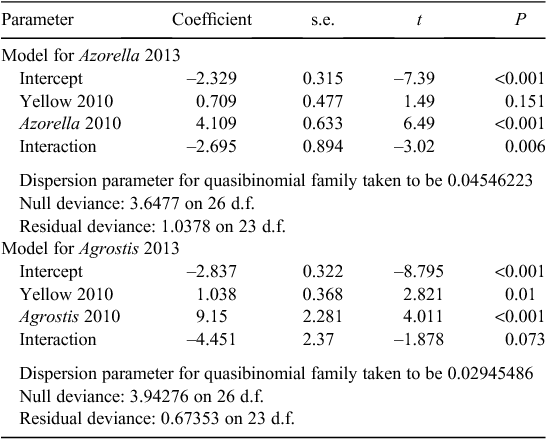
|
|
Azorella live cover had an increasingly significant relationship with the wind-exposure variable as time elapsed (2010, r28 = 0.334, P = 0.072; 2011, r14 = 0.494, P = 0.052; 2012, r28 = 0.4, P = 0.028; 2013, r25 = 0.430, P = 0.025). In all four years, the less exposed the site was, the greater the live cover. Grey damage began to appear on the windward surfaces of cushions two years after the passage of the chlorosis (Fig. 8d).
Change in live Azorella cover between 2010 and 2013 was negatively related to change in Agrostis cover (r25 = –0.571, P = 0.002). The generalised linear model for percentage Agrostis cover in 2013 had both yellow damage in 2010 and Agrostis cover in 2010 as significant components, but not the interaction (Table 3). Between 2010 and 2013, Agrostis cover increased by an average of 4% where there was no yellow damage in 2010, whereas where there was yellow damage in 2010, there was an increase of 12%. The response of Agrostis was highly heterogeneous (Fig. 9). Examination of the photographs suggested that Agrostis expansion, where it occurred, partly consisted of growth of established individuals and partly consisted of establishment of new individuals on cushion surfaces (e.g. Fig. 8).
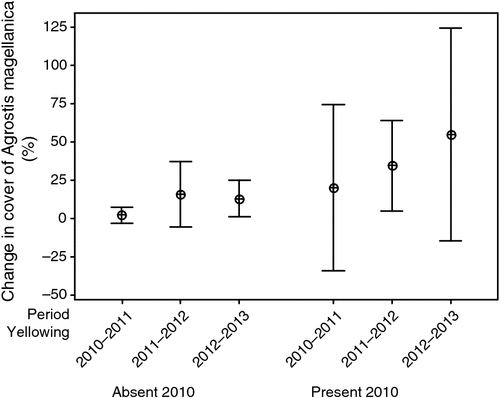
|
Observations on the process of dieback
The general pattern of progression of dieback associated with yellow damage was that a small (2–10 cm), diffuse yellow stippling appeared in a zone up to 50 cm in front of a 5–10-cm wide line of yellow leaves (Fig. 2d). In the wake of the front, the majority of the cushion was brown, although individual branchlets and small patches of cushions were usually left in an apparently healthy green state (Fig. 8b–d). This process was observed both on individual cushions and on cushion-banked terraces.
Between February 2010 and January 2011, examples of the yellowing front were seen to progress between 30 and 100 cm, the progression being at a greater speed in lower-profile plants than in higher relief cushions. The disease front appeared to progress at similar speeds in the inter-cushion spaces. Rosettes of Pleurophyllum hookeri Buchan. greater than 10 cm in diameter died with the passage of the yellow line, although smaller rosettes survived. Some areas of cushion did not come out of winter senescence, suggesting that dieback was active in dormant cushions. By February 2013, many cushions with yellow damage in 2010 and 2011 had some expansion of live foliage, although most of the previously live cushion foliage was dead and decaying.
The twelve plants with yellow lines or patches that were excavated in 2009 had rotted tap roots. Several instances of a lesion between healthy and dead tissue in the tap root were also observed (Fig. 8e). Live cushion shoots in dieback areas in March 2013 had imbricately packed dead leaves and leaf scars from previous seasonal cycles (Fig. 8f). These characteristics differed from those of young seedlings in rocky areas, which had short branches split off from a basal node, below which a well developed tap root was present (Fig. 8g).
Discussion
Types of damage and dieback
Two of the three distinct damage types evident in 2009–2010 were also evident in the 2013 photographs, speck damage having largely healed. The series of photographs between 2010 and 2013 (Fig. 8) showed that yellow damage could be a precursor to grey damage, especially on the parts of cushions exposed to strong winds, as further indicated by the increasing significance of the correlation between percentage live cushion and wind exposure between 2010 and 2013. We cannot determine whether all grey damage develops from yellow damage, but we can conclude that recovery from yellow damage disproportionately occurs on the sheltered parts of cushions, and that a lack of recovery is expressed in grey damage.
Although yellow damage had a low incidence (16%) in the randomly located 1 × 1-m short term-change plots and averaged only 12% of the surface area of cushions at the 10 spatial-comparison sites (cf. 3% for speck damage and 28% for grey damage), it was present on all cushions that exhibited an increase in dieback between spring and autumn 2009–2010 and was associated with all of the high level of ongoing dieback between 2010 and 2013 in the medium term-change plots.
Possible causes of types of damage
Much of our data are consistent with exposure to strong winds as a major contributor to grey damage, including its preferential occurrence in fjaeldmark, on gravelly surfaces and in areas with high levels of exposure (Tables 1, 2), and its concentration on the windward sections of the cushions (Fig. 6). An association between wind exposure and damage has been widely observed in cushion plants (Taylor 1955a; Ashton and Gill 1965; Molau 1996; le Roux and McGeoch 2008). In our study area, wind damage might have been at least partly facilitated by the dieback that followed the chlorosis of yellow damage. Additionally or alternatively, the apparent wind damage may have been caused by increased drought stress, as has been suggested as a possibility for other Macquarie Island species by Scott and Kirkpatrick (2013) and for another Azorella species by le Roux et al. (2005).
The localised clipping of leaves characteristic of speck damage strongly suggested that rabbit-grazing was its cause. Consistent with our directional data (Fig. 6), rabbits are known to minimise their exposure to strong winds (Southern 1940, 1948; Rowley 1957; Wheeler et al. 1981; Fraser 1992; Ballinger and Morgan 2002). Speck damage was strongly associated with herbfield, rather than fjaeldmark, reflecting the known relative abundance of rabbits (Copson et al. 1981). New speck damage was not noted after 2011, which coincides with the completion of aerial baiting for the Macquarie Island Pest Eradication Project, with no live rabbit observed on the island since November 2011 (Parks and Wildlife Service 2012).
Rabbits could not have been the primary cause of yellow damage (Figs 2d, 8a–d) because yellow damage persisted after rabbit extermination. Yellow damage was prominent on sheltered parts of the cushions as well as exposed parts, so wind was unlikely to have been its primary cause. Frost and drought are unlikely to be the causes of yellow damage because it had no consistent association with topographic position or the tops of cushions, and no associated moss dieback was observed.
The patchy incidence in relation to environment of yellow damage is consistent with the presence of a soil-borne pathogen (Gilligan 1995). Such patchiness is largely a function of the variability in pathogen infectivity and host susceptibility in the primary and secondary infection phases (Burdon et al. 1989; Kleczkowski et al. 1997). The association of yellow damage with lesions and rotted tap roots further supports this hypothesis. A yellow discoloration of leaf tissue has been previously observed in pathogen-affected plants (Tamada and Baba 1973; Trapero-Casas and Jiménez-Díaz 1985). Brown discolorations have been observed in other cases (Scortichini and Lazzari 1996; Unger et al. 2005; Bobev et al. 2009), and a variety of discolorations ranging from yellow to brown in still others (Johnson and Littrell 1969; Fradin and Thomma 2006).
Is Azorella on a path to extinction?
Our data did not indicate local extinction of Azorella, with the passage of the yellow front being followed by recovery (Fig. 8a–d). The individual shoots that survived dieback could be surviving branches of the parent plant, previously split off vegetative individuals or small genetically distinct individuals that have established within the parent cushion (Fig. 8f).
Given that there were no environmental or vegetation differences between the medium term-change plots with yellow damage in 2010 and those without damage at the time, the putative pathogen has not realised its potential range, at least based on the variables measured in the present study. Yet, it did not attack cushions in 13 of the 14 plots from which it was absent in 2010. These data, combined with the observations of yellow lines in cushions by scientists in the 1950s and the 1980s, could mean that the widely observed cushion death of 2008–2010 represented a peak in fluctuations of a pathogen that is widespread on the island. However, the climate of the island is changing (Adams 2009), possibly in a way that favours the organism that may cause the necrosis preceded by yellow damage, so a precautionary approach is required.
Is Agrostis replacing Azorella?
There was no indication in our data that Agrostis was displacing Azorella in plots where cushions were healthy throughout the period 2010–2013. However, Agrostis has proven effective in occupying the dead parts of cushions. Whether this strong shift in vegetation dominance is likely to persist is uncertain. Further long-term observation of our plots is desirable to determine whether the replacement of Azorella by Agrostis will be persistent. Persistence may vary in response to the severity of the environment and cushion size, as for the closely related species, Azorella selago Hook. (le Roux and McGeoch 2008; le Roux et al. 2013).
Conclusions
We conclude that there is strong circumstantial evidence that the fast-spreading dieback associated with yellow damage is caused by a pathogen, and that specks were mechanical damage caused by grazing by the recently eradicated rabbits. Grey damage is strongly associated with wind exposure, but wind may not always be its primary cause. The dieback associated with yellow damage does not appear to cause local extinction of Azorella, but is associated with a decline in cushion cover and an expansion of Agrostis.
Acknowledgements
Dr Dana Bergstrom from the AAD made it possible for JAR to collect data and provided advice in the early stages of the project. Dr Phillippa Bricher helped with the random selection of the 1 × 1-m plots and provided advice to JAR. Parks and Wildlife Service staff and Nick Fitzgerald assisted with the establishment and monitoring of the temporal monitoring sites.
References
Adams N (2009 ) Climate trends at Macquarie Island and expectations of future climate change in the sub-antarctic. Papers and Proceedings of the Royal Society of Tasmania 143 1 8Ashton DH, Gill AM (1965) Pattern and process in a Macquarie Island feldmark. Proceedings of the Royal Society of Victoria 79, 235–245.
Ballinger A, Morgan DG (2002) Validating two methods for monitoring population size of the European rabbit (Oryctolagus cuniculus). Wildlife Research 29, 431–437.
| Validating two methods for monitoring population size of the European rabbit (Oryctolagus cuniculus).Crossref | GoogleScholarGoogle Scholar |
Bergstrom DM, Lucieer A, Kiefer K, Wasley J, Belbin L, Pedersen TK, Chown SL (2009) Indirect effects of invasive species removal devastate World Heritage island. Journal of Applied Ecology 46, 73–81.
| Indirect effects of invasive species removal devastate World Heritage island.Crossref | GoogleScholarGoogle Scholar |
Bobev SG, van Poucke K, Maes M (2009) First report of Phytophthora citricola on Cornus mas in Bulgaria. Plant Disease 93, 551
| First report of Phytophthora citricola on Cornus mas in Bulgaria.Crossref | GoogleScholarGoogle Scholar |
Bricher P, Lucieer A, Shaw J, Terauds A, Bergstrom DM (2013) Mapping sub-antarctic cushion plants using random forests to combine very high resolution satellite imagery and terrain modelling. PLoS ONE 8, e72093
| Mapping sub-antarctic cushion plants using random forests to combine very high resolution satellite imagery and terrain modelling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht12js73K&md5=daa39f2f206ccbd285f6a0bd57781777CAS | 23940805PubMed |
Burdon JJ, Jarosz AM, Kirby GC (1989) Pattern and patchiness in plant-pathogen interactions – causes and consequences. Annual Review of Ecology and Systematics 20, 119–136.
Chapuis JL, Bousses P, Barnaud G (1994) Alien mammals, impact and management in the French sub-antarctic islands. Biological Conservation 67, 97–104.
| Alien mammals, impact and management in the French sub-antarctic islands.Crossref | GoogleScholarGoogle Scholar |
Copson GR, Whinam J (1998) Response of vegetation on subantarctic Macquarie Island to reduced rabbit grazing. Australian Journal of Botany 46, 15–24.
| Response of vegetation on subantarctic Macquarie Island to reduced rabbit grazing.Crossref | GoogleScholarGoogle Scholar |
Copson GR, Whinam J (2001) Review of ecological restoration programme on subantarctic Macquarie Island: pest management progress and future directions. Ecological Management & Restoration 2, 129–138.
| Review of ecological restoration programme on subantarctic Macquarie Island: pest management progress and future directions.Crossref | GoogleScholarGoogle Scholar |
Copson GR, Brothers NP, Skira IJ (1981) Distribution and abundance of the rabbit, Oryctolagus cuniculus (L.), at subantarctic Macquarie Island. Australian Wildlife Research 8, 597–611.
| Distribution and abundance of the rabbit, Oryctolagus cuniculus (L.), at subantarctic Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Cribari-Neto F, Zeileis A (2010) Beta regression in R. Journal of Statistical Software 34, 1–24.
Fradin EF, Thomma BPHJ (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Molecular Plant Pathology 7, 71–86.
| Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVWgsr8%3D&md5=751d44eace1c5fb3975b7f7e0dc6a0cdCAS | 20507429PubMed |
Fraser KW (1992) Emergence behaviour of rabbits, Oryctolagus cuniculus, in central Otago. New Zealand Journal of Zoology 228, 615–623.
Gilligan CA (1995) Modeling soil-borne pathogen – reaction-diffuse models. Canadian Journal of Plant Pathology 17, 96–108.
| Modeling soil-borne pathogen – reaction-diffuse models.Crossref | GoogleScholarGoogle Scholar |
Hauri H, Schröter C (1914) Versucheiner Uebersicht der siphonogamen Polsterpflanzen. Engler’s Botanisches Jahrbuch 50, 618–656.
Johnson AW, Littrell RH (1969) Effect of Meloidogyne incognita, M. hapla, and M. javanica on the severity of Fusarium wilt of Chrysanthemum. Journal of Nematology 1, 122–125.
Kirkpatrick JB 2009 The importance of integrating science and management: lessons from terrestrial vegetation change on Macquarie and Heard Islands. Papers and Proceedings of the Royal Society of Tasmania 143 25 32
Kirkpatrick JB, Harwood CE (1980) The vegetation of an infrequently burned Tasmanian mountain region. Proceedings of the Royal Society of Victoria 91, 79–107.
Kirkpatrick JB, Bridle K, Lynch AJJ (2002) Changes in vegetation and landforms at Hill One, Tasmania. Australian Journal of Botany 50, 753–759.
| Changes in vegetation and landforms at Hill One, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Kleczkowski A, Gilligan CA, Bailey DJ (1997) Scaling and spatial dynamics in plant–pathogen systems: from individuals to populations. Proceedings. Biological Sciences 264, 979–984.
| Scaling and spatial dynamics in plant–pathogen systems: from individuals to populations.Crossref | GoogleScholarGoogle Scholar |
Kleier C, Rundel PW (2004) Microsite requirements, population structure and growth of the cushion plant Azorella compacta in the tropical Chilean Andes. Austral Ecology 29, 461–470.
| Microsite requirements, population structure and growth of the cushion plant Azorella compacta in the tropical Chilean Andes.Crossref | GoogleScholarGoogle Scholar |
le Roux PC, McGeoch MA (2008) Spatial variation in plant interactions across a severity gradient in the sub-antarctic. Oecologia 155, 831–844.
| Spatial variation in plant interactions across a severity gradient in the sub-antarctic.Crossref | GoogleScholarGoogle Scholar | 18253754PubMed |
le Roux PC, McGeoch MA, Nyakatya MJ, Chown SL (2005) Effects of a short term climate change experiment on a sub-antarctic keystone species. Global Change Biology 11, 1628–1639.
| Effects of a short term climate change experiment on a sub-antarctic keystone species.Crossref | GoogleScholarGoogle Scholar |
le Roux PC, Shaw JD, Chown SL (2013) Ontogenetic shifts in plant interactions vary with environmental severity and affect population structure. New Phytologist 200, 241–250.
| Ontogenetic shifts in plant interactions vary with environmental severity and affect population structure.Crossref | GoogleScholarGoogle Scholar | 23738758PubMed |
Lebouvier M, Laparie M, Hullé M, Marais A, Cozic Y, Lalouette L, Vernon P, Candresse T, Frenot Y, Renault D (2011) The significance of the sub-antarctic Kerguelen Islands for the assessment of the vulnerability of native communities to climate change, alien insect invasions and plant viruses. Biological Invasions 13, 1195–1208.
| The significance of the sub-antarctic Kerguelen Islands for the assessment of the vulnerability of native communities to climate change, alien insect invasions and plant viruses.Crossref | GoogleScholarGoogle Scholar |
Marr DL (1997) Impact of a pollinator transmitted disease on reproduction in healthy Silene acaulis. Ecology 78, 1471–1480.
Minitab Inc (2010) ‘Minitab users guide release 16.’ (Minitab: State College, PA)
Molau U (1996) Impacts on flowering, growth, and vigour in an Arctic-alpine cushion plant, Diapensia lapponica, under different snow cover regimes. Ecological Bulletins 45, 210–219.
Noguchi Y (1979) Deformation of trees in Hawaii and its relation to wind. Journal of Ecology 67, 611–628.
| Deformation of trees in Hawaii and its relation to wind.Crossref | GoogleScholarGoogle Scholar |
Núñez L, Grosjean M (2003) Biodiversity and human impact during the last 11 000 years in north-central Chile. In ‘How landscapes change’. (Eds GA Bradshaw, PA Marquet) pp. 7–17. (Springer-Verlag: Berlin)
Parks and Wildlife Service (2012) ‘Macquarie dispatch. Macquarie Island Pest Eradication Project Newsletter, Issue 10.’ Available at http://www.parks.tas.gov.au/index.aspx?base=13001. [Verified March 2014]
Phiri EE, McGeoch MA, Chown SL (2009) Spatial variation in structural damage to a keystone plant species in the sub-antarctic: interactions between Azorella selago and invasive house mice. Antarctic Science 21, 189–196.
| Spatial variation in structural damage to a keystone plant species in the sub-antarctic: interactions between Azorella selago and invasive house mice.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2012) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna). Available at http://www.R-project.org/. [Verified March 2014]
Robertson A (1987) The use of trees to study wind. Arboricultural Journal 11, 127–143.
| The use of trees to study wind.Crossref | GoogleScholarGoogle Scholar |
Rowley I (1957) Observations on evening rabbit activity in relation to weather and sunset. Wildlife Research 2, 168–169.
| Observations on evening rabbit activity in relation to weather and sunset.Crossref | GoogleScholarGoogle Scholar |
Rundel PW, Palma B (2000) Preserving the unique puna ecosystems of the Andean Altiplano: a descriptive account of Lauca National Park, Chile. Mountain Research and Development 20, 262–271.
| Preserving the unique puna ecosystems of the Andean Altiplano: a descriptive account of Lauca National Park, Chile.Crossref | GoogleScholarGoogle Scholar |
Scortichini M, Lazzari M (1996) Systemic migration of Pseudomonas syringae pv. avellanae in twigs and young trees of hazelnut and symptom development. Journal of Phytopathology 144, 215–219.
| Systemic migration of Pseudomonas syringae pv. avellanae in twigs and young trees of hazelnut and symptom development.Crossref | GoogleScholarGoogle Scholar |
Scott JJ, Kirkpatrick JB (1994) Effects of human trampling on the sub-antarctic vegetation of Macquarie Island. The Polar Record 30, 207–220.
| Effects of human trampling on the sub-antarctic vegetation of Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Scott JJ, Kirkpatrick JB (2008) Rabbits, landslips and vegetation change on the coastal slopes of subantarctic Macquarie Island, 1980–2007: implications for management. Polar Biology 31, 409–419.
| Rabbits, landslips and vegetation change on the coastal slopes of subantarctic Macquarie Island, 1980–2007: implications for management.Crossref | GoogleScholarGoogle Scholar |
Scott JJ, Kirkpatrick JB (2013) Changes in the cover of plant species associated with climate change and grazing pressure on the Macquarie Island coastal slopes, 1980–2009. Polar Biology 36, 127–136.
| Changes in the cover of plant species associated with climate change and grazing pressure on the Macquarie Island coastal slopes, 1980–2009.Crossref | GoogleScholarGoogle Scholar |
Selkirk PM 2012 Plateau vegetation on sub-antarctic Macquarie Island. Papers and Proceedings of the Royal Society of Tasmania 146 71 79
Selkirk PM, Seppelt RD, Selkirk DR (1990) ‘Subantarctic Macquarie Island: environment and biology.’ (Cambridge University Press: Cambridge, UK)
Shaw J, Terauds A, Bergstrom D (2011) Rapid commencement of ecosystem recovery following aerial baiting on sub-antarctic Macquarie Island. Ecological Management & Restoration 12, 241–244.
| Rapid commencement of ecosystem recovery following aerial baiting on sub-antarctic Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychological Methods 11, 54–71.
| A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables.Crossref | GoogleScholarGoogle Scholar | 16594767PubMed |
Southern HN (1940) The ecology and population dynamics of the wild rabbit (Oryctolagus cuniculus). Annals of Applied Biology 27, 509–526.
| The ecology and population dynamics of the wild rabbit (Oryctolagus cuniculus).Crossref | GoogleScholarGoogle Scholar |
Southern HN (1948) Sexual and aggressive behaviour in the wild rabbit. Behaviour 1, 173–194.
| Sexual and aggressive behaviour in the wild rabbit.Crossref | GoogleScholarGoogle Scholar |
Tamada T, Baba T (1973) Beet necrotic yellow vein virus from rhizomania affected sugar beet in Japan. Annals of the Phytopathological Society of Japan 39, 325–332.
| Beet necrotic yellow vein virus from rhizomania affected sugar beet in Japan.Crossref | GoogleScholarGoogle Scholar |
Taylor BW (1955a) Terrace formation on Macquarie Island. Journal of Ecology 43, 133–137.
| Terrace formation on Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Taylor BW (1955b) ‘The flora, vegetation and soils of Macquarie Island. ANARE scientific report, series B, vol 2.’ (Antarctic Division, Department of External Affairs: Melbourne)
Threatened Species Scientific Committee (2010) Advice to the Minister for Environment Protection, Heritage and the Arts from the Threatened Species Scientific Committee (the Committee) on amendment to the list of threatened species under the Environmental Protection and Biodiversity Conservation Act 1999 (EPBC Act). Department of Environment Protection, Heritage and the Arts, Canberra.
Threatened Species Section (2009) ‘Notesheet for Azorella macquariensis (Macquarie cushions).’ (Department of Primary Industries and Water, Hobart, Tas.)
Trapero-Casas A, Jiménez-Díaz RM (1985) Fungal wilt and root rot diseases of chickpea in southern Spain. Phytopathology 75, 1146–1151.
| Fungal wilt and root rot diseases of chickpea in southern Spain.Crossref | GoogleScholarGoogle Scholar |
Unger CH, Kleta S, Jandl G, von Tiedemann A (2005) Suppression of the defence-related oxidative burst in bean leaf tissue and bean suspension cells by the necrotrophic pathogen Botrytis cinerea. Journal of Phytopathology 153, 15–26.
| Suppression of the defence-related oxidative burst in bean leaf tissue and bean suspension cells by the necrotrophic pathogen Botrytis cinerea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhvVCktbg%3D&md5=5842231f05c878098193019fea09542aCAS |
Wheeler SH, King DR, Robinson MH (1981) Habitat and warren utilisation by the European rabbit, Oryctolagus cuniculus (L.), as determined by radio-tracking. Australian Wildlife Research 8, 581–588.
| Habitat and warren utilisation by the European rabbit, Oryctolagus cuniculus (L.), as determined by radio-tracking.Crossref | GoogleScholarGoogle Scholar |
Whinam J, Chilcott N (1999) Impacts of trampling on alpine environments in central Tasmania. Journal of Environmental Management 57, 205–220.
| Impacts of trampling on alpine environments in central Tasmania.Crossref | GoogleScholarGoogle Scholar |
Whinam J, Chilcott N (2003) Impacts after four years of experimental trampling on alpine/sub-alpine environments in western Tasmania. Journal of Environmental Management 67, 339–351.
| Impacts after four years of experimental trampling on alpine/sub-alpine environments in western Tasmania.Crossref | GoogleScholarGoogle Scholar | 12710922PubMed |
Wooldridge GL, Musselman RC, Sommerfeld RA, Fox DG, Connell BH (1996) Mean wind patterns and snow depths in an alpine–subalpine ecosystem as measured by damage to coniferous trees. Journal of Applied Ecology 33, 100–108.
| Mean wind patterns and snow depths in an alpine–subalpine ecosystem as measured by damage to coniferous trees.Crossref | GoogleScholarGoogle Scholar |