Linear Trinuclear Copper(ii) Complexes Derived from the Nucleophilic Addition Products of Dicyanonitrosomethanide [C(CN)2(NO)]–: Syntheses, Structures, and Magnetic Properties*
Mohd. R. Razali A , Aron Urbatsch A , Stuart K. Langley A , Jonathan G. MacLellan A , Glen B. Deacon A , Boujemaa Moubaraki A , Keith. S. Murray A and Stuart R. Batten A BA School of Chemistry, Monash University, Vic. 3800, Australia.
B Corresponding author. Email: stuart.batten@monash.edu.au
Australian Journal of Chemistry 65(7) 918-925 https://doi.org/10.1071/CH12100
Submitted: 16 February 2012 Accepted: 9 April 2012 Published: 19 July 2012
Abstract
Two novel trinuclear CuII complexes have been synthesised from the nucleophilic addition derivatives of the small cyano anion, dicyanonitrosomethanide (dcnm). The reaction of CuII with the water adduct ligand, carbamoylcyanonitrosomethanide (ccnm) and teaH3 (triethanolamine) in a basic MeOH/MeCN solution results in the formation of [Cu3(acnm)2(teaH2)2]·2MeOH (1) (acnm = amidocarbonyl(cyano)nitrosomethanide and teaH2– = singly deprotonated triethanolamine). The reaction of CuII with dicyanonitrosomethanide (dcnm) and m-xylenediamine in a basic MeOH/MeCN solution results in the formation of [Cu3(cimm)2(a3acnm)2]·6MeCN (2) (cimm = cyano(imido(methoxy)methyl)nitrosomethanide and a3acnm = {amino(3-aminomethylphenyl)methylimino}methyl(cyanonitrosomethanide)). Both complexes display linear trinuclear CuII metallic cores. Solid state DC magnetic susceptibility studies were performed on 1 and 2. Compound 1 revealed very strong antiferromagnetic interactions between central and terminal Cu atoms, while compound 2 displayed ferromagnetic interactions because of the orthogonal relationship of the terminal 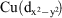 and the central
and the central 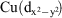 ‘magnetic’ orbitals, which contrasts with these orbitals being coplanar in 1 thus providing strong superexchange pathways involving Cu-N-O-Cu moieties.
‘magnetic’ orbitals, which contrasts with these orbitals being coplanar in 1 thus providing strong superexchange pathways involving Cu-N-O-Cu moieties.
References
[1] (a) D. R. Turner, A. S. R. Chesman, K. S. Murray, G. B. Deacon, S. R. Batten, Chem. Commun. (Camb.) 2011, 47, 10189.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWqurvF&md5=1636714966d47432e23f40560c28adbaCAS |
(b) M. Hvastijová, J. Kohout, J. W. Buchler, R. Boča, J. Kožíšek, L. Jäger, Coord. Chem. Rev. 1998, 175, 17.
| Crossref | GoogleScholarGoogle Scholar |
[2] S. R. Batten, K. S. Murray, Coord. Chem. Rev. 2003, 246, 103.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpt1Gisbg%3D&md5=008f5e9e9834f84d1f71b3f1a7d6a342CAS |
[3] A.-Q. Wu, F.-K. Zheng, W.-T. Chen, L.-Z. Cai, G.-C. Guo, J.-S. Huang, Z.-C. Dong, Y. Takano, Inorg. Chem. 2004, 43, 4839.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltl2mtrk%3D&md5=7a7c7c1957ff76076346f3cd8da58d7eCAS |
[4] C.-F. Hsu, S.-H. Lin, H.-H. Wei, Inorg. Chem. Commun. 2005, 8, 1128.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1arsLrE&md5=4718ed811d3b64116411b7ba5f0b7e8cCAS |
[5] (a) N. Arulsamy, D. Bohle, J. Org. Chem. 2000, 65, 1139.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1Wlsg%3D%3D&md5=10b26b48b866452c3024cf60416110a1CAS |
(b) M. Hvastijová, J. Kohout, J. W. Buchler, R. Boča, J. Kožíšek, L. Jäger, Coord. Chem. Rev. 1998, 175, 17.
| Crossref | GoogleScholarGoogle Scholar |
[6] E. K. Izgorodina, A. S. R. Chesman, D. R. Turner, G. B. Deacon, S. R. Batten, J. Phys. Chem. B 2010, 114, 16517.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVegtbbM&md5=d700014abadf82ce2155cbb465219583CAS |
[7] A. S. R. Chesman, D. R. Turner, K. S. Murray, B. Moubaraki, G. B. Deacon, S. R. Batten, Chem.– Eur. J. 2009, 15, 5203.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtVKhurk%3D&md5=43fe57f4a8c83bf9c62c12d5204d5337CAS |
[8] (a) A. S. R. Chesman, D. R. Turner, K. S. Murray, B. Moubaraki, G. B. Deacon, S. R. Batten, Eur. J. Inorg. Chem. 2010, 59.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFehsg%3D%3D&md5=890471fe2b600dec741c85fdc0c836a0CAS |
(b) T. Y. Silva, A. M. Duda, T. Glowiak, I. O. Fritsky, V. M. Amirkhanov, A. A. Mokhir, H. Kozlowski, J. Chem. Soc., Dalton Trans. 1997, 273.
| Crossref | GoogleScholarGoogle Scholar |
[9] A. S. R. Chesman, D. R. Turner, G. B. Deacon, S. R. Batten, Chem. Asian J. 2009, 5, 626.
[10] (a) S. K. Langley, K. Berry, B. Moubaraki, K. S. Murray, Dalton Trans. 2009, 973.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOlsbw%3D&md5=54db1b6c570af5fa9ca46885ae4af4ccCAS |
(b) S. K. Langley, B. Moubaraki, K. Berry, K. S. Murray, Dalton Trans. 2010, 39, 4848.
| Crossref | GoogleScholarGoogle Scholar |
[11] (a) G. Longo, Gazz. Chim. Ital. 1931, 61, 575.
| 1:CAS:528:DyaA38XmtFWk&md5=54883ac66f00be65726c90422423f3adCAS |
(b) M. Hvastijová, J. Kohout, J. W. Buchler, R. Boča, J. Kožíšek, L. Jäger, Coord. Chem. Rev. 1998, 175, 17.
| Crossref | GoogleScholarGoogle Scholar |
[12] D. S. Bohle, B. J. Conklin, C.-H. Hung, Inorg. Chem. 1995, 34, 2569.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXltlyks7w%3D&md5=c54ea1743411ee0a8d4def3bb1303be2CAS |
[13] T. M. McPhillips, S. E. McPhillips, H. J. Chiu, A. E. Cohen, A. M. Deacon, P. J. Ellis, E. Garman, A. Gonzalez, N. K. Sauter, R. P. Phizackerley, S. M. Soltis, P. Kuhn, J. Synchrotron Radiat. 2002, 9, 401.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xotleluro%3D&md5=380f70c3cee5e196922dc18c1fbabce4CAS |
[14] W. Kabsch, J. Appl. Cryst. 1993, 26, 795.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXptFeltw%3D%3D&md5=044d3a66cf8d76e0fc6868b1694ef3bbCAS |
[15] APEXII, v2.1.0, Bruker AXS Ltd., Madison, Wisconsin, 2005.
[16] G. M. Sheldrick, Acta Crystallogr. A 2008, 64, 112.
| Crossref | GoogleScholarGoogle Scholar |
[17] L. J. Barbour, J. Supramol. Chem. 2001, 1, 189.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlOlsb8%3D&md5=b8ca8c15e12e7249c9068cf6390b143aCAS |
[18] I. Krabbes, W. Seicter, T. Breuning, P. Otschik, K. Gloe, Z. Anorg. Chem. 1999, 625, 1562.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvFGks7g%3D&md5=1852f68426354700776a19c687fe5fd0CAS |
[19] L. Mei, J. Li, L. S. Tai, L. S. Song, L. Q. Rong, Z. S. Ming, Inorg. Chem. Commun. 2010, 13, 1009.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1Wnsbo%3D&md5=b3a48f09e5ec68f31f889bc2403e918aCAS |
[20] (a) W. Mazurek, K. J. Berry, K. S. Murray, M. J. O’ Connor, M. R. Snow, A. G. Wedd, Inorg. Chem. 1982, 21, 3071.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xks1Ggu78%3D&md5=5e3d357842dbeb8babc4e03f02b87f78CAS |
(b) F. Birkelbach, M. Winter, U. Flőrke, H.-J. Haupt, C. Butzlaff, M. Lengen, E. Bill, A. X. Trautwein, K. Wieghardt, P. Chaudhuri, Inorg. Chem. 1994, 33, 3990.
| Crossref | GoogleScholarGoogle Scholar |
[21] R. Veit, J. J. Girerd, O. Kahn, F. Roberts, Y. Jeannin, Inorg. Chem. 1986, 25, 4175.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XlvVaktbg%3D&md5=1b33234a659853bb979a119f78748c90CAS |
[22] P. Chaudhuri, M. Winter, B. P. C. Della Védova, E. Bill, A. Trautwein, S. Gehring, P. Fleischauer, B. Nuber, J. Weiss, Inorg. Chem. 1991, 30, 2148.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhvFyntL4%3D&md5=c0959b2b07157197b2ef40eeded5b246CAS |
[23] P. Chaudhuri, Coord. Chem. Rev. 2003, 243, 143.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXns1Witb0%3D&md5=11f7a05edf108f5230e317918f4f04d6CAS |
[24] Z. Boulsourani, V. Tangoulis, C. P. Raptopoulou, V. Psycharis, C. Dendrinou-Samara, Dalton Trans. 2011, 40, 7946.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptleqsbw%3D&md5=7ef5be963d6dd99a752aa59f9f0c35a8CAS |


