Synthesis and Evaluation of a Biocompatible Macromolecular Gadolinium Compound as a Liver-Specific Contrast Agent for MRI
Youyang Zhan A B , Rong Xue A , Mengchao Zhang C , Chuanling Wan A B , Xiaojing Li A D , Fengkui Pei A , Changjiang Sun C and Lin Liu CA Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China.
B University of Chinese Academy of Sciences, Beijing 100049, China.
C China-Japan Union Hospital, Jilin University, Changchun 130033, China.
D Corresponding author. Email: xjli@ciac.ac.cn
Australian Journal of Chemistry 70(3) 307-313 https://doi.org/10.1071/CH16347
Submitted: 7 June 2016 Accepted: 1 September 2016 Published: 26 October 2016
Abstract
A new macromolecular biocompatible gadolinium chelate complex (PAI-N2-DOTA-Gd) as a liver-specific magnetic resonance imaging (MRI) contrast agent was synthesised and evaluated. An aspartic acid–isoleucine copolymer was chemically linked with Gd-DOTA via ethylenediamine to give PAI-N2-DOTA-Gd. In vitro, the T1-relaxivity of PAI-N2-DOTA-Gd (14.38 mmol–1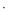 L
L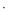 s–1, 0.5 T) was much higher than that of the clinically used Gd-DOTA (4.96 mmol–1
s–1, 0.5 T) was much higher than that of the clinically used Gd-DOTA (4.96 mmol–1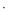 L
L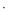 s–1, 0.5 T), with obvious imaging signal enhancement. In the imaging experiments in vivo, PAI-N2-DOTA-Gd exhibited good liver selectivity, and had a greater intensity enhancement (68.8 ± 5.6 %) and a longer imaging window time (30–70 min), compared to Gd-DOTA (21.1 ± 5.3 %, 10–30 min). Furthermore, the in vivo histological studies of PAI-N2-DOTA-Gd showed a low acute toxicity and desirable biocompatibility. The results of this study indicate that PAI-N2-DOTA-Gd is a feasible liver-specific contrast agent for MRI.
s–1, 0.5 T), with obvious imaging signal enhancement. In the imaging experiments in vivo, PAI-N2-DOTA-Gd exhibited good liver selectivity, and had a greater intensity enhancement (68.8 ± 5.6 %) and a longer imaging window time (30–70 min), compared to Gd-DOTA (21.1 ± 5.3 %, 10–30 min). Furthermore, the in vivo histological studies of PAI-N2-DOTA-Gd showed a low acute toxicity and desirable biocompatibility. The results of this study indicate that PAI-N2-DOTA-Gd is a feasible liver-specific contrast agent for MRI.
References
[1] P. Verwilst, S. Park, B. Yoon, J. S. Kim, Chem. Soc. Rev. 2015, 44, 1791.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFSmtL4%3D&md5=b059c26d9576bf29b1d6f9b8ac55649dCAS | 25622561PubMed |
[2] M. P. Lowe, Aust. J. Chem. 2002, 55, 551.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XoslKntL8%3D&md5=624592bb41d93fab2cc0597f9cb43692CAS |
[3] P. Caravan, J. J. Ellison, T. J. McMurry, R. B. Lauffer, Chem. Rev. 1999, 99, 2293.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlt12rsrg%3D&md5=45033f07efe2afd92dc3a0389c6a63c4CAS | 11749483PubMed |
[4] E. Terreno, W. Dastru, D. D. Castelli, E. Gianolio, S. G. Crich, D. Longo, S. Aime, Curr. Med. Chem. 2010, 17, 3684.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVGqu7nM&md5=9fc14bec21d251a8859df343035bc230CAS | 20846110PubMed |
[5] E. J. Werner, A. Datta, C. J. Jocher, K. N. Raymond, Angew. Chem. Int. Ed. Engl. 2008, 47, 8568.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVShsrzI&md5=99cdf6f401db1f32cc157fbe616070d3CAS | 18825758PubMed |
[6] P. Hermann, J. Kotek, V. Kubicek, I. Lukes, Dalton Trans. 2008, 3027.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXms1yht7Y%3D&md5=d7f95fd69ffdbe468f1bb153fb8a9533CAS | 18521444PubMed |
[7] U. T. Lam, R. Yoganathan, A. G. Carr, R. Mammucari, N. R. Foster, Aust. J. Chem. 2012, 65, 40.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFelt7s%3D&md5=f6b0ac45e914c077a32ecf111d94b73aCAS |
[8] K. Chan, W. Wong, Coord. Chem. Rev. 2007, 251, 2428.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeqs73I&md5=eb423210bafc123f14ccdc5e31179f50CAS |
[9] A. J. L. Villaraza, A. Bumb, M. W. Brechbiel, Chem. Rev. 2010, 110, 2921.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkt1amtA%3D%3D&md5=3a4b51b564ae4f8471b24d55226ec35bCAS |
[10] G. Z. Liu, M. K. Tse, M. R. Hill, D. F. Kennedy, C. J. Drummond, Aust. J. Chem. 2011, 64, 617.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFyiu7k%3D&md5=28d39cbd1498001ac3f49e60fcb7b41eCAS |
[11] E. Terreno, D. Delli Castelli, A. Viale, S. Aime, Chem. Rev. 2010, 110, 3019.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFChurw%3D&md5=ba7ed9787cbb35e3155470c1f464c69fCAS | 20415475PubMed |
[12] J. A. Park, Y. J. Lee, I. O. Ko, T. J. Kim, Y. Chang, S. M. Lim, K. M. Kim, J. Y. Kim, Biochem. Biophys. Res. Commun. 2014, 455, 246.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFShtbvF&md5=7d36af104cc609eb841448f4a3f40713CAS | 25449282PubMed |
[13] Y. Miyake, Y. Kimura, N. Orito, H. Imai, T. Matsuda, A. Toshimitsu, T. Kondo, Tetrahedron 2015, 71, 4438.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXnt1Wjsrs%3D&md5=9961b5d340e381b6544d28264b53f262CAS |
[14] G. Bort, S. Catoen, H. Borderies, A. Kebsi, S. Ballet, G. Louin, M. Port, C. Ferroud, Eur. J. Med. Chem. 2014, 87, 843.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslags7%2FL&md5=77772fa6698dbeddbecac760c7952d0aCAS | 25440885PubMed |
[15] R. Patil, P. R. Gangalum, S. Wagner, J. Portilla-Arias, H. Ding, A. Rekechenetskiy, B. Konda, S. Inoue, K. L. Black, J. Y. Ljubimova, E. Holler, Macromol. Biosci. 2015, 15, 1212.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpt1OgtbY%3D&md5=be3b42db3924e99945f8bd31d90da05bCAS | 26036700PubMed |
[16] L. Zhu, Y. Yang, K. Farquhar, J. Wang, C. Tian, J. Ranville, S. G. Boyes, ACS Appl. Mater. Interfaces 2016, 8, 5040.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtV2msLc%3D&md5=1a9d2b2bc4ebe6c5469546c8ac9dbc6cCAS | 26790986PubMed |
[17] Y. Chen, Q. Zhu, Y. Tian, W. Tang, F. Pan, R. Xiong, Y. Yuan, A. Hu, Polym. Chem. 2015, 6, 1521.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitValu7jJ&md5=25cf9b7ae93c437961dd12c8d90d0556CAS |
[18] K. C. Yu, Y. Hamdan, F. X. Wan, Y. X. Li, K. X. Huang, J. L. Zhou, Aust. J. Chem. 2007, 60, 218.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjvFKju7g%3D&md5=7fd5aa9dcb7493b15529ebd4629a870cCAS |
[19] B. Nottelet, V. Darcos, J. Coudane, Eur. J. Pharm. Biopharm. 2015, 97, 350.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFCqur3L&md5=0f4ad9ea8677f86cca273b086bcd309dCAS | 26614557PubMed |
[20] B. Porsio, L. Lemaire, S. El Habnouni, V. Darcos, F. Franconi, X. Garric, J. Coudane, B. Nottelet, Polymer 2015, 56, 135.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVWhurrJ&md5=fef33eb35800656f745144b11e8b897fCAS |
[21] G. P. Yan, L. Robinson, P. Hogg, Radiography 2007, 13, e5.
| Crossref | GoogleScholarGoogle Scholar |
[22] J. Bryson, J. W. Reineke, T. M. Reineke, Macromolecules 2012, 45, 8939.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1KnurjK&md5=9fefb68039e5c7a7f4c902eab6233020CAS | 23467737PubMed |
[23] L. N. Goswami, Q. Cai, L. Ma, S. S. Jalisatgi, M. F. Hawthorne, Org. Biomol. Chem. 2015, 13, 8912.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFOlu7jM&md5=05ab0b223a2396c1512cb0c85d4083bdCAS | 26204958PubMed |
[24] Y. Xiao, R. Xue, T. You, X. J. Li, F. K. Pei, Magn. Reson. Imaging 2015, 33, 822.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmtlelsro%3D&md5=41c144cd08eca635d3024d640830608bCAS | 25839395PubMed |
[25] Y. Xiao, R. Xue, T. You, X. J. Li, F. K. Pei, X. Wang, H. Lei, Carbohydr. Res. 2014, 395, 9.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1Gru73J&md5=70de6a2265589ddf127868e04e2a965bCAS | 24995911PubMed |
[26] H. Yim, S. G. Yang, Y. S. Jeon, I. S. Park, M. Kim, D. H. Lee, Y. H. Bae, K. Na, Biomaterials 2011, 32, 5187.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtlehtrs%3D&md5=f4ba518dc44420a43fc086540c5539a2CAS | 21561660PubMed |
[27] S. Carron, Q. Y. Li, L. Vander Elst, R. N. Muller, T. N. Parac-Vogt, J. A. Capobianco, Dalton Trans. 2015, 44, 11331.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXptFClsLY%3D&md5=59f104c5e3ba6b4a207ab0246697b1feCAS | 26011519PubMed |
[28] C. Guanci, R. Pinalli, S. Aime, E. Gianolio, L. Lattuada, G. B. Giovenzana, Tetrahedron Lett. 2015, 56, 1994.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjslChtbo%3D&md5=dccb8a5f4d010134c9788fa7c75f85fcCAS |
[29] X. Lin, Q. Zhang, J. Chen, X. Kong, L.-S. Long, C. Wang, W. Lin, RSC Adv. 2015, 5, 2914.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvF2rs7%2FF&md5=2e4b914f33767bd4cc6b83a364e24a7fCAS |
[30] M. Perrier, A. Gallud, A. Ayadi, S. Kennouche, C. Porredon, M. Gary-Bobo, J. Larionova, C. Goze-Bac, M. Zanca, M. Garcia, I. Basile, J. Long, J. de Lapuente, M. Borras, Y. Guari, Nanoscale 2015, 7, 11899.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXnt1WntLs%3D&md5=00ba8bec88785e293db26ce51f119338CAS | 25967733PubMed |
[31] G. Sun, J. Feng, F. Jing, F. Pei, M. Liu, J. Magn. Magn. Mater. 2003, 265, 123.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXms1Slsrs%3D&md5=078d94c8ccc9b940b98d82d282f48462CAS |
[32] D. Ling, W. Park, S. J. Park, Y. Lu, K. S. Kim, M. J. Hackett, B. H. Kim, H. Yim, Y. S. Jeon, K. Na, T. Hyeon, J. Am. Chem. Soc. 2014, 136, 5647.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXlsFKqtbg%3D&md5=dcdf5546b7d8f77b95b02c01f228d824CAS | 24689550PubMed |
[33] Y. Song, Y. J. Kang, H. Jung, H. Kim, S. Kang, H. Cho, Sci. Rep. 2015, 5, 15656.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslans7nI&md5=ef50b9b0e247a409abd7e65551b63dc2CAS | 26493381PubMed |
[34] J. Li, Y. Hu, J. Yang, W. Sun, H. Cai, P. Wei, Y. Sun, G. Zhang, X. Shi, M. Shen, J. Mater. Chem. B 2015, 3, 5720.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpsFensLw%3D&md5=57dbf73b1ef594a18d9fb3639494a18cCAS |


