Wood Protection Properties of Quaternary Ammonium Arylspiroborate Esters Derived from Naphthalene 2,3-Diol, 2,2′-Biphenol and 3-Hydroxy-2-naphthoic Acid
Jenny M. Carr A , Peter J. Duggan B G , David G. Humphrey A E , James A. Platts C and Edward M. Tyndall D FA CSIRO Materials Science and Engineering, Private Bag 33, Clayton South MDC, Vic. 3169, Australia.
B CSIRO Molecular and Health Technologies, Private Bag 10, Clayton South, Vic. 3169, Australia.
C School of Chemistry, Cardiff University, Cardiff CF10 3AT, UK.
D Centre for Green Chemistry, Monash University, Melbourne, Vic. 3800, Australia.
E Present address: Arch Wood Protection (Aust) Pty Ltd, Unit 3, Aerolink Business Park, 85–91 Keilor Park Drive, Tullamarine, Vic. 3043, Australia.
F Present address: Biota Holdings Ltd, 10/585 Blackburn Road, Notting Hill, Vic. 3168, Australia.
G Corresponding author. Email: peter.duggan@csiro.au
Australian Journal of Chemistry 63(10) 1423-1429 https://doi.org/10.1071/CH10132
Submitted: 19 March 2010 Accepted: 13 May 2010 Published: 1 October 2010
Abstract
In continuation of a program aimed at developing a boron-based, high performing and environmentally benign wood preservative suitable for outdoor use, three lipophilic tetra-n-butylammonium spiroborates, tetra-n-butylammonium bis[naphthalene-2,3-diolato(2-)-O,O′]borate 4, tetra-n-butylammonium bis[2,2′-biphenolato(2-)-O,O′]borate 5 and tetra-n-butylammonium bis[3-hydroxy-2-naphthoato(2-)-O,O′]borate 6 were prepared and tested. The higher molecular weight and lipophilicity of these borates compared with related borates previously examined correlates, in the case of 5 and 6, with significantly enhanced leach resistance while termiticidal activity has been maintained. The racemic spiroborate derived from 2,2′-biphenol 5, in particular, appears to be close to an optimum balance between ease of synthesis, solubility, hydrolytic stability and termiticidal activity.
Introduction
Wood has a low energy and environmental cost compared with alternatives such as plastic, concrete, aluminium or steel. If produced using responsible forestry practices, wood thus has a highly favourable environmental profile, which together with its practical advantages continues to make its use as a renewable building material highly attractive. As a natural product, however, wood is susceptible to decay by wood-destroying fungi and insects and so it is necessary to preserve this material in order to extend its useful life. The single most significant development in wood preservation occurred in 1933, with the invention of copper chrome arsenate (CCA), which although not popularized until the 1970s, has become the cheapest and most widespread preservative system used today.[1,2] Concerns over the carcinogenic properties of the arsenic contained in CCA, however, has led to significant restrictions on the use of CCA-treated timber in Europe, North America, parts of Asia and more recently Australia. Since March 2006, CCA has been banned in Australia for use in timber that has frequent and intimate human contact, such as that used for playground equipment, picnic tables, handrails, decking boards, garden furniture and exterior seating.[3]
Preservatives based on boron oxides are regarded as highly feasible green alternatives to CCA because they have a low mammalian toxicity while maintaining good activity against wood-degenerating termites and fungi.[4,5] As wood preservatives, borates also offer the advantages of being odourless, colourless and at certain minimum retentions, fire retardant. Boron compounds, such as boric acid and sodium tetraborate, have been used successfully for over 30 years in Australia, New Zealand and Europe, and enjoy significant and increasing use today. They are, however, restricted to indoor applications or need to be used in combination with other systems,[6] because the borate systems used commercially are highly water soluble and are readily leached when the treated timber comes into contact with moisture.
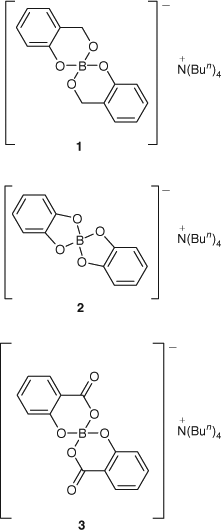
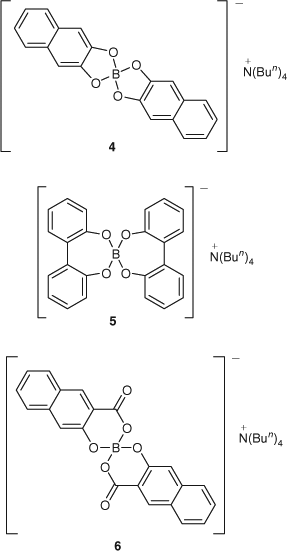
As part of a program aimed at the development of leach resistant boron-based wood preservatives, the synthesis, biocidal activity and permanence in timber of a series of spiroborate esters are being investigated. The first three borate esters to be investigated in this way are shown in Fig. 1.[7,8] All three of these esters were found to be more resistant to aqueous leaching from timber than boric acid, with the salicylate, 3, performing best in this regard. The complexation of borate in this way was expected to lead to a drop in biocidal activity,[5] as it should restrict the release of the biologically active free borate. All three spiroborates, however, were actually found to be more active than boric acid in both fungal and termite assays. Consideration of the lipophilicity of these compounds based on calculated polar surface areas and measured hydrolytic stability led to the observation that the termiticidal activity of these compounds correlated with lipophilicity rather than susceptibility to hydrolysis, whereas leach resistance correlated with hydrolytic stability rather than lipophilicity. It was concluded that important timber preservative properties such as permanence, delivery to the biological site of action in the wood decaying organism, and activity at this biological site have conflicting physiochemical requirements, and the best preservative will meet a balance between these properties. In order to understand these findings further, the compounds shown in Fig. 2, which are more lipophilic analogues of 2 and 3, have been prepared and tested for termiticidal activity and leach resistance, and the results compared with calculated lipophilicities and measured hydrolytic stabilities. The method used to increase the lipophilicity of these borates involved the extension of the borate ligands by one phenyl moiety each, but in such a way that the chemical reactivity should be largely unaffected. Compounds 4 and 6 are direct analogues of 2 and 3, respectively, where the ligand has been extended by a fused phenyl ring. Compound 5 is related to the borate derived from catechol 2 but is distinct in that it contains 7-membered borate rings and is stereoisomeric, having a chiral axis at each 2,2′-biphenolate ligand.
|
|
Results
Synthesis of Borate Esters
The three spiroborate esters used in this investigation 4–6, were prepared following methods similar to those previously described;[8] two equivalents of the dihydric compound (naphthalene-2,3-diol, 2,2′-biphenol and 3-hydroxy-2-naphthenoic acid, respectively) were added to an aqueous solution of tetra-n-butylammonium borate and the product collected and recrystallized from an organic solvent. Following this approach, analytically pure bis[naphthalene-2,3-diolato(2-)-O,O′]borate 4, bis[2,2′-biphenolato(2-)-O,O′]borate 5 and bis[3-hydroxy-2-naphthoato(2-)-O,O′]borate 6 were obtained as tetra-n-butylammonium salts in 35%, 64% and 50% yield, respectively.
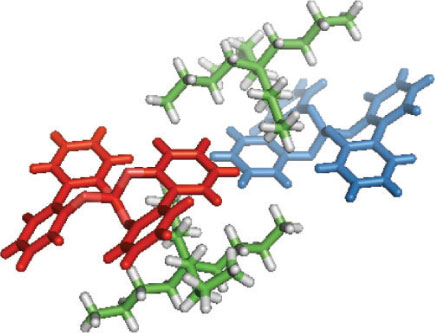
The borate derived from 2,2′-biphenol can exist in three diastereomeric forms; meso-5, (S,S)-5 and (R,R)-5, however the proton and carbon NMR spectra obtained of the recrystallized product showed only a single set of signals, suggesting that this purified product was either meso-5 or a racemic mixture of (S,S)-5 and (R,R)-5, i.e. rac-5. X-ray crystallography performed on a single crystal of 5 provided a structure in close agreement with that recently published by Wuest and coworkers,[9] in that the crystal belonged to the monoclinic space group P21/c, with the asymmetric unit comprising a tetra-n-butylammonium cation with a single bis(2,2′-biphenolato)borate anion. The crystal was found to correspond to rac-5 with opposite enantiomers alternating along the c axis. The arrangement of (S,S)-5 and (R,R)-5 within the crystal, as determined by X-ray crystallography, is shown in Fig. 3.
|
Protection of Wood Against Termite Attack
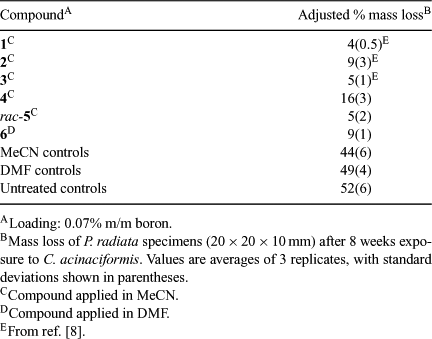
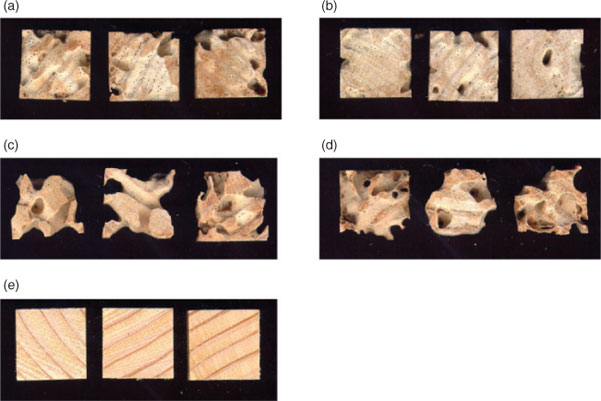
The activity of 4–6 against C. acinaciformis was examined in a termite bioassay using P. radiata sapwood specimens treated with 0.07% m/m boron as previously described.[8] After 8 weeks, the specimens were recovered and the extent of attack determined by weight loss. The results are summarized in Table 1 and selected images of the blocks after treatment are shown in Fig. 4. It can be seen that good protection against termite attack is provided by compounds 4–6, relative to solvent and untreated controls, with the performance of rac-5 and 6 being in the same range of that previously reported for the borates bearing monocyclic ligands 1–3.* All three of these previously investigated compounds have been shown to be more active than boric acid in termite bioassays. The spiroborate derived from naphthalene-2,3-diol, 4, was not quite as effective as rac-5 and 6.
|
|
Boron Fixation
The resistance of 4–6 to leaching from timber by water, or boron fixation, was determined using a standard leaching test as previously described.[8] The procedure involves saturating the preservative-treated specimens of uniform dimension with water and shaking them in jars containing a fixed volume of water at 35°C for 5 days, with daily changes of water. The resistance to leaching of each compound is then determined through chemical analysis of both leached and unleached samples. The leaching results obtained with 4–6 are shown in Table 2 together with those previously determined for 1–3. It can be seen that the borates derived from 2,2′-biphenol rac-5 and 3-hydroxy-2-naphthenoic acid 6 provided strong boron fixation relative to boric acid and the previously investigated borates, with compound 6 in particular providing almost complete retention of boron in the timber. This result is particularly impressive given the relatively severe conditions employed in the leaching test.

|
Lipophilicity Calculations
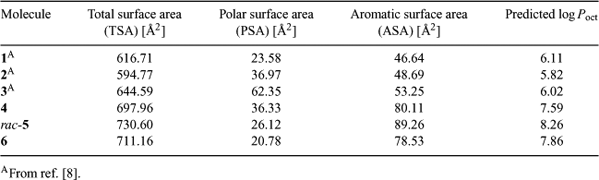
The lipophilicity of the spiroborates is expected to influence both their termiticidal bioavailability and permanence in timber, or leach resistance. The experimental determination of lipophilicity typically involves partitioning the test compound between octanol and water to provide a log P-value. The susceptibility to hydrolysis of many borate esters, however, might complicate such an experiment so a theoretical method for predicting log P-values[10] has therefore been employed. As previously described,[8] the method uses calculated total, polar and aromatic molecular surface areas to generate a predicted log Poct.† The results of these calculations for compounds 4–6 are shown in Table 3, together with those previously determined for compounds 1–3. As expected, the calculated log Poct for compounds 4–6 are significantly higher than those determined for compounds 1–3, with the borate derived from 2,2′-biphenol rac-5 giving the highest predicted log Poct.
|
Stability of Borate Esters – Equilibrium Constants for Formation
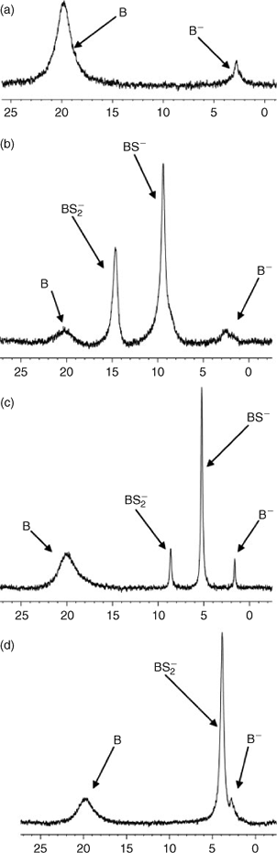
Attempts to measure the hydrolysis rates of pre-formed spiroborates did not produce suitable data, mainly because many spiroborates examined in this way showed no or very limited hydrolysis under all conditions employed, including elevated temperatures. Instead, the relative stability of the spiroborates 4–6 was determined through examination of ester formation using a 11B NMR method specifically developed for the purpose. The method has been described in detail elsewhere, including an extensive discussion on NMR peak assignments.[8] It involves incubating d6-DMSO solutions of boric acid, tetra-n-butylammonium hydroxide and the appropriate dihydric substrate in equimolar concentrations at 30°C for 24 h, then recording a 11B NMR spectrum at 128.4 MHz. Fig. 5a shows a spectrum obtained with a 1:1 solution of boric acid and tetra-n-butylammonium hydroxide. Figs 5b–d show typical spectra obtained when one equivalent of naphthalene-2,3-diol, 2,2′-biphenol or 3-hydroxy-2-naphthenoic acid, respectively is included in the solution. Peaks corresponding to all boron-containing species involved in the equilibrium can be seen in spectra of solutions prepared with naphthalene-2,3-diol and 2,2′-biphenol. In line with results previously obtained with salicylic acid, however, the 2:1 borate ester formed with 3-hydroxy-2-naphthenoic acid 6 appears to be very stable – such that no 1:1 borate ester could be detected in this case.
|
Integration of the NMR signals due to free borate (B and B–) and the 1:1 (BS–) and 2:1 borate esters (BS2–) allows the concentration of all species involved in the equilibrium to be determined. This was not possible in the case of 3-hydroxy-2-naphthenoic acid, however, as the 1:1 borate ester could not be detected. The calculated concentrations obtained from solutions containing naphthalene-2,3-diol and 2,2′-biphenol are shown in Table 4. These values can be used to estimate the magnitude of the equilibrium constants involved in the ester formation process and thus provide a measure of the relative stability of the borate esters involved.[8] The following expressions can be used to describe the equilibria involved:
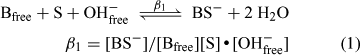
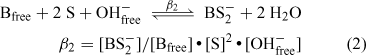
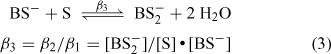

|
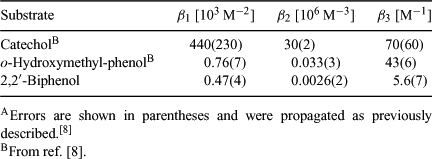
where [Bfree] is the concentration of free borate = ([B] + [B–]), [OHfree–] is the concentration of free hydroxide ions, [BS–] is the concentration of the 1:1 ester, [BS2–] is the concentration of the 2:1 ester and [S] is the concentration of free substrate. The calculated [S] for the naphthalene diol solution was near zero (Table 4) and although suggesting significant ester formation, this precluded the calculation of the corresponding equilibrium constants for this case. Thus the data shown in Table 4 can only be used to estimate β1, β2 and β3 for the equilibria involving 2,2′-biphenol. These results are shown in Table 5, along with corresponding data previously determined with catechol and o-hydroxymethyl-phenol. It should be noted that the method used here was developed for the rapid comparison of the stability of a range of borates, with equilibrium constants being calculated at a single stoichiometry. A more accurate determination of β1–3 would require the collection of data from solutions containing a wide range of substrate to boron ratios, and the measurement or estimation of solution acidity and substrate ionization constants.
|
The data obtained for borates 4–6 together with that previously reported for 1–3 indicate that of the six spiroborate esters studied in detail, 3 and 6 are the most stable (large β2 and very large β3) and rac-5 is the least stable. Although estimates of β2 and β3 could not be obtained for the borate derived from 2,3-naphthalene diol 4, the ratio of the various boron-containing species present in the solution containing this diol resemble closely those found for catechol (data not shown),[8] implying that spiroborates 2 and 4 possess very similar stabilities. The high stability of 2:1 salicylate-type esters 3 and 6 may arise from the strong delocalization of electron density through the carbonyl and aromatic π systems and the favourable planar geometry of the free substrate. Presumably good orbital overlap and partial charge delocalization is also possible with the catechol-type borates 2 and 4, and together with entropic advantages provided by the rigid arrangement of the oxygen atoms in the precursor diols, may account for their improved stability over borates 1 and rac-5.
This investigation is the first to report on the stabilities of 1:1 and 2:1 esters derived from 2,2′-biphenol, which both exist in significant quantities in the DMSO system studied. Attempts to form seven-membered ring borate esters from 1,4-diols reported in the literature have shown that esterification is more favourable for diols with low internal rotational freedom. For example, 1,2-(dihydroxymethyl)benzene was observed to form a 1:1 borate ester, whereas no ester was observed for butane-1,4-diol, even at high diol-borate ratios.[11] Although the DMSO system used here cannot be compared directly with the aqueous systems described in the literature, the facile formation of 1:1 and 2:1 esters from 2,2′-biphenol relative to more flexible 1,4-diols can be attributed to an entropic advantage of this substrate’s rigid aromatic structure. The flexible nature of 2,2′-biphenol and its arrangement of oxygen atoms do not match the very strong entropic advantages for borate formation possessed by the catechol-type diols, which might account for the lower stability of rac-5, relative to 2 and 4.
Discussion
The main aim of this study was to measure the effect of increasing the lipophilicity of spiroborates on termiticidal activity and permanence in timber. Relative to previously studied spiroborates 2 and 3, lipophilicity enhancement was achieved by adding a second benzene ring to each borate ligand. This was done by choosing naphthyl analogues 4 and 6, and the biphenyl analogue rac-5. These three spiroborates were readily prepared in an aqueous medium through the addition of the relevant dihydric substrate to a solution of tetra-n-butylammonium borate. The borate ester derived from 2,2′-biphenol rac-5 was obtained as a racemic mixture of the (S,S) and (R,R) enantiomers. It is possible that these stereoisomers would have differing biological activity but for the current application, this appears to be unlikely as this particular spiroborate probably readily hydrolyzes on ingestion by the target organism. The lower stability of rac-5 in moist media may also lead to ready racemisation within the timber substrate.
As expected, the predicted lipophilicities of 4–6 are significantly higher than their direct analogues 2 and 3, with calculated log Poct’s for 4–6 in the range of 7.6–8.3 cf. 5.8–6.0 for 2 and 3. This increase in molecular size and lipophilicity had a limited effect on termidicial activity for rac-5 and 6, which retained good potency relative to 2 and 3, whereas the spiroborate derived from 2,3-naphthalene diol 4 showed a slight drop-off in activity. It is well known from drug discovery research that the relationship between biological activity and lipophilicity can often follow a bell-shaped relationship, with the insolubility of the more lipophilic bioactives in aqueous media ultimately limiting their efficacy. This could account for the slightly lower activity of 4. It is difficult to make definitive conclusions about the termiticidal activities of 2–3 relative to 4–6, however, because the organic precursors of 4–6 possess inherent termiticidal and/or termitistat activity.
The modifications that led to rac-5 and 6 also produced impressive improvements in leach resistance, with 97% of boron from 6 and 56% of the boron from rac-5 remaining in the timber substrate under the severe conditions employed in the test. Interestingly, 4 gave an equivalent result to its direct catechol analogue 2 in this test. The results obtained using a 11B NMR method to quantify the boron-containing species in moist d6-DMSO suggest that the stabilities of the naphthyl borates 4 and 6 are very similar to their direct analogues 2 and 3, whereas the spiroborate derived from 2,2′-biphenol rac-5 is significantly less stable.
Conclusions
The optimal borate wood preservative will possess high biological activity, be highly resistant to leaching from timber and be easy to prepare and apply. The biological activity and leach resistance properties are expected to reflect a balance between hydrophobicity and resistance to hydrolysis, although exactly how all of these properties are related is still unclear. The results from earlier work[8] suggested that the most hydrophobic borates would show the most biological activity and the most stable would be most resistant to leaching. These apparent correlations are not evident in the results obtained here with the significantly more lipophilic borates 4–6. While these compounds apparently have very similar lipophilicities, they show diverse termiticidal activity and permanence, and even though rac-5 is the least stable of the six spiroborates investigated so far, it shows excellent leach resistance. The fact that the dihydric ligands in 4–6 have some inherent termiticidal and/or termitistat activity makes further interpretation of the termite data obtained with these borate esters difficult.
Overall, the modifications that produced rac-5 and 6 led to excellent permanence in timber while retaining good termite activity. Both of these compounds warrant further investigation, but the slightly higher bioactivity and the better solubility properties of rac-5‡ make it the most promising lead.
Experimental
General
The general experimental methods used in this study were the same as those previously described.[7] Termite bioassays and accelerated leaching tests were conducted according to industry standards,[12] and are described in detail elsewhere[8] and in the associated supplementary information, as are the methods used for lipophilicity calculations and stability measurements.
Tetra-n-butylammonium Bis[naphthalene-2,3-diolato(2-)-O,O′]borate 4
Boric acid (2.0 g, 32 mmol) was dissolved with warming in an aqueous solution of tetra-n-butyl ammonium hydroxide (32 mmol, 21.2 mL 40% w/v solution in H2O) and a further 10 mL water. Naphthalene-2,3-diol (10.4 g, 64 mmol) was added in portions to the stirring solution at 30–40°C over a period of 30 min. An off-white precipitate formed which was collected by filtration and further product was obtained by allowing the filtrate (a purple solution) to concentrate under ambient conditions. The crude product was dried at 110°C then recrystallized from THF to give the title spiroborate (6.4 g, 35%) as off-white flakes, mp 236–238°C. νmax (KBr)/cm–1 2965m, 2874w, 1606w, 1465s, 1250s, 1224w, 1161m, 1091s, 1047vs, 934m, 900m, 849m, 742m, 703m. δH (300 MHz, d6-DMSO) 0.93 (t, 3J 7.2, 12H, N(CH2CH2CH2CH3)4), 1.28 (sextet, 6J 7.2, 8H, N(CH2CH2CH2CH3)4), 1.46–1.62 (m, 8H, N(CH2CH2CH2CH3)4), 3.13 (t, 3J 8.4, 8H, N(CH2CH2CH2CH3)4), 6.86 (s, 4H, H-1), 7.14 (dd, J6,5 6.0, J6,8 3.3, 4H, H-6), 7.56 (dd, J5,6 6.0, J5,7 3.3, 4H, H-5). δC (75 MHz, d6-DMSO) 13.6 (N(CH2CH2CH2CH3)4), 19.3 (N(CH2CH2CH2CH3)4), 23.1 (N(CH2CH2CH2CH3)4), 57.5 (N(CH2CH2CH2CH3)4), 102.0 (C-1), 121.7 (C-6), 125.7 (C-5), 129.2 (C-8-C(q)-C-1), 152.4 (C-2). δB (160 MHz, d6-DMSO) 14.2. m/z (ESI) 327.1 (M–, C20H12BO4–, 100%). Anal. Calc. for C36H48BNO4: C 75.9, H 8.5, N 2.5. Found: C 75.8, H 8.3, N 2.4%.
Tetra-n-butylammonium Bis[2,2′-biphenolato(2-)-O,O′]borate, rac-5
Boric Acid (2.4 g, 39 mmol) was dissolved with warming in an aqueous solution of tetrabutyl-n-ammonium hydroxide (39 mmol, 25.4 mL 40% w/v solution in H2O) and a further 10 mL water. The resulting solution was heated to 60°C and 2,2′-biphenol (14.5 g, 78 mmol) was added in portions with stirring over 10 min. The resulting slurry was heated with stirring to 80°C for 30 min to yield an off-white, water-insoluble solid. The mixture was allowed to cool, then filtered and the crude product was dried in air then dissolved in boiling methanol (800 mL). The solution was allowed to cool to RT and then left for several days, after which time the pure spiroborate (11.7 g) crystallized as large colourless blocks. The filtrate was reduced under reduced pressure on the rotary evaporator to a total volume of approx. 200 mL by which time a precipitate was observed. The mixture was then heated to boiling then allowed to cool, yielding more of the spiroborate (3.9 g) as white needles (combined yield 64%), mp 285–287°C (lit.[9] 229°C). νmax (KBr)/cm–1 2961 m, 2874w, 1493 m, 1479 m, 1297w, 1262s, 1096w, 1038s, 989vs, 950s, 754s, 725 m. δH (300 MHz; d6-DMSO) 0.91 (t, 3J 7.2, 12H, N(CH2CH2CH2CH3)4), 1.27 (sextet, 6J 7.2, 8H, N(CH2CH2CH2CH3)4), 1.45–1.65 (m, 8H, N(CH2CH2CH2CH3)4), 3.14 (t, 3J 8.4, 8H, N(CH2CH2CH2CH3)4), 6.87 (dd, J3,5 1.2, J3,4 8, 2H, H-3), 6.95 (ddd, J5,3 1.2, J5,4 7.5, J5,6 1.2, 2H, H-5), 7.23 (ddd, J4,5 7.5, J4,3 8, J4,6 1.8, 2H, H-4), 7.32 (dd, J6,4 1.8, J6,5 7.5, 2H, H-6). δC (75 MHz, d6-DMSO) 13.5 (N(CH2CH2CH2CH3)4), 19.2 (N(CH2CH2CH2CH3)4), 23.1 (N(CH2CH2CH2CH3)4), 57.5 (N(CH2CH2CH2CH3)4), 119.7 (C-3, C-3′), 121.9 (C-5, C-5′), 127.5 (C-4, C-4′), 128.0 (C-6, C-6′), 131.6 (C-1, C-1′), 156.9 (C-2, C-2′). δB (160 MHz, d6-DMSO) 8.2. m/z (ESI) 379.2 (M–, C24H16BO4–, 100%), 184.8(10). Anal. Calc. for C40H52BNO4: C 77.3, H 8.4, N 2.2. Found: C 77.3, H 8.7, N 2.1%.
Crystal Data: Single crystal from THF. C40H52O4NB, M = 621.64, monoclinic, a = 11.1640(2), b = 16.3623(3), c = 19.2273(4) Å, β = 90.660(1)°, U = 3512.0(1) Å3, T = 123(2) K, space group P21/c (no. 14), Z = 4, µ(Mo-Kα) = 0.074 mm–1, 48741 reflections measured, 8368 unique (Rint = 0.1101) which were used in all calculations. The final wR(F2) was 0.1168 (all data).
Tetra-n-butylammonium Bis[3-hydroxy-2-naphthoato(2-)-O,O′]borate 6
Boric Acid (0.20 g, 3.2 mmol) was dissolved with warming in an aqueous solution of tetra-n-butylammonium hydroxide (3.2 mmol, 2.1 mL 40% w/v solution in H2O) and a further 5 mL water. The resulting solution was heated to 60°C and 3-hydroxy-2-naphthenoic acid (1.2 g, 6.4 mmol) was added in portions with stirring over 10 min. The resulting slurry was heated with stirring to 80°C for 30 min to yield an off-white solid. The mixture was filtered and the crude product was dried in air at RT and recrystallized from boiling THF/CH3CN (1:1) to give the title spiroborate (1.0 g, 50%) as a straw-coloured crystalline solid, mp 223–224°C. νmax (KBr)/cm–1 2962m, 2936m, 2875w, 1702vs, 1688vs, 1633s, 1604m, 1499m, 1459s, 1382w, 1348vs, 1291s, 1253vs, 1216s, 1172w, 1151m, 1134m, 1092s, 1072s, 1011m, 959m, 791w, 771w, 742w. δH (300 MHz d6-DMSO) 0.92 (t, 3J 7.2, 12H, N(CH2CH2CH2CH3)4), 1.28 (sextet, 6J 7.2, 8H, N(CH2CH2CH2CH3)4), 1.47–1.63 (m, 8H, N(CH2CH2CH2CH3)4), 3.14 (t, 3J 8.4, 8H, N(CH2CH2CH2CH3)4), 7.22 (s, 2H, H-4), 7.32 (ddd, J7,6 6.9, J7,8 8, J7,5 1.2, 2H, H-7), 7.48 (ddd, J6,7 6.9, J6,5 8.3, J6,8 1.2, 2H, H-6), 7.73 (d, J5,6 8.3, 2H, H-5), 7.97 (d, J8,7 8, 2H, H-8), 8.47 (s, 2H, H-1). δC (75 MHz, d6-DMSO) 13.5 (N(CH2CH2CH2CH3)4), 19.2 (N(CH2CH2CH2CH3)4), 23.1 (N(CH2CH2CH2CH3)4), 57.5 (N(CH2CH2CH2CH3)4), 111.6 (C-4), 117.4 (C-2), 123.2 (C-7), 125.8 (C-5), 126.9 (C-8-C(q)-C-10), 128.0 (C-6), 129.0 (C-8), 123.0 (C-1), 137.1 (C-5-C(q)-C-4), 154.7 (C-3), 163.4 (CO). δB (160 MHz, d6-DMSO) 3.4. m/z (ESI) 383.2 (M–, C22H12BO6–, 30%), 186.6(100), 142.6(15), 616.6(10), 812.7(5). Anal. Calc. for C38H48BNO6: C 73.0, H 7.7, N 2.2. Found: C 73.2, H 8.0, N 2.1%.
Accessory Publication
Details of the validation of AM1 for use in log Poct calculations are available from the Journal’s website.
Acknowledgements
This work was funded by the Australian Research Council, Monash University and the former CSIRO Forestry and Forest Products. E.M.T. is a recipient of an Australian Postgraduate Award. Kate Cavanagh is thanked for technical assistance.
[1]
[2]
D. Humphrey,
Rev. Inorg. Chem. 2002, 22, 1.
[accessed February 2010].
[4]
A. Peylo,
H. Willeitner,
Holz Roh-Werkst. 2001, 58, 476.
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |

[12]
* In contrast to results obtained with 1–3, control experiments run with naphthalene-2,3-diol, 2,2′-biphenol and TBA 3-hydroxy-2-naphthenoate in the cellulose paper termite bioassay,[8] suggest that these compounds actually have higher termiticidal and/or termitistat activity than boric acid, but less than the most active borate esters. Hence the termiticidal activity of 4–6 cannot be entirely attributed to the boron content of these compounds.
† As described in ref. [8] several semi-empirical methods were tested for their ability to reproduce the X-ray structure of spiroborate 1. AM1 was found to give the correct spiroborate structure, with BO bond lengths within 0.05 Å of experimental values. This procedure was repeated for spiroborate 5 and similar agreement was found. See Accessory Publication.
‡ Spiroborate 6 is insoluble in many solvents and was applied to timber in DMF.


