N-Heterocyclic Carbenes
Anthony J. ArduengoA Department of Chemistry, The University of Alabama, 3068 Shelby Hall, Tuscaloosa, Alabama 35487-0336, USA. Email: aj@ajarduengo.net

Anthony J. Arduengo, III is presently the Saxon Professor of Chemistry at the University of Alabama in Tuscaloosa. He completed his B.Sc.in chemistry cum laude at the Georgia Institute of Technology in 1974 and his Ph.D. two years later from the same institution. His independent research career began in DuPont's CRD and he has moved several times during his career between academics and industry. He has held positions with the University of Illinois, DuPont, the Technical University – Braunschweig, Germany, and the University of Alabama. Research activities in his group focus broadly in the area of compounds with unusual valency. His present research programs include efforts directed toward chemical hydrogen storage, materials for nonlinear optical applications, and organic photovoltaics. |
Australian Journal of Chemistry 64(8) 1106-1108 https://doi.org/10.1071/CH11249
Published: 19 August 2011
Scientific research offers many rewards and benefits that are highly sought in any activity – sharing ideas, interests, and experiences with like-minded individuals; the excitement of the quest for an understanding of the unknown and the function of the surrounding world; the satisfaction of achievement; and the generation of new knowledge upon which others may build. The study of the compounds with unusual valency has been and continues to be just such a productive and rewarding activity.
In chemistry, the study of compounds with unusual valency offers researchers a rapid path ‘out of the box’ of the science's conventional wisdom. The traditional grasp of how things are, how things function, and what one should anticipate from molecule–molecule interactions and reactions, or matter–energy interactions is best tested in this realm of the unusual. Accepted ideas are most easily pushed to their limits (and beyond) when applied to that which appears unusual by accepted standards.
Carbenes fall within this area of compounds with unusual valency. These compounds contain a divalent carbon that, formally, violates the octet rule. Carbene chemistry has its roots in much simpler times when there was no real cause to be alarmed or concerned about such compounds because there was no octet rule to violate. Hence early chemical research seeking to isolate and study stable carbenes was reasonable. The octet rule has its origins at the turn of the 20th century in Abegg’s rule[ 1 ] and Lewis's cubical atom.[ 2 ] However, recognizable ‘carbene’ research started some sixty five years earlier with the attempts of Dumas to isolate and characterize methylene (Scheme 1).[ 3 ]
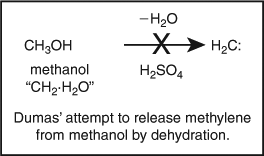
|
The progress of carbene research from its origins has been recounted elsewhere and is left to the reader to retrace in detail;[ 4 ] here I only intend to briefly summarize those earlier events. The typically high reactivity of carbenes and lack of suitable spectroscopy methods or laboratory techniques to handle these apparently transient species led to a widespread view that carbenes were too reactive to be isolated as stable entities, but were, nonetheless, important intermediates in some chemical transformations. This view of extreme reactivity could be further supported by reliance on the octet rule. By the late 20th century, the view of carbenes was beginning to change again. Experimental techniques had improved substantially and new spectroscopic techniques, beyond the reach of early carbene researchers, were becoming common-place. There was hope that transient carbene species could at least be captured briefly and studied if not placed in a bottle. Beginning in 1960 Wanzlick reported the chemistry of imidazolines and (to a lesser extent) imidazoles in the quest for stable carbenes.[ 5 ] Unfortunately, Wanzlick's efforts were contemporaneous with other rather spectacular experimental mishaps that laid false claims to the isolation of simple singlet carbenes.[ 6 ] Wanzlick's experiments were perhaps nearly capable of producing a ‘bottle-able’ carbene,[ 7 ] but the scientific environment and culture in his time was still not welcoming to his views. Publications suggesting flaws or oversights in his work kept a truly ‘bottle-able’ carbene just beyond the community's grasp.[ 8 ] Nonetheless, Wanzlick, Öfele, Lappert, Huttner and others published chemistry that showed that in situ generated imidazol(in)-2-ylidenes give rise to interesting new compounds (Scheme 2).
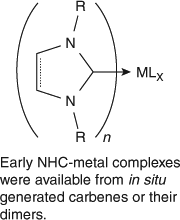
|
Chemistry of the main group elements sulfur and phosphorus opened another opportunity to approach the quest for carbenes. Seppelt[ 9 ] and Bertrand[ 10 ] employed the elements of sulfur and phosphorus to construct molecules in which the conceptual ‘resonance’ relationship between α-dicarbenes and acetylenes could be perturbed by the introduction of the heavier main group elements. These pioneering studies provided thiaacetylenes[ 9 ] and phosphaacetylenes[ 10 ] that exhibited ‘carbene-like’ reactivity, thus offering the first entries into persistent singlet carbenes (Scheme 3).
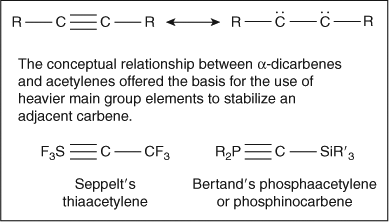
|
However, an easily produced and easily handled carbene remained elusive until August 27, 1990,[ 11 ] when researchers in DuPont's Central Research and Development Department managed to place a stable crystalline carbene in a bottle (Scheme 4).
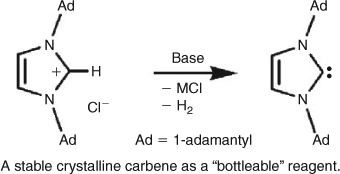
|
The ease of working with carbenes as reagents that could be taken out of a bottle and studied at will greatly facilitated the rapid growth of this area of chemistry. Indeed, the growth in this area has been described in various reviews as ‘explosive’ and the once elusive carbene is now viewed as ‘plentiful and versatile,’ at least for nucleophilic singlet carbenes. The versatility of nucleophilic singlet carbenes is documented with chemistry involving elements from all sections of the Periodic Table. A search of the literature reveals over a 100 reviews of this chemistry already published with the primary literature in the thousands of articles. This science now is too vast to be recited in this Foreword, but a few general areas can be mentioned with leading references. Combined with transition metals, the almost ubiquitous nucleophilic singlet carbene has opened new venues in catalysis,[ 12 ] while nucleophilic singlet carbenes themselves have found application in ‘organic catalysis’.[ 13 ] Nucleophilic singlet carbenes have found application in hydrogen storage and activation.[ 14 ] Nucleophilic singlet carbenes have been studied with the aid of a wide variety of spectroscopic methods including detailed nuclear magnetic resonance[ 15 ] and photo-electron spectroscopy.[ 16 ] X-ray crystallography has revealed numerous solid state structures for once elusive carbenes and an experimentally determined electron density has been measured.[ 17 ]
This issue of the Australian Journal of Chemistry offers a glimpse of a ‘Research Front’ on N-heterocyclic carbenes (NHCs). Contributions include highlights on ‘amidic’ NHCs[ 18 ] and ‘abnormal’ NHCs,[ 19 ] a review on mesoionic triazolylidene ‘click’ carbene ligands,[ 20 ] synthetic work related to nucleophilic carbene-catalyzed cycloaddition reactions,[ 21 ] NHC-promoted Rauhut-Currier reactions, [ 22 ] a gallium ‘isoelectronologue’ of heterocyclic carbenes,[ 23 ] an ionic liquid-derived imidazol-2-ylidene and its metal complexes,[ 24 ] an approach to hydrogen storage and activation by nucleophilic singlet carbenes,[ 25 ] new reactions of rhodium complexes of six-membered ring NHCs,[ 26 ] and NHCs on gold surfaces.[ 27 ] I anticipate that readers of the Australian Journal of Chemistry will find these offerings enlightening and useful.
Finally, I'd like to close my comments by returning to my opening remark about ‘sharing ideas, interests, and experiences with like-minded individuals.’ This aspect of science has been truly rewarding for me personally. Carbene research has put me in touch with many of the legendary scientists who pioneered the work on which this area now builds. It also offers opportunities to meet dynamic scientists who now push this effort forward on various research fronts. From these latter interactions some new collaborations have evolved, while earlier collaborations were re-purposed to address the new issues arising from evolving carbene chemistry. These interactions have proved both stimulating and satisfying. Chemistry in this area has also served to strengthen long standing professional friendships. Prior to our involvement with nucleophilic singlet carbenes, much of the effort of the young scientists in my own research group focussed on unusual valency with main group elements other than carbon. A mutual interest in phosphorus chemistry first brought me in contact with Guy Bertrand before the involvement of both our groups in carbene chemistry. Even though we had a shared interest in phosphorus chemistry, Guy and I entered carbene research from different venues. Guy's entry from an academic perspective reflected his clever application of bonding capabilities at a phosphorus centre to coax stability out of a neighbouring carbene centre. The entry of my group into nucleophilic carbene chemistry was from an industrial perspective based on insights into chemistry employed to produce catalysts for automotive paint cross-linkers. Our friendship originally grew out of a shared interest in phosphorus chemistry and has been strengthened through sharing our different experiences and approaches to carbene chemistry. My path as an explorer in chemistry, although complicated, moving more than once between academe and industry, has benefited from many such lasting and evolving friendships. Along this path, these friendships and intellectual partnerships have influenced my research focus and interests, and enabled contributions that would have not otherwise been likely. The reader of this Research Focus has an opportunity to see the experiences and insights offered by the issue's contributors. I hope that many of you will find here a reason to launch new research efforts and ideas to refine those already underway.
References
[1] R. Abegg, Z. Anorg, Chem. 1904, 39, 330.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaD28XhtFOmt7c%3D&md5=2cee0628b4b2165363ffd9c2ca3e2d6fCAS |
[2] G. N. Lewis, J. Am. Chem. Soc. 1916, 38, 762.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaC28XlvFSl&md5=b2c83a235e6d7207d18c9b0c79a08971CAS |
[3] J. B. Dumas, E. Péligot, Ann. Chim. Phys 1835, 58, 5.
[4] (a) W. Kirmse, Angew. Chem. Int. Ed. Engl. 2010, 49, 87981.
| Crossref | GoogleScholarGoogle Scholar |
(b) W. Kirmse, Angew. Chem. Int. Ed. 2004, 43, 1767.
| Crossref | GoogleScholarGoogle Scholar |
(c) C. Wentrup, Science 2001, 292, 1846.
| Crossref | GoogleScholarGoogle Scholar |
(d) A. J. Arduengo, Acc. Chem. Res. 1999, 32, 913.
| Crossref | GoogleScholarGoogle Scholar |
(e) A. J. Arduengo III, R. Krafczyk, Chem. Unserer Zeit 1998, 32, 6.
| Crossref | GoogleScholarGoogle Scholar |
(f) M. Regitz, Angew. Chem. Int. Ed. Engl. 1996, 35, 725.
| Crossref | GoogleScholarGoogle Scholar |
[5] H.-W. Wanzlick, E. Schikora, Angew. Chem. 1960, 72, 494.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXhtFaju7Y%3D&md5=08866075190a2f926cbb4b4ff4487936CAS |
[6] M. Schmeisser, H. Schröter, Angew. Chem. 1960, 72, 349.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3cXhtFOntr0%3D&md5=d37eeb42180c9a901330d444e4a50524CAS |
[7] A. J. Arduengo III, J. R. Goerlich, R. Krafczyk, W. Marshall, Angew. Chem. Int. Ed. Engl. 1998, 37, 1963.
| Crossref | GoogleScholarGoogle Scholar |
[8] D. M. Lemal, R. A. Lovald, K. I. Kawano, J. Am. Chem. Soc. 1964, 86, 2518.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXktl2rur4%3D&md5=8c425af8c1329c8523866cf52603df5cCAS |
[9] B. Poetter, K. Seppelt, A. Simon, E. M. Peters, B. Hettich, J. Am. Chem. Soc. 1985, 107, 980.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXnvVaqsg%3D%3D&md5=553674e1833be9eae7bfa5b1260d9353CAS |
[10] A. Igau, H. Grützmacher, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 1988, 110, 6463.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlt12itL0%3D&md5=679da5c86cba2752e7ea604c213d45c0CAS |
[11] (a) The successful deprotonation of 1,3-diadamantylimidazolium chloride with sodium hydride (dimsyl anion-catalyzed) in tetrahydrofuran was started on Friday, August 24, 1990 and worked up on Monday, August 27, 1990 to provide the first stable crystalline carbene.
(b) A. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361.
| Crossref | GoogleScholarGoogle Scholar |
[12] W. A. Herrmann, C. Köcher, Angew. Chem. Int. Ed. Engl. 1997, 36, 2162.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnsVCrs7Y%3D&md5=620fb1b77a30abb92ea3649b3b9e81cfCAS |
[13] (a) P.-C. Chiang, J. W. Bode “N-heterocyclic carbenes as organic catalysts.” RSC Catalysis Series 2011, 6 (N-Heterocyclic Carbenes), 399–435.
(b) D. A. DiRocco, T. Rovis, Science of Synthesis, Stereoselective Synthesis 2011, 2, 835.
[14] P. A. Chase, D. W. Stephan, Angew. Chem. Int. Ed. 2008, 47, 7433.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Shu7jO&md5=a7a8064d9d7f7c746e461ac8c0977fc7CAS |
[15] A. J. Arduengo III, D. A. Dixon, K. K. Kumashiro, C. Lee, W. P. Power, K. W. Zilm, J. Am. Chem. Soc. 1994, 116, 6361.
| Crossref | GoogleScholarGoogle Scholar |
[16] A. J. Arduengo, H. Bock, H. Chen, M. Denk, D. A. Dixon, J. C. Green, W. A. Herrmann, N. L. Jones, M. Wagner, R. West, J. Am. Chem. Soc. 1994, 116, 6641.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXltlCgsrs%3D&md5=475c65e57d8863f8e1cca1f2fb924f84CAS |
[17] A. J. Arduengo III, H. V. R. Dias, D. A. Dixon, R. L. Harlow, W. T. Klooster, T. F. Koetzle, J. Am. Chem. Soc. 1994, 116, 6812.
| Crossref | GoogleScholarGoogle Scholar |
[18] U. Siemeling, Aust. J. Chem. 2011, 64, 1108.
[19] A. Krüger, M. Albrecht, Aust. J. Chem. 2011, 64, 1112.
[20] J. D. Crowley, A.-L. Lee, K. J. Kilpin, Aust. J. Chem. 2011, 64, 1117.
[21] S. J. Yan, L. Candish, I. Martínez, D. W. Lupton, Aust. J. Chem. 2011, 64, 1147.
| Crossref | GoogleScholarGoogle Scholar |
[22] R. L. Atienza, K. A. Scheidt, Aust. J. Chem. 2011, 64, 1157.
[23] S. L. Choong, W. D. Woodul, A. Stasch, C. Schenk, C. Jones, Aust. J. Chem. 2011, 64, 1172.
[24] M. L. Cole, M. R. Gyton, J. B. Harper, Aust. J. Chem. 2011, 64, 1132.
[25] J. W. Runyon, O. Steinhof, H. V. R. Dias, J. C. Calabrese, W. J. Marshall, A. J. Arduengo III, Aust. J. Chem. 2011, 64, 1164.
[26] A. Binobaid, K. J. Cavell, M. S. Necheav, B. M. Kariuki, Aust. J. Chem. 2011, 64, 1140.
[27] T. Weidner, J. E. Baio, A. Mundstock, C. Große, C. Bruhn, U. Siemeling, Aust. J. Chem. 2011, 64, 1176.


