Mass Spectrometry and its Applications in Life Sciences
Costel C. DarieBiochemistry and Proteomics Group, Department of Chemistry and Biomolecular Science, Clarkson University, 8 Clarkson Avenue, Potsdam, NY, 13699-5810, USA. Email: cdarie@clarkson.edu

Dr Costel C. Darie is a biochemist and currently Assistant Professor and leader of the Biochemistry and Proteomics Group within the Department of Chemistry and Biomolecular Science at Clarkson University. He received his B.S. and M.S. in biochemistry from Iasi, Romania, and his Ph.D. in biochemistry from Freiburg, Germany. Dr Darie's main research interests are in new proteomics approaches for biomarker discovery and identification of post-translational modifications and protein–protein interactions. His research is also focussed on the investigation of one particular protein – the tumour differentiation factor protein. |
Australian Journal of Chemistry 66(7) 719-720 https://doi.org/10.1071/CH13284
Published: 24 July 2013
Abstract
Deciphering the biological and clinical significance of the proteins is investigated by mass spectrometry in a relatively new field, named proteomics. Mass spectrometry is, however, also used in chemistry for many years. In this Research Front we try to show the potential use of mass spectrometry in chemical, environmental and biomedical research and also to illustrate the applications of mass spectrometry in proteomics.
The purpose of this Research Front is two-fold: to show the potential use of mass spectrometry in chemical, environmental and biomedical research and also to illustrate the applications of mass spectrometry in proteomics.
The sequencing of the human genome has been completed. Other genomes were also sequenced, with even more to come. We sadly must realise that we are not as complex as we wished or liked to believe. With less than 30000 genes, we are not complicated. However, gene splicing (one gene can produce more proteins), protein post-translational modifications (phosphorylation, glycosylation, truncation), and protein–protein interactions make the human genome (and other organisms’ genomes) more complicated. Deciphering the biological and clinical significance of all these gene products (i.e. proteins) is investigated by mass spectrometry in a relatively new field, named proteomics.
What is proteomics? First, it is a word; invented in 1994 in Australia, by Marc Wilkins.[1] Second, it is the study of proteins; from a cellular compartment, organelle, cell, tissue, organ, or organism. These proteins from these specific places are called proteomes: membrane proteomes, organellar proteomes, extracellular proteomes, etc. Third, proteomics is a mass spectrometry-based discipline; new and on the rise.[1–6]
A PubMed search using the keywords mass spectrometry, mass spectrometry AND chemistry or proteomics clearly demonstrated the importance of mass spectrometry in chemical research, as well as the establishment of proteomics as a rather mature discipline (Fig. 1). In fact, the same trend is observed in the Chemical Engineering News, as demonstrated in the May 2013 edition. [7]
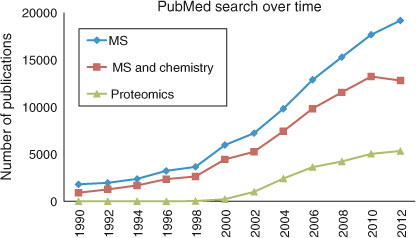
|
In this Research Front, the authors emphasise the power of mass spectrometry and then focus on mass-spectrometry-based proteomics. Initially, Sokolowska et al.[8] describe mass spectrometry as a research tool, along with the main components of a mass spectrometer and the types of experiments that one can do with it. The focus was proteins: identification of proteins, protein–protein interactions, protein modifications, and proteomics, also explained in other publications.[9–19] Once the reader becomes familiar with mass spectrometry and proteomics, specific examples are given by Ngounou Wetie et al,[20] on how one can use mass spectrometry to identify proteins and protein modifications.[5,12–14,21–25] The power of this manuscript lies not in describing the modifications per se, which are well known (i.e. phosphorylation, glycosylation, acetylation), but rather in how to identify these modifications using a mass spectrometer and what are the steps that we have to take to complete the tasks. In addition, the approaches and rationales used to identify a protein modification can be easily applied in other proteomics subfields such as secretomics, transductomics, glycomics, or disulfide proteomics. This is well reflected in the next three papers, where the authors describe part of their work that reflects their expertise in glycomics and its applications in biomedicine (Flangea et al),[26–29] in glycomic-based biomarker discovery (Shetty and Philip),[30–35] or in glycomic signature (Perdivara et al).[36–38] Quantitative proteomics is also elegantly revealed by Shetty and Philip.[35] The same mass spectrometry principles are then applied in designing and using cross-linkers for study of proteins and protein–protein interactions (Calabrese and Pukala),[39] already demonstrated in previous studies.[40–42] Here we can see a combination of chemistry, biology, and mass spectrometry within the same project and the same laboratory. A similar project, but with focus on monitoring chemical reactions (both intermediates and products), both qualitative and quantitative is presented by Xu and Melman.[43–46] Finally, application of mass spectrometry in monitoring the environment in the Great Lakes is presented in the last publication by Crimmins and colleagues.[47–49]
Of course, it will not be possible to cover many of the topics related to mass spectrometry, proteins, protein modifications, monitoring chemical syntheses, or monitoring chemicals, simply because the topic is way too large. Just look around, and you will see millions and millions of reasons of why mass spectrometry can be used and applied in so many fields. Therefore, I urge you to read and learn from the papers from this short but comprehensive Research Front.
References
[1] R. Aebersold, M. Mann, Nature 2003, 422, 198.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvFKgs7s%3D&md5=5bca9334fa0a1d79721bb915fee77311CAS | 12634793PubMed |
[2] M. Mann, Nat. Rev. Mol. Cell. Biol. 2006, 7, 952.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1KisrrF&md5=b4fc6c071b63dd7c17fa63f0e6686fb6CAS | 17139335PubMed |
[3] D. S. Spellman, K. Deinhardt, C. C. Darie, M. V. Chao, T. A. Neubert, Mol. Cell. Proteomics 2008, 7, 1067.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1ynsbg%3D&md5=ebed1e28768a3211b4fadc76f934f8efCAS | 18256212PubMed |
[4] M. E. Mason, J. L. Koch, M. Krasowski, J. Loo, Proteome Sci. 2013, 11, 2.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjslyku78%3D&md5=8901f1ca593705f27de351782f437183CAS | 23317283PubMed |
[5] A. G. Ngounou Wetie, I. Sokolowska, A. G. Woods, U. Roy, J. A. Loo, C. C. Darie, Proteomics 2013, 13, 538.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSmu7k%3D&md5=403ca867a3f1594bbfa3dbd5b8a134e2CAS | 23193082PubMed |
[6] V. B. Wang, S. L. Chua, B. Cao, T. Seviour, V. J. Nesatyy, E. Marsili, S. Kjelleberg, M. Givskov, T. Tolker-Nielsen, H. Song, J. Say Chye Loo, PloS One 2013, 8, e63129.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXoslamtL4%3D&md5=90ca07480e89b44362a925e7c594d23cCAS | 23700414PubMed |
[7] C. Henry Arnaud, Chem. Engineering News 2013, 91, 11.
[8] I. Sokolowska, A. G. Ngounou Wetie, A. G. Woods, C. C. Darie, Aust. J. Chem. 2013, 66, 721.
| Crossref | GoogleScholarGoogle Scholar |
[9] P. E. Florian, A. Macovei, C. Lazar, A. L. Milac, I. Sokolowska, C. C. Darie, R. W. Evans, A. Roseanu, N. Branza-Nichita, J. Med. Virology 2013, 85, 780.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkt1Gmurw%3D&md5=f15f95e78b465da3dde18caa1913a581CAS |
[10] U. Roy, I. Sokolowska, A. G. Woods, C. C. Darie, Biotechnol. Appl. Biochem. 2012, 59, 445.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitFyitQ%3D%3D&md5=1355a7d2a73912c6050cacfd685cdb7eCAS | 23586953PubMed |
[11] I. Sokolowska, C. Dorobantu, A. G. Woods, A. Macovei, N. Branza-Nichita, C. C Darie, Proteome Sci 2012, 10, 47.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFChtrs%3D&md5=d46170cbbd43db7df8dbc7140d6e1d7eCAS | 22857383PubMed |
[12] I. Sokolowska, M. A. Gawinowicz, A. G. Ngounou Wetie, C. C. Darie, Electrophoresis 2012, 33, 2527.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Sku7jI&md5=79535b10dc93b0d1b05c2b47f29db37fCAS | 22899260PubMed |
[13] I. Sokolowska, A. G. Ngounou Wetie, U. Roy, A. G. Woods, C. C. Dari, Biochimica et Biophysica Acta 2013, advance article.
| 23632316PubMed |
[14] I. Sokolowska, A. G. Ngounou Wetie, A. G. Woods, C. C. Darie, J. Lab. Automation 2012, 17, 408.
| 1:CAS:528:DC%2BC3sXhtV2msbk%3D&md5=67550f2be3900b208f5ec683d5821814CAS |
[15] I. Sokolowska, A. G. Woods, M. A. Gawinowicz, U. Roy, C. C. Dari, Cell. Mol. Life Sci. 2012, advance article.
| 23076253PubMed |
[16] I. Sokolowska, A. G. Woods, M. A. Gawinowicz, U. Roy, C. C. Darie, FEBS J. 2012, 279, 2579.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFKnurw%3D&md5=02be31291f892a5c9724b1919fcc4c3dCAS | 22613557PubMed |
[17] I. Sokolowska, A. G. Woods, M. A. Gawinowicz, U. Roy, C. C. Darie, J Biol. Chem. 2012, 287, 1719.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtlGmug%3D%3D&md5=bc401abead6a94971ba7f615ace294e2CAS | 22130669PubMed |
[18] A. G. Woods, I. Sokolowska, C. C. Darie, Biochem. Biophys. Res. Comm. 2012, 419, 305.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVOgtbk%3D&md5=3de55c4aff6cb6cc18384945c8745d97CAS | 22342715PubMed |
[19] A. G. Woods, I. Sokolowska, R. Taurines, M. Gerlach, E. Dudley, J. Thome, C. C. Darie, J. Cell. Mol. Med. 2012, 16, 1184.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1SjsbzP&md5=bca4d8a09b45764a98c5afd03f26f5faCAS | 22304330PubMed |
[20] A. G. Ngounou Wetie, I. Sokolowska, A. G. Woods, C. C. Darie, Aust. J. Chem. 2013, 66, 734.
| Crossref | GoogleScholarGoogle Scholar |
[21] A. G. Ngounou Wetie, I. Sokolowska, A. G. Woods, U. Roy, K. Deinhardt, C. C. Darie, Cell. Mol. Life Sci. 2013, advance article.
| 23579629PubMed |
[22] A. G. Ngounou Wetie, I. Sokolowska, A. G. Woods, K. L. Wormwood, S. Dao, S. Patel, B. D. Clarkson, C. C. Darie, J. Lab. Automation 2013, 18, 19.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXislyiur8%3D&md5=92a91bc87268270939c2b3f24ab4c30fCAS |
[23] C. C. Darie, M. L. Biniossek, M. A. Gawinowicz, Y. Milgrom, J. O. Thumfart, L. Jovine, E. S. Litscher, P. M. Wassarman, J. Biol. Chem. 2005, 280, 37585.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtF2isrjF&md5=783650d50c4014450dd2bf44cfe99a72CAS | 16157586PubMed |
[24] C. C. Darie, M. L. Biniossek, L. Jovine, E. S. Litscher, P. M. Wassarman, Biochem. 2004, 43, 7459.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvFCgtLw%3D&md5=c8b72313de3e196edcbba93636110e70CAS |
[25] C. C. Darie, K. Deinhardt, G. Zhang, H. S. Cardasis, M. V. Chao, T. A Neubert, Proteomics 2011, 11, 4514.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlOnu7bP&md5=ca62d41343e1fd51dcdf8dd64b7aac4eCAS | 21932443PubMed |
[26] C. Flangea, C. Mosoarca, C. Cozma, M. Galusca, M. Przybylski, A. D. Zamfir, Electrophoresis 2013, 34, 1572.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXms1eru78%3D&md5=c89c4359361b260b974aa5215b0bf3b5CAS | 23483567PubMed |
[27] C. Flangea, A. J. Petrescu, D. G. Seidler, C. V. Munteanu, A. D. Zamfir, Electrophoresis 2013, 34, 1581.
| 1:CAS:528:DC%2BC3sXntV2nsbk%3D&md5=13f4213414aa56613a9169157a965178CAS | 23494731PubMed |
[28] A. D. Zamfir, C. Flangea, A. Serb, A. M. Zagrean, A. M. Rizzi, E. Sisu, Methods in Mol. Biol. 2013, 951, 145.
| Crossref | GoogleScholarGoogle Scholar |
[29] I. Flangea, D. Fabris, Z. Vukelic, A. D. Zamfir, Aust. J. Chem. 2013, 66, 781.
| Crossref | GoogleScholarGoogle Scholar |
[30] V. Shetty, J. Hafner, P. Shah, Z. Nickens, R. Philip, Clin. Proteomics 2012, 9, 10.
| Crossref | GoogleScholarGoogle Scholar | 22856521PubMed |
[31] V. Shetty, P. Jain, Z. Nickens, G. Sinnathamby, A. Mehta, R. Philip, Omics: J Integrative Biol. 2011, 15, 705.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlWit7nM&md5=536ac80b3e6e12fcdbe6723b85541b7cCAS |
[32] V. Shetty, Z. Nickens, P. Shah, G. Sinnathamby, O. J. Semmes, R. Philip, Anal. Chem. 2010, 82, 9201.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1CmurzF&md5=5a0541c3964f9acb3cf085678fdf1003CAS | 20923142PubMed |
[33] V. Shetty, Z. Nickens, J. Testa, J. Hafner, G. Sinnathamby, R. Philip, J. Proteomics 2012, 75, 3270.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xms12rsbY%3D&md5=71d2022d58a999d0eb8ceb0a25638e09CAS | 22504797PubMed |
[34] V. Shetty, G. Sinnathamby, Z. Nickens, P. Shah, J. Hafner, L. Mariello, S. Kamal, G. Vlahovic, H. K. Lyerly, M. A. Morse, R. Philip, J. Proteomics 2011, 74, 728.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFGnsb8%3D&md5=44b594bb4c27a079f869d9d8eb788d8eCAS | 21362506PubMed |
[35] V. Shetty, R. Philip, Aust. J. Chem. 2013, 66, 770.
| Crossref | GoogleScholarGoogle Scholar |
[36] I. Perdivara, L. Perera, M. Sricholpech, M. Terajima, N. Pleshko, M. Yamauchi, K. B. Tomer, J. Am. Soc. Mass Spectrometry 2013, 24, 1072.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptFSrtL0%3D&md5=7efa4447ff34aa9e9a1e09a9ce3a16b6CAS |
[37] M. Sricholpech, I. Perdivara, M. Yokoyama, H. Nagaoka, M. Terajima, K. B. Tomer, M. Yamauchi, J. Biol. Chem. 2012, 287, 22998.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptlWmtrY%3D&md5=c9ff9547cda7684288d46321693be4fdCAS | 22573318PubMed |
[38] I. Perdivara, M. Yamauchi, K. B. Tomer, Aust. J. Chem. 2013, 66, 760.
| Crossref | GoogleScholarGoogle Scholar |
[39] A. Calabrese, T. Pukala, Aust. J. Chem. 2013, 66, 749.
| Crossref | GoogleScholarGoogle Scholar |
[40] A. N. Calabrese, N. J. Good, T. Wang, J. He, J. H. Bowie, T. L. Pukala, J. Am. Soc. Mass Spectrometry 2012, 23, 1364.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVGhsLvF&md5=c6247dbb32d748b05a325bb7fd649aa6CAS |
[41] A. N. Calabrese, T. Wang, J. H. Bowie, T. L. Pukala, Rapid Comm. Mass Spectrometry 2013, 27, 238.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVCktr3I&md5=a2cea68af0dac5e76f1c89a3c46e24efCAS |
[42] T. L. Pukala, T. Urathamakul, S. J. Watt, J. L. Beck, R. J. Jackway, J. H. Bowie, Rapid Comm. Mass Spectrometry 2008, 22, 3501.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOhu7vF&md5=cf0de5bb8862343ae25cdc1b192dfd2aCAS |
[43] Z. Jin, G. Guven, V. Bocharova, J. Halamek, I. Tokarev, S. Minko, A. Melman, D. Mandler, E. Katz, ACS Appl. Mat. Interfaces 2012, 4, 466.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1OjurfI&md5=573f86e5025d3d2fe782f0d3ad19e147CAS |
[44] N. LeTourneau, P. Vimal, D. Klepacki, A. Mankin, A. Melman, Bioorganic Med. Chem. Letters 2012, 22, 4575.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xpt1Shu74%3D&md5=8665f970edcfaf7f2bf0ec49f1becbe4CAS |
[45] R. P. Narayanan, G. Melman, N. J. Letourneau, N. L. Mendelson, A. Melman, Biomacromolecules 2012, 13, 2465.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvVKksrw%3D&md5=a12deb08d6c49a46e343af48d785cf92CAS | 22775540PubMed |
[46] X. X. Liu, A. Melman, Aust. J. Chem. 2013, 66, 791.
| Crossref | GoogleScholarGoogle Scholar |
[47] F. Chang, J. J. Pagano, B. S. Crimmins, M. S. Milligan, X. Xia, P. K. Hopke, T. M. Holsen, Sci. Total Env. 2012, 439, 284.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1WntLzN&md5=f0174fa17fce36fd492ed4b88b4576baCAS |
[48] B. S. Crimmins, J. J. Pagano, X. Xia, P. K. Hopke, M. S. Milligan, T. M. Holsen, Env. Sci. Techn. 2012, 46, 9890.
| 1:CAS:528:DC%2BC38Xht1Kqu7jE&md5=e21f7035ac3c97f2f0a59acdf0b41da0CAS |
[49] B. S. Crimmins, J. J. Pagano, M. S. Milligan, T. M. Holsen, Aust. J. Chem. 2013, 66, 798.
| Crossref | GoogleScholarGoogle Scholar |


