Halomethyllithium Carbenoids: Versatile Reagents for the Homologation of Electrophilic Carbon Units
Vittorio Pace AA Department of Drug and Natural Product Synthesis, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria. Email: vpace@farm.ucm.es

Vittorio Pace received a degree in Pharmacy from the University of Perugia (Italy) in 2005 and an MSc in Drug Design and Development from the University of Pavia (Italy) in 2008. He obtained a PhD in Chemistry under the guidance of Professor A. R. Alcántara at the Complutense University of Madrid (2010). He then undertook postdoctoral training at the University of Vienna (Austria) with Professor W. Holzer and at the University of Manchester (UK) with Professor D. J. Procter. In October 2013, he moved to the University of Stockholm (Sweden) for a third postdoctoral position with Professor B. Olofsson. |
Australian Journal of Chemistry 67(2) 311-313 https://doi.org/10.1071/CH13416
Submitted: 10 August 2013 Accepted: 17 October 2013 Published: 28 November 2013
Background
Organometallic compounds presenting a lithium atom and at least one electronegative atom (e.g. halogen) are referred as carbenoids.[1] In this sense, it is indicative of the choice of the term ‘carbenoid’ by Closs and Moss to define such species, which show a reactivity ‘qualitatively analogous to those of carbenes without necessarily being free divalent carbon species’.[2] Their chemistry is characterized by the intrinsic dichotomy (ambiphilicity) between nucleophilic and electrophilic behaviour.[3] Depending on the experimental conditions, they can selectively exhibit one of these properties: usually, at low temperatures they act as nucleophiles, whereas at higher temperatures their electrophilic reactivity comes into play. This fact can be rationalized by the resonance structures depicted in Scheme 1, in which the extreme ionization of the polar bonds could lead, in principle, to the carbanionic (1a) or carbocationic (1b) species (Scheme 1). In-depth spectroscopic investigations support such a behaviour based on the high s character of the C–Li bond and on the high p character of the C–X bond.[4] In this scenario, monohalomethyllithium reagents (i.e. LiCH2X, X = Cl, Br, I) have received particular attention, and since their introduction by Köbrich as nucleophilic agents in the 1960s (Scheme 2),[5] have become a versatile tool to perform homologation reactions as an alternative to diazomethane-based procedures[6] or sulfur ylides (e.g. Corey–Chaykovski-type chemistry).[7] Significantly, fluoromethyllithium has been reported to be chemically too unstable, and thus its application in organic processes has been very limited.[8]
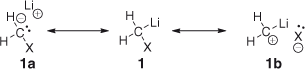
|
|
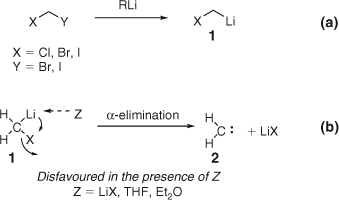
Key features of the Li carbenoids chemical reactivity can be summarized as follows: (i) they are immediately formed on treatment of a dihalomethane (ICH2Cl, ICH2Br, CH2Br2, CH2I2) with an alkyllithium base, as a consequence of the faster rate of halogen–Li exchange compared with the possible attack of the organolithium on a given electrophilic substrate (Scheme 2a)[9]; (ii) to ensure complete formation of the carbenoids and thus, minimize competing attack of the organolithium on the electrophile, it is wise to use a slight excess (~0.2–0.4 equiv.) of dihalomethane respect to RLi[10]; (iii) their pronounced thermal instability requires negative temperatures (<–78°C) to accomplish the reactions[4a] (for a remarkable exception, see below); (iv) because of the facile decomposition via α-elimination they undergo, reactions are performed in the presence of the electrophile[11]; (v) the stability of this species is enhanced by both Lewis bases (e.g. THF or diethyl ether) and LiX salts, which, through the coordination to the lithium atom of the carbenoid, disrupt the internal Li–X interaction responsible for α-elimination with consequent decomposition of the carbenoid to the carbene 2 (Scheme 2b).[12]
The effective thermal stability of LiCH2Cl has been a topic of intense debate in the literature. In this sense, Köbrich considered it the most unstable of the three monohalocarbenoids (except LiCH2F), because of decomposition at temperatures above –110°C.[5] This behaviour was also confirmed by Villieras in the 1980s.[12] However, studies published in the late 1980s[9] and in the 1990s[13] point out its stability at temperatures up to –78°C, and thus it is now well recognized that working at this non-prohibitive temperature does not compromise the efficiency of the reactions in which such species are involved. Though various alkyllithium bases have been employed for generating the carbenoids, MeLi–LiBr seems to provide the best results because of the aforementioned stabilization of the carbenoid with LiBr[9,10] and of its lower basicity compared with other organolithiums (e.g. n-BuLi) that can further react with the so-formed carbenoids.[11]
Application of Halomethyllithiums in Organic Synthesis
Homologation of Carbonyl-type Derivatives
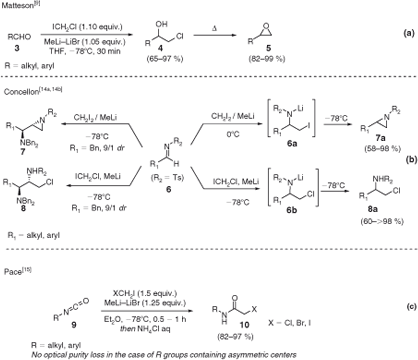
Owing to their inherent electrophilicity, carbonyl-type compounds have been extensively homologated with lithium carbenoids. The addition of LiCH2X to an aldehyde or a ketone (3) after mild acidic treatment provides the corresponding halohydrin (4) in high yields (Scheme 3a).[9] Interestingly, when the reaction is allowed to reach room temperature before quenching, an intramolecular nucleophilic displacement takes place, thus directly affording the epoxide (5). Concellón and coworkers conveniently exploited such a strategy for the (diastereoselective) preparation of aziridines (7–7a) and β-chloroamines (8–8a) starting from activated N-arylsulfonamido imines 6 (Scheme 3b).[14] Remarkably, iodomethyllithium used for these aziridination reactions could be efficiently prepared at 0°C, thus representing the highest temperature known for reaction involving such species. According to the authors, the reaction proceeds through the addition of the carbenoids to the N-tosyl-protected imine 6 to provide the intermediate lithiated haloamides (6a–6b), which then can undergo a spontaneous heterocyclization to 7a (i.e. expulsion of the good leaving group iodine), or rather, stop at the β-chloroamine 8a because of the non-optimal leaving ability of the chlorine. Very recently, Pace and coworkers reported the treatment of isocyanates 9 with LiCH2X to access versatile N-haloacetamides 10 (Scheme 3c).[15]
|
Homologation of Carboxylic Acid Derivatives
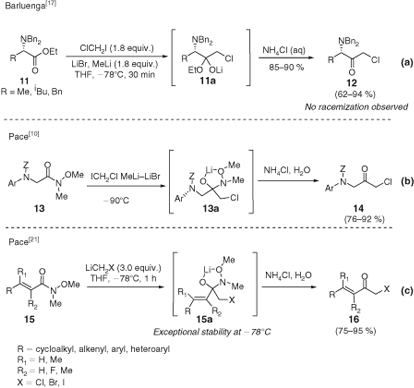
Most of the success of these species have in the organic community is due to their use in the preparation of α-amino-α′-haloketones starting from α-amino esters, which represent valuable scaffolds for the synthesis of HIV-inhibitors.[16] In this sense, Barluenga and coworkers reported the chloromethylation of N-protected amino esters 11 to the corresponding α-haloketones 12 without loss of optical purity during the transformation (Scheme 4a).[17] However, the feasibility of the procedure is conditioned by the electronic behaviour of the nitrogen protecting group.[18] In this sense, a general method has been developed by Pace et al. who employed Weinreb amides[19] 13 as the electrophilic starting materials (Scheme 4b, c).[10,20] Remarkably, the use of such amides could be successfully extended to the synthesis of α′-halo-α,β-unsaturated ketones 16, for which the use of esters provided exclusively the double-addition products (i.e. carbinols).[21] In this regard, the high stability under the reaction conditions of the tetrahedral intermediate 15a arising from the addition of the carbenoids to a Weinreb amide, which prevents a deleterious double addition of the carbenoid, should be stressed.
|
Conclusions and Outlook
Monohalomethyllithium carbenoids are clearly synthetically useful methylene transfer agents commonly employed in synthetic operations both at academic and industrial levels. Remarkably, they allow the direct incorporation of a (reactive) halomethylenic fragment into an electrophile, thus overcoming additional steps needed for the transformation of intermediate diazoketones or β-oxo dimethylsulfoxonium ylides into the desired adducts. The good nucleophilicity of these lithium species accounts for the exclusive 1,2-addition to α,β-unsaturated systems (e.g. esters), thus complementing the 1,4-addition observed with sulfur ylides.[22] Though the preparation of these reagents requires cryogenic temperatures and the use of pyrophoric reagents, the hazardous risks can be considered less problematic than those involving the use of the carcinogenic and explosive diazomethane.
References
[1] M. Braun, in The Chemistry of Organolithium Compounds (Eds Z. Rappoport, I. Marek) 2004, Vol. 2, pp. 829–899 (Wiley-VCH: Chichester).[2] G. L. Closs, R. A. Moss, J. Am. Chem. Soc. 1964, 86, 4042.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXkslKhsLw%3D&md5=eb71e632dd1d19a536347097a8482873CAS |
[3] V. Capriati, S. Florio, Chem. Eur. J. 2010, 16, 4152.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXks12qurc%3D&md5=f59a491b54af3702b76ded0054530e15CAS | 20222089PubMed |
[4] (a) H. Siegel, in Topics in Current Chemistry, Vol. 106 1982, pp. 55–78 (Springer: Heidelberg).
(b) H. Hermann, J. C. W. Lohrenz, A. Kühn, G. Boche, Tetrahedron 2000, 56, 4109.
| Crossref | GoogleScholarGoogle Scholar |
[5] G. Köbrich, A. Akhtar, F. Ansari, W. E. Breckoff, H. Büttner, W. Drischel, R. H. Fischer, K. Flory, H. Fröhlich, W. Goyert, H. Heinemann, I. Hornke, H. R. Merkle, H. Trapp, W. Zündorf, Angew. Chem. Int. Ed. Engl. 1967, 6, 41.
| Crossref | GoogleScholarGoogle Scholar |
[6] (a) G. Maas, Angew. Chem. Int. Ed. 2009, 48, 8186.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht12htLjJ&md5=ab35aa6c7001f72d38e59b7ecee8b9e9CAS |
(b) V. Pace, G. Verniest, J. V. Sinisterra, A. R. Alcántara, N. De Kimpe, J. Org. Chem. 2010, 75, 5760.
| Crossref | GoogleScholarGoogle Scholar |
[7] E. J. Corey, M. Chaykovsky, J. Am. Chem. Soc. 1962, 84, 867.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XktVelt7w%3D&md5=2b5d3c8ad12ed08ba0b8c5f5ac7257ebCAS |
[8] G. Boche, J. C. W. Lohrenz, Chem. Rev. 2001, 101, 697.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtl2ksbs%3D&md5=e63285870e47c01670c9da3c90e0c515CAS | 11712501PubMed |
[9] K. M. Sadhu, D. S. Matteson, Tetrahedron Lett. 1986, 27, 795.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhvValtw%3D%3D&md5=b51fcee7f94dddf2fc58946e53794cbaCAS |
[10] V. Pace, W. Holzer, G. Verniest, A. R. Alcántara, N. De Kimpe, Adv. Synth. Catal. 2013, 355, 919.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtlaqs7o%3D&md5=bb82a56746e58628437b1e751426aae2CAS |
[11] D. C. Kapeller, F. Hammerschmidt, J. Am. Chem. Soc. 2008, 130, 2329.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1KgtQ%3D%3D&md5=0be2f83254e864820c05b94f17bcf8ecCAS | 18217759PubMed |
[12] R. Tarhouni, B. Kirschleger, M. Rambaud, J. Villieras, Tetrahedron Lett. 1984, 25, 835.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlslWgsrc%3D&md5=5a283e4cfd72ba6669bd210a91cededcCAS |
[13] J. Barluenga, J. M. Fernández-Simón, J. M. Concellón, M. Yus, J. Chem. Soc., Perkin Trans. 1 1989, 77.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktl2is78%3D&md5=82863caef560db4d9f2674168f927516CAS |
[14] (a) J. M. Concellón, H. Rodríguez-Solla, C. Simal, Org. Lett. 2008, 10, 4457.
| Crossref | GoogleScholarGoogle Scholar | 18788813PubMed |
(b) J. M. Concellón, H. Rodríguez-Solla, P. L. Bernad, C. Simal, J. Org. Chem. 2009, 74, 2452.
| Crossref | GoogleScholarGoogle Scholar |
[15] V. Pace, L. Castoldi, W. Holzer, Chem. Commun. 2013, 8383.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlakurjK&md5=7de8628bfcef602a7e59beab1ffc3d2fCAS |
[16] (a) K. Izawa, T. Onishi, Chem. Rev. 2006, 106, 2811.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVemu7w%3D&md5=9c986d095c7f216e437a64494e4a9e7aCAS | 16836300PubMed |
(b) V. Pace, L. Castoldi, M. Pregnolato, Mini Rev. Med. Chem. 2013, 13, 988.
| Crossref | GoogleScholarGoogle Scholar |
[17] J. Barluenga, B. Baragaña, J. M. Concellón, J. Org. Chem. 1995, 60, 6696.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXotleltrw%3D&md5=51f92a6288e40c07bb1a4c79ce73d7d0CAS |
[18] (a) T. Onishi, N. Hirose, T. Nakano, M. Nakazawa, K. Izawa, Tetrahedron Lett. 2001, 42, 5883.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFOlu7s%3D&md5=a90a68001ed26fc0e3539d8810cd82c5CAS |
(b) T. Onishi, T. Nakano, N. Hirose, M. Nakazawa, K. Izawa, Tetrahedron Lett. 2001, 42, 5887.
| Crossref | GoogleScholarGoogle Scholar |
[19] (a) S. Nahm, S. M. Weinreb, Tetrahedron Lett. 1981, 22, 3815.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XosFSitg%3D%3D&md5=e58ef66b56c5cd5ce272cf2920f7c3bcCAS |
(b) S. Balasubramaniam, I. S. Aidhen, Synthesis 2008, 3707.
(c) V. Pace, L. Castoldi, A. R. Alcantara, W. Holzer, RSC Adv. 2013, 3, 10158.
| Crossref | GoogleScholarGoogle Scholar |
[20] (a) V. Pace, L. Castoldi, M. J. Hernáiz, A. R. Alcántara, W. Holzer, Tetrahedron Lett. 2013, 54, 4369.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVCrtbbK&md5=0996cfb3ea97340c1d359bab258a8c29CAS |
(b) V. Pace, W. Holzer, Tetrahedron Lett. 2012, 53, 5106.
| Crossref | GoogleScholarGoogle Scholar |
(c) V. Pace, Á. Cortés-Cabrera, V. Ferrario, J. V. Sinisterra, C. Ebert, L. Gardossi, P. Braiuca, A. R. Alcántara, J. Mol. Catal., B Enzym. 2011, 70, 23.
| Crossref | GoogleScholarGoogle Scholar |
(d) V. Pace, Á. Cortés-Cabrera, M. Fernández, J. V. Sinisterra, A. R. Alcántara, Synthesis 2010, 3545.
(e) V. Pace, Synlett 2010, 2825.
| Crossref | GoogleScholarGoogle Scholar |
(f) V. Pace, F. Martínez, M. Fernández, J. V. Sinisterra, A. R. Alcántara, Adv. Synth. Catal. 2009, 351, 3199.
| Crossref | GoogleScholarGoogle Scholar |
[21] V. Pace, L. Castoldi, W. Holzer, J. Org. Chem. 2013, 78, 7764.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVWhtb%2FM&md5=15a2102e9ac24958158b4c1c1f2e3889CAS | 23805887PubMed |
[22] E. J. Corey, M. Chaykovsky, J. Am. Chem. Soc. 1964, 86, 1640.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXktVCjsrw%3D&md5=e30cf96c297c087177df1c368915055eCAS |


