Pulsed Gradient Spin-Echo NMR Studies of the Interactions of Platinum Complexes
Benjamin J. Pages AA Nanoscale Organisation and Dynamics Group, University of Western Sydney, Penrith, NSW 2751, Australia. Email: 16948730@student.uws.edu.au

Benjamin Pages completed his BSc (Advanced Science) with Distinction in 2012 at the University of Western Sydney and was awarded the Dean's Medal. He is currently undertaking a BSc(Hons) degree at the University of Western Sydney under the supervision of Professors Janice Aldrich-Wright and William S. Price and Dr Gang Zheng. His research interests include the development of novel platinum(II) metallointercalators and the use of new techniques to study their biological activity. |
Australian Journal of Chemistry 67(2) 314-315 https://doi.org/10.1071/CH13496
Submitted: 18 September 2013 Accepted: 28 October 2013 Published: 21 November 2013
Background and Preparation
Platinum complexes (PCs) are known for their chemotherapeutic properties since the discovery of the anticancer drug cisplatin.[1] Thousands of biologically active PCs have since been synthesised, however the mechanism by which their cytotoxicity is exerted is often ambiguous.[2–4] Among the many spectroscopic techniques that have been used to elucidate the activity of these complexes,[5–7] pulsed gradient spin-echo (PGSE) nuclear magnetic resonance (NMR) spectroscopy is increasingly popular among chemists and biochemists. This technique can be used to determine the translational diffusion coefficient (D) and effective hydrodynamic radius (rS) of compounds in solution, characteristics that can reveal information regarding compound size and chemical behaviour. In a PGSE experiment, the sample is subjected to two gradient magnetic pulses, each of which induces a phase shift in the nuclear spins of the sample. If diffusion does not occur during the time between the pulses, the second pulse will realign the spins and the NMR signal is maximised. However, if diffusion occurs, the spins will not completely realign and the signal will be attenuated.[8–10] Conducting the experiment at varying gradient strengths allows the generation of a curve that can be fitted to calculate D (Fig. 1).
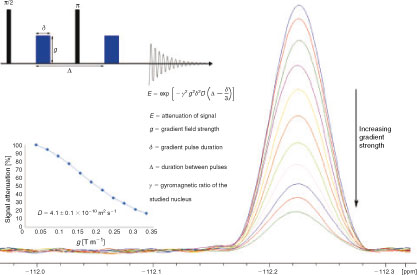
|
PGSE NMR is non-invasive and is also sensitive to concentrations and diffusion coefficients as low as ~0.1 mM and 10–15 m2 s–1, respectively.[10–12] It has been used to probe aggregation, ion pairing, and reaction mechanisms of metal complexes;[13–15] yet, its use to study PCs is limited. Examples of recent and future applications are presented below.
Applications
Complex Structure and Aggregation
The simplest application of PGSE NMR is for the calculation of rS values and the detection of aggregation of PCs in solution. Measurements of 1H and 31P NMR signals of several PCs using PGSE NMR showed that both ligand geometry and molecular weight affect the rS values of PCs.[16] Aggregation studies were performed on [(5,6-dimethyl-1,10-phenanthroline)(1S,2S-diaminocyclohexane)platinum(II)]2+ (56MESS) and [Pt(terpyridine)Cl]+. Measurements of D over a range of concentrations showed that these compounds aggregate into nanorods of 0.45–3.9 nm in length (Fig. 2). This concentration-dependent aggregation may influence the biological activity of PCs.[17]
|
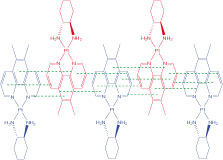
Host–Guest Interactions
PGSE NMR was used to study the host–guest association of 56MESS and other derivatives with substituted calix[4]arene and β-cyclodextrin.[18] The diffusion of 56MESS, [(5,6-dimethyl-1,10-phenanthroline)(1R,2R-diaminocyclohexane)platinum(II)]2+ (56MERR), and [(5,6-dimethyl-1,10-phenanthroline)(1,2-diaminoethane)platinum(II)]2+ (56MEEN) decreased markedly upon addition of the host compounds, indicating that association had occurred (Table 1).
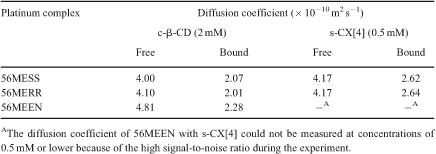
|
Interactions with Biomolecules
PGSE NMR has been used sparingly for characterising interactions between PCs and biomolecules. The association of complexes 56MESS, 56MERR, and 56MEEN with bovine serum albumin (BSA) was determined previously.[19] Diffusion experiments were performed whereby a ‘two-site’ model was used; this assumed that PCs could bind to any n identical sites of BSA, as represented by:
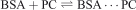
For each PC, n was ~10 and the average apparent association constant (Kapp) of the sites was ~102–103 M–1. Circular dichroism (CD) and fluorescence studies suggested that n was ~1 and Kapp was ~105–106 M–1. However, these techniques measured discreet changes in the CD and fluorescence of BSA respectively, whereas PGSE NMR results were representative of the general diffusion of BSA–PC. It is possible that the Kapp values determined by CD and fluorescence were representative of the strongest BSA–PC binding, whereas those determined by PGSE NMR were an average of all binding sites, including those with comparatively low affinity, resulting in an overall lower Kapp. PGSE NMR may provide novel insights into the subtle interactions of these complexes with DNA considering the differences in the n and Kapp values determined for BSA–PC interactions. This will be the focus of future studies.
References
[1] B. Rosenberg, L. Van Camp, J. E. Trosko, V. H. Mansour, Nature 1969, 222, 385.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXktVWquro%3D&md5=4b92af9171f0ab713fe8844e6d9a3863CAS | 5782119PubMed |
[2] W. D. McFadyen, L. P. G. Wakelin, I. A. G. Roos, V. A. Leopold, J. Med. Chem. 1985, 28, 1113.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXktlajsbw%3D&md5=0c6c302979e1c4acae5023da6ec6be09CAS | 4020833PubMed |
[3] H. Baruah, C. L. Rector, S. M. Monnier, U. Bierbach, Biochem. Pharmacol. 2002, 64, 191.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xlt12ktro%3D&md5=41eb7dabac9c1f2b9b5f7449e0f4c934CAS | 12123739PubMed |
[4] K. B. Garbutcheon-Singh, P. Leverett, S. Myers, J. R. Aldrich-Wright, Dalton Trans. 2013, 42, 918.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVOrtbzP&md5=105031f9d66a7ce0f3a03200c5325344CAS | 23018340PubMed |
[5] A. Casini, A. Guerri, C. Gabbiani, L. Messori, J. Inorg. Biochem. 2008, 102, 995.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvVyjt7g%3D&md5=0d6c502ebbaec9c269cdbb0c4624cbb6CAS | 18289690PubMed |
[6] C. Sanchez-Cano, M. Huxley, C. Ducani, A. E. Hamad, M. J. Browning, C. Navarro-Ranninger, A. G. Quiroga, A. Rodger, M. J. Hannon, Dalton Trans. 2010, 39, 11365.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVOktrvE&md5=e25d2595ad038284a53e0c8d349c789bCAS | 21031219PubMed |
[7] Y. Song, K. Suntharalingam, J. Yeung, M. Royzen, S. J. Lippard, Bioconjugate Chem. 2013, 24, 1733.
| 1:CAS:528:DC%2BC3sXht12gsrrM&md5=ff039fa3c27f0441d033aa41189c5640CAS |
[8] W. S. Price, in Encyclopedia of Nuclear Magnetic Resonance (Eds D. M. Grant and R. K. Harris) 2002, Vol 9, pp. 364–374 (Wiley: New York).
[9] W. S. Price, Aust. J. Chem. 2003, 56, 855.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsV2jtrY%3D&md5=59e550eca239e6fbc38099e8ef1ecfe4CAS |
[10] NMR Studies of Translational Motion: Principles and Applications (Ed. W. S. Price) 2009 (Cambridge University Press: Cambridge).
[11] P. S. Pregosin, P. G. A. Kumar, I. Fernández, Chem. Rev. 2005, 105, 2977.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXntVOmtrg%3D&md5=3495382df2ec3fc4883a1a9ebf768bfdCAS | 16092825PubMed |
[12] G. Zheng, W. S. Price, Environ. Sci. Technol. 2012, 46, 1675.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFGj&md5=4df07e901728238ee5580d09517b7ebeCAS | 22211466PubMed |
[13] S. Bolaño, G. Ciancaleoni, J. Bravo, L. Gonsalvi, A. Macchioni, M. Peruzzini, Organometallics 2008, 27, 1649.
| Crossref | GoogleScholarGoogle Scholar |
[14] P. S. Pregosin, Pure Appl. Chem. 2009, 81, 615.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvVynt78%3D&md5=fad9a0b9eeec54b2f6d956c48f094290CAS |
[15] D. Li, I. Keresztes, R. Hopson, P. G. Williard, Acc. Chem. Res. 2009, 42, 270.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFelsLzF&md5=ffca42b1c9b17b09e9cef91379e418d7CAS | 19105594PubMed |
[16] D. Nama, P. G. A. Kumar, P. S. Pregosin, Magn. Reson. Chem. 2005, 43, 246.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsFOjsro%3D&md5=dcdfd118a30f77e8b3dfd16e984e269cCAS | 15609369PubMed |
[17] A. M. Krause-Heuer, N. J. Wheate, W. S. Price, J. Aldrich-Wright, Chem. Commun. 2009, 1210.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVChsLY%3D&md5=73b8a94ad2bbf8b90fdd38a171aaafdaCAS |
[18] A. M. Krause-Heuer, N. J. Wheate, M. J. Tilby, D. G. Pearson, C. J. Ottley, J. R. Aldrich-Wright, Inorg. Chem. 2008, 47, 6880.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFKjs7c%3D&md5=6a5a13af8bc0234c55cf5eed73f43706CAS | 18597414PubMed |
[19] A. Krause-Heuer, W. Price, J. Aldrich-Wright, J. Chem. Biol. 2012, 5, 105.
| Crossref | GoogleScholarGoogle Scholar | 23667391PubMed |


