Tailoring Substrate Hydrophilicity Using Grafted Polypeptide Nanocoatings*
Steven Harris Wibowo A , Adrian Sulistio A , Edgar H. H. Wong A , Anton Blencowe A B and Greg G. Qiao A CA Department of Chemical and Biomolecular Engineering, The University of Melbourne, Parkville, Melbourne, Vic. 3010, Australia.
B Present address: Mawson Institute, Division of Information Technology, Engineering and the Environment, University of South Australia, Mawson Lakes, SA 5095, Australia.
C Corresponding author. Email: gregghq@unimelb.edu.au
Australian Journal of Chemistry 67(4) 598-602 https://doi.org/10.1071/CH13519
Submitted: 27 September 2013 Accepted: 18 December 2013 Published: 17 February 2014
Abstract
Peptide nanocoatings with tailored surface-wetting properties were formed on a range of organic (cellulose and cotton) and inorganic (glass) substrates via surface-initiated ring-opening polymerization of amino acid N-carboxyanhydride derivatives. The film thickness, surface roughness, and wettability can be tuned by controlling the polymerization time and the type of N-carboxyanhydride derivative used (i.e. lysine or valine). Whereas poly(l-lysine) coatings are hydrophilic, poly(l-valine) coatings exhibit water-repellent properties. The functional polypeptide nanocoatings can potentially be applied to waterproof woven fabrics, macromolecular separation technologies, biodiagnostic sensors, and sustained drug-release wound dressings.
Introduction
Engineered functional nanocoatings with tailored wetting and fouling characteristics are essential for various applications including self-cleaning and anti-fouling surfaces,[1–5] microfluidic devices,[6] (bio)chemosensors,[7] filtration systems,[8,9] biomedical implants,[10] and therapeutic delivery.[11,12] Specifically in the biomedical field, nanocoatings should ideally be biocompatible and biodegradable, which makes the utilization of synthetic polypeptides as coating materials an attractive option. To date, commercial coating technologies rely on conventional techniques such as spray- and dip-coating.[13,14] Although these approaches allow rapid deposition over large areas, applications on complex porous substrates and/or colloidal systems are hampered by pore blockage and aggregation resulting from build-up of excess coating material. In comparison, the layer-by-layer (LbL) approach has been utilized to immobilize enzymes, proteins, and synthetic polypeptides with various functionalities and hydrophobicities.[12,15–19] Despite the fine control over film properties, the LbL technique involves multistep processing and relies on the availability of complementary functionalized polypeptides. In general, the formation of peptide-based films via the LbL approach relies on electrostatic interactions between two oppositely charged peptides.[15,19] Therefore, the incorporation of non-ionic hydrophobic peptide segments into LbL-generated films remains challenging. Furthermore, hydrophobic peptides are typically insoluble in most solvents, which hampers their use as building blocks for films and coatings.
Peptide film fabrication using the grafting-from approach through surface-initiated ring-opening polymerization (ROP) of amino acid N-carboxyanhydride (NCA) derivatives has been gaining popularity in recent years. The formation of polypeptide films via the grafting-from approach has been developed to modify the surface wettability,[20] pH-responsive behaviour,[20,21] and biocompatibility[22] of a range of planar and colloidal substrates.[23–25] Peptide film formation using the grafting-from approach precludes the need to pre-synthesize the peptides and/or polymers, and therefore avoids insolubility issues associated with hydrophobic peptides. Modification of surface wettability by grafted peptide films has been achieved by the formation of peptide films composed of hydrophilic polypeptides bearing charged side chain functionalities, namely poly(glutamic acid) and polylysine.[20] However, the grafting-from of hydrophobic amino acid NCA derivatives (such as l-valine NCA) to tune wetting characteristics is yet to be demonstrated. The incorporation of hydrophobic peptides within the film will enable tunable wetting characteristics that are desirable for biomedical applications, such as in the development of sterile waterproof wound dressings or as antifouling coatings on medical implants.
Therefore, the aim of this study was to develop a facile method for the synthesis of surface-bound peptide coatings with tunable surface wetting properties. In order to achieve this, the surface-initiated ROP of amino acid NCA derivatives was employed as a bottom-up approach to fabricate surface-bound peptide films on various substrates including glass, cotton fabric, and cellulose paper. Judicious selection of amino acid NCA derivatives enabled the hydrophobic–hydrophilic composition of the polypeptide films to be tuned. This method also allows subsequent chain extension with another amino acid NCA derivatives to obtain amphiphilic block co-polypeptide films with switchable wetting characteristics (Scheme 1).
In this study, all substrates were functionalized with hyperbranched poly(ethylene imine) (PEI) to introduce free amine groups on the surface that could initiate ROP of NCA derivatives; l-valine NCA (Val-NCA) or carboxybenzyl (CBz)-protected l-lysine NCA (Lys-NCA) were employed to form poly(l-valine) (PVal) and poly(CBz-l-lysine) (PZLL) grafts respectively. After polymerization, the CBz groups were removed from the PZLL grafts using HBr to afford hydrophilic poly(l-lysine) (PLL) brushes.
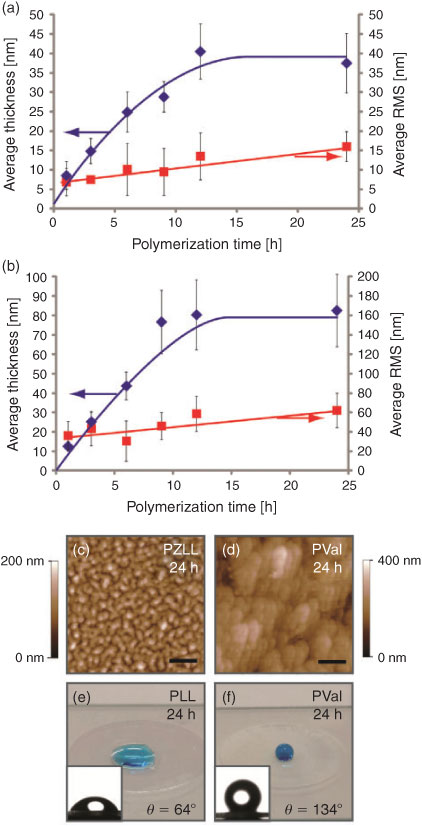
Initially, film formation was conducted on commercial glass coverslips pretreated with PEI. The glass coverslips were incubated in amino acid NCA (Lys-NCA or Val-NCA) solutions (0.75 M in anhydrous N,N-dimethylformamide (DMF)) for predetermined times. Our previous study using PEI as an NCA-ROP initiator revealed the production of non-grafted polypeptides in solution, potentially resulting from the initiation of NCA-ROP via the ‘activated monomer mechanism’ from the secondary amines of PEI.[25] As such, in the present study, extensive washing and ultrasonication steps were conducted to ensure removal of potential non-grafted polypeptide from the surface (refer to the Supplementary Material for detailed procedure). The resulting films were analysed via atomic force microscopy (AFM) to determine their thickness and morphology. The formation of PZLL films on the glass coverslips showed an asymptotic film growth from 8.0 ± 3.5 nm after 1 h to a maximum film thickness of 38 ± 7.6 nm after 24 h (Fig. 1a), as determined by AFM scratch analysis. The surface root-mean-square (RMS) roughness of the film also increased with polymerization time (6.9 ± 3.5 to 16.0 ± 3.9 nm) (Fig. 1a). The PVal films were also analysed in a similar fashion and revealed a film growth from 13 ± 2.3 nm after 1 h to 83 ± 18 nm after 24 h, which was also accompanied by an increase in the surface roughness (36.2 ± 14.8 to 62.3 ± 17.8 nm) (Fig. 1b). Interestingly, AFM analysis (Fig. 1c, d) revealed different morphologies for the PZLL and PVal films after 24 h polymerization. The PVal film appears to be significantly rougher with coalesced morphology whereas the PZLL film appears to have a granular morphology. This granular morphology corresponds with the formation α-helix PZLL grafts, as previously reported for other peptide chains with α-helix secondary structures, such as poly(benzyl-l-glutamate) (PBLG) grafts.[26,27] In comparison, the coalesced granular morphology of the PVal film is consistent with the formation of hydrophobic peptide grafts.[26]
|
To study the wetting characteristics of the PZLL and PVal coatings on glass coverslips, water contact angles were measured before and after peptide grafting. The water contact angles of untreated and PEI-coated substrates were determined to be 24° and 60° respectively (see Fig. S1a, b, available as Supplementary Information for this paper). After the grafting of PZLL, the contact angle increased to 81°, which indicates an increase in surface hydrophobicity (Fig. S1c), possibly as a result of the CBz protecting groups along the PZLL brushes. The subsequent removal of CBz protecting groups using HBr afforded hydrophilic PLL films, as indicated by the reduction in the water contact angle to 64° (Fig. 1e), which is similar to the value observed on a PEI-coated glass coverslip. In comparison, the PVal coating rendered the glass surface hydrophobic, with a contact angle of 134° (Fig. 1f). This demonstrates that the surface wetting characteristics of polypeptide films can be turned through careful selection of amino acid building blocks.
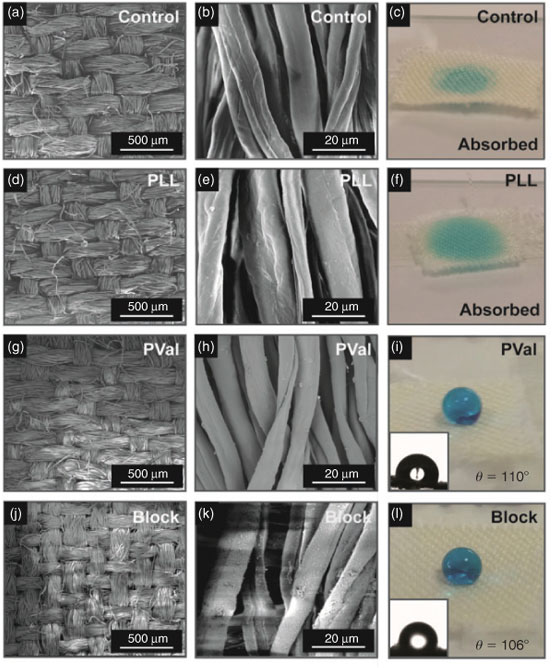
The versatility of this approach to afford peptide coatings on other substrates was demonstrated by grafting Lys-NCA and Val-NCA from cotton fabrics. Scanning electron microscopy (SEM) and water contact angle analysis were used to ascertain the change in surface morphology and wetting characteristics of the cotton following grafting. Commercially available, non-treated cotton is hydrophilic and therefore absorbs water immediately on contact (Fig. 2a–c). After grafting, SEM images of PLL-coated cotton revealed a slight increase in surface roughness (Fig. 2d, e). As the PLL grafts are hydrophilic, the coatings did not provide any barrier towards water absorption (Fig. 2f). In contrast, successful formation of PVal grafts was shown to prevent water from being absorbed, with an immediate water contact angle of 110° (Fig. 2i). SEM images of the PVal coating revealed an increase in surface roughness compared with the PLL-coated fabric (Fig. 2g, h), which was consistent with the results previously obtained on glass coverslips.
|
To further demonstrate the versatility of this technique, successive chain extension of PLL grafts with Val-NCA (0.75 M initial concentration, 24 h) was conducted to extend the formation of the original film into block co-polypeptide coatings (Scheme 1). The addition of PVal as the second block increases the surface roughness and imparts surface hydrophobicity (contact angle of 106°) to the cotton (Fig. 2j–k), whereas the underlying poly-l-lysine block provides amine functionalities for potential ionic or covalent conjugation.[20,21] Such block polypeptide films are promising coatings for waterproof wound dressings, whereby the underlying amine groups can be functionalized with therapeutic cargo (e.g. anti-inflammatory drugs through various conjugation chemistries) that can be released directly into the wound area, while the PVal outer layer creates a hydrophobic barrier to keep the wound dry and avoid adherence of the healing wound to the dressing.
Paper technology has been widely applied in analytical and clinical chemistry for solid–liquid separation and chromatography. To demonstrate the applicability of the peptide grafting-from approach to this field, ROP of Val-NCA (0.75 M initial concentration) to form a hydrophobic coating on cellulose-based filter paper (Whatman no. 542) was conducted. Specifically, a kinetic study was conducted and an increase in water contact angle from 32 ± 1.3° to 140 ± 1.0° was observed as the reaction time was increased from 1 to 24 h (Fig. S2). Additionally, the water absorption rate into the filter paper (i.e. the time taken for a water droplet to be completely absorbed into the filter paper) increased with increasing polymerization time. For example, after 3 h of polymerization, the PVal-coated filter paper was capable of resisting water absorption for 3 min before the water droplet started to diffuse into the filter paper. In contrast, after 24 h of polymerization, the PVal-coated filter paper resisted water absorption until the water droplet had completely evaporated (~40 min). These results suggest that longer polymerization times produce thicker PVal films with higher surface roughness on filter paper, analogous to the kinetic studies on the glass substrates. It is well known that an increase in surface roughness causes air pockets to be trapped between the water and substrate surface, leading to significantly lowered solid–liquid adhesion forces, which consequently increases the apparent contact angle.[28,29] As seen in the SEM images (Fig. S3), an increase in surface roughness after 24 h of polymerization was evident when compared with untreated filter paper. Therefore, it is likely that the higher surface roughness contributed to the observable increase in water contact angle and water absorption resistance.
In conclusion, this study demonstrates that the grafting-from approach using surface-initiated ROP of amino acid NCA derivatives is a versatile method to form peptide nanocoatings that can tune the wetting characteristics of various inorganic (glass) and organic (cotton and cellulose) substrates. Kinetic studies on commercial glass coverslips reveal an increase in film thickness and surface roughness with increasing polymerization time. From water contact angle measurements, poly(l-lysine) coatings exhibit hydrophilic behaviour whereas poly(l-valine) coatings increase the surface hydrophobicity of the substrate. Therefore, through the conscientious selection of hydrophobic or hydrophilic NCA derivatives, the wetting characteristics of surfaces can be tailored. Film formation on organic porous substrates such as cotton and filter paper indicate that poly(l-valine) coatings increase the surface hydrophobicity and water absorption resistance of the material, which can be tuned with polymerization time or the material can even be made waterproof. Studies are currently under way to elucidate the role of secondary structure in the properties of polypeptide coatings. Investigations on the formation of multiblock peptide coatings conjugated with bioactive (macro)molecules for applications in targeted biomedical applications such as biodiagnostic sensors and sustained drug-release are also underway.
Experimental Method
Peptide nanocoating fabrication: Carboxybenzyl-protected l-lysine NCA and l-valine NCA were synthesized via the phosgenation method as reported previously.[25] Clean glass coverslips (12-mm diameter) were washed with deionized water and soaked in PEI solution (1 mL per substrate, 1 mg mL–1 in 0.5 M NaCl solution) for 30 min. The amine-functionalized substrates were then washed in Milli-Q water (3 × 10 mL), anhydrous THF (3 × 10 mL), and anhydrous DMF (3 × 10 mL) to remove unbound PEI. Subsequently, the substrates were soaked in NCA solution (1 mL per substrate, 0.75 M in anhydrous DMF) and allowed to react under reduced pressure. After a predetermined reaction period, coated substrates were washed with DMF (3 × 5 mL) and then soaked in DMF (1 mL per substrate) for 15 h to remove any non-grafted materials. Samples were then sonicated for 5 min before further washing in DMF (3 × 5 mL), deionized water (3 × 5 mL), and methanol (3 × 5 mL). The samples were finally dried under vacuum before AFM, SEM, and contact angle analysis. Refer to the Supporting Information for detailed experimental procedures.
Supplementary Material
Supplementary figures, and detailed experimental methods and materials are available on the Journal’s website.
* Steven Harris Wibowo was invited to contribute to the 34th Australasian Polymer Symposium (APS) series following receipt of the Treloar Prize (Best Poster Presentation) at the 34th APS (7–10 July 2013).
Acknowledgement
The authors acknowledge the Australian Research Council under the Future Fellowship (Grant no. FT110100411, to G.G.Q.) for financial support of this work.
References
[1] J. Wang, M. I. Gibson, R. Barbey, S.-J. Xiao, H.-A. Klok, Macromol. Rapid Commun. 2009, 30, 845.| Crossref | GoogleScholarGoogle Scholar | 21706667PubMed |
[2] V. A. Ganesh, H. K. Raut, A. S. Nair, S. Ramakrishna, J. Mater. Chem. 2011, 21, 16304.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12gs7rK&md5=7634a7d84f658c619c23dc7abb75b113CAS |
[3] A. C. Engler, A. Shukla, S. Puranam, H. G. Buss, N. Jreige, P. T. Hammond, Biomacromolecules 2011, 12, 1666.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVGjtr8%3D&md5=7a8aaeac34a377d68f741bf05a247441CAS | 21443181PubMed |
[4] B. B. Hsu, S. Y. Wong, P. T. Hammond, J. Chen, A. M. Klibanov, Proc. Natl. Acad. Sci. USA 2011, 108, 61.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXms1GrtA%3D%3D&md5=ee65955e0639b26ab8e18269ba41c2cfCAS | 21173278PubMed |
[5] S. Y. Wong, Q. Li, J. Veselinovic, B.-S. Kim, A. M. Klibanov, P. T. Hammond, Biomaterials 2010, 31, 4079.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFKisrg%3D&md5=c7ea3695e7876c68a2107e864adede54CAS | 20163855PubMed |
[6] T. Saitoh, A. Sekino, M. Hiraide, Anal. Chim. Acta 2005, 536, 179.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtl2mtL0%3D&md5=18ec662e5e5ef4a5f34f067998e1fbaaCAS |
[7] Z. Li, X. Wang, G. Wen, S. Shuang, C. Dong, M. C. Paau, M. M. F. Choi, Biosens. Bioelectron. 2011, 26, 4619.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsl2itr8%3D&md5=234b7667e2b63b34be2fcfc54a1a1717CAS | 21612909PubMed |
[8] C. R. Crick, I. P. Parkin, Chemistry 2010, 16, 3568.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjslGrs7w%3D&md5=3af3a31955c0a366d31c0d2575c161b0CAS | 20209527PubMed |
[9] T. Sun, L. Feng, X. Gao, L. Jiang, Acc. Chem. Res. 2005, 38, 644.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkt1aju7k%3D&md5=14b4720706560215b58bfb6bb78e224aCAS | 16104687PubMed |
[10] P. Li, J. Biomed. Mater. Res. A 2003, 66A, 79.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlslWns78%3D&md5=482787e27303abac9b215314821ba9c3CAS |
[11] J. F. Quinn, A. P. R. Johnston, G. K. Such, A. N. Zelikin, F. Caruso, Chem. Soc. Rev. 2007, 36, 707.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkslCltLw%3D&md5=fb253feca5af0ca77937275f8729dcd2CAS | 17471396PubMed |
[12] A. N. Zelikin, A. L. Becker, A. P. R. Johnston, K. L. Wark, F. Turatti, F. Caruso, ACS Nano 2007, 1, 63.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptVejtr0%3D&md5=73abe5c5532871e9236ac17dcf341138CAS | 19203131PubMed |
[13] I. B. Rietveld, K. Kobayashi, H. Yamada, K. Matsushige, Soft Matter 2009, 5, 593.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1yjtLo%3D&md5=676961527cd8f18e731f496d915b6237CAS |
[14] H. Liu, S. Szunerits, M. Pisarek, W. Xu, R. Boukherroub, ACS Appl. Mater. Interfaces 2009, 1, 2086.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2mtLfL&md5=c972c45ee8ac0ceebd3025367bdd6c5dCAS | 20355837PubMed |
[15] A. L. Becker, A. P. R. Johnston, F. Caruso, Small 2010, 6, 1836.
| 1:CAS:528:DC%2BC3cXhtFKksLnE&md5=6e108ae38f6b4412288f717487a5d74bCAS | 20715072PubMed |
[16] F. Caruso, H. Möhwald, J. Am. Chem. Soc. 1999, 121, 6039.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjslWmtLo%3D&md5=844e590509a08e60313f4c9f67b638bcCAS |
[17] C. J. Ochs, T. Hong, G. K. Such, J. Cui, A. Postma, F. Caruso, Chem. Mater. 2011, 23, 3141.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXns1Kgur8%3D&md5=76361947333f2c446bdb7638f0a4e80fCAS |
[18] Y. Min, P. T. Hammond, Chem. Mater. 2011, 23, 5349.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFKhu7nO&md5=d035efbb05ed40a92bffa98ba8d3b21eCAS |
[19] A. Shukla, K. E. Fleming, H. F. Chuang, T. M. Chau, C. R. Loose, G. N. Stephanopoulos, P. T. Hammond, Biomaterials 2010, 31, 2348.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsV2ksg%3D%3D&md5=bda29970872abf4c310dfb24649fc7c0CAS | 20004967PubMed |
[20] F. Audouin, M. Fox, R. Larragy, P. Clarke, J. Huang, B. O’Connor, A. Heise, Macromolecules 2012, 45, 6127.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVCmtLnI&md5=964e57e503fb78d7d94b19e139f2e04aCAS |
[21] T. Borase, M. Iacono, S. I. Ali, P. D. Thornton, A. Heise, Polymer Chemistry 2012, 3, 1267.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvV2nt7o%3D&md5=f38cd9479381bba50cbbf022b89124b6CAS |
[22] G. Marcelo, A. Munoz-Bonilla, J. Rodriguez-Hernandez, M. Fernandez-Garcia, Polymer Chemistry 2013, 4, 558.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtl2ruw%3D%3D&md5=f1c8fbb35c1b3a734bb5a2b63da5c9feCAS |
[23] A. Heise, H. Menzel, H. Yim, M. D. Foster, R. H. Wieringa, A. J. Schouten, V. Erb, M. Stamm, Langmuir 1997, 13, 723.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtFeisbs%3D&md5=ca62798891238af79ebf98f4c70700deCAS |
[24] T. Jaworek, D. Neher, G. Wegner, R. H. Wieringa, A. J. Schouten, Science 1998, 279, 57.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivFymtA%3D%3D&md5=76c6f4617265181f7c7a11da9a1fe7b3CAS | 9417021PubMed |
[25] S. H. Wibowo, E. H. H. Wong, A. Sulistio, S. N. Guntari, A. Blencowe, F. Caruso, G. G. Qiao, Adv. Mater. 2013, 25, 4619.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosVCjs74%3D&md5=5008bfe97353671b1eabcd806e6c0223CAS | 23722350PubMed |
[26] N. H. Lee, L. M. Christensen, C. W. Frank, Langmuir 2003, 19, 3525.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhslSks70%3D&md5=6a027e256e089265921c84f5a261b2acCAS |
[27] Y.-C. Chang, C. W. Frank, Langmuir 1998, 14, 326.
| Crossref | GoogleScholarGoogle Scholar |
[28] S. N. Guntari, A. C. H. Khin, E. H. H. Wong, T. K. Goh, A. Blencowe, F. Caruso, G. G. Qiao, Adv. Funct. Mater. 2013, 23, 5159.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvVOjtr0%3D&md5=751444becab89993f3e779d600493590CAS |
[29] M. Nosonovsky, B. Bhushan, Curr. Opin. Colloid Interface Sci. 2009, 14, 270.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFWjsLw%3D&md5=e2b4387860ab9d616e8fb58ec4ed049aCAS |



