Acetylenes from Aldehydes. Preparation of Ethynylphenols and Phenylacetylenes by Flash Vacuum Pyrolysis of Isoxazolones*
Curt Wentrup A B C , Maria Wiedenstritt A and Hans-Wilhelm Winter AA Fachbereich Chemie der Philipps-Universität Marburg, D-35037 Marburg, Germany.
B School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Qld 4072, Australia.
C Corresponding author. Email: wentrup@uq.edu.au
Australian Journal of Chemistry 68(8) 1233-1236 https://doi.org/10.1071/CH15234
Submitted: 29 April 2015 Accepted: 20 May 2015 Published: 5 June 2015
Abstract
The flash vacuum pyrolysis method of synthesis of acetylenes from aldehydes via isoxazolones is a convenient method for the preparation of a variety of derivatives, including kinetically unstable, sensitive compounds such as the ethynylphenols.
As acetylenes have numerous important uses in organic synthesis and materials science, efficient syntheses of acetylenes are highly desirable.[1–3] Particularly noteworthy is the advent of ‘click chemistry’, which has made terminal acetylenes the molecules of choice for linking large molecular moieties.[3d–g] We have previously reported the synthesis of a variety of alkynes 3 in high yields using flash vacuum pyrolysis (FVP) of 4-methylideneisoxazol-5(4H)-ones 1, which themselves are readily synthesized from aldehydes and 3-methylisoxazol-5(4H)-ones. This permitted the preparation of kinetically unstable but thermodynamically stable compounds such as ethynylpyrroles, -thiophens, -furans, and -indoles 3a–f (Scheme 1).[1,4,5] Computational studies have confirmed that these isoxazolone decompositions take place via transient vinylidenes 2.[6] A 1,2-H shift affords the alkynes 3.[6] Although a 1,2-R shift is also possible, this has a higher activation barrier.[2,7] 3-Alkylidene-1-azirines may also be involved as transient intermediates.[6] In addition, ethynylamines RR′N–C≡C–H and RNH–C≡C–H and ketenimines R–N=C=CH2 have also been generated by FVP of 4-aminomethyleneisoxazolones.[8]
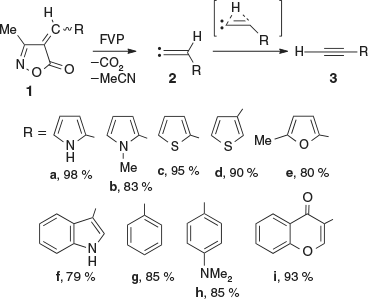
|
However, isoxazolones with large substituents can become too involatile for conventional FVP. For this reason, we developed falling solid flash vacuum pyrolysis (FS-FVP),[1,9] which allows the efficient, rapid, automated, and usually high-yielding pyrolysis of solids in high vacuum. We have recently applied this method to the synthesis of a variety of arylacetylenes, including compounds 4–8 (Scheme 2).[1]
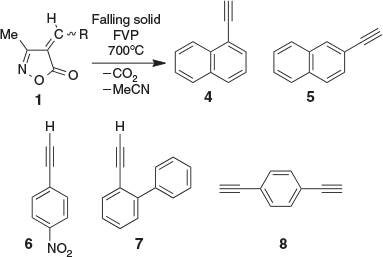
|
Ethynylphenols are known, but they have not been widely used in synthesis, and only very few methods of preparation have been reported.[10–12] We were interested in easy access to ethynylphenols and have therefore investigated the synthesis of the title compounds by FVP. The isoxazolones 10 (Scheme 3) were synthesized from 3-methylisoxazolone 9 and the appropriate substituted benzaldehyde using the previously reported procedure.[1,4] Compounds 10 were subjected to FVP at 800°C and ~10−3 hPa. The acetylene products 11 were isolated in liquid-nitrogen cold traps, purified by distillation and/or sublimation, and characterized spectroscopically and by comparison with literature data. The ethynylphenols 11a,b were obtained in good yields (67–87 %) but should be stored in the cold and dark under exclusion of air to prevent polymerization. While the ethynylphenols 11a,b have been prepared previously by wet methods,[10–12] the FVP yields are superior for these compounds, and the advantage of this method is that the products are formed rapidly and immediately isolated at low temperature, where they can be kept till needed for other synthetic reactions. Ethynylphenols are sensitive compounds that polymerize at room temperature. Once isolated, they should be stored in the cold under N2.
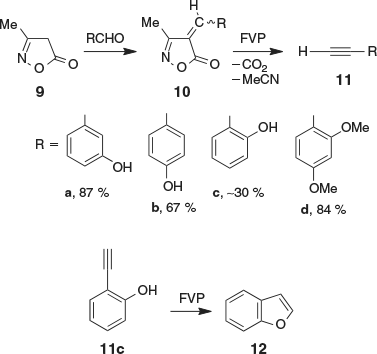
|
However, the o-ethynylphenol 11c was only obtained in ~30 % yield by FVP of 10c because the majority of 11c cyclized to benzofuran 12 at 800°C. When 10c was pyrolyzed at 1000°C, almost pure benzofuran 12 was obtained together with only a very small amount of 11c (Scheme 3). The fact that 11c can be obtained at all in this pyrolysis is thanks to the low pressure and short contact times in our apparatus. In contrast, Bloch and Orvane reported that o-ethynylphenol is completely converted into 12 at 800°C (the pressure was not reported, but it is likely that it was higher than ours, resulting in longer contact times and a more facile secondary reaction at the same nominal temperature).[13] It is not known whether the cyclization 11c → 12 involves addition of the OH group to the vinylidene 2 or to the acetylene function. The addition of weakly nucleophilic phenols to acetylenes usually requires transition metal catalysis, e.g. with HgII,[14] PdII,[15] AuIII,[16] or CuI.[17] We envisage that this unwanted cyclization could be suppressed by protecting the OH group by silylation. The cyclization of 11c to 12 suggests that 1-buten-3-yn-1-ols of the type HO–CH=CH–C≡C–R may also cyclize to furans under FVP conditions. A potential reaction of this sort may take place in the previously reported rearrangement of allenic ketones (4-arylbuta-2,3-dienones) to furans at 700–750°C[18] (see Scheme S1, Supplementary Material).
In contrast to the ethynylphenols, the methoxyphenylacetylenes are relatively stable and can be handled under normal laboratory conditions. Thus, 2,4-dimethoxyphenylacetylene 11d was obtained in 84 % yield by FVP of 10d. The related preparations of o- and p-methoxyphenylacetylenes in 75–95 % yields were described previously.[5]
In conclusion, the FVP method of synthesis of acetylenes from aldehydes via isoxazolones is a convenient method for the preparation of a variety of derivatives, including kinetically unstable, highly sensitive compounds such as the ethynyl derivatives of five-membered heterocycles (Scheme 1)[4] and the ethynylphenols (Scheme 3).
Experimental
The apparatus and methods for FVP have been described.[1,9] The apparatus illustrated in fig. 3b in ref. [9] was used. The vacuum was maintained at ~10−3 hPa with a Balzers two-stage oil pump. Starting materials were sublimed into the pyrolysis tube and pyrolyses were carried out at the temperatures given in the text. Pyrolysis products were isolated on cold fingers cooled with liquid N2.
4-(m-Hydroxyphenylmethylidene)-3-methylisoxazol-5(4H)-one 10a
m-Hydroxybenzaldehyde (6.10 g, 0.05 mol) was dissolved in 200 mL of dry CHCl3 and this solution was added dropwise with stirring to a solution of 3-methylisoxazolone (5.1 g, 0.05 mol) in 10 mL of dry CHCl3 in an ice bath under N2. The resulting mixture was stirred for 24 h in the dark. The light-yellow precipitate was filtered and recrystallized twice from ethanol/methanol 1 : 1 and further purified by sublimation at 170°C and 10−2 hPa to yield 6.70 g (66 %) of 10a, mp 234–235°C. δH (DMSO-d6) 9.9 (s, 1H), 8.0 (s, 1H), 7.9 (m, 1H), 7.6 (m, 1H), 7.2 (m, 2H), 2.3 (s, 3H). νmax (KBr)/cm−1 3300, 1730, 1610, 1595, 1560, 1470, 1310, 1270, 1230, 890, 780, 765, 680. m/z (EI) 203 (M+, 40 %), 145 (12), 134 (16), 133 (82), 118 (15), 89 (11). Anal. Calc. for C11H9NO3: C 65.03, H 4.46, N 6.89. Found: C 64.76, H 4.34, N 6.66 %.
4-(p-Hydroxyphenylmethylidene)-3-methylisoxazol-5(4H)-one 10b
This compound was prepared in the same manner as 10a from p-hydroxybenzaldehyde (2.44 g, 0.02 mol) to afford a yield of 2.90 g (71 %) of 10b, mp 216–218°C. δH (DMSO-d6) 11.0 (br s, 1H, OH), 8.5 (d, J 9, 2H), 7.8 (s, 1H), 7.0 (d, J 9, 2H), 2.2 (s, 3H). νmax (KBr)/cm−1 3500–3200, 1725, 1590, 1510, 1230, 1180, 860, 775. m/z (EI) 203 (M+, 100 %), 145 (21), 118 (8), 89 (5). Anal. Calc. for C11H9NO3: C 65.03, H 4.46, N 6.89. Found: C 65.20, H 4.38, N 6.97 %.
4-(o-Hydroxyphenylmethylidene)-3-methylisoxazol-5(4H)-one 10c
This compound was prepared in the same manner as 10a from o-hydroxybenzaldehyde (1.22 g, 0.01 mol) to afford a yield of 1.42 g (70 %) of 10c as yellow crystals, mp 168–170°C. δH (DMSO-d6) 10.5 (s, 1H), 8.1–6.9 (m, 4 H), 7.9 (s, 1H), 2.2 (s, 3H). νmax (KBr)/cm−1 3400, 2250 (br), 1760, 1740, 1720, 1695, 1610, 1460, 1370, 1350 (m), 1270, 1165, 995, 905, 765. Anal. Calc. for C11H9NO3: C 65.03, H 4.46, N 6.89. Found: C 65.02, H 4.32, N 6.81 %.
4-(2,4-Dimethoxyphenylmethylidene)-3-methylisoxazol-5(4H)-one 10d
This compound was prepared in the same manner as 10a from 2,4-dimethoxybenzaldehyde (1.66 g, 0.01 mol) to afford a yield of 1.00 g (40 %), mp 258°C. δH (DMSO-d6) 8.2 (s, 1H), 7.1–6.9 (m, 3H), 3.9 (s, 3H), 4.0 (s 3H), 2.3 (s, 3H). νmax (KBr)/cm−1 2850, 1730, 1580, 1550, 1320, 1270, 1205, 860, 820, 760, 730. m/z (EI) 247 (M+, 100 %), 216 (37), 177 (56), 173 (10), 162 (23), 147 (13), 119 (14). Anal. Calc. for C13H13NO4: C 63.16, H 5.30, N 5.66. Found: C 63.20, H 5.41, N 5.64 %.
m-Ethynylphenol 11a
Compound 10a (161 mg, 0.79 mmol) was sublimed into the FVP apparatus at 170–180°C and pyrolyzed at 800°C and 10−3 hPa. The products were isolated in a liquid-N2 cold trap. After the end of the experiment, the CO2 and acetonitrile were removed under vacuum, and the remaining light-yellow, liquid product was distilled at 50°C and 1 hPa to yield 81 mg (87 %) of 10a as a colourless liquid. δH (DMSO-d6) 7.2–6.7 (m, 4H), 4.90 (br s, 1H, OH), 3.05 (s, 1H). νmax (KBr)/cm−1 3600–3000 (br), 3280, 2090, 1565–1610, 1465, 1430, 1135, 850, 770, 675. m/z (EI) 118 (M+, 100 %), 89 (18), 63 (10). HRMS m/z 118.0420; calcd for C8H6O 118.0419. The properties are in agreement with literature data.[10,12]
p-Ethynylphenol 11b
Compound 10b (300 mg) was sublimed into the FVP apparatus at 150–160°C and pyrolyzed at 800°C and 10−3 hPa. The products were isolated in a liquid-N2 cold trap. After the end of the experiment, the CO2 and acetonitrile were removed under vacuum at −40°C, and the remaining brown, crystalline product was sublimed at 25°C and 10−1 hPa to yield 117 mg (67 %) of 11b as a white solid, mp 56–58°C. δH (DMSO-d6) 7.45 (d, 2H, J 9), 6.80 (d, 2H, J 9), 4.85 (br s, 1H, OH), 3.01 (s, 1H). νmax (KBr)/cm−1 3400–3200 (br), 3270, 2097, 1610, 1590, 1500, 1440, 1225, 830, 820, 680. m/z (EI) 118 (M+, 100 %), 89 (22), 63 (11). HRMS m/z 118.0417; calcd for C8H6O 118.0419. The properties were in agreement with literature data.[11,12]
o-Ethynylphenol 11c and Benzofuran 12
(a) Compound 10c (150 mg) was sublimed into the FVP apparatus at 140°C and pyrolyzed at 800°C and 10−3 hPa. The products were isolated in a liquid-N2 cold trap. After the end of the experiment, the CO2 and acetonitrile were removed under vacuum at −40°C, and the remaining yellow-brown oil was distilled bulb to bulb to yield a ~1 : 2 mixture of 11c and 12.
Data for 11c: δH (CDCl3) 7.4 (m, 1H), 7.3 (m, 1H), 7.0 (m, 1H), 6.8 (m, 1H), 5.9 (s, 1H, OH), 3.5 (s, 1H, CC–H). νmax (KBr)/cm−1 3400 (br, OH), 2120 (vw, CCH), 1535 (w), 1340 (m), 1200 (w), 1150 (w), 940 (s), 830 (s), 810 (s). The properties were in agreement with literature data[12,19]
(b) Compound 10c was subjected to FVP at 1000°C. The IR and 1H NMR spectra of the product indicated that it was almost pure benzofuran 12 containing very little o-ethynylphenol 11c. Benzofuran 12 was identified by comparison of IR and NMR[20] spectra with those of an authentic sample.
2,4-Dimethoxyphenylacetylene 11d
Compound 9c (200 mg) was sublimed into the FVP apparatus at 170–180°C and pyrolyzed at 750°C and 10−3 hPa. The products were isolated in a liquid-N2 cold trap. After the end of the experiment, the CO2 and acetonitrile were removed under vacuum, leaving 80 mg (84 %) of crude 11c, which was purified by column chromatography (silica gel, CHCl3). The first fraction afforded 11c as an oil (50 mg, 52 %). δH (DMSO-d6) 7.4 (d, J 9, 1H), 6.5 (m, 1H), 6.4 (d, J 2, 1H), 4.0 (s, 1H), 3.9 (s, 3H), 3.8 (s, 3H). νmax (KBr)/cm−1 3280, 2960, 2100, 1601, 1570, 1405, 1275, 1200, 810. m/z (EI) 162 (M+, 100 %), 119 (15), 91 (11), 65 (10). HRMS m/z 162.0670; calcd for C10H10O2 162.0681. The properties were in agreement with literature data.[21]
Supplementary Material
Scheme S1 showing the potential cyclization of 1-buten-3-yn-1-ols to furans under FVP conditions is available on the Journal’s website.
Acknowledgement
This work was supported by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and The University of Queensland.
References
[1] C. Wentrup, J. Becker, H.-W. Winter, Angew. Chem. Int. Ed. 2015, 54, 5702.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXnvV2qu7s%3D&md5=b7dd16af7032c9dffce3d82f6ee2c1c5CAS |
[2] C. Wentrup, H.-W. Winter, D. Kvaskoff, J. Phys. Chem. A 2015, 119,
| Crossref | GoogleScholarGoogle Scholar |
[3] (a) Acetylenes in synthesis: Science of Synthesis: Houben-Weyl Methods of Molecular Transformations (Ed. H. Hopf) 2008, Vol. 43 (Thieme: Stuttgart).
(b) I. V. Alabugin, B. Gold, J. Org. Chem. 2013, 78, 7777.
| Crossref | GoogleScholarGoogle Scholar |
(c) In materials science: E. Chernick, R. Tykwinski, J. Phys. Org. Chem. 2013, 26, 742.
| Crossref | GoogleScholarGoogle Scholar |
(d) In click chemistry: H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. Engl. 2001, 40, 2004.
| Crossref | GoogleScholarGoogle Scholar |
(e) M. Meldal, C. W. Tornøe, Chem. Rev. 2008, 108, 2952.
| Crossref | GoogleScholarGoogle Scholar |
(f) J. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 2007, 36, 1249.
| Crossref | GoogleScholarGoogle Scholar |
(g) M. G. Finn, V. V. Fokin, Chem. Soc. Rev. 2010, 39, 1231.and subsequent papers in this thematic issue.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. Wentrup, H.-W. Winter, Angew. Chem. Int. Ed. Engl. 1978, 17, 609.
| Crossref | GoogleScholarGoogle Scholar |
[5] C. Wentrup, W. Reichen, Helv. Chim. Acta 1976, 59, 2615.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXivFCgug%3D%3D&md5=2a7b707439467d26d26cca74fbab8272CAS |
[6] H. S. Rzepa, C. Wentrup, J. Org. Chem. 2013, 78, 7565.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpvFagtb8%3D&md5=b490bc6d532dddc8525aaf0bdfc17de5CAS | 23795601PubMed |
[7] I. D. Mackie, R. P. Johnson, J. Org. Chem. 2009, 74, 499.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOmtrvO&md5=47cc0a0998cf3fcd5b9ce76ed82c6021CAS | 19067566PubMed |
[8] (a) H.-W. Winter, C. Wentrup, Angew. Chem. Int. Ed. Engl. 1980, 19, 720.
| Crossref | GoogleScholarGoogle Scholar |
(b) C. Wentrup, H. Briehl, P. Lorencak, U. J. Vogelbacher, H.-W. Winter, A. Maquestiau, R. Flammang, J. Am. Chem. Soc. 1988, 110, 1337.
| Crossref | GoogleScholarGoogle Scholar |
[9] C. Wentrup, Aust. J. Chem. 2014, 67, 1150.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVynt77E&md5=4bae9731de0c1c8a40cb2940537ce510CAS |
[10] F. Bohlmann, K.-D. Albrecht, G. Schmidt, Chem. Ber. 1966, 99, 2822.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XkvF2isr4%3D&md5=d65e877a327498735675a131f833de33CAS |
[11] I. L. Kotlyarevskii, M. I. Bardamova, Izvest. Akad. Nauk SSSR, Ser. Khim. 1964, 11, 2073.
[12] T. Pirali, S. Gatti, R. Di Brisco, S. Tacchi, R. Zaninetti, E. Brunelli, A. Massarotti, G. Sorba, P. L. Canonico, L. Moro, A. A. Genazzani, G. C. Tron, R. A. Billington, ChemMedChem 2007, 2, 437.The IR spectra of 2-, 3-, and 4-ethynylphenols reported by these authors cannot be correct with absorptions claimed at 2360 and 1705 cm−1; however, the NMR data appear to be correct.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvV2jtr4%3D&md5=44d846cc537cc860877850fde2841459CAS | 17278167PubMed |
[13] R. Bloch, P. Orvane, Tetrahedron Lett. 1981, 22, 3597.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XltFKgsQ%3D%3D&md5=981095a74953e7877f5f5b0df179eddeCAS |
[14] V. Franzen, in Friedel-Crafts and Related Reactions (Ed. G. A. Olah ) 1964, Vol. 2, pp. 413–476 (Wiley Interscience: New York, NY).
[15] A. Arcadi, S. Cacchi, M. Del Rosario, G. Fabrizi, F. Marimelli, J. Org. Chem. 1996, 61, 9280.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xntlerurw%3D&md5=99124ed0fb5f067bf42b6dc8f6ea7c03CAS |
[16] (a) A. S. K. Hashmi, E. Enns, T. M. Frost, S. Schäfer, W. Frey, F. Rominger, Synthesis 2008, 2707.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht12hsrbI&md5=b851645aebeecc7af99b615540cdff1bCAS |
(b) H. Huang, Y. Zhou, H. Liu, Beilstein J. Org. Chem. 2011, 7, 897.
| Crossref | GoogleScholarGoogle Scholar |
[17] G. W. Kabalka, L.-L. Zhou, L. Wang, R. M. Pagni, Tetrahedron 2006, 62, 857.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlCiurnJ&md5=3e49b1c3d2804b28ade0b30048808ad7CAS |
[18] R. Koch, H. M. Berstermann, C. Wentrup, J. Org. Chem. 2014, 79, 65.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFegsrjK&md5=d1cbd85be67f43ea0f3b5481e5cecca7CAS | 24328255PubMed |
[19] T. Visser, J. H. van der Maas, J. Chem. Soc., Perkin Trans. 2 1988, 1649.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtlCmtLc%3D&md5=c13bf3f3c8a36afcb71c873c01ff2c68CAS |
[20] R. J. Abraham, M. Reid, J. Chem. Soc., Perkin Trans. 2 2002, 1081.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjvFSqtLg%3D&md5=f7e8a7e12eb055bb9042c381b9ff114bCAS |
[21] S. F. Nielsen, A. Kharazmi, S. B. Christensen, Bioorg. Med. Chem. 1998, 6, 937.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVans74%3D&md5=f9eada7eef89fcd7028290db26ee5039CAS | 9730229PubMed |
* This paper is the third part of a trilogy on acetylenes from isoxazolones by flash vacuum pyrolysis. The first two are reported in references [1] and [2]. The three papers celebrate the award of an honorary doctorate by the University of Pau, France, the award of the David Craig Medal of the Australian Academy of Science, and the award of the Arthur J. Birch Medal of the Organic Division of the Royal Australian Chemical Institute to the corresponding author, all in 2014.


