Natural Products Version?
Russell A. BarrowResearch School of Chemistry, Australian National University, Acton, ACT 2601, Australia. Email: rab@anu.edu.au

Russell Barrow is a group leader (Organic Chemistry of Natural Systems) at the Research School of Chemistry, Australian National University. After completing B.Sc.(Hons.) and Ph.D. degrees at The University of Melbourne, he undertook post-doctoral research at The University of Hawaii, focusing on the isolation and synthesis of natural products. His current research interests include the chemical ecology of pollination strategies employed by Australian insects and the chemistry supporting the traditional use of fungi in Papua New Guinea, where he has an adjunct appointment at the University of Goroka. |
Australian Journal of Chemistry 69(2) 127-128 https://doi.org/10.1071/CHv69n2_FO
Published: 8 February 2016
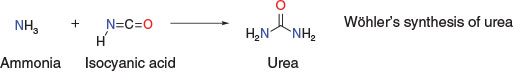
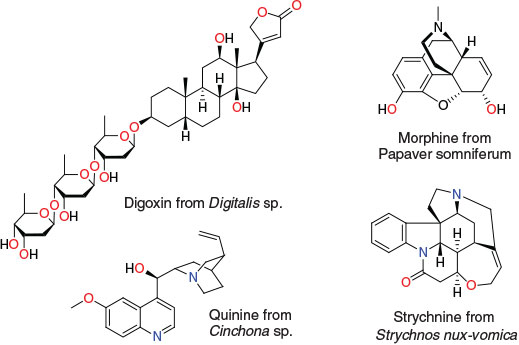
Organic chemistry is often regarded as originating with the chance discovery involving the synthesis of urea by Friedrich Wöhler in 1828 (Scheme 1). However, the study of organic compounds from natural sources – natural products chemistry – is the foundation of organic chemistry and had been ongoing for hundreds of years, not only in the form of traditional remedies such as digitalis (Withering, 1785), but also with the isolation and use of organic molecules including morphine (Sertürner, 1803), strychnine (Caventou and Pelletier, 1818), and quinine (Caventou and Pelletier, 1820), all predating Wöhler’s famed synthesis of urea (Chart 1).
|
|
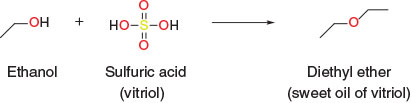
The earliest organic synthesis I am aware of involves the production of diethyl ether,[1] a molecule named by Augustus Frobenius in 1729, first used as an anaesthetic by Crawford Long in 1842, but regularly prepared synthetically since at least the 13th century. Diethyl ether, or ‘sweet oil of vitriol’ as it was known before Frobenius, was prepared synthetically by combining ethanol with sulfuric acid (vitriol) (Scheme 2).
|
What Wöhler’s synthesis did do was prove that organic molecules could be prepared from inorganic starting materials, contradicting the ‘vital force’ paradigm put forward by Jacob Berzelius requiring that organic molecules were the products of organisms.[2] Rather than establishing the field of organic chemistry, Wöhler’s discovery expanded it to include organic synthesis along with natural products chemistry as branches of organic chemistry.
What defines natural products chemistry in the 21st century?
Natural products chemistry in the 21st century is central to studies across multiple disciplines and branches within those disciplines. It is responsible for revealing complex molecular targets to challenge synthetic techniques, discovering target molecules of interest in agriculture and medicine and allowing insights into the ecological roles secondary metabolites play in biological systems. Arguably the greatest factor contributing to advances in natural products chemistry in recent years has been the capacity to identify and manipulate the biosynthetic gene clusters responsible for the in vivo production of these secondary metabolites,[3] thus giving rise to ‘Natural Products Version 2.0’ as coined by Walsh.[4]
Over the centuries, natural products chemistry has evolved from the use of crude preparations of plant materials to the isolation of pure compounds of unknown structures, through hard fought determination of molecule architectures from degradative studies of pure compounds to modern day instrumental techniques that facilitate the rapid isolation and identification of molecules at milligram and sub-milligram levels.[5]
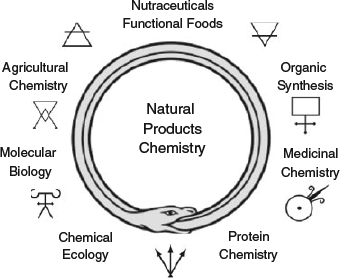
The ouroboros, used by our alchemical forefathers to represent the cyclical nature of their work and for millennia before them as a symbol representing ‘… something existing in or persisting from the beginning with such force or qualities it cannot be extinguished’,[6] is used here to represent the ever transforming and evolving study of natural products chemistry (Fig. 1). This will see a new generation of scientists trained across the disciplines of chemistry and biology, a change that is ongoing and reflected in the papers in this research front.
|
References
[1] C. Priesner, AMBIX 1986, 33, 129.| Crossref | GoogleScholarGoogle Scholar |
[2] Jöns Jacob Berzelius was credited with creating the term ‘organic chemistry’, dividing molecules up into those that required an organism to create them (organic) and those which did not (inorganic). https://en.wikibooks.org/wiki/Organic_Chemistry/Foundational_concepts_of_organic_chemistry/History_of_organic_chemistry (accessed 23 January 2016).
[3] For a recent review see P. J. Rutledge, G. L. Challis, Nat. Rev. Microbiol. 2015, 13, 509.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. T. Walsh, M. A. Fischbach, J. Am. Chem. Soc. 2010, 132, 2469.
| Crossref | GoogleScholarGoogle Scholar |
[5] T. F. Molinski, Nat. Prod. Rep. 2010, 27, 321.
| Crossref | GoogleScholarGoogle Scholar |
[6] https://en.wikipedia.org/wiki/Ouroboros (accessed 20 January 2016).


