
Australian Journal of Chemistry
Volume 70 Number 2 2017
Australasian Peptide Chemistry
This special issue of the Journal contains a selection of papers which showcase the cutting edge research being conducted in peptide chemistry today.
CH16511Unique Functional Materials Derived from β-Amino Acid Oligomers

As demonstrated in the recent literature, β-peptides can self-assemble into a range of materials. This highlight explores recent developments in the preparation of functional materials involving β-peptides, wherein the self-assembly of β-peptides is unperturbed by side chain functionalisation and leads to the formation of stable gels and scaffolds that can support cellular growth.
CH16425Atomic Force Microscopy Studies of the Interaction of Antimicrobial Peptides with Bacterial Cells
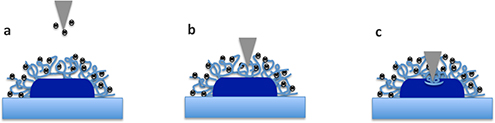
Many antimicrobial peptides (AMPs) are membrane-active but their mode of action remains elusive. Most studies have been conducted with synthetic membrane systems but, more recently, atomic force microscopy has been utilized to reveal the effect of AMPs on bacterial cells and provide great insight into how this class of peptides lyse cells.
CH16532Macrocyclic Peptidomimetics Prepared by Ring-Closing Metathesis and Azide–Alkyne Cycloaddition
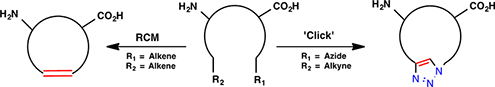
Ring-closing metathesis (RCM) and copper(i)-catalyzed azide–alkyne cycloaddition (CuAAC) are powerful strategies to introducing a macrocycle. This review focusses on the current synthetic approaches to access biologically active macrocycles that target important therapeutic aims for conditions such as cancer, cataracts, HIV, and neurological diseases.
CH16589Synthesis and Protein Engineering Applications of Cyclotides
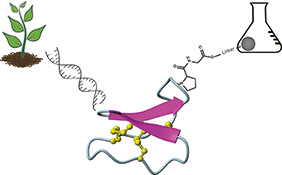
Cyclotides are a group of cysteine-rich peptides derived from plants. Their exceptional stability based on their head-to-tail cyclized backbone and cystine knot, together with their tolerance to sequence modifications, make them promising drug design scaffolds into which novel bioactive sequences may be grafted. These grafting applications are achievable by solid-phase peptide synthesis. This article describes recent developments in the chemical synthesis and enzyme-mediated cyclization of cyclotides.
CH16407Synthesis of Multivalent [Lys8]-Oxytocin Dendrimers that Inhibit Visceral Nociceptive Responses
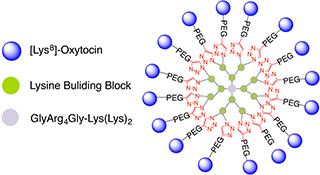
We demonstrate efficient ‘click’ synthesis of large dendrimeric oxidatively folded peptides with up to 16 copies of [Lys8]-oxytocin that maintain potency and structure.
CH16499An Efficient Chemical Synthesis of Lassomycin Enabled by an On-Resin Lactamisation–Off-Resin Methanolysis Strategy and Preparation of Chemical Variants
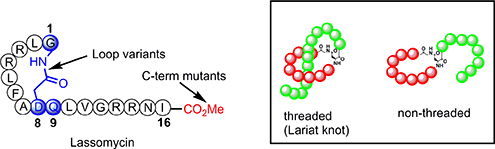
Lasso peptides contain a unique 3-D topography (known as a lariat knot) and are touted as novel scaffolds for biomedical applications. The synthesis of the non-threaded lasso peptide lassomycin was accomplished by solid-phase peptide chemistry and a novel way to install the C-terminal ester on the fully unprotected peptide.
CH16501Synthesis of Multicomponent Peptide-Based Vaccine Candidates against Group A Streptococcus
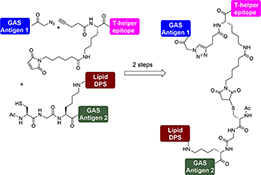
A stepwise conjugation technique was employed to synthesise a multiantigenic vaccine candidate against Group A streptococcus (GAS) through azide–alkyne cycloaddition and mercapto–maleimide reactions. The final vaccine candidate consists of four different components: two GAS antigens, a T-helper epitope, and the lipidic moiety dipalmitoyl serine (DPS).
CH16510Molecular Insights into the Interaction Between the SPRY Domain-Containing SOCS Box Protein SPSB2 and Peptides Based on the Binding Motif from iNOS
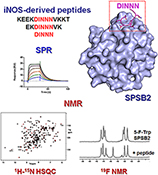
Surface plasmon resonance and NMR spectroscopy were used to gain insights into the binding of iNOS-derived peptides to SPSB2. The complex interplay of peptide sequence and protein binding will inform efforts to design peptide therapeutics to disrupt the iNOS–SPSB interaction.
CH16567Synthesis of Side-Chain Modified Peptides Using Iterative Solid Phase ‘Click' Methodology
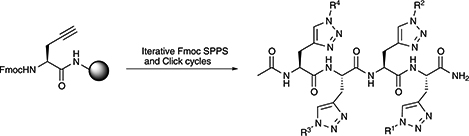
The introduction of an iterative ‘click’ step into solid phase peptide synthesis protocols provides a method for rapid functionalisation of peptide side chains with a range of diverse moieties.
CH16626Total Solid-Phase Synthesis of Biologically Active Drosophila Insulin-Like Peptide 2 (DILP2)
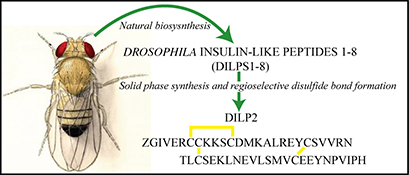
The fruit fly insulin-like peptide 2, DILP2, is one of seven closely related peptides that each act upon the same insulin-like receptor. It was successfully chemically assembled by regioselective disulfide bond formation within its two synthetic chains and shown to potently stimulate receptor phosphorylation and downstream signalling.
CH16591Alpha Helix Nucleation by a Simple Cyclic Tetrapeptide
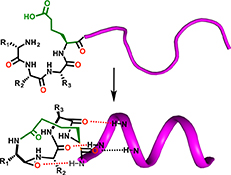
The simple cyclic tetrapeptide cyclo-(1,4)-[Ala-Arg-Ala-homoGlu]-NH2 (3) is shown to adopt an unusual α-turn structure. The latter structure is not α-helical but can nucleate α-helicity when attached to the N-terminus of either model peptides or two biologically relevant peptides. This new N-terminal helix-capping macrocycle provides simple and rapid synthetic access to α-helical peptide structures.
CH16659Fluorescent Ion Efflux Screening Assay for Determining Membrane-Active Peptides
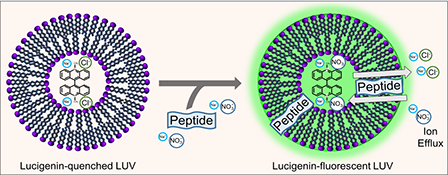
The emergence of antibiotic-resistant microbes and the lack of new antibiotics require development of new antimicrobial molecules and screening assays for them. We provide a proof of concept that a large unilamellar vesicle method used to study chloride ion efflux can be adapted to identify membrane-active antimicrobial peptides and screen their relative activity.
CH16559Synthesis of Norfijimycin A with Activity against Mycobacterium tuberculosis
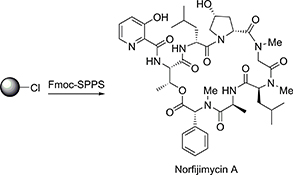
Fijimycin A is a cyclic depsipeptide that has been shown to possess activity against a range of pathogenic bacteria. This communication describes the total synthesis of norfijimycin A, a simplified analogue of the marine natural product fijimycin A. The natural product analogue was shown to possess significant activity against Mycobacterium tuberculosis, the etiological agent of tuberculosis.



