Limitations in Electrochemical Determination of Mass-Transport Parameters: Implications for Quantification of Electrode Kinetics Using Data Optimisation Methods
Elena Mashkina A , Alan M. Bond A B and Alexandr N. Simonov A BA School of Chemistry and the ARC Centre of Excellence in Electromaterials Science, Monash University, Clayton, Vic. 3800, Australia.
B Corresponding authors. Email: alan.bond@monash.edu; alexandr.simonov@monash.edu
Australian Journal of Chemistry 70(9) 990-996 https://doi.org/10.1071/CH17241
Submitted: 5 May 2017 Accepted: 4 June 2017 Published: 29 June 2017
Abstract
Voltammetric quantification of the electrode kinetics for the quasi-reversible reaction 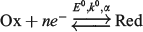 requires detailed experiment–theory comparisons. Ideally, predicted data derived from the theoretical model are fitted to the experimental data by adjusting the reversible potential (E0), heterogeneous electron transfer rate constant at E0 (k0), and charge transfer coefficient α, with mass-transport and other parameters exactly known. However, parameters relevant to mass transport that include electrode area (A), diffusion coefficient (D), and concentration (c), are usually subject to some uncertainty. Herein, we examine the consequences of having different combinations of errors present in A, D, and c in the estimation of E0, k0, and α on the basis of the a.c. (alternating current) voltammetric experiment–theory comparisons facilitated by the use of a computer-assisted parameter optimisation algorithm. In most cases, experimentally reasonable errors (<10 %) in the mass-transport parameters do not introduce significant errors in recovered E0, k0, and α values. However, a pernicious situation may emerge when a slight overestimation of A, D or c is included in the model and results in erroneous identification of a reversible redox process as a quasi-reversible one with a report of apparently quantifiable kinetic parameters k0 and α.
requires detailed experiment–theory comparisons. Ideally, predicted data derived from the theoretical model are fitted to the experimental data by adjusting the reversible potential (E0), heterogeneous electron transfer rate constant at E0 (k0), and charge transfer coefficient α, with mass-transport and other parameters exactly known. However, parameters relevant to mass transport that include electrode area (A), diffusion coefficient (D), and concentration (c), are usually subject to some uncertainty. Herein, we examine the consequences of having different combinations of errors present in A, D, and c in the estimation of E0, k0, and α on the basis of the a.c. (alternating current) voltammetric experiment–theory comparisons facilitated by the use of a computer-assisted parameter optimisation algorithm. In most cases, experimentally reasonable errors (<10 %) in the mass-transport parameters do not introduce significant errors in recovered E0, k0, and α values. However, a pernicious situation may emerge when a slight overestimation of A, D or c is included in the model and results in erroneous identification of a reversible redox process as a quasi-reversible one with a report of apparently quantifiable kinetic parameters k0 and α.
References
[1] R. S. Nicholson, I. Shain, Anal. Chem. 1964, 36, 706.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXktV2ms7s%3D&md5=d26dc31bb86f1bd2f6ec880aa40fe9a0CAS |
[2] R. S. Nicholson, Anal. Chem. 1965, 37, 1351.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XisFSksQ%3D%3D&md5=24ea942e94bd73847c501eceefc416c4CAS |
[3] A. J. Bard, L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications (2nd edn) 2001 (John Wiley & Sons, Inc.: New York, NY).
[4] (a) C. J. Miller, in Physical Electrochemistry, Principles, Methods and Applications (Ed. I. Rubinstein) 1995, pp. 27–79 (Marcel Dekker: New York, NY).
(b) N. S. Hush, J. Electroanal. Chem. 1999, 470, 170.
| Crossref | GoogleScholarGoogle Scholar |
[5] (a) A. M. Bond, N. W. Duffy, S.-X. Guo, J. Zhang, D. Elton, Anal. Chem. 2005, 77, 186A.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkslamu70%3D&md5=d5a49f0b782dbc4110e71bc8ce5e0925CAS |
(b) A. M. Bond, D. Elton, S. Guo, E. A. Mashkina, A. N. Simonov, J. Zhang, Electrochem. Commun. 2015, 57, 78.
| Crossref | GoogleScholarGoogle Scholar |
[6] A. M. Bond, E. A. Mashkina, A. N. Simonov, in Developments in Electrochemistry: Science Inspired by Martin Fleischmann (Eds D. Pletcher, Z.-Q. Tian, D. E. Williams) 2014, pp. 21–47 (John Wiley & Sons, Ltd.: Chichester, UK).
[7] (a) R. L. McCreery, Chem. Rev. 2008, 108, 2646.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1Wjsb8%3D&md5=39657dc283838a5b976c2506937c3efbCAS |
(b) A. M. Bond, N. W. Duffy, D. Elton, B. D. Fleming, Anal. Chem. 2009, 81, 8801.
| Crossref | GoogleScholarGoogle Scholar |
(c) R. G. Compton, C. E. Banks, Understanding Voltammetry (2nd edn) 2011 (Imperial College Press: London).
(d) S. C. S. Lai, A. N. Patel, K. McKelvey, P. R. Unwin, Angew. Chem. Int. Ed. 2012, 124, 5501.
| Crossref | GoogleScholarGoogle Scholar |
[8] A. N. Simonov, G. P. Morris, E. A. Mashkina, B. Bethwaite, K. Gillow, R. E. Baker, D. J. Gavaghan, A. M. Bond, Anal. Chem. 2014, 86, 8408.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFOqtb3P&md5=cfd7af05578ee5d893479d44c7f8d6abCAS |
[9] (a) A. N. Simonov, W. Grosse, E. A. Mashkina, B. Bethwaite, J. Tan, D. Abramson, G. G. Wallace, S. E. Moulton, A. M. Bond, Langmuir 2014, 30, 3264.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtlSjsrw%3D&md5=f22b2425a8686da2bfc5d961cbd9d722CAS |
(b) E. A. Mashkina, A. N. Simonov, A. M. Bond, J. Electroanal. Chem. 2014, 732, 86.
| Crossref | GoogleScholarGoogle Scholar |
(c) A. N. Simonov, J. F. Boas, M. A. Skidmore, E. A. Mashkina, C. M. Forsyth, M. Bown, A. M. Bond, Inorg. Chem. 2015, 54, 4292.
| Crossref | GoogleScholarGoogle Scholar |
[10] J. Janisch, A. Ruff, B. Speiser, C. Wolff, J. Zigelli, S. Benthin, V. Feldmann, H. A. Mayer, J. Solid State Electrochem. 2011, 15, 2083.and references therein
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12gsbnJ&md5=cd478d7751d5310602d6da1173af5c46CAS |
[11] G. P. Morris, A. N. Simonov, E. A. Mashkina, R. Bordas, K. Gillow, R. E. Baker, D. J. Gavaghan, A. M. Bond, Anal. Chem. 2013, 85, 11780.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1ygsLnP&md5=6ded27654298205f59846e574e97caa6CAS |
[12] T. Peachey, E. Mashkina, C.-Y. Lee, C. Enticott, D. Abramson, A. M. Bond, D. Elton, D. J. Gavaghan, G. P. Stevenson, G. F. Kennedy, Philos. Trans. R. Soc. A 2011, 369, 3336.and references therein.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1eqsrbL&md5=f852fda469130ab650b9e5e4560ba13bCAS |
[13] G. F. Kennedy, A. M. Bond, A. N. Simonov, Curr. Opin. Electrochem. 2017, 1, 140.
| Crossref | GoogleScholarGoogle Scholar |
[14] ElchSoft Simulation Software and Experience 2011. Available at http://www.elchsoft.com
[15] M. C. Buzzeo, R. G. Evans, R. G. Compton, ChemPhysChem 2004, 5, 1106.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnsVejtL8%3D&md5=338c99576a633e7a59708ef57b90a582CAS |
[16] K. B. Oldham, J. C. Myland, A. M. Bond, Electrochemical Science and Technology: Fundamentals and Applications 2012 (John Wiley & Sons, Inc.: Chichester, UK).
[17] K. Ngamchuea, S. Eloul, K. Tschulik, R. G. Compton, J. Solid State Electrochem. 2014, 18, 3251.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVGqs7nO&md5=bf60b1127b7a32f95908ae164352f579CAS |
[18] A. N. Simonov, E. Mashkina, P. J. Mahon, K. B. Oldham, A. M. Bond, J. Electroanal. Chem. 2015, 744, 110.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjsVanu7s%3D&md5=07eadff2971522054bfd1cbbc7edcaaaCAS |
[19] C. L. Bentley, A. M. Bond, A. F. Hollenkamp, P. J. Mahon, J. Zhang, in Electrochemistry in Ionic Liquids (Ed. A. A. J. Torriero) 2015, Vol. 1, Ch. 5, pp. 143–168 (Springer International Publishing: Cham, Switzerland).
[20] J. C. Myland, K. Oldham, J. Solid State Electrochem. 2014, 18, 3259.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFShu7zL&md5=60bdd8ee0e71b2771f659f94b6bc077fCAS |
[21] A. N. Simonov, G. P. Morris, E. A. Mashkina, B. Bethwaite, K. Gillow, R. E. Baker, D. J. Gavaghan, A. M. Bond, Anal. Chem. 2016, 88, 4724.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XltlOns7o%3D&md5=8140754e94ed77fd8b66b3e352cfbc8aCAS |
[22] J. Li, C. L. Bentley, A. M. Bond, J. Zhang, Anal. Chem. 2016, 88, 2367.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XpsFahug%3D%3D&md5=c4587d8b87d480b647b74ca5e8ff9941CAS |


