Wheat physiology: a review of recent developments
R. A. FischerCSIRO Plant Industry, PO Box 1600, Canberra, ACT 2601, Australia. Email: tony.fischer@csiro.au

Tony Fischer was born in January 1940 and grew up on a wheat–sheep farm in the Riverina of NSW, where he has still maintained a close links until recently. He completed his secondary education in Melbourne, with B. Agric. Sci., (University of Melbourne), graduating in 1961. He then obtained a Master’s of Agricultural Science degree (also from University of Melbourne) in 1964, and a PhD in Plant Physiology from the University of California (Davis) in 1967. Tony has worked for NSW Department of Agriculture at Wagga Wagga, CSIRO in Canberra, CIMMYT (Centro Internacional de Mejoramiento de Maiz y Trigo) in Mexico (1970–95, 1988–95), and finally for ACIAR (The Australian Centre for International Agricultural Research) from 1995 to 2005. He is currently an Honorary Research Fellow at CSIRO Plant Industry. Whilst at CIMMYT, Tony was the Director of the Wheat Program from 1988 to 1995 and later has been a member of the Board of Trustees of GRDC, ICARDA and IRRI. His research interests have always been the physiology, agronomy and genetic improvement of wheat, which has been gradually expanded to include the role of crop science in international agricultural development. Tony has published over 100 peer-reviewed papers. Tony was awarded the CM Donald Medal in 2004, the William Farrer Memorial Medal in 2007 and Member of the Order of Australia (AM) in 2007 for services to agricultural research. |
Crop and Pasture Science 62(2) 95-114 https://doi.org/10.1071/CP10344
Submitted: 25 October 2010 Accepted: 18 January 2011 Published: 17 February 2011
Abstract
This review focuses on recent advances in some key areas of wheat physiology, namely phasic development, determination of potential yield and water-limited potential yield, tolerance to some other abiotic stresses (aluminium, salt, heat shock), and simulation modelling. Applications of the new knowledge to breeding and crop agronomy are emphasized. The linking of relatively simple traits like time to flowering, and aluminium and salt tolerance, in each case to a small number of genes, is being greatly facilitated by the development of molecular gene markers, and there is some progress on the functional basis of these links, and likely application in breeding. However with more complex crop features like potential yield, progress at the gene level is negligible, and even that at the level of the physiology of seemingly important component traits (e.g., grain number, grain weight, soil water extraction, sensitivity to water shortage at meiosis) is patchy and generally slow although a few more heritable traits (e.g. carbon isotope discrimination, coleoptile length) are seeing application. This is despite the advent of smart tools for molecular analysis and for phenotyping, and the move to study genetic variation in soundly-constituted populations. Exploring the functional genomics of traits has a poor record of application; while trait validation in breeding appears underinvested. Simulation modeling is helping to unravel G × E interaction for yield, and is beginning to explore genetic variation in traits in this context, but adequate validation is often lacking. Simulation modelling to project agronomic options over time is, however, more successful, and has become an essential tool, probably because less uncertainty surrounds the influence of variable water and climate on the performance of a given cultivar. It is the ever-increasing complexity we are seeing with genetic variation which remains the greatest challenge for modelling, molecular biology, and indeed physiology, as they all seek to progress yield at a rate greater than empirical breeding is achieving.
Introduction
A review honouring Australia’s first wheat breeder, William Farrer, in a journal titled Crop and Pasture Science cannot avoid considering wheat physiology’s delivery of useful impact at the crop level, even though plant physiology today spans from the molecular level of functional genomics, through individual organ and plant studies, to that of the crop. Increasingly physiologists appear to expect applications from their research, but the path leading to impact on crop productivity is often more difficult than anticipated. Attention to such impact will be a secondary theme of this review.
‘Recent’ is defined here as the last 10 years or so, but a brief excursion into the history of wheat physiology, is warranted. Much of it is to be found in the predecessor to this Journal, namely the Australian Journal of Agricultural Research (1950–2008), and some bias towards the role of Australian research is admitted. Farrer was in fact one of the first to think and write about adaptive traits in wheat (e.g. Farrer 1898). Interest in numerical components of wheat yield blossomed in the 1920s and 1930s in the UK, with work by F. L. Engledow and S. M. Wadham, moving on, in the 1950s, to crop growth analysis under the guidance of D. J. Watson and G. N. Thorne, and in the following decade, to source-sink yield analysis by breeder J. Bingham. In Australia, H. C. Trumble and A. E. V. Richardson did important early work on wheat transpiration, before C. M. Donald put crop physiology definitively on the map (e.g. Donald 1962), pointing out that plant behaviour in the highly competitive crop community is usually quite different to that of plants in isolation. In 1963 the Canberra Phytotron opened, and for the next 30 years the environmental control of wheat growth and development was extensively researched therein, especially by L. T. Evans and I. F. Wardlaw. At Wagga Wagga, in New South Wales, A. T. Pugsley was sorting out the major genes behind phasic development, W. V. Single was grappling with wheat, nitrogen (N) and frost, and R. A. Fischer with haying-off; and in 1969 H. A. Nix published the first physiologically based simulation model of grain yield in the wheat crop. From about then onwards, wheat being Australia’s major crop, the funding for wheat physiology research grew notably, as did the published results. At the same time, research on wheat physiology languished somewhat in Europe, only to be picked up elsewhere in the new world, initially Canada and the USA, then Mexico and Argentina, followed lately by a revival of interest in Europe. Much of these developments are captured in the reviews by Evans et al. (1975), Slafer et al. (1999), Passioura (2002), Fischer (2007) and Reynolds et al. (2009).
In reviewing recent research, it is impossible to ignore the boom in molecular biology and functional genomics research. Much adopts a bottom-up or reverse genetics approach, working from the gene level upwards towards function, typically starting with change in gene expression. It is not surprising that it is proving very difficult to reach an understanding of function at higher levels of organisation, especially that of plant phenotypes and crop performance in the field, through this route. However, the alternative, top-down forward genetics approach, starting with observable genetic variation at higher levels of organisation, is bringing some progress at the level of genetic control. Thus, some attention will be given to the latter approach, where relevant, in this review of the physiology of the wheat plant and crop. This will follow under the headings of phenology, potential yield (PY), water-limited PY, effects of some other abiotic stresses (aluminium, salinity, heat shock), advances in simulation modelling, and concluding remarks. Attention will focus on common or hexaploid wheat (Triticum aestivum L.) unless otherwise stated.
Wheat phenology
Days to anthesis
Genetic variation in flowering (anthesis) date and crop duration are primary considerations in adaptation (Evans 1993); these are part of the crop’s phenology or phasic development which describes the occurrence of key growth and development events. Date of anthesis (AN) is the first appearance of dehisced anthers (if a crop, in 50% of spike-producing culms); time to AN is more accurately described as degree-days above a temperature base, usually of 0°C. Duration is under strong genetic control from a few alleles of a relatively limited number of genes affecting sensitivity to photoperiod (Ppd), to vernalising cold (Vrn), and to earliness per se (Eps) 1 . For these reasons, it has been the favourite subject of geneticists and physiologists for almost 100 years. The classic work on wheat genes by Pugsley (1968, 1972), and on cultivar responsiveness and environmental control by his colleague, Syme (1968), set the scene for a flood of physiological genetic research on the subject (see reviews by Slafer and Rawson 1994 and Slafer et al. 2009). Thus, it is not surprising that molecular biology has had an impact in this area of wheat physiology. Initially isolines and chromosome substitution lines developed laboriously by geneticists and cytogeneticists provided the material for quantification of genetic effects (e.g. Pugsley 1972; Worland 1996; Stelmakh 1998). However, in the last 20 years or so, quantitative trait loci (QTL) analyses has contributed to gene localisation, and in the last decade, perfect molecular markers for several key photoperiod and vernalisation alleles have greatly assisted isoline development and/or characterisation of cultivars. At the same time, the actual biochemistry of gene action in wheat has yielded somewhat to the powerful tools of functional genomics (e.g. Dwivedi et al. 2008; Trevaskis 2010).
González et al. (2005a) usefully summarised early work with Ppd-B1 and Ppd-D1 iso- and chromosome substitution lines: the sensitive alleles delayed anthesis/heading in long days at high latitudes around 2.5 days (Ppd-B1) or 5 days (Ppd-D1), but in short days at low latitudes, as much as 13 days (Ppd-B1) 2 . Worland (1996) had previously shown that the Ppd-D1-sensitive allele delayed heading too much for the adaptation of winter wheats in southern Europe, but that the smaller delays in the UK were inconsequential for performance. Immediately preceding the arrival of accurate molecular markers, Dyck et al. (2004) revealed in spring wheat isolines sown across latitudes 40–58°N in North America that the Ppd-D1-sensitive allele, to be found in most spring-planted spring wheats at high latitude, delayed heading on average 3 days. van Beem et al. (2005) determined by test crossing to known sources the sensitive/insensitive alleles at the four Vrn loci across 51 cultivars largely from CIMMYT, but did not quantify effects. However, they did measure genetic differences in Eps and responsiveness to temperature, using fully vernalised seed, grown under 24-h photoperiod: at 16/6 or 23/12°C, days to anthesis ranged from 59 to 74, and 45 to 63 days, respectively. Recently the first gene for Eps appears to have been located on chromosome A1 in Triticum monococcum L. (Eps-Am1), with evidence that a similar gene may be present in hexaploid wheat (Lewis et al. 2008). In one of the earliest studies on Vrn alleles with molecular markers (and chromosome substitution lines), Iqbal et al. (2007a , 2007b ) quantified the delaying effect on AN of the vernalisation-sensitive alleles at each of the three homeologous locii (Vrn-A1, Vrn-B1, Vrn-D1) across Canadian spring cultivars grown at 54°N. Effects were additive, but also interactive, with the allele at Vrn-A1 the strongest; having all sensitive alleles give a winter wheat which was usually far too late, while the genotype with all insensitive alleles was too early and only suited to the most northern short season locations.
Molecular markers permitted Eagles et al. (2009) to identify alleles at four key loci in around 120 Australian cultivars from the 19th Century up until 2007 and in 18 CIMMYT cultivars. This review will adopt their allele nomenclature for the photoperiod gene Ppd-D1 (insensitive allele a, or sensitive allele b), and the vernalisation genes Vrn-A1, Vrn-B1 and Vrn-D1 (all having dominant-insensitive allele a, or sensitive allele v; Vrn-A1 has a second dominant-insensitive allele, b). The classification often corresponded quite well to field performance, for example, of the maturity classes of varieties in Western Australia in the mid 1990s (Table 1). These classes govern the recommended optimal sowing date, such that varieties all reach flowering in a common optimum period in September (Anderson et al. 1996). Table 1 adds the Triple Dirk isolines, developed originally by Pugsley (1968, 1972) and widely used in research globally ever since. Not shown is his Triple Dirk F, which Yoshida et al. (2010) showed to have an insensitive allele at Vrn-D4 (at which gene all other isolines are vernalisation sensitive); these authors also confirmed that in the presence of the weaker insensitive a alleles (Vrn-B1, Vrn-D1, Vrn-D4) there remains a small residual vernalisation response.

|
More recently, Eagles et al. (2010) have related days to heading to the allelic composition (for Ppd-D1 and Vrn-A1, Vrn-B1 and Vrn-D1 only) of 1085 genotypes across 128 late April to early July sowings at many sites in south-eastern Australia (latitude 34–37°S). There were 8524 observations in this unbalanced dataset, from which allelic effects on days to heading were estimated for an early June sowing. Effects were estimated with considerable accuracy (s.e.d. <0.7 days). In photoperiod-insensitive genotypes (Ppd-D1 a), a single vernalisation sensitive v allele delayed heading on average 2.3 days, but the Vrn-D1 allele was the most powerful (3.1 days) and the Vrn-B1 allele the least (1.6 days). Furthermore, effects were not exactly additive (i.e. epistasis) such that substituting a single insensitive a allele hastened heading most (4.1 days) when compared to the winter type (vvv), and hardly at all (0.4 days) when it gave rise to a totally spring genotype (aaa). The analysis also indicated the effects of v alleles were greater when the post-sowing environment was warmer (despite the fact that all vernalisation requirements would have been satisfied naturally within 48 days of sowing even at the warmest site-years), notably so when comparing the winter type with those with only one a allele (i.e. avv, vav, vva). This explained the greater delay in flowering with winter wheats (vvv) the warmer the site. Finally the photoperiod-sensitive allele (Ppd-D1 b) was estimated to delay heading on average 7 days compared to the insensitive one (Ppd-D1 a), an effect which was greatest in fully vernalisation-insensitive genotypes (aaa, 11.8 days).
Eagles et al. (2010) has been highlighted because it shows the way forward: the value of more exactly identifying key alleles, and the power of modern statistics for deducing patterns from complex unbalanced data. But the identified alleles explained only 45% of the genetic variance in days to heading (main effect of genotypes), while there was also a genotype × sowing-site variance component equal to 20% of the main effect. Probably other major genes are involved (e.g. Ppd-B1, Vrn-D4), and even unknown major genes and alleles 3 , such as those controlling Eps. In addition the dataset covered a relatively narrow range of latitude and sowing dates. Taking a wider dataset of 24 winter wheat and five spring wheat cultivars grown across 12 years and 82 global locations ranging from latitude 19 to 61° in the International Winter Wheat Performance Nursery, White et al. (2008) had earlier considered the allelic classification at Ppd-D1, Vrn-A1, Vrn-B1, and Vrn-D1 in an effort to predict days to flowering in the set. Using the simulation model CERES version 4.0.2.0, cultivar parameters for photoperiod sensitivity (P1D) and vernalisation sensitivity (P1V) were fitted across 105 site-years, following which P1D parameter values were fitted to the known photoperiod alleles, and P1V to the vernalisation ones, to give relationships which could be used in CERES as an allele-based algorithm. Validation across 257 additional sowings (at separate sites), showed that using the original parameters explained only 27% of the genetic variation, compared to even less (17%) with the allele-based approach. In conclusion, using molecular markers to identify the major development alleles and thus to predict AN, has progressed rapidly recently, and obviously will be an exciting area for the near future. The challenge will be to improve predictive accuracy, for breeders usually target environments where only a few days difference in AN can be significant for performance, and yet they have handled the issue empirically with relative ease since William Farrer himself.
Beyond degree-days to anthesis
A new challenge would be to develop cultivars for which date of AN was the same (or at least always optimal) regardless of sowing date. Given the uncertainty surrounding sowing (germination) date in dryland cropping at intermediate latitudes with autumn and winter planting, this would mean farmers need not hold a suite of varieties of different maturity classes as shown in Table 1. A degree of vernalisation responsiveness is obviously needed with early sowings when photoperiods are longer, and in general gives the least delay in flowering per day delay in sowing [typically around 0.3 day, Syme (1968)], but this is not enough.
A second and greater novel challenge with the new knowledge is to ask whether it matters which combination of major gene alleles produces a given date of AN (see also below). For the wheat physiologist, phasic development not only includes AN, but also the timing of key events within this period such as floral initiation, terminal spikelet (TS), end of tillering/start of stem elongation (SE), flag leaf emergence, and meiosis, and that of events during grain filling (Slafer et al. 2009). Vernalisation of sensitive cultivars accelerates events to floral initiation and sometimes TS, while longer photoperiods appear to accelerate development right up to flowering, and increased temperature accelerates all development (Slafer and Rawson 1994; Slafer et al. 2009), although the base temperature for a constant degree-day response appears to increase, and clearly exceeds 0°C for the later stages of development (e.g. Fischer 1985). The relationship between the duration of the various phases and the production and survival of numerical components of grains m–2 (tillers, spikes, spikelets, florets, grains) has been soundly established (e.g. Slafer and Whitechurch 2001; González et al. 2005a ). Lately most interest has focused on the period in the crop most critical for the determination of grains (per m2), namely that of spike growth (dry matter accumulation in spike structure), with the view to manipulating development genetically so as to lengthen the period around spike growth, preferably at the expense of earlier periods, thus not changing time to AN.
Fischer (1984) defined the spike growth period as that when the spikes in a crop accumulate the last 95% of final dry weight excluding grains (for a single culm, approximately the interval from penultimate leaf emergence, well after TS/SE, to AN (see Fig. 1)). Only photoperiod and temperature appear to directly control the duration of spike growth as defined, since they appear to also control the duration of the subtending longer period, TS/SE to AN (Fischer 1985; Slafer and Rawson 1994), and even the last part of this period, namely flag leaf emergence to AN (e.g. Whitechurch and Slafer 2002). Reduced duration with artificially extended photoperiod in this period (and before the period, via a memorised effect) had been shown in the field in Mexico (Wall 1979; Fischer 1985), and later confirmed in the Canberra Phytotron (Miralles et al. 2000) and in the field in Argentina (Whitechurch and Slafer 2002; González et al. 2003, 2005a ). With Chinese Spring chromosome substitution lines, the TS–AN period appeared to be sensitive to photoperiod in the presence of the Ppd-B1b-sensitive allele, but not the Ppd-A1b or Ppd-D1b ones; in shorter photoperiods the period was notably longer only with Ppd-B1b (Whitechurch and Slafer 2002). However, the same Argentine laboratory found a longer duration (and sensitivity to photoperiod) with the Ppd-D1b-sensitive allele and little response to the Ppd-B1one in Mercia vernalised isolines (González et al. 2005a ). These latter authors admit to many remaining uncertainties in understanding the genetic control of duration of this critical phase.
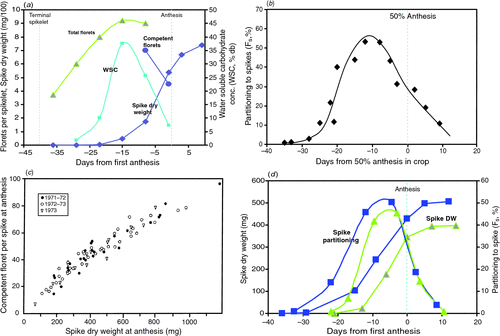
|
One weakness in the above studies is that actual spike growth duration is not a development phase, and was not measured, although it is clearly only the later portion of the TS/SE to AN phase. Indeed it has been too loosely defined since its beginnings in Fischer (1984). In reality spike growth is sigmoidal and continues for a few days after AN (see Fig. 1). The author (unpubl. data) has shown that in a single culm the growth duration, defined now as the interval between 5 and 95% final dry weight, as suggested in Abbate et al. (1997), for the main shoot of the cultivar Yecora 70, is 300 degree-days (above 4.5°C) at 11-h photoperiod and only 200 degree-days at 17-h; commencement was well after SE, at 22 and 17 days before AN, respectively, and termination 3 days after AN (mean temperature (Tmean) was 16.4°C). Abbate et al. (1997) reported a similar duration in field crops, and suggested the duration to be 4.5 phyllocrons. Serrago et al. (2008) also measured growth duration directly, albeit somewhat differently, and showed it to be reduced by longer photoperiod in two cultivars, but not in another (but see below). In summary we remain far from the goal of Fischer (1985) and Slafer and Rawson (1994) of boosting yield through lengthening the duration of spike growth at the expense of earlier periods. Whether via exploiting natural genetic variation in the independent photoperiod sensitivities of different periods, as the early work of Wall (1979) and others (see Slafer and Rawson 1994) suggest may exist, or such variation in Eps, or via exploiting the burgeoning functional genomic knowledge pertaining to photoperiod sensitivity (e.g. Dwivedi et al. 2008), it remains a very worthwhile challenge.
Potential yield
PY is defined as the yield achieved by an adapted cultivar in the absence of manageable biotic and abiotic stresses, in particular lack of water, and in the presence of a given representative natural resource base of climate and soil. In many situations around the world, PY progress drives farm yield growth hence its importance (Fischer and Edmeades 2010). Solar radiation, temperature and photoperiod, interacting with genotype, in turn determine PY.
Flowering date and crop duration
While phasic development provides the temporal framework within which the crop develops, climate and cropping system constraints, along with empirical selection, have generally optimised the dates of sowing, AN and maturity within which PY is realised. Relatively simple considerations of Tmean and solar radiation seem to underpin the flowering date for maximum yield in wheat. At low latitudes (~<30°), optimum flowering date is a compromise, following closely upon the early spring maximum in photothermal quotient [PTQ = solar radiation/(Tmean – 4.5°)] for greatest grains/m2 (GN), but tempered by the need to maximise weight per grain (GW), which is inversely related to post-flowering temperatures (e.g. Ortiz Monasterio et al. 1994). At intermediate latitudes (~30–40°), with autumn-sown spring wheats, where winters are cold enough to notably slow growth, these relationships still appear to hold (e.g. Stapper and Fischer 1990b ). At even higher latitudes, where severe winter cold and winter wheats predominate, at least in humid northern Europe where grain filling is mild, for example in south-east UK, PY appears to be relatively insensitive to variation in date of AN around an optimum of early June (Foulkes et al. 2004). In the UK solar radiation peaks in June, Tmean in July–August, but spring temperatures are too low for the above PTQ to apply; nevertheless, May is probably the month with the best growth per unit of development time. At the highest latitudes for wheat, where spring-sown spring wheats prevail (e.g. Canada), the situation is distinct: sowing as early as soil warming allows and flowering as late as permitted by autumn frost appears to be optimum, because maximising days from sowing to flowering is linked to higher yield in such environments (e.g. Iqbal et al. 2007b ).
Extra total duration before flowering is important with high-latitude spring wheats because the time to build leaf area and grain sites is inevitably short. How important it is in the other three situations mentioned is a misunderstood issue. Fischer (1985) working with irrigated spring wheat in Mexico (latitude 27°N) suggested that a longer duration to AN, giving full light interception at earlier stages of development, may increase leaf and tiller production, and total dry matter at flowering, but does not increase spike dry weight (SDW). The latter is maximised as long as full light interception is reached before the onset of spike growth (penultimate leaf emergence, see above); with well managed crops, full light interception is easily achieved before this point in the crop’s development (unless extra early cultivars are used). Studies with well watered autumn-sown spring cultivars of differing times to flowering in southern Australia appear to confirm this (e.g. Stapper and Fischer 1990a ; Gomez-Macpherson and Richards 1995): the extra early growth with longer cycle wheats is also associated with greater lodging risk and perhaps greater respiratory losses later on, although it does open up the possibility of early grazing without yield loss, and does bring larger root systems, but this may not carry a net benefit under humid conditions. In intermediate winter-cold environments, such as humid south-western Victoria, a more intermediate duration and sowing date combination may be optimum (Rivkin and O’Leary 2010). Finally, where winter growth is severely constrained by low temperatures such as most winter wheat environments, sowing date is more a question of being early enough to guarantee good winter survival; spring growth can still be excessive and N is managed to avoid this. Thus, it appears that, except with high-latitude spring wheats, increased sowing to AN duration and the resultant increased crop biomass at AN may not be critical for maximising yield, with sowing date therefore driven by other considerations.
Grain number
The consideration of grain yield as the product of grains/m2 (GN) and final GW has become common. It has the advantage of two components separated somewhat temporally and easy to determine, although care needs to be taken regarding the fate of small grains in mechanical threshing. In many cases yield variation and yield progress is associated with GN changes which, more recently, have been linked to dry weight accumulation in spikes (g/m2) at flowering. This framework, which actually traces back to Bingham (1969), was further developed by Fischer (1984), has been adopted in recent reviews (Fischer 2007; Miralles and Slafer 2007; Reynolds et al. 2009), and will be used here. This strong emphasis therein on dry matter accumulation and partitioning up to flowering was challenged recently by Sinclair and Jamieson (2006) who saw the crops’ acquisition of reduced N as equally or more fundamental to yield determination, than that of assimilate from photosynthesis driving GN. However, under potential conditions, where, by definition yield is relatively unresponsive to N, there is no evidence to support this notion, and even under conditions of N stress, GN at least is more directly related to SDW than to spike N (see rebuttal in Fischer 2008). Thus:

where SDWa is the dry weight of spikes at anthesis (per unit area basis), Ds is the duration of spike growth, and CGR and Fs are, respectively, the rate of crop dry accumulation and the fraction of this dry matter growth partitioned to the spike averaged over the spike growth period (as defined above). Also:

where the ratio of grains : SDWa, initially proposed as a cultivar-specific trait (Fischer 1984), is now termed the spike fertility index (SFI). It can be usefully disaggregated as well:
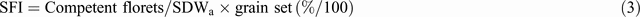
in which competent florets have plump anthers just before AN, and grain set, refers to those competent florets which progress through pollination, fertilisation and early grain survival to bear grains at maturity. Grain set is important, and can fall significantly below 100% under stress, and even sometimes under apparently favourable conditions, especially in older cultivars (e.g. Evans et al. 1972), but will not be discussed further in the context of PY. Figure 1a illustrates the partitioning of dry matter to the growing spike, the associated water-soluble carbohydrate (WSC) changes, and the formation of competent florets in the simple situation of a single main stem, while Fig. 1b shows partitioning in wheat crops, and Fig. 1c the relationship of competent florets to SDWa across spikes taken from crops of a given variety.
From the above model, traits Ds and CGR capture the important environmental determinants of GN. Thus, major climatic influences are seen in the simple PTQ ratio during spike growth mentioned earlier, with Ds inversely proportional to Tmean, and CGR proportional to solar radiation and relatively-stable radiation-use efficiency (RUE, crops under PY conditions usually intercept all the incident solar radiation before the onset of the spike growth stage). However, this simplification ignores several generally weaker influences of climate on RUE, such as low vapour pressure deficit (vpd) and a high ratio of diffuse to total solar radiation improving RUE and thus CGR (see review by Stockle and Kermanian 2009), as well as possible independent effects of minimum temperature (Tmin). In addition, the influence of photoperiod on Ds should be included, as seen in Serrago et al. (2008) and illustrated experimentally in Fig. 1d . The positive effect of lower Tmin (but not approaching freezing levels) has received attention lately in rice, but may also operate in wheat (Lobell et al. 2005), possibly because of greater respiratory losses on warmer nights, but data are lacking.
Despite the strong association between yield progress through breeding and GN, genetic effects on the above determinants of GN are less widely reported. Following Eqn 1, Ds did not vary in a tall versus short isogenic comparison (see references in Fischer 2007) nor in the comparison of modern Argentine wheats (Abbate et al. 1998), so apart from Serrago et al. (2008) above, cultivar effects on Ds have yet to be described. However, large Argentine cultivar differences in the duration of the phase (TS/SE to AN) in °C (at least 2-fold for a given photoperiod) were reported by Whitechurch et al. (2007), and these may reflect differences in Ds, which falls largely within TS/SE to AN. Also, González et al. (2005a) showed a highly significant relationship between SDW at flowering and solar radiation intercepted over the TS to AN phase, when its duration was varied by photoperiod extension across photoperiod-sensitive alleles in the Mercia background.
For CGR during spike growth, most studies find no genetic differences (e.g. Sayre et al. 1997; Abbate et al. 1998). However, a positive effect of genetic progress (year of release) on RUE between start of SE and AN, and on SDWa, was seen in UK winter wheats released between 1972 and 1995 (Shearman et al. 2005). Indirect evidence for a similar relationship underlying spring wheat GN and yield comes from positive correlations of pre-anthesis stomatal conductance (gs) and light-saturated photosynthetic rate (Pmax) with GN progress in north-west Mexico (Fischer et al. 1998), and from similar pre-anthesis conductance/Pmax correlations with yield in a warmer Mexican environment (Reynolds et al. 2000). Short erect leaves tending to have a higher specific leaf area and specific leaf N, common in the latest winter and spring cultivars (Fischer et al. 1998; Shearman et al. 2005), could also be contributing to the CGR increase through higher RUE. These observations hinting at increased GN associated with photosynthesis and CGR immediately before flowering have support from similar results with modern versus older rice cultivars in Japan (see Fischer and Edmeades 2010).
Dwarfing genes clearly enhanced Fs, the partitioning of dry matter to growing spikes (e.g. Fischer 1984, 2007; Abbate et al. 1998; Slafer et al. 1999). The explanation is that shorter stems, growing at the same time as spikes, compete less for limited assimilate, permitting spikes to acquire more, but the real control mechanisms are likely to be much more complex (Fischer and Stockman 1986; Bancal 2008). On the other hand, for a given genotype, Fischer (2007) demonstrated under controlled conditions that Fs was quite stable across different total assimilation amounts, and even different potential spike sizes, as caused by early photoperiod effects on spikelet number. Abbate et al. (1998) found only small differences in Fs (range 0.29–0.34) among modern semi-dwarf Argentine cultivars. Reynolds et al. (2001) reported that the GN advantage associated with LR19 from Agropyron was associated with a proportional increase in Fs.
Significant genetic variation in SFI was first noted in modern Argentine cultivars (Abbate et al. 1998); values ranged from 61 to 106 grains/g, entirely explaining GN variation among five cultivars. Shearman et al. (2005) also reported genetic variation in SFI (range 73–129 grains/g) but no significant increase with year of release, nor association with GN. It is also evident that there can be some environmental effects on SFI. In particular, in controlled environments, heavy shading in the critical stage just before AN reduced the number of competent florets per unit spike weight at AN (Fischer and Stockman 1980). This probably also happens in the field, especially in lower order tillers, which are already disadvantaged in the canopy, as was clearly shown by Wall (1979). Several other issues can further complicate the study of SFI: in the field crop AN takes place over several days across the main culms and tillers, blurring precision with respect to stage of development. Second, a spike does not finish growing until several days after first anthesis (see Fig. 1a, b ), by which time grains are beginning to grow; such grains need to be removed if spike weight is to be correctly determined 4 . Finally, it is tempting to use chaff weight at maturity to calculate SFI (e.g. Stapper and Fischer 1990a , who found fairly consistent cultivar differences on this basis), but chaff weight can be 20–50% greater than spike weight at AN, both in controlled environments and in the field (Wall 1979; Fischer and Stockman 1980; Stockman et al. 1983), for reasons that need to be better understood.
The close link between dry matter accumulation in the growing spike, floret survival (for many more florets are initiated than ever survive to competency, see Fig. 1a ), and competent floret number, exists whether spike weight is varied by shading, dwarfing genes, photoperiod, and photoperiod × photoperiod-sensitive alleles (Fischer and Stockman 1980, 1986; González et al. 2003, 2005a , 2005b ); even photoperiod shortening in the field is reported to increase duration and spike size (M. Vasquez, unpubl. data). Subsequently there has been detailed exploration of this relationship by González et al. (2005c), Bancal (2008, 2009) and Ghiglione et al. (2008). There seems little doubt that distal florets, especially in basal and distal spikelets, are the most vulnerable, and that florets begin visibly to ‘die’ early in the spike growth period; death begins at ~10% final spike weight when SDW accumulation is approaching the maximum rate. It is around the time that WSC concentration in the spike normally peaks (Fischer and Stockman 1980; Bancal 2008; Ghiglione et al. 2008; also Fig. 1a ). This appears to confirm the link between floret survival and carbohydrate supply. Subsequently, florets continue to ‘die’ up until close to AN, at differing rates and durations of floret failure which are poorly understood. Ghiglione et al. (2008) found large differences in the expression of many genes associated with the greater levels of floret death under long compared to short photoperiods, but was unable to conclude much about the causality of death. In a departure from shading and photoperiod treatments, Ugarte et al. (2010) applied differing red : far red light ratios to spaced wheat plants over the whole period from SE to AN; there were interesting but difficult-to-interpret effects on rate of floret development and spike growth.
In conclusion, much research recently has focused for obvious reasons on GN determination: exploring the relationship to SDWa seems to remain a sound approach. It agrees with the observation that the final GN always equates to only a small percentage of the initiated florets, and with the notion that a floret competent to bear a grain represents a significant relatively fixed dry matter investment in spike structure, something which can, however, vary between cultivars. Searching for underlying mechanisms and even molecular controls of floret survival (e.g. Ghiglione et al. 2008) has been unsuccessful and indeed seems futile if the dry matter cost at anthesis of competent florets remains fixed. It may be more rewarding to note that the spike growth period is only the latter part of the TS/SE to AN phase, and to manipulate assimilate supply, and other aspects of the environment (e.g. temperature, red/far red radiation, ethylene), over sub-periods within the period, as in Fischer and Stockman (1980) and Stockman et al. (1983). Examining the nature of spike sink strength (essentially ratio Fs above) and the basis of genetic differences in this ratio, may also be more fruitful for achieving SDWa increase. On the other hand, any changes which increase GN via increased SFI should note the tendency for a trade-off between SFI and potential grain size (e.g. Fischer and HilleRisLambers 1978; Dreccer et al. 2009).
Grain weight
Final GW in wheat is traditionally considered as the product of the duration of linear grain growth and the rate of this growth. Following Bingham (1969), Fischer (1984) proposed it to be the result of an interplay between the potential GW (the sink), being the GW reached when the assimilate supply is not limiting grain growth, and the actual supply of assimilates per grain during grain filling (the source). For wheat crops under PY conditions, it is often reported that grain-filling source exceeds the sink capacity of grains; this was clearly the case with older cultivars, but still seems to be so with the most modern cultivars (see below). At the same time GW is under the strong influence of grain-filling Tmean (a negative relationship with slope of 2–7%/C for Tmean between 15 and 28°C (Wardlaw and Wrigley 1994)), and of cultivar, although genetic yield progress has generally not raised GW. There is also a weak positive GW response to grain-filling solar radiation independent of temperature (Fischer 1984). The negative effect of temperature is related to a shortening of grain filling (in days, not in degree-days), which is not fully compensated for by an increase in grain growth rate. Cultivar differences in GW tend, however, to be largely related to differences in filling rate.
The above simple notions, including that of a cultivar-specific potential GW, have proved useful, but now need to accommodate the fact that recent research has shown GW to be affected by conditions before anthesis, in particular spike temperature in the period between booting to AN, and especially heading to AN: even though the duration of leaf area during grain filling was unaffected, higher temperature just before AN reduced carpel size at AN, and then GW (Calderini et al. 1999, 2001; Ugarte et al. 2007). There was a positive relationship between GW and carpel size, which seems to apply not only as a result of temperature variation immediately pre-anthesis (Calderini et al. 1999), but also with cultivar differences (Calderini and Reynolds 2000; Calderini et al. 2001), with variation in floret position along the spikelet rachilla, and with variation in their apparent assimilate supply in the period (e.g. pre-anthesis floret removal treatments in Calderini and Reynolds 2000). The GW versus carpel weight relationships tended to be curvilinear downwards; temperature and floret thinning treatments a week after AN had no effect on GW in this work (Calderini and Reynolds 2000). Potential GW therefore appears to be determined by carpel size and not solely by endosperm cell division occurring in the week or so after AN as was believed earlier. Work has continued, seeking to relate GW to early grain expansion and hence to the size of the pericarp, already present in the carpel, and following the expression of expansin-coding genes in elongating pericarp cells, expression which correlated well with early grain expansion (Lizana et al. 2010).
While potential GW may be determined by events up to about a week either side of AN, realisation of this potential across all grains in the crop (the grain-filling sink) depends on an adequate supply of assimilates during the grain-filling period (the source), both from current assimilate and WCS stored at AN in the crop, principally in stems and sheathes. Arguments about the importance of source versus sink during grain filling in wheat are legion (Evans 1993; Sinclair and Jamieson 2006; Fischer 2008) and the outcome will obviously depend at least to some extent on the weather before relative to that after AN. While low radiation (as simulated by post-anthesis shading) and high temperature can reduce GW by tipping the source-sink balance towards source limitation, in most wheat studies GW is quite insensitive to artificial manipulation of source/sink, such that in the control crop, sink limitation appears to dominate during grain filling (Borras et al. 2004; Miralles and Slafer 2007). A component of this apparent insensitivity is seen in the increased Pmax during grain filling when GN was artificially increased in four modern varieties (Reynolds et al. 2005).
The apparently low level of source limitation during grain filling in commercial cultivars under potential conditions is probably ultimately related to the market penalty for grains which are not plump, but the physiological mechanisms could be multiple. Thus, as breeders have lifted GN and PY, they may have (unwittingly) increased WSC levels at anthesis; these reserves can be translocated relatively efficiently to the grain and buffer GW against reduced current assimilate as argued by Borras et al. (2004). This appears to have happened in UK winter wheats lately: WSC content rose significantly with genetic yield progress, at a rate of ~20 mg for each extra grain, and the most modern varieties have ~4 t/ha WSC at around anthesis (Shearman et al. 2005). Considerable research is now underway on WSC reserves, which tend to peak at the onset of grain filling, when most measurements are focused. Spring wheat populations revealed a large range in WSC concentration at the onset of grain growth, a moderate to high narrow-sense heritability, complex genetic control across up to 10 QTL, and an association with larger GW, less grain shrivelling, but in some backgrounds, also earliness (Rebetzke et al. 2008; Dreccer et al. 2009). Borras et al. (2004) also suggest that the insensitivity of GW to source variation could be due to the early establishment in wheat of the potential or maximum GW, at a time when GN is also being determined, thereby facilitating adjustment of the grain-filling sink to the potential source. Another likely factor is that green area and photosynthetic activity are maintained longer into the grain-filling period in modern varieties [as is widely recognised in modern maize hybrids, Fischer and Edmeades (2010)]. Certainly RUE levels during grain filling have improved (e.g. Miralles and Slafer 1997), and some modern varieties appear to show better ‘stay green’ (Christopher et al. 2008). Finally it appears that grain-filling photosynthetic activity can actually be increased by a larger GN sink (see above). It would also seem very likely that any gain in post-anthesis assimilate production has required greater levels of leaf N late in the life of the canopy, something which might constrain the N harvest index (HI) (N in grain as a % of total N uptake by the crop).
Homeostasis of propagule size (e.g. GW in wheat) is a strong force in nature (Sadras and Denison 2009). This appears to have persisted through yield improvement by breeding: genetic variation in GW is common and an easy selection target, but it has not generally contributed to higher PY. In reviewing this general phenomenon Egli (2006) points out, as did Borras et al. (2004) above, that temporal overlap in the determination of GN and potential GW aids compensation or trade-off between these components. The indication of a strong negative genotypic relationship between potential GW and SFI in Fischer and HilleRisLambers (1978), suggests one such compensatory mechanism. Another could arise when GN increase is associated with more grains per spikelet [i.e. more grains in rachilla positions 3 and 4 with lower potential GW (Miralles and Slafer 1995)]. But such relationships do not explain why there seems to be overcompensation, such that in most comparisons across wheat genotypes, as GW increases, GN falls faster, so that PY also falls. However, before concluding that future breeding progress will be a question of continuing to maintain GW rather than increase it, it is worth noting that recent PY progress in spring wheat at CIMMYT appears also to be associated with GW increase (Aisawi et al. 2010). Whether their large-grained parent Baviacora or Babax (Sayre et al. 1997) is an exception to the rule, or the beginning of a new rule, is unknown, but the GN-GW nexus appears also to have been weakened in the CIMMYT-derived Seri-Babax mapping population studied intensively in Queensland (Rattey et al. 2009).
Harvest index and lodging resistance
Following Donald and Hamblin (1976), crop physiologists tended to relate wheat grain yield to total biomass and HI. This model suffers physiologically because many processes are integrated into these two components; nevertheless its simplicity and the moderate heritability of HI are advantages. Fischer (2007) recently pointed out that the highest values of HI in winter wheats (~0.50), and especially spring wheat (0.45), leave scope for some improvement, if an upper limit of 0.62 (Austin 1980) is accepted, and if we note that modern varieties of rice and maize are approaching an HI of 0.55 (Fischer and Edmeades 2010). But as the height of modern wheat varieties settles at an apparent optimum of ~70–100 cm (Flintham et al. 1997), there is already a tendency for recent PY breeding progress to be less linked solely to HI, and more to both HI and biomass, or even biomass alone (e.g. Shearman et al. 2005; Aisawi et al. 2010). In addition a recent thorough physical analysis of dry matter costs of reducing stem lodging in heavy wheat crops to a tolerable risk, limits HI to closer to 0.50, and involves extra dry matter investment in surface roots for reduced root lodging risk (Berry et al. 2007); in addition, for a given yield level and HI, a larger spike size and corresponding culm diameter, via reducing culm number, is more efficient for reducing lodging than more smaller spikes borne by narrower culms. There seems little doubt that further PY increase in wheat will depend more on increased biomass, necessarily accompanied by a HI, which is as high as possible: culling progeny with HI below 0.40 or 0.45 would seem a sensible breeding strategy, and rapid determination of HI thus a worthwhile goal.
Water-limited potential yield
A simple model
The prevailing paradigm for understanding water-limited potential yield (PYw) starts with the quantity of water available for the crop, namely the available water in the root zone at sowing plus rainfall on the crop. Assuming there is no in-crop run-off or deep drainage, nor any available water in the soil at maturity, this quantity equals crop evapotranspiration (ET). By definition, water limitation implies that ET is no more than say two-thirds of potential ET for the crop. PYw is then usefully described by three components:

where Es is soil evaporation in the crop, so that ET – Es is transpiration (T), TE the transpiration efficiency, and HI. These relatively independent components are reasonably well understood (see review of Passioura and Angus 2010). The original proponents of Eqn 4 had shown that in southern Australia, Es was typically ~100 mm, with PYw rising in linear proportion to additional ET at a rate of 20 kg/ha.mm (French and Schultz 1984). Sadras and Angus (2006) suggest that the slope for modern varieties could now be approaching 22 kg/ha.mm.
The distribution of rainfall during the crop cycle can be more important than Eqn 4 suggests, especially in low water-holding capacity soils, such that if water stress is evident at AN, and there is no further rain, post-anthesis stress will be severe and HI very low. Approximately 30% of ET must occur after AN for the maximum HI, as determined by cultivar and other aspects of climate, can be reached. Use of the wheat simulation model APSIM (see later) has further improved consideration of the effect of rainfall distribution, and simulations with historical weather suggests that PYw is best represented by a boundary function of about the same slope as determined by French and Schultz (1984), but which cannot be reached in all years because of poor rainfall distribution or water losses through deep drainage, run-off, or very late rain events (Hochman et al. 2009b ).
Traits for improved PYw
The phasic development framework for dry conditions have been broadly determined empirically, initially for Australia by William Farrer himself, but there is need for greater flexibility than for PY because sowing date is governed more by rainfall occurrence, and nowadays, seasonal climate forecasts (see later) can drive tactical adjustments by the farmer. PYw is usually very sensitive to sowing date delays: improved pre-sowing agronomy and better seeding machinery have helped guarantee early seeding and germination. Physiology has also contributed with a natural herbicide-resistance trait permitting dry seeding, and hopefully will further assist with the search for long coleoptile wheats which can emerge from deep moisture-seeking seed placement (Richards 2006). An unstressed plant height of 70–100 cm is optimum for PYw, and most wheat cultivars achieve this with one of the Norin-10 dwarfing genes (Rht-B1b, Rht-D1b), but these GA-insensitive semi-dwarf wheats have short coleoptiles and do not emerge well from deep seeding especially in warm soils. Alternative GA-sensitive dwarfing genes with longer coleoptiles are being sought and tested with success (Rebetzke et al. 2007). Finally, optimum flowering date for PYw may be earlier than that for PY because of climate considerations, even though it brings an increased risk of spike frosting in mid-latitude spring wheat environments like Australia or Argentina. Researchers are again targeting resistance to spike frosting, but progress has been very slow in wheat although somewhat promising in barley (ACPFG 2010a ); quantification of the likely benefit through simulation modelling would be helpful.
Significant soil evaporation (Es) occurs whenever solar radiation reaches wet soil (once the soil surface dries Es drops markedly and is less radiation-dependent): Es is thus an important wasteful component in rain-fed cropping systems (ranging from 30 to 70% of ET), obviously being smaller where cover, whether by the crop or by surface residue, is less. For a given cover, the Es saving relative to no cover is greater when rains are frequent, potential evaporation is low and soil texture is heavy (Gregory et al. 2000). A rapid approach to substantial cover by the crop itself can therefore reduce Es losses in winter-rainfall environments. As well it needs to be noted that partial cover and a dry soil surface can cause significant energy transfer from the soil to the canopy and its atmosphere (e.g. Gregory et al. 2000), further favouring higher green cover and pointing to the importance of micrometeorology to our full understanding. Fischer (1979) was surprised by marginal water-use efficiencies for dry matter as high as 150 kg/ha.mm with growth (cover) enhancing treatments (earlier sowing, higher fertiliser and greater seed density) at Wagga Wagga and Tamworth. In heavy-textured infertile soils in the dry winter-rainfall environment of Syria (<300 mm annual rainfall), N and P fertiliser so boosted crop cover that the replacing of Es by T alone explained much of the substantial yield increase (Cooper et al. 1987). Similarly it is postulated that selection for greater early vigour in wheat, for which there is substantial genetic variation, can significantly boost growth at little overall cost in ET (Richards and Lukacs 2002).
Equation 4 assumes the crop uses all available soil water. Sometimes, however, available water is found at maturity deep in the root zone, even when the crop has been severely stressed. Such water could have been used during grain filling very efficiently since it is not subject to evaporative losses and all assimilation then goes to the grain: thus Kirkegaard et al. (2007) measured grain efficiencies of up to 60 kg/ha.mm when crops were subirrigated at depth during grain filling. Unused deep water points to insufficient deep roots arising from physical or chemical (salinity, acidity, high boron) restrictions in the subsoil. To the extent that the water is replenished between crops or in wet years, [which is not always the case (Lilley and Kirkegaard 2007)], it is a wasted resource. Genetic tolerance of roots to high boron is believed to benefit subsoil water extraction where subsoil boron is high (Millar et al. 2007); molecular characterisation is being pursued (ACPFG 2010b ). Variation in root depth and water extraction between genotypes has been shown by Manschadi et al. (2006) and Lopes and Reynolds (2010); it is also evident that longer cycle wheat tend to have deeper roots (Fig. 2a ). Interest in this previously neglected area of wheat physiology is now high (e.g. Palta and Watt 2009).
|
Strictly speaking TE refers to the ratio of photosynthesis to transpiration, but in Eqn 4 it refers to net dry matter accumulation (above ground) relative to crop transpiration. It is strongly controlled by an inverse relationship to daytime vpd, and is notably greater for crops with the C4 photosynthetic pathway than C3 crops like wheat. Nevertheless, Farquhar and Richards (1984) found TE in wheat to show useful cultivar variation, which in accord with theory, was related inversely to 13C isotope discrimination (Δ). This knowledge fired research in the area, and some 20 years later cultivars began to be released with the high TE trait (Richards 2006). It was learnt that in wheat, high TE is mostly associated with lower gs and Pmax. This trade-off meant high TE was only superior in environments where growth tended to rely more on soil stored moisture and Es was low [conversely Δ is positively related to PY (e.g. Fischer et al. 1998)]. Several issues remain: little research has compared high TE/low gs genotypes with low TE/high gs cultivars on a scale sufficiently large to be fully relevant to farmer fields. One such attempt (Condon et al. 2002), based on 10-ha fields of each type, was frustrated by the poorer growth of the former, but did suggest that T (and hence TE) differences driven by gs differences, were relatively less than seen in leaf gas exchange studies because of the uncoupling of the crops from the atmosphere (i.e. the presence of a significant atmospheric resistance to energy and gas interchange within and immediately above the crop canopy), again pointing to the importance of micrometeorology. Another issue is suggested here: in most latitudes crop growth before flowering is often occurring at suboptimal temperatures for leaf expansion, if not for photosynthesis [although frost can have lasting effects on the latter (Koh et al. 1978)]; there must be implications for TE but little research now focuses on this, or on the underlying genetic variability.
The final component in Eqn 4, HI, is especially sensitive to water stress just before flowering and again during grain filling. The former is related to the sensitivity of pollen viability at the young microspore stage, which actually occurs at around flag leaf emergence in any culm, ~10 days before AN. Some of the effect of this stress may operate via reduced photosynthesis, as reflected in reduced final SDWa, but there is little doubt that many cultivars show an additional depression in GN per spike due to male sterility (Fischer 1973), effectively reducing SFI; Fig. 2b illustrates this for a drought-affected wheat crop. The physiology of the sterility has been related to changes in histology, hormones, and gene expression (Koonjul et al. 2005; Ji et al. 2010). Importantly the latter paper found repeatable genotypic differences (as did Fischer (1980)), and related susceptibility to an inability to maintain carbohydrate supply to the anthers, as reflected inter alia in changes in their fructan-transferase gene expression.
The second aspect of HI currently under intense scrutiny is the role of WSC storage at AN as a useful trait in grain-filling terminal drought. As already mentioned under PY, the WSC content shows genetic variation, and van Herwaarden and Richards (2002) were able to relate the grain yield of cultivars under dry conditions to the WSC levels at AN. However, definitive confirmation of the benefit of this trait under terminal drought from the recombinant inbred populations (RIL) of Rebetzke et al. (2008) and Dreccer et al. (2009) has yet to surface. Lopes and Reynolds (2010) recently suggested that WSC at AN might be competitive with deep roots (it must also surely be competitive with spike growth); also it is apparent that the amounts of WSC may be quite small if there is water shortage before flowering (Dreccer et al. 2009). Interestingly, van Herwaarden et al. (1998a ) were able to advance understanding of the grain shrivelling or ‘haying-off’ phenomenon in high N crops under terminal drought, by showing that WSC reserves were actually reduced in such crops.
Many other traits have been proposed as important for understanding and advancing PYw in wheat (e.g. Tambussi et al. 2007; Reynolds and Tuberosa 2008). Many of these and the above-mentioned traits are also discussed in detail in Richards et al. (2010), who go on to consider the vital issue of proper strategies for their validation and subsequent utilisation in breeding, an issue often neglected in the past.
What does PYw variation in the real world tell us?
Many years ago, physiologists started looking at grain yield variation and trait associations among cultivars, then there were comparisons of isolines with and without given traits, and lately RIL and bulk segregant analyses have come to dominate. With each step the linking of yield variation, through traits to genes, has been advanced, but at the same time, greater complexity has been revealed. Recent studies with an elite × elite CIMMYT population (Seri-Babax) of more than 160 RILs are illustrative (Olivares-Villegas et al. 2007; Rattey et al. 2009). Averaged over 3 years at a single location in north-west Mexico under controlled terminal drought (RIL mean yield range 100–450 g/m2), the former reference showed yield to be weakly correlated with days to AN (phenotypic r = –0.26), strongly with height (r = 0.67) and canopy temperature (r = –0.72 to –0.78 for the average of measurements on 5–7 days), and very strongly with GN (r = 0.97); it was postulated that differences in soil water extraction explained these associations, and later work with extremes from the population confirmed this (Lopes and Reynolds 2010). This could be considered a reasonably satisfactory result. When Rattey et al. (2009) tested the material in south-east Queensland over three locations and 5 years (only six environments, with yield range 202–660 g/m2), the mean yield of the RILs ranged from ~350 to 500 g/m2, and were correlated with days to AN (genotypic r = –0.50), GN (0.52), HI (0.47), and biomass (0.36) and weakly with GW (0.22) and WSC at anthesis (0.25). Canopy temperature relationships with yield were weaker than those in Mexico (A. Rattey, pers. comm.). McIntyre et al. (2010) went on to map yield and traits in the Queensland study, identifying many significant QTL, but none of better than weak explanatory value. The weaker relationships seen in Queensland are likely to be more realistic of a breeding program target, reflecting natural drought and soil variation, and suggest no simple path to improved PYw. On the positive side, it does seem the population produced some RILs combining high GN and GW, and yielding significantly more than the parents and the best local checks.
Other abiotic stresses
Wheat is subject to many other abiotic stresses (salinity, aluminium and boron toxicity, water logging, high temperature, low temperature and frost, ozone, pre-harvest rain, etc.), all of which reduce yield (and/or quality) to a significant extent in different parts of the world. The physiology of response to and tolerance of most of these appears to be simpler than that for water stress, even if some may be linked to water stress (e.g. salinity), and genetic differences are easier to demonstrate. It is therefore to be expected that physiology has progressed further in understanding and exploiting these differences, especially as agronomic solutions tend to be unavailable, or expensive (e.g. drainage, liming). Space permits brief attention to only three examples, the first two of which reveal excellent progress and good chances of impact, a welcome contrast with the frustrations of advancing PYw.
Aluminium tolerance
Selection of wheat progeny tolerant of high levels of the soluble trivalent aluminium cation in the rhizosphere, a common problem of acid soils, has been practised for many years, often via solution culture screening. The role of malate secretion by root tips in this tolerance was recognised by Delhaize et al. (1993), malate precipitating the aluminium cation. Molecular markers for a major resistance gene were located (Riede and Anderson 1996), and the gene itself was identified (TaALMT1) in tolerant wheat and sequenced by Sasaki et al. (2004), the first ever such plant gene. In an unusual reversal of the chronological order of things, the gene has been transformed into Arabidopsis where it is effective, and into barley, while its overexpression in a susceptible wheat cultivar leads to improved tolerance in an acid soil with a high % of exchangeable aluminium (Pereira et al. 2010). The effective allele appears to have a stronger promoter region leading to greater efflux of malate when activated by aluminium (Raman et al. 2008). A second gene, from the MATE family, has recently been found and sequenced in wheat: it is also aluminium activated but leads to citrate excretion (Ryan et al. 2009). Tolerance has been exploited in conventional breeding, while the transgenic approach offers further options.
Salinity
Salinity refers to high sodium chloride in the root zone and it imposes both an instant osmotic stress similar to water stress which reduces leaf growth, as well as a slowly developing stress due to toxic levels of Na+ in key leaf tissues which reduce photosynthesis and hastens senescence (Munns and Tester 2008). Bread wheat is moderately tolerant of salinity as a species, durum wheat less so, barley more so. Physiological studies of genotypic differences in tolerance in wheat point to the importance of differences in Na+ exclusion from leaves. Initially a single gene (Kna1) from bread wheat and known to exclude Na+ was located on 4DL (Dubcovsky et al. 1996). Lately extensive screening and QTL analysis of appropriate populations (tolerant × susceptible) have revealed two genes which exclude Na+ in durum wheat leaves, Nax1 and Nax2. Both genes derive from a wheat ancestor T. monococcum, but apparently an accession not involved in modern durum varieties. Nax1 is found on chromosome 2AL, removes Na+ from the root and leaf sheath xylem, and appears to be a member of the HKT (high affinity K+ transporter) family (Huang et al. 2006). Nax2 is found on 5AL and removes Na+ from the xylem in the roots; it appears to be homeologous to Kna1 of bread wheat (ancient chromosome translocations account for the different chromosome groups today) and is also a member of the HKT family (Byrt et al. 2007).
In accord with the above work on wheat, the amphiploid between the barley wild relative, highly salt-tolerant Hordeum marinum, and bread wheat, has intermediate salt tolerance, associated with intermediate leaf exclusion of Na+ and intermediate leaf concentrations of glycinebetain and proline protectants (Islam et al. 2007). In another wheat-wide cross, some fertile wheat-like progeny from the somatic hybridisation with the salt-tolerant wheat wild relative Thinopyron ponticum Podp., appear to have salt tolerance when compared to the winter wheat parent, both in the laboratory and field, but the mechanism of tolerance may not be Na+ exclusion from the leaves (Chen et al. 2004).
The second major mechanism by which crops withstand salinity is tolerance to high leaf Na+, the apparent basis of cultivated barley’s tolerance. There is some evidence for genotypic variation in this trait in durum wheat but its importance is yet to be fully understood (Munns et al. 2006).
The development of transgenic salt-tolerant wheats, expressing a vacuolar Na+/H+ antiporter gene from Arabidopsis (AtNHX1), is noteworthy because it was taken through to successful field testing: the best lines appear to carry no yield penalty in the absence of salinity, yet outperform the original parent notably in its presence (Xue et al. 2004); both leaves and roots accumulate less Na+ and more K+ than the check in the presence of salinity.
In contrast to wheat performance with water limitation, the simpler trait, performance under salinity, has thus yielded somewhat to a combination of physiology and functional genomics. Much, however, remains to be done: Nax1 and Nax2 are currently being validated in modern durum cultivar backgrounds in saline fields (R. Munns, pers. comm.), while the wide cross and GM tolerances from China seem not to have had the apparent early progress confirmed. It is also ironic that salinity-tolerant bread wheat cultivars performing well in saline farmers’ fields in the north-west IndoGangetic Plains and in Egypt were developed empirically some time ago, but little is known of the underlying mechanisms of tolerance (Munns et al. 2006).
Heat shock tolerance
The negative effect of raised Tmean (chronic heat) on yield and yield components has already been mentioned. Here reference is made to the negative effect of shorter periods (1–4 consecutive days) of maximum temperature (Tmax > around 32°C) during the grain-filling period, noting that such heat shocks can be common at middle and low latitudes, and are expected to become more common. Heat shock can reduce GW, and also sometimes GN if it is soon after AN, and can cause serious damage to wheat quality. Asseng et al. (2011) showed that there are already on average 1–5 days with Tmax >34°C during grain filling at locations across the Australian wheat belt. Their modelling suggested a yield reduction of ~0.2 t/ha for each such day, because of an assumed dramatic acceleration of leaf senescence in proportion to the number of such shocks, something probably influenced by the level of soil water and warranting further experimental validation because, in contrast to this mechanism, it has earlier been proposed that heat shock specifically inhibited soluble starch synthase in the grain, and not assimilate supply (Jenner 1994).
Not surprisingly, there is growing interest in genotypic differences in heat shock tolerance but problems arise with screening for such shock tolerance. These include the possible effects of preceding temperatures (hardening) as in Spiertz et al. (2006), exposure of roots to excessive heat when testing plants in pots (van Herwaarden et al. 1998b ), and the influence of soil water supply and vpd. Heat shocks are usually accompanied by dry winds and high vpd, and the escape effect provided by plant cooling relative to the air temperature can be substantial provided soil water is readily available as seen in Table 2 showing the influence of soil water (and of Tmax) with a single heat shock. Canopy and spike cooling by 5°C or more is not uncommon with irrigated wheat (Amani et al. 1996; Fischer et al. 1998): the effect is dependent on transpiration and is proportional to vpd and gs.
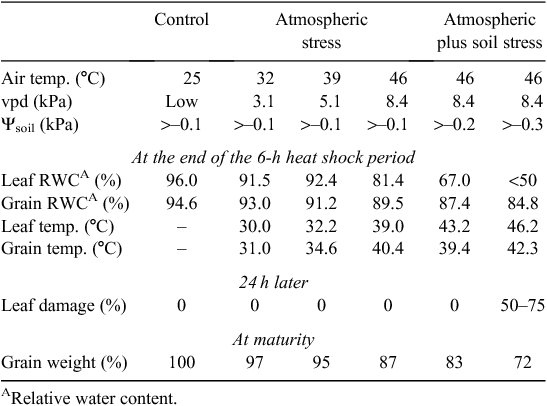
|
Nothwithstanding the uncertainties of heat shock screening, wheat cultivars appear to differ in the sensitivity of GW to this abiotic stress (Stone and Nicolas 1994; Wardlaw et al. 2002; Yang et al. 2002; Spiertz et al. 2006). Looking specifically at a susceptible (Karl 92) and a tolerant cultivar (Halberd) grown at 20/18°C, exposed to 1–2 days of heat (38/25°C) at 10 days after pollination, Hays et al. (2007) found 25% grain abortion and 10% GW reduction in cultivar Karl, but no response whatsoever in Halberd, responses which were associated with a large increase in ethylene production in grains and leaves with heat in the susceptible cultivar. These researchers have gone on to identify, in a RIL population of Halberd × Susceptible, several QTLs significantly associated with heat tolerance (Mason et al. 2010), but they are still some way from gene identification.
Simulation modelling
Simulation modelling of the wheat crop has advanced greatly since the early efforts of Nix and Fitzpatrick (1969), in line with increased physiological understanding, more transparent model structures, and much more powerful computing capacity. A comparison by Jamieson et al. (1998) of five wheat models against carefully measured wheat crops under a wide range of water treatments in New Zealand (yields from 3.5 to 9.9 t/ha) showed that the AFRCWHEAT2 model from the UK to be best for yield prediction [root mean squared deviation (RMSD) = 0.64 t/ha, c.f. CERES 0.90 t/ha, and Sirius 0.90 t/ha]. In Australia, APSIM-Wheat was developed from CERES and now dominates (Asseng et al. 1998, 2011; Wang et al. 2003; Hochman et al. 2009a, 2009b; Ludwig and Asseng 2010). The model uses daily time steps and includes a phenological framework, leaf expansion, crop growth driven by either radiation interception and RUE or transpiration and TE (whichever is most limiting), root penetration and layered soil water and N uptake, water and N stress indices feeding back into leaf area and RUE, and finally estimates of GN and GW. Yield predictions are reasonable, e.g. RMSD of 0.40 t/ha for yield range of 1–4 t/ha in Western Australia (Asseng et al. 1998) and 0.74 t/ha for a range 1–7 t/ha in Queensland (Wang et al. 2003). The latest official version of APSIM-Wheat is to be found at: www.apsim.info/Wiki/Wheat.ashx, accessed 21 January 2011. Good progress has also been made in modelling grain protein content of wheat (Martre et al. 2006; Jamieson et al. 2010). All the models referred to so far are one-dimensional, but Evers et al. (2010) have attempted to build a 3D-architectural model of the wheat crop, tracking individual leaves and tillers in space; however, success was limited and such complexity may not be necessary for most uses. Recently Jamieson et al. (2010) have again extensively reviewed the structure and performance of wheat modelling, pointing to many worthwhile applications while recognising scope for further improvement. Independently Hall and Sadras (2009) have pointed to three specific areas for model improvement, namely root morphology and function, biomass partitioning, and crop response to extreme temperatures.
APSIM-Wheat is certainly sufficiently accurate and user friendly to inform farm management decisions, especially in dryland situations where rain is uncertain, and yield response risk needs to be quantified, and adjusted according to somewhat skilful seasonal forecasts and unfolding seasonal weather; in this role in Australia it has been renamed Yield Prophet (Hochman et al. 2009b ). Model accuracy across a sample of 334 crops of ‘elite’ farmers over 2004–07, however, remains an issue; farmers want 0.5 t/ha accuracy while currently the RMSD of model versus observed is 0.8 t/ha, and there was a bias towards yield underestimate at high yield levels (Hochman et al. 2009a , Fig. 3a ). Incorrect inputs (climate and especially key soil properties) and ignored biotic stresses (e.g. weeds, disease) could be part of the error; inadequate physiology is presumably the rest. Interestingly, the model, using ‘best bet’ values for plant density, N and time of sowing, gives an estimate of PYw against which to benchmark the farmers’ yields; the latter averaged 77% of PYw (Hochman et al. 2009b ).
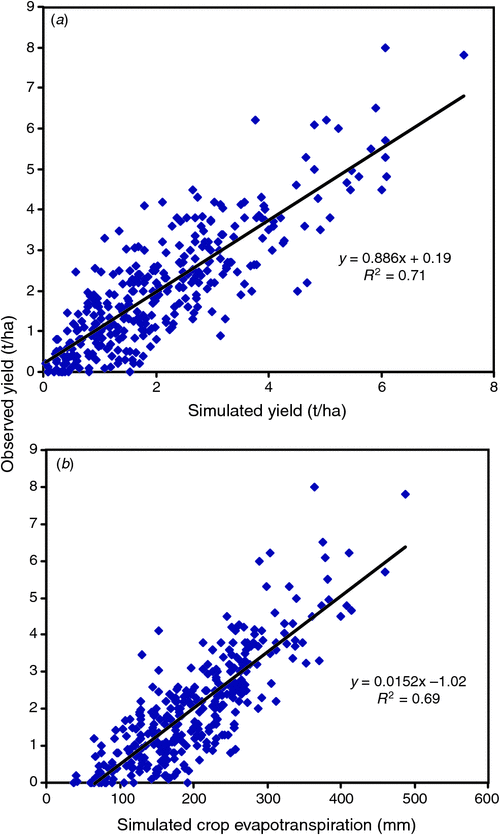
|
Yield Prophet simulated yield explains little more of the actual yield variation than did simulated ET alone (r 2 of 0.71 versus 0.69, Fig. 3b ). Admittedly simulated ET values should take care of yield responses related to lost water through deep drainage or soil water left at maturity. Nonetheless it is a surprising result, for APSIM’s simulation of yield attempts to allow for the trading of soil evaporation for transpiration when, for example, higher soil N stimulated greater leaf area index (LAI), for the timing of rainfall via effects of plant water stress at critical stages, and for the influence of vpd on TE. The authors suggest that the substantial gap in both predictions may be partly due to ignoring the negative effects of extreme spring temperatures (frost and heat), in accord with Hall and Sadras (2009) above. Also the assumption of no biotic stresses is a weakness (although other evidence suggests these effects reduced yield no more than 5% for the crops sampled). There is little doubt, however, that simulation models which deal satisfactorily with biotic stress, in particular foliar disease are needed for better crop management.
There are several other common uses of wheat simulation models including the extrapolation in time and space of results from agronomic experiments, the exploration of likely effects on yield of past and future climate scenarios, and, in breeding, the understanding of G × E (and × management (M)) and the prediction of trait change effects on yield. Because validation is very difficult in these situations, model inaccuracies become more worrying. The reasonable yield predictions highlighted above often hide the fact that key internal physiological parameters like GN, GW and LAI are poorly modelled; compensating errors seem common because of the upper limits to growth and yield imposed by resource supply, and in fact one model does reasonably well ignoring GN and GW altogether (Jamieson et al. 1998, 2010). With climate change-related applications of wheat modelling (e.g. Ludwig and Asseng 2010; Asseng et al. 2011), there are added uncertainties, about future climates themselves, especially rainfall, and about likely adaptation of agronomy and of cultivars to higher CO2 levels and to the new climates, and more caution is urged.
Notwithstanding abundant physiological uncertainties, the newest application of modelling is the linking of genes (in fact alleles of genes) to phenotype (G-to-P) through incorporating trait physiology into models like APSIM. Indeed this is seen by many as a key way forward for crop breeding, including the genetic engineering of yield. A courageous start has been made with genes in models of other crops like beans, soybeans, and barley, and with wheat phenology mentioned earlier (White et al. 2008), while APSIM is the vehicle by which the task is being approached in sorghum (Hammer et al. 2005), maize (Messina et al. 2009), and very recently, wheat (Chenu et al. 2010). But the initial efforts on traits and yield in wheat have been less ambitious. For example, Asseng and van Herwaarden (2003) have attempted to simulate the effect of a hypothetical increase in pre-grain-filling assimilates (a trait for which there is genetic variation) on wheat yield at a dry location in southern New South Wales: there was some validation against observations (but not of the genetic effect), and the positive simulated effects on yield at intermediate yield levels (1.5–4 t/ha) were plausible. Recent efforts on modelling traits to yield in wheat have been more ambitious. Thus, Ludwig and Asseng (2010) simulated five hypothetical trait combinations related to early vigour in wheat across three locations in Western Australia, two soil types, two N levels, and 50 years of historic weather, with and without many climate change scenarios. Even staying with historic climate and CO2, interactions abound and it becomes daunting enough to describe the modelled effects on yield of trait changes, let alone explain them, although greater early vigour, as created in the model, seems to boost yield except at the wettest coolest site on heavy soil. However, the absence of any trait validation is a major blow to credibility. This wheat case, using modern computer power to tackle huge simulation tasks, does not differ from those of other crops above, illustrated by Messina et al. (2009) who modelled the effects of variation in five hypothetical traits (in all combinations) with APSIM-Maize. In all cases there is a serious lack of validation of the consequences for yield of hypothesised trait changes, and of the linking physiology; there is also daunting complexity. What is lacking is a simpler stepwise approach which could start with, for example, modelling the effect a single Ppd allele change, a change for which trait (flowering date) and phenotype (yield) data are already available from winter wheat Ppd/ppd isolines (e.g. Worland 1996; Foulkes et al. 2004; González et al. 2005a ). In the meantime G-to-P simulation modelling will undoubtedly remain crop modellers’ greatest challenge. For readers who wish to see the latest courageous attempt to simulate the effect of real genetic variation in key traits (via QTL) on grain yield (in maize), Chenu et al. (2009) is recommended.
Concluding remarks
Today research on wheat physiology is undertaken usually with a stated view to impact, whether from improved crop agronomy or better cultivars: at the least, greater physiological understanding is invoked as showing the way forward. But resultant predictions are usually outputs not impacts, for while there has been steady progress in physiological understanding, impacts remain illusive. There may, however, be emerging greater confidence in physiological applications in agronomy than in breeding, although most scientists remain aware of the possibilities also of useful G × M interactions (Fischer 2009).
With agronomy, the questions relate to the strategic and tactical management of soil and soil water, the crop planting date, its density, row spacing and fertilisation, and the management of biotic stresses, all done so as to maximise economic return at acceptable risk levels. Given the importance and uncertainty of weather in this endeavour, especially in rain-fed wheat cropping, capturing the physiological understanding through simulation modelling has become accepted as an essential tool, something strengthened by considering seasonal weather forecasts of improving skill (e.g. Moeller et al. 2008). Model calibration and validation against agronomic inputs is generally satisfactory, but should never be neglected and needs ongoing attention from physiologists, especially bearing in mind the new management opportunities that innovative agronomic technologies (e.g. precision seeding, nanotechnology) and new cultivars (e.g. herbicide resistance, adaptation to wide rows) can create. Better knowledge of root systems and rhizospheres may soon also need to be considered, and management models including biotic stresses are lacking.
It is with genetic improvement that the gap between physiological aspirations and impact is greatest, undoubtedly because the complexity of the path from gene through physiology to phenotype and yield, a route which far exceeds the complexities in going from agronomic management to yield. The examples given in wheat phenology and aluminium and salinity tolerance, where major genes and simple environmental cues, dominate, offer glimmers of hope for linking physiology to impact through manipulation of marked alleles for desirable and predictable effects in the field. This becomes much more difficult for quantitative traits, such that seeking understanding at deeper levels than that of trait physiology may be counterproductive, and selection based at the trait level, already fraught by unanticipated trade-offs, will remain a better option. This seems to be the current experience with breeding for PY and PYw: in the examples given, the genes and alleles involved may remain indecipherable, but this does not preclude progress through seeking and assembling apparently desirable traits, some of which may associate with robust low cost molecular markers; but others may be more readily assessable through low cost direct measurement (e.g. remote sensing). It should be remembered that the greatest threats to world food security will come soon, in the next 20 years, and seeking to explain at the molecular level all the trait phenomena may be a costly distraction from seeking to exploit the traits. There are of course exceptions to the frustrations of chasing alleles, like selection of short wheats with longer coleoptiles, where direct selection for the major new dwarfing alleles may be the most efficient way forward. But it goes without saying that the use of simulation modelling to predict from the gene level to the quantitative phenotype will remain extremely difficult for a long time to come, while modelling from trait change to yield will be difficult enough and needs to proceed in adequately validated successive steps of increasing complexity.
This review has not canvassed genetic engineering for PY or PYw gain because, to date and despite claims to the contrary, there are no well validated field successes, and because the approach reflects excessive naivety with respect to the complex physiology of yield determination. Success from genetic engineering for yield potential is most likely to arise largely by chance, just as it does in conventional breeding, both in Farrer’s time, and even today, with conventional breeding still managing to raise wheat yields 0.5–1% per annum!
References
Abbate PE, Andrade FH, Culot JP, Bindraban PS (1997) Grain yield in wheat: effects of radiation during spike growth period. Field Crops Research 54, 245–257.| Grain yield in wheat: effects of radiation during spike growth period.Crossref | GoogleScholarGoogle Scholar |
Abbate PE, Andrade FH, Lazaro L, Bariffi JH, Berardocco HG, Inza VH, Marturano F (1998) Grain yield increase in recent Argentine wheat cultivars. Crop Science 38, 1203–1209.
| Grain yield increase in recent Argentine wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
ACPFG (2010a) Australian Centre for Plant Functional Genomics: frost tolerance. Available at: www.acpfg.com.au/index.php?id=12
ACPFG (2010b) Australian Centre for Plant Functional Genomics: genetic variation for boron tolerance in barley. Available at: www.acpfg.com.au/index.php?id=19
Aisawi K, Foukes J, Reynolds M, Mayes S (2010) The physiological basis of genetic progress in yield potential of CIMMYT wheat varieties from 1966 to 2009. In ‘Abstracts 8th International Wheat Conference’. 1–4 June 2010, St Petersburg, Russia. pp. 349–350. (N I Vavilov Research Institute: St Petersburg, Russia)
Amani I, Fischer RA, Reynolds MP (1996) Canopy temperature depression associated with yield of irrigated spring wheats in a hot climate. Journal of Agronomy & Crop Science 176, 119–129.
| Canopy temperature depression associated with yield of irrigated spring wheats in a hot climate.Crossref | GoogleScholarGoogle Scholar |
Anderson WK, Heinrich A, Abbotts R (1996) Long-season wheats extend sowing opportunities in the central wheat belt of Western Australia. Australian Journal of Experimental Agriculture 36, 203–208.
| Long-season wheats extend sowing opportunities in the central wheat belt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Asseng S, Foster I, Turner NC (2011) The impact of temperature variability on wheat yields. Global Change Biology 17, 997–1012.
| The impact of temperature variability on wheat yields.Crossref | GoogleScholarGoogle Scholar |
Asseng S, Keating BA, Fillery IRP, Gregory PJ, Bowden JW, Turner NC, Palta JA, Abrecht DG (1998) Performance of the APSIM-wheat model in Western Australia. Field Crops Research 57, 163–179.
| Performance of the APSIM-wheat model in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Asseng S, van Herwaarden AF (2003) Analysis of the benefits to wheat yield from assimilates stored prior to grain filling in a range of environments. Plant and Soil 256, 217–229.
| Analysis of the benefits to wheat yield from assimilates stored prior to grain filling in a range of environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1ClsLY%3D&md5=4e61f13fb6a4f37846fc2f6d19ce748dCAS |
Austin RB (1980) Physiological limitations to cereal yields and ways of reducing them by breeding. In ‘Opportunities for increasing crop yields’. (Eds RG Hurd, PV Biscoe, C Dennis) pp. 3–19. (Pitman: London)
Bancal P (2008) Positive contribution of stem growth to grain number per spike in wheat. Field Crops Research 105, 27–39.
| Positive contribution of stem growth to grain number per spike in wheat.Crossref | GoogleScholarGoogle Scholar |
Bancal P (2009) Early development and enlargement of wheat floret primordia suggest a role of partitioning within spike to grain set. Field Crops Research 110, 44–53.
| Early development and enlargement of wheat floret primordia suggest a role of partitioning within spike to grain set.Crossref | GoogleScholarGoogle Scholar |
Berry PM, Sylvester-Bradley R, Berry S (2007) Ideotype design for lodging-resistant wheat. Euphytica 154, 165–179.
| Ideotype design for lodging-resistant wheat.Crossref | GoogleScholarGoogle Scholar |
Bingham J (1969) The physiological determinants of grain yield in cereals. Agricultural Progress 44, 30–42.
Borras L, Slafer GA, Otegui ME (2004) Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research 86, 131–146.
| Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal.Crossref | GoogleScholarGoogle Scholar |
Byrt CS, Platten D, Spielmayer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to N+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiology 143, 1918–1928.
| HKT1;5-like cation transporters linked to N+ exclusion loci in wheat, Nax2 and Kna1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFWjurs%3D&md5=6d73090a51efc4294ab930f58694ccfcCAS | 17322337PubMed |
Calderini DF, Abeledo LG, Savin R, Slafer GA (1999) Final grain weight in wheat as affected by short periods of high temperature during pre- and post-anthesis under field conditions. Australian Journal of Plant Physiology 26, 453–458.
| Final grain weight in wheat as affected by short periods of high temperature during pre- and post-anthesis under field conditions.Crossref | GoogleScholarGoogle Scholar |
Calderini DF, Reynolds MP (2000) Changes in grain weight as a consequence of de-graining treatments at pre- and postanthesis in synthetic hexaploid lines of wheat (Triticum durum × T. tauschii). Australian Journal of Plant Physiology 27, 183–191.
Calderini DF, Savin R, Abelodo LG, Reynolds MP (2001) The importance of the period immediately preceding anthesis for grain weight determination in wheat. Euphytica 119, 199–204.
| The importance of the period immediately preceding anthesis for grain weight determination in wheat.Crossref | GoogleScholarGoogle Scholar |
Chen S, Xia G, Quan T, Xiang F, Jin Y, Chen H (2004) Introgression of salt-tolerance from somatic hybrids between common wheat and Thinopyron ponticum. Plant Science 167, 773–779.
| Introgression of salt-tolerance from somatic hybrids between common wheat and Thinopyron ponticum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmt1Wlu7g%3D&md5=f15de403ffbb6260c6607341da2cf1d2CAS |
Chenu K, Chapman SC, Tardieu F, McLean G, Welcker C, Hammer GL (2009) Simulating the yield impacts of organ-level quantitative loci associated with drought response in maize: a ‘gene-to-phenotype’ modelling approach. Genetics 183, 1507–1523.
| Simulating the yield impacts of organ-level quantitative loci associated with drought response in maize: a ‘gene-to-phenotype’ modelling approach.Crossref | GoogleScholarGoogle Scholar | 19786622PubMed |
Chenu K, Hammer GL, Dreccer F, Chapman SC (2010) Environmental characterisation to aid crop improvement in drought-prone environments. In ‘Proceedings of Agro2010, XI European Society of Agronomy Congress’. 29 Aug.–3 Sept. 2010, Montpellier, France. (Eds J Wery, I Shili-Touzi, A Perrin) pp. 79–80. (Agropolis Productions: Montpellier, France)
Christopher JT, Manschadi AM, Hammer GL, Borrell AK (2008) Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Australian Journal of Agricultural Research 59, 354–364.
| Developmental and physiological traits associated with high yield and stay-green phenotype in wheat.Crossref | GoogleScholarGoogle Scholar |
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water-use efficiency and crop yield. Crop Science 42, 122–131.
| Improving intrinsic water-use efficiency and crop yield.Crossref | GoogleScholarGoogle Scholar | 11756262PubMed |
Cooper PM, Gregory PJ, Tully D, Harris HC (1987) Improving water use efficiency of annual crops in the rainfed farming systems of West Asia and North Africa. Experimental Agriculture 23, 113–158.
| Improving water use efficiency of annual crops in the rainfed farming systems of West Asia and North Africa.Crossref | GoogleScholarGoogle Scholar |
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiology 103, 695–702.
Donald CM (1962) In search of yield. Journal of Australian Institute of Agricultural Science 28, 171–178.
Donald CM, Hamblin J (1976) The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Advances in Agronomy 28, 361–405.
| The biological yield and harvest index of cereals as agronomic and plant breeding criteria.Crossref | GoogleScholarGoogle Scholar |
Dreccer F, van Herwaarden AF, Chapman SC (2009) Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration. Field Crops Research 112, 43–54.
| Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration.Crossref | GoogleScholarGoogle Scholar |
Dubcovsky J, Santa Maria G, Epstein E, Luo MC, Dvorak J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theoretical and Applied Genetics 92, 448–454.
| Mapping of the K+/Na+ discrimination locus Kna1 in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XltFOjtLg%3D&md5=b25e3a11e6f204857cd7f20562da9301CAS |
Dwivedi S, Perotti E, Ortiz R (2008) Towards molecular breeding of reproductive traits in cereal crops. Plant Biotechnology Journal 6, 529–559.
| Towards molecular breeding of reproductive traits in cereal crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVWmtrbE&md5=f11771ea3a305ce6f56d3368749ad5e7CAS | 18507792PubMed |
Dyck JA, Matus-Cadiz MA, Hucl P, Talbert L, Hunt T, Dubuc JP, Nass H, Clayton G, Dobb J, Quick J (2004) Agronomic performance of hard red spring wheat isolines sensitive and insensitive to photoperiod. Crop Science 44, 1976–1981.
| Agronomic performance of hard red spring wheat isolines sensitive and insensitive to photoperiod.Crossref | GoogleScholarGoogle Scholar |
Eagles HA, Cane K, Kuchel H, Hollamby GJ, Vallance N, Eastwood RF, Gororo NN, Martin PJ (2010) Photoperiod and vernalization gene effects in southern Australian wheat. Crop & Pasture Science 61, 721–730.
| Photoperiod and vernalization gene effects in southern Australian wheat.Crossref | GoogleScholarGoogle Scholar |
Eagles HA, Cane K, Vallance N (2009) The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop & Pasture Science 60, 646–657.
| The flow of alleles of important photoperiod and vernalisation genes through Australian wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosVymtbo%3D&md5=121be24edf831d335034b36e085a2dd9CAS |
Egli DB (2006) The role of seed in the determination of yield in grain crops. Australian Journal of Agricultural Research 57, 1237–1247.
| The role of seed in the determination of yield in grain crops.Crossref | GoogleScholarGoogle Scholar |
Evans LT (1993) ‘Crop evolution, adaptation and yield.’ (Cambridge University Press: Cambridge, UK)
Evans LT, Bingham J, Roskhams MA (1972) The pattern of grain set within ears of wheat. Australian Journal of Biological Sciences 25, 1–8.
Evans LT, Wardlaw IF, Fischer RA (1975) Wheat. In ‘Crop physiology; some case histories’. (Ed. LT Evans) pp. 101–149. (CUP: London)
Evers JB, Vos J, Yin X, Romero P, van der Putten PEL, Struik PC (2010) Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation. Journal of Experimental Botany 61, 2203–2216.
| Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGnsrw%3D&md5=65d85bf8d913af6412e002797e63d090CAS | 20231326PubMed |
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11, 539–552.
| Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFSju7w%3D&md5=fefc22cee2e0f0974bec890502744bf3CAS |
Farrer WJ (1898) The making and improvement of wheats for Australian conditions. Agricultural Gazette NSW 9, 131–250.
Fischer RA (1973) The effect of water stress at various stages of development on yield processes in wheat. In ‘Plant response to climatic factors. Proceedings Uppsala Symposium 1970’. pp. 233–241. (UNESCO: Paris)
Fischer RA (1979) Growth and water limitation to dryland wheat yield in Australia: a physiological framework. Journal of Australian Institute of Agricultural Science 45, 83–94.
Fischer RA (1980) Influence of water stress on crop yield in semiarid regions. In ‘Adaptation of plants to water and high temperature stress’. (Eds NC Turner, PJ Kramer) pp. 323–340. (John Wiley and Sons: New York)
Fischer RA (1984) Wheat. In ‘Proceedings of symposium on potential productivity of field crops under different environments’. September 1980. (Eds WH Smith, SJ Banks) pp. 129–154. (IRRI: Los Baños, Philippines)
Fischer RA (1985) Number of kernels in wheat crops and the influence of solar radiation and temperature. Journal of Agricultural Science, Cambridge 105, 447–461.
| Number of kernels in wheat crops and the influence of solar radiation and temperature.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2007) Understanding the physiological basis of yield potential in wheat. Journal of Agricultural Science, Cambridge 145, 99–113.
| Understanding the physiological basis of yield potential in wheat.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2008) The importance of grain or kernel number in wheat: a reply to Sinclair and Jamieson. Field Crops Research 105, 15–21.
| The importance of grain or kernel number in wheat: a reply to Sinclair and Jamieson.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2009) Farming systems of Australia: exploiting the synergy between genetic improvement and agronomy. In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 23–54. (Academic Press: San Diego, CA)
Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Science 50, S85–S98.
| Breeding and cereal yield progress.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, HilleRisLambers D (1978) Effect of environment and cultivar on source limitation to grain weight in wheat. Australian Journal of Agricultural Research 29, 443–458.
| Effect of environment and cultivar on source limitation to grain weight in wheat.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Rees D, Sayre KD, Lu Z, Condon AG, Larque Saavedra A (1998) Wheat yield progress is associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475.
| Wheat yield progress is associated with higher stomatal conductance and photosynthetic rate, and cooler canopies.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Stockman Y (1980) Kernel number per spike in wheat (Triticum aestivum L.): responses to preanthesis shading. Australian Journal of Plant Physiology 7, 169–180.
| Kernel number per spike in wheat (Triticum aestivum L.): responses to preanthesis shading.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXktVCjs7s%3D&md5=f65b6a86f8a92c50aec2e442de19cf77CAS |
Fischer RA, Stockman Y (1986) Increased kernel number in Norin10-derived dwarf wheat: evaluation of the cause. Australian Journal of Plant Physiology 13, 767–784.
| Increased kernel number in Norin10-derived dwarf wheat: evaluation of the cause.Crossref | GoogleScholarGoogle Scholar |
Flintham JE, Bőrner A, Worland AJ, Gale MD (1997) Optimizing wheat grain yield: effects of Rht (gibberelin-insensitive) dwarfing genes. Journal of Agricultural Science, Cambridge 128, 11–25.
| Optimizing wheat grain yield: effects of Rht (gibberelin-insensitive) dwarfing genes.Crossref | GoogleScholarGoogle Scholar |
Foulkes MJ, Sylvester-Bradley R, Worland AJ, Snape JW (2004) Effects of a photoperiod-response gene Ppd-D1 on yield potential and drought resistance in UK winter wheat. Euphytica 135, 63–73.
| Effects of a photoperiod-response gene Ppd-D1 on yield potential and drought resistance in UK winter wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvFehsLk%3D&md5=f6cd322fcddf37bb390d55491bffbaf5CAS |
French RJ, Schultz JE (1984) Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate. Australian Journal of Agricultural Research 35, 743–764.
| Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate.Crossref | GoogleScholarGoogle Scholar |
Ghiglione HO, González FG, Serrago R, Maldonado SB, Chilcott C, Cura JA, Miralles DJ, Zhu T, Casal JJ (2008) Autophagy regulated by day length determines the number of fertile florets in wheat. The Plant Journal 55, 1010–1024.
| Autophagy regulated by day length determines the number of fertile florets in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Srs77J&md5=31061420a699db22e8570e9c920db7bbCAS | 18547393PubMed |
Gomez-Macpherson H, Richards RA (1995) Effect of sowing time on yield and agronomic characteristics of wheat in south-eastern Australia. Australian Journal of Agricultural Research 46, 1381–1399.
| Effect of sowing time on yield and agronomic characteristics of wheat in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2003) Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats. Field Crops Research 81, 17–27.
| Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats.Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2005a) Pre-anthesis development and number of fertile florets in wheat as affected by photoperiod sensitivity genes Ppd-D1 and Ppd-B1. Euphytica 146, 253–269.
| Pre-anthesis development and number of fertile florets in wheat as affected by photoperiod sensitivity genes Ppd-D1 and Ppd-B1.Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2005b) Photoperiod during stem elongation in wheat: is its impact on fertile floret and grain number determination similar to that of radiation? Functional Plant Biology 32, 181–188.
| Photoperiod during stem elongation in wheat: is its impact on fertile floret and grain number determination similar to that of radiation?Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2005c) Floret development and survival in wheat plants exposed to contrasting photoperiod and radiation environments during stem elongation. Functional Plant Biology 32, 189–197.
| Floret development and survival in wheat plants exposed to contrasting photoperiod and radiation environments during stem elongation.Crossref | GoogleScholarGoogle Scholar |
Gregory PJ, Simmonds LP, Pilbeam CJ (2000) Soil type, climate regime, and the response of water use efficiency to crop management. Agronomy Journal 92, 814–820.
| Soil type, climate regime, and the response of water use efficiency to crop management.Crossref | GoogleScholarGoogle Scholar |
Hall AJ, Sadras VO (2009) Wither crop physiology? In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 545–570. (Academic Press: London)
Hammer GL, Chapman S, van Oosterom E, Podlich DW (2005) Trait physiology and crop modelling as a framework to link phenotypic complexity to underlying genetic systems. Australian Journal of Agricultural Research 56, 947–960.
| Trait physiology and crop modelling as a framework to link phenotypic complexity to underlying genetic systems.Crossref | GoogleScholarGoogle Scholar |
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Science 172, 1113–1123.
| Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFGitLw%3D&md5=6eece471b047cb4771b4e44a012c4dd3CAS |
Hochman Z, Holzworth D, Hunt JR (2009a) Potential to improve on-farm wheat yield and WUE in Australia. Crop & Pasture Science 60, 708–716.
| Potential to improve on-farm wheat yield and WUE in Australia.Crossref | GoogleScholarGoogle Scholar |
Hochman Z, van Rees H, Carberry PS, Hunt JR, McCown RL, Gartmann A, Holzworth D, van Rees S, Dalgliesh NP, Long W, Peake AS, Poulton PL (2009b) Re-inventing model-based decision support with Australian dryland farmers. 4. Yield prophet helps farmers monitor and manage crops in a variable climate. Crop & Pasture Science 60, 1057–1070.
| Re-inventing model-based decision support with Australian dryland farmers. 4. Yield prophet helps farmers monitor and manage crops in a variable climate.Crossref | GoogleScholarGoogle Scholar |
Huang SB, Spielmayer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiology 142, 1718–1727.
| A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCns7jE&md5=3c00714501e0a6175beb5111e3d3a811CAS | 17071645PubMed |
Iqbal M, Navabi A, Salmon DF, Yang R-C, Murdoch BM, Moore SS, Spaner D (2007a) Genetic analysis of flowering time in high latitude spring wheat: genetic analysis of earliness in wheat. Euphytica 154, 207–218.
| Genetic analysis of flowering time in high latitude spring wheat: genetic analysis of earliness in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhs1Ckt74%3D&md5=34f4065f391131b4bf7d00eb84efcaa2CAS |
Iqbal M, Navabi A, Yang R-C, Salmon DF, Spaner D (2007b) The effect of vernalization genes on earliness and related agronomic traits of spring wheat in northern growing regions. Crop Science 47, 1031–1039.
| The effect of vernalization genes on earliness and related agronomic traits of spring wheat in northern growing regions.Crossref | GoogleScholarGoogle Scholar |
Islam S, Malik AI, Islam AKMR, Colmer TD (2007) Salt tolerance in a Hordeum marinum–Triticum aestivum amphiploid, and its parents. Journal of Experimental Botany 58, 1219–1229.
| Salt tolerance in a Hordeum marinum–Triticum aestivum amphiploid, and its parents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktlWjsbs%3D&md5=f7eaaf28c6c5a735f46fac7deb0eec5aCAS | 17283374PubMed |
Jamieson PD, Asseng S, Chapman SC, Dreccer MF, White JW, McMaster GS, Porter JR, Semenov MA (2010) Modelling wheat production. In ‘The world wheat book’. (Eds M van Ginkel, A Bonjean, W Angus) p. 40. (Lavoisier Publishing: Paris)
Jamieson PD, Porter JR, Goudriaan J, Ritchie JT, van Keulen H, Stol W (1998) A comparison of the models AFRCWHEAT2, CERES-Wheat, Sirius, SUCROS2 and SWHEAT with measurements from wheat grown under drought. Field Crops Research 55, 23–44.
| A comparison of the models AFRCWHEAT2, CERES-Wheat, Sirius, SUCROS2 and SWHEAT with measurements from wheat grown under drought.Crossref | GoogleScholarGoogle Scholar |
Jenner CF (1994) Starch synthesis in the kernel of wheat under high-temperature conditions. Australian Journal of Plant Physiology 21, 791–806.
| Starch synthesis in the kernel of wheat under high-temperature conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkslequrk%3D&md5=7abea65d8a625d29b36c7bb9e572cddbCAS |
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, Richards RA, Dolferus R (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell & Environment 33, 926–942.
| Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVagsb4%3D&md5=7c3d9bbb42fbd9bee97e69bc958ad0bbCAS | 20199626PubMed |
Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315.
| Impact of subsoil water use on wheat yield.Crossref | GoogleScholarGoogle Scholar |
Koh S, Kumara A, Murata Y (1978) Studies on dry matter production in wheat plant. Japanese Journal of Crop Science 47, 69–74.
Koonjul PK, Minhas JS, Nunes C, Sheoran IS, Saini HS (2005) Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat. Journal of Experimental Botany 56, 179–190.
Lewis S, Faricelli ME, Appendino ML, Valárik M, Dubcovsky J (2008) The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat. Journal of Experimental Botany 59, 3595–3607.
| The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SisLnL&md5=6c3a7445b78ef4f6eedf959a409cf949CAS | 18836186PubMed |
Lilley JM, Kirkegaard JA (2007) Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales. Australian Journal of Agricultural Research 58, 1115–1128.
| Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lizana XC, Riegel R, Gomez LD, Herrera J, Isla A, McQueen-Mason SJ, Calderini DF (2010) Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.). Journal of Experimental Botany 61, 1147–1157.
| Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Wnsr8%3D&md5=939fb6cae828051d0bcab0c655cd507dCAS | 20080826PubMed |
Lobell DB, Ortiz-Monasterio JI, Asner GP, Matson PA, Naylor RL, Falcon WP (2005) Analysis of wheat yield and climatic trends in Mexico. Field Crops Research 94, 250–256.
| Analysis of wheat yield and climatic trends in Mexico.Crossref | GoogleScholarGoogle Scholar |
Lopes MS, Reynolds M (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology 37, 147–156.
| Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat.Crossref | GoogleScholarGoogle Scholar |
Ludwig F, Asseng S (2010) Potential benefits of early vigor and changes in phenology in wheat to adapt to warmer and drier climates. Agricultural Systems 103, 127–136.
| Potential benefits of early vigor and changes in phenology in wheat to adapt to warmer and drier climates.Crossref | GoogleScholarGoogle Scholar |
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837.
| The role of root architectural traits in adaptation of wheat to water-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVClsbY%3D&md5=53fa5a7332edec01a8ec7f1e7815efb8CAS |
Martre P, Jamieson PD, Semenov MA, Zyskowski RF, Porter JR, Triboi E (2006) Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. European Journal of Agronomy 25, 138–154.
| Modelling protein content and composition in relation to crop nitrogen dynamics for wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1KjsLY%3D&md5=1a1c805161e7b3f23394dfa3a4f115f2CAS |
Mason RE, Mondal S, Beecher FW, Pacheco A, Jampala B, Ibrahim AMH, Hays DB (2010) QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica 174, 423–436.
| QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress.Crossref | GoogleScholarGoogle Scholar |
McIntyre C, Mathews KL, Rattey A, Chapman SC, Drenth J, Ghaderi M, Reynolds M, Shorter R (2010) Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions. Theoretical and Applied Genetics 120, 527–541.
| Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotV2iug%3D%3D&md5=0bd43acec3b2517fd59dbcfa9e2dfc24CAS | 19865806PubMed |
Messina C, Hammer G, Dong Z, Podlich D, Cooper M (2009) Modelling crop improvement in a G × E × M framework via gene-trait-phenotype relationships. In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 235–265. (Academic Press: London)
Millar AL, Rathjen AJ, Cooper DS (2007) Genetic variation for subsoil toxicities in high pH soils. In ‘Wheat production in stressed environments. Proceedings of the 7th International Wheat Conference’. Mar del Plata, Argentina, 27 Nov.–2 Dec. 2005. (Eds HT Buck, JE Nisi, N Salamon) pp. 395–401. (Springer: Dordrecht, The Netherlands)
Miralles DJ, Richards RA, Slafer GA (2000) Duration of the stem elongation period influences the number of fertile florets in wheat and barley. Australian Journal of Plant Physiology 27, 931–940.
Miralles DJ, Slafer GA (1995) Yield, biomass and yield components in dwarf, semi-dwarf and tall isogenic lines of spring wheat under recommended and late sowing dates. Plant Breeding 114, 392–396.
| Yield, biomass and yield components in dwarf, semi-dwarf and tall isogenic lines of spring wheat under recommended and late sowing dates.Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Slafer GA (1997) Radiation interception and radiation use efficiency of near isogenic wheat lines with different height. Euphytica 97, 201–208.
| Radiation interception and radiation use efficiency of near isogenic wheat lines with different height.Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Slafer GA (2007) Sink limitations to yield in wheat: how could it be reduced? The Journal of Agricultural Science 145, 139–149.
| Sink limitations to yield in wheat: how could it be reduced?Crossref | GoogleScholarGoogle Scholar |
Moeller C, Smith I, Asseng S, Ludwig F, Telcik N (2008) The potential value of seasonal forecasts of rainfall categories – case studies from the wheatbelt in Western Australia’s Mediterranean region. Agricultural and Forest Meteorology 148, 606–618.
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany 57, 1025–1043.
| Approaches to increasing the salt tolerance of wheat and other cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xis1GlsrY%3D&md5=6038ea9b30256e36c82dbdebe1a5406dCAS | 16510517PubMed |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=83aef80d0ef3da5f4666ad0d9f4c8724CAS | 18444910PubMed |
Nix HA, Fitzpatrick EA (1969) An index of crop water stress related to wheat and grain sorghum yields. Agricultural Meteorology 6, 321–337.
| An index of crop water stress related to wheat and grain sorghum yields.Crossref | GoogleScholarGoogle Scholar |
Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive attributes in the Seri-Babax hexaploid wheat population. Functional Plant Biology 34, 189–203.
| Drought-adaptive attributes in the Seri-Babax hexaploid wheat population.Crossref | GoogleScholarGoogle Scholar |
Ortiz-Monasterio JI, Dhillon SS, Fischer RA (1994) Date of sowing effects on grain yield and yield components of irrigated spring wheat cultivars and relationships with radiation and temperature in Ludhiana, India. Field Crops Research 37, 169–184.
| Date of sowing effects on grain yield and yield components of irrigated spring wheat cultivars and relationships with radiation and temperature in Ludhiana, India.Crossref | GoogleScholarGoogle Scholar |
Palta J, Watt M (2009) Vigorous crop root systems: form and function for improving the capture of water and nutrients. In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 309–325. (Academic Press: London)
Passioura JB (2002) Environmental biology and crop improvement. Functional Plant Biology 29, 537–546.
| Environmental biology and crop improvement.Crossref | GoogleScholarGoogle Scholar |
Passioura JB, Angus JF (2010) Improving productivity of crops in water-limited environments. Advances in Agronomy 106, 37–75.
| Improving productivity of crops in water-limited environments.Crossref | GoogleScholarGoogle Scholar |
Pereira JF, Zhou G, Delhaize E, Richardson T, Zhou M, Ryan P (2010) Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany 106, 205–214.
| Engineering greater aluminium resistance in wheat by over-expressing TaALMT1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVWjsLY%3D&md5=d171d142333510ec5acdc57ce3a4409aCAS | 20338951PubMed |
Pugsley AT (1968) Genetic studies of phasic development and their application to wheat breeding. In ‘Proceedings 3rd International Wheat Genetics Symposium’. Canberra, ACT. pp. 288–293. (Australian Academy of Science: Canberra)
Pugsley AT (1972) Additional genes inhibiting winter habit in wheat. Euphytica 21, 547–552.
| Additional genes inhibiting winter habit in wheat.Crossref | GoogleScholarGoogle Scholar |
Raman H, Ryan PR, Raman R, Stodart BJ, Zhang K, Martin P, Wood R, Sasaki T, Yamamoto Y, Mackay M, Hebb DM, Delhaize E (2008) Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theoretical and Applied Genetics 116, 343–354.
| Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Shug%3D%3D&md5=245ef0496bfc2947e2a0d26b5419b08cCAS | 18046532PubMed |
Rattey A, Shorter R, Chapman S, Dreccer F, van Heerwarden A (2009) Variation for and relationships among biomass and grain yield component traits conferring improved yield and grain weight in an elite wheat population grown in variable yield environments. Crop & Pasture Science 60, 717–729.
| Variation for and relationships among biomass and grain yield component traits conferring improved yield and grain weight in an elite wheat population grown in variable yield environments.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Ellis MH, Bonnett DG, Richards RA (2007) Molecular mapping of genes for coleoptile growth in bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics 114, 1173–1183.
| Molecular mapping of genes for coleoptile growth in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkslWnsrc%3D&md5=d5f73c8232a6a7bc84dab9e4f7204caeCAS | 17294164PubMed |
Rebetzke GJ, van Herwaarden A, Jenkins C, Ruuska S, Tabe L, Lewis D, Weiss M, Fettell N, Richards RA (2008) Quantitative trait loci for water soluble carbohydrates and associations with agronomic traits in wheat. Australian Journal of Agricultural Research 59, 891–905.
| Quantitative trait loci for water soluble carbohydrates and associations with agronomic traits in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFaisrfE&md5=8853115fd8e5efa3ac32bce106d99ad2CAS |
Reynolds M, Foulkes JM, Slafer GA, Berry P, Snape JW, Angus WJ (2009) Raising yield potential in wheat. Journal of Experimental Botany 60, 1899–1918.
| Raising yield potential in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFSjtbc%3D&md5=8be4cde7c7cab29e60911ed16b1607e5CAS | 19363203PubMed |
Reynolds M, Tuberosa R (2008) Translational research impacting on crop productivity in drought-prone environments. Current Opinion in Plant Biology 11, 171–179.
| Translational research impacting on crop productivity in drought-prone environments.Crossref | GoogleScholarGoogle Scholar | 18329330PubMed |
Reynolds MP, Calderini DF, Condon AG, Rajaram S (2001) Physiological basis of yield gains in wheat associated with the Lr19 translocation from Agropyron elongatum. Euphytica 119, 139–144.
| Physiological basis of yield gains in wheat associated with the Lr19 translocation from Agropyron elongatum.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Delgado MI, Gutiérrez-Rodriguez M, Larqué-Saavedra A (2000) Photosynthesis of wheat in a warm, irrigated environment. I: genetic diversity and crop productivity. Field Crops Research 66, 37–50.
| Photosynthesis of wheat in a warm, irrigated environment. I: genetic diversity and crop productivity.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Pellegrineschi A, Skovmand B (2005) Sink-limitation to yield and biomass: a summary of some investigations in spring wheat. Annals of Applied Biology 146, 39–49.
| Sink-limitation to yield and biomass: a summary of some investigations in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Richards RA (2006) Physiological traits used in the breeding of new cultivars for water-scarce environments. Agricultural Water Management 80, 197–211.
| Physiological traits used in the breeding of new cultivars for water-scarce environments.Crossref | GoogleScholarGoogle Scholar |
Richards RA, Lukacs Z (2002) Seedling vigour in wheat – sources of variation for genetic and agronomic improvement. Australian Journal of Agricultural Research 53, 41–50.
| Seedling vigour in wheat – sources of variation for genetic and agronomic improvement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhsVGrtL0%3D&md5=82c56f4466f890ddb79a0f2cb5c910ffCAS |
Richards RA, Rebetzke GJ, Watt M, Condon AG, Spielmeyer W, Dolferus D (2010) Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Functional Plant Biology 37, 85–97.
| Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment.Crossref | GoogleScholarGoogle Scholar |
Riede CR, Anderson JA (1996) Linkage of RFLP markers to an aluminium tolerance gene in wheat. Crop Science 36, 905–909.
| Linkage of RFLP markers to an aluminium tolerance gene in wheat.Crossref | GoogleScholarGoogle Scholar |
Rivkin P, O’Leary G (2010) Phase durations in wheat will need to be optimized for crops in the high rainfall zone of south-west Victoria to achieve yield potential. In ‘Proceedings 15th ASA Conference’. 15–19 Nov., Lincoln, New Zealand. Available at: www.agronomy.asa.org.au
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology 149, 340–351.
| A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1Wqt7w%3D&md5=93a81e686b478813a1a7fa7221143435CAS | 19005085PubMed |
Sadras VO, Angus JF (2006) Benchmarking water-use efficiency of rainfed wheat in dry environments. Australian Journal of Agricultural Research 57, 847–856.
| Benchmarking water-use efficiency of rainfed wheat in dry environments.Crossref | GoogleScholarGoogle Scholar |
Sadras VO, Denison RF (2009) Do plants compete for resources? An evolutionary viewpoint. New Phytologist 183, 565–574.
| Do plants compete for resources? An evolutionary viewpoint.Crossref | GoogleScholarGoogle Scholar | 19413690PubMed |
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal 37, 645–653.
| A wheat gene encoding an aluminum-activated malate transporter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXislyltr4%3D&md5=40f91584bdd78482667dec2bf34a6db9CAS | 14871306PubMed |
Sayre KD, Rajaram S, Fischer RA (1997) Yield potential progress in short wheats in northwest Mexico. Crop Science 37, 36–42.
| Yield potential progress in short wheats in northwest Mexico.Crossref | GoogleScholarGoogle Scholar |
Serrago RA, Miralles DJ, Slafer GA (2008) Floret fertility in wheat as affected by photoperiod during stem elongation and removal of spikelets at booting. European Journal of Agronomy 28, 301–308.
| Floret fertility in wheat as affected by photoperiod during stem elongation and removal of spikelets at booting.Crossref | GoogleScholarGoogle Scholar |
Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ (2005) Physiological processes associated with wheat yield progress in the UK. Crop Science 45, 175–185.
Sinclair TR, Jamieson PD (2006) Grain number, wheat yield, and bottling beer: an analysis. Field Crops Research 98, 60–67.
| Grain number, wheat yield, and bottling beer: an analysis.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Araus JL, Richards RA (1999) Physiological traits that increase the yield potential of wheat. In ‘Wheat: ecology and physiology of yield determination’. (Eds EH Satorre, GA Slafer) pp. 379–415. (Food Products Press: New York)
Slafer GA, Kantolic AG, Appendino ML, Miralles DJ, Savin R (2009) Crop development: genetic control, environmental modulation and relevance to genetic improvement of crop yield. In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 277–308. (Academic Press: London)
Slafer GA, Rawson HM (1994) Sensitivity of wheat phasic development to major environmental factors: a re-examination of some assumptions made by physiologists and modellers. Australian Journal of Plant Physiology 21, 393–426.
| Sensitivity of wheat phasic development to major environmental factors: a re-examination of some assumptions made by physiologists and modellers.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Whitechurch EM (2001) Manipulating wheat development to improve adaptation. In ‘Application of physiology in wheat breeding’. (Eds MP Reynolds, JI Ortiz-Monasterio, A McNab) pp. 160–170. (CIMMYT: Mexico, DF)
Spiertz JHJ, Hamer RJ, Xu H, Primo-Martin C, Don C, van der Putten PEL (2006) Heat stress in wheat (Triticum aestivum L.): effects on grain growth and quality traits. European Journal of Agronomy 25, 89–95.
| Heat stress in wheat (Triticum aestivum L.): effects on grain growth and quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1Kjsbo%3D&md5=5d09705f494b0136c8f5f1d3c6ee2f8eCAS |
Stapper M, Fischer RA (1990a) Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. II. Growth yield and nitrogen use. Australian Journal of Agricultural Research 41, 1021–1041.
| Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. II. Growth yield and nitrogen use.Crossref | GoogleScholarGoogle Scholar |
Stapper M, Fischer RA (1990b) Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. III. Potential yields and optimum flowering dates. Australian Journal of Agricultural Research 41, 1043–1056.
| Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. III. Potential yields and optimum flowering dates.Crossref | GoogleScholarGoogle Scholar |
Stelmakh AF (1998) Genetic systems regulating flowering response in wheat. Euphytica 100, 359–369.
| Genetic systems regulating flowering response in wheat.Crossref | GoogleScholarGoogle Scholar |
Stockle C, Kermanian AR (2009) Crop radiation capture and use efficiency: a framework for crop growth analysis. In ‘Crop physiology: applications for genetic improvement and agronomy’. (Eds V Sadras, D Calderini) pp. 145–170. (Academic Press: London)
Stockman YM, Fischer RA, Brittain EG (1983) Assimilate supply and floret development within the wheat spike. Australian Journal of Plant Physiology 10, 585–594.
| Assimilate supply and floret development within the wheat spike.Crossref | GoogleScholarGoogle Scholar |
Stone PJ, Nicolas ME (1994) Wheat cultivars vary widely in their responses of grain yield and quality to short periods of postanthesis heat-stress. Australian Journal of Plant Physiology 21, 887–900.
| Wheat cultivars vary widely in their responses of grain yield and quality to short periods of postanthesis heat-stress.Crossref | GoogleScholarGoogle Scholar |
Syme JR (1968) Ear emergence of Australian, Mexican and European wheats in relation to time of sowing and their response to vernalization and daylength. Australian Journal of Experimental Agriculture and Animal Husbandry 8, 578–581.
| Ear emergence of Australian, Mexican and European wheats in relation to time of sowing and their response to vernalization and daylength.Crossref | GoogleScholarGoogle Scholar |
Tambussi E, Bort J, Araus J (2007) Water use efficiency in C3 cereals under Mediterranean conditions: a review of physiological aspects. Annals of Applied Biology 150, 307–321.
| Water use efficiency in C3 cereals under Mediterranean conditions: a review of physiological aspects.Crossref | GoogleScholarGoogle Scholar |
Trevaskis B (2010) The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Functional Plant Biology 37, 479–487.
| The central role of the VERNALIZATION1 gene in the vernalization response of cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmt12ksrk%3D&md5=67bbdef88569360371ad1283c715e9b5CAS |
Ugarte C, Calderini DF, Slafer GA (2007) Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Research 100, 240–248.
| Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale.Crossref | GoogleScholarGoogle Scholar |
Ugarte CC, Trupkin SA, Ghiglione H, Slafer G, Casal JJ (2010) Low red/far red ratios delay spike and stem growth in wheat. Journal of Experimental Botany 61, 3151–3162.
| Low red/far red ratios delay spike and stem growth in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVWkt70%3D&md5=b634834c4fdfd65018a14a7dc3e98994CAS | 20497971PubMed |
van Beem J, Mohler V, Lukman R, van Ginkel M, William M, Crossa J, Worland AJ (2005) Analysis of genetic factors influencing the development rate of globally important CIMMYT wheat cultivars. Crop Science 45, 2113–2119.
| Analysis of genetic factors influencing the development rate of globally important CIMMYT wheat cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVOrt7fF&md5=0e2b54522d99ec80d513716eca1135c4CAS |
van Herwaarden AF, Angus JF, Richards RA, Farquhar GD (1998a) ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser – II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research 49, 1083–1094.
| ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser – II. Carbohydrate and protein dynamics.Crossref | GoogleScholarGoogle Scholar |
van Herwaarden AF, Richards RA (2002) Water-soluble carbohydrate accumulation in stems is related to breeding progress in Australian wheats. In ‘Plant breeding for the 11th Millennium: Proceedings of the 12th Australian Plant Breeding Conference’. 15–20 Sept. 2002, Perth, WA. (Ed. JA McComb) pp. 878–882. (Australian Plant Breeding Association, Inc.: Perth)
van Herwaarden AF, Richards RA, Farquhar GD, Angus JF (1998b) ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser – III. The influence of water deficit and heat shock. Australian Journal of Agricultural Research 49, 1095–1110.
| ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser – III. The influence of water deficit and heat shock.Crossref | GoogleScholarGoogle Scholar |
Wall PC (1979) An analysis of factors limiting grain numbers and yield of spring wheat in a low-latitude environment. PhD Thesis, University of Reading, UK.
Wang E, van Oosterom E, Meinke H, Robertson M, Hunt N, Keating B, Probert M (2003) The new APSIM-Wheat model – performance and future improvements. In ‘Proceedings of the 11th Australian Agronomy Conference’. Geelong, Vic. Available at: www.regional.org.au/au/asa/2003/p/2/wang.htm
Wardlaw IF, Blumenthal C, Larroque O, Wrigley CW (2002) Contrasting effects of chronic heat stress and heat shock on kernel weight and flour quality in wheat. Functional Plant Biology 29, 25–34.
| Contrasting effects of chronic heat stress and heat shock on kernel weight and flour quality in wheat.Crossref | GoogleScholarGoogle Scholar |
Wardlaw IF, Wrigley CW (1994) Heat tolerance in temperate cereals: an overview. Australian Journal of Plant Physiology 21, 695–703.
| Heat tolerance in temperate cereals: an overview.Crossref | GoogleScholarGoogle Scholar |
White JW, Herndl M, Hunt LA, Payne TS, Hoogenboom G (2008) Simulation-based analysis of effects of Vrn and Ppd loci on flowering in wheat. Crop Science 48, 678–687.
| Simulation-based analysis of effects of Vrn and Ppd loci on flowering in wheat.Crossref | GoogleScholarGoogle Scholar |
Whitechurch EM, Slafer GA (2002) Contrasting Ppd alleles in wheat: effects on sensitivity to photoperiod in different phases. Field Crops Research 73, 95–105.
| Contrasting Ppd alleles in wheat: effects on sensitivity to photoperiod in different phases.Crossref | GoogleScholarGoogle Scholar |
Whitechurch EM, Slafer GA, Miralles DJ (2007) Variability in the duration of stem elongation in wheat and barley genotypes. Journal of Agronomy & Crop Science 193, 138–145.
| Variability in the duration of stem elongation in wheat and barley genotypes.Crossref | GoogleScholarGoogle Scholar |
Worland AJ (1996) The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 89, 49–57.
| The influence of flowering time genes on environmental adaptability in European wheats.Crossref | GoogleScholarGoogle Scholar |
Xue Z-Y, Zhi D-Y, Xue G-P, Zhang H, Zhao Y-X, Xia G-M (2004) Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Science 167, 849–859.
| Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmt1Wksrs%3D&md5=ffaafd9939c9cadeef03f56f33fde026CAS |
Yang J, Sears RG, Gill BS, Paulsen GM (2002) Genotypic influences in utilization of assimilate sources during maturation of wheat under chronic heat and heat shock stress. Euphytica 125, 179–188.
| Genotypic influences in utilization of assimilate sources during maturation of wheat under chronic heat and heat shock stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksFehsr0%3D&md5=e399fb25224479ae25de046d621c2fadCAS |
Yoshida T, Nishida H, Zhu J, Nitcher R, Distelfeld A, Akashi Y, Kato K, Dubcovsky J (2010) Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat. Theoretical and Applied Genetics 120, 543–552.
| Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotV2hsw%3D%3D&md5=450e963c792e8aaa012259dd840634e1CAS | 19847391PubMed |
1This review assumes a specific gene occupies a given position or locus on one of the wheat homeologous chromosomes and codes for a protein; different alleles of the gene produce slightly different proteins or different amounts of a given protein leading to measurable phenotypic changes. Specific known genes are named, abbreviated, and italicised according to standard nomenclature, beginning with capitals, but dominance or recessiveness are not symbolised in any way.
2Since earliness seems to be a more stable state, as seen with photoperiod and vernalisation-insensitive cultivars in warm natural environments, responses to development alleles seem more sensibly expressed relative to the early phenotype, thus as delays in development; an approach adopted here.
3There can be significant sequence variation beyond the region of any allele targeted by a given molecular marker.
4The study of Abbate et al. (1998) sampled crops at 7–9 days after 50% anthesis (GS65), and removed developing grains. Spearman et al. (2005) sampled at GS61, which may be too soon for SDWa in a crop. For individual culms at 16.4°C, grain weight as a % of non-grain spike weight increases 3.6% per day from the 2nd to 9th day after first anthesis (R. A. Fischer, unpubl. data).


