Carbon storage value of native vegetation on a subhumid–semi-arid floodplain
Rhiannon Smith A B and Nick Reid AA Ecosystem Management, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
B Corresponding author. Email: rsmith66@une.edu.au
Crop and Pasture Science 64(12) 1209-1216 https://doi.org/10.1071/CP13075
Submitted: 25 February 2013 Accepted: 26 November 2013 Published: 18 December 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
The protection of carbon (C) stores in the form of remnant native vegetation and soils is crucial for minimising C emissions entering the atmosphere. This study estimated C storage in soils, woody vegetation, dead standing vegetation, coarse woody debris, herbaceous vegetation, litter and roots in plant communities commonly encountered on cotton farms. River red gum was the most valuable vegetation type for C storage, having up to 4.5% C content in the surface (0–5 cm) soil, a total-site C store of 216 ± 28 t ha–1 (mean ± s.e.) and a maximum value of 396.4 t C ha–1. Grasslands were the least C-dense, with 36.4 ± 3.72 t C ha–1. The greatest proportion of C in river red gum sites was in standing woody biomass, but in all other vegetation types and especially grasslands, the top 0–30 cm of the soil was the most C-rich component. Aboveground woody vegetation determined total-site C sequestration, as it strongly influenced all other C-storing components, including soil C. This study illustrates the value of native vegetation and the soil beneath for storing large amounts of C. There is a case for rewarding farmers for maintaining and enhancing remnant vegetation to avoid vegetation degradation and loss of existing C stores.
Additional keywords: carbon accounting, rangelands, soil, woody vegetation.
Introduction
Accelerated global climate change is one of the most pressing environmental issues facing the world today (IPCC 2007). Increases in greenhouse gas (GHG) concentrations (including carbon dioxide, CO2) in the atmosphere are the primary cause of accelerated climate change (Crowley 2000; Houghton 2007). Carbon dioxide is one of the most important GHGs and in 2004 accounted for 77% of total anthropogenic GHG emissions (Olivier et al. 2005). Human-induced CO2 emissions currently exceed the ability of natural systems to sequester C (Houghton 2007). Hence, atmospheric concentrations of CO2 have increased by ~35% since 1850 (Houghton 2007). Scenarios resulting from climate change entail varying degrees of impact on human wellbeing through increases in sea level, changes in fresh water availability and agricultural production, and subsequent global water and food shortages, as well as deleterious effects on human health (Millennium Ecosystem Assessment Board 2005; Stokes and Howden 2008, 2010). Mass extinctions and severe biodiversity loss are probable consequences of climate change, resulting in altered ecosystem composition, structure and function, and flow-on impacts on ecosystem service provision (Millennium Ecosystem Assessment Board 2005; Fischlin et al. 2007; Norton and Reid 2013).
Extensive land-use change (e.g. conversion of forest and woodlands to agriculture) has made a sizeable contribution to anthropogenic GHG emissions through the release of significant carbon (C) stores (Houghton and Hackler 2000; Beedlow et al. 2004; Houghton 2007; Lal 2008). Currently, protection of existing C stores in old-growth forests does not gain credit under the Kyoto Protocol (United Nations 1998). Only anthropogenic effects on ecosystems and changes in C stocks since 1990 are considered mandatory (Article 3.3, United Nations 1998). There is considerable evidence to show that protection of existing C stores from clearing, especially in old-growth forest ecosystems, is a key mechanism to avoid and reduce C emissions worldwide (Harmon et al. 1990; Schulze et al. 2000; Jandl et al. 2007; Luyssaert et al. 2008). Structurally diverse vegetation including woody plants and a range of age classes stores more C in both soils and vegetation than timber plantations, grasslands, pastures and crops (Harmon et al. 1990; Schulze et al. 2000; Eldridge and Wilson 2002; Luyssaert et al. 2008; Wilson et al. 2009; Young et al. 2009).
Measurement of C storage and sequestration is currently being undertaken at all scales, from individual soil aggregates to whole continents, and in a wide range of ecosystems, from deserts to tropical rainforests (e.g. Brown 2002; Wilson et al. 2009; Pan et al. 2011; McSherry and Ritchie 2013). Countries that have ratified the Kyoto Protocol, in particular, are seeking information on both emissions and sequestration potential for C-accounting purposes. Research into C sequestration and storage has generally centred on higher rainfall zones, especially tropical areas, with little consideration given to the importance of semi-arid rangelands (Follett and Reed 2010; Pacala et al. 2001; Schuman et al. 2002). Data on C sequestration in semi-arid woodlands is lacking in Australia (Keith et al. 2000), which is surprising considering these ecosystems cover a large portion of Australia, and small changes in land management practices over such a large area have the potential to sequester large amounts of C.
The aim of this study was to quantify the C storage value of various types of native vegetation commonly encountered on farms on inland, semi-arid floodplains. The C store in different components of native vegetation and soil at a site was estimated to determine the relative importance of each ecosystem component in contributing to C storage. The objective was to determine differences in C storage for multiple ecosystem components and vegetation types, and hence determine their value in providing a C-storage service. Carbon storage, rather than sequestration, was the focus of this study for two reasons. First, considerable uncertainty surrounds the direct measurement of C sequestration (Schulze et al. 2000; Brown 2002; García-Oliva and Masera 2004; Lövbrand 2004). Second, retention of C already stored in ecosystems is important, as indicated by the amount of C emissions attributed to land conversion (IPCC 2007).
Methods
Study region
The lower Namoi floodplain in northern New South Wales, Australia, is dominated by agriculture, including irrigated and dryland cropping and grazing of native pastures. The study region has a semi-arid, subtropical climate with unreliable and sporadic rainfall throughout the region. Mean annual rainfall declines from east to west across the region, varying from 600 mm near Boggabri (–30.7000°, 150.0333°; 246 m amsl) to 400 mm near Walgett (–30.0167°, 148.1167°; 136 m amsl), with a slight summer dominance (Stannard and Kelly 1977). Mean maximum summer temperatures increase across the region from east to west, ranging between 33 and 36°C, while winter mean minima are 3−4°C (BOM 2008). The floodplain soils are mainly Vertosols (Isbell 1996), mostly black, grey or brown clays, typically with self-mulching characteristics, slightly to strongly alkaline, and with free CaCO3 at varying depths (Stannard and Kelly 1977).
Many cotton properties on inland floodplains in eastern Australia have river or creek frontage and areas of native vegetation, including river red gum (Eucalyptus camaldulensis) forests, coolibah (E. coolabah), black box (E. largiflorens) and myall (Acacia pendula) woodlands, and native and derived grasslands. Up to 40% of the average cotton farm is native vegetation (Inovact Consulting 2012). Cotton properties therefore have the potential to contribute to regional C stores through protection of existing stands of remnant native vegetation.
Vegetation patterns on the lower Namoi floodplain are largely dictated by the presence and movement of water across the floodplain (Kearle et al. 2002; Smith 2010). In general, on the cracking clay soils, woody vegetation occurs in lower lying areas and along drainage lines, while large expanses of sparsely timbered natural grassland once occurred in drier, less frequently flooded areas. Land-use history and management of native vegetation varies across the region. Areas of coolibah and black box woodland were ring-barked in the 1900s (J. Moore, pers. comm., 2006), and dead standing trees remain at many formerly wooded sites. Some stands of coolibah and black box were allowed to coppice after being ring-barked and now exist as stunted, multiple-stemmed trees. In other areas, timber has been clear-felled or thinned to encourage pasture growth, allow cropping, or cut for firewood and fence posts. Approximately 7% of the region is woody vegetation (Smith 2010), while areas of natural or derived grasslands, usually grazed, are interspersed among cropping paddocks.
Field sampling
Sixty-one sites on cotton farms and travelling stock routes on the floodplain between Boggabri and Walgett (study area 7163 km2) were surveyed in 2008. Cotton farms were the focus of the study as they covered the range of floodplain positions from regularly flooded riparian areas to current and relict floodplains and rarely flooded rises. Six commonly occurring native vegetation types in the region were targeted: (i) river red gum-dominated riparian forests and woodlands; (ii) coolibah woodland; (iii) weeping myall woodland and open woodland; (iv) black box woodland; (v) native tree and shrub plantings; and (vi) native grasslands and derived grasslands (i.e. those derived by clearing the over-storey of trees and shrubs to leave the herbaceous layer). River red gum communities occurred along the banks of the Namoi River and graded into coolibah-dominated vegetation, which occurred along lesser streams and in slight depressions on the floodplain. Black box communities occurred in the western (low-rainfall) end of the region, usually on relict floodplains that are now rarely flooded. Grasslands occurred across the floodplain, especially on the heaviest clays, although derived grasslands occurred throughout the floodplain. Myall communities were generally only encountered on slight rises. Some of the derived grassland sites were probably once myall-dominated woodlands, as myall trees are easy to clear given their small stature. Other vegetation types occur in the region, but the vegetation types studied here were the most common.
Aboveground woody vegetation biomass
Biomass was sampled in quadrats stratified by vegetation type. Quadrats varied in size (25 by 25 m to 1 ha) according to tree density. All trees found in 1-ha quadrats were measured in open situations (e.g. grasslands and open woodlands), and a minimum of 15–20 trees in dense vegetation. The diameter of trees was measured at breast height (1.3 m) over bark (DBHOB) using a metal diameter tape or electronic digital callipers. For trees or shrubs where this measurement was not possible or sensible due to low branching habit or small height, height was measured using height poles, or the diameter at 30 cm above ground level (diam30) was measured. Tree DBHOB was measured with 0.1-cm accuracy and height was measured to the nearest 0.1 m. Dead standing material was also assessed in this manner. Where the dead tree was reduced to no more than a hollow stem, biomass was calculated using a method similar to that used for estimation of coarse woody debris (CWD) biomass (see below).
Allometric equations were used to estimate tree biomass based on tree DBHOB, diam30 or height. The equations used were developed by Snowdon et al. (2000) and are used by the Australian Greenhouse Office. Five equations were used for shrubs, woodland trees (DBHOB and diam30), native plantations and native sclerophyll forest, respectively (Snowdon et al. 2000):
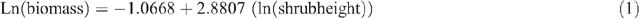
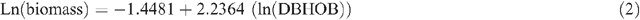
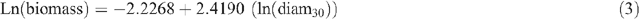
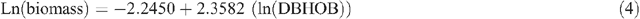
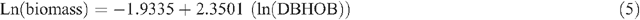
Tree hollow development and large branch fall was noted, and an estimate of the proportion of the tree that was missing was deducted during tree biomass calculations.
Litter and aboveground herbaceous vegetation biomass
Litter and herbaceous biomass was sampled in autumn and spring 2008 using a modified BOTANAL (Tothill et al. 1978) method. Litter and total herbaceous vegetation biomass were ranked using the BOTANAL method in 20 quadrats of 50 cm by 50 cm located at 4-m intervals around the perimeter of a representative 20 m by 20 m quadrat at each site. Weather conditions in the months preceding sampling were favourable for maximum herbaceous vegetation growth; therefore, the data estimate the maximum vegetation biomass potential at sites under prevailing management. In converting biomass dry weight to mass of C, it was assumed that 50% of the herbaceous vegetation biomass was C (Snowdon et al. 2000).
To convert BOTANAL ranks to yield and percentage biomass composition by species, regression relationships were developed using calibration quadrats representing the range of conditions and species encountered. Calibration quadrats were scored on a daily basis and harvested at the end of each of the two survey periods. Twenty calibration quadrats of 50 cm by 50 cm were set up at each of three locations across the floodplain (2 survey periods × 3 locations × 20 calibration quadrats). Litter was harvested by hand and stored separately before harvest of standing biomass 2 cm above ground level using secateurs. Litter was defined as dead, unattached plant material and it included twigs up to 5 cm in diameter. Harvested biomass and litter samples were dried for 72 h at 60°C before calculation of total herbage mass and litter mass. One or two observers were used, and for each observer, daily regression relationships were developed for herbaceous vegetation biomass and litter to account for variation in the observer’s biomass estimates during the survey period.
Coarse woody debris
The length and end diameters of each piece of CWD were measured using callipers and a metal tape in order to generate a volume. The CWD included any woody material with a diameter >5 cm. The percentage of each piece that was missing was estimated and its status as sound or rotten was recorded. Samples of sound and rotten wood were collected across the study area for river red gum, coolibah and myall, and the average density was used to convert volume to mass. Where CWD crossed the quadrat boundary, only the portion of wood inside the quadrat was considered. Quadrat size varied from 10 m by 10 m for the site with the largest amount (18.1 t C ha–1) of CWD to 1 ha at more open sites. The size of the quadrat was determined by the density of CWD; the biggest quadrat that was logistically possible to survey was used at each site.
Soils
Soil C was sampled using a manual soil-coring device as per current standards (McKenzie et al. 2000, 2002), with soil cores divided into four depth increments, 0–5, 5–10, 10–20 and 20–30 cm. A depth of 30 cm was chosen to be consistent with soil C sampling protocols (McKenzie et al. 2000, 2002). At each site, a quadrat of 25 m by 25 m was located in a representative area of the vegetation type and management history of interest. Where possible, sites were at least 30 m from boundaries with other land uses and different management histories. Nine cores were collected at each site and stratified according to the proportion of different cover types, including trees, shrubs, herbs, grasses, litter and bare ground. Cores were stored in zip-lock plastic bags in cool conditions for up to 1 week and then stored at 4°C for up to 2 weeks before air-drying.
Soil samples were bulked by depth at each site and ground using a mechanical grinder to pass a 2-mm sieve after macro-organic matter (leaves, plant roots, etc.) was removed. Moisture content was determined on a subsample (after drying at 105°C for 48 h) for subsequent calculation of oven-dry bulk density. Another subsample was crushed and passed through a 0.5-mm sieve for analysis of percentage total organic C (TOC) content using a NA 1500 Solid Sample Analyzer (Carlo Erba Reagents, Milan, Italy). Where soil pH was >7.5, a subsample treated to remove carbonates using 2% orthophosphoric acid was measured immediately after the corresponding untreated subsample. Where treatment to remove carbonates had no effect on the percentage C measured, the average percentage C value of the treated and untreated samples was used in further analysis. Where treatment removed carbonates, the percentage C value of the treated sample alone was used.
Roots and total site C
Root biomass was estimated using recommended root : shoot ratios of 0.25 × aboveground biomass for woody vegetation (Snowdon et al. 2000) and 0.5 × aboveground vegetation for herbaceous vegetation (Mooney 1972). Total site C was determined by summing the C content of each component, expressed on a per-hectare basis.
Statistical analyses
Parametric analysis of variance (AOV) in Statistix 8 (Analytical Software 2003) was used to examine differences in C contribution by woody vegetation, herbaceous vegetation, litter, CWD, dead standing wood, roots, soil and total site C for each of the six vegetation types. Data were transformed to achieve normality; where normality of data could not be achieved using log or square-root transformations, rank-transformed data were used. Significant differences between vegetation types for each C component were determined using least significant difference (l.s.d.). Spearman rank correlations were generated to highlight relationships between vegetation characteristics and C contribution in woody vegetation, herbaceous vegetation, litter, CWD, dead standing wood, and soil across all vegetation types. Spearman’s correlations were used, as data were not normally distributed. Differences were regarded as significant where P ≤ 0.05.
Results
River red gum
The largest quantity of C stored was found in river red gum vegetation (Table 1). River red gum sites generally consisted of large, old-growth trees as reflected by high mean and maximum DBHOB values (Table 2). More than half of the C stored in river red gum sites came from woody vegetation (Fig. 1). Spearman correlations showed that the C stored in soil, litter, CWD and dead standing woody components was positively correlated with aboveground woody C (Table 3). Litter, CWD, dead standing tree and root biomasses were also higher in river red gum sites than in other vegetation types. River red gum soils also had the highest TOC values and consistently had higher mean TOC down the profile to 30 cm depth (F5,55 = 8.04, P < 0.001) compared with other vegetation types (Fig. 2).

|
Coolibah
Coolibah sites had less than half the total C values of river red gum sites (Table 1). This was due to aboveground woody vegetation, on average, being less than half that of river red gum sites, although this difference was not significant (after rank transformation). Coolibah trees were generally about half the size of river red gums in DBHOB (Table 2). The woody vegetation and soil C components were similar and contributed ~40% each to total C in coolibah sites (Fig. 1). All other C-storing components made up ~20% of total C storage at coolibah sites; this value was similar across most of the wooded vegetation types.
Black box, myall and planted vegetation
In black box, myall and planted vegetation, the largest proportion of total site C was contained in the upper 30 cm of the soil (Fig. 1). Woody vegetation contributed ~30% of C to the overall store. Only minor amounts of C were found in herbaceous vegetation, litter, CWD, dead standing woody vegetation and plant roots, these components together averaging 7–18% across vegetation types. Myall, black box and planted sites had similar mean and maximum DBHOB values, which were considerably less than for river red gum or coolibah (Table 2). However, the mean number of trees at each site was greatest in planted sites. Planted sites had the largest stores of C in litter of the six vegetation types, but variability in litter accumulation was high. Planted sites also had the highest herbaceous biomass of the wooded vegetation types, but this varied considerably depending on the density and age of the planting.
Grassland
Grassland vegetation contained the least C of all of the vegetation types (Table 1). This was due to the lack of woody vegetation at most grassland sites. Where trees were present in grassland sites, they tended to be relatively large with mean DBHOB similar to river red gum and coolibah sites (Table 2). Given the low woody biomass values in grassland sites, litter, CWD, dead standing trees, roots and TOC contributed very little to total C storage. Spearman’s correlations showed that aboveground herbaceous C was negatively correlated with aboveground woody C (Table 3). Grasslands had, on average, the highest proportion of C in aboveground herbaceous biomass.
Discussion
Four major results of our study are emphasised here. First, woody vegetation was a strong determinant of total site C storage. Woody biomass was positively correlated with litter, CWD, dead standing trees and TOC. Even when hollow formation was accounted for, sites with multiple, old-growth trees stored more C than sites with fewer or no old-growth trees. This result is consistent with numerous studies from around the world (e.g. Harmon et al. 1990; Schulze et al. 2000; Young et al. 2005; Jandl et al. 2007; Luyssaert et al. 2008; Wilson et al. 2008), and underscores the value of woody vegetation in C storage and sequestration.
Second, we showed that C is partitioned differently within distinct ecosystems. The relative importance of the various C-storing components depends on the abundance of woody vegetation. In ecosystems with lots of woody biomass, C was stored equally between the woody biomass and associated components (CWD, litter, dead standing trees), and the soil. However, in ecosystems with less woody biomass, TOC was the dominant C store. In grassland sites, >90% of total site C was stored in the top 30 cm of the soil profile. In general, larger tree basal area resulted in lower herbaceous vegetation biomass. At sites with abundant woody biomass, herbaceous vegetation therefore contributed little to total site C, as found by other researchers (Jackson and Ash 1998; Scanlan and Burrows 1990; Scholes 2003; Scholes and Archer 1997). However, in open woodland and grassland ecosystems, herbaceous vegetation may provide a significant proportion of C inputs to the soil.
Third, within C-storing components, ecosystems differed in the way in which C was partitioned. For example, the high concentration of TOC in the surface 0–5 cm of the soil profile in river red gum and perhaps coolibah sites indicated that the soils received greater C inputs at the surface than from deeper in the soil profile. This result is consistent with data presented by Jobbágy and Jackson (2000), who reported that the majority of soil C in forested ecosystems is found in the surface soil, more so than in grasslands. This is due to the way in which different vegetation types partition biomass. In grasslands, a high proportion of annual production occurs below ground, and this is the greatest input of C to the soil, whereas in wooded ecosystems the dominant C input from the vegetation to the soil is through litter deposition (Jobbágy and Jackson 2000).
Finally, our study confirmed that C is distributed unevenly on floodplains, being concentrated in riparian areas where nutrients are abundant, floods are frequent, and access to water is not a limiting factor. This is reflected in the dimensions (height and DBH) of the dominant species, i.e. river red gum and coolibah trees achieve greater dimensions than black box and myall. The turnover times for litter and woody debris also play a role in C accumulation in each of the vegetation communities. Woody debris and litter derived from eucalypts has a higher C : N ratio, and therefore has a longer residence time, than litter derived from grasses (Snowdon et al. 2005). As such, some vegetation types are disproportionately important for C storage. This result is consistent with studies elsewhere that show greater net primary productivity (NPP) in riparian areas compared with other parts of the floodplain (Naiman et al. 2005). While each of the vegetation types surveyed here has different values in terms of biodiversity conservation, forage production and other ecosystem services, given the value of riparian areas in storing large amounts of C, appropriate management and protection of these areas should be a priority in strategies to mitigate climate change.
We have demonstrated that woodlands such as those occurring on cotton farms represent a significant C store, and a small but significant and often overlooked C sink (Burrows et al. 2002). Burrows et al. (2002) estimated the mean net aboveground annual C increment for 57 savanna woodland sites across Queensland (including the Darling Downs region) at 0.53 t C ha–1 (0.53 × 3.667 = 1.94 t CO2(e)) each year. Grace (2008) calculated ~446.5 t CO2(e) is emitted each year on an average, mixed farming enterprise (100 ha grazed pastures, 100 ha of dryland cropping and 200 ha of irrigated crops such as cotton) in the Darling Downs region of Queensland; ~230 ha of woodland vegetation would be required to offset C emissions and achieve a C-neutral enterprise on the case study farm. Approximately 40% of the average Australian cotton farm is under native vegetation (Inovact Consulting 2012). Using the data of Burrows et al. (2002) and Grace (2008), the average Australian cotton farm should be C-neutral, and may be accumulating a small amount of C.
Policy implications
This study and many others (e.g. Harmon et al. 1990; Schulze et al. 2000; Jandl et al. 2007; Luyssaert et al. 2008) illustrate the importance of existing native vegetation, especially old-growth woodlands and forests, in storing large amounts of C. There is a case for the development of incentives for landholders in the form of C credits for protection and appropriate management of these stores in order to avoid large amounts of C entering the atmosphere. Currently, landholders who protect areas of remnant native vegetation on their land are unable to access C credits under the rules of the Kyoto Protocol. In Australia, the Carbon Farming Initiative (www.climatechange.gov.au/reducing- carbon/carbon-farming-initiative) clearly states that ‘the establishment of a conservation covenant in perpetuity over native vegetation’ is not an eligible C-abatement activity and does not attract C credits. However, ‘the protection of native forest from clearing where the landholder received consent to clear before 1 July 2010’ is an eligibly abatement activity under strict conditions. The data presented here show that landholders are providing a valuable service to the wider community by maintaining remnant native vegetation on their land. If this effort is not recognised and rewarded, market and other forces could drive landholders to transform these areas for farming and crop production at times when land-use controls over vegetation clearance are relaxed (Norton and Reid 2013).
Conclusion
Presence of woody vegetation biomass was the most important determinant for C storage in remnant native vegetation on floodplain farms, both as a C sink in its own right, and in generating litter, CWD, dead standing wood, root and soil C. Riparian vegetation dominated by river red gum was of greatest value in terms of total C storage, reflecting a worldwide pattern of high NPP in riparian vegetation compared with other vegetation types in similar environments.
Acknowledgements
Associate Professor Brian Wilson, Dr Matthew Tighe, David Tongway and David Carr gave advice on methods in the early phase of this research. Leanne Lisle helped with laboratory analysis of soil samples. Scott Purcell, Fiona Norrie, Tegan Smith, Mark Dahm, Dr Megan Good, Dr Guy Roth and Dr Peter Berney helped with fieldwork. Funding for this research was provided by the Cotton Catchment Communities Cooperative Research Centre, Cotton Research and Development Corporation, Namoi Catchment Management Authority and the School of Environmental and Rural Sciences at the University of New England.
References
Analytical Software (2003) ‘Statistix 8 User’s Manual.’ (Analytical Software: Tallahassee, FL)Beedlow PA, Tingley DT, Phillips DL, Hogsett WE, Olszyk DM (2004) Rising atmospheric CO2 and carbon sequestration in forests. Frontiers in Ecology and the Environment 2, 315–322.
BOM (2008) ‘National Archives of Australia: Rainfall and climate files.’ (Climate and Consultancy Section in the NSW Office of the Bureau of Meteorology: Sydney)
Brown S (2002) Measuring carbon in forests: current status and future challenges. Environmental Pollution 116, 363–372.
| Measuring carbon in forests: current status and future challenges.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovVersLc%3D&md5=e20d04925883af8542634d22c12654beCAS | 11822714PubMed |
Burrows W, Henry B, Back P, Hoffmann M, Tait L, Anderson E, Menke N, Danaher T, Carter J, McKeon G (2002) Growth and carbon stock change in eucalypt woodlands in northeast Australia: ecological and greenhouse sink implications. Global Change Biology 8, 769–784.
| Growth and carbon stock change in eucalypt woodlands in northeast Australia: ecological and greenhouse sink implications.Crossref | GoogleScholarGoogle Scholar |
Crowley TJ (2000) Causes of climate change over the past 1000 years. Science 289, 270–277.
| Causes of climate change over the past 1000 years.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXltFejsbo%3D&md5=619213b0214e1b5d2ef33083562868baCAS | 10894770PubMed |
Eldridge DJ, Wilson BR (2002) Carbon storage in soil and vegetation in paired roadside sites in the box woodlands of eastern Australia. Australian Forestry 65, 268–272.
| Carbon storage in soil and vegetation in paired roadside sites in the box woodlands of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Fischlin A, Midgley GF, Price JT, Leemans R, Copal B, Turley C, Rounsevell MDA, Duke OP, Tarazona J, Velichko AA (2007) Ecosystems, their properties, goods and services. In ‘Climate change 2007: Impacts, adaption and vulnerability. Contribution of the Working Group II to the 4th Assessment Report of the Intergovernmental Panel on Climate Change (IPCC)’. (Eds ML Parry, OF Canziani, IP Palutikof, PJ van der Linden, CE Hanson) pp. 211–72. (Cambridge University Press: Cambridge, UK)
Follett RF, Reed DA (2010) Soil carbon sequestration in grazing lands: societal benefits and policy implications. Rangeland Ecology and Management 63, 4–15.
| Soil carbon sequestration in grazing lands: societal benefits and policy implications.Crossref | GoogleScholarGoogle Scholar |
García-Oliva F, Masera OR (2004) Assessment and management issues related to soil carbon sequestration in land-use, land-use change, and forestry (LULUCF) projects under the Kyoto protocol. Climatic Change 65, 347–364.
| Assessment and management issues related to soil carbon sequestration in land-use, land-use change, and forestry (LULUCF) projects under the Kyoto protocol.Crossref | GoogleScholarGoogle Scholar |
Grace P (2008) A cotton farm’s carbon and greenhouse footprint. In ‘14th Australian Cotton Conference’. 12–15 August 2008, Broadbeach, Australia. (Cotton Australia: Sydney)
Harmon M, Ferrel WK, Franklin JF (1990) Effects on carbon storage of conversion of old-growth forests to young forests. Science 247, 699–702.
| Effects on carbon storage of conversion of old-growth forests to young forests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhs1Citbs%3D&md5=f944fb4a169dca094097ab28f72483c5CAS | 17771887PubMed |
Houghton RA (2007) Balancing the global carbon budget. Annual Review of Earth and Planetary Sciences 35, 313–347.
| Balancing the global carbon budget.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtlKhuro%3D&md5=103f91b8e62fb224468c2666c1fa7529CAS |
Houghton RA, Hackler JL (2000) Changes in terrestrial carbon storage in the United States. 1: the roles of agriculture and forestry. Global Ecology and Biogeography 9, 125–144.
| Changes in terrestrial carbon storage in the United States. 1: the roles of agriculture and forestry.Crossref | GoogleScholarGoogle Scholar |
Inovact Consulting (2012) ‘Australian cotton industry: Third environmental assessment.’ (Cotton Research and Development Corporation: Narrabri, NSW)
IPCC (2007) ‘Climate change 2007: Fourth Assessment Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Eds RK Pachauri, A Reisinger) (IPCC: Geneva)
Isbell RF (1996) ‘The Australian Soil Classification.’ Revised edn (CSIRO Publishing: Melbourne)
Jackson J, Ash AJ (1998) Tree–grass relationships in open eucalypt woodlands of northeastern Australia: influence of trees on pasture productivity, forage quality and species distribution. Agroforestry Systems 40, 159–176.
| Tree–grass relationships in open eucalypt woodlands of northeastern Australia: influence of trees on pasture productivity, forage quality and species distribution.Crossref | GoogleScholarGoogle Scholar |
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137, 253–268.
| How strongly can forest management influence soil carbon sequestration?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnvFCgsA%3D%3D&md5=498a0291167c3c875cf2a549f4f08ec4CAS |
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10, 423–436.
| The vertical distribution of soil organic carbon and its relation to climate and vegetation.Crossref | GoogleScholarGoogle Scholar |
Kearle A, Gosper C, Achurch H, Laity T (2002) ‘Darling Riverine Plains background report: Darling Riverine Plains bioregion.’ (Western Regional Assessments Unit, NSW National Parks and Wildlife: Dubbo, NSW)
Keith H, Barrett D, Keenan R (2000) ‘Review of allometric relationships for estimating woody biomass for New South Wales, the Australian Capital Territory, Victoria, Tasmania and South Australia.’ (Australian Greenhouse Office: Canberra, ACT)
Lal R (2008) Carbon sequestration. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 815–830.
| Carbon sequestration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXislGgtbw%3D&md5=97c44f607657ab96dd0448cad4e0a129CAS | 17761468PubMed |
Lövbrand E (2004) Bridging political expectations and scientific limitations in climate risk management—on the uncertain effect of international carbon sink policies. Climatic Change 67, 449–460.
| Bridging political expectations and scientific limitations in climate risk management—on the uncertain effect of international carbon sink policies.Crossref | GoogleScholarGoogle Scholar |
Luyssaert S, Schulze ED, Borner A, Knohl A, Hessenmoller D, Law BE, Ciais P, Grace J (2008) Old-growth forests as global carbon sinks. Nature 455, 213–215.
| Old-growth forests as global carbon sinks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtV2qtLzE&md5=15f306be9aa271c2aa7f9a8b878c9460CAS | 18784722PubMed |
McKenzie N, Ryan P, Fogarty P, Wood J (2000) ‘Sampling, measurement and analytical protocols for carbon estimation in soil, litter and coarse woody debris.’ National Carbon Accounting System Technical Report No. 14. (Australian Greenhouse Office: Canberra, ACT)
McKenzie N, Henderson B, McDonald W (2002) ‘Monitoring soil change, principles and practices for Australian conditions.’ Technical Report 18/02. (CSIRO Land and Water: Canberra, ACT)
McSherry ME, Ritchie ME (2013) Effects of grazing on grassland soil carbon: a global review. Global Change Biology 19, 1347–1357.
| Effects of grazing on grassland soil carbon: a global review.Crossref | GoogleScholarGoogle Scholar | 23504715PubMed |
Millennium Ecosystem Assessment Board (2005) ‘Ecosystems and human well-being. Synthesis.’ (Island Press: Washington, DC)
Mooney HA (1972) The carbon balance of plants. Annual Review of Ecology and Systematics 3, 315–346.
| The carbon balance of plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXht1Whsbo%3D&md5=c7d0a62377889227d3ad329f7e10fa0fCAS |
Naiman RJ, Décamps H, McClain ME (2005) ‘Riparia: Ecology, conservation, and management of streamside communities.’ (Elsevier Inc.: San Diego, CA)
Norton D, Reid N (2013) ‘Nature and farming: Sustaining native biodiversity in agricultural landscapes.’ (CSIRO Publishing: Melbourne)
Olivier JGJ, van Aardenne JA, Dentener F, Ganzeveld L, Peters JAHW (2005) Recent trends in global greenhouse gas emissions: regional trends 1970–2000 and spatial distribution of key sources in 2000. Environmental Sciences 2, 81–99.
| Recent trends in global greenhouse gas emissions: regional trends 1970–2000 and spatial distribution of key sources in 2000.Crossref | GoogleScholarGoogle Scholar |
Pacala SW, Hurtt GC, Baker D, Peylin P, Houghton RA, Birdsey RA, Heath L, Sundquist ET, Stallard RF, Ciais P, Moorcroft P, Caspersen JP, Shevliakova E, Moore B, Kohlmaier G, Holland E, Gloor M, Harmon ME, Fan SM, Sarmiento JL, Goodale CL, Schimel D, Field CB (2001) Consistent land- and atmosphere-based U.S. carbon sink estimates. Science 292, 2316–2320.
| Consistent land- and atmosphere-based U.S. carbon sink estimates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzmtlWmsQ%3D%3D&md5=492093c34c9492c2bbab3e95166dba17CAS | 11423659PubMed |
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala S, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333, 988–993.
| A large and persistent carbon sink in the world’s forests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWrtr%2FE&md5=b20403c442bc3ad17c7255a9e1a8b7c9CAS | 21764754PubMed |
Scanlan JC, Burrows WH (1990) Woody overstorey impact on herbaceous understorey in Eucalyptus spp. communities in central Queensland. Austral Ecology 15, 191–197.
| Woody overstorey impact on herbaceous understorey in Eucalyptus spp. communities in central Queensland.Crossref | GoogleScholarGoogle Scholar |
Scholes RJ (2003) Convex relationships in ecosystems containing mixtures of trees and grass. Environmental and Resource Economics 26, 559–574.
| Convex relationships in ecosystems containing mixtures of trees and grass.Crossref | GoogleScholarGoogle Scholar |
Scholes RJ, Archer SR (1997) Tree–grass interactions in savannas. Annual Review of Ecology and Systematics 28, 517–544.
| Tree–grass interactions in savannas.Crossref | GoogleScholarGoogle Scholar |
Schulze ED, Wirth C, Heimann M (2000) Climate change: managing forests after Kyoto. Science 289, 2058–2059.
| Climate change: managing forests after Kyoto.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmvVCnu7g%3D&md5=6f842ffbde1b5f0a686ed2e3bcb68cb6CAS |
Schuman GE, Janzen HH, Herrick JE (2002) Soil carbon dynamics and potential carbon sequestration by rangelands. Environmental Pollution 116, 391–396.
| Soil carbon dynamics and potential carbon sequestration by rangelands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovVersbs%3D&md5=49d9eb0763c72ecc2201b999e8f94520CAS |
Smith R (2010) Biodiversity and ecosystem services associated with remnant native vegetation in and agricultural floodplain landscape. PhD Thesis, University of New England, Armidale, NSW, Australia.
Snowdon P, Eamus D, Gibbons P, Khanna P, Keith H, Raison J, Kirschbaum M (2000) ‘Synthesis of allometrics, review of root biomass and design of future woody biomass sampling strategies.’ Technical Report No. 17. (Australian Greenhouse Office: Canberra, ACT)
Snowdon P, Ryan P, Raison J (2005) ‘Review of C:N ratios in vegetation, litter and soil under Australian native forests and plantations.’ Technical Report No. 24. (Australian Greenhouse Office: Canberra, ACT)
Stannard ME, Kelly ID (1977) ‘The irrigation potential of the lower Namoi Valley.’ (Water Resources Commission: Sydney)
Stokes C, Howden M (Eds) (2008) ‘An overview of climate change adaption in Australian primary industries—impacts, options and priorities.’ Report prepared for the National Climate Change Research Strategy for Primary Industries. (CSIRO: Canberra, ACT)
Stokes C, Howden M (2010) ‘Adapting agriculture to climate change: Preparing Australian agriculture, forestry and fisheries for the future.’ (CSIRO: Canberra, ACT)
Tothill JC, Hargreaves JNG, Jones RM (1978) ‘A comprehensive sampling and computing procedure for estimating pasture yield and composition 1. Field sampling.’ Tropical Agronomy Technical Memorandum No. 8. (CSIRO Division of Tropical Crops and Pastures: Brisbane, Qld)
United Nations (1998) ‘Kyoto Protocol to the United Nations Framework Convention on Climate Change.’ (UNFCCC: Bonn, Germany) Available at: http://unfccc.int/resource/docs/convkp/kpeng.pdf
Wilson BR, Growns I, Lemon J (2008) Land-use effects on soil carbon and other soil properties on the NW slopes of NSW: Implications for soil condition assessment. Australian Journal of Soil Research 46, 359–367.
| Land-use effects on soil carbon and other soil properties on the NW slopes of NSW: Implications for soil condition assessment.Crossref | GoogleScholarGoogle Scholar |
Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecology Letters 12, 452–461.
| Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments.Crossref | GoogleScholarGoogle Scholar |
Young R, Wilson BR, McLeod M, Alston C (2005) Carbon storage in the soils and vegetation of contrasting land uses in northern New South Wales, Australia. Australian Journal of Soil Research 43, 21–31.
| Carbon storage in the soils and vegetation of contrasting land uses in northern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtl2ku7c%3D&md5=3857109cba57604626a31d151276fdbbCAS |
Young RR, Wilson B, Harden S, Bernardi A (2009) Accumulation of soil carbon under zero tillage cropping and perennial vegetation on the Liverpool Plains, eastern Australia. Australian Journal of Soil Research 47, 273–285.
| Accumulation of soil carbon under zero tillage cropping and perennial vegetation on the Liverpool Plains, eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlWrtbY%3D&md5=2eca64de6afabc18fdfc0bee3724bb61CAS |






