Three decades of cotton disease surveys in NSW, Australia
K. A. Kirkby A C , P. A. Lonergan A and S. J. Allen BA NSW Department Primary Industries, 21888 Kamilaroi Highway, Narrabri, NSW 2390, Australia.
B Cotton Seed Distributors Ltd (Visiting Scientist with CSIRO), ‘Shenstone’ Culgoora Road, Wee Waa, NSW 2388, Australia.
C Corresponding author. Email: karen.kirkby@dpi.nsw.gov.au
Crop and Pasture Science 64(8) 774-779 https://doi.org/10.1071/CP13143
Submitted: 26 April 2013 Accepted: 19 August 2013 Published: 29 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Three decades of disease survey data have shown Verticillium wilt was one of the first major diseases of cotton recorded in the 1984–85 season. Survey reports the mean incidence was 4.1% in the 1984–85 season and rose to 16.6% in the 1989–90 season. Prior to 1984 all commercial varieties of cotton available in Australia were susceptible to bacterial blight and the disease was common. The adoption of the resistant varieties contributed to a dramatic decline in the incidence of bacterial blight and the removal of bacterial blight as a significant pathogen to Australian cotton crops by 1992.
Survey results showed the incidence of black root rot increased on farms with a long history of growing cotton during the 1990s. Fusarium wilt of cotton was first reported in New South Wales (NSW) in 1994. The disease is now widespread, being confirmed on 86 NSW farms in six of the eight cotton production areas in NSW. These four significant plant disease ‘problems’ have challenged the cotton industry in NSW.
Data provided by the surveys have indicated the relative importance of each of the diseases present and the impact of cultural practices and the adoption of new varieties on disease distribution, incidence and severity. The results have therefore been used to support and justify requests for research funding and have contributed to the development of Integrated Disease Management strategies.
The NSW Department of Primary Industries continues to monitor the distribution of disease and the incidence and severity present in commercial cotton crops in all production areas of NSW. The aim of this paper is to highlight four significant cotton diseases in Australia and show relationships between cultural practices and declining and increasing incidence of disease.
Additional keywords: bacterial blight, black root rot, Fusarium wilt, Verticillium wilt.
Introduction
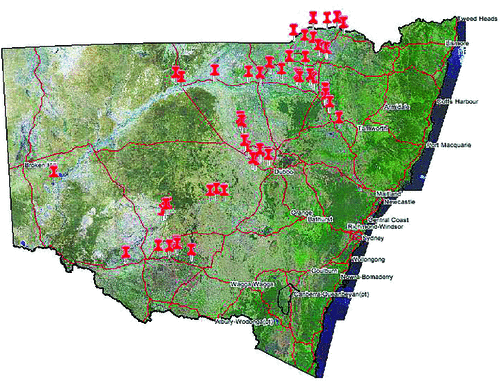
Cotton (Gossypium hirsutum L.) production areas of New South Wales (NSW) are confined to latitudes between 28°S and 35°S. The majority of the crop is furrow irrigated, on alkaline clay soils, located on or near flood plains, associated with river systems draining to the west. The NSW Department of Primary Industries has been making quantitative assessments of disease incidence and severity in cotton for three decades, with the first commencing in 1983–84. As a result of the recent expansion of cotton production into southern NSW, the number of fields surveyed has increased from ~40 to 112, covering a distance of over 20 000 km per year (see Fig. 1).
|
The standard cotton production practices used in Australia favour the dispersal and survival of plant pathogens. Furrow irrigation and tail-water recirculation disperse pathogens efficiently. Reduced or minimum tillage increased pathogen survival season to season. The introduction of permanent bed systems facilitates optimum placement of developing seedlings directly above pathogens in the soil. Pathogen dispersal increased due to frequent movement of machinery, vehicles and people.
Changing farming practices and genetic technology has meant there continues to be a need to monitor diseases on commercial cotton farms. Survey results are maintained in a database, which includes information on field history, crop establishment and cultural practices. Disease data is entered and stored on the Filemaker (Filemaker Pro 11.0 version 3 1984–2011, Filemaker, Inc., Santa Clara, CA, USA) database.
Environmental conditions have had a big impact on cotton production in Australia. Years of drought contributed to a 60% reduction in crop size in the 2003–04 season (Cotton Australia 2013). The 2007–08 cotton crop was the smallest in more than 30 years, a direct result of the drought. Production peaked at 578 500 ha in the 2010–11 season, with NSW growing 66% of the total crop (Cotton Australia 2013).
Method
Cotton disease surveys are conducted early in the season, 3–6 weeks after sowing and before the first irrigation and again after the final irrigation but before defoliation. In order to carry out quantitative assessment of diseases in NSW cotton crops, a systematic method of sampling was initiated following the step-point method described in Nehl et al. (2004b). Information collected from each field included cropping history, ground preparation, variety, seeding rate, sowing date, carryover of crop residues, survey date and crop growth stage as well as the incidence and severity of those diseases present within crops.
After 1996, the disease survey method was standardised (Nehl et al. 2004b). A total of 200 plants from each field were sampled using a step-point method across two transects. The first sampling was taken 50 m into the field at the tail drain end where a GPS coordinate was recorded. The second sampling was done by walking across 10 m and up the row 20 m. This pattern was repeated until 10 sampling sites had been surveyed (Fig. 2).

|
During the early season surveys, at each sampling point, the number of plants present along 1 m of the row was recorded. Seedling mortality was derived from the proportion of surviving plants in 1 m divided by the number of seeds planted/m. Quantitative assessment of black root rot severity was done using the method described by Nehl et al. (2004b). Briefly, at each of the 10 sampling points, 10 plants were carefully removed from soil and tap roots inspected for symptoms of black root rot. Severity was rated on a scale of 0–10 where 0 = total absence of disease symptoms and 10 = tap root completely blackened. Plants were also examined for the presence of Rhizoctonia, Pythium and exotic diseases.
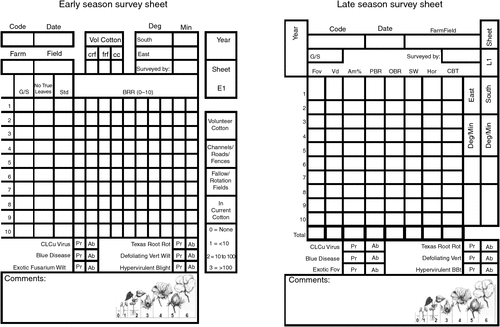
During late season surveys, sampling was similar to that described for early season surveys; however, stems were cut near the base of 10 plants and assessed for symptoms of Verticillium wilt, Fusarium wilt, boll rot, leaf spots, cotton bunchy top and presence/absence of exotic disease. Score cards (Fig. 3a, b) were used to record all relevant information for each field. Disease identification was confirmed where necessary using microscopy and standard laboratory isolation techniques.
|
In 2007–08 the Australian cotton industry adopted a National Biosecurity Plan that included surveillance for the presence/absence of priority pests (exotic strains of Verticillium wilt and Fusarium wilt, hypervirulent strains of bacterial blight, blue disease, cotton leaf curl disease and Texas root rot).
Results and discussion
Throughout the three decades of disease surveys in NSW, Verticillium wilt, bacterial blight, black root rot and Fusarium wilt diseases have been particularly significant.
Verticillium wilt
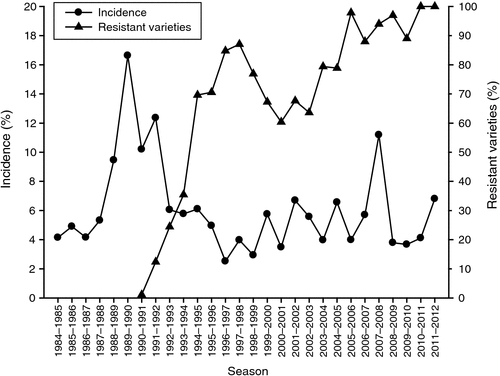
The disease Verticillium wilt is caused by the pathogen Verticillium dahliae Kleb. It was first recorded in Australia in 1959 (Anon. 1959) and later reported in cotton crops in the Namoi Valley by Evans and Paull (1967). Allen and Nehl (1999) reported the standard practice for irrigated cotton was to slash the crop after harvest, disc in crop residues and relist the field into hills for the next cotton crop. During the late 1980s the rapid adoption of permanent bed systems with reduced tillage led to an increase in the carryover of crop residues from season to season and an increase in the average incidence of Verticillium wilt followed. Mean incidence reached a peak of 16.6% of plants affected in NSW in 1989–90 (Fig. 4).
|
Release of resistant varieties in 1990 resulted in an immediate and steady decline in incidence to less than 3%. A subsequent drop in the use of resistant varieties was accompanied by a rise in the average incidence of the disease (Fig. 4). Between 2001–02 and 2007–08 the mean incidence ranged from 5.7 to 11.2%. The 2010–11 and 2011–12 seasons were characterised by wet starts and cool conditions, favouring the disease. Consequently disease severity was higher than previous years. Average incidence for those seasons was 4.1 and 6.8%, respectively.
Bacterial blight
Bacterial blight of cotton is caused by Xanthomonas axonopodis pv. malvacearum (Xam) (Kado 2010). Bacterial blight is a major disease of cotton worldwide causing up to 70% yield losses during severe epidemics (Kirkpatrick and Rothrock 2001).
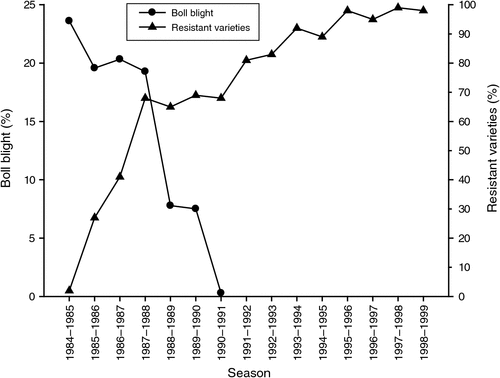
Prior to 1984 all commercial varieties of cotton available in Australia were susceptible to bacterial blight and the disease was widespread. For the susceptible varieties the cotton industry formed the Blight Investigation Group, which developed a seed production strategy to reduce seed infestation to less than 0.03% by 5 years. Australian varieties with resistance to this pathogen were released in 1985–86 and within three seasons these varieties were being grown in 65% of fields. The rapid adoption of the resistant varieties and the reduction in seedborne transmission contributed to a dramatic decline in the incidence of bacterial blight and the removal of bacterial blight as a significant pathogen to Australian cotton crops by 1992 (Fig. 5). Since 1990 bacterial blight on bolls has only been observed on the susceptible Pima cultivar Gossypium barbadense. Trace amounts were observed in a field of Pima S7 in the Bourke region in the 2001–02 season.
|
Black root rot
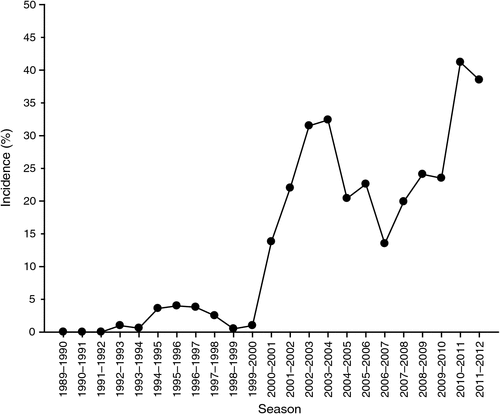
Black root rot of cotton, caused by Thielaviopsis basicola (Berk. and Broome) Ferraris was first observed in Australia in 1989 (Allen 1990). Nehl et al. (2004a) reported the exponential increased incidence of black root rot on farms with a long history of growing cotton during the 1990s. The disease has now been recorded in all cotton-producing areas of Australia and on 95% of the farms visited during annual disease surveys in NSW. The average incidence in commercial crops during 2000–01 to 2003–04 increased rapidly to over 30%. Incidence declined over the next three seasons before rising again to 41.2% in the 2010–11 season. The incidence was 38.5% in the 2011–12 season (Fig. 6).
|
Lower mean incidence recorded in previous seasons can be attributed to drier weather and years of drought before the wet start of 2010–11. Long-term survey data has shown the rapid rate of introduction and dispersal of the pathogen throughout the industry, particularly into the cooler southern regions of NSW.
Fusarium wilt
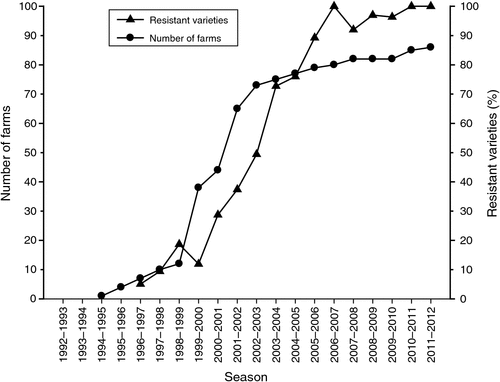
Fusarium wilt of cotton, caused by Fusarium oxysporum Schlect. f.sp. vasinfectum (Atk.) Snyd. and Hans. (Fov), was first reported in NSW in 1994 (Kochman 1995). By the end of 1999, the disease was present in six of the eight cotton production areas in NSW. Fusarium wilt has now been confirmed on 86 farms in NSW (Fig. 7). According to Kochman et al. (1994), the isolates in Australia appear to be distinct from those overseas. DNA fingerprinting techniques and Vegetative Compatibility Group (VCG) analyses identified three strains unique to Australia: VCG 01111 (known as the Darling Downs strain), which is widespread and detected in disease surveys within NSW. VCG 01112 (known as the Boggabilla strain) has only been found on a few farms in northern NSW (Smith et al. 2006). More recently a third strain known as the Mungindi strain has been identified in the Macintyre Valley. These strains have been allocated to Race 6 of the pathogen using their reaction on a set of differential hosts. More recently Races 1, 2 and 6 have been placed together and designated as ‘Group A’.
|
Prior to 2002, the number of farms in NSW where Fusarium wilt was confirmed, appeared to be rapidly increasing and some projections estimated that the disease would be on 90% of farms in NSW by 2010 (Nehl et al. 2004a). However, since 2002, the rate of spread had slowed considerably (Fig. 6). Possible reasons for the reduced spread to new farms include (i) the severe drought conditions, (ii) the use of new varieties with better host plant resistance, (iii) the reticence of some growers to report that the disease had been detected on their farm, and (iv) the impact of increased attention to farm hygiene (Come Clean, Go Clean). Survey results have recorded pathogen distribution, adoption of resistant varieties and the effect of Integrated Disease Management strategies or drought on incidence and severity of the disease.
Conclusions
Results of three decades of data collected from NSW disease surveys has illustrated the different trends of the four major diseases of cotton in Australia. Increased understanding of pathogen survival and transmission, plant genetics and better management strategies have all contributed to reducing the effects of disease. Biosecurity of the Australian cotton industry has also been enhanced with the inclusion of surveillance for the presence/absence of exotic diseases. To date there have been no priority pests reported in NSW.
Survey results were first published in the Annual Plant Disease Survey from 1983–84 to 1987–88 (Fahy and Allen 1985), then in the Cotton Irrigator, then since 1990–91 in the CSD Variety Trial Results (Allen 1991) and also printed in the Cotton Pest Management Guide (Smith et al. 2013). Over time, more field/farm history information has been collected such as hormone damage to crops and more recently the development of an assessment card for volunteer/ratoon cotton on farms.
The development of the Industry Biosecurity Plan for the cotton industry and the industry’s recent expansion into southern NSW has presented new opportunities for further application of the annual disease surveys. The surveys document the spread of diseases established in northern NSW into southern NSW and surveillance for the ‘priority exotic pests’ indentified in the biosecurity plan.
Acknowledgements
The authors wish to acknowledge the contribution to the disease surveys over the years by Gus Shaw, David Nehl, Chris Anderson, Susanna Driessen, Alison Seyb, Karyl-Lee West, Scott Mason, Tony Pattison, Tracey Mor, Bethany Cooper and Sharlene Roser. The authors also acknowledge and thank the cotton industry growers for their participation in the disease surveys.
References
Allen SJ (1990) Thielaviopsis basicola, a new record on cotton in Australia. Australasian Plant Pathology 19, 24–25.| Thielaviopsis basicola, a new record on cotton in Australia.Crossref | GoogleScholarGoogle Scholar |
Allen SJ (1991) ‘Cotton pathology. CSD variety trial results.’ (Cotton Seed Distributors: Wee Waa, NSW)
Allen SJ, Nehl DB (1999) The dilemma between sustainable soil management and controlling soilborne diseases of cotton. In ‘First Australasian Soilborne Disease Symposium’. (Ed. RC Magarey) pp. 9–11. (Watson Ferguson Company Ltd: Brisbane)
Anon. (1959) NSW Department of Agriculture, Annual Report. p.81.
Cotton Australia (2013) Statistics. Available at: www.cottonaustralia.com.au (accessed 1 March 2013).
Evans G, Paull RE (1967) Incidence and distribution of Verticillium wilt in cotton crops of the Namoi Valley. Australian Institute of Agricultural Science and Technology 33, 210–212.
Fahy P, Allen SJ (1985) A survey of bacterial blight incidence in cotton over three seasons. In ‘New South Wales Department of Agriculture, Biology Branch Plant Disease Survey (1983–84)’. (Department of Agriculture New South Wales: Rydalmere)
Kado CI (2010) ‘Plant bacteriology.’ (American Phytopathological Society: St Paul, MN)
Kirkpatrick TL, Rothrock CS (2001) ‘Compendium of cotton diseases.’ (The American Phytopathological Society: St Paul, MN)
Kochman JK (1995) Fusarium wilt in cotton – a new record in Australia. Australasian Plant Pathology 24, 74
| Fusarium wilt in cotton – a new record in Australia.Crossref | GoogleScholarGoogle Scholar |
Kochman JK, Pegg KG, Davis RD, Moore NY, Bentley S (1994) Fusarium wilt in cotton on the Darling Downs in Queensland. In ‘Proceedings of the 7th Australian Cotton Conference’. 10–12 August 1994, Broadbeach, Gold Coast, Qld. pp. 265–269. (Australian Cotton Grower Research Association (ACGRA))
Nehl DB, Allen SJ, Lonergan PA (2004a) Disease surveys in cotton: a finger on the pulse. In ‘Proceedings of the Third Australasian Soilborne Diseases Symposium’. (Eds KM Ophel Keller, BH Hall) pp. 190–191. (South Australian Research and Development Institute: Adelaide)
Nehl DB, Allen SJ, Mondal AH, Lonergan PA (2004b) Black root rot: a pandemic in Australian cotton. Australasian Plant Pathology 33, 87–95.
| Black root rot: a pandemic in Australian cotton.Crossref | GoogleScholarGoogle Scholar |
Smith L, Kochman JK, Swan L, Lehane J, Carrick D, Salmond G, Clapham G (2006) Management of Fusarium wilt of cotton. In ‘13th Australian Cotton Conference’. 8–19 August 2006, Broadbeach, Gold Coast, Qld. (Australian Cotton Growers Research Association (ACGRA): Orange, NSW)
Smith L, Lehane J, Kirkby KA, Lonergan PA, Cooper BR, Allen SJ (2013) Cotton pathology 2011–12. In ‘Cotton Pest Management Guide 2012–2013’. (Ed. S Maas) pp. 128–131. (Cotton Research and Development Corporation: Narrabri, NSW)


