Crop sequences in Western Australia: what are they and are they sustainable? Findings of a four-year survey
Martin Harries A D , Geoffrey C. Anderson B and Daniel Hüberli CA Department of Agriculture and Food, Western Australia, 20 Gregory Street, Geraldton, WA 6530, Australia.
B Department of Agriculture and Food, Western Australia, 75 York Road, Northam, WA 6401, Australia.
C Department of Agriculture and Food, Western Australia, 3 Baron-Hay Court, Perth, WA 6151, Australia.
D Corresponding author. Email: martin.harries@agric.wa.gov.au
Crop and Pasture Science 66(6) 634-647 https://doi.org/10.1071/CP14221
Submitted: 6 August 2014 Accepted: 18 February 2015 Published: 29 May 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
A survey was conducted of commercial broadacre paddocks in the south-west cropping zone of Western Australia from 2010 to 2013. In total, 687 paddock years of data were sampled from 184 paddocks. The land use of each paddock was recorded together with measurements of weed density, the incidence of soilborne pathogen DNA, and soil inorganic nitrogen (nitrate and ammonium). The dynamics of these biophysical variables were related to the crop and pasture sequences employed.
Wheat was the most frequent land use (60% of paddock years), followed by canola and pasture (12% each), and lupins and barley (6% each). Four crop species, wheat, canola, barley and lupins, accounted for 84% of land use. By region, wheat, canola, barley and lupin accounted for 90% of land use in the Northern Agricultural Region (NAR), 83% in the Central Agricultural Region (CAR) and 78% in the Southern Agricultural Region (SAR). Conversely, pasture usage in the SAR was 21%, compared with 12% in the CAR and 7% in the NAR.
Over the surveyed paddocks, weed density, soilborne pathogens and soil N were maintained at levels suitable for wheat production. The inclusion of land uses other than wheat at the frequency reported maintained the condition of these biophysical variables.
Additional keywords: agronomy, brassicas, break crops, canola, crop sequence, cropping systems, fungal root rots, rotation, R. solani.
Introduction
Annual crops and pastures are grown in the south-west cropping zone of Western Australia (WA), which receives annual average rainfall >275 mm. This region has hot, dry summers and cool, wet winters, commonly referred to as a Mediterranean climate. Cropping is the dominant land use in areas receiving annual average rainfall of 275–450 mm and grazing in areas that receive >450 mm. Cropping is therefore dominant on ~14 Mha in the region and grazing on ~12 Mha. Fluctuations in commodity prices can change the proportion of land cropped or grazed; the trend over recent decades is to increased frequency of cropping in areas with annual rainfall of 450–550 mm.
The inclusion of legume break crops and pastures in rotation can improve wheat production. Suppression of wheat pathogens, control of difficult weeds, and the addition of soil nitrogen (N) via rhizobial symbiosis are documented as the main benefits (Angus et al. 2008; Kirkegaard et al. 2008; Peoples et al. 2009). Canola (Brassica napus) also provides benefits to the following wheat crop through disease suppression and weed control, and (although not a legume) may alter soil N dynamics (Angus et al. 2011; Ryan et al. 2006). Within the south-west cropping zone, the yield boost to a subsequent wheat crop from a range of break crop species ranges from 0.60 t ha–1 following lupins (Lupinus angustifolius) to 0.45–0.30 t ha–1 following field peas (Pisum sativum), canola, oats (Avena sativa) and fallow, as estimated by analysing results of 167 crop sequence experiments conducted from 1974 to 2007 (Seymour et al. 2012).
Historically, much research has aimed at increasing the number of legume species available in the south-west for commercial production. In the 1960s and 1970s, narrow-leafed lupins were domesticated (Cowling et al. 1998). A major impetus for the domestication of narrow-leafed lupins was their adaptation to acid soils, which are widespread in the south-west region (Gazey et al. 2013). Hence, tolerance to soil acidity and associated nutrient toxicities have been, and continue to be, important determinants of crop and pasture sequence options across a large proportion of soils in the south-west. The first suitable cultivars were released in the late 1970s, with lupins becoming an important grain legume crop. Many benefits of lupins to the farming systems of WA have since been recognised: high-protein cash crop; high value of residue for grazing; non-host of many wheat pathogens; improved N nutrition for following, non-legume crops; and elevated nutrient recycling rates. Compared with the growing of wheat following wheat, equivalent wheat yields could be obtained following lupins by applying 20–47 kg ha–1 less N in situations where residual N explained all of the benefits of growing lupin in the previous year (Rowland et al. 1988).
In the 1990s, several novel legume species, including Desi and Kabuli chickpeas (Cicer arietinum), lentils (Lens culinaris), narbon beans (Vicia narbonensis) and grass peas (Lathyrus sativus), were introduced to WA. These added to the already utilised species, field peas, faba beans (Vicia faba) and vetch (Vicia spp.). For each of the species mentioned above, comprehensive agronomic production guides were developed (White et al. 2005a, 2005b).
By 1999, 2.1 Mha in WA was sown to the grain legumes: narrow-leafed lupins, chickpeas, field peas, faba beans and lentils. However, the area sown to these species had declined to 0.6 Mha by 2010 (ABS 2011), in part due to difficulty in controlling disease and weed pests such as various ascochyta complexes, anthracnose and weeds resistant to herbicides, specifically annual ryegrass (Lolium rigidum) and wild radish (Raphanus raphanistrum).
Canola production over the same period has increased from 0.39 to 1.1 Mha in 2010 (ABS 2011). Genetic gains, including improved blackleg (Leptosphaeria maculans) resistance, rapid plant genetic development enabling production in lower rainfall zones, the introduction of hybrid plant types, and tolerance to a wider range of herbicides, including glyphosate, have contributed to this increase.
Changes in farming systems in WA have also occurred within animal production. Droughts in 2006 and 2007 instigated destocking, particularly in the Northern Agricultural Region (NAR), with sheep numbers declining from 23 million in the late 1990s to 13.9 million in 2010 (ABS 2011).
Research by Robertson et al. (2010) used a deterministic model, MIDAS, to evaluate inclusion rates of break crops to achieve maximum whole-farm profit. The modelled area of break crops at maximum profit was higher than that found in farm surveys. The authors suggested that this discrepancy might be explained by lower break-crop yields realised by farmers and/or a reduced boost to cereal yields following break crops than assumed in the model. It was concluded that further research was required to better quantify costs and benefits of break crops in WA farming systems.
We use a survey methodology to gain insights into the most common crop and pasture sequences being used, and investigate the changes in biotic stresses, specifically weeds, disease and soil N, observed from break crops currently occurring in farmers’ paddocks. We discuss sustainability of common crop sequences in terms of the agricultural ecosystem.
Materials and methods
Study paddocks
Paddocks were identified through consultation with farm production groups and each landholder. Paddocks were selected to encompass a range of crop and pasture sequences by targeting two or three soil types on each farm common to the area and likely to require the implementation of differing crop and pasture sequences. We selected 184 paddocks across a large geographical area encompassing 14 agroecological zones (Fig. 1). The grainbelt region is commonly divided into Northern, Central and Southern agricultural regions (NAR, CAR and SAR) with associated agroecological zones (Agzones): NAR, Agzones with numbers 1 and 2; CAR, Agzones with numbers 3 and 4; and SAR, Agzones with number 5. Seventy paddocks surveyed from the NAR and 65 from the CAR in 2010. An additional 49 paddocks from the SAR were included in 2011, with 184 surveyed in total to 2013. Wheat was grown in all paddocks in the first year of monitoring, followed by farmer-specified sequences in the following years. Other land use observed included barley (Hordeum vulgare), canola, chickpeas, faba beans, fallow, field peas, lupins, oaten hay, oats, pasture and vetch. Pastures were further divided into regenerating pasture and sown pasture.
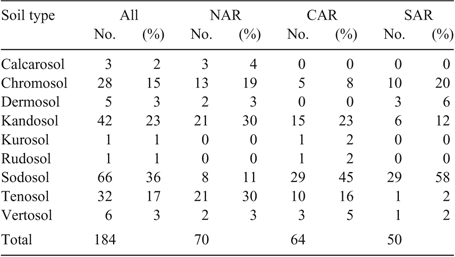
Soil types for the experimental sites were described using the Australian Soil Classification system (Isbell 2002). Sodosol was the most frequent soil type (36% of paddocks), followed by Kandosol (23%), Tenosol (17%) and Chromosol (15%) (Table 1). Soil types represented at low frequency included Vertosol, Dermosol, Calcarosol, Kurosol and Rudosol.
|
Monthly rainfall data observed over the study period were derived from the Australian Bureau of Meteorology weather station closest to each paddock. Examples of rainfall over the study area included the following towns with associated Meteorological Station ID number and AgZone: Yuna 8147 (L1), Buntine 8018 (L2), Hyden 10568 (L4), Mingenew 8088 (M1), Coorow 8037 (M2), Cunderdin 10035 (M3), Wickepin 10654 (M4), Jerramungup 10707 (M5C), Amelup 10502 (M5W), Badgingarra 9037 (H2), Kojonup 10582 (H5W) (Table 2).

|
Sampling methods
Samples of soil, plants and weed seed, as well as measurements of weed populations, were taken from a 1-ha geo-referenced area that was representative of each paddock, as advised by the farmer. The 1-ha area started at least 30 m from the edge of the paddock to avoid edge effects and was divided into four pseudo-replicates, 25 m by 100 m, to ensure consistent sampling. Paddocks were sampled and/or monitored four times during the year: before sowing, 4–6 weeks after emergence, at anthesis, and at crop maturity. Soil samples for chemical analysis were collected before sowing in February and March. The soil layer 0–0.1 m was sampled using a 0.01-m-diameter AccuCore soil probe (Spurr Soil Probes, Adelaide, S. Aust.), with 11 samples collected in a zigzag pattern within each of the four pseudo-replicates; these 44 samples were bulked for analysis in each year. In addition, soil samples were collected from soil layers below 0.1 m derived from four 0.048-m-diameter soil cores, and samples were bulked for analysis. In 2010, the soil depths 0.1–0.5, 0.5–0.7 and 0.7–1.0 m were used. In 2011, soil layers below 0.1 m were separated into soil horizons, enabling classification using the Australian Soil Classification scheme (Isbell 2002). In 2012 and 2013, soil depths of 0.1–0.2, 0.2–0.3, 0.3–0.5 and 0.5–0.9 m were used.
Weed species and density were recorded from five quadrats per pseudo-replicate (20 quadrats). Each quadrat was 0.1 m2 (0.33 m by 0.33 m). Alternatively, if weeds were dense and it was not practical to count using the quadrat method, transects were taken. The numbers of weeds within a transect of 0.5 m by 10 m were counted and ranked according to an exponential scale described by Rew et al. (2000). As with quadrats, this was done 20 times within the sample area. All weed measurements were taken twice a year at 4–6 weeks after emergence and at anthesis. Ryegrass seed samples were collected immediately before harvest from paddocks where sufficient ryegrass plants survived in-season control. Samples were sent to the Herbicide Resistance Testing Service at Charles Sturt University for dose-response testing at three rates of six herbicides: Hoegrass (active ingredient (a.i.) diclofop-methyl 500 g L–1) at 188, 375 and 750 g a.i.; Select (a.i. clethodim 240 g L–1) at 60, 120 and 240 g a.i.; Logran (a.i. triasulfuron 750 g kg–1) at 13, 26 and 53 g a.i.; atrazine (900 g L–1) at 900, 1800 and 3600 g a.i.; trifluralin (480 g L–1) at 408, 816 and 1633 g a.i.; and Roundup (a.i. glyphosate 360 g L–1) at 324, 648 and 1296 g a.i. Results from the maximum label rate treatment are reported as the frequency of plants within the following categories: <10% survivors, no resistance detected; 10–20% survivors, resistance developing; and >20% survivors, resistant. Weed data are presented as percentage change from either the first paddock measurement or the first measurement of each sampling year to the measurement collected at anthesis.
In order to monitor soilborne pathogen levels over the growing season, additional soil samples were taken as described above for soil chemical analysis. These samples were taken from within the previous year’s seeding row at 4–6 weeks after seeding and immediately before harvest. All samples were air-dried and stored away from sunlight, as described in McKay et al. (2008). The concentrations of root pathogen DNA in soil, including Rhizoctonia solani AG-8, Gaeumannomyces graminis var. tritici (take-all) and Pratylenchus neglectus (root-lesion nematode), were measured using a commercial DNA assay testing service (PreDicta B) provided by the South Australian Research and Development Institute at Urrbrae, South Australia. Experimental error associated with this method is discussed in Ophel-Keller et al. (2008), and we report the data in broad risk-categories and as aggregations of all result values. Units provided by this testing service are log(pg DNA g–1 soil + 1) for the fungal pathogens discussed and individuals g–1 soil for nematodes. Soilborne pathogen data are presented as percentage change from either the first paddock measurement or the first measurement of each sampling year to the measurement collected immediately before harvest. The incidence of Sclerotinia sclerotiorum within broad-leafed crops was scored at anthesis from 40 plants in each survey area. Plants were visually assessed for stem-rot symptoms and the presence of sclerotia was determined by a canola pathologist.
Soil nitrate (NO3–) and ammonium (NH4+), soil pH in 0.01 m CaCl2 (pHCa), exchangeable aluminium (Alex, %), electrical conductivity (EC) and soil carbon (C, %) (Rayment and Lyons 2011) were measured using the samples of the soil layer 0–0.9 m collected in autumn. A detection limit of 0.1 cmol Al kg–1 was applied to the data to account for the presence of colloidal material in the soil extractants. The proportion of Al (Alex%) occupied by the effective cation exchange capacity was used to indicate Al concentration of the soil solution. When interpreting these results, the EC of the soil was taken into account to predict the effect of the Al on plant growth. The ranges used to interpret Al toxicity are based on those within Upjohn et al. (2005). When EC was <0.7 dS m–1, the critical Al concentration (level where plant growth is reduced 10%) for highly sensitive plants was considered to be 9–16% Alex, sensitive plants 17–20% Alex, tolerant plants 21–32% Alex and highly tolerant plants 33–43% Alex.
Soil inorganic N data are presented as NO3– and total inorganic N (NO3– plus NH4+) in the soil profile using the units kg N ha–1. These values were calculated using measured NO3– and NH4 concentrations (mg N kg–1), estimated soil bulk density and sampling depth. Estimations of soil bulk density of 1.5 g cm–3 for Tenosols and the coarse-texture layer of Chromosols and Sodosols, and 1.2 g cm–3 for Kandosols and clay layers within Chromosols and Sodosols, were used (McArthur 2004). In some instances, subsoil could not be sampled because of the inability of machinery to penetrate; this occurred at seven or eight sites in 2010, 2012 and 2013. However, the frequency of shallow soil depth of sampling (<0.5 m) was greater in 2011 (33 sites). This was due to division of sampling layers into soil horizons to enable soil classification. The sampling depth of the soil profile referred to here is 0–1.0 m in 2010 and 0–0.9 m in 2011–13. The impact of sampling to a depth to 1.0 m compared with 0.9 m is small because of the low NO3– content at 0.9–1.0 m. Shallow sampled profiles (<0.5 m) were not included in the analyses of soil profile NO3– or total inorganic N.
Statistical analyses
Soil data collected from 0–0.1 m depth were averaged over the sampling years 2010–13. Soil data collected from below 0.1 m depth were averaged over the sampling years 2012 and 2013. Soil profile inorganic N and soil NO3– data are presented for individual years. Statistical analyses were then conducted on the data included, calculating the average, median and frequency distribution using defined target values. Frequency distributions are presented using the averages of samples collected between 2010 and 2013 for the soil layer 0–0.1 m and for samples collected in 2012 and 2013 for deeper sampling soil layers. Frequency distributions of soil profile inorganic N (kg N ha–1) are presented for individual years.
Soil organic C frequencies were calculated using the following values: <1%, 1–2% and >2%. Broad soil-profile inorganic N target ranges of <60, 60–100 and >100 kg N ha–1 were used (Bell et al. 2013). Soil pHCa data frequencies were calculated using the values <4.8, 4.8–5.5 and >5.5, based on levels defined by Gazey et al. (2013). Soil Alex% data frequencies were calculated using the following values: <16%, 16–20% and >20%. EC values were divided into <0.07, 0.07–0.23, 0.23–0.50 and >0.50 dS m–1, with EC >0.5 dS m–1 used as an indicator of subsoil salinity for wheat.
The range and standard error of the mean are presented for grass weeds, R. solani AG8, P. neglectus and soil profile inorganic N content (kg N ha–1).
Results
Rainfall
Rainfall received in each Agzone was highly variable between the monitoring years (Table 2). Long-term average growing-season (1 April–31 October) rainfall (GSR) across all Agzones ranged from 253 to 551 mm. GSR within the survey period was often less than the long-term average. In 2010 and 2012, GSR was less than Agzone long-term average for all Agzones, and in 2013, this occurred in eight of 11 Agzones. By contrast, in 2011, GSR was greater than long-term average for eight Agzones.
Land usage
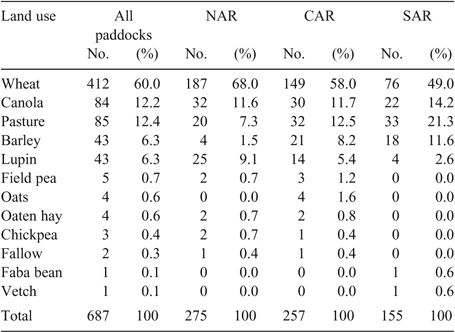
Wheat was the most frequent land use, occupying 60% of paddock years (Table 3). The amount of wheat used was greater in the NAR (68%) than the CAR (58%) and the SAR (49%). There were also regional differences in the use of break crops, with lupins accounting for 9% in the NAR, 5% in the CAR and 3% in the SAR. Barley accounted for 1% in the NAR, 8% in the CAR and 12% in the SAR. Canola was the most commonly used break crop, accounting for 12% in the NAR and CAR and 14% in the SAR. Four species—wheat, canola, barley and lupins—accounted for 84% of land use. By region, wheat, canola, barley and lupins accounted for 90% of land use in the NAR, 83% in the CAR and 78% in the SAR. Pastures made up 12% of overall land use. There were regional differences in pasture usage, with 7% in the NAR, 12% in the CAR and 21% in the SAR (Table 3).
|
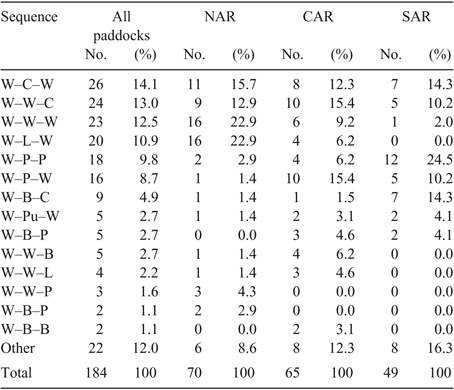
The differences in land use described above resulted in different crop and pasture sequences employed across regions. The wheat–wheat–wheat sequence occurred in 23% of NAR paddocks, 9% of CAR paddocks and 2% of SAR paddocks. The wheat–pasture–pasture sequence occurred in 3% of NAR paddocks, 6% of CAR paddocks, and 25% of SAR paddocks. The top six 3-year sequences (wheat–canola–wheat, wheat–wheat–canola, wheat–wheat–wheat, wheat–lupins–wheat, wheat–pasture–pasture, wheat–pasture–wheat) totalled 69% of sequences across all regions (Table 4).
|
Paddocks in the NAR had less sequence diversity than SAR paddocks. Twice as many NAR paddocks (61%) had wheat included every second year compared with SAR paddocks.
Weeds
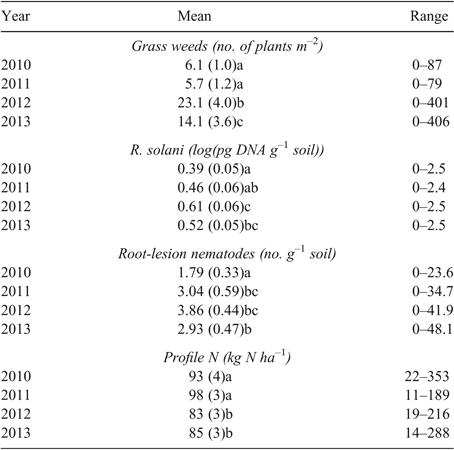
Averaged across all paddocks, there were 13 grass weeds m–2 at 3–4 weeks after crop or pasture establishment, before the application of post-emergent selective herbicides. Seasonal conditions affected weed numbers, with yearly averages ranging from 6 to 23 weeds m–2 at 3–4 weeks after establishment (Table 5). Significant differences occurred in the weed dynamics during the growing season for the major land uses. Canola was the most effective land use at reducing grass weed densities over the growing season. On average, grass weed density declined from 24 plants m−2 at 3–4 weeks after establishment to 3 plants m−2 at anthesis when canola was grown. Grass weed density increased under wheat crops, from an average density of 8.5 plants m−2 at 3–4 weeks after establishment to 13.6 plants m−2 at anthesis (Table 6).
|
Ryegrass populations from 108 paddocks were tested for herbicide resistance. Most paddocks (81%) contained ryegrass populations resistant to Hoegrass (diclofop-methyl). The frequency of resistance found was greatest in the NAR (97%), followed by the CAR (86%) and the SAR (64%). Most paddocks (90%) contained ryegrass populations resistant to Logran (triasulfuron). The frequency of resistance was greatest in the NAR (96%), followed by the CAR (88%) and SAR (87%). Overall, 8% of paddocks contained populations that were either resistant or developing resistance to Select (clethodim). The proportion of resistant populations was 3% in the NAR, 11% in the CAR and 0% in the SAR. One paddock in the CAR was found with ryegrass resistant to trifluralin. No populations were found to be resistant to atrazine or glyphosate.
Disease
Plant pathogen results were grouped into yield loss categories based on log(pg DNA g–1 soil) for fungal root pathogens and P. neglectus g–1 soil (McKay et al. 2008). Of R. solani assays, 75% were either negative or below detection limit (i.e. <1.0 log(pg DNA g–1 soil), with no yield loss predicted; 16% were at 1.0–1.7 log(pg DNA g–1 soil), low risk with predicted yield loss 0–10%; 6% were at 1.7–2.1 log(pg DNA g–1 soil), medium risk with predicted yield loss 5–20%; and 3% were at >2.1 log(pg DNA g–1 soil), high risk with predicted yield loss 10–50%. Averaged across all paddocks, R. solani DNA soil concentration increased over the period of the study by log 0.13 pg g–1 soil, a small but statistically significant amount (Table 5). It should also be noted that R. solani levels >1 log(pg DNA g–1 soil) increased from 73% in 2010 to 83% in 2013, indicating an increase in paddocks within the low-yield-loss risk category. Land use had a significant effect on the amount of R. solani DNA detected. The concentration of R. solani DNA increased under all land uses except canola and faba beans; however, there was only one paddock year of faba beans. Multiplication rates were highest under the cereal crops (oats, wheat and barley) (Table 6). Most (95%) take-all (G. graminis var. tritici) assays were below the PreDicta B assay detection limit at pre-sowing sampling and 92% were below detection at pre-harvest sampling. From the autumn sampling, 3% of assays contained 0.8–1.2 log(pg DNA g–1 soil), that is, low risk with predicted yield loss 10–30% of yield in 10% of paddocks. None were in the medium-risk category and 2% of assays contained >2.0 log(pg DNA g–1 soil), that is, high risk with predicted yield loss 10–30% of yield in >44% of paddocks. Half (50%) of P. neglectus DNA assays were either negative or below detection limit (<0.3 nematodes g–1 soil); 22% were low-risk (>0 0.3–2.0 nematodes g–1 soil), with predicted yield loss <15%; 24% of assays were medium-risk (2.0–15 nematodes g–1 soil), with predicted yield loss 5–30%; 3% of assays were high-risk (>15 nematodes g–1 soil), with predicted yield loss 10–50%. The concentration of P. neglectus DNA increased from 2010 to 2011 and remained at about the same level for the rest of the survey period (Table 5). The range of results widened throughout the years of the survey. Land use affected multiplication rate. Use of grain legume species decreased the concentration of DNA, whereas under canola, cereal crops and pastures, DNA increased (Table 6). In 2011 and 2012, incidences of S. sclerotiorum were similar. Averaged over 2011 and 2012, S. sclerotiorum was observed in 24% of canola paddocks, with an average of 21% of plants affected. In 2013, this increased to 39% of paddocks, with 50% of plants affected. Averaged over 2011 and 2012, S. sclerotiorum was observed in 15% of lupin paddocks, with an average of 14% of plants affected. In 2013, this increased to 29% of paddocks, with 13% of plants affected.
Nitrogen
Soil profile NO3– and total inorganic N were concentrated in the soil layer 0–0.3 m, with 65–71% of the soil profile NO3– and inorganic N contained within this soil layer (Table 7). Soil profile NO3– average values ranged from 46 to 83 kg NO3-N ha–1 for the four sampling years. Soil profile (0–0.9 m) total inorganic (TN) N average values ranged from 70 to 106 kg N ha–1 over the four sampling years. Soil profile NO3– accounted for 49–90% of the soil profile total inorganic N (Table 7). Soils in the C% category of <0.75% consistently had lower levels of soil profile NO3– and total inorganic N compared to soils with greater soil C%. Soil profile N content <60 kg ha–1 was more frequent in 2012 (30%) and 2013 (39%) than in 2010 (20%) and 2011 (19%). Profile N content >100 kg ha–1 was more frequent (30–44%) in other years than in 2010 (7%), due to low amounts of fallow-period rainfall in 2009–10. In the dataset, instances of high soil-profile N due to crop sequences occur (Table 6). For example, soil-profile inorganic N was relatively high at 112 kg N ha–1 following pasture grown in 2011, 140 kg N ha–1 following faba beans grown in 2012, and 131 kg N ha–1 following fallow in 2012. However, there were also instances where inclusion of a legume did not result in elevated N levels in the profile the following autumn. For example, soil profile inorganic N following lupins was 86 kg N ha–1 in 2012 and 77 kg N ha–1 in 2013; following pasture grown in 2012, 72 kg N ha–1; and following chickpeas grown in 2011, 55 kg N ha–1.
Carbon
Soil C measurements were strongly related to rainfall or biomass production. Average soil C at 0–10 cm was lower in the NAR and CAR (0.9–1.0%) than the SAR (2.4%). There was a higher frequency of soils with C levels <1.0% in the NAR (73%) compared with the CAR (26%) and SAR (1%). The frequency of soils with C levels >2.0% was 3% in the NAR, 10% in the CAR and 59% in the SAR (Table 8).
Soil acidity and electrical conductivity
Overall, 75% of paddocks were classified as acidic soil, with pH <5.5 within the soil layer 0–10 cm and/or pH <4.8 below 0.1 m. The frequency distribution of soil pHca indicates that the 0.1–0.3 m soil layer is more frequently acidic than other depths averaged over all paddocks and all years (Table 8). There was a high frequency (63%) of soils with pHca <5.5 in the soil layer 0–0.1 m. Also, 29% of soils registered soil pHca <4.8 in the soil layer 0.1–0.2 m. There was a lower frequency (8–20%) of soils with pHca <4.8 in the soil layers below 0.2 m. Regional differences were observed, with 40% of NAR paddocks acidic in the soil layer 0–0.1 m, compared with the CAR (63%) and SAR (85%). The frequency of acid soil in the soil layer 0.1–0.2 m was higher in the NAR (48%) than the CAR (28%) and SAR (5%) (Table 8).
As soil pHCa declined, soil Alex% increased (Fig. 2) and soil EC decreased (Fig. 3), and soils with pHCa <5.0 had EC values <0.07 dS m–1. The highest correlation occurred between soil pHCa and soil Alex% for the soil layer 0.1–0.2 m (Fig. 2b): y = 0.23 + (545508 × exp(–2.392.64x), r2 = 0.73. Soil Alex% in the soil layer 0–0.1 m was <16% for all paddocks (Table 8). This decreased to 89% of paddocks in the soil layer 0.1–0.2 m, and ranged between 90 and 97% of paddocks in the soil layers below 0.2 m. Soil Alex% at 0.1–0.2 m was >20% for 8% of paddocks. Regional differences in frequency of paddocks with soil Alex% >20% at 0.1–0.2 m were observed: NAR (12%), CAR (11%) and SAR (0%) (Table 8).

|

|
Discussion
The frequency of each crop and pasture species used within the monitored paddocks is consistent with Australian Bureau of Statistics data (ABS 2011), Planfarm Bankwest benchmark data (Kirk 2012) and survey results of Robertson et al. (2010). In the medium-rainfall NAR, Planfarm Bankwest data indicate that wheat, lupins and canola combined occupy 79% of land area, compared with 82% from the paddocks surveyed. In the medium-rainfall SAR, Planfarm Bankwest data indicate that wheat, barley and canola combined occupy 67% of land area, compared with 75% from the paddocks surveyed. Hence, our survey, adds to the evidence in the literature that crop and pasture sequences in the south-west of WA have changed in the past decade and that wheat, canola, barley, lupins and pasture combined comprise well over 90% of land use. Agronomic developments within each species are likely to have contributed to these adjustments in land use. The decline in production of grain-legume crops in WA is documented to have occurred in large part because of an inability to manage weeds and diseases adequately. Ascochyta complexes have been a major impediment to production, particularly within chickpea and field pea crops. Ascochyta rabiei was first detected in 70 chickpea crops in WA during 1999; at this time, all cultivars available were susceptible to this pathogen (Galloway and MacLeod 2003). Siddique and Sykes (1997) concluded that the expansion of field peas would be limited because black spot (Mycosphaerella pinodes, Phoma medicaginis var. pinodella and Ascochyta pisi) ‘cannot be controlled economically with fungicides and at present there are no useful resistant varieties’, a situation that has not changed.
Early in the development of agronomic practices for lupins in WA, it was recognised that options for post-emergent broadleaf herbicides were limited (Gilbey 1984); again, this situation has not changed. Control of grass weeds is also an increasing challenge, with the increasing frequency of ryegrass populations resistant to grass-selective herbicides such as clethodim (Owen et al. 2005).
By contrast, many agronomic improvements have been realised within canola. Potter et al. (2009) compared canola varieties released between 1978 and 2007 and found that, on average, yield increased by 24 kg ha–1 year–1, oil content increased 37–40%, and time to flower decreased by 7–15 days, depending on trial location. This change in phenology to more rapid flowering has enabled production in lower rainfall zones. Blackleg (Leptosphaeria maculans) was a major disease of canola in Australia in the 1980s and 1990s (Salisbury et al. 1995). Successful breeding programs have produced varieties with resistance to this pathogen. When comparing varieties released from 1978 to 2007 within blackleg disease nurseries, Potter et al. (2009) found that all varieties released before 2002 had survival rates <30%, the most recently released varieties had survival rates up to 60%, and yet-to-be-released lines up to 90%. These developments, combined with the introduction of hybrid plant types and cultivars with tolerance to a wider range of herbicides, including glyphosate, may all have contributed to the increased area sown to canola.
Over the past decade, technological developments within wheat agronomy have influenced the ability to manage disease, weeds and N nutrition. Within the area of disease control, the adoption of conservation tillage (Llewellyn et al. 2012) has affected the spectrum of soilborne pathogens. For example, the incidence of take-all and cereal cyst nematode (CCN) has declined in the past 30 years (MacLeod et al. 2008; Khangura et al. 2013), and incidence of these pathogens was very low within monitored paddocks, at 5% for take-all and <1% for CCN (data not presented). Conversely, when a 2005–07 survey was compared with a 1976–82 survey, rhizoctonia root rot, fusarium crown rot and root-lesion nematode had increased and they were found to be the most serious impediments to intensive cereal production (Khangura et al. 2013). This is consistent with the findings of Roget (1995), who reported that a lower tillage farming system increased the incidence of R. solani. In 2013, the new seed-dressing fungicides EverGol Prime (a.i. penflufen) and Vibrance (a.i. sedaxane) were registered for suppression of R. solani, and liquid in-furrow fungicides are expected to be registered for 2015. The seed-dressing and in-furrow fungicides have been shown to improve yields of wheat and barley in trials in WA and South Australia by an average 5% and 10–13%, respectively (Hüberli et al. 2014). Knife-point tillage and GPS guidance has enabled sowing between previous stubble, which has been shown to decrease the severity of crown rot (Verrell et al. 2009). The management of wheat foliar pathogens has also changed, with the price of the most commonly used foliar fungicides decreasing; for example, tebuconazole (430 g L–1) cost AU$143 L–1 in 2010, compared with $10.60 in 2013. With regard to N nutrition, liquid N formulations that were first commercially available in 2001 are now routinely applied. Timing of N inputs can now be closely matched to crop demand or crop yield potential by using sophisticated yield-prediction tools such as Yield Prophet or APSIM and plant tissue testing. In addition, liquid formulations of N can be tank-mixed with fungicides and herbicides, improving timeliness and reducing costs of application. Integrated weed-control methods have also been researched and results heavily promoted. These include use of sequences such as pasture phases with spray-topping (the application of non-selective herbicides in spring to reduce weed-seed production) to decrease weed populations from very high levels, increased seeding rates to increase crop competitiveness, delayed sowing to control the first weed cohorts before sowing the crop, and a range of methods by which to destroy viable weed seed at harvest (Walsh and Powles 2007).
The technologies discussed above are likely performing in part some of the functions for which a break crop has traditionally been used, such as disease suppression, weed control and N nutrition.
Carbon
Soil C measurements are consistent with other reports indicating that soil C content in the south-west is strongly related to rainfall or biomass production (National Land and Water Resources Audit 2001). That is, soil C is lower in Agzones 1, 2 and 3 (0.9–1.0%) than Agzone 5 (2.4%), due to higher temperature, lower rainfall and greater frequency of cropping. Carbon and N cycles are tightly coupled, with the C : N ratio of agricultural soils maintained within a constant narrow range, with an average of 10 : 1 (Carlyle et al. 2010). The adoption of conservation tillage (Llewellyn et al. 2012) has resulted in the accumulation of wheat residues (Anderson 2009), with resulting increases in the more labile soil C fractions (Roper et al. 2010). The increased C input achieved in the past two decades has slowed the rate of decrease in soil C (Wang et al. 2013). If fewer legumes are utilised in the long term, the likelihood of negative N balances is increased, which is a concern because this may cause organic C decline.
Nitrogen
The wide range in soil C, climatic conditions (rainfall and temperatures) and crop sequences used in this study resulted in a wide range of soil profile NO3–, (46–83 kg NO3-N ha–1) and total inorganic N, (71–106 kg N ha–) (Table 7). Inorganic N was concentrated within the 0–0.3 m soil layer; 67–71% of soil profile inorganic N was contained within this soil layer because there was insufficient fallow rainfall to leach mineralised N to deeper soil layers. The dominant form of inorganic N in the soil was NO3–, accounting for 49–90% of the soil profile inorganic N content. This is consistent with observations by Anderson et al. (1998). The measured soil profile NO3– of 67–81 kg NO3-N ha–1 and total inorganic N of 87–105 kg N ha– for soils with soil C content ranging between 1.0% and 1.5% represent a relatively high inorganic N status of the soil in a minimum-tillage cropping system with cereals and canola as frequent land uses (Table 4). For a soil with 1.2% soil C, Anderson et al. (1998) observed soil profile NO3– to range between 31 and 73 kg NO3-N ha–1 following wheat, 68–97 kg NO3-N ha–1 following lupin, and 40–106 kg NO3-N ha–1 following pasture, where the sample depth was 1.5 m. In some instances, the use of a legume species elevated N levels in the following autumn measurement, but in many, it did not. This could occur because of lack of N2 fixation, poor nodulation, poor growth or luxury soil N; however, we did not measure these variables. Another reason this could occur is the low N mineralisation over the fallow, due to low rainfall in this period. The impact of N mineralisation following summer fallow rainfall was studied by Murphy et al. (1998), who observed an additional 2.7–2.9% (24–26 kg N ha–1) of soil organic N mineralised over a 14-day period following application of 45 mm of simulated rainfall in February. In the present study, soil N levels were higher after pasture, lupin and field peas sown in 2011 than in 2012, indicating a fallow-season effect. Legume residues from the previous year also contribute to growing-season mineralisation (Anderson et al. 1998). Hence, it is likely that soil-profile measurements taken in autumn underestimated the N benefits from previously grown legumes.
Soil pH and Al
Soil acidity (low pH and high Al) is an important determinant of soil suitability for many of the break crops used in WA (White et al. 2005a, 2005b). We observed that 75% of paddocks had pHCa <5.5 in the soil layer 0–0.1 m and/or pHCa 4.8 for soil layers <0.1 m (Table 8). There was a greater occurrence of pHCa <4.8 in the soil layer 0.1–0.2 m in the NAR and a greater occurrence of pHCa <5.5 in the soil layer 0–0.1 m in the SAR. These results are similar to those of a 93 000-point survey of soil pHca across the south-west, conducted by Gazey et al. (2013).
As expected, Alex% correlated with pHca, and this measure was used in conjunction with EC to make a further estimate of the number of paddocks where crop selection would be limited by Al toxicity as described by Upjohn et al. (2005). Using this approach, 8% of paddocks were identified as having Alex% >20% in the soil layer 0.1–0.2 m. In general, Al sensitivity (tolerance) classifications of crops are as follows: highly sensitive crops are some barley varieties, faba beans, lentils and chickpeas; sensitive crops are some wheat, barley and canola varieties; tolerant crops are some wheat varieties; and highly tolerant crops are lupins, oats, triticale and cereal rye (Upjohn et al. 2005). These results indicate a risk of acid-sensitive species being inadvertently sown to inappropriate soil conditions if subsoil pHca is not measured, particularly in the NAR.
Weeds
Overall grass-weed numbers were low, indicating that growers were, in most instances, employing effective weed-control strategies. The density of grass weeds at seedling stage changed significantly between years. In 2010, grass density was ~6.0 plants m−2. This was in part due to very dry conditions throughout the 2010 season for most of the survey area (Table 2). The fecundity of weeds was not measured, but weed seedset was likely poor in 2010, which was reflected in the low seedling density of weeds in 2011, again ~6 plants m−2. The 2011 seasonal conditions were much more favourable across the sampling region (Table 2). Again, weed seedset was not measured but the increase in seedling density of grass weeds in 2012 to 23 plants m−2 is likely to be related to plant fecundity, because there is a low level of seed dormancy in annual ryegrass, the most common grass species observed (Ellery et al. 2003; Cheam and Lee 2005). Seasonal conditions in 2013 were exceptional. Early rainfall in April was followed by an extended period without rain, of up to 8 weeks in some paddocks within the NAR. This coincided with application of post-emergent herbicides, and after this period, spring conditions were mild. Weed numbers from seedling to anthesis increased from 14 to 23 plants m−2, indicating that poor weed control was achieved from applied in-crop herbicides. Density of grass weeds at anthesis in other years ranged from 3.5 to 10 plants m−2. Although seasonal conditions had a major influence on weed dynamics, differences in weed control were achieved within different land uses. On average over all years, wheat was sown into paddocks with low weed density. Despite this, weed numbers increased over the growing season (Table 6). Canola was the best crop for weed control. When sown into paddocks with a high weed challenge, weed numbers were reduced to 11% of initial populations (Table 6). Pasture, barley, lupins and oats also decreased weed numbers over the growing season.
Results of testing for herbicide resistance indicated that, for most paddocks surveyed, herbicides other than Hoegrass (diclofop-methyl) and Logran (triasulfuron) would control ryegrass. The frequencies of ryegrass populations resistant to Select (clethodim), trifluralin, atrazine and Roundup (glyphosate) were lower than reported by the Australian Herbicide Resistance Initiative (AHRI) (Owen et al. 2005). It should be noted that the survey and assessment methods differ between these two studies. AHRI surveys sample from roadside paddocks, whereas most of our survey points are well within farm boundaries. Reporting standards also differ. AHRI surveys categorise 1–19% of plants surviving the label rate of herbicide as developing resistance; the testing service we used categorise 10–20% of plants surviving the label rate of herbicide as developing resistance. The number of samples and geography also differ. The low frequency of paddocks with resistance to Select (clethodim) may explain the high level of ryegrass control within lupin crops.
Diseases
Rhizoctonia solani AG8 has a wide host range, and rotation of crop and pastures has not been found to be effective in reducing inoculum (Kataria and Verma 1992). Traditionally, the only management strategy recommended has been deep tillage, below 10 cm (Jarvis and Brennan 1986), which, as previously mentioned, has become less popular. Fungicides registered in 2013 provide a new means of control (Hüberli et al. 2014). In addition, DNA levels of this pathogen are shown to be reduced under canola, and root disease in the subsequent cereal crop is also reduced (Gupta et al. 2012; Hüberli et al. 2013). In this study, we have confirmed these results, recording reductions in R. solani DNA over the growing season within canola crops, indicating that canola is a poor host. Importantly, the excellent weed control in canola crops reduced the risk of R. solani being hosted by alternative species. The increase of canola to ~10% of land use across the survey area may provide a new management tool for R. solani. In particular, there is an opportunity for targeted inclusion of canola to the small proportion of paddocks with medium (6%) or high (3%) risk of yield loss to R. solani. Further studies are required with current canola cultivars to confirm the R. solani response detected in this survey and in the trial work by others (Gupta et al. 2012; Hüberli et al. 2013).
The frequency and concentration of take-all DNA (G. graminis var. tritici) was low. This is consistent with observations over the past few decades and has been attributed to effective grass-weed control within crops (MacLeod et al. 2008).
The incidence of detection of P. neglectus DNA was high at 50%, which is the same as reported by MacLeod et al. (2008). However, the concentration of DNA was low, with only 3% of paddocks predicted to have a high yield loss of 10–50%. DNA multiplication rates over single growing seasons were affected by the species sown. The results followed the expected trends based on the host range of P. neglectus. The grain legume species (lupins, field peas, faba beans and vetch) decreased DNA concentration over the growing season. Notably, DNA concentration increased to 1.6 times the pre-sowing levels when canola was sampled pre-harvest. Hence, the risk of P. neglectus levels increasing is higher with canola replacing the pulse crops. Geographic distribution was uniform across all regions (data not presented); however, the risk of increased P. neglectus levels may be higher in the SAR, where barley rather than lupin is the second most frequently used break crop.
Sclerotinia sclerotiorum is a major pathogen of canola worldwide, and as such, the increase in its incidence is a concern for the sustainability of canola production in WA. The expression of S. sclerotiorum is dependent on environmental conditions, and in 2013, seasonal conditions were conducive. As outlined by Khangura et al. (2014), many canola crops were sown early in the sowing window (in April), as a consequence of rain at that time. Spring conditions were wetter than average in many districts; hence, many canola crops were bulky with humid canopy conditions. The results of this survey suggest that the conclusions of Kirkegaard et al. (2006), who suggested that cost-effective control of S. sclerotiorum would be required for canola to persist as a reliable break crop in New South Wales, also apply to WA. Fungicide control products were registered in 2013 (210 g L–1 of prothioconazole, 210 g L–1 of tebuconazole), and these provide an economically viable management option (Khangura et al. 2014). However, it has been suggested that a range of integrated management options, including extending the period between canola crops to more than 1 year, may be required (Khangura et al. 2014).
Conclusion
This survey indicates that current WA farming systems continue to be dominated by wheat and that the break crop of choice has changed from legume to canola. This change in break-crop preference will likely alter paddock biology; for example, with a greater area of canola, R. solani is likely to decrease but P. neglectus increase. Whichever crop and pasture sequences are used, the management of biophysical variables in this farming system is an ongoing challenge, and undoubtedly, instances will occur where weeds, disease and N nutrition limit yield potential. The data presented show that over the survey period, break crops were used effectively to manage common weeds and soil pathogens and there are no biological indicators detected within these paddocks that would suggest this to be a fundamentally unsustainable farming system.
Acknowledgements
This study was financially supported by the Grain Research and Development Corporation (GRDC) through project DAW00213. We acknowledge the support of many grain growers who hosted experiments on their properties, and staff from the Mingenew–Irwin Group, Liebe Group, Western Australian No-tillage Farmers Association, Facey Group and DAFWA for field monitoring. We also thank Ravjit Khangura for sclerotinia ratings, Angela Stuart-Street and Dennis van Gool for soil characterisation and Jo Walker for coordinating field monitoring.
References
ABS (2011) Agricultural commodities. Australian Bureau of Statistics. Available at: www.abs.gov.au/ausstatsAnderson GC (2009) The impact of tillage practices and crop residue (stubble) retention in the cropping system of Western Australia. Bulletin No. 4786. Department of Agriculture and Food, Western Australia, South Perth, W. Aust.
Anderson GC, Fillery IRP, Dunin FX, Dolling PJ, Asseng S (1998) Nitrogen and water flows under pasture–wheat and lupin–wheat rotations in deep sands in Western Australia. 2. Drainage and nitrate leaching. Australian Journal of Agricultural Research 49, 345–361.
| Nitrogen and water flows under pasture–wheat and lupin–wheat rotations in deep sands in Western Australia. 2. Drainage and nitrate leaching.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFGqsbk%3D&md5=6411fc797fa11010271379c9b918eb98CAS |
Angus J, Kirkegaard J, Peoples M, Ryan M, Ohlander (2008) The value of break crops for wheat. In ‘Proceedings 14th Australian Agronomy Conference’. 21–25 September 2008. Adelaide, S. Aust. (Australian Society of Agronomy/The Regional Institute: Gosford, NSW) Available at: www.regional.org.au/au/asa/2008/concurrent/rotations/5786_angusjf.htm
Angus J, Kirkegaard J, Peoples M, Ryan M, Ohlander L, Hufton L (2011) A review of break crop benefits of brassicas. In ‘17th Australian Research Assembly on Brassicas’. 15–17 August 2011, Wagga Wagga, NSW. pp. 123–127. (Department of Primary Industries: Orange, NSW)
Bell M, Strong W, Elliott D, Walker C (2013) Soil nitrogen–crop response calibration relationships and criteria for winter cereal crops grown in Australia. Crop & Pasture Science 64, 442–460.
| Soil nitrogen–crop response calibration relationships and criteria for winter cereal crops grown in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlakt73I&md5=59c248f4fb65347cbd3e5727227d68feCAS |
Carlyle JC, Charmley E, Baldock JA, Polglase PJ, Keating B (2010) Agricultural greenhouse gases and mitigation options. In ‘Adapting agriculture to climate change: preparing Australian agriculture, forestry and fisheries for the future’. (Eds C Stokes, M Howden) pp. 229–244. (CSIRO Publishing: Melbourne)
Cheam A, Lee S (2005). Survival of annual ryegrass seed at various soil depths. In ‘Crop Update Weeds 2005’. pp. 51–52. (Department of Agriculture Western Australia/GRDC)
Cowling WA, Huyghe C, Swiecicki W (1998) Lupin breeding. In ‘Lupins as crop plants: biology, production and utilisation’. (Eds JS Gladstones, C Atkins, J Hamblin) pp. 93–120. (CAB International: Wallingford, UK)
Ellery A, Moore A, Nedelkos S, Chapman R (2003) Predicting annual ryegrass dormancy from climatic variables. In ‘Crop Update Weeds 2003’. pp. 12–13. (Department of Agriculture Western Australia/GRDC)
Galloway J, MacLeod WJ (2003) Didymella rabiei, the teleomorph of Ascochyta rabiei, found on chickpea stubble in Western Australia. Australasian Plant Pathology 32, 127–128.
| Didymella rabiei, the teleomorph of Ascochyta rabiei, found on chickpea stubble in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gazey C, Andrew J, Griffin T (2013) Soil acidity. In ‘Report card on sustainable natural resource use in agriculture—status and trend in the agricultural areas of the south-west of Western Australia’. (Department of Agriculture and Food, Western Australia: South Perth, W. Aust.)
Gilbey DJ (1984) Weed control in grain legumes in Western Australia. In ‘Proceedings 7th Australian Weed Conference’. 17–21 Sept 1984, Perth, W. Aust. (Ed. RW Madin) pp. 319–327.
Gupta V, Roget D, McKay A, Ophel-Keller K, Wilhelm N, MacLeod B, Kirkegaard J (2012) Rhizoctonia fact sheet. Grains Research and Development Corporation, Canberra, ACT.
Hüberli D, Connor M, Miyan S, MacLeod W, Desbiolles J, Bogacki P, McKay A (2013) Integrated disease management options to control rhizoctonia bare-patch in cereals. In ‘Crop Updates 2013’. 25–26 February, Perth, W. Aust. (Department of Agriculture and Food Western Australia/GRDC) Available at: http://researchrepository.murdoch.edu.au/13686/
Hüberli D, Connor M, Miyan S, MacLeod W, Bogacki P, Desbiolles J, Correll R, Gupta V, Battaglia R, McKee K, May L, Forsyth L, Roget D, McKay A (2014) Liquid banding of fungicide increases yields of cereals in paddocks with rhizoctonia bare-patch. In ‘North Mallee Farm Improvement Group Crop Updates 2014’. 20 March. Salmon Gums, W. Aust. pp. 24–27. (Department of Agriculture and Food Western Australia/GRDC) Available at: http://researchrepository.murdoch.edu.au/21390/
Isbell RF (2002) ‘The Australian Soil Classification.’ Revised edn (CSIRO Publishing: Melbourne)
Jarvis RJ, Brennan RF (1986) Timing and intensity of surface cultivation and depth of cultivation affect rhizoctonia patch and wheat yield. Australian Journal of Experimental Agriculture 26, 703–708.
| Timing and intensity of surface cultivation and depth of cultivation affect rhizoctonia patch and wheat yield.Crossref | GoogleScholarGoogle Scholar |
Kataria HR, Verma PR (1992) Rhizoctonia solani damping-off and root rot in oilseed rape and canola. Crop Protection 11, 8–13.
| Rhizoctonia solani damping-off and root rot in oilseed rape and canola.Crossref | GoogleScholarGoogle Scholar |
Khangura RK, MacNish GC, MacLeod WJ, Vanstone VA, Hansbury CD, Loughman R, Speijers JE (2013) Current status of cereal root diseases in Western Australia under intensive cereal production and their comparison with the historical survey conducted during 1976–1982. Journal of Phytopathology 161, 828–840.
| Current status of cereal root diseases in Western Australia under intensive cereal production and their comparison with the historical survey conducted during 1976–1982.Crossref | GoogleScholarGoogle Scholar |
Khangura RK, Van Burgel A, Salam M, Aberra M, MacLeod WJ (2014) Why sclerotinia was so bad in 2013? Understanding the disease and management options. In ‘Crop Updates 2014’. 24–25 February, Perth, W. Aust. (Department of Agriculture and Food Western Australia/GRDC) Available at: www.giwa.org.au/pdfs/2014/Presented_Papers/Khangura%20et%20al%20presentation%20paper%20CU2014%20-DR.pdf
Kirk G (2012) Planfarm Bankwest benchmarks 2012–2013. Planfarm Pty Ltd, Geraldton, W. Aust. Available on request at: http://planfarm.com.au/contact.aspx
Kirkegaard JA, Robertson MJ, Hamblin P, Sprague SJ (2006) Effect of blackleg and sclerotinia stem rot on canola yield in the high rainfall zone of southern New South Wales, Australia. Australian Journal of Agricultural Research 57, 201–212.
| Effect of blackleg and sclerotinia stem rot on canola yield in the high rainfall zone of southern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Christen O, Krupinsky J, Layzell D (2008) Break crop benefits in temperate wheat production. Field Crops Research 107, 185–195.
| Break crop benefits in temperate wheat production.Crossref | GoogleScholarGoogle Scholar |
Llewellyn RS, D’Emden FH, Kuehne G (2012) Extensive use of no-tillage in grain growing regions of Australia. Field Crops Research 132, 204–212.
| Extensive use of no-tillage in grain growing regions of Australia.Crossref | GoogleScholarGoogle Scholar |
MacLeod W, Vanstone V, Khangura R, Beard C (2008) Root disease under intensive cereal production systems. Bulletin No. 4732. Department of Agriculture and Food, Western Australia, South Perth, W. Aust.
McArthur WM (2004) Reference soils of south-western Australia. Department of Agriculture, Western Australia, for the Australian Soil Science Society, WA Branch, South Perth, W. Aust.
McKay A, Roget D, Hannam R, Ophel-Keller K (2008) ‘Root disease risk management resource manual.’ (PIRSA Publishing: Adelaide, S. Aust.)
Murphy DV, Sparling GP, Fillery IRP, McNeill AM, Braunberger P (1998) Mineralisation of soil organic nitrogen and microbial respiration after simulated summer rainfall events in an agricultural soil. Australian Journal of Soil Research 36, 231–246.
| Mineralisation of soil organic nitrogen and microbial respiration after simulated summer rainfall events in an agricultural soil.Crossref | GoogleScholarGoogle Scholar |
National Land and Water Resources Audit (2001) Australian agriculture assessment nutrient management in Australian agriculture. Commonwealth of Australia, Canberra, ACT. Available at: http://nrmonline.nrm.gov.au/downloads/mql:892/content
Ophel-Keller K, McKay A, Hartley D, Herdina , Curran J (2008) Development of a routine DNA-based testing service for soil borne diseases in Australia. Australasian Plant Pathology 37, 243–253.
| Development of a routine DNA-based testing service for soil borne diseases in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktVChtro%3D&md5=8407230895afd239b93c023ab4da8e79CAS |
Owen M, Walsh MJ, Powles SB (2005) Frequency of herbicide resistance in annual ryegrass across the WA wheatbelt. Australian Herbicide Initiative. Available at: http://ahri.uwa.edu.au/page/Research/Surveys/ryegrass#RGbacktotop (accessed 12 December 2013).
Peoples MB, Brockwell J, Herridge DF, Rochester IJ, Alves BJR, Urquiaga S, Boddey RM, Dakora FD, Bhattaria S, Maskey SL, Sampet ES (2009) The contribution of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48, 1–17.
| The contribution of nitrogen-fixing crop legumes to the productivity of agricultural systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFenur4%3D&md5=2304260acfc7b9a875c100c876d8a978CAS |
Potter T, Burton W, Edwards J, Wratten N, Mailer R, Seberry D (2009) Comparison of historical canola varieties in Australia. In ‘Proceedings 16th Australian Research Assembly on Brassicas’. Ballarat, Vic. (Eds W Burton, R Norton, A Worthy) pp. 110–115. (Australian Oilseeds Federation: Sydney)
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods—Australasia.’ Australian Soil and Land Survey Handbook Series. (CSIRO Publishing: Melbourne)
Rew LJ, Alston CL, Harden S, Felton WL (2000) Counts versus categories: choosing the more appropriate weed scoring method. Australian Journal of Experimental Agriculture 40, 1121–1129.
| Counts versus categories: choosing the more appropriate weed scoring method.Crossref | GoogleScholarGoogle Scholar |
Robertson MJ, Lawes RA, Bathgate A, Byrne F, White P, Sands R (2010) Determinants of the proportion of break crops on Western Australian broadacre farms. Crop & Pasture Science 61, 203–213.
| Determinants of the proportion of break crops on Western Australian broadacre farms.Crossref | GoogleScholarGoogle Scholar |
Roget DK (1995) Decline in root rot (Rhizoctonia solani AG-8) in wheat in a tillage and rotation experiment at Avon, South Australia. Australian Journal of Experimental Agriculture 35, 1009–1013.
| Decline in root rot (Rhizoctonia solani AG-8) in wheat in a tillage and rotation experiment at Avon, South Australia.Crossref | GoogleScholarGoogle Scholar |
Roper MM, Gupta VVSR, Daniel V, Murphy DV (2010) Tillage practices altered labile soil organic carbon and microbial function without affecting crop yields. Australian Journal of Soil Research 48, 274–285.
| Tillage practices altered labile soil organic carbon and microbial function without affecting crop yields.Crossref | GoogleScholarGoogle Scholar |
Rowland IC, Mason MG, Hamblin J (1988) Effects of lupins and wheat on the yield of subsequent wheat crops grown at several rates of applied nitrogen. Australian Journal of Experimental Agriculture 28, 91–97.
| Effects of lupins and wheat on the yield of subsequent wheat crops grown at several rates of applied nitrogen.Crossref | GoogleScholarGoogle Scholar |
Ryan MH, Kirkegaard JA, Angus JF (2006) Brassica crops stimulate soil mineral N accumulation. Australian Journal of Soil Research 44, 367–377.
| Brassica crops stimulate soil mineral N accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFKgsLw%3D&md5=d85334e6ba8ed470cc3a0f64865523daCAS |
Salisbury PA, Ballinger DJ, Wratten N, Plummer KM, Howlett BJ (1995) Blackleg disease on oilseed brassica in Australia: a review. Australian Journal of Experimental Agriculture 35, 665–672.
| Blackleg disease on oilseed brassica in Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Seymour M, Kirkegaard JA, Peoples MB, White PF, French RJ (2012) Break-crop benefits to wheat in Western Australia: insights from over three decades of research. Crop & Pasture Science 63, 1–16.
| Break-crop benefits to wheat in Western Australia: insights from over three decades of research.Crossref | GoogleScholarGoogle Scholar |
Siddique KHM, Sykes J (1997) Pulse production in Australia past, present and future. Australian Journal of Experimental Agriculture 37, 103–111.
| Pulse production in Australia past, present and future.Crossref | GoogleScholarGoogle Scholar |
Upjohn B, Fenton G, Conyers M (2005) Soil acidity and liming. Agfact AC. 19, 3rd edn. Department of Primary Industries, Orange, NSW.
Verrell A, Simpfendorfer S, Nash P, Moore K (2009) Can inter-row sowing be used in continuous wheat systems to control crown rot and increase yield? In ‘Proceedings 13th Annual Symposium on Precision Agriculture in Australasia’. (Eds MG Trotter, EB Garraway, DW Lamb) p. 101. (University of New England: Armidale, NSW)
Walsh MJ, Powles SB (2007) Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems. Weed Technology 21, 332–338.
| Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1WhtbY%3D&md5=4fa197f43d75ec303f15680b2fe50407CAS |
Wang G, Huang Y, Wang E, Yu Y, Zhang W (2013) Modeling soil organic carbon change across Australian wheat growing areas, 1960–2010. PLoS One 8, e63324
| Modeling soil organic carbon change across Australian wheat growing areas, 1960–2010.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosFCqtbk%3D&md5=1e2857a5095d3e39e5654f04f96ab716CAS | 23696813PubMed |
White P, Harries M, Seymour M, Burgess P (2005a) Producing pulses in the Northern Agricultural Region. Bulletin No. 4656. Department of Agriculture and Food, Western Australia, South Perth, W. Aust.
White P, Seymour M, Burgess P, Harries M (2005b) Producing pulses in the Southern Agricultural Region. Bulletin No. 4645. Department of Agriculture and Food, Western Australia, South Perth, W. Aust.






