Role of magnesium fertilisers in agriculture: plant–soil continuum
Mehmet Senbayram A C , Andreas Gransee B , Verena Wahle C and Heike Thiel B DA Department of Plant Nutrition and Soil Science, University of Harran, SanliUrfa, TR-63000, Turkey.
B K+S KALI GmbH, Bertha-von-Suttner-Straße 7, 34131 Kassel, Germany.
C Institute of Applied Plant Nutrition, University of Goettingen, Carl-Sprengel-Weg 1, 37075 Göttingen, Germany.
D Corresponding author. Email: heike.thiel@k-plus-s.com
Crop and Pasture Science 66(12) 1219-1229 https://doi.org/10.1071/CP15104
Submitted: 26 March 2015 Accepted: 16 September 2015 Published: 21 December 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
In this review, we summarise factors contributing to plant availability of magnesium (Mg) in soils, the role of Mg in plant physiological processes related to yield formation and abiotic stress tolerance, and soil and fertiliser parameters related to Mg leaching in fertilised soils. Mg is a common constituent in many minerals, comprising 2% of Earth’s crust; however, most soil Mg (90–98%) is incorporated in the crystal lattice structure of minerals and thus not directly available for plant uptake. Plants absorb Mg from the soil solution, which is slowly replenished by soil reserves. Duration and intensity of weathering, soil moisture, soil pH, and root–microbial activity in soil are key factors that determine plant-available Mg release from soils. On the other hand, the amount of Mg released from soil minerals is generally small compared with the amounts needed to sustain high crop yield and quality. Thus, in many agro-ecosystems, application of Mg fertilisers is crucial. Magnesium is involved in many physiological and biochemical processes; it is an essential element for plant growth and development and plays a key role in plant defence mechanisms in abiotic stress situations. An early effect of Mg deficiency in plants is the disturbed partitioning of assimilates between roots and shoots because the supply of sink organs with photosynthetic products is impaired, and sugars accumulate in source leaves. Thus, optimal supply of Mg is required to improve crop tolerance to various stresses and to increase yield and quality parameters of harvested products. Unlike other cations, Mg is very mobile in soils because it is less bound to the soil charges. Therefore, Mg losses by leaching might occur in sandy soils with high water conductivity. Leaching of Mg in soils when applied with various water-soluble fertilisers may also vary depending on the fertiliser’s chemical composition, granule size, and effect on soil pH and cation balance, as we discuss in detail.
Additional keywords: antagonism, mineral fertilisers, soil texture, solubility.
Introduction
Global human population has doubled during the last 45 years and this trend will continue in the coming decades. The availability of adequate potable water and water reserves for agricultural purposes is already critical in many regions, regardless of change in global climate. In the very near future, a strong increase in crop production will be needed to meet food and energy demands alongside preservation of the ecological and energy-related resources of our planet. One of the major challenges for agriculture will be to maintain the crop yields in harsher environments (e.g. seasonal drought, heat and excess light energy) and/or enhance crop yields in more resource-efficient systems (Reynolds et al. 2012). Minimising the ‘yield gap’ and increasing yield stability under different stress conditions are of strategic importance to guarantee food for the future (Cattivelli et al. 2008). Recent research advances in crop physiology and genomics have led to new insights into stress tolerance and provided breeders with new knowledge and tools for plant improvement (Tuberosa and Salvi 2006). In parallel, innovative site-specific agricultural management techniques and precise crop-nutrient management also need further attention so that crop plants with higher yield potential can be grown under unfavourable conditions and can achieve their potential yield under given environmental conditions. Better understanding of crop strategies to deal with stress situations and of factors that control response mechanisms is key to developing innovative techniques.
For successful farming practice and optimal crop-nutrient management, fundamental principles of mineral plant nutrition must be considered, such as physical, chemical and biological processes in plants and soils. The International Plant Nutrition Institute (IPNI), which performs agricultural projects in collaboration with scientists from various countries, aims to develop a simple management concept (BMP, best management practise) to enhance specific crop performance under certain environmental conditions. The 4R Nutrient Stewardship technology initiated by IPNI is one of the most advanced tools for decision making with site- and crop-specific application. There is direct interaction between applying the right nutrient source, at the right rate, right time and right place (4R), and profitable impacts for good crop growing, soil health and decreased pollution of the environment via enhanced nutrient-use efficiency.
Magnesium (Mg) is involved in many physiological and biochemical processes; it is an essential element for plant growth and development and plays a key role in plant defence mechanisms in abiotic stress situations (Cakmak and Kirkby 2008; Cakmak and Yazici 2010; Cakmak 2013; Gransee and Führs 2013; Huber and Jones 2013; Mengutay et al. 2013). The most commonly known function of Mg in plants is probably its role as the central atom of the chlorophyll molecule in the light-absorbing complex of chloroplasts and its contribution to photosynthetic fixation of carbon dioxide (Cakmak and Kirkby 2008; Cakmak and Yazici 2010; Gerendás and Führs 2013). However, the Mg bond to chlorophyll makes up only a small part of the total Mg fraction. Depending on the Mg status of the plant, ~20% (Marschner 2012; Gransee and Führs 2013) and up to 35% (Cakmak and Kirkby 2008; Cakmak and Yazici 2010) of the element is localised in the chloroplast, and the remaining Mg is present in more mobile forms (Marschner 2012). Because of its high phloem mobility, Mg can easily be translocated to active growing parts of the plant where it is needed for chlorophyll formation, enzyme activation for protein biosynthesis, and phloem export of photosynthates to ensure vegetative and generative growth. Therefore, first visual deficiency symptoms generally occur on older leaves (Cakmak and Kirkby 2008; White and Broadley 2009; Gransee and Führs 2013). Thus, even slight Mg deficiency may affect biomass formation and plant susceptibility to environmental stresses by diminishing several biochemical and physiological processes.
Soil magnesium
Magnesium is a common constituent in many minerals, comprising 2% of Earth’s crust. However, most soil Mg (90–98%) is incorporated in the crystal lattice structure of minerals and, thus, not directly available for plant uptake. Owing to high variation in Mg content of source material and the degree of weathering, the total content of Mg in soils varies considerably, between 0.05% and 0.5% (Grimme 1991; Maguire and Cowan 2002; Gransee and Führs 2013). Many common soil minerals contain Mg, including amphibole, biotite, chlorite, dolomite, montmorillonite, olivine, pyroxene, serpentine and vermiculite. Stores of bioavailable Mg originate from inputs by mineral weathering. Therefore, soils that have developed from coarse-grained rocks low in these minerals tend to be low in Mg.
Soil Mg is often subdivided into four fractions: rapidly exchangeable, slowly exchangeable (acid-soluble), organic complexed, and structural forms (Mayland and Wilkinson 1989) (Fig. 1). The last of these accounts for differences in bioavailability; plants absorb Mg from the soil solution, which is buffered by the readily exchangeable form, which, in turn, is slowly replenished by the soil reserves. Soil texture is a key variable that affects plant-available Mg. Because Mg is located in clay minerals and associated with cation exchange sites on clay surfaces, clayey soils generally contain adequate Mg for plant requirements, whereas sandy soils are frequently deficient in Mg (Mayland and Wilkinson 1989). Several ferromagnesian minerals (e.g. olivine, pyroxene, amphibole, and mica) are major Mg sources in basic igneous rocks (Chu and Johnson 1985). Secondary minerals such as magnesite, talc, and the serpentine group are the weathering products of these primary minerals. Salmon (1963) suggested that the main sources of Mg that can be made available in soils are secondary minerals, particularly clay minerals, mica, and chlorite. To become soluble, Mg adsorbed on a clay particle needs to be replaced by other cations, such as potassium (K+) and hydrogen (H+), from the soil solution. Stahlberg (1960) determined the amount of slowly exchangeable Mg released from several Swedish topsoils (boiling them in 1 n HCl) and summarised that vermiculite and chlorite were the main sources of this acid-soluble Mg. However, there was a poor correlation between exchangeable Mg and total or acid-soluble Mg in soils (Prince et al. 1947; Stahlberg 1960; Baker 1971). Similarly, Hailes et al. (1997a) observed that exchangeable Mg was not significantly (P > 0.05) correlated with organic carbon, and only 45% of the variation in exchangeable Mg could be explained by a combination of pH and clay content. Therefore, plant-available Mg concentrations cannot be accurately predicted solely on the basis of the parent material composition, because of differences in mineral weathering rates depending on various environmental conditions. Duration and intensity of weathering, soil moisture, soil pH, and root and microbial activity in soil are other key factors that determine the plant-available Mg release from soils (Mayland and Wilkinson 1989).

|
Soil pH and magnesium availability
Soil pH has a direct effect on release of Mg from clay minerals, as well as on plant Mg uptake. Chan et al. (1979) and Hailes et al. (1997b) showed that Mg that is still exchangeable at a soil pH <6.0 becomes non-exchangeable when soil pH is increased to >6.5. Similarly, Sumner et al. (1978) reported that when the pH of Ultisols increased from 5.5 to 7.5, soil exchangeable Mg dropped >50%. Because plant uptake of Mg can be hampered by an excess concentration of other cations (e.g. H+), higher soluble Mg concentrations in soil solution do not necessarily mean that this Mg is plant-available (Metson 1974). In acid soils (pH <5), high levels of exchangeable aluminium, which is harmful for plants, are also released. Additionally, the soil solution is saturated with H+ ions instead of base cations at the site of rhizosphere (Metson 1974). In this context, the decrease in plant availability of Mg at low soil pH is a consequence of the increasing inability to build up and maintain a sufficient pH and hence electrochemical gradient across the plasma membrane of root cells (Schubert et al. 1990; Gransee and Führs 2013). In conclusion, at low pH, soil exchangeable Mg concentration may increase; however, the dominance of H+ at the site of rhizosphere may interfere with the uptake of Mg, causing Mg deficiency and hampering yield and quality of agricultural products (Mayland and Wilkinson 1989).
Magnesium supply, crop yield formation and crop quality in a changing environment
Effects of magnesium on photosynthesis and transport of photosynthates
Magnesium is well described for its important role in chlorophyll synthesis. In addition, Mg plays a key role in several plant physiological processes through its key function in phloem loading, being a co-factor and allosteric modulator for >300 enzymes (including Calvin cycle, kinases, RNA polymerases and ATPases), and in chelation to nucleotidyl phosphate forms (Cowan 2002; Shaul 2002; Verbruggen and Hermans 2013). Therefore, Mg is crucial for the transport of assimilates from source leaves to sink organs, and thus, an early symptom of Mg-deficiency stress in plants is the disturbed partitioning of assimilates between roots and shoots, resulting in increased accumulation of these assimilates in source leaves and reduced growth rate of sink organs (Cakmak and Kirkby 2008; Cakmak 2013). Here, the root system and other developing plant parts (young leaves and grain) as a heavy sink for assimilates suffer from the impaired phloem loading. The limited availability of carbohydrates and other important assimilates leads to a reduction in root growth, promoting the risk of water and nutrient deficiencies from reduced exploration of soil volume, causing less access to soil resources (Cakmak and Kirkby 2008; Cakmak 2013; Gransee and Führs 2013).
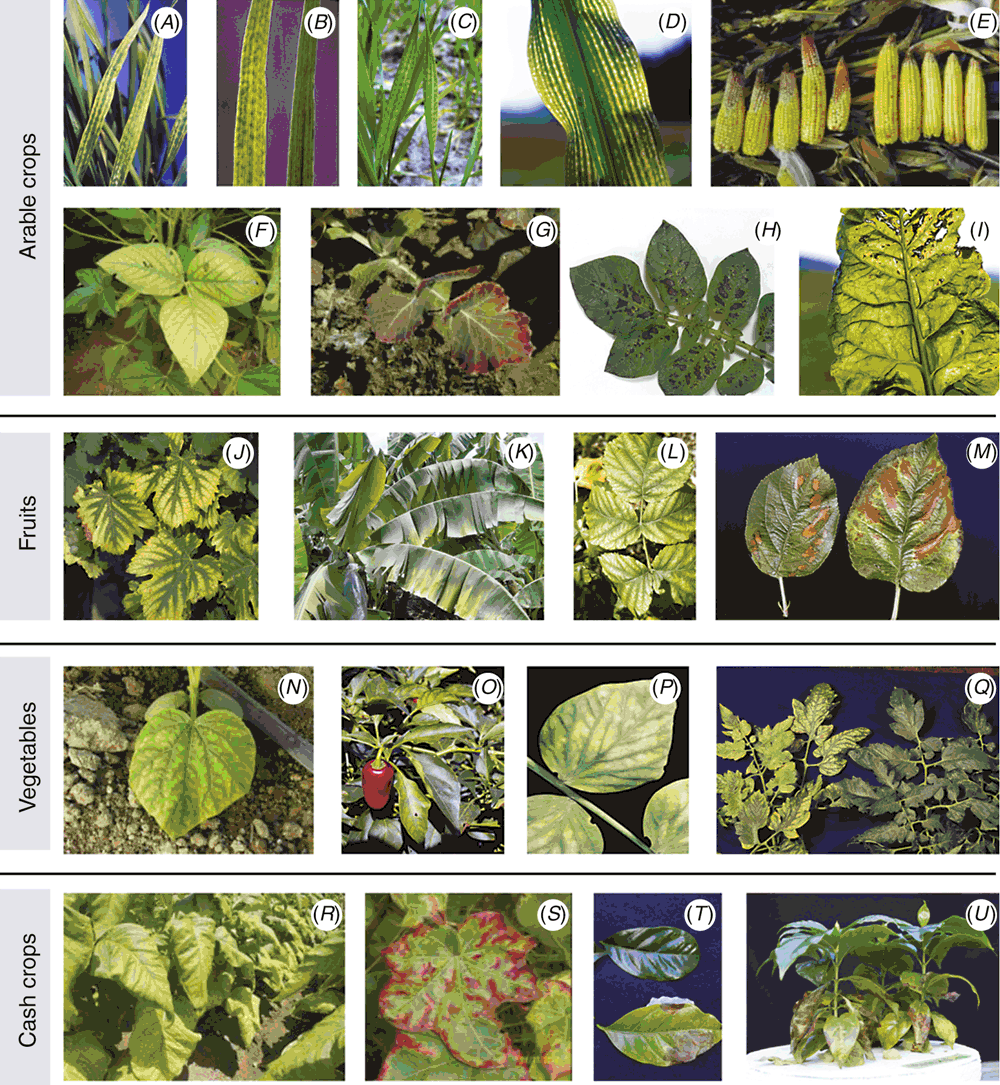
Disturbance in carbon partitioning may be regarded as a latent deficiency symptom (Gransee and Führs 2013) and it occurs long before visible symptoms such as interveinal chlorosis (e.g. in wheat or maize; Cakmak and Yazici 2010). Because of impaired phloem loading, accumulation of carbohydrates in leaves suffering Mg deficiency often causes a decrease in CO2 fixation by Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), in very early stages of the deficiency. This occurs by two main mechanisms: (i) feedback inhibition of sucrose synthesis; and (ii) accumulation of starch in the chloroplast, affecting CO2 conductance of the chloroplast membrane and causing lower CO2 partial pressure at the catalytic site of Rubisco (Araya et al. 2006). Starch synthesis and accumulation in the chloroplasts is known to cause deformation of the chloroplasts and a decrease in the rate of CO2 diffusion from the membrane (Nafziger and Koller 1976; Keenan et al. 2010). Thus, imbalance between light capture and its utilisation typically occurs, in which the non-utilisation triggers the production of reactive oxygen species (ROS) in Mg-deficient plants (Cakmak and Yazici 2010). Whether enhanced ROS would serve as signalling molecules and/or could cause oxidative damage to the chlorophyll molecules depends on the delicate equilibrium between ROS production, and their scavenging (Asada and Takahashi 1987; Elstner 1991; Mittler 2002). As shown by some examples in various crops (Fig. 2), one of the early visual symptoms of leaves suffering Mg deficiency is interveinal chlorosis, meaning chlorophyll degradation due to excessive ROS production (Mengutay et al. 2013). In almost all cases, the reason for such chlorosis in Mg-deficient leaves is excessive ROS damage, not lack of Mg for chlorophyll synthesis.
Magnesium enhances nutrient utilisation
Reducing the environmental issues while increasing unit productivity of nitrogen (N) fertiliser is the aim in modern agricultural practice (Grzebisz et al. 2010). Vegetative and generative plant growth greatly depend on the plant’s ability to take up N in amounts necessary to cover metabolic requirements at every stage of its lifecycle (Andrieu et al. 1997; Grzebisz 2013). Plant access to N and/or its utilisation can be negatively affected by physiological disorders of plants under other nutrient deficiencies (e.g. Mg deficiency). Because Mg is involved in simultaneously controlling processes responsible for photosynthesis, assimilate production and partitioning among plant parts, it seems to be a major player in N uptake and its utilisation (Gastal and Lemaire 2002; Shaul 2002; Rubio et al. 2003; Cakmak and Kirkby 2008; Grzebisz 2013). The review by Grzebisz (2013) compared various crops (sugar beets, cereals and maize) for their ability to take up more Mg in deficient soils and estimated its effect on N-use efficiency (NUE) and yield components. For example, Grzebisz et al. (2010) reported that, compared with other crops tested, tuber and root crops (especially sugar beet) showed the best response to Mg fertiliser supply, with the greatest increase in crop yield from enhanced NUE (Grzebisz et al. 2010). In N-limited environments, sufficient Mg supply was also reported to enhance N uptake (soil + fertiliser) (Grzebisz et al. 2010; Grzebisz 2013). This is due to: (i) the importance of Mg in assimilate translocation from source to sink organs, which increases root growth and may enhance crop–microbe competition for N uptake (Casson and Lindsey 2003; Cakmak and Kirkby 2008); and/or (ii) enhanced NUE from better transport of amino acids and increased rate of photosynthesis (Andrieu et al. 1997; Grzebisz et al. 2010).
Magnesium nutrition and crop stress resistance
In the field, crops face multiple stress factors that may negatively affect metabolism, growth, and yield formation of the cultivated plants—abiotic stresses (e.g. drought, salinity, cold or high light events) and biotic stresses. Furthermore, climate models predict that incidences and duration of drought and heat-stress events will increase in some parts of the world, and indicate that such conditions will have a dramatic impact on agricultural production and farming practices in coming decades (Brouder and Volenec 2008). Because of its fundamental roles in plants, Mg nutrition affects the resistance of crops to most abiotic and biotic stresses, both directly and indirectly.
Under drought conditions, a common consequence is limited nutrient uptake (including Mg) from the soil, because in dry soils, diffusion and mass flow of nutrients to the root are hampered (Engels and Kirkby 2001). Additionally, root growth is inhibited in dry soils and this may lead to a further reduction in nutrient uptake. Plant roots may counteract this physical problem by increasing root hair length and enhancing the secretion of a gelatinous substance called ‘mucilage’ with high water-holding capacity (Carminati and Vetterlein 2013). As discussed above, suboptimal Mg nutrition affects phloem loading, causing an accumulation of sucrose in photosynthetically active tissues and a poor energy supply of roots. Therefore, we may speculate that plants suffering from Mg deficiency will be more sensitive to soil-water deficit, because root exudation (e.g. mucilage) and root elongation require enormous assimilate transport from the source organs (e.g. photosynthesising leaves).
Under Mg deficiency, sugar accumulation in the leaves causes feedback inhibition of Rubisco and lowers the rate of photosynthesis. The light reaction is also dependent on Mg providing charge balance. Inhibition of photosynthesis by drought or Mg deficiency results in a misallocation of electrons to oxygen, thereby producing ROS, which cause oxidative stress (Cakmak 2005). Heat stress, high light intensity and atmospheric drought often co-occur with soil-water deficit (Braun et al. 1996). Similar to Mg deficiency, heat stress causes peroxidative damage in chloroplasts, excessive ROS production and enhanced antioxidative defence enzymes (Gong et al. 1997; Dash and Mohanty 2002; Jiang and Huang 2002). Harmful effects of excess heat stress on arable crops are pronounced when plants are simultaneously exposed to low Mg supply, and adequate Mg nutrition is critical in plant response to heat-stress events (Mengutay et al. 2013). Therefore, to maintain growth and yield formation, the amount of fertiliser needed to meet the crop Mg requirement is higher under drought than under well-watered conditions. Selecting plant genotypes with higher potential to take up more Mg and with higher Mg-utilisation efficiency should be considered as an option to secure optimum yield under stress conditions.
Application of foliar Mg fertilisers is also reported to ameliorate the nutritional status of crops subjected to Mg deficiency. It has been demonstrated to increase the chlorophyll concentration and vegetative yield of plants (Neuhaus et al. 2014). However, little is known about the plant’s ability to take up nutrients from the leaves treated with foliar fertilisers (Eichert et al. 2008). More research is needed to reveal the leaf Mg uptake potential of various crops or genotypes. Under drought situations, nutrient uptake from the leaves is limited because of closed stomata or changes in leaf morphology (e.g. thicker leaves and waxy leaf surface). In agricultural systems with higher risk of drought periods, application of Mg fertiliser, in particular in combination with other nutrients, is suggested to enhance fertiliser-use efficiency (Römheld and Kirkby 2010).
Magnesium interactions with other nutrients
Once the Mg ion has reached the root surface, it can be taken up into the root cells by building up an electrochemical gradient through pumping protons out of the cytoplasm, which allows the passive influx of Mg into the root cells (Barber 1984; Mayland and Wilkinson 1989). However, especially in sandy soils, application of high rates of K or ammonium (NH4+) fertiliser often enhances the risk of Mg deficiency (Mulder 1956). High concentrations of these cations in the soil solution interfere with Mg uptake by plants (called nutrient antagonism). Usually, such events do not occur when the soil contains more exchangeable Mg than exchangeable K (Mulder 1956; Metson 1974; Seggewiss and Jungk 1988). The simplified drawing in Fig. 3 shows how antagonism between Mg and K possibly occurs in soils containing various concentrations of Mg and K. In the case of a higher Mg concentration in the soil solution, generally K uptake is not disturbed (Fig. 3c). Typically, the amount of K in the soil solution is much lower than Mg concentration; therefore, plants have been developed specific K-transport systems in the root cells to ensure sufficient K uptake when its concentration in the soil solution is critically low (Horie et al. 2011). These specific K transporters cannot be blocked by other nutrients. By contrast, the Mg transporters are non-specific and can be passed by other cations such as K. Therefore, when K concentration in the soil–root interface is high, plant ability to take up sufficient Mg is limited. Seggewiss and Jungk (1988) and Wilkinson et al. (1990) reported that Mg uptake at the root surface was inhibited by K concentrations >20 µmol L–1. Additionally, ratios of Mg accumulated in shoots to whole plant Mg content are reportedly negatively correlated with plant K concentrations in roots (Huang et al. 1990). This suggests that excessive K concentration in roots may depress the rate of net Mg translocation from roots to shoots. However, further studies are needed to confirm this hypothesis.
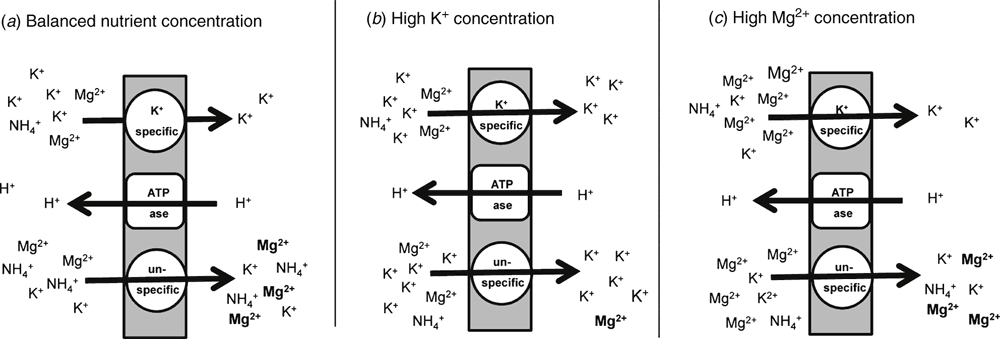
|
As a competing ion in cation exchange reactions, calcium (Ca) in excess may also interfere (similar to K) with Mg uptake (Metson 1974; Mayland and Wilkinson 1989; Wilkinson et al. 1990). This does not take place at low concentrations of Ca in the soil solution (Fageria 1973, 2009). Although Mg has a higher mobility in the soil than Ca (van der Heijden et al. 2013), Mg uptake has been repeatedly shown to be lower in soils with high Ca concentration in the soil solution (Ferguson and Clarkson 1976; Wilkinson et al. 1990; Diem and Godbold 1993; Gransee and Führs 2013). Similar to K and Ca antagonisms with Mg, NH4 nutrition has been shown to decrease Mg uptake through (i) its acidifying character when assimilated, and (ii) ion competition at the adsorbing surfaces of the roots (Mulder 1956). Therefore, in soils with limited plant-available Mg, use of NO3-based fertilisers is recommended because they do not interfere with Mg uptake (Mulder 1956). Lasa et al. (2000) showed that NH4 nutrition results in an inhibition of growth in sunflowers in a sandy soil; however, additional Mg supply mitigated the adverse effect of NH4 supply. Therefore, we may summarise that the use of NH4-based N fertilisers enhances crop Mg demands compared with plants supplied with NO3-based fertilisers.
Magnesium fertilisation
The amount of Mg released from soil minerals is mostly small compared with the amounts needed to sustain high crop yield and quality. Additionally, conditions such as high or low soil pH, drought and high levels of competing cations (e.g. K+, NH4+ and Ca2+) may reduce plant availability of Mg even if its concentration in soil solution is high (Fig. 3). Therefore, on soils with limited plant-available Mg for optimal crop production, the application of Mg fertilisers is crucial. Soil analysis and specific crop requirements need to be considered for accurate fertiliser management. For example, critical soil test value for 90% relative yield was defined as 0.21 cmol(+) kg–1 of exchangeable Mg or 7% Mg saturation in a glasshouse experiment, whereas the critical (90% yield) plant-tissue Mg concentration (whole shoots) was 0.15% (Hailes et al. 1997b). However, further experimental work is needed to define critical soil test values for specific crops under field conditions.
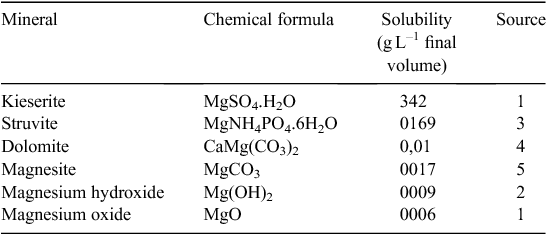
Common Mg fertilisers are generally distinguished into two classes: soluble sources and semi-soluble sources. Minerals such as dolomite are semi-soluble Mg sources. These minerals are often used as Mg fertiliser with minimum processing. On the other hand, several naturally occurring soil minerals (such as kieserite) mined from the ground contain Mg in hydrated form (MgSO4) and are used as soluble Mg fertilisers; these include magnesium sulfate monohydrate and/or magnesium sulfate heptahydrate (Kawamura and Rao 2007). The release rates of available Mg to soil solution from various mineral fertilisers are determined by their physical and chemical compositions, which are based on particle size and water solubility (Mayland and Wilkinson 1989; Härdter et al. 2004; Loganathan et al. 2005).
Water solubility of Mg in fertilisers mainly depends on its chemical composition, such as oxide, sulfate, carbonate, nitrate, chloride, phosphate or silicate (Mayland and Wilkinson 1989). Solubilities of various Mg-containing minerals are listed in Table 1. Minerals containing Mg in the form of MgSO4 are completely soluble and thus most suitable for Mg-deficient soils. Alternatively, synthetic forms of MgSO4 produced by a chemical reaction are also commercially available. Here, magnesium oxide reacts with sulfuric acid, producing a synthetic MgSO4 (SMS) (Kawamura and Rao 2007). Purification, by separation from extraneous materials, and fine grinding (particularly for the less soluble sources) are the usual methods to prepare Mg-deriving sources (Metson 1974).
|
The roles of Mg and the specific requirement for Mg supply differ with crop type. Below, we review three very different crops: grassland, wheat (Triticum aestivum L.) and potato (Solanum tuberosum L.).
Grassland
Continued inputs of N, K and Ca fertilisers into grassland and the removal of soil Mg via leaching and transfer in animal products without sufficient resupply generally cause significant decline in available Mg in grassland soils. Therefore, Mg fertiliser applications to pastures are necessary to ensure balanced nutrient supply, not only to the plant (mixed-herbage pasture) but also to the animals. This is of great importance for livestock, because it reduces the risk of low pasture Mg intakes by grazing dairy and beef cows, which can lead to hypomagnesaemic grass tetany (hypomagnesaemia), a condition that usually occurs soon after calving. Hypomagnesaemia is a major cause of lower milk production, affecting ~30–50% of dairy herds in the major dairying areas of New Zealand (O’Connor et al. 1987; Loganathan et al. 2005). Edmeades (2004) cited data showing a significant decline in soil Mg levels in New Zealand pastoral soils from the 1980s to 2000.
Wheat
Magnesium is important for both product quality and yield of cereals (Beringer and Forster 1981; Grzebisz 2013). Carbohydrate translocation and therefore optimal grain filling is positively supported by an optimal amount of available Mg. Thousand-grain weight, one of the most important wheat-grain quality parameters, is generally negatively affected in Mg-deficient soils (Marschner 2012; Grzebisz 2013). Processing behaviour is another grain-quality parameter (i.e. milling performance during flour production) known to be directly related to plant Mg content (Greffeuille et al. 2006; Gerendás and Führs 2013). In a field trial, wheat was supplied with MgSO4 next to a basic NPK fertilisation, and it was clearly showed that Mg fertilisation increased grain yield, 1000-grain weight, crude protein and raw gluten in single grains (Al’shevskii and Derebon 1982). For Mg fertiliser management, specifically in sandy soils, Mg removal from grain and/or straw may need to be considered in order to keep adequate reserves of soil Mg.
Potato
Potato is a crop with a wide spectrum of quality parameters. Because of its usage in many forms (e.g. fresh consumption, processing and starch production), quality control is difficult and Mg is known to be a key element of potato quality (Talburt and Smith 1997; Hiltrop 1999). We can summarise the effect of Mg on potato yield and quality with four main points.
First, starch content and thus the mealiness grade of cooking potatoes vary because of enhanced assimilation and carbohydrate translocation (Smith 1977; Talburt and Smith 1997; Feltran et al. 2004). Therefore, the involvement of Mg in photosynthesis and assimilate translocation has a direct effect (Cepl 1994; Poberezny and Wszelaczynska 2011; Affleck et al. 2012).
Second, tuber firmness and resistance against mechanical stresses occurring during harvest, transport and storage are major quality parameters. Increased firmness, which is positively influenced by Mg supply, reduces the risk of bruising and various forms of discoloration (Klein et al. 1982).
Third, colouring of potatoes is most important for potato attractiveness. Discolorations of crude pulp and black spot incidence are induced by an enzymatic process, by harvest or transport, as well by processing of crude pulp. During processing, polyphenol oxidases in potato tubers interfere with free phenolic compounds. In this process, polyphenol oxidases catalyse the formation of diphenols from monophenols, changing finally to dark melanins (Mulder 1949; Mondy et al. 1965; Mondy and Koch 1978; Klein et al. 1981; Muneta 1981). The role of Mg in these reactions is not clear, but the role of Mg in assimilation and carbohydrate translocation indicates that an improved Mg supply should have a positive effect.
Fourth, another process is the production of glycoalkaloids, correlating with the greening of tubers (Maga and Fitzpatrick 1980). These alkaloids are described as toxic substances but the positive response of their concentration in leaves and stems lead to an increase of N supply (Love et al. 1994; Rogozinska and Wojdyla 1999), and the significance of Mg for N metabolism has prompted several investigators to study the influence of Mg supply on glycoalkaloid accumulation in potato tubers. Mondy and Ponnampalam (1985) and Klein et al. (1982) reported an increased N and protein concentration. Additionally, Evans and Mondy (1984) and Mondy and Ponnampalam (1985) described a significant increase in glycoalkaloid content after Mg application. Within these results, a hypothesis is raised that increased chlorophyll synthesis, as well as stimulation of sugar metabolism and/or changes in amino acid production, are part of this effect.
Magnesium fertiliser leaching potential
Unlike other cations, Mg is very mobile in soils because it is less bound to the soil charges. This results in a relatively high abundance of this element in the soil solution and thus a higher risk of leaching (Maguire and Cowan 2002; Shaul 2002; Gardner 2004; Gransee and Führs 2013). Leaching of Mg should be less severe in soils under a crop than under bare fallow but may increase when fertilisers are added. The potential for applied Mg to be taken up by crop plants and not lost via leaching greatly depends on the solubility of Mg fertilisers (Loganathan et al. 1999; Mitchell et al. 2000; Härdter et al. 2004). Applications of Mg fertilisers known as slow-release fertilisers (e.g. dolomite, magnesite and calcined magnesite) may mitigate leaching risks but not deliver sufficient plant-available Mg to crops. On the other hand, application of soluble Mg fertilisers (e.g. kieserite and SMS) may lead to Mg losses by leaching when applied to sandy soils with high water conductivity (e.g. sandy soils), specifically in wet seasons (Härdter et al. 2004; Loganathan et al. 2005).
In several studies, various Mg fertiliser sources were tested in different soils to examine Mg plant uptake and losses through leaching (Durrant and Draycott 1976; Heming and Hollis 1995; Härdter et al. 2004; Hanly et al. 2005). It is commonly accepted that there is almost no Mg leaching risk of slow-release Mg fertilisers (dolomite or fertilisers contains Mg in the form of Mg oxide). Efficiency of slow-released Mg fertilisers may be slightly higher, especially in acid soil conditions, and/or when applied in ground forms (Härdter 1992; Heming and Hollis 1995; Härdter et al. 2004). During the critical vegetative periods (such as shooting or flowering in wheat), crop nutrient requirements are at their maximum. Thus, it is important that applied fertilisers release sufficient plant-available nutrients during such critical periods when crop nutrient demand is high. In this context, water-soluble Mg fertilisers (such as kieserite and SMS) typically release more plant-available Mg in a relatively short period and are therefore more effective to secure crop Mg demand in Mg-deficient soils. On the other hand, SMS may cause higher risk of Mg leaching under certain conditions, such as in sandy soils and after heavy rain showers (Durrant and Draycott 1976; Heming and Hollis 1995; Härdter et al. 2004; Hanly et al. 2005; Loganathan et al. 2005). Härdter et al. (2004) reported that maize grown in a pot experiments had 19.6% greater Mg uptake and 10.6% higher yield with kieserite (water-soluble) treatment than MgO (slow-release Mg source) in both sandy and loamy soils. However, the same authors concluded that in soils with water-soluble Mg fertilisers applied, 9% and 22% of applied Mg could be lost via leaching during heavy rain showers.
Magnesium leaching of various water-soluble fertilisers may also vary depending on their chemical composition, granule size, and effect of soil pH and cation balance. Härdter et al. (2004) compared SMS and kieserite at two application rates and found that Mg leaching was ~33% lower in soils treated with kieserite than SMS, which they attributed to slower dissolution properties of kieserite than SMS. Slower release of Mg in kieserite may lead to a greater Mg adsorption in soil and thus lower leaching potential.
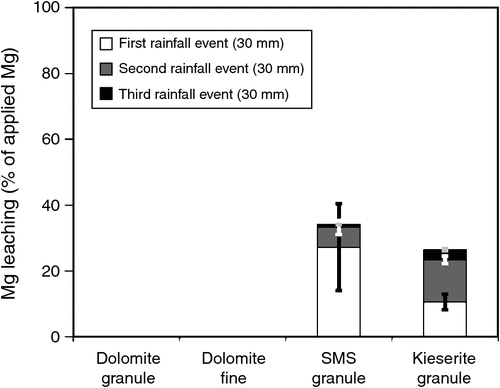
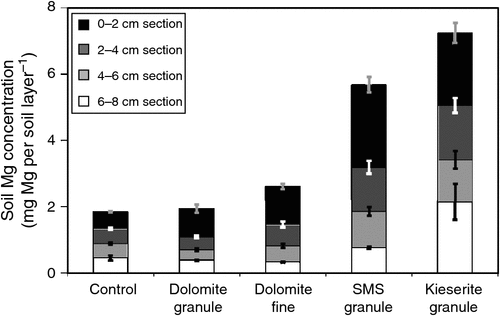
A similar experiment was done in another incubation trial at the Institute of Applied Plant Nutrition, Göttingen, where sandy soil with low soil pH was placed in small lysimeters. Briefly, after soil sampling (from Ahlten, in Hannover, Germany), soil was air-dried and sieved to 2 mm. Sieved soil (60 g) was packed into mini-lysimeters (8 cm height by 3 cm diameter) to a bulk density of 1.1 g cm–3 (similar to field conditions; for method details, see Senbayram et al. 2015). Non-treated control was compared with three fertiliser treatments: dolomite, SMS and kieserite (single granule in each fertiliser treatment placed on top of the soil). In an additional treatment, fine-ground dolomite was applied to the soil surface. Three rainfall simulation events were applied by using a peristaltic pump, to simulate rain at 10 mm h–1. Each event consisted of 30 mL rainfall within 3 h, and leachates were collected for Mg analysis. Three weeks after onset of treatments, soil exchangeable Mg at soil depth sections 0–2, 2–4, 4–6, and 6–8 cm were analysed by the CaCl2 extraction method. Data from the experiment are presented in Figs 4 and 5, and clearly show no significant Mg leaching in either dolomite granule or dolomite fine treatment (known as non-soluble or slow release Mg fertiliser) compared with the non-fertilised control treatment, even after the third rainfall event (Fig. 4). Therefore, even fine-ground dolomite fertiliser does not cause detectable Mg leaching, even under extreme storm events. This is in line with the previous reports (Loganathan et al. 2005; Härdter et al. (2004). Soil analysis of exchangeable Mg showed that Mg concentration only in the top layer of soil (0–2 cm soil section) increased slightly in the dolomite granule treatment compared with the non-fertilised control soil. On the other hand, grinding dolomite caused greater release of Mg, and soil net exchangeable Mg content was ~4-fold higher in the dolomite fine treatment than the dolomite granule treatment. However, even in the dolomite fine treatment, total net exchangeable Mg in 0–8 cm soil section was 87% lower than with kieserite 3 weeks after the onset of treatments (Fig. 5). This result may suggest that dolomite or its ground form has very limited capacity to supply sufficient Mg under deficient conditions. When comparing soluble Mg fertilisers, kieserite and SMS differed greatly in leaching behaviour. Concentrations of Mg in the leachate of SMS-treated pots increased drastically (2.5-fold that of the kieserite treatment) after the first rainfall event (Fig. 4). However, after the second rainfall event, about twice as much Mg was leached in the kieserite treatment than the SMS treatment. Overall, 34% and 27% of applied Mg was lost via leaching in SMS and kieserite treatments.
|
|
Compared with the above findings, Härdter et al. (2004) reported lower total Mg loss via leaching (16.7% and 22.3% of the applied Mg in kieserite and SMS treatment) from water-soluble Mg fertilisers. This was most likely due to differences in the size of the lysimeters. Soil columns were 20 cm high in the study of Härdter et al. (2004), whereas in the presently reported study, the lysimeters height was only 8 cm. However, both studies showed that Mg lost via leaching was significantly lower in soils with applied kieserite than SMS (21–33% lower). There is a clear indication that the more rapidly soluble SMS displaced larger amounts of cations from the exchange complex, causing greater losses of these nutrients than kieserite.
Conclusion
In this review, we have summarised current knowledge regarding the importance of Mg nutrition in plant growth and quality under changing climate and discussed the factors controlling Mg availability and leaching in soil. We can summarise three take-home messages.
First, the amount of Mg released from soil minerals is commonly not sufficient in sandy soils compared with the amounts needed to sustain high crop yield and quality. Especially in sandy soils, application of high rates of K or NH4+ fertilisers often enhances the risk of Mg deficiency.
Second, Mg fertiliser supply in many agricultural systems is often inadequate mainly through lack of knowledge and/or economic reasons. Precise site- and crop-specific Mg fertiliser management practices need to be developed as recommended by IPNI 4R Nutrient Stewardship.
Third, unlike other cations, Mg is very mobile in soils because it is less bound to the soil charges. This results in a relatively high abundance of this element in the soil solution and thus higher risk of leaching. Mg-leaching properties of various water-soluble fertilisers may also vary depending on their chemical composition, granule size, and effect of soil pH and cation balance. Theoretically, the ideal Mg fertiliser would contain both high- and low-soluble Mg sources in agricultural systems where higher risk of leaching loss is expected.
References
Affleck I, Sullivan JA, Tarn R, Yada R (2012) Stability of eight potato genotypes for sugar content and French fry quality at harvest and after storage. Canadian Journal of Plant Science 92, 87–96.| Stability of eight potato genotypes for sugar content and French fry quality at harvest and after storage.Crossref | GoogleScholarGoogle Scholar |
Al’shevskii NG, Derebon YG (1982) Effect of magnesium fertilizers on yield and quality of winter wheat. Khimiya v Sel’skom Khozyaistve 4, 17–20.
Andrieu B, Allirand JM, Jaggard KW (1997) Ground cover and leaf area index of maize and sugar beet crops. Agronomie 17, 315–321.
| Ground cover and leaf area index of maize and sugar beet crops.Crossref | GoogleScholarGoogle Scholar |
Araya T, Noguchi K, Terashima I (2006) Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant & Cell Physiology 47, 644–652.
| Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlsFaitbw%3D&md5=5c2130406e308729820c71c846ef245bCAS |
Asada K, Takahashi M (1987) Production and scavenging of active oxygen photosynthesis. In ‘Photoinhibition. Topics in photosynthesis. Vol. 9’. (Eds DJ Kyle, CB Osmond, CJ Arntzen) pp. 227–287. (Elsevier: Amsterdam)
Baker DE (1971) A new approach to soil testing. Soil Science 112, 381–391.
| A new approach to soil testing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XksV2quw%3D%3D&md5=570a6db19174ffa606fd272219d6014dCAS |
Barber SA (1984) ‘Soil nutrient bioavailability.’ (John Wiley & Sons: New York)
Bénézeth P, Saldi G, Dandurand JL, Schott J (2011) Experimental determination of the solubility product of magnesite at 50 to 200°C. Chemical Geology 286, 21–31.
Beringer H, Forster H (1981) Einfluß variierter Mg‐ernährung auf tausendkorngewicht und P‐fraktionen des gerstenkorns. Journal of Plant Nutrition and Soil Science 144, 8–15.
Bhuiyan MIH, Mavinic DS, Beckie RD (2007) A solubility and thermodynamic study of struvite. Environmental Technology 28, 1015–1026.
Braun H, Lichter A, Haberlein I (1996) Kinetic evidence for protein complexes between thioredoxin and NADP-malate dehydrogenase and presence of a thioredoxin binding site at the N-terminus of the enzyme. European Journal of Biochemistry 240, 781–788.
| Kinetic evidence for protein complexes between thioredoxin and NADP-malate dehydrogenase and presence of a thioredoxin binding site at the N-terminus of the enzyme.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvFKltbc%3D&md5=89fbab843e84bafd636631b91156feadCAS | 8856084PubMed |
Brouder SM, Volenec JJ (2008) Impact of climate change on crop nutrient and water use efficiencies. Physiologia Plantarum 133, 705–724.
| Impact of climate change on crop nutrient and water use efficiencies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit7k%3D&md5=3657ee920967fa65156b529b4b968b38CAS | 18507815PubMed |
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science 168, 521–530.
| The role of potassium in alleviating detrimental effects of abiotic stresses in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsFCntL8%3D&md5=bf0b9024a813f2fc133bd99db0455909CAS |
Cakmak I (2013) Magnesium in crop production, food quality and human health. Plant and Soil 368, 1–4.
| Magnesium in crop production, food quality and human health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovFClt70%3D&md5=43ccd926be2f1777438c404b1a5742e4CAS |
Cakmak I, Kirkby EA (2008) Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiologia Plantarum 133, 692–704.
| Role of magnesium in carbon partitioning and alleviating photooxidative damage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit7g%3D&md5=f6171fa0071edcd49dbb7a844858be0dCAS | 18724409PubMed |
Cakmak I, Yazici AM (2010) Magnesium—a forgotten element in crop production. Better Crops 94, 23–25.
Carminati A, Vetterlein D (2013) Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Annals of Botany 112, 277–290.
| Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSiur7L&md5=f2f84364572971d6cc3e604fc62c7397CAS | 23235697PubMed |
Casson S, Lindsey K (2003) Genes and signalling in root development. New Phytologist 158, 11–38.
| Genes and signalling in root development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1Wlsbs%3D&md5=59e5d25b63acfe1e32e11574ce530c09CAS |
Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C, Tondelli A, Stanca AM (2008) Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Research 105, 1–14.
| Drought tolerance improvement in crop plants: An integrated view from breeding to genomics.Crossref | GoogleScholarGoogle Scholar |
Cepl J (1994) Analysis of the effect of potassium and magnesium fertilization on some indicators of the potato yield and quality. Rostlinna Vyroba 40, 899–905.
Chan KY, Davey BG, Geering HR (1979) Adsorption of magnesium and calcium by a soil with variable charge. Soil Science Society of America Journal 43, 301–304.
| Adsorption of magnesium and calcium by a soil with variable charge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXlt1altbs%3D&md5=e4bd003770c838b046bf2e84fa2aed23CAS |
Chu C, Johnson L (1985) Relationship between exchangeable and total magnesium in Pennsylvania soils. Clays and Clay Minerals 33, 340–344.
| Relationship between exchangeable and total magnesium in Pennsylvania soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXkvVOjs7g%3D&md5=c30e7aed30af4b0b7986634cb1824315CAS |
Cowan JA (2002) Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 15, 225–235.
| Structural and catalytic chemistry of magnesium-dependent enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvFCgtLs%3D&md5=ae716e4c65d3e4e911a88d9c0d18a171CAS | 12206389PubMed |
D’Ans J, Lax E (1949) Löslichkeit. In ‘Taschenbuch für Chemiker und Physiker’. 2nd edn pp. 910–975. (Springer Verlag: Berlin)
Dash S, Mohanty N (2002) Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. Journal of Plant Physiology 159, 49–59.
| Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhslOksbc%3D&md5=0fae5cd0cef7601edebd96618d04944dCAS |
Diem B, Godbold DL (1993) Potassium, calcium and magnesium antagonism in clones of Populus trichocarpa. Plant and Soil 155/156, 411–414.
| Potassium, calcium and magnesium antagonism in clones of Populus trichocarpa.Crossref | GoogleScholarGoogle Scholar |
Durrant MJ, Draycott AP (1976) Improvements in calcined magnesite as a magnesium fertiliser. The Journal of Agricultural Science 86, 543–552.
| Improvements in calcined magnesite as a magnesium fertiliser.Crossref | GoogleScholarGoogle Scholar |
Edmeades DC (2004) The magnesium requirements of pastures in New Zealand: a review. New Zealand Journal of Agricultural Research 47, 363–380.
| The magnesium requirements of pastures in New Zealand: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpvFOisrY%3D&md5=cad7b29f7282a001caa53d36c3e363dfCAS |
Eichert T, Kurtz A, Steiner U, Goldbach HE (2008) Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water‐suspended nanoparticles. Physiologia Plantarum 134, 151–160.
| Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water‐suspended nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFChtbrL&md5=b3149c953eb1ebeccdccfd0f0167c469CAS | 18494856PubMed |
Elstner EF (1991) Mechanisms of oxygen activation in different compartments of plant cells. In ‘Active oxygen/oxidative stress and plant metabolism’. (Eds EJ Pell, KL Steffen) (American Society of Plant Physiologists: Rockville, MD, USA)
Engels C, Kirkby EA (2001) Cycling of nitrogen and potassium between shoot and roots in maize as affected by shoot and root growth. Journal of Plant Nutrition and Soil Science 164, 183–191.
| Cycling of nitrogen and potassium between shoot and roots in maize as affected by shoot and root growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtlOrt70%3D&md5=adf7f105d0d51a7ef8cca0dfd382fbc1CAS |
Evans D, Mondy NI (1984) Effect of magnesium fertilization on glycoalkaloid formation in potato tubers. Journal of Agricultural and Food Chemistry 32, 465–466.
| Effect of magnesium fertilization on glycoalkaloid formation in potato tubers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXitVynuro%3D&md5=179a655b452e78974ec882fa2a86f823CAS |
Fageria NK (1973) Uptake of nutrients by rice plants from dilute solutions. PhD thesis, Catholic University of Louvain, Belgium.
Fageria NK (2009) ‘The use of nutrients in crop plants.’ (CRC Press, Taylor and Francis Group: London)
Feltran JC, Lemos LB, Vieites RL (2004) Technological quality and utilization of potato tubers. Scientia Agricola (Piracicaba, Braz) 61, 593–597.
Ferguson IB, Clarkson DT (1976) Simultaneous uptake and translocation of magnesium and calcium in barley (Hordeum vulgare L.) roots. Planta 128, 267–269.
| Simultaneous uptake and translocation of magnesium and calcium in barley (Hordeum vulgare L.) roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28Xhtlaktrk%3D&md5=9a1ff597e2604158225c8b7bf6f855c9CAS | 24430757PubMed |
Gardner WK (2004) Changes in soils irrigated with saline groundwater containing excess bicarbonate. Australian Journal of Soil Research 42, 825–831.
| Changes in soils irrigated with saline groundwater containing excess bicarbonate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpslGlu7Y%3D&md5=63547baa0992de0d473f2bc6fe58b9d0CAS |
Gastal F, Lemaire G (2002) N uptake and distribution in crops: an agronomical and ecophysiological perspective. Journal of Experimental Botany 53, 789–799.
| N uptake and distribution in crops: an agronomical and ecophysiological perspective.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFSntL0%3D&md5=0437d8fe5c67cd087f188628a27de3e7CAS | 11912222PubMed |
Gerendás J, Führs H (2013) The significance of magnesium for crop quality. Plant and Soil 368, 101–128.
| The significance of magnesium for crop quality.Crossref | GoogleScholarGoogle Scholar |
Gong M, Li YJ, Dai X, Tian M, Li ZG (1997) Involvement of calcium and calmodulin in the acquisition of heat-shock induced thermotolerance in maize seedlings. Journal of Plant Physiology 150, 615–621.
| Involvement of calcium and calmodulin in the acquisition of heat-shock induced thermotolerance in maize seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjsVWlu70%3D&md5=7be65a5b916bb6ff5aacc5de9d997dfcCAS |
Gransee A, Führs H (2013) Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant and Soil 368, 5–21.
| Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Ggu74%3D&md5=f86750f7d8454d22cf661cde86262316CAS |
Greffeuille V, Abecassis J, Lapierre C, Lullien-Pellerin V (2006) Bran size distribution at milling and mechanical and biochemical characterization of common wheat grain outer layers: a relationship assessment. Cereal Chemistry 83, 641–646.
| Bran size distribution at milling and mechanical and biochemical characterization of common wheat grain outer layers: a relationship assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yqtrjK&md5=611fdb6f64f191a3a15957d15fdc737bCAS |
Grimme H (1991) Magnesium in landwirtschaftlichen und forstlichen Ökosystemen. KALI-BRIEFE 20, 525–538.
Grzebisz W (2013) Crop response to magnesium fertilization as affected by nitrogen supply. Plant and Soil 368, 23–39.
| Crop response to magnesium fertilization as affected by nitrogen supply.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Ghu74%3D&md5=0e601555c538daeba4102c30294f4a85CAS |
Grzebisz W, Przygocka-Cyna K, Szczepaniak W, Diatta JB, Potarzycki J (2010) Magnesium as a nutritional tool of nitrogen efficient management—plant production and environment. Journal of Elementology 15, 771–788.
Hailes KJ, Aitken RL, Menzies NW (1997a) Magnesium in tropical and subtropical soils from north-eastern Australia. I. Magnesium fractions and interrelationships with soil properties. Australian Journal of Soil Research 35, 615–627.
| Magnesium in tropical and subtropical soils from north-eastern Australia. I. Magnesium fractions and interrelationships with soil properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1Crsrg%3D&md5=8d2ea99e259c105f5d40b5a5bdfa7be3CAS |
Hailes KJ, Aitken RL, Menzies NW (1997b) Magnesium in tropical and subtropical soils from north-eastern Australia. II. Response by glasshouse-grown maize to applied magnesium. Australian Journal of Soil Research 35, 629–641.
| Magnesium in tropical and subtropical soils from north-eastern Australia. II. Response by glasshouse-grown maize to applied magnesium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1Crsrk%3D&md5=0ac83770b460dd1810f31de646875be3CAS |
Hanly JA, Loganathan P, Currie LD (2005) Effect of serpentine rock and its acidulated products as magnesium fertilisers for pasture, compared with magnesium oxide and Epsom salts, on a Pumice Soil. 1. Dry matter yield and magnesium uptake. New Zealand Journal of Agricultural Research 48, 451–460.
| Effect of serpentine rock and its acidulated products as magnesium fertilisers for pasture, compared with magnesium oxide and Epsom salts, on a Pumice Soil. 1. Dry matter yield and magnesium uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovVyltQ%3D%3D&md5=35b092b819c443ae611520cc58520f2cCAS |
Härdter R (1992) Magnesium—The forgotten macronutrient. In ‘IFAFADINAP Regional Fertiliser Conference for Asia and the Pacific’. 29 Nov.–2. Dec. 1992, Bali, Indonesia. p. 16 (International Fertiliser Association: Paris)
Härdter R, Rex M, Olovius K (2004) Effects of different Mg fertiliser sources on the magnesium availability in soils. Nutrient Cycling in Agroecosystems 70, 249–259.
| Effects of different Mg fertiliser sources on the magnesium availability in soils.Crossref | GoogleScholarGoogle Scholar |
Helgeson HC (1969) Thermodynamics of hydrothermal systems at elevated temperatures and pressures. American Journal of Science 267, 729
Heming SD, Hollis JF (1995) Magnesium availability from kieserite and calcined magnesite on five soils of different pH. Soil Use and Management 11, 105–109.
| Magnesium availability from kieserite and calcined magnesite on five soils of different pH.Crossref | GoogleScholarGoogle Scholar |
Hiltrop P (1999) Rohstoffqualitäten für Pommes frites und andere vorgebackene Kartoffelprodukte. In ‘Die erzeugung von kartoffeln zur industriellen verarbeitung’. (Ed. P Schuhmann) (Agrimedia GmbH: Bergen, Germany)
Horie T, Brodsky DE, Costa A, Kaneko T, Schiavo FL, Katsuhara M, Schroeder JI (2011) K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiology 156, 1493–1507.
| K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWksLg%3D&md5=33e2eb2fcb92c673d840a5fe76bb3d99CAS | 21610181PubMed |
Huang JW, Grunes DL, Welch RM (1990) Magnesium, nitrogen form, and root temperature effects on grass tetany potential of wheat forage. Agronomy Journal 82, 581–587.
| Magnesium, nitrogen form, and root temperature effects on grass tetany potential of wheat forage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlsFWgt70%3D&md5=43f25d7c738ba81f3d0c9b5586dd7e16CAS |
Huber DM, Jones JB (2013) The role of magnesium in plant disease. Plant and Soil 368, 73–85.
| The role of magnesium in plant disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Ggt7Y%3D&md5=4f8407388073c3d614d64c897aaba463CAS |
Jiang YW, Huang BR (2002) Protein alterations in tall fescue in response to drought stress and abscisic acid. Crop Science 42, 202–207.
| Protein alterations in tall fescue in response to drought stress and abscisic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsVantbw%3D&md5=e6ac25fbeea532eebc6352f42c0b8445CAS |
Kawamura Y, Rao M (2007) Magnesium sulfate: chemical and technical assessment. Compendium of Food Additive Specifications, Joint FAO/WHO Expert Committee on Food Additives. 68th Meeting 2007, pp. 25–27. www.fao.org/docrep/010/a1447e/a1447e00.htm (accessed 9 December 2010)
Keenan T, Sabate S, Gracia C (2010) The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods. Global Change Biology 16, 1019–1034.
| The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods.Crossref | GoogleScholarGoogle Scholar |
Klein LB, Chandra S, Mondy NI (1981) Effect of magnesium fertilization on the quality of potatoes: yield, discolouration, phenols, and lipids. Journal of Agricultural and Food Chemistry 29, 384–387.
| Effect of magnesium fertilization on the quality of potatoes: yield, discolouration, phenols, and lipids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXhtlynsrY%3D&md5=3ddd5c99995a47020a32f3e979531cf0CAS |
Klein LB, Chandra S, Mondy NI (1982) Effect of magnesium fertilization on the quality of potatoes: total nitrogen, nonprotein nitrogen, protein, amino acids, minerals, and firmness. Journal of Agricultural and Food Chemistry 30, 754–757.
| Effect of magnesium fertilization on the quality of potatoes: total nitrogen, nonprotein nitrogen, protein, amino acids, minerals, and firmness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XktlOqu7g%3D&md5=79569b7ad6171a2e5e80639bf1a78451CAS |
Lasa B, Frechilla S, Aleu M, González-Moro B, Lamsfus C, Aparicio-Tejo PM (2000) Effects of low and high levels of magnesium on the response of sunflower plants grown with ammonium and nitrate. Plant and Soil 225, 167–174.
| Effects of low and high levels of magnesium on the response of sunflower plants grown with ammonium and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotVGitw%3D%3D&md5=b80c6f35afae7f1aa257f55d565e1837CAS |
Loganathan P, Payn TW, Mitchell AD, Tillman RW (1999) A sequential extraction method for the determination of dissolution of magnesium from fertilizers applied to Pumice Soils. Communications in Soil Science and Plant Analysis 30, 199–211.
| A sequential extraction method for the determination of dissolution of magnesium from fertilizers applied to Pumice Soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtlGitb0%3D&md5=f5f59f8389b9a04a42ac527c706f6b5bCAS |
Loganathan P, Hanly JA, Currie LD (2005) Effect of serpentine rock and its acidulated products as magnesium fertilisers for pasture, compared with magnesium oxide and Epsom salts, on a Pumice Soil. 2. Dissolution and estimated leaching loss of fertiliser magnesium. New Zealand Journal of Agricultural Research 48, 461–471.
| Effect of serpentine rock and its acidulated products as magnesium fertilisers for pasture, compared with magnesium oxide and Epsom salts, on a Pumice Soil. 2. Dissolution and estimated leaching loss of fertiliser magnesium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovVylug%3D%3D&md5=af21676da3049c27d5afd61c680b5574CAS |
Love SL, Herrman TJ, Thomson-Johns A, Baker TP (1994) Effect and interaction of crop management factors on the glycoalkaloid concentration of potato tubers. Potato Research 37, 77–85.
| Effect and interaction of crop management factors on the glycoalkaloid concentration of potato tubers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmslKlu7k%3D&md5=895288bad27c38118d2d7ee43331c0ccCAS |
Maga JA, Fitzpatrick TJ (1980) Potato alkaloids. Critical Reviews in Food Science and Nutrition 12, 371–405.
| Potato alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXls1Kitb8%3D&md5=569fdd945f5b7fbcf42e03a2ec307020CAS | 6996922PubMed |
Maguire ME, Cowan JA (2002) Magnesium chemistry and biochemistry. Biometals 15, 203–210.
| Magnesium chemistry and biochemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvFCgtL0%3D&md5=e63db3e54dd8c0cf1dbb7ea8d04dd7b3CAS | 12206387PubMed |
Marschner H (2012) ‘Marschner’s mineral nutrition of higher plants.’(Ed. P Marschner) (Academic Press: London)
Mayland HF, Wilkinson SR (1989) Soil factors affecting magnesium availability in plant–animal systems: a review. Journal of Animal Science 67, 3437–3444.
Mengutay M, Ceylan Y, Kutman UB, Cakmak I (2013) Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant and Soil 368, 57–72.
| Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnslCns7k%3D&md5=a024cea31f960aedbfd5b43b2cfb64bfCAS |
Metson AJ (1974) Magnesium in New Zealand soils. I. Some factors governing the availability of soil magnesium: A review. New Zealand Journal of Experimental Agriculture 2, 277–319.
| Magnesium in New Zealand soils. I. Some factors governing the availability of soil magnesium: A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXkt1WrsA%3D%3D&md5=cc7228db8b69715a57f7d2440e7adf04CAS |
Mitchell AD, Loganathan P, Payn TW, Tillman RW (2000) Magnesium fertiliser dissolution rates in pumice soils under Pinus radiata. Australian Journal of Soil Research 38, 753–767.
| Magnesium fertiliser dissolution rates in pumice soils under Pinus radiata.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktlGisrk%3D&md5=c13d4fcb831c348c15e4dfedfcfef1edCAS |
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410.
| Oxidative stress, antioxidants and stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntVWnu7Y%3D&md5=c9a3775b6fda926bee60a56998752866CAS | 12234732PubMed |
Mondy NI, Koch RL (1978) Influence of nitrogen fertilization on potato discoloration in relation to chemical composition. I. Lipid, potassium and dry matter content. Journal of Agricultural and Food Chemistry 26, 666–669.
| Influence of nitrogen fertilization on potato discoloration in relation to chemical composition. I. Lipid, potassium and dry matter content.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhvVyqtb4%3D&md5=f7d85be3b0be05443502b9f90d62652cCAS |
Mondy NI, Ponnampalam R (1985) Effect of magnesium fertilizers on total glycoalkaloids and nitrate-N in Katahdin tubers. Journal of Food Science 50, 535–536.
| Effect of magnesium fertilizers on total glycoalkaloids and nitrate-N in Katahdin tubers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhs1Grt70%3D&md5=066ded78667ade0e0a19fcb530228b82CAS |
Mondy NI, Bourne A, Breslow B, Mattick LR (1965) The effect of boron on the lipid content and discoloration of potatoes. Journal of Food Science 30, 420–425.
| The effect of boron on the lipid content and discoloration of potatoes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXktFaqu7s%3D&md5=b7a1f3467fdc721d54e6e6735deb2941CAS |
Mulder EG (1949) Mineral nutrition in relation to the biochemistry and physiology of potatoes. Plant and Soil 2, 59–121.
| Mineral nutrition in relation to the biochemistry and physiology of potatoes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG3cXkslaisg%3D%3D&md5=1f160f112ed5dedb2e43d96be53044eaCAS |
Mulder EG (1956) Nitrogen–magnesium relationships in crop plants. Plant and Soil 7, 341–376.
| Nitrogen–magnesium relationships in crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1cXpvVGnsw%3D%3D&md5=18435d9944ff98ad6d237efa087ccc34CAS |
Muneta P (1981) Comparisons of inhibitors of tyrosine oxidation in the enzymatic blackening of potatoes. American Journal of Potato Research 58, 85–92.
| Comparisons of inhibitors of tyrosine oxidation in the enzymatic blackening of potatoes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXhsFGhsL4%3D&md5=cfa205a6213403318275b53202948c09CAS |
Nafziger E, Koller H (1976) Influence of leaf starch concentration on CO2 assimilation in soybean. Plant Physiology 57, 560–563.
| Influence of leaf starch concentration on CO2 assimilation in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XktValtbk%3D&md5=1f2f7986c41aa5aabbecf03493009915CAS | 16659526PubMed |
Neuhaus C, Geilfus CM, Muehling KH (2014) Increasing root and leaf growth and yield in Mg-deficient faba beans (Vicia faba) by MgSO4 foliar fertilization. Journal of Plant Nutrition and Soil Science 177, 741–747.
| Increasing root and leaf growth and yield in Mg-deficient faba beans (Vicia faba) by MgSO4 foliar fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Gmtb%2FF&md5=a2eed622c82abbdf765c3e313a25c9e2CAS |
O’Connor MB, Pearce MG, Gravett IM, Towers NR (1987) Fertilising with magnesium to prevent hypomagnesaemia (grass staggers) in dairy cows. In ‘Proceedings of the Ruakura Farmers Conference’. pp. 47–49. (Ministry of Agriculture and Fisheries: Wellington, New Zealand)
Poberezny J, Wszelaczynska E (2011) Effect of bioelements (N, K, Mg) and long-term storage of potato tubers on quantitative and qualitative losses Part II. Content of dry matter and starch. Journal of Elementology 16, 237–246.
Prince A, Zimmerman M, Bear F (1947) The magnesium-supplying powers 20 New-Jersey soils. Soil Science 63, 69–78.
| The magnesium-supplying powers 20 New-Jersey soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaH2sXhslOjtw%3D%3D&md5=97811aeba4f8c6f496d4ebbef0e8379aCAS |
Reynolds MP, Hellin J, Govaerts B, Kosina P, Sonder K, Hobbs P, Braun H (2012) Global crop improvement networks to bridge technology gaps. Journal of Experimental Botany 63, 1–12.
| Global crop improvement networks to bridge technology gaps.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yms7%2FN&md5=8806ce2b4a34dbea85e43beb89e3dfc2CAS | 21926090PubMed |
Rogozinska I, Wojdyla T (1999) Der Einfluß variierter Düngung, einer Pflanzenschutz-Anwendung und der Lagerung auf den Glykoalkaloid-Gehalt in Kartoffelknollen zweier Sorten. Potato Research 42, 79–88.
| Der Einfluß variierter Düngung, einer Pflanzenschutz-Anwendung und der Lagerung auf den Glykoalkaloid-Gehalt in Kartoffelknollen zweier Sorten.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkvFejt7w%3D&md5=95e6e92c7284fa281338a41efdbb59ffCAS |
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant and Soil 335, 155–180.
| Research on potassium in agriculture: needs and prospects.Crossref | GoogleScholarGoogle Scholar |
Rubio G, Zhu J, Lynch J (2003) A critical test of the prevailing theories of plant response to nutrient availability. American Journal of Botany 90, 143–152.
| A critical test of the prevailing theories of plant response to nutrient availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1Sks7g%3D&md5=ef36816bdc42ebca80eea6946320fa27CAS | 21659090PubMed |
Salmon R (1963) Magnesium relationships in soils and plants. Journal of the Science of Food and Agriculture 14, 605–610.
| Magnesium relationships in soils and plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXhvFelsQ%3D%3D&md5=a70efebcc4b7150683d1ca70d29ba305CAS |
Schubert S, Schubert E, Mengel K (1990) Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans (Vicia faba). Plant and Soil 124, 239–244.
| Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans (Vicia faba).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltlWisLw%3D&md5=6adb1e4611328360bab0e95ecbfe3310CAS |
Seeger M, Otto W, Flick W, Bickelhaupt F, Akkerman OS (2011) Magnesium compounds (Ullmann’s Encyclopedia of Industrial Chemistry) (Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim)
Seggewiss B, Jungk A (1988) Einfluss der kaliumdynamik im wurzelnahen boden auf die magnesiumaufnahme von pflanzen. Zeitschrift für Pflanzenernährung und Bodenkunde 151, 91–96.
| Einfluss der kaliumdynamik im wurzelnahen boden auf die magnesiumaufnahme von pflanzen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkt1aiu7Y%3D&md5=a1c2c96ee9eb9920768203425efd7495CAS |
Senbayram M, Bol R, Dixon L, Fisherd A, Stevens C, Quinton J, Fangueiro D (2015) Potential use of rare earth oxides as tracers of organic matter in grassland. Journal of Plant Nutrition and Soil Science 178, 288–296.
| Potential use of rare earth oxides as tracers of organic matter in grassland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkvVSnsbo%3D&md5=6686e0cdb0c9843867277d772c4b47d0CAS |
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15, 307–321.
| Magnesium transport and function in plants: the tip of the iceberg.Crossref | GoogleScholarGoogle Scholar |
Smith O (1977) ‘Potatoes: production, storing, processing.’ pp. 606–607. (Avi Publishing Co.: Westport, CT, USA)
Stahlberg S (1960) Release of magnesium from clay fractions of minerals and soils. Acta Agriculturae Scandinavica 10, 221–225.
Sumner ME, Farina PMW, Hurst VJ (1978) Magnesium fixation—a possible cause of negative yield responses to lime applications. Communications in Soil Science and Plant Analysis 9, 995–1007.
| Magnesium fixation—a possible cause of negative yield responses to lime applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXntFSgsg%3D%3D&md5=de31cec243985e34fc35ecb1f1540a17CAS |
Talburt W, Smith O (1997) ‘Potato processing.’ 4th edn (AVI-Van Nostrand Reinhold Co.: New York)
Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends in Plant Science 11, 405–412.
| Genomics-based approaches to improve drought tolerance of crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1KjurY%3D&md5=4e9771ef5e89af1f789c175facbac4afCAS | 16843036PubMed |
van der Heijden G, Legout A, Midwood AJ, Craig C, Pollier B, Ranger J, Dambrine E (2013) Mg and Ca root uptake and vertical transfer in soils assessed by an in situ ecosystem-scale multi-isotopic (26Mg & 44Ca) tracing experiment in a beech stand (Breuil-Chenue, France). Plant and Soil 369, 33–45.
| Mg and Ca root uptake and vertical transfer in soils assessed by an in situ ecosystem-scale multi-isotopic (26Mg & 44Ca) tracing experiment in a beech stand (Breuil-Chenue, France).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVyqsLjP&md5=81cc5ebbc6283ee4d4518dffaad96f80CAS |
Verbruggen N, Hermans C (2013) Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant and Soil 368, 87–99.
| Physiological and molecular responses to magnesium nutritional imbalance in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Gntrw%3D&md5=65c1e475e36c007c42bfb2bb32133978CAS |
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets- iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182, 49–84.
| Biofortification of crops with seven mineral elements often lacking in human diets- iron, zinc, copper, calcium, magnesium, selenium and iodine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVKhtbw%3D&md5=bc00d739e8f944c687ed9fe0c40fb43eCAS | 19192191PubMed |
Wilkinson SR, Welch RM, Mayland HF, Grunes DL (1990) Magnesium in plants uptake, distribution, function, and utilization by man and animals. Metal Ions in Biological Systems 26, 33–56.
Zörb C, Senbayram M, Peiter E (2014) Potassium in agriculture-status and perspectives. Journal of Plant Physiology 171, 656–669.
| Potassium in agriculture-status and perspectives.Crossref | GoogleScholarGoogle Scholar | 24140002PubMed |