Phenotypic and metabolic variation among spring Brassica napus genotypes during heat stress
C. B. Koscielny A , J. Hazebroek B and R. W. Duncan C DA DuPont Pioneer, Plant Breeding Research & Development, Carman, MB, R0G 0J0, Canada.
B DuPont Pioneer, Analytical & Genomics Technologies, 7300 NW 62nd Avenue, Johnston, IA, 50131-1004, USA.
C Department of Plant Science, University of Manitoba, 222 Agriculture Building, Winnipeg, MB, R3T 2N2, Canada.
D Corresponding author. Email: rob.duncan@umanitoba.ca
Crop and Pasture Science 69(3) 284-295 https://doi.org/10.1071/CP17259
Submitted: 24 February 2017 Accepted: 12 December 2017 Published: 14 February 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Heat stress can frequently limit the yield of Brassica napus L. grown in Canada because of the often unavoidable concurrence of high temperatures and flowering. Ten B. napus inbred genotypes, an open-pollinated B. napus commercial cultivar and a B. juncea genotype were grown in a greenhouse and subjected to two temperature regimes in a growth chamber for 14 days during flowering: control 22°C/10°C and high 31°C/14°C (day/night). Floral buds were sampled at the end of the 14-day treatments, and an untargeted metabolomic assessment was completed using gas chromatography–mass spectrometry. Flower duration, number of flowers, number of pods, biomass, number of seeds and seed weight were recorded. Yield was reduced by 55% in the heat treatment during winter and by 41% during the subsequent autumn experimental run. Of the 12 genotypes, five were classified as heat-tolerant and four as heat-susceptible based on the calculated heat susceptibility index across two experiments. In total, 25 metabolic markers were identified that discriminated between the heat-tolerant and -susceptible genotypes exposed to the heat treatment. The variation identified within this set of germplasm has provided evidence that variation exists within B. napus to enable genetic gain for heat tolerance.
Additional keywords: Brassicas, canola, metabolic marker, GC–MS.
Introduction
Heat stress is an important global abiotic stress that limits the production of many crops (Hall 1992; Mittler et al. 2012; Bita and Gerats 2013; Jha et al. 2014; Liu et al. 2015). Average temperatures have increased by 0.6°C in the past 100 years (Root et al. 2003), and with a projected rate of increase of 0.5°C–2.8°C by the end of the 21st Century (Meehl et al. 2005; Van Vuuren et al. 2008), breeding for thermotolerance will be critical to maintaining or increasing genetic gain.
Brassica napus L. is an economically critical oilseed grown globally (FAOSTAT 2013). In Canada alone, it has been reported to contribute Can$26.7 billion to the economy (Canola Council of Canada 2016). Brassica napus is a cool-season allotetraploid (AACC, 2n = 38) originating from two progenitor species, B. rapa L. (AA = 2n = 20), and B. oleracea L. (CC 2n = 18) (Nagaharu 1935). Research suggests that B. napus is most sensitive heat stress during gametogenesis and reproduction (Morrison 1993; Angadi et al. 2000; Young et al. 2004). Various studies have indicated that high-temperature stress can occur in B. napus at >25°C. These effects have been investigated using treatments as high as 35°C (Gan et al. 2004) and with control temperatures (day/night) of 20°C/18°C (Angadi et al. 2000) and 23°C/18°C (Young et al. 2004). Flowering of B. napus in western Canada occurs ~40–50 days after planting. Depending on planting date, the reproductive phase can align with the high temperatures in July, exacerbating the impact of heat (Kutcher et al. 2010).
An essential requirement to enable genetic gain in thermotolerance within B. napus is the identification of genetic variation. Thermotolerance variation between Brassica species has been reported, with B. rapa found to be the most sensitive followed by B. napus and B. juncea (Angadi et al. 2000). Annisa et al. (2013) reported genetic variation within a global set of B. rapa accessions. Although it may be possible to exploit the genetic variation for thermotolerance within other Brassica species and introgress heat tolerance to B. napus, this method could negatively affect favourable agronomic and quality traits required to meet canola quality classification. If genetic variation within the primary gene pool exists and exploited, this would expedite the process of improving thermotolerance within B. napus.
In attempting to discover variation within germplasm to a trait such as thermotolerance, it is important to remove underlying genetic effects that may not be related to the trait of interest. Fischer and Maurer (1978) developed a drought-susceptibility index that accounted for genetic differences between wheat genotypes under control v. water-limited environments. This index has since been utilised to measure variation in response to different abiotic stresses including heat in various crops (Bayuelo-Jiménez et al. 2002; Pradhan and Prasad 2015; Trapp et al. 2016).
Phenotyping abiotic stress traits can be resource-intensive (Araus and Cairns 2014). Because of the high costs associated with phenotyping, breeders often attempt to discover secondary or supplementary traits (Campos et al. 2004; Passioura 2012). Physiological traits such as carbon isotope discrimination (CID) have shown promise in the ability to screen wheat genotypes for water-use efficiency and yield simultaneously (Monneveux et al. 2006; Zhang et al. 2014). Under periods of heat stress, plants increase transpiration in an effort to maintain an optimum temperature (Crawford et al. 2012). Given the association between CID and stomatal conductance (Fischer et al. 1998), the relationship between heat stress and CID in B. napus should be explored.
Abiotic stresses such as salt, low temperature, high temperature and drought have been shown to alter dramatically the metabolome of multiple species (Kaplan et al. 2004; Rizhsky et al. 2004; Almeselmani et al. 2006; Babu and Rangaiah 2008; Guy et al. 2008). Untargeted metabolomic studies have shown promise in their ability to discover metabolic markers in response to heat stress in Agrostis (bentgrass) (Xu et al. 2013) and in rice (Li et al. 2015). Exploring this metabolic variation may identify metabolic markers that could then be utilised to increase the accuracy and/or capacity of phenotypic screening (Peng et al. 2015). In a review, Fernandez et al. (2016) list the several metabolic markers from grain yield under drought stress in maize (Obata and Fernie 2012) to chip quality in potatoes (Steinfath et al. 2010) that have the potential to enhance phenotypic data and enable earlier selection decisions.
We conducted a greenhouse and growth chamber study, with the aims of (i) determining whether genetic variation exists within spring B. napus in response to heat stress during reproduction, and (ii) identifying potential secondary or supplementary traits that could be used in a high-throughput manner to identify genotypes with increased thermotolerance. The phenotypic, physiological and metabolic measurements will provide a path forward for future thermotolerance research in B. napus.
Materials and methods
Experimental material, design and treatments
Twelve genotypes were used in the research: 11 spring B. napus genotypes comprising five ogu INRA restorer lines (PM03, PM63, PM68, PM69, PM88), five maintainer lines (PB27, PB36, PB56, PB82, PB98) and one commercial open-pollinated cultivar (46A65); and a B. juncea genotype (45J10). The plants were sown in the greenhouse at the University of Manitoba, Winnipeg, MB, Canada, and the experimental treatments were conducted in a growth chamber (Econair GRC-10; BioChambers, Winnipeg, MB).
A split-plot experimental design with five replicates and two temperature treatments was used for this controlled environment experiment. Temperature treatment was considered the main effect and genotype the subplot, with each plant treated as a replicate. Owing to space constraints in the growth chamber (the same growth chamber was used in all cases), the treatments and experiments were planted sequentially in the greenhouse. Therefore, for the first (winter) experimental run, plants were planted on 6 January 2014 and the control plants on 24 January 2014. The second (autumn) experimental run was planted during the subsequent autumn, with the controls planted on 8 September 2014 and the heat-treatment plants on 15 October 2014. Plants were transferred to the growth chamber for 14 days for treatments.
The two growth-chamber treatments were control 22°C/10°C and heat stress 31°C/14°C (day/night) (Fig. 1), with a photoperiod of 16 h day/8 h night and a photosynthetic photon flux density ~432 µmol m–2 s–1.
Greenhouse settings were photoperiod 16 h day/8 h night, with average temperatures of 23°C/20°C and 22°C/19°C (day/night) for the winter and autumn experiments, respectively.
Plant husbandry
Genotypes were planted in a growth room into germinating mix (Sunshine Mix #4; Sun Gro Horticulture, Agawam, MA, USA), and at the two-leaf stage, five uniform plants of each genotype were transplanted into five 1.5-L pots and placed in a green house. Each pot was filled with 2 : 2 : 1 soil : sand : peat moss. A water solution (2.8 g L–1) of 20N-20P-20K-0S fertiliser (20% total N, 20% available P2O5, 20% soluble K2O, 0% S) (Plant Products, Leamington, ON) was applied at the cotyledon stage, followed by 5.6 g L–1 of 10N-52P-10K-0S at transplanting, with a final application of 2.8 g L–1 of 20N-20P-20K-0S at the rosette stage. All fertiliser solutions were applied until the soil reached field capacity. Intercept 60WP greenhouse insecticide (active ingredient 60% imidacloprid; Bayer Crop Science, Calgary, AB) was used to control piercing/sucking insects (application concentration 0.13 g L–1, 60 mL per pot).
Plants were watered to field capacity every second day until the rosette stage, at which time they were watered daily. All plants were grown in the greenhouse until BBCH growth stage 53 (buds at the same height as the newest leaves) (Hess et al. 1997). When a plant had reached BBCH 53, it was transferred from the greenhouse to the growth chamber for 14 days; this ensured that all plants entered the growth chamber at the same growth stage. During the growth-chamber treatments, plants were watered in the morning and supplemental watering occurred in the afternoon to eliminate drought as a potential limiting factor. At the end of the 14-day treatment, plants were transferred back to the greenhouse. At physiological maturity (seeds on the main raceme starting to change colour), pots were watered every second day until all seeds had turned black, at which point watering ceased.
Data collection
Number of days to first flower was measured from planting date to first open flower, and number of days to last flower was recorded based on planting date until the last flower had opened. Based on this information, flower duration was calculated by subtracting the number of days to first flower from number of days to last flower. When plants were removed from the growth chamber, all racemes were tagged at the point in which the flowers were open. Flowers and pods that formed below those tags were labelled as growth chamber (GC) and flowers and pods that formed above the tags were labelled as greenhouse (GH). Number of flowers indicates all flowers that originated on the plant, including the developed pods. Siliques that contained at least one seed were counted as pods. Pod : flower ratios were calculated to give the proportion of flowers that successfully produced seed. Pollen numbers were counted by collecting the anthers from five dehiscing flowers from each plant at the end of the 14-day growth-chamber treatment. These anthers were submerged in 1 mL distilled water in a 2-mL microtube and shaken to release pollen. Immediately following mixing, a 10-µL aliquot of solution was placed on a hemocytometer. Four counts were taken within the 4 × 4 grids in each corner by using an Olympus CH2 light microscope (Olympus, Tokyo), and the mean was recorded.
Seed yield (g) was measured separately for the pods formed labelled as GC and GH, and the total seed yield was the combined weight. Seeds were also counted to determine the number of seeds for both the GC and GH portions of the plants. Number of seed per pod was calculated by dividing number of seeds by number of pods, and 1000-kernel weight (g) was calculated by dividing the yield by number of seeds and multiplying by 1000. Biomass (g) was measured by taking the weight of aboveground biomass after the plant had completely dried in the greenhouse; the seed weight was then subtracted from this value. Harvest index was calculated by dividing seed yield by biomass.
Heat susceptibility and heat intensity indices
Heat susceptibility index (HSI) and heat intensity index (HII) were calculated for seed yield and yield components conforming to the model outlined by Fischer and Maurer (1978). For HSI:
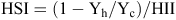
where Yh is the response of the genotype when exposed to high temperatures in the growth chamber and Yc is the response of the same genotype when exposed to the control temperatures in the growth chamber. For HII:
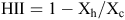
where Xh is the average of all entries when exposed to high temperatures in the growth chamber and Xc is the average response of all entries when exposed the control temperatures. If a genotype received an HSI >1 then it was more sensitive to the heat treatment than the mean response of all entries. If the index was <1 then the genotype was more tolerant than the mean response of all entries.
Carbon isotope discrimination
Carbon isotope composition (δ13C) for all genotypes and all treatments was measured on the stem tissue collected at harvest. Farquhar and Richards (1984) demonstrated that stem, leaf and seed samples, while differing in δ13C concentration, were highly correlated with one another, indicating that any plant tissue has the potential to be utilised in measuring δ13C. Samples were ground into a fine powder, then a 3.5-µg sample was placed in a tin capsule and sent to the University of Saskatchewan where the δ13C was determined (Bichel 2013). The δ13C was calculated by comparing the 13C : 12C ratio on each sample (Rs) to the Pee Dee Belemnite (PDB) international standard (RPDB), using the formula:

The CID was determined by using the formula below and was the difference between the δ13C in the plant (δ13Cp) and the δ13C of the air (δ13Ca), with δ13Ca assumed to be –8% (Zhang et al. 2014):
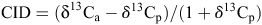
Metabolomics
Metabolites were extracted from growing buds with dry weights of 1.86–16.81 mg (4.99 mg mean), with differences in weight mainly due to differential bud size. Metabolites were extracted and analysed following an established protocol (Asiago et al. 2012) with minor deviations. Samples were analysed with an 6890A gas chromatograph (Agilent, Palo Alto, CA, USA) coupled to a Pegasus HT time-of-flight mass spectrometer (LECO, St. Joseph, MI, USA). The detector voltage was set at 1625–1675 V. Genotypes were distributed across analytical batches to ensure that genotype was not confounded with analytical batch (day of analysis).
Raw Leco.peg data files were converted into.netcdf (Andi) formats by using ChromaTof software version 4.50.8.0 (LECO) and were processed with Refiner MS software version 8.1 (Genedata, Basel, Switzerland) as described in Asiago et al. (2012). All peaks within the same retention index window (0.75 retention index units) that corresponded to the same compound were combined into a one group based on normalised Euclidean distance measurements between the individual intensity profiles across all samples. The resulting data matrices consisted of intensities for each of the 264 m/z value and retention index combinations (peak groups) for each sample. Subsequent data normalisation and multivariate statistical analyses were performed with Genedata Analyst version 8.1, MATLAB version R2013a (MathWorks, Natick, MA, USA), and PLS Toolbox version 7.8.2 (Eigenvector Research, Wenatchee, WA, USA).
Statistical analyses
Homogeneity of variance and normality of distribution were tested using ASReml 3 (VSN International, Hemel Hempstead, UK) and entries were removed from the analysis if the total seed yield of an entry was >3 standard deviations from its mean. Data was analysed by using ASReml 4 to calculate best linear unbiased predictions (BLUPs) with the following model:
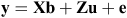
where the data vector y represents the observed phenotype, b is the fixed terms for the matrix X, u is the random terms for matrix Z, with e representing the error matrix. Overall mean and treatments were fixed effects, while experimental run (winter, autumn), genotype, experiment × treatment, treatment × genotype and experiment × treatment × genotype were treated as random effects along with replicate, which was nested within experiment and treatment. The selection of model terms was assessed using the Akaike information criterion. Microsoft Excel (Microsoft, Redmond, WA, USA) was used to calculate the Pearson r correlation between seed yield and yield components. Regressions were calculated using Microsoft Excel 2010 and R (R Foundation for Statistical computing, Vienna).
Results
Effect of treatment on yield and yield components
The main treatment effect was not significant for seed yield when both experimental runs (winter, autumn) were combined; however, there was a significant experiment × treatment × genotype interaction when tested with a likelihood ratio test (21.2, P < 0.005). An assessment of the residuals v. the fitted values was homogenous across both experimental runs; therefore, the combined analysis was used to investigate further the two 3-way interactions (Gilmour et al. 2009). When referring to the winter or autumn experimental runs henceforth, it will be to refer to the treatment × genotype interactions within these experimental repetitions as determined by the combined analysis.
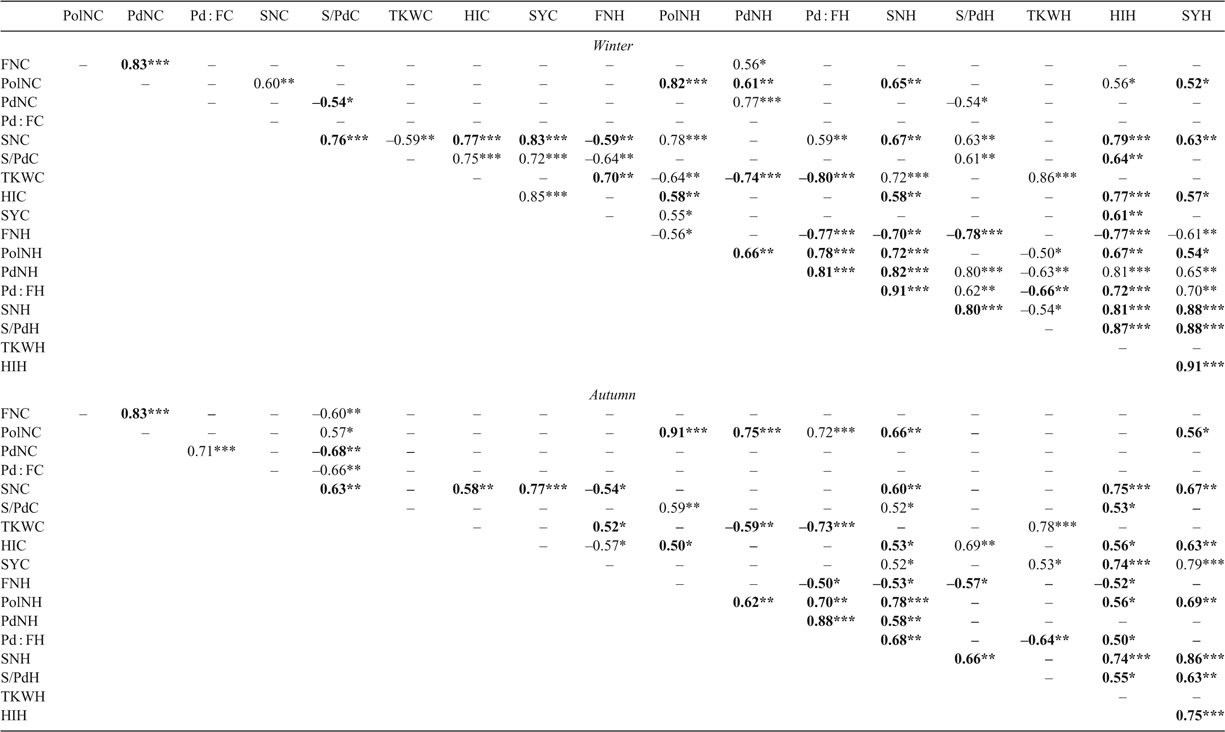
Within each 3-way interaction, all traits collected were significantly affected by the heat treatment, with the exception of flower duration and 1000-kernal weight in the autumn experimental run (Table 1). Trends across treatments were consistent for all traits except biomass. Flower duration was longer in the heat treatment for both experimental runs (by 4 days in winter and 1 day in autumn), due to a greater number of flowers produced on the heat-treated plants (43 in winter and 25 in autumn). However, this increased number of flowers did not translate into more pods, and there were fewer pods on the heat-treated plants (by 34 in winter and 22 in autumn) and a lower pod : flower ratio (decrease of 0.25 in winter and 0.16 in autumn). Pollen number declined significantly in the heat treatment for both experimental runs, dropping by 56% in the winter experiment and 44% in the autumn experiment. Number of seeds per pod decreased in both experiments, by seven seeds per pod. Seed number declined in the heat treatment in both experimental runs, by 68% (winter) and 53% (autumn). The 1000-kernal weight increased in the heat treatment, by 45% in winter and 24% in autumn. Total seed yield declined by 55% (winter) and 41% (autumn) for the heat-treated plants (Table 1). The yield from flowers that opened in the growth chamber contributed to 51% and 52% of the total yield for the control treatment in the winter and autumn runs respectively, but only 37% and 43% for the heat treatment.
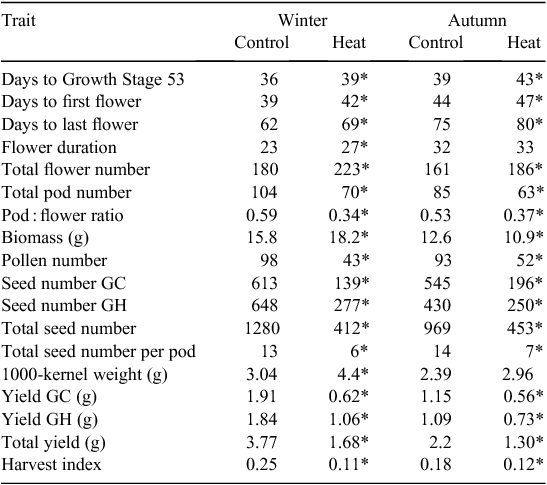
|
Yield component analysis
Total yield had a significant positive relationship between the both treatments within the autumn experimental run (Pearson r = 0.65). There was no significant relationship for total yield between treatments in the winter experimental run, indicating that genotypes were responding differently across treatments (Table 2). Total seed number and harvest index were related to total yield in all treatments and experimental runs. Interestingly, pollen number from the control treatment was significantly related to total yield, pod number, pod : flower ratio and seed number within the heat treatment of both experimental runs, but not to the same traits within the control treatment with the exception of pod : flower ratio in the winter run.
Heat susceptibility index
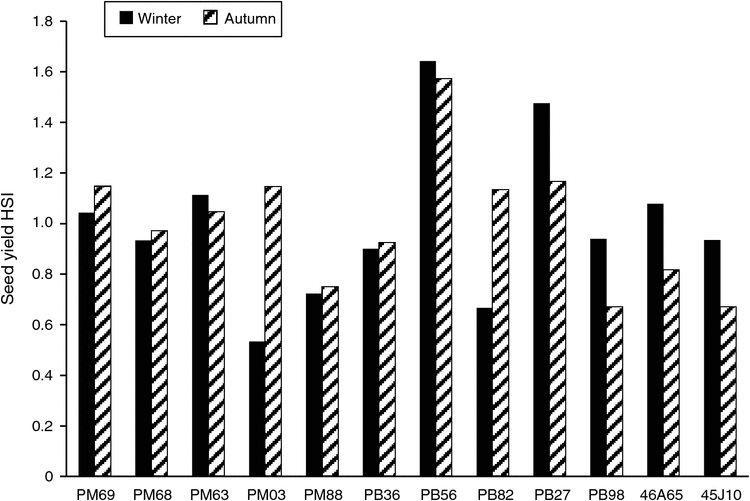
The HII was for 56% the winter experimental run and 41% for autumn. Total seed yield HSI values represent the impact of the heat stress treatment on seed yield within the context of the mean treatment effect on all genotypes (Fig. 2). The relationship of the HSI values across both experimental runs was r = 0.50 (P < 0.1), with entries 45J10, PB98, PB36, PM68 and PM88 all having HSI <1 for total seed yield in both runs, indicating a greater thermotolerance than the mean of the population. Entries PB27, PB56, PM63 and PM69 all had HSI >1 for total seed yield during both runs, indicating lower thermotolerance than the mean of the population. Three entries (PB82, PM03, 46A65) did not exhibit a consistent HSI across both experimental runs and they were therefore left unclassified.
To compare the ability of genotypes to compensate, total seed yield HSI was plotted against GC seed yield HSI for both experimental runs (Fig. 3). PB98 and PM88 were located in the lower left quadrant for both winter (Fig. 3a) and autumn (Fig. 3b), which demonstrates their ability to set seed during the heat treatment and minimise the impact of the stress on total yield compared with the rest of the group. In both runs, PM68 had a GC HSI of 1.26 and 1.49, which made it the second-worst performing genotype in the group; however, its total HSI was 0.51 and 0.97 in winter and autumn, respectively. This indicates that once the heat stress was removed, PM68 was able to compensate by setting seed in the greenhouse. PB56 and PM63 were in the upper right quadrant for both winter and autumn, indicating that they were less able to set seed during the heat treatment and unable to compensate for this reduction in seed set when moved back to the greenhouse. In the autumn run (Fig. 3b), PB36 was able to set more seed during the heat stress treatment than during control treatment in the growth chamber, with an HSI of –0.18, and was classified as heat-tolerant overall for both experimental runs. PM03 and PB82 showed the most dramatic differences between both runs, whereas PB98, PM88, PB56 and PM63 each remained within the same quadrant across both runs.
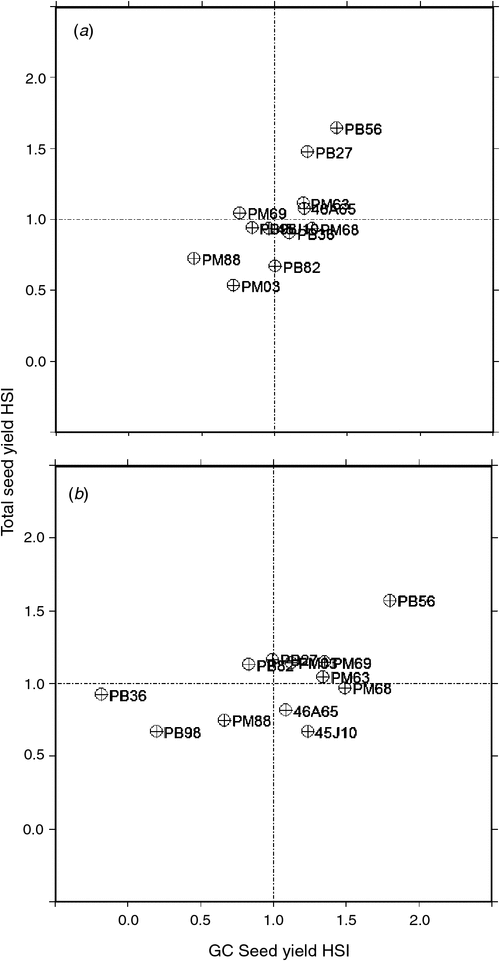
|
Total HSI was used to apply a final classification to the genotypes. The abilities to tolerate the heat stress and/or compensate once the stress is removed are of value in dealing with transient heat stress. Entries 45J10, PB98, PB36, PM68 and PM88 were classified as heat-tolerant, and entries PB27, PB56, PM63 and PM69 were classified as heat-susceptible. These classifications were used for the subsequent metabolomic data analysis.
Carbon isotope discrimination
There was significant main effect for CID (P < 0.05) and an entry × experiment interaction (P < 0.005), but the treatment × entry × year interaction was not significant. The relationship of CID across the treatments in both years was significant, with Pearson r values of 0.99 and 0.98, demonstrating consistency across treatments. All treatment combinations were reviewed to determine whether there were significant relationships between seed yield and CID within these genotypes, but none were found. Total seed number and 1000-kernel weight were significant across both experimental runs and treatments CID (Supplementary material table 1, available at the journal’s website).
Floral bud metabolite content
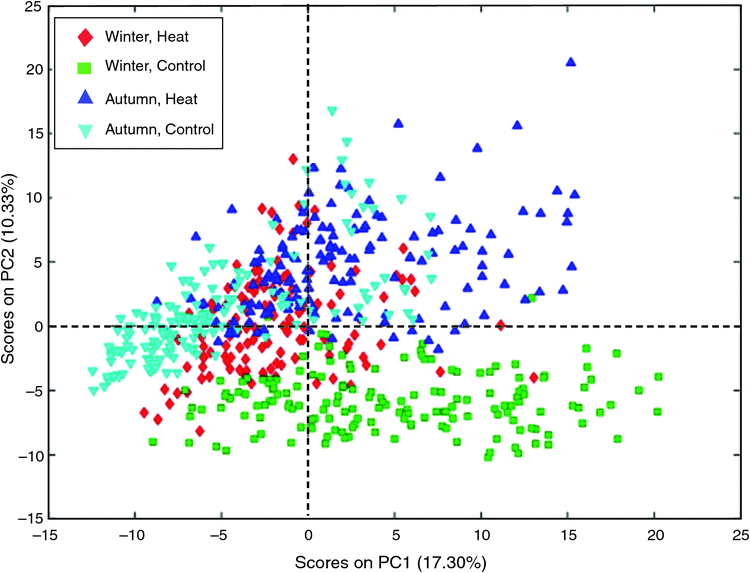
An unsupervised principal component analysis (PCA) with floral bud GC–MS metabolomics data was able to identify a significant treatment effect in both runs, with 27.63% of the total variation in the data (17.30% PC1 and 10.33% PC2) (Fig. 4). There was little overlap in the scores plot between the control-treated plants of both runs; however, the heat treatment caused the metabolomic profile of entries from the two runs to converge (Fig. 4).
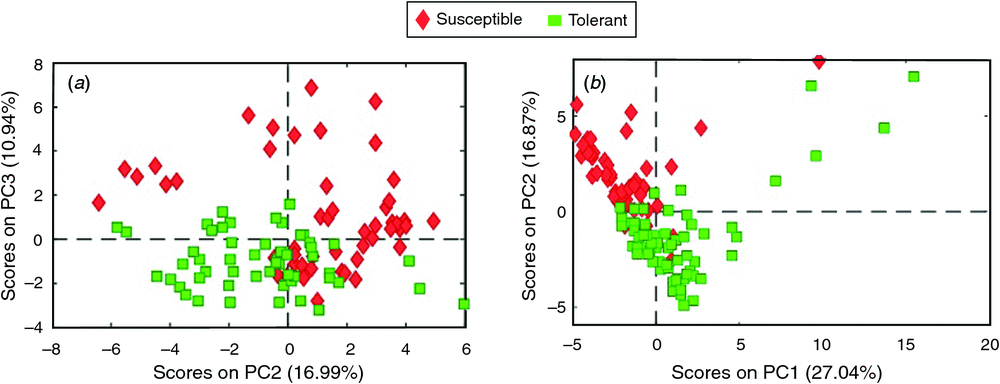
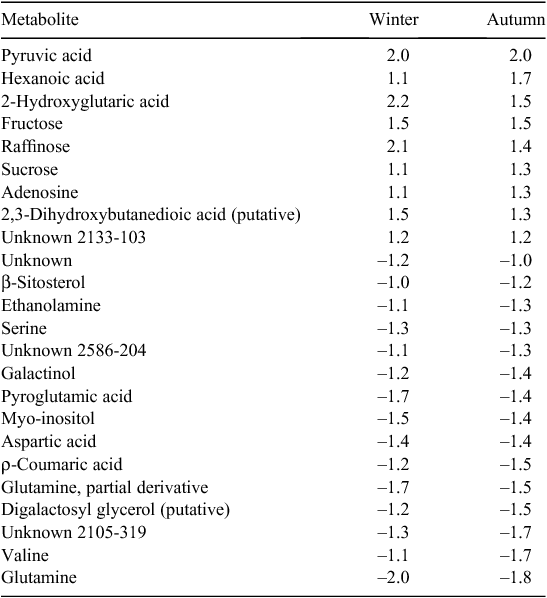
Principal component analysis using all metabolites failed to discriminate lines based on their heat-tolerance classification in the winter run. However, PCA analysis gave a distinct grouping of the tolerant and susceptible genotypes within the heat treatment in the autumn experimental run, although this classification was apparent only with higher principal components (PC4 5.53% and PC5 4.34%) (data not shown). The top 24 loadings (metabolites) that were distinct between the tolerant and susceptible lines were identified (Table 3). These 24 metabolites alone were then used in a subsequent PCA for each run separately (heat treatment only) (Fig. 5). In the autumn experimental run, the tolerant and susceptible genotypes were distinguished with PC1 (27.04%) and PC2 (16.87%), as expected. However, these top 24 loadings derived from the autumn run were also effective in separating the tolerant and susceptible genotypes in the winter experimental run by PC2 (16.99%) and PC3 (10.94%). These results depict a common association between heat tolerance and a limited set of metabolites, and may explain why PCA failed to distinguish heat-tolerant from heat-susceptible genotypes when using all metabolomics data in the winter experimental run.
|
The fold change in relative abundance of each of the 24 aforementioned metabolites between the heat-tolerant and heat-susceptible genotypes is detailed in Table 3. Nine metabolites increased and 15 decreased in the heat-tolerant genotypic class compared with the heat-susceptible genotypic class. Fold changes for each metabolite were consistent across experimental runs, with a maximum fold change difference of 0.7. These metabolites represent diverse pathways, strongly suggesting a broad differential metabolic response of the buds to heat stress between the two groups of plants.
Discussion
The overall effect of the high temperature treatment on the 12 genotypes was evident in the yield reduction, with genotypic variability evident for total seed yield HSI. The discovery of informative metabolic markers for heat-stress tolerance shows promise and warrants further investigation. This information has the potential to expedite the development of increased heat tolerance within B. napus by allowing breeders to exploit this variation within the primary gene pool, thereby minimising the disruption of carefully selected genetic structures. The metabolic markers provide the ability to enhance phenotypic information to improve overall selection for increased heat tolerance.
The effect of experimental run was significant when the data were combined; however, homogeneity of the residuals v. fitted values allowed the data to be combined, providing increased statistical power in detecting differences within the 3-way interaction (treatment × genotype × experiment). The consistency of trends across both experimental runs for phenotypic traits provided evidence that the impact of the heat treatment on all genotypes was similar in the winter and autumn runs. The consistent susceptible and tolerant classification of nine genotypes across the two experimental runs demonstrates that the majority of the genotypes exhibit a consistent response to heat stress.
When yield components were compared within and across treatments, seed number and harvest index were related to seed yield, which aligns with previous data within B. napus (Diepenbrock 2000). The increase in the number of days to flower ending and consequently flower duration is contrary to some field studies (Jumrani and Bhatia 2014), but aligns with indoor heat-stress studies conducted on B. napus (Angadi et al. 2000; Ramsahoi 2013). This increased flower duration is likely due to fewer seeds setting, which minimised the sink strength during control growing conditions in the greenhouse. Numerous studies have indicated that high temperatures affect the pollen production in soybean and in rice (Prasad et al. 2006; Salem et al. 2007; Rang et al. 2011). The relationship between pollen number in the control treatment and yield in the heat treatment suggests that pollen number may be a limiting factor in heat-stressed B. napus. The lack of relationship between pollen number in the control treatment and control yield as well as pollen number in the heat treatment and yield in the heat treatment indicates that other limiting factors are playing a role in limiting yield. If an automated, high-throughput method could be utilised for increasing the rate of measuring pollen number (Costa and Yang 2009), screening genotypes for pollen number may provide insight into their ability to withstand high temperatures. Because of the high degree of variability reported in the literature regarding pollen germination in B. napus (20%, Morrison et al. 2016; 37%, Singh et al. 2008; 59.2%, Young et al. 2004), this trait was not explored.
This is the first report of HSI within B. napus and it clearly demonstrates that genetic variation exists among B. napus genotypes. Brassica juncea had previously been reported as more heat-tolerant than B. napus (Woods et al. 1991), and although the present data demonstrate this genotype to be better than the mean of the population, some B. napus genotypes showed similar or better heat tolerance. The use of HSI provided the ability to separate overall genetic potential from the response to heat stress by indicating the magnitude of the response to the treatment in relation to the overall population response. This collection of genotypes demonstrated that it is possible to apply selection pressure for heat tolerance, enabling breeders to make positive genetic gain.
Genotypic differences for CID have been reported in numerous crop species including B. napus (Matus et al. 1995; Chen et al. 2011; Kottmann et al. 2014; Dhanapal et al. 2015; Mora et al. 2015). Easlon et al. (2013) demonstrated a relationship between CID and water-use efficiency in Arabidopsis; however, given the absence of any relationship between yield and CID in either the control or heat-stress treatment in this study, further exploration into the relationship of CID to heat stress appears unwarranted. Previous research in B. napus exploring the relationship between yield and CID was also unable to demonstrate any significant relationship (Matus et al. 1995).
The metabolome of heat-treated plants enabled classification of heat tolerance among the B. napus genotypes assayed, and this classification of heat tolerance with selected metabolic markers was apparent in both experimental runs. The differential expression of each of these metabolites between susceptible and tolerant genotypes was remarkably similar in the two experimental runs. Together, these consistencies indicate the robustness of the response and potential utility during the selection of tolerant genotypes.
Multiple chemical classes of metabolites were represented among the top loadings for heat tolerance within the heat treatment. Among the nine metabolites with higher abundances in tolerant genotypes were two simple sugars, fructose and sucrose, along with raffinose, a common trisaccharide. Of the 15 metabolites with lower abundances with heat tolerance, seven were amino acids and two biosynthetic precursors of raffinose, myo-inositol and galactinol. Taken together, these results suggest that heat tolerance among these Brassica genotypes is associated with elevated simple sugar content rather than amino acid synthesis and/or protein breakdown. The ability of plants to alter their metabolome in response to different abiotic stresses has been reported in several species (Rizhsky et al. 2004; Wahid 2007; Witt et al. 2012). Sun et al. (2016) differentiated metabolic profiles in maize depending on the stress imposed and, from multiple sampling time points, elucidated the most discriminatory sampling time for the stress imposed. The present work provides evidence that the metabolomic information collected from plants may provide the ability to determine not only the differences among genotypes, but also more accurately define the severity and timing of the stress to aid in the interpretation of the phenotypic response. Of the elevated metabolites within our heat-tolerant genotypes, most have been previously reported. Fructose and sucrose have been shown to be involved in heat-stress response within tomato (Sato et al. 2006) and chickpea (Kaushal et al. 2013), with each declining when plants were subjected to heat stress. Similar to our results on the floral buds, Firon et al. (2006) demonstrated that pollen collected from tomato plants under high temperatures exhibited different sugar concentrations depending upon sensitivity to heat, with the more tolerant genotypes maintaining a higher sugar content within the anther wall than the susceptible genotypes. Raffinose has also been reported to be involved in protecting plants against heat stress by minimising oxidative damage (Nishizawa et al. 2008). Heat stress is known to increase free amino acid content (Guy et al. 2008; Obata and Fernie 2012); the fact that seven of the metabolites showing an increase in the susceptible genotypes were amino acids suggests that these susceptible genotypes experienced greater stress with the heat treatment imposed. The ability to accumulate or maintain osmoprotectants and primary energy sources and minimise the breakdown of proteins to amino acids within the floral buds may demonstrate the metabolic response necessary to minimise the impact of heat stress for tolerant genotypes (Wahid et al. 2007). The aforementioned studies that differentiated the metabolome of genotypes or species based on their response to high temperatures screened only single susceptible and tolerant lines. Even within a species, different genotypes under control conditions have been reported to show detectable differences within their respective metabolomes (Asiago et al. 2012; Smolikova et al. 2016). The present research was able to link a group of potential markers associated with heat tolerance by using five tolerant and four susceptible genotypes, which further strengthens the potential use of these markers across a broader set of germplasm.
Conclusion
There was a clear trend among a subset of the genotypes across both experimental runs with regard to heat tolerance. This variation should be explored further and exploited before introgression of more exotic germplasm or consideration of transgenic approaches. Use of elite B. napus genotypes would avoid the pitfalls of inadvertent introgression of deleterious alleles or stringent regulatory implications. Further studies need to be conducted utilising the most tolerant and sensitive lines in this set of material to determine the main cause of the heat tolerance or susceptibility within B. napus.
In exploiting the existing thermotolerance variation, the use of metabolic markers can be further explored to supplement phenotypic data and enhance genetic gain. The area of metabolic markers needs further examination by different analytical methods (i.e. liquid chromatography–MS) to capture information on an expanded group of metabolites, as well as searching for metabolite quantitative trait loci to increase predictive power and throughput while decreasing labour and costs. Through continued exploration of the metabolome, we may be able not only to identify metabolic markers present in stressed plants, but also to define precisely the level of stress and potentially discover constitutive metabolic markers that predict performance of a genotype to an abiotic stress even when the stress is not imposed.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by DuPont Pioneer. Sincere appreciation to Judith Nugent-Rigby, Sarika Saini, Victoria Mungall, Teresa Harp, Stuart Gardner, Raymond Cowley and Erin Lemky for their technical support in this research.
References
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Science 171, 382–388.| Protective role of antioxidant enzymes under high temperature stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmslyksrc%3D&md5=2c89bf4aacfc6c98484c5b229ad762b6CAS |
Angadi SV, Cutforth HW, Miller PR, McConkey BG, Entz MH, Brandt SA, Volkmar KM (2000) Response of three Brassica species to high temperature stress during reproductive growth. Canadian Journal of Plant Science 80, 693–701.
| Response of three Brassica species to high temperature stress during reproductive growth.Crossref | GoogleScholarGoogle Scholar |
Annisa , Chen S, Turner NC, Cowling WA (2013) Genetic variation for heat tolerance during the reproductive phase in brassica rapa. Journal of Agronomy and Crop Science 199, 424–435.
| Genetic variation for heat tolerance during the reproductive phase in brassica rapa.Crossref | GoogleScholarGoogle Scholar |
Araus JL, Cairns JE (2014) Field high-throughput phenotyping: The new crop breeding frontier. Trends in Plant Science 19, 52–61.
| Field high-throughput phenotyping: The new crop breeding frontier.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1CrtbjL&md5=280628e0fdc824e32e3213d18f78e6b5CAS |
Asiago VM, Hazebroek J, Harp T, Zhong C (2012) Effects of genetics and environment on the metabolome of commercial maize hybrids: A multisite study. Journal of Agricultural and Food Chemistry 60, 11498–11508.
| Effects of genetics and environment on the metabolome of commercial maize hybrids: A multisite study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsF2ktLzJ&md5=96c2c6dc45d036eeb5139779f42791acCAS |
Babu RN, Rangaiah DV (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Australian Journal of Crop Science 2, 40–48.
Bayuelo-Jiménez JS, Debouck DG, Lynch JP (2002) Salinity tolerance in Phaseolus species during early vegetative growth. Crop Science 42, 2184–2192.
| Salinity tolerance in Phaseolus species during early vegetative growth.Crossref | GoogleScholarGoogle Scholar |
Bichel A (2013) Applied soybean and maize residue contributions to soil organic matter in a temperate soybean/maize intercropping system. MSc Dissertation, University of Waterloo, Waterloo, ON, Canada.
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 4, 273
| Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops.Crossref | GoogleScholarGoogle Scholar |
Campos H, Cooper M, Habben JE, Edmeades GO, Schussler JR (2004) Improving drought tolerance in maize: A view from industry. Field Crops Research 90, 19–34.
| Improving drought tolerance in maize: A view from industry.Crossref | GoogleScholarGoogle Scholar |
Canola Council of Canada (2016) An industry inspired. 2016 Annual Report. Canola Council of Canada. Available at: http://www.canolacouncil.org/media/584651/ccc-ar2016_inspired.pdf
Chen J, Chang SX, Anyia AO (2011) The physiology and stability of leaf carbon isotope discrimination as a measure of water-use efficiency in barley on the Canadian prairies. Journal of Agronomy & Crop Science 197, 1–11.
| The physiology and stability of leaf carbon isotope discrimination as a measure of water-use efficiency in barley on the Canadian prairies.Crossref | GoogleScholarGoogle Scholar |
Costa CM, Yang S (2009) Counting pollen grains using readily available, free image processing and analysis software. Annals of Botany 104, 1005–1010.
| Counting pollen grains using readily available, free image processing and analysis software.Crossref | GoogleScholarGoogle Scholar |
Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA (2012) High temperature exposure increases plant cooling capacity. Current Biology 22, R396–R397.
| High temperature exposure increases plant cooling capacity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntlaqtrY%3D&md5=a2d0735d2ee47923ed056851be8f7ed7CAS |
Dhanapal AP, Ray JD, Singh SK, Hoyos-Villegas V, Smith JR, Purcell LC, King CA, Cregan PB, Song Q, Fritschi FB (2015) Genome-wide association study (gwas) of carbon isotope ratio (δ13c) in diverse soybean [Glycine max (l.) Merr.] genotypes. Theoretical and Applied Genetics 128, 73–91.
| Genome-wide association study (gwas) of carbon isotope ratio (δ13c) in diverse soybean [Glycine max (l.) Merr.] genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFWhu77I&md5=afdc2e7504a0e8cf2337c241c9e69963CAS |
Diepenbrock W (2000) Yield analysis of winter oilseed rape (Brassica napus l.): A review. Field Crops Research 67, 35–49.
| Yield analysis of winter oilseed rape (Brassica napus l.): A review.Crossref | GoogleScholarGoogle Scholar |
Easlon H, Nemali K, Richards J, Hanson D, Juenger T, McKay J (2013) The physiological basis for genetic variation in water use efficiency and carbon isotope composition in Arabidopsis thaliana. Photosynthesis Research 119, 1–11.
FAOSTAT (2013) Crops. Food and Agriculture Organization of the United Nations. Available at: http://faostat3.fao.org/browse/Q/QC/E
Farquhar G, Richards R (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Functional Plant Biology 11, 539–552.
Fernandez O, Urrutia M, Bernillon S, Giauffret C, Tardieu F, Le Gouis J, Langlade N, Charcosset A, Moing A, Gibon Y (2016) Fortune telling: Metabolic markers of plant performance. Metabolomics 12, 158
| Fortune telling: Metabolic markers of plant performance.Crossref | GoogleScholarGoogle Scholar |
Firon N, Shaked R, Peet MM, Pharr DM, Zamski E, Rosenfeld K, Althan L, Pressman E (2006) Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Scientia Horticulturae 109, 212–217.
| Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmt1yms7o%3D&md5=e500a4c85b75c42725c7337beb16842fCAS |
Fischer R, Maurer R (1978) Drought resistance in spring wheat cultivars. I. Grain yield responses. Australian Journal of Agricultural Research 29, 897–912.
| Drought resistance in spring wheat cultivars. I. Grain yield responses.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475.
| Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies.Crossref | GoogleScholarGoogle Scholar |
Gan Y, Angadi SV, Cutforth H, Potts D, Angadi VV, McDonald CL (2004) Canola and mustard response to short periods of temperature and water stress at different developmental stages. Canadian Journal of Plant Science 84, 697–704.
| Canola and mustard response to short periods of temperature and water stress at different developmental stages.Crossref | GoogleScholarGoogle Scholar |
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ‘ASReml user guide release 3.0.’ pp. 278–355. (VSN International: Hemel Hempstead, UK)
Guy C, Kaplan F, Kopka J, Selbig J, Hincha DK (2008) Metabolomics of temperature stress. Physiologia Plantarum 132, 220–235.
Hall AE (1992) Breeding for heat tolerance. In ‘Plant breeding reviews’. pp. 129–168. (John Wiley & Sons: New York)
Hess M, Barralis G, Bleiholder H, Buhr L, Eggers T, Hack H, Stauss R (1997) Use of the extended bbch scale - general for the descriptions of the growth stages of mono- and dicotyledonous weed species. Weed Research 37, 433–441.
| Use of the extended bbch scale - general for the descriptions of the growth stages of mono- and dicotyledonous weed species.Crossref | GoogleScholarGoogle Scholar |
Jha UC, Bohra A, Singh NP (2014) Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breeding 133, 679–701.
| Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance.Crossref | GoogleScholarGoogle Scholar |
Jumrani K, Bhatia VS (2014) Impact of elevated temperatures on growth and yield of chickpea (Cicer arietinum L.). Field Crops Research 164, 90–97.
| Impact of elevated temperatures on growth and yield of chickpea (Cicer arietinum L.).Crossref | GoogleScholarGoogle Scholar |
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology 136, 4159–4168.
| Exploring the temperature-stress metabolome of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtVakug%3D%3D&md5=429bd9bee4d8b155319ab0d37b8c1aa8CAS |
Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique KHM, Nayyar H (2013) Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Functional Plant Biology 40, 1334–1349.
| Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslyqt7fL&md5=85491360d5330dc8f614ac4c7061ba1aCAS |
Kottmann L, Schittenhelm S, Giesemann A (2014) Suitability of carbon isotope discrimination, ash content and single mineral concentration for the selection of drought-tolerant winter rye. Plant Breeding 133, 579–587.
| Suitability of carbon isotope discrimination, ash content and single mineral concentration for the selection of drought-tolerant winter rye.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslaltLbO&md5=2c7c6335e7d7fc9292515244b535bdeeCAS |
Kutcher HR, Warland JS, Brandt SA (2010) Temperature and precipitation effects on canola yields in Saskatchewan, Canada. Agricultural and Forest Meteorology 150, 161–165.
| Temperature and precipitation effects on canola yields in Saskatchewan, Canada.Crossref | GoogleScholarGoogle Scholar |
Li X, Lawas LMF, Malo R, Glaubitz U, Erban A, Mauleon R, Heuer S, Zuther E, Kopka J, Hincha DK, Jagadish KSV (2015) Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant, Cell & Environment 38, 2171–2192.
| Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFGrt77K&md5=57da46de31eb8bdf5c9bda0bb4faf60aCAS |
Liu J, Feng L, Li J, He Z (2015) Genetic and epigenetic control of plant heat responses. Frontiers in Plant Science 6, 267
Matus A, Slinkard A, Van Kessel C (1995) Carbon isotope discrimination: Potential for indirect selection for seed yield in canola. Crop Science 35, 1267–1271.
| Carbon isotope discrimination: Potential for indirect selection for seed yield in canola.Crossref | GoogleScholarGoogle Scholar |
Meehl GA, Washington WM, Collins WD, Arblaster JM, Hu A, Buja LE, Strand WG, Teng H (2005) How much more global warming and sea level rise? Science 307, 1769–1772.
| How much more global warming and sea level rise?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXit1yrs7c%3D&md5=51803773639f03d5f3c696cb8de4679cCAS |
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends in Biochemical Sciences 37, 118–125.
| How do plants feel the heat?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFOjtLo%3D&md5=ddb8172d9d57cf6545eddaf0865282b1CAS |
Monneveux P, Rekika D, Acevedo E, Merah O (2006) Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Science 170, 867–872.
| Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsFKktb0%3D&md5=161d4b5a7b27c7fb59f8a7c32a72877fCAS |
Mora F, Castillo D, Lado B, Matus I, Poland J, Belzile F, von Zitzewitz J, del Pozo A (2015) Genome-wide association mapping of agronomic traits and carbon isotope discrimination in a worldwide germplasm collection of spring wheat using snp markers. Molecular Breeding 35, 69
Morrison MJ (1993) Heat stress during reproduction in summer rape. Canadian Journal of Botany 71, 303–308.
| Heat stress during reproduction in summer rape.Crossref | GoogleScholarGoogle Scholar |
Morrison MJ, Gutknecht A, Chan J, Miller SS (2016) Characterising canola pollen germination across a temperature gradient. Crop & Pasture Science 67, 317–322.
| Characterising canola pollen germination across a temperature gradient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XnsF2rsrg%3D&md5=88f46a9d2e2b49845cf36d5346afc3e9CAS |
Nagaharu U (1935) Genome analysis in brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japanese Journal of Botany 7, 389–452.
Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology 147, 1251–1263.
| Galactinol and raffinose constitute a novel function to protect plants from oxidative damage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslyisL0%3D&md5=3d0cfa3b0846c654c558aab2c587e54eCAS |
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cellular and Molecular Life Sciences 69, 3225–3243.
| The use of metabolomics to dissect plant responses to abiotic stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtlait7nJ&md5=602a5f1ce186b43279b04723f8146a6fCAS |
Passioura JB (2012) Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Functional Plant Biology 39, 851–859.
| Phenotyping for drought tolerance in grain crops: When is it useful to breeders?Crossref | GoogleScholarGoogle Scholar |
Peng B, Li H, Peng XX (2015) Functional metabolomics: From biomarker discovery to metabolome reprogramming. Protein & Cell 6, 628–637.
| Functional metabolomics: From biomarker discovery to metabolome reprogramming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFCgtrnJ&md5=ebb3ffe8113efae4edcf5698962cea2fCAS |
Pradhan GP, Prasad PVV (2015) Evaluation of wheat chromosome translocation lines for high temperature stress tolerance at grain filling stage. PLoS One 10, e0116620
| Evaluation of wheat chromosome translocation lines for high temperature stress tolerance at grain filling stage.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Boote KJ, Allen LH, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research 95, 398–411.
| Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Crossref | GoogleScholarGoogle Scholar |
Ramsahoi L (2013) Alleviating heat stress in spring canola (Brassica napus L.) with foliar boron treatment. PhD Thesis, University of Guelph, Guelph, Ontario, Canada.
Rang Z, Jagadish S, Zhou Q, Craufurd P, Heuer S (2011) Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environmental and Experimental Botany 70, 58–65.
| Effect of high temperature and water stress on pollen germination and spikelet fertility in rice.Crossref | GoogleScholarGoogle Scholar |
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134, 1683–1696.
| When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsFKmsbs%3D&md5=9bd66878c03c0ee9acb9aa11ab0613adCAS |
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421, 57–60.
| Fingerprints of global warming on wild animals and plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovVc%3D&md5=8f806fe853fe622d5854e58b5173acf9CAS |
Salem MA, Kakani VG, Koti S, Reddy KR (2007) Pollen-based screening of soybean genotypes for high temperatures. Crop Science 47, 219–231.
| Pollen-based screening of soybean genotypes for high temperatures.Crossref | GoogleScholarGoogle Scholar |
Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, Ikeda H (2006) Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Annals of Botany 97, 731–738.
| Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFahtrs%3D&md5=52871c706dadc167a1c1410831d97c1aCAS |
Singh SK, Kakani VG, Brand D, Baldwin B, Reddy KR (2008) Assessment of cold and heat tolerance of winter-grown canola (Brassica napus L.) cultivars by pollen-based parameters. Journal of Agronomy & Crop Science 194, 225–236.
| Assessment of cold and heat tolerance of winter-grown canola (Brassica napus L.) cultivars by pollen-based parameters.Crossref | GoogleScholarGoogle Scholar |
Smolikova GN, Shavarda AL, Alekseichuk IV, Chantseva VV, Medvedev SS (2016) The metabolomic approach to the assessment of cultivar specificity of Brassica napus l. Seeds. Russian Journal of Genetics: Applied Research 6, 78–83.
| The metabolomic approach to the assessment of cultivar specificity of Brassica napus l. Seeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xht1SktLbJ&md5=100116f39c9fe5c32db49334bfdf1e23CAS |
Steinfath M, Strehmel N, Peters R, Schauer N, Groth D, Hummel J, Steup M, Selbig J, Kopka J, Geigenberger P, van Dongen JT (2010) Discovering plant metabolic biomarkers for phenotype prediction using an untargeted approach. Plant Biotechnology Journal 8, 900–911.
| Discovering plant metabolic biomarkers for phenotype prediction using an untargeted approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlSgtrfF&md5=80576719a70162ffdd65da313c7b85adCAS |
Sun CX, Li MQ, Gao XX, Liu LN, Wu XF, Zhou JH (2016) Metabolic response of maize plants to multi-factorial abiotic stresses. Plant Biology 18, 120–129.
| Metabolic response of maize plants to multi-factorial abiotic stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitVentbvK&md5=89e856717e339218bd15b37a3da578a7CAS |
Trapp JJ, Urrea CA, Zhou J, Khot LR, Sankaran S, Miklas PN (2016) Selective phenotyping traits related to multiple stress and drought response in dry bean. Crop Science 56, 1460–1472.
| Selective phenotyping traits related to multiple stress and drought response in dry bean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXnvVSntLs%3D&md5=2022222945ebc698d4d118c1eb57378fCAS |
Van Vuuren DP, Meinshausen M, Plattner G-K, Joos F, Strassmann KM, Smith SJ, Wigley TML, Raper SCB, Riahi K, de la Chesnaye F, den Elzen MGJ, Fujino J, Jiang K, Nakicenovic N, Paltsev S, Reilly JM (2008) Temperature increase of 21st century mitigation scenarios. Proceedings of the National Academy of Sciences of the United States of America 105, 15258–15262.
| Temperature increase of 21st century mitigation scenarios.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1GntrfN&md5=8c0b714753b94f0f7b6a9a3afe0715e7CAS |
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. Journal of Plant Research 120, 219–228.
| Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts.Crossref | GoogleScholarGoogle Scholar |
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: An overview. Environmental and Experimental Botany 61, 199–223.
| Heat tolerance in plants: An overview.Crossref | GoogleScholarGoogle Scholar |
Witt S, Galicia L, Lisec J, Cairns J, Tiessen A, Araus JL, Palacios-Rojas N, Fernie AR (2012) Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Molecular Plant 5, 401–417.
| Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xks1Sjsro%3D&md5=4ef3a1569ced8162ebdec8ffdb829bcaCAS |
Woods DL, Capcara JJ, Downey RK (1991) The potential of mustard (Brassica juncea (L.) Coss) as an edible oil crop on the Canadian prairies. Canadian Journal of Plant Science 71, 195–198.
| The potential of mustard (Brassica juncea (L.) Coss) as an edible oil crop on the Canadian prairies.Crossref | GoogleScholarGoogle Scholar |
Xu Y, Du H, Huang B (2013) Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling. Crop Science 53, 1626–1635.
| Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling.Crossref | GoogleScholarGoogle Scholar |
Young LW, Wilen RW, Bonham‐Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro‐ and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 55, 485–495.
| High temperature stress of Brassica napus during flowering reduces micro‐ and megagametophyte fertility, induces fruit abortion, and disrupts seed production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXms1entg%3D%3D&md5=fb1e7b2b173d4f595d0aaa4721c8a753CAS |
Zhang G, Aiken R, Martin TJ (2014) Relationship between carbon isotope discrimination and grain yield of rainfed winter wheat in a semi-arid region. Euphytica 204, 39–48.