Comments on ‘Possible contribution of triboelectricity to snow–air interactions’
B. Van Dam A and D. Helmig A BA Institute of Arctic and Alpine Research, University of Colorado at Boulder, Boulder, CO 80309-0450, USA.
B Corresponding author. Email: detlev.helmig@colorado.edu

Brie Van Dam earned a B.Sc.E. degree in Earth Systems Science and Engineering from the University of Michigan's Engineering School in 2007. She is currently a graduate research assistant at the Institute of Arctic and Alpine Research in Boulder, CO. investigating the dependencies and controls on exchanges of ozone and nitrogen oxides at the snow-atmosphere boundary. She has been involved in several field campaigns measuring fluxes of these atmospheric constituents at Summit, Greenland, and Toolik Lake, AK, and this research will form the core of her Ph.D. thesis in Atmospheric and Oceanic Science at the University of Colorado, Boulder. |

Detlev Helmig is a Research Professor with the Institute of Arctic and Alpine Research at the University of Colorado, Boulder. He received his education in Environmental and Analytical Chemistry at the Universities of Bochum and Duisburg in Germany before moving to the US in 1989. His research is on surface gas fluxes, and atmospheric transport and chemistry. Particular current foci are snow cover effects on surface gas exchanges of ozone, nitrogen oxides and volatile organic compounds and studies of the dependencies and controls of ozone deposition fluxes to the ocean. |
Environmental Chemistry 9(2) 116-118 https://doi.org/10.1071/EN11151
Submitted: 1 December 2011 Accepted: 20 December 2011 Published:
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
There have been numerous new discoveries of chemical activity at the snow–atmosphere interface over the last 20 years. These observations have stimulated an increasing body of research on snow chemical systems. The findings from this research have led to a general consensus that photochemical processes are the determining control of this snow chemistry. Traditional gas-phase chemical reactions have fallen short of fully explaining observed behaviour. Heterogeneous and quasi-liquid layer chemistry has been postulated to play a role in accounting for the discrepancies. The Tkachenko and Kozachkov[1] article is therefore a timely presentation of new ideas for further elucidating snowpack chemical mechanisms. In the following discussion we present recent data relevant to testing hypothesis proposed by Tkachenko and Kozachkov.[1]
Some of the hypotheses presented in this paper build upon data from measurements of ozone (O3) in interstitial air of the deep, glacial snowpack at Summit, Greenland, that were presented in our previous work.[2] These experiments reported on positive O3 atmosphere–snowpack gradients, i.e. depleted O3 within the snowpack, and their dynamical behaviour dependent on environmental conditions. We have since taken this research to other locations and further investigated the dependencies of O3 within snow on a variety of parameters. A particular interest of this new research was to further investigate snowpack chemistry in environments with different snowpack conditions (year-round dry, polar snow; seasonal mid-latitude snowpack; snow over permafrost; snow over frozen freshwater lakes). We have also conducted a further 2 years of experiments at Summit,[3] where the measurement depth in the snowpack was increased to 2.5 m and experiments were extended year round to include winter observations. Another environment with polar year-round snowpack over glacial ice was investigated at South Pole, Antarctica.
From the comparison with these other sites it has become obvious that the Summit snowpack is somewhat unique in that (1) O3 levels within the snowpack are generally higher than observed at other locations, and (2) interstitial air O3 shows a much more dynamic dependence on time of day and season than at the other locations that we have investigated. This behaviour might be in agreement with some hypotheses presented by Tkachenko and Kozachkov as they propose that O3 production by the triboelectrical effect would be most pronounced at sites characterised by a thick, glacial snowpack, low humidity, periodic high wind speeds, and low temperatures[1]; all conditions that are typical for Summit.
Our series of experiments have shown that different processes dominate snowpack chemistry in non-glacial snowpack. For example, one definite influence on O3 reactions in seasonal, midlatitude snowpack is the role of NO emitted from microbial processes in the subniveal soil.[4,5] These influences were evident at a high alpine site at Niwot Ridge, CO, in the Rocky Mountains[4]; snowpack under a canopy at the University of Michigan Biological Station (B. Seok, D. Helmig, M. W. Williams, C. Vogel, P. Curtis, unpubl. data); and snowpack over permafrost at Toolik Field Station, AK (B. Van Dam, D. Helmig, R. Honrath, L. Kramer, C. Toro, unpubl. data).
For the Summit snowpack, Helmig et al. noted that O3 atmosphere–snowpack concentration gradients show three characteristic features: (1) diurnal cycles; (2) seasonal variations; and (3) a dependency on wind speed.[2] Tkachenko and Kozachkov propose that the electrification of snow under the influence of wind initiates various free radical processes that can result in O3 production in the snow.[1] This theory is used to explain the third characteristic listed above. Tkachenko and Kozachkov claim that the decrease of the gradient seen under high winds is primarily a result of ozone production in the snowpack driven by the triboelectrification of snow under wind and that this produced ozone compensates for the loss from photochemical destruction.[1]
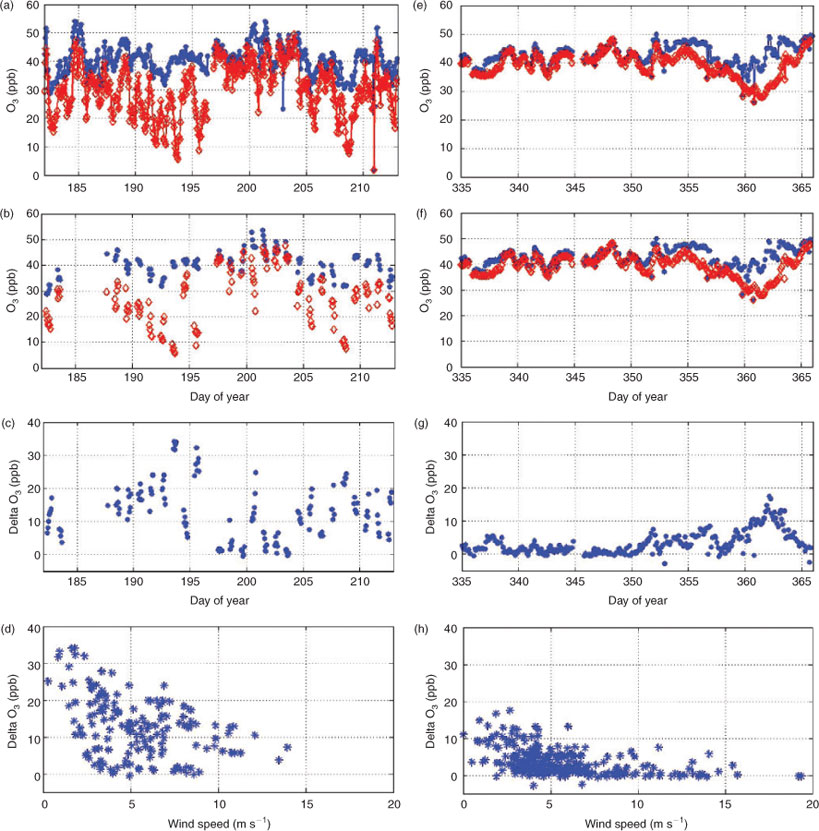
To address this hypothesis we re-examined data from our 2008–10 snowpack chemistry experiment at Summit. Fig. 1a–d show summer data from July 2009 and Fig. 1e–f show winter data from December 2009. All data shown are filtered for clean air conditions (eliminating potentially polluted air from the direction of main camp). Figs 1a and 1e show the absolute concentrations of O3 above the snow surface (blue dots) and within the snowpack (red diamonds). In July, the snowpack O3 measurements are from air sampled at a depth of 0.25 m, and in December, the snowpack measurements are from a depth of 0.30 m.
Fig. 1b and 1f show the same data except filtered for radiation. For July, we only looked at cases when radiation was high, greater than 400 W m–2, and in December, we only considered cases in darkness, i.e. when radiation was less than 4 W m–2, Figs 1c and 1g show the ozone atmosphere–snowpack gradient time series (calculated from the ambient O3 measurement minus the snowpack O3 measurement) for these filtered data. These data clearly illustrate that the measured atmosphere–snowpack O3 gradient is significantly larger during the higher radiation summer conditions (compared to low radiation conditions in winter). During summer >80 % of the ambient ozone levels are destroyed inside the snowpack during late afternoon hours. Lastly, Figs 1d and 1h show the O3 gradient v. wind speed measured from our meteorological tower at a height of 7 m. Both summer and winter data illustrate the dependence of the atmosphere-snowpack gradient on wind speed. The highest positive gradients occur under low winds, both during the summer and winter conditions. Under no measured conditions are negative gradients (higher O3 within the snowpack) recorded.
According to Tkachenko and Kozachkov, ‘ozone production under wind occurs in quantities that are comparable with its photochemical loss and this is the reason why the concentration gradient decreases’.[1] From this statement it would be expected that O3 production from triboelectrification under windy conditions would compensate for the up to 80 % ozone loss observed in the summer. Taking this one step further, one would expect that during the darkness of winter (when photochemical ozone destruction is absent) under windy conditions this triboelectrification-initiated ozone production would supersede the ozone loss, and that this ozone production should result in an ozone enhancement inside the snowpack. The wintertime data presented in Fig. 1e–h, however, do not show any O3 enhancement within the snow. Even under the highest wind conditions, ozone in air below the snow surface remains consistently below ambient air levels. Consequently, there is no evidence for any O3 production occurring within the snow under any wind conditions. Clearly these data do not support the hypothesis proposed by Tkachenko and Kozachkov.[1] Therefore, our hypothesis remains that snowpack ventilation by wind pumping is the cause of decreased O3 gradients during high winds. Support for this explanation has also been shown in other work,[6] where wind pumping effects on other trace gas gradients and fluxes in the seasonal snowpack of Niwot Ridge, CO, were investigated.
Further elucidation of several points raised by the Tkachenko and Kozachkov paper[1] would be beneficial to advance further discussion. First, the paper unfortunately does not describe to what depth within the snowpack the proposed corona discharge-initiated ozone production mechanism would penetrate, and whether the vertical scale of these electrical effects within the snowpack is similar to what we show in the observational data from Summit. Second, the authors propose several reactions relating to O3 within the snow under the influence of corona discharge. These proposed reactions include both an ozone source (reactions 7–9 in Tkachenko and Kozachkov[1]) and an ozone sink (reaction 11[1]), yet the article neglects to comment on the interplay between these proposed ozone sources and sinks.
The Tkachenko and Kozachkov[1] publication provides interesting thoughts that may initiate further stimulating discussion among polar snow scientists. We hope that further experimental evidence can be provided to address the role of triboelectricity to snow–air interactions as proposed by these authors.[1]
References
Tkachenko E. Y., Kozachkov S. G. ( ). Possible contribution of triboelectricity to snow–air interactions.. Environmental Chemistry| Possible contribution of triboelectricity to snow–air interactions.Crossref | GoogleScholarGoogle Scholar | [Published online ahead of print 13 September 2011].
Helmig D., Bocquet F., Cohen L., Oltmans S. (2007). Ozone uptake to the polar snowpack at Summit, Greenland.. Atmos. Environ. 41, 5061.
| Ozone uptake to the polar snowpack at Summit, Greenland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns1Kru7o%3D&md5=156163a479d5d807d55154484941c760CAS | [Published online ahead of print 21 December 2006].
B. Van Dam, D. Helmig, R. Honrath, J. Hueber, B. Seok, C. Toro, L. Kramer, L. Ganzeveld, W. Neff, Exchange of ozone at the atmosphere-snowpack interface at Summit, Greenland, in 2010 State of the Arctic Meeting, Miami, FL, 16–19 March 2010. Available at http://soa.arcus.org/abstracts/atmosphere-snowpack-ozone-exchanges-summit-greenland-and-their-role-polar-tropospheric-ozo [Verified 10 January 2012].
Bocquet F., Helmig D., Oltmans S. (2007). Ozone in interstitial air of the mid-latitude, seasonal snowpack at Niwot Ridge, Colorado.. Arct. Antarct. Alp. Res. 39, 375
| Ozone in interstitial air of the mid-latitude, seasonal snowpack at Niwot Ridge, Colorado.Crossref | GoogleScholarGoogle Scholar |
Helmig D., Seok B., Williams M. W., Hueber J., Sanford R. J. (2009). Fluxes and chemistry of nitrogen oxides in the Niwot Ridge, Colorado snowpack.. Biogeochemistry 95, 115
| Fluxes and chemistry of nitrogen oxides in the Niwot Ridge, Colorado snowpack.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVWhtLs%3D&md5=2f5cad3baa74fddf63f09c1f9b5ab13eCAS |
Seok B., Helmig D., Williams M. W., Liptzin D., Chowanski K., Hueber J. (2009). An automated system for continuous measurements of trace gas fluxes through snow: an evaluation of the gas diffusion method at a subalpine forest site, Niwot Ridge, Colorado.. Biogeochemistry 95, 95
| An automated system for continuous measurements of trace gas fluxes through snow: an evaluation of the gas diffusion method at a subalpine forest site, Niwot Ridge, Colorado.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVWhtLk%3D&md5=26bd01e3f93e67ce20604dc814e3a3f2CAS |