When are metal complexes bioavailable?
Chun-Mei Zhao A B , Peter G.C. Campbell C and Kevin J. Wilkinson D EA School of Environmental Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, P.R. China.
B Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, Guangzhou 510275, P.R. China.
C Institut National de la Recherche Scientifique – Eau, Terre et Environnement (INRS-ETE), 490 Rue de la Couronne, Quebec, QC, G1K 9A9, Canada.
D Biophysical Environmental Chemistry Group, Department of Chemistry, University of Montreal, CP 6128 Succursale Centre-ville, Montreal, QC, H3C 3J7, Canada.
E Corresponding author: Email: kj.wilkinson@umontreal.ca

Chun-Mei Zhao is an associate professor at Sun Yat-Sen University. Her research interests include an examination of the environmental behaviour of trace metals and nanoparticles in the aquatic environment. She is especially interested by the influence of environmental factors on bioavailability and toxicity of trace metals and nanoparticles. Currently, her research is focussed on the speciation and bioavailability of rare earth elements in freshwater ecosystems and those affected by mining. |

Peter Campbell completed a Ph.D. at Queen’s University (Kingston, ON) in organometallic chemistry prior to spending 2 years at Monash University working with Professor John Swan in the area of organophosphorus chemistry. In 1970, he took up a position at the Institut National de la Recherche Scientifique (Université du Québec, INRS-ETE), where he is currently a Professor. Peter was elected to the Academy of Sciences of the Royal Society of Canada in 2002. His research interests focus on metals in the aquatic environment and include elements of analytical chemistry, geochemistry and ecotoxicology, where he has made a very important and sustained impact. This special issue is a recognition of the very important body of work of Peter Campbell. |

Kevin J. Wilkinson is a Professor at the Université de Montréal. His research is aimed at gaining a molecular-level understanding of contaminant bioavailability and mobility. Kevin is especially interested in examining the mobility and bioavailability of both metals and engineered nanomaterials in the environment. His research group also focuses on the development of analytical techniques designed to better understand environmental processes, including those looking at trace metal speciation or nanoparticle detection. Kevin is currently an Editor of Environmental Chemistry. |
Environmental Chemistry 13(3) 425-433 https://doi.org/10.1071/EN15205
Submitted: 29 September 2015 Accepted: 23 November 2015 Published: 2 March 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Environmental context. The concentration of a free metal cation has proved to be a useful predictor of metal bioaccumulation and toxicity, as represented by the free ion activity and biotic ligand models. However, under certain circumstances, metal complexes have been shown to contribute to metal bioavailability. In the current mini-review, we summarise the studies where the classic models fail and organise them into categories based on the different uptake pathways and kinetic processes. Our goal is to define the limits within which currently used models such as the biotic ligand model (BLM) can be applied with confidence, and to identify how these models might be expanded.
Abstract. Numerous data from studies over the past 30 years have shown that metal uptake and toxicity are often best predicted by the concentrations of free metal cations, which has led to the development of the largely successful free-ion activity model (FIAM) and biotic ligand model (BLM). Nonetheless, some exceptions to these classical models, showing enhanced metal bioavailability in the presence of metal complexes, have also been documented, although it is not yet fully understood to what extent these exceptions can or should be generalised. Only a few studies have specifically measured the bioaccumulation or toxicity of metal complexes while carefully measuring or controlling metal speciation. Fewer still have verified the fundamental assumptions of the classical models, especially when dealing with metal complexes. In the current paper, we have summarised the exceptions to classical models and categorised them into five groups based on the fundamental uptake pathways and kinetic processes. Our aim is to summarise the mechanisms involved in the interaction of metal complexes with organisms and to improve the predictive capability of the classic models when dealing with complexes.
Introduction
In the aquatic environment, metals are found in numerous forms including the hydrated free ion, inorganic complexes (with ligands such as Cl–, OH–, CO32–), organic complexes (with simple organic molecules of biogenic or anthropogenic origin, and with natural organic matter, NOM); colloidal and other solid-phase forms may also be present. Metal partitioning among the different forms is a dynamic process that depends on the type and concentration of ligands, temperature, pH and redox conditions of the medium.[1–3] In natural aquatic systems, many metals are mainly found as complexes and in colloidal forms, often at concentrations that are much greater than that of the free metal ion.[4] Nonetheless, the general consensus for cationic metals is that the concentration of the free metal ion is the best predictor for both metal bioaccumulation and toxicity in aquatic systems.[2,5] The strong observed dependency of biological effects on the concentration (activity) of the free ion has been attributed to the rapid equilibration of the metal forms in solution with toxicologically sensitive sites on the biological surface,[2,6,7] which is the basis of both the Free Ion Activity model (FIAM) and Biotic Ligand model (BLM). A general misconception is that the FIAM and BLM imply that the free ion is the only toxic species in solution. In fact, this is not necessarily the case; the models simply rely on the direct (linear) relationship between the free ion and the surface-bound metal species responsible for biological effects, a relationship that prevails when the species are at equilibrium. Note that whereas the equilibrium assumption is likely valid for metals equilibrating among microorganisms and inorganic ligands in the water column, it is less clear whether it will be valid in more complex media such as soils, sediments and biofilms or in the presence of heterogeneous ligands such as humic substances.[3]
For uptake and biological effects to occur, the metal must first be transported from the bulk solution to the biological surface (Fig. 1). During transport, many of the metal complexes are dynamic, implying that they undergo numerous complexation–dissociation reactions in the time that it takes to reach the surface of the organism. Generally speaking, metal equilibration (adsorption–desorption) with the surface of the organism will occur next, followed by the facilitated transport of the metal into the organism (uptake). In the FIAM/BLM, the transport of metal across the biological membrane (internalisation) is assumed to be rate-limiting, with biological effects resulting directly from the binding of metals to the ‘biotic ligands’. In such a case, the metals in solution are at an apparent equilibrium (steady-state conditions) with the biological surface and it follows that uptake (proportional to the concentration of metal bound to surface-bound metal transporters) and even toxicity (proportional to metal uptake flux) can be related back to the trace metal speciation in solution (by the equilibrium stability constants, K).
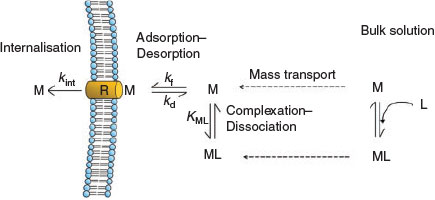
|
Metal uptake and toxicity are not always well predicted by the concentration of free metal ion.[2,3,7,8] Indeed, there are several classes of exceptions whereby metal complexes are known to contribute to bioavailability: (i) intact metal complexes are internalised by the organism (either by simple diffusion across the lipid bilayer or by transport across the lipid bilayer via a ligand transport site); (ii) metal complexes react with sensitive sites (i.e. biotic ligands) on the organism surface (ternary complex formation); (iii) metal complexes dissociate and increase the local free-metal-ion concentrations in the boundary layer close to the organism surface. In the present paper, we summarise the studies where the classic models appear to fail and organise them into five categories based on the uptake pathways and kinetic processes involved. Our aim is to summarise the mechanisms involved in the interaction of metal complexes with organisms and to improve the predictive capability of the classic models when dealing with complexes.
Five documented cases showing the bioavailability of metal complexes
(1) Passive diffusion of lipophilic complexes
It has been known for many years that uncharged, lipophilic metal complexes can passively diffuse through the lipid bilayer of biological membranes. The passive diffusion of metal complexes has been observed for complexes involving synthetic organic ligands, including 8-hydroxyquinoline (Ox), diethyldithiocarbamate (DDC) and ethylxanthate (XANT),[9–11] and for inorganic metal complexes such as HgCl20.[12] The passive diffusion of AgCl0 remains questionable. Indeed, although several authors have attributed increased bioaccumulation to the passive diffusion of AgCl0 in marine diatoms and salmonids, for the green alga Chlamydomonas reinhardtii, Fortin and Campbell were able to show that enhanced Ag biouptake resulted from increased mass transport and dissociation of the complex under conditions of diffusion limitation (see Dissociation of labile metal complexes section below).[13–15]
In these cases, metal complexes are thought to be taken up in a similar manner to hydrophobic organic contaminants, which are simply partitioned between the water and the phospholipid bilayer of the cell membrane. In such a case, metal complexes could diffuse into the cytosol where the metal could dissociate from the complex and bind with intracellular ligands.[10] This implies that the bioaccumulation of lipophilic metal complexes would not simply be dependent on complex permeability (or the octanol–water partition coefficient, Kow, of the complex) but rather would be driven to some extent by the re-equilibration of the complex within the cell. For example, whereas (CH3)2Hg and Hg0 have higher hydrophobicity than HgCl2 and CH3HgCl, their bioaccumulation by a marine alga (Thalassiosira weissflogii) is lower, presumably because elemental mercury and dimethylmercury do not readily form complexes with intracellular ligands.[12]
Generally speaking, the observed uptake rates of lipophilic metal complexes are usually at least an order of magnitude higher than their hydrophilic metal counterparts,[10,11,16,17] which makes this mechanism potentially important for certain metals. Furthermore, uptake is sensitive to the pH of the medium, with a decreased bioaccumulation of the lipophilic metal complexes at low pH, potentially due to a reduction in the electrostatic repulsion between adjacent phospholipids, leading to their tighter packing and a decreased membrane permeability.[11,18,19]
With the exception of what is known about such hydrophobic metal species as the methylated forms of Hg, Sn, As and Sb[20–22] and the hydrophobic complexes described above, it is currently unclear to what extent lipophilic ligands are present in natural systems. Owing in large part to the analytical difficulties associated with measurements of (likely low concentrations of) hydrophobic complexes in aquatic systems, few data are available to estimate metal uptake fluxes for these species. One exception is the work of Turner and Mawji who used C-18 and liquid–liquid extractions to quantify and characterise the hydrophobic fraction of dissolved metals in natural waters.[23] Their octanol–water partition coefficients indicated that the hydrophobicity of metal complexes was both pH- and metal-dependent,[24,25] reaching a maximum in the range of pH 7–8, where the overall charge of the metal complexes was thought to be very low. Some of the metal complexes with dissolved organic matter (DOM) were also shown to be hydrophobic, and their prevalence was related to the nature, origin and concentration of the DOM[26] (Box 1).
| Box 1. Research priorities for the uptake of lipophilic metal complexes |
|
(2) Uptake of hydrophilic complexes through a ligand transporter system
Hydrophilic metal complexes cannot passively diffuse through biological membranes, but some have been found to enter the cell using transport systems meant for ligands. This mechanism has been observed for ligands with established biological functions and their own specialised channels or transporters.[27,28] For example, for some bacteria, citrate, which can be used as a carbon source,[29,30] is taken up by citrate transporters (e.g. CitM and CitH). Furthermore, citrate has been shown to be transported in the form of a complex with Ca2+, Cd2+, Co2+, Mg2+, Mn2+, Ni2+, Pb2+, Sr2+ or Zn2+, transport rates being related to the specific size of the metal ion.[30] Metals taken up by this pathway can enter cells at a faster rate than metals entering by the cation transporters. In observations made using the green alga Pseudokirchneriella subcapitata, Cd uptake was 2 times faster in the presence of citrate than when similar concentrations of free Cd were buffered by nitrilotriacetic acid (NTA).[31] Furthermore, for a constant concentration of Cd2+ (0.25 µM), growth inhibition increased from 50 % of control values to 70 % of control values as the concentration of the Cd–citrate complexes increased from 0.077 to 1.47 µM.
Iron complexes with siderophores are another class of species that can be directly incorporated into cells by transporters that are specific to the siderophores. In the open ocean, where the concentration of free iron is extremely low (<10–18 M), many bacteria and fungi have evolved specific pathways in order to acquire iron for growth. Numerous microbes (nearly all fungi) can excrete siderophores, which have an extremely high affinity for iron (log K ~1019 to 1023)[32] and will complex FeIII extracellularly. In this case, the uptake of FeIII–siderophore complexes is mediated by transport proteins that are found only in microorganisms.[33]
For other inorganic ligands, such as thiosulfate and phosphate, similar situations have been observed. For example, silver–thiosulfate complexes are taken up by algal cells (Chlamydomonas reinhardtii) at higher fluxes than free Ag.[34] In this case, evidence that uptake was occurring over the sulfate transport system was provided by the observation uptake was significantly inhibited by the addition of sulfate. Nonetheless, the toxicity induced by the silver–thiosulfate complexes was not as important as that due to silver ions,[35] again demonstrating the importance of the intracellular fate of metal complexes once assimilated. Finally, although it is extremely difficult to distinguish transport of the complexes from that of a co-transport of inorganic phosphate and the metal ions, some bacteria (Escherichia coli[36]; Arthrobacter sp.[37]) are thought to internalise metals in the form of neutral metal phosphate complexes (MeHPO4) by their phosphate transporters. In this case, divalent metal complexes (e.g. Cd and Zn) exerted additional toxicity on the bacteria, which also depended on the pH (greater toxicity was observed at higher pH values).[37] Clearly, the role of ligands such as phosphate, which is independently required as a nutrient element by some organisms, requires further study.
In a final example of a ligand transporter being used by metal complexes, it has been observed in plants and using molecular modelling that some metalloids, especially arsenic (As) and antimony (Sb), can be transported through the aquaglyceroporins, which are channels that are specific for the transport of water, glycerol and other small, uncharged solutes (similar conformations prevail among As(OH)3, Sb(OH)3 and glycerol).[38,39] Similarly, owing to its structural similarities to phosphate, AsV, in the form of AsO43–, is known to be accumulated through phosphate transporters in E. coli, yeast and mammals.[40,41] Owing to this dependency on transporters that are designed for nutrients, the toxicity induced by the metalloids is highly dependent on nutrient exposure concentrations and conditions (Box 2).
| Box 2. Research priorities for the uptake of metal complexes by ligand transporters |
|
(3) Formation of ternary metal complexes at a metal uptake site
It is often assumed that the biotic ligand, <–R>, binds to the free metal ion, either directly or by ligand exchange, to yield <M–R>. However, several cases of bioavailability enhancements, resulting from the apparent formation of a ternary complex on the biological surface, <L–M–R>, have also been documented.[42–45] In these cases, toxicity or bioaccumulation data were best explained by taking into account the interaction of a metal complex with the biotic ligand <L–M–R>, most often in addition to the contribution of the free metal ion <M–R>. An underlying assumption is that the site of toxic action occurs on the biological surface and not intracellularly.
Direct physical evidence for the presence of a ternary complex on the biological surface is difficult to obtain. Chemical extractions using strong ligands such as EDTA[46] cannot always distinguish unambiguously between a surface-bound and internalised metal. This especially appears to be the case for the trivalent metals. Techniques such as solid-state nuclear magnetic resonance spectroscopy and synchrotron-based X-ray microscopy have been used to probe for the presence of ternary complexes on biological surfaces. For example, the formation of AlFx ternary complexes on cells obtained from fish gills (Micropterus salmoides) was demonstrated by 19F nuclear magnetic resonance,[42] whereas Hg LIII-edge X-ray absorption near-edge spectroscopy (XANES) showed that ternary complexes involving Hg–EDTA and the biological surface were unlikely. Experiments using radiolabelled ligands can also show when ligands are not taken up by the organism.[45,47] In such cases, either an equilibrium or a kinetic explanation can be put forward to account for enhanced uptake. For example, for cases where metal adsorption–desorption kinetics are rate-limiting, the formation of the mixed-ligand complex at the cell surface (especially for inorganic ligands, such as hydroxide and chloride) can accelerate the water loss rate of the metal, thus increasing the rate of biouptake (mechanism 5 below).[48] Otherwise, the biological effect can simply be related to the total concentration of metal bound to the biotic ligands on the surface of the organism (i.e. the sum of the equilibrium concentrations of <M–R> and <L–M–R>). In this case, the use of an equilibrium model (such as the BLM) that uses multiple equilibrium constants (KMR, KLMR, etc.) would be an appropriate means by which to predict biological effects.
The formation of ternary complexes has been postulated to occur for both divalent metals, including Pb[43,49,50] and Zn,[44,51] and trivalent metals such as Al,[42,52] Sc,[53] Eu[47] and Tm.[45] Indeed, Aristilde et al.[44] suggested that the formation of ternary complexes may be universal, irrespective of the specificity of the metal or ligand. They found a linear relationship between an enhancement of Zn uptake and the concentration of Zn–cysteine complexes in the presence of cysteine and EDTA (cysteine still inhibits Zn uptake if only the cysteine is present, which rules out the possibility of mechanism 2). Zhao and Wilkinson[45] found that an observed increase in Tm uptake (above what was observed in the absence of complexes) could be related to the concentrations of the dominant Tm species (including both 1 : 1 and 1 : 2 complexes with malate and citrate). As would be expected for a fixed ligand concentration, the contribution of the complexes is generally more significant for low concentrations (proportions) of free ion, where the metal complexes predominate. As is often the case for metal uptake of the free cation, the formation of the ternary surface complexes also appears to follow a saturable Michaelis–Menten-like function.[45]
It is unclear to what extent the nature of the metal–ligand complex plays a role with respect to its ability to form ternary complexes at the biological surface. In order for ternary complexes to contribute to biouptake, the dissociation of L from <L–Tm–R> needs to occur at a faster rate than the dissociation of Tm–L from the same sites. In other words, in the ternary complex L–M–R, the M–L bond must be weaker than the M–R bond. It follows that weaker metal complexes or those with a greater affinity with the metal uptake site are likely to contribute more to bioaccumulation. Such determinations would be consistent with results showing that algae grown in Zn-limited environments and having developed an enhanced uptake capacity can use Zn–cysteine complexes more efficiently than Zn-replete algae, potentially owing to the increased affinity of the Zn transporter.[44,54] Nonetheless, for metals that are very strongly bound to biotic ligands, metal dissociation kinetics are likely to be limiting.
In the presence of NOM, such as humic or fulvic acids, the role of ternary complexes must be evaluated with great care. Although Lamelas et al. found that Pb biouptake was clearly enhanced in the presence of NOM,[43] i.e. Pb uptake was greater than what would have been expected given the free Pb2+ concentration, alternative explanations to the formation of a ternary complex can be proposed. As mentioned above, it is sometimes difficult to distinguish between surface-bound and internalised metals using chemical extraction techniques. In addition, purely physiological effects caused by increased nutrient availability or decreased light in the presence of NOM also need to be evaluated.[55–58] Furthermore, NOM adsorption to biological surfaces can increase their overall negative surface charge,[59] thereby enhancing their overall electrostatic attraction for cations. Nonetheless, even when taking these other explanations into account, Lamelas and Slaveykova estimated that most (57–95 %) of the Pb internalisation in the presence of 10 mg L–1 of humic acid could be attributed to the formation of ternary complexes ([Pb2+], nanomolar to micromolar scale). However, for Cd under similar experimental conditions, ternary complexes did not appear to contribute to biouptake[49] (Box 3).
| Box 3. Research priorities concerning the role of ternary surface metal complexes |
|
(4) Dissociation of labile metal complexes
The basic assumption of the equilibrium models (FIAM and BLM) is that metal species in the bulk solution are in (pseudo)-equilibrium with the surface of the organism. In other words, metal biouptake is rate-limiting and the concentrations of each of the metal species in solution are the same as those in the boundary layer at the biological interface. In contrast to this assumption, several examples in both defined media and natural waters have been documented for which the mass transport of the metal to the cell surface is the rate-limiting step.[60–62] In these cases, biologically reactive metal species (e.g. free Mz+) are depleted in the diffusion layer next to the organism. To restore equilibrium, ‘labile’A metal complexes are able to dissociate, thus providing an extra source of free ions to the organism. According to this scenario, both physical transport (size of the complex) and chemical reactions (kinetic lability of the metal complex) will then affect metal bioaccumulation,[3] which will not necessarily be proportional to the concentration of the free ion in the bulk medium, but rather some combination of the free ion and labile metal complexes.
A diffusive limitation is usually identified by comparing the metal internalisation flux with the calculated maximum diffusive flux.[14,61,63] Diffusive limitation can also be inferred from fluxes determined by the diffusive gradients in thin films (DGT) technique,[64] from free-metal-ion gradients close to biological surfaces, as measured by microelectrode arrays,[65,66] or from changes in biouptake under different flow conditions.[64,67,68] In the environment, a mass transport limitation (and thus potential contribution of labile complexes) is often observed: (i) for very low concentrations of metals (picomolar to nanomolar levels)[14,61,64]; (ii) in constrained media such as sediments, soils or biofilms, where metals have much lower diffusion coefficients[69]; or (iii) for nutrients and metals with high biouptake fluxes (e.g. PO43–, Ag).[14,70] For example, for Chlamydomonas reinhardtii, metal uptake occurs in the order: Ag > Pb ≈ Cu > Cd ≈ Zn ≈ Mn, with an Ag uptake flux that is nearly one order of magnitude higher than that for Pb.[71] Indeed, as mentioned earlier, AgCl complexes were shown to contribute to biouptake for C. reinhardtii[14] and the authors concluded that the increased bioaccumulation was due to the lability of AgCln(n–1)– complexes, rather than to passive diffusion of the uncharged metal species through the biological membrane. This interpretation was supported by subsequent reanalysis of the data by Pinheiro et al.[72]
Under conditions of a diffusion limitation, both the ligand properties and the size of the organisms will influence metal bioavailability through their influence on mass transport. Thus, for a given stability constant, a metal that is bound to a small ligand will be more bioavailable than the same metal bound by a macromolecule. Interestingly, this implies that the bioavailability of Ag nanoparticles will be reduced in line with the reduction of their diffusive flux (which would scale with the reciprocal particle diameter).[73] Because organisms in biofilms and macro-organisms generally have less efficient mass transport than unicellular microorganisms (planar diffusion instead of radial diffusion),[74,75] they are also more likely to encounter diffusive limitation. Because labile complexes can contribute to metal uptake under a mass-transport limitation, equilibrium models (e.g. BLM) are not useful predictors of metal accumulation or toxicity under these conditions (Box 4).
| Box 4. Research priorities for metal bioavailability due to a transport limitation |
|
(5) Dissociation of metal complexes – kinetically controlled uptake (ligand exchange)
Complex lability is dependent on the intrinsic characteristics of the metal ion, i.e. on the water loss rate constant of the hydrated free ion (k–w, Eigen–Wilkins mechanism).[76,77] Metals with lower k–w values such as Al, CrIII, FeIII, Ni and Co are likely to react slowly with the biotic ligand/transport site and the dissociation rate of these metals from their transporters back into the boundary layer could be lower than their internalisation rates. In this case, metal uptake would be kinetically controlled.[6,48] Unfortunately, for conventional experiments conducted with chelators, it is often difficult to distinguish metal uptake under kinetic control from a thermodynamically controlled uptake. Elegant experiments have been performed by Hudson and Morel,[78] who used pulse-chase experimentsB to show that Fe uptake was kinetically controlled for two species of marine phytoplankton (the dissociation rate constant of the Fe from the biotic ligand was lower than the Fe internalisation rate constant), and in direct contradiction to the equilibrium assumption of the FIAM and BLM. In that case, the kinetically controlled metal uptake rate depended on the total concentration of inorganic metal, mainly Fe(OH)2+, Fe(OH)3 and Fe(OH)4–; these hydroxo-complexes have faster ligand exchange rates than Fe3+ (Eigen–Wilkins mechanism).[78]
Note that the intrinsic properties of the biotic ligand are critical to whether metal uptake is thermodynamically or kinetically controlled.[6] The kinetically controlled uptake of Fe was observed with Fe-stressed algae that had a large number of strong-affinity transporters, but this was not the case for Fe-replete algae.[78] In a more recent example, Thomas et al.[79] demonstrated increased bioavailability of Hg in the presence of Hg–EDTA complexes and suggested that this enhanced uptake was likely due to a ligand exchange reaction between the complex (Hg–EDTA) and the biotic ligand R on the surface of E. coli. Because no spectroscopic evidence could be obtained for the presence of a ternary complex <EDTA–Hg–R>, their results suggested that the presence of EDTA may have accelerated the exchange of Hg with the uptake site. There remain very few well-documented cases of kinetically controlled uptake of a trace metal (Box 5).
| Box 5. Research priorities for cases where metal uptake is kinetically controlled |
|
Conclusion
As seen above, our knowledge with respect to metal bioavailability has greatly evolved over the past 25 years, progressing largely (but not entirely) from equilibrium concepts that mainly considered the contribution of the free ion[1] to dynamic systems where several factors, including the intrinsic properties of the metal and ligand,[34,80] their respective concentrations, organism characteristics such as size,[64,81] media properties and the kinetic behaviour of the metal–ligand system[78,82] are considered. In many cases, equilibrium models are a reasonable simplification of the real-world system, such that simple relationships should be obtained that relate metal speciation to biological effects. Indeed, the BLM has often been an extremely useful construct for explaining the effect of trace-metal complexation or metal–metal interactions in well-controlled, (often) laboratory-based experiments. Nonetheless, as the five classes of exceptions demonstrate, for many systems, metal complexes will need to be taken into account and kinetic aspects considered when one attempts to relate trace-metal speciation to biological effects.
In all cases, speciation measurements will be the key to making well-founded predictions of biological effects. However, just as physicochemical conditions and organism characteristics affect metal bioavailability, these same factors will also determine which chemical species are detected by a chemical sensor (its size, accumulation rate, etc.). In other words, in order to improve our ability to predict the effects of metals in the environment, it will be necessary to improve our theoretical understanding of both speciation techniques and metal bioaccumulation. Furthermore, the FIAM and BLM consider the interaction between metal and organism largely from a chemical perspective, even though metal uptake can also be affected by the physiology of an organism. For example, because stronger binding between the metal and transporter can affect its dissociation rate, an organism in metal-replete media could theoretically shift from pseudo-equilibrium conditions where metal complexes are not bioavailable to a kinetically controlled uptake where the biouptake rates are determined by the rate of metal exchange between complex in solution and the biotic ligand. Similarly, whereas the role of NOM is often thought of in terms of its ability to complex metals, physiological changes induced by the interaction of the NOM with the organism cannot be ignored.[55,83–85]
The overwhelming advantage of equilibrium models such as the BLM is their simplicity in taking into account the effects of complexation and cation competition on trace metal uptake and toxicity. However, for many of the examples where trace metal complexes appeared to be bioavailable, equilibrium conditions may not exist. In such cases, the construction of a dynamic model, taking into account mass transport and chemical kinetics, would be necessary to improve our predictive capacity of metal bioavailability. Whereas this may prove to be essential for some environmental media (such as soils, sediments and biofilms), it is clear that such models would require a much larger number of input parameters and may be difficult to implement within the perspective of environmental regulation. The identification of rate-limiting steps and further research into some of the likely exceptions (e.g. trivalent metals, hydrophobic complexes) will be an important first step for advancing our knowledge of trace metal bioavailability and producing effective tools to identify the associated risks (Fig. 2).
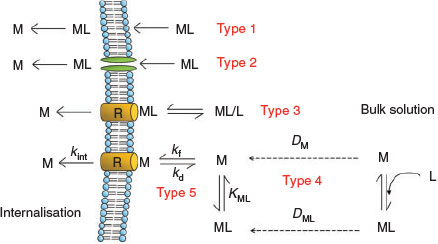
|
References
[1] F. M. M. Morel, J. G. Hering, Principles and Applications of Aquatic Chemistry 1993 (Wiley-Interscience: New York).[2] P. G. C. Campbell, Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model, in Metal Speciation and Bioavailability in Aquatic Systems (Eds A. Tessier, D. Turner) 1995, pp. 45–102 (Wiley: New York).
[3] J. Buffle, K. J. Wilkinson, H. P. van Leeuwen, Chemodynamics and bioavailability in natural waters. Environ. Sci. Technol. 2009, 43, 7170.
| Chemodynamics and bioavailability in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGgu7vE&md5=75009c5e3e556f229bfafa16171b8b71CAS | 19848118PubMed |
[4] J. Buffle, Complexation Reactions in Aquatic Systems: An Analytical Approach 1990 (Ellis Horwood: Chichester, UK).
[5] M. A. Anderson, F. M. M. Morel, R. R. L. Guillard, Growth limitation of a coastal diatom by low zinc ion activity. Nature 1978, 276, 70.
| Growth limitation of a coastal diatom by low zinc ion activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhs1arsr8%3D&md5=88cf581bfcd7fd958f6ab20a8eae5a90CAS |
[6] R. J. M. Hudson, F. M. M. Morel, Trace metal transport by marine microorganisms: implications of metal coordination kinetics. Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 129.
| Trace metal transport by marine microorganisms: implications of metal coordination kinetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktVCksrw%3D&md5=38260017b07a23801fe6a7f20052ac8fCAS |
[7] V. I. Slaveykova, K. J. Wilkinson, Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environ. Chem. 2005, 2, 9.
| Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisV2it7Y%3D&md5=350cf3f1e5d78b9c8b2ae03d2b205afaCAS |
[8] D. de Paiva Magalhães, M. R. da Costa Marques, D. F. Baptista, D. F. Buss, Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69.
| Metal bioavailability and toxicity in freshwaters.Crossref | GoogleScholarGoogle Scholar |
[9] J. E. Poldoski, Cadmium bioaccumulation assays. Their relationship to various ionic equilibria in Lake Superior water. Environ. Sci. Technol. 1979, 13, 701.
| Cadmium bioaccumulation assays. Their relationship to various ionic equilibria in Lake Superior water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXlt1yktbs%3D&md5=ca821d3a43f9d1309ac8066d31e77be5CAS |
[10] J. T. Phinney, K. W. Bruland, Uptake of lipophilic organic Cu, Cd, and Pb complexes in the coastal diatom Thalassiosira weissflogii. Environ. Sci. Technol. 1994, 28, 1781.
| Uptake of lipophilic organic Cu, Cd, and Pb complexes in the coastal diatom Thalassiosira weissflogii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlslansrc%3D&md5=6f6591bcb5d9ce940ad71f78074cb240CAS | 22175916PubMed |
[11] A. Boullemant, M. Lavoie, C. Fortin, P. G. C. Campbell, Uptake of hydrophobic metal complexes by three freshwater algae: unexpected influence of pH. Environ. Sci. Technol. 2009, 43, 3308.
| Uptake of hydrophobic metal complexes by three freshwater algae: unexpected influence of pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktFemtbg%3D&md5=86a38a2a33dfc323c9649cebee8483a3CAS | 19534151PubMed |
[12] R. P. Mason, J. R. Reinfelder, F. M. M. Morel, Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 1996, 30, 1835.
| Uptake, toxicity, and trophic transfer of mercury in a coastal diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XisFansL4%3D&md5=46064b14e2ee19b1dcdf54dc77464bb7CAS |
[13] J. R. Reinfelder, S. I. Chang, Speciation and microalgal bioavailability of inorganic silver. Environ. Sci. Technol. 1999, 33, 1860.
| Speciation and microalgal bioavailability of inorganic silver.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXisVyjtL8%3D&md5=88662943c0607b282350cfcffd82b2f9CAS |
[14] C. Fortin, P. G. C. Campbell, Silver uptake by the green alga Chlamydomonas reinhardtii in relation to chemical speciation: influence of chloride. Environ. Toxicol. Chem. 2000, 19, 2769.
| Silver uptake by the green alga Chlamydomonas reinhardtii in relation to chemical speciation: influence of chloride.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtVeqtw%3D%3D&md5=0ed2228ed1d58cc10bdd51b82c742e42CAS |
[15] N. R. Bury, C. Hogstrand, Influence of chloride and metals on silver bioavailability to Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) yolk-sac fry. Environ. Sci. Technol. 2002, 36, 2884.
| Influence of chloride and metals on silver bioavailability to Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) yolk-sac fry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjslyjurk%3D&md5=ba76824e3cc3cc3d61e0a371919b0cf6CAS | 12144263PubMed |
[16] J. T. Phinney, K. W. Bruland, Trace metal exchange in solution by the fungicides Ziram and Maneb (dithiocarbamates) and subsequent uptake of lipophilic organic zinc, copper and lead complexes into phytoplankton cells. Environ. Toxicol. Chem. 1997, 16, 2046.
| Trace metal exchange in solution by the fungicides Ziram and Maneb (dithiocarbamates) and subsequent uptake of lipophilic organic zinc, copper and lead complexes into phytoplankton cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmsFCjs7s%3D&md5=bb7a97986680acf6b1223ce8399b3d39CAS |
[17] P. L. Croot, B. Karlson, J. T. van Elteren, J. J. Kroon, Uptake of 64Cu–oxine by marine phytoplankton. Environ. Sci. Technol. 1999, 33, 3615.
| Uptake of 64Cu–oxine by marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlslOms7c%3D&md5=fbea2b3097172d73f0f54279a74ba0c9CAS |
[18] C. A. Puckett, R. J. Ernst, J. K. Barton, Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159.
| Exploring the cellular accumulation of metal complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFehsrc%3D&md5=e37e72d5e1346da2189d2ffaa5365430CAS | 20104335PubMed |
[19] M. Lavoie, S. Le Faucheur, A. Boullemant, C. Fortin, P. G. C. Campbell, The influence of pH on algal cell membrane permeability and its implications for the uptake of lipophilic metal complexes. J. Phycol. 2012, 48, 293.
| The influence of pH on algal cell membrane permeability and its implications for the uptake of lipophilic metal complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnsFGhsbc%3D&md5=4ede33afaa18c5993a81dfc889c16864CAS |
[20] T. Hamasaki, H. Nagase, Y. Yoshioka, T. Sato, Formation, distribution, and ecotoxicity of methylmetals of tin, mercury, and arsenic in the environment. Crit. Rev. Environ. Sci. Technol. 1995, 25, 45.
| Formation, distribution, and ecotoxicity of methylmetals of tin, mercury, and arsenic in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkvVCrs7c%3D&md5=7a23cecd003f247806e0425dfdddbfb5CAS |
[21] S. Andres, J.-M. Laporte, R. P. Mason, Mercury accumulation and flux across the gills and the intestine of the blue crab (Callinectes sapidus). Aquat. Toxicol. 2002, 56, 303.
| Mercury accumulation and flux across the gills and the intestine of the blue crab (Callinectes sapidus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtlSktrw%3D&md5=bbe5d2033178df8156a273adcce98827CAS | 11856578PubMed |
[22] E. Dopp, L. M. Hartmann, A. M. Florea, A. W. Rettenmeier, A. V. Hirner, Environmental distribution, analysis, and toxicity of organometal(loid) compounds. Crit. Rev. Toxicol. 2004, 34, 301.
| Environmental distribution, analysis, and toxicity of organometal(loid) compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlt1Cnu7o%3D&md5=42ac5993eeef0b020afa686d4f95ff37CAS | 15239389PubMed |
[23] A. Turner, E. Mawji, Hydrophobicity and octanol–water partitioning of trace metals in natural waters. Environ. Sci. Technol. 2004, 38, 3081.
| Hydrophobicity and octanol–water partitioning of trace metals in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs1ensL0%3D&md5=925b2ba9c96a197d130eb71326b082e0CAS | 15224739PubMed |
[24] A. Turner, E. Mawji, Octanol-solubility of dissolved and particulate trace metals in contaminated rivers: implications for metal reactivity and availability. Environ. Pollut. 2005, 135, 235.
| Octanol-solubility of dissolved and particulate trace metals in contaminated rivers: implications for metal reactivity and availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsleisL8%3D&md5=8e2b8e0961a0716500fe1ae005d58f5eCAS | 15734583PubMed |
[25] A. Turner, E. Mawji, Hydrophobicity and reactivity of trace metals in the low-salinity zone of a turbid estuary. Limnol. Oceanogr. 2005, 50, 1011.
| Hydrophobicity and reactivity of trace metals in the low-salinity zone of a turbid estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1Chsbw%3D&md5=198efc2a4357a13c86e5a26710e76b99CAS |
[26] A. Turner, I. Williamson, Octanol–water partitioning of chemical constituents in river water and treated sewage effluent. Water Res. 2005, 39, 4325.
| Octanol–water partitioning of chemical constituents in river water and treated sewage effluent.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGhsLbP&md5=cc601110fc8cc24135f458e29416f1c8CAS | 16225903PubMed |
[27] P. Bell, M. J. McLaughlin, G. Cozens, D. P. Stevens, G. Owens, H. South, Plant uptake of 14C-EDTA, 14C-citrate, and 14C-histidine from chelator-buffered and conventional hydroponic solutions. Plant Soil 2003, 253, 311.
| Plant uptake of 14C-EDTA, 14C-citrate, and 14C-histidine from chelator-buffered and conventional hydroponic solutions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsFKru7c%3D&md5=36003d971199447d7d5b62b92d6c721cCAS |
[28] H. Svennerstam, S. Jämtgård, I. Ahmad, K. Huss-Danell, T. Näsholm, U. Ganeteg, Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytol. 2011, 191, 459.
| Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFSqtr%2FF&md5=8c69398141adb171996f6d2387f3ab9fCAS | 21453345PubMed |
[29] B. P. Krom, J. B. Warner, W. N. Konings, J. S. Lolkema, Complementary metal ion specificity of the metal–citrate transporters CitM and CitH of Bacillus subtilis. J. Bacteriol. 2000, 182, 6374.
| Complementary metal ion specificity of the metal–citrate transporters CitM and CitH of Bacillus subtilis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnvVGhu74%3D&md5=c3d646bd822e1ecf47f174ae34101bf5CAS | 11053381PubMed |
[30] V. S. Blancato, C. Magni, J. S. Lolkema, Functional characterization and Me2+ ion specificity of a Ca2+–citrate transporter from Enterococcus faecalis. FEBS J. 2006, 273, 5121.
| Functional characterization and Me2+ ion specificity of a Ca2+–citrate transporter from Enterococcus faecalis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yqs7zJ&md5=dc30f20a599f33eb49a47b515dc069c5CAS | 17042778PubMed |
[31] O. Errecalde, M. Seidl, P. G. C. Campbell, Influence of a low-molecular-weight metabolite (citrate) on the toxicity of cadmium and zinc to the unicellular green alga Selenastrum capricornutum: an exception to the free-ion model. Water Res. 1998, 32, 419.
| Influence of a low-molecular-weight metabolite (citrate) on the toxicity of cadmium and zinc to the unicellular green alga Selenastrum capricornutum: an exception to the free-ion model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXns1OgtQ%3D%3D&md5=dd82fb5343e4d6ea2ed3840ba6635c0eCAS |
[32] D. A. Hutchins, A. E. Witter, A. Butler, G. W. Luther, Competition among marine phytoplankton for different chelated iron species. Nature 1999, 400, 858.
| Competition among marine phytoplankton for different chelated iron species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlslGls74%3D&md5=7d41b1d81ff3e0f8ce78a9dd0f580ed0CAS |
[33] J. B. Neilands, Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723.
| Siderophores: structure and function of microbial iron transport compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXpsVGmurw%3D&md5=f5f5a4b71f562621d11be31c0de38d0dCAS | 7592901PubMed |
[34] C. Fortin, P. G. C. Campbell, Thiosulfate enhances silver uptake by a green alga: role of anion transporters in metal uptake. Environ. Sci. Technol. 2001, 35, 2214.
| Thiosulfate enhances silver uptake by a green alga: role of anion transporters in metal uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXivVCgsLo%3D&md5=b935df4904b0c3e55c0729c47d2d1c54CAS | 11414021PubMed |
[35] V. P. Hiriart-Baer, C. Fortin, D.-Y. Lee, P. G. C. Campbell, Toxicity of silver to two freshwater algae, Chlamydomonas reinhardtii and Pseudokirchneriella subcapitata, grown under continuous culture conditions: influence of thiosulphate. Aquat. Toxicol. 2006, 78, 136.
| Toxicity of silver to two freshwater algae, Chlamydomonas reinhardtii and Pseudokirchneriella subcapitata, grown under continuous culture conditions: influence of thiosulphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltVeksbs%3D&md5=7dae7ef6a9c73e8c658131eb5e66ede9CAS | 16621059PubMed |
[36] H. W. van Veen, T. Abee, G. J. J. Kortstee, W. N. Konings, A. J. B. Zehnder, Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 1994, 33, 1766.
| Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXht12hsbg%3D&md5=161fb04376fb7c2314defb15dad62803CAS | 8110778PubMed |
[37] J. G. Moberly, A. Staven, R. K. Sani, B. M. Peyton, Influence of pH and inorganic phosphate on toxicity of zinc to Arthrobacter sp. isolated from heavy-metal-contaminated sediments. Environ. Sci. Technol. 2010, 44, 7302.
| Influence of pH and inorganic phosphate on toxicity of zinc to Arthrobacter sp. isolated from heavy-metal-contaminated sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFGit7k%3D&md5=7bc373ceba520acafa740305dfa34f5eCAS | 20553043PubMed |
[38] G. P. Bienert, M. Thorsen, M. D. Schüssler, H. R. Nilsson, A. Wagner, M. J. Tamás, T. P. Jahn, A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008, 6, 26.
| A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes.Crossref | GoogleScholarGoogle Scholar | 18544156PubMed |
[39] A. Porquet, M. Filella, Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chem. Res. Toxicol. 2007, 20, 1269.
| Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVelu74%3D&md5=fca02d59a2146142a4788a66a284739cCAS | 17713961PubMed |
[40] H.-C. Yang, H.-L. Fu, Y.-F. Lin, B. P. Rosen, Pathways of arsenic uptake and efflux, in Metal Transporters (Eds S. Lutsenko, J.M. Arguello) 2012, Vol. 69, pp. 323–358 (Academic Press: San Diego, CA)
[41] R. Mukhopadhyay, H. Bhattacharjee, B. P. Rosen, Aquaglyceroporins: generalized metalloid channels. Biochimica et Biophysica Acta (BBA) – General Subjects 1840, 2014, 1583.
[42] K. J. Wilkinson, P. M. Bertsch, C. H. Jagoe, P. G. C. Campbell, Surface complexation of aluminium on isolated fish gill cells. Environ. Sci. Technol. 1993, 27, 1132.
| Surface complexation of aluminium on isolated fish gill cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXit1Oks7o%3D&md5=3fd5b84931c090ac9f1d9dc02164be21CAS |
[43] C. Lamelas, K. J. Wilkinson, V. I. Slaveykova, Influence of the composition of natural organic matter on Pb bioavailability to microalgae. Environ. Sci. Technol. 2005, 39, 6109.
| Influence of the composition of natural organic matter on Pb bioavailability to microalgae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvVKks7s%3D&md5=84910a2d77986b5722afa20633c072e2CAS | 16173570PubMed |
[44] L. Aristilde, Y. Xu, F. M. M. Morel, Weak organic ligands enhance zinc uptake in marine phytoplankton. Environ. Sci. Technol. 2012, 46, 5438.
| Weak organic ligands enhance zinc uptake in marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xltl2ht7c%3D&md5=ffaa065c4e60bbf80aa23b525f29d3afCAS | 22494184PubMed |
[45] C.-M. Zhao, K. J. Wilkinson, Biotic ligand model does not predict the bioavailability of rare earth elements in the presence of organic ligands. Environ. Sci. Technol. 2015, 49, 2207.
| Biotic ligand model does not predict the bioavailability of rare earth elements in the presence of organic ligands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVCgtbg%3D&md5=0938f9fd2a2fc5896c43ffc3c3383272CAS | 25611881PubMed |
[46] C. S. Hassler, V. I. Slaveykova, K. J. Wilkinson, Discriminating between intra- and extracellular metals using chemical extractions. Limnol. Oceanogr. Methods 2004, 2, 237.
| Discriminating between intra- and extracellular metals using chemical extractions.Crossref | GoogleScholarGoogle Scholar |
[47] G. Yang, Q.-G. Tan, L. Zhu, K. J. Wilkinson, The role of complexation and competition in the biouptake of europium by a unicellular alga. Environ. Toxicol. Chem. 2014, 33, 2609.
| The role of complexation and competition in the biouptake of europium by a unicellular alga.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslKnsb%2FI&md5=4e281cd28b1603a06dfad4ff1581f284CAS | 25132226PubMed |
[48] R. J. M. Hudson, Which aqueous species control the rates of trace metal uptake by aquatic biota? Observations and predictions of non-equilibrium effects. Sci. Total Environ. 1998, 219, 95.
| Which aqueous species control the rates of trace metal uptake by aquatic biota? Observations and predictions of non-equilibrium effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlslWltrw%3D&md5=e65588742d315cd03a9c2941cf8d76beCAS |
[49] C. Lamelas, V. I. Slaveykova, Comparison of CdII, CuII, and PbII biouptake by green algae in the presence of humic acid. Environ. Sci. Technol. 2007, 41, 4172.
| Comparison of CdII, CuII, and PbII biouptake by green algae in the presence of humic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvVCltLk%3D&md5=43610ac85161168563d63d916c41998fCAS | 17612207PubMed |
[50] C. Lamelas, J. P. Pinheiro, V. I. Slaveykova, Effect of humic acid on CdII, CuII, and PbII uptake by freshwater algae: kinetic and cell wall speciation considerations. Environ. Sci. Technol. 2009, 43, 730.
| Effect of humic acid on CdII, CuII, and PbII uptake by freshwater algae: kinetic and cell wall speciation considerations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVSqtg%3D%3D&md5=1574edbb755920600a17eb55e9127dbeCAS | 19245009PubMed |
[51] A. Gramlich, S. Tandy, E. Frossard, J. Eikenberg, R. Schulin, Availability of zinc and the ligands citrate and histidine to wheat: does uptake of entire complexes play a role? J. Agric. Food Chem. 2013, 61, 10409.
| Availability of zinc and the ligands citrate and histidine to wheat: does uptake of entire complexes play a role?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFGhu7%2FI&md5=b89497f503f1acadf7b7d8dab5a5a028CAS | 24147770PubMed |
[52] K. J. Wilkinson, P. G. C. Campbell, P. Couture, Effect of fluoride complexation on aluminium toxicity towards juvenile Atlantic salmon (Salmo Salar). Can. J. Fish. Aquat. Sci. 1990, 47, 1446.
| Effect of fluoride complexation on aluminium toxicity towards juvenile Atlantic salmon (Salmo Salar).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlslSmtw%3D%3D&md5=a9d37987b01cc22cabb08cc288ef600fCAS |
[53] A. Crémazy, P. G. C. Campbell, C. Fortin, The biotic ligand model can successfully predict the uptake of a trivalent ion by a unicellular alga below pH 6.50 but not above: possible role of hydroxo-species. Environ. Sci. Technol. 2013, 47, 2408.
| The biotic ligand model can successfully predict the uptake of a trivalent ion by a unicellular alga below pH 6.50 but not above: possible role of hydroxo-species.Crossref | GoogleScholarGoogle Scholar | 23360212PubMed |
[54] W. G. Sunda, S. A. Huntsman, Feedback interactions between zinc and phytoplankton in seawater. Limnol. Oceanogr. 1992, 37, 25.
| Feedback interactions between zinc and phytoplankton in seawater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltVOqsrg%3D&md5=41e2fc3e4a865ad5150e972c47f5aa52CAS |
[55] L. Parent, M. R. Twiss, P. G. C. Campbell, Influences of natural dissolved organic matter on the interaction of aluminium with the microalga Chlorella: a test of the free-ion model of trace metal toxicity. Environ. Sci. Technol. 1996, 30, 1713.
| Influences of natural dissolved organic matter on the interaction of aluminium with the microalga Chlorella: a test of the free-ion model of trace metal toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhvVKnsr4%3D&md5=1ceb420d84eeb732b494566f3d52dfdeCAS |
[56] E. Granéli, P. Carlsson, C. Legrand, The role of C, N and P in dissolved and particulate organic matter as a nutrient source for phytoplankton growth, including toxic species. Aquat. Ecol. 1999, 33, 17.
| The role of C, N and P in dissolved and particulate organic matter as a nutrient source for phytoplankton growth, including toxic species.Crossref | GoogleScholarGoogle Scholar |
[57] J. Shapiro, Chemical and biological studies on the yellow organic acids of lake water. Limnol. Oceanogr. 1957, 2, 161.
[58] S. A. Green, N. V. Blough, Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol. Oceanogr. 1994, 39, 1903.
| Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXks1SrurY%3D&md5=2d0d7b28b8552fedac612da825e7c973CAS |
[59] P. G. C. Campbell, M. R. Twiss, K. J. Wilkinson, Accumulation of natural organic matter on the surfaces of living cells: implications for the interaction of toxic solutes with aquatic biota. Can. J. Fish. Aquat. Sci. 1997, 54, 2543.
| Accumulation of natural organic matter on the surfaces of living cells: implications for the interaction of toxic solutes with aquatic biota.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhsVGqtrk%3D&md5=a6657d107a28bfe5930861bbfcf6597bCAS |
[60] L. P. Sanford, S. M. Crawford, Mass transfer versus kinetic control of uptake across solid–water boundaries. Limnol. Oceanogr. 2000, 45, 1180.
| Mass transfer versus kinetic control of uptake across solid–water boundaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlvFCrt7g%3D&md5=25ab3d92e6d2f32636791fba7227d4b1CAS |
[61] C. S. Hassler, K. J. Wilkinson, Failure of the biotic ligand and free-ion activity models to explain zinc bioaccumulation by Chlorella kesslerii. Environ. Toxicol. Chem. 2003, 22, 620.
| Failure of the biotic ligand and free-ion activity models to explain zinc bioaccumulation by Chlorella kesslerii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlCkurs%3D&md5=d36b149a26d7863fcd8e6cae7bae126fCAS | 12627651PubMed |
[62] S. Meylan, R. Behra, L. Sigg, Influence of metal speciation in natural freshwater on bioaccumulation of copper and zinc in periphyton: a microcosm study. Environ. Sci. Technol. 2004, 38, 3104.
| Influence of metal speciation in natural freshwater on bioaccumulation of copper and zinc in periphyton: a microcosm study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs1ensLg%3D&md5=6357850bd9a82c5a199a955966c1b64cCAS | 15224742PubMed |
[63] S. Jansen, R. Blust, H. P. Van Leeuwen, Metal speciation dynamics and bioavailability: ZnII and CdII uptake by mussel (Mytilus edulis) and carp (Cyprinus carpio). Environ. Sci. Technol. 2002, 36, 2164.
| Metal speciation dynamics and bioavailability: ZnII and CdII uptake by mussel (Mytilus edulis) and carp (Cyprinus carpio).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitVOjtrc%3D&md5=ac0650cbbf81387d35583f12d4639332CAS | 12038825PubMed |
[64] F. Degryse, E. Smolders, R. Merckx, Labile Cd complexes increase Cd availability to plants. Environ. Sci. Technol. 2006, 40, 830.
| Labile Cd complexes increase Cd availability to plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlarsrfK&md5=cd86a770c906b0380699551230062719CAS | 16509325PubMed |
[65] I. A. Newman, Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ. 2001, 24, 1.
| Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlygu7g%3D&md5=098e6a724f026c510fca688d386d1efeCAS | 11762438PubMed |
[66] J. Harskamp, M. O’Donnell, E. Berkelaar, Determining the fluxes of Tl+ and K+ at the root surface of wheat and canola using TlI and K ion-selective microelectrodes. Plant Soil 2010, 335, 299.
| Determining the fluxes of Tl+ and K+ at the root surface of wheat and canola using TlI and K ion-selective microelectrodes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFOrt7zL&md5=f835fd239903bd4188acb840904ba676CAS |
[67] E. W. Koch, Hydrodynamics, diffusion-boundary layers and photosynthesis of the seagrasses Thalassia testudinum and Cymodocea nodosa. Mar. Biol. 1994, 118, 767.
| Hydrodynamics, diffusion-boundary layers and photosynthesis of the seagrasses Thalassia testudinum and Cymodocea nodosa.Crossref | GoogleScholarGoogle Scholar |
[68] F. I. M. Thomas, M. J. Atkinson, Ammonium uptake by coral reefs: effects of water velocity and surface roughness on mass transfer. Limnol. Oceanogr. 1997, 42, 81.
| Ammonium uptake by coral reefs: effects of water velocity and surface roughness on mass transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtVCltr4%3D&md5=d07de90b1fbe295beaecfbac4c177e82CAS |
[69] T. N. P. Bosma, P. J. M. Middeldorp, G. Schraa, A. J. B. Zehnder, Mass transfer limitation of biotransformation: quantifying bioavailability. Environ. Sci. Technol. 1997, 31, 248.
| Mass transfer limitation of biotransformation: quantifying bioavailability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntVyjsrs%3D&md5=07e1f0584c747c0209e923ffd6a69804CAS |
[70] G. Mierle, Kinetics of phosphate transport by Synechococcus leopoliensis (Cyanophyta): evidence for diffusion limitation of phosphate uptake. J. Phycol. 1985, 21, 177.
| Kinetics of phosphate transport by Synechococcus leopoliensis (Cyanophyta): evidence for diffusion limitation of phosphate uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXks1yjtL8%3D&md5=47e7230f9a506615e3d49d4d52297b9eCAS |
[71] P. Sánchez-Marín, C. Fortin, P. G. C. Campbell, Copper and lead internalisation by freshwater microalgae at different carbonate concentrations. Environ. Chem. 2013, 10, 80.
| Copper and lead internalisation by freshwater microalgae at different carbonate concentrations.Crossref | GoogleScholarGoogle Scholar |
[72] J. P. Pinheiro, J. Galceran, H. P. van Leeuwen, Metal speciation dynamics and bioavailability: bulk depletion effects. Environ. Sci. Technol. 2004, 38, 2397.
| Metal speciation dynamics and bioavailability: bulk depletion effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhslCltL8%3D&md5=cce1f8457aba09f579081a6506488dcdCAS | 15116846PubMed |
[73] S. Leclerc, K. J. Wilkinson, Bioaccumulation of nanosilver by Chlamydomonas reinhardtii – nanoparticle or the free ion? Environ. Sci. Technol. 2014, 48, 358.
| Bioaccumulation of nanosilver by Chlamydomonas reinhardtii – nanoparticle or the free ion?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvV2mtL%2FO&md5=7fd03d15999d7461cf35ccbc5a4e1fbeCAS | 24320028PubMed |
[74] J. P. Pinheiro, H. P. van Leeuwen, Metal speciation dynamics and bioavailability. 2. Radial diffusion effects in the microorganism range. Environ. Sci. Technol. 2001, 35, 894.
| Metal speciation dynamics and bioavailability. 2. Radial diffusion effects in the microorganism range.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnvVOnsA%3D%3D&md5=c228096eb9a69820bd1f7387761ba4dcCAS | 11351532PubMed |
[75] K. J. Wilkinson, J. Buffle, Critical evaluation of physicochemical parameters and processes for modelling the biological uptake of trace metals in environmental (aquatic) systems, in Physicochemical Kinetics and Transport at Biointerfaces (Eds H. P. van Leeuwen, W. Köster) 2004, pp. 445–533 (Wiley: Chichester, UK).
[76] M. Eigen, R. G. Wilkins, The kinetics and mechanism of formation of metal complexes, in Mechanisms of Inorganic Reactions (Eds J. Kleinberg, R. K. Murmann, R. T. M. Fraser, J. Bauman) 1965, pp. 55–80 (American Chemical Society: Washington, DC).
[77] R. G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes 1991 (Wiley-VCH: Weinheim, Germany).
[78] R. J. M. Hudson, F. M. M. Morel, Iron transport in marine phytoplankton: kinetics of cellular and medium coordination reactions. Limnol. Oceanogr. 1990, 35, 1002.
| Iron transport in marine phytoplankton: kinetics of cellular and medium coordination reactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitFersrg%3D&md5=7d4daf9d36893993bf347db39879ada9CAS |
[79] S. A. Thomas, T. Tong, J.-F. Gaillard, HgII bacterial biouptake: the role of anthropogenic and biogenic ligands present in solution and spectroscopic evidence of ligand exchange reactions at the cell surface. Metallomics 2014, 6, 2213.
| HgII bacterial biouptake: the role of anthropogenic and biogenic ligands present in solution and spectroscopic evidence of ligand exchange reactions at the cell surface.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslekt7vL&md5=34e1339d7b003bca64d9ca83ccf878d9CAS | 25322360PubMed |
[80] J. Buffle, Z. Zhang, K. Startchev, Metal flux and dynamic speciation at (bio)interfaces. Part 1: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ. Sci. Technol. 2007, 41, 7609.
| Metal flux and dynamic speciation at (bio)interfaces. Part 1: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1ymtr3E&md5=fb4284759a26f24ddba7b97ff29754c3CAS | 18075065PubMed |
[81] D. Tran, A. Boudou, J.-C. Massabuau, How water oxygenation level influences cadmium accumulation pattern in the Asiatic clam Corbicula fluminea: a laboratory and field study. Environ. Toxicol. Chem. 2001, 20, 2073.
| How water oxygenation level influences cadmium accumulation pattern in the Asiatic clam Corbicula fluminea: a laboratory and field study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmt12ltLs%3D&md5=64467611ca4be9616e4d83de701bc702CAS | 11521837PubMed |
[82] H. P. van Leeuwen, Metal speciation dynamics and bioavailability: inert and labile complexes. Environ. Sci. Technol. 1999, 33, 3743.
| Metal speciation dynamics and bioavailability: inert and labile complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvV2jtLc%3D&md5=86d6b6545e1a559828b61a166366fe08CAS |
[83] F. Galvez, A. Donini, R. C. Playle, D. S. Smith, M. J. O’Donnell, C. M. Wood, A matter of potential concern: natural organic matter alters the electrical properties of fish gills. Environ. Sci. Technol. 2008, 42, 9385.
| A matter of potential concern: natural organic matter alters the electrical properties of fish gills.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlKqtL%2FO&md5=a3c7f61beb9554b1c168caac267995e1CAS | 19174920PubMed |
[84] C. E. W. Steinberg, NOM as natural xenobiotics, in Advances in the Physicochemical Characterization of Dissolved Organic Matter: Impact on Natural and Engineered Systems (Ed. F. Rosario-Ortiz) 2014, pp. 115–144 (American Chemical Society: Washington, DC).
[85] C. E. W. Steinberg, S. Kamara, V. Y. Prokhotskaya, L. Manusadžianas, T. A. Karasyova, M. A. Timofeyev, Z. Jie, A. Paul, T. Meinelt, V. F. Farjalla, A. Y. O. Matsuo, B. Kent Burnison, R. Menzel, Dissolved humic substances – ecological driving forces from the individual to the ecosystem level? Freshw. Biol. 2006, 51, 1189.
| Dissolved humic substances – ecological driving forces from the individual to the ecosystem level?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFyqtrk%3D&md5=028a267c917955f3999fe5ab9a0c0a71CAS |
A Labile complexes are those that can frequently associate and dissociate over the time frame of the transport from the bulk solution to the cell surface,[82] i.e. metal flux provided by complex dissociation >> maximum diffusion flux. More precise physicochemical definitions and equations are provided in Pinheiro and van Leeuwen.[74]
B Cells are first exposed to a radioactive metal (pulse) that is replaced by the same non-radiolabelled metal (chase). When uptake of radioactive metal is observed during the chase phase, it is possible to conclude that the rate of surface complex dissociation is less important than the internalisation rate (i.e. equilibrium was not attained among the metal species in solution and the biotic ligands).


