Similar irradiance-elicited plasticity of leaf traits in saplings of 12 tropical rainforest tree species with highly different leaf mass to area ratio
Sabrina Coste A , Jean-Christophe Roggy A , Gregory Sonnier A and Erwin Dreyer B CA AgroParisTech-ENGREF, INRA, Unité Mixte de Recherches CIRAD-ENGREF-INRA-CNRS ‘Ecologie des Forêts de Guyane’, Campus Agronomique de Kourou, F 97387 Kourou, Guyane, France.
B INRA, Nancy-Université, UMR 1137 ‘Ecologie et Ecophysiologie Forestières’, IFR 110 ‘Ecosystèmes Forestiers, Agroressources, Biomolécules et Alimentation’, F 54280 Champenoux, France.
C Corresponding author. Email: dreyer@nancy.inra.fr
Functional Plant Biology 37(4) 342-355 https://doi.org/10.1071/FP09119
Submitted: 25 May 2009 Accepted: 11 January 2010 Published: 26 March 2010
Abstract
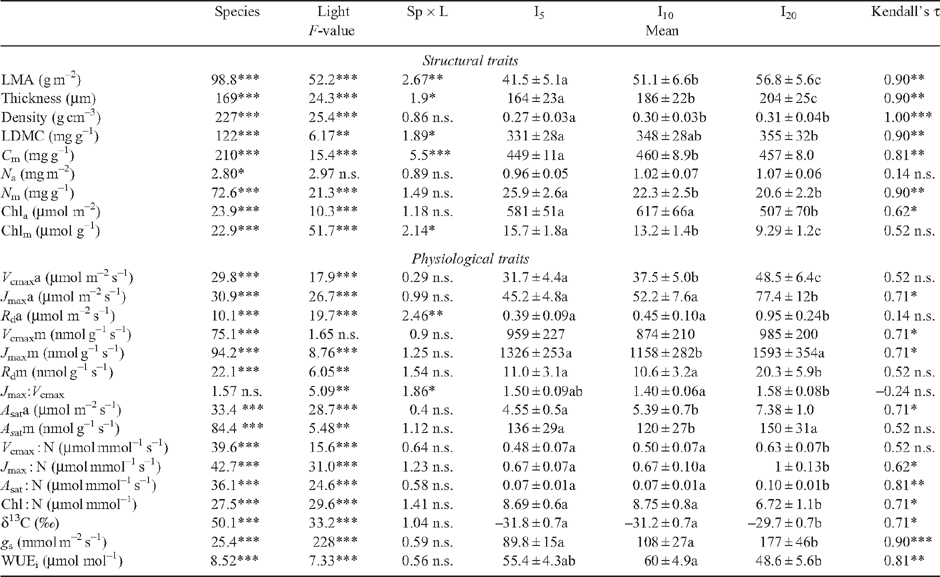
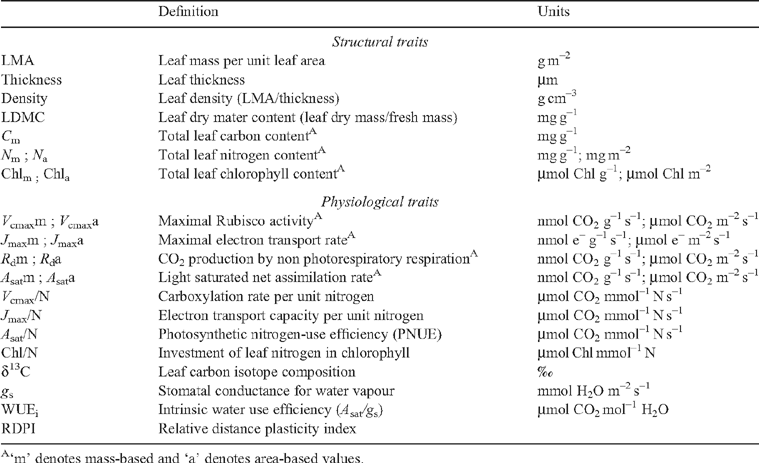
Leaf traits of tropical tree species display an important inter-specific diversity, as detected for instance in the large range of values of leaf mass : area ratio (LMA). They also demonstrate a large irradiance-elicited plasticity, but there is still debate whether this plasticity differs among species. To address this question, leaf traits were recorded on saplings from 12 rainforest tree species in French Guiana, grown under approximately 5, 10 and 20% relative irradiance. Fifteen structural and physiological leaf traits related to photosynthesis were measured. The irradiance-elicited plasticity was quantified using a relative distance plasticity index. A large inter-specific diversity was detected for all leaf traits. A principal component analysis opposed species with a large mass-based photosynthesis, respiration, N content and photosynthetic nitrogen use efficiency, to species with a large leaf mass : area ratio, LMA. The two pioneer species used in this study displayed the largest photosynthetic capacity (and lowest LMA) and ranked at one end of the species continuum. Relative irradiance affected almost all traits with the exception of mass-based photosynthesis. A weak interaction was found between species and relative irradiance and the species ranking was maintained among relative irradiance treatments for the majority of the traits. A principal component analysis of the values of relative-distance plasticity index failed to reveal any consistent patterns of traits or species. We concluded that irradiance-elicited plasticity of leaf traits was similar among species irrespective of LMA and successional status, despite the occurrence of a large inter-specific diversity for the investigated traits.
Additional keywords: functional diversity, light availability, photosynthetic nitrogen use efficiency, photosynthetic capacity, tropical rainforest.
Acknowledgements
S. Coste was supported by a PhD grant from the French Ministry for Higher Education and Research and by a grant for technical cooperation in overseas regions of France. The authors are indebted to Pascal Imbert, Saintano Dufort, Marcel Blaise, Jean-Louis De Kerpeztron and Henry Grootfaam (UMR Ecofog, Kourou) for their help throughout the experiment and to Claude Brechet and Jacqueline Marchand (UMR EEF, INRA, Nancy) for carbon, nitrogen and δ13C analysis, Christopher Baraloto (UMR Ecofog, Kourou) contributed to the delicate process of botanical identification and selection of the species while Jean-Yves Goret helped establish and maintain the experiment under the shading nets. Useful discussions with Eric Marcon, Christopher Baraloto and Heidy Schimann (UMR ECOFOG) and with Pierre Montpied and Daniel Epron (UMR EEF, INRA, Nancy) are gratefully acknowledged.
Baraloto C,
Goldberg DE, Bonal D
(2005) Performance trade-offs among tropical tree seedlings in contrasting microhabitats. Ecology 86, 2461–2472.
| Crossref | GoogleScholarGoogle Scholar |

Bazzaz FA, Pickett STA
(1980) Physiological ecology of tropical succession: a comparative review. Annual Review of Ecology and Systematics 11, 287–310.
| Crossref | GoogleScholarGoogle Scholar |

Bernacchi CJ,
Singsaas EL,
Pimentel C,
Portis AR, Long SP
(2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment 24, 253–259.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bloor JMG, Grubb PJ
(2003) Growth and mortality in high and low light: trends among 15 shade-tolerant tropical rain forest tree species. Journal of Ecology 91, 77–85.
| Crossref | GoogleScholarGoogle Scholar |

Bloor JMG, Grubb PJ
(2004) Morphological plasticity of shade-tolerant tropical rainforest tree seedlings exposed to light changes. Functional Ecology 18, 337–348.
| Crossref | GoogleScholarGoogle Scholar |

Bonal D,
Born C,
Brechet C,
Coste S,
Marcon E,
Roggy J-C, Guehl JM
(2007) The successional status of tropical rainforest tree species is associated with differences in leaf carbon isotope discrimination and functional traits. Annals of Forest Science 64, 169–176.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bradshaw AD
(1965) Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13, 115–155.
| Crossref | GoogleScholarGoogle Scholar |

Cernusak LA,
Aranda J,
Marshall JD, Winter K
(2007) Large variation in whole-plant water-use efficiency among tropical tree species. New Phytologist 173, 294–305.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cornelissen JHC,
Lavorel S,
Garnier E,
Diaz S, Buchmann N ,
et al
.
(2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51, 335–380.
| Crossref | GoogleScholarGoogle Scholar |

Coste S,
Roggy J-C,
Imbert P,
Born C,
Bonal D, Dreyer E
(2005) Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiology 25, 1127–1137.
|
CAS |
PubMed |

Coste S,
Baraloto C,
Leroy C,
Marcon E,
Renaud A,
Richardson AD,
Roggy JC,
Schimann H,
Uddling J, Hérault B
(2010) Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Annals of Forest Science ,

Ethier GJ, Livingston N
(2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell & Environment 27, 137–153.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Evans JR, von Caemmerer S
(1996) Update on photosynthesis: carbon dioxide diffusion inside the leaves. Plant Physiology 110, 339–346.
|
CAS |
PubMed |

Evans JR,
Kaldenhoff R,
Genty B, Terashima I
(2009) Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Farquhar GD, Richards RA
(1984) Isotopic composition of plant carbon correlates with water-use efficiency in wheat genotypes. Australian Journal of Plant Physiology 11, 539–552.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Farquhar GD,
von Caemmerer S, Berry JA
(1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Fetcher N,
Strain BR, Oberbauer SF
(1983) Effects of light regime on the growth, leaf morphology, and water relations of seedlings of two species of tropical trees. Oecologia 58, 314–319.
| Crossref | GoogleScholarGoogle Scholar |

Frak E,
Le Roux X,
Millard P,
Dreyer E,
Jaouen G,
Saint-Joanis B, Wendler R
(2001) Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant, Cell & Environment 24, 1279–1288.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Givnish TJ
(1988) Adaptation to sun and shade: a whole plant perspective. Australian Journal of Plant Physiology 15, 63–92.
| Crossref | GoogleScholarGoogle Scholar |

Givnish TJ,
Montgomery RA, Goldstein G
(2004) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. American Journal of Botany 91, 228–246.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Le Roux X,
Grand S,
Dreyer E, Daudet FA
(1999) Parameterization and testing of a biochemically based photosynthesis model for walnut trees (Juglans regia) and seedlings. Tree Physiology 19, 481–492.
| PubMed |

Lusk CH,
Reich PB,
Montgomery RA,
Ackerly DD, Cavender-Bares J
(2008) Why are evergreen leaves so contrary about shade? Trends in Ecology & Evolution 23, 299–303.
| Crossref | GoogleScholarGoogle Scholar |

Markesteijn L,
Poorter L, Bongers F
(2007) Light-dependent leaf trait variation in 43 tropical dry forest species. American Journal of Botany 94, 515–525.
| Crossref | GoogleScholarGoogle Scholar |

Meir P,
Kruijt B,
Broadmeadow M,
Barbosa E,
Kull O,
Carswell F,
Nobre A, Jarvis PG
(2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant, Cell & Environment 25, 343–357.
| Crossref | GoogleScholarGoogle Scholar |

Miner BG,
Sultan SE,
Morgan SG,
Padilla DK, Relyea RA
(2005) Ecological consequences of phenotypic plasticity. Trends in Ecology & Evolution 20, 685–692.
| Crossref | GoogleScholarGoogle Scholar |

Molino J-F, Sabatier D
(2001) Tree diversity in tropical rain forests: a validation of the intermediate disturbance hypothesis. Science 294, 1702–1704.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Montpied P,
Granier A, Dreyer E
(2009) Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. Journal of Experimental Botany 60, 2407–2418.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Nicotra A,
Chazdon RL, Schlichting CD
(1997) Patterns of genotypic variation and phenotypic plasticity of light responses in two tropical Piper (Piperaceae) species. American Journal of Botany 84, 1542–1552.
| Crossref | GoogleScholarGoogle Scholar |

Niinemets Ü
(2006) The controversy over traits conferring shade-tolerance in trees: ontogenetic changes revisited. Journal of Ecology 94, 464–470.
| Crossref | GoogleScholarGoogle Scholar |

Niinemets Ü, Tenhunen JD
(1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade tolerant species Acer saccharum. Plant, Cell & Environment 20, 845–866.
| Crossref | GoogleScholarGoogle Scholar |

Piel C,
Frak E,
Le Roux X, Genty B
(2002) Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut. Journal of Experimental Botany 53, 2423–2430.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Pons TL, Pearcy RW
(1994) Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiologia Plantarum 92, 636–644.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Poorter L
(2007) Are species adapted to their regeneration niche, adult niche, or both? American Naturalist 169, 433–442.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Poorter H, Evans JR
(1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116, 26–37.
| Crossref | GoogleScholarGoogle Scholar |

Poorter L, Werger MJA
(1999) Light environment, sapling architecture, and leaf display in six rain forest tree species. American Journal of Botany 86, 1464–1473.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Poorter L,
Oberbauer SF, Clark DB
(1995) Leaf optical properties along a vertical gradient in a tropical rain forest canopy in Costa Rica. American Journal of Botany 82, 1257–1263.
| Crossref | GoogleScholarGoogle Scholar |

Poorter H,
Niinemets Ü,
Poorter L,
Wright IJ, Villar R
(2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182, 565–588.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Popma J,
Bongers F, Werger MJA
(1992) Gap-dependence and leaf characteristics of trees in a tropical lowland rain forest in Mexico. Oikos 63, 207–214.
| Crossref | GoogleScholarGoogle Scholar |

Reich PB, Walters MB
(1994) Photosynthesis-nitrogen relations in Amazonian tree species. II: Variation in nitrogen vis-a-vis specific leaf area influences mass and area-based expressions. Oecologia 97, 73–81.
| Crossref | GoogleScholarGoogle Scholar |

Reich PB,
Ellsworth DS,
Walters MB,
Vose JM,
Gresham C,
Volin JC, Bowman WD
(1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80, 1955–1969.
| Crossref |

Rice SA, Bazzaz FA
(1989) Growth consequences of plasticity of plant traits in response to light conditions. Oecologia 78, 508–512.
| Crossref | GoogleScholarGoogle Scholar |

Rijkers T,
Pons TL, Bongers F
(2000) The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Functional Ecology 14, 77–86.
| Crossref | GoogleScholarGoogle Scholar |

Roggy J-C,
Nicolini E,
Imbert P,
Caraglio Y,
Bosc A, Heuret P
(2005) Links between tree structure and functional leaf traits in the tropical forest tree Dicorynia guianensis Amshoff (Caesalpiniaceae). Annals of Forest Science 62, 553–564.
| Crossref | GoogleScholarGoogle Scholar |

Roussel M,
Dreyer E,
Montpied P,
Le Provost G,
Guehl JM, Brendel O
(2009) The diversity of 13C isotope discrimination in a Quercus robur full-sib family is associated with differences in intrinsic water use efficiency, transpiration efficiency, and stomatal conductance. Journal of Experimental Botany 60, 2419–2431.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rozendaal DMA,
Hurtado VH, Poorter L
(2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Functional Ecology 20, 207–216.
| Crossref | GoogleScholarGoogle Scholar |

Valladares F, Niinemets U
(2008) Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology and Systematics 39, 237–257.
| Crossref | GoogleScholarGoogle Scholar |

Valladares F,
Wright SJ,
Lasso E,
Kitajima K, Pearcy RW
(2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81, 1925–1936.
| Crossref |

Valladares F,
Sanchez-Gomez D, Zavala MA
(2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94, 1103–1116.
| Crossref | GoogleScholarGoogle Scholar |

Warren CR, Adams MA
(2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant, Cell & Environment 29, 192–201.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Warren CR,
Dreyer E, Adams MA
(2003) Photosynthesis–Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees – Structure and Function 17, 359–366.
|
CAS |

Wright IJ,
Reich PB,
Westoby M,
Ackerly DD, Baruch Z ,
et al
.
(2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wright IJ,
Reich PB,
Cornelissen JHC,
Falster DS, Garnier E ,
et al
.
(2005) Assessing the generality of global leaf trait relationships. New Phytologist 166, 485–496.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

|