Salinity drives host reaction in Phaseolus vulgaris (common bean) to Macrophomina phaseolina
Ming Pei You A C , Timothy D. Colmer A B and Martin J. Barbetti A BA School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B The UWA Institute of Agriculture, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Corresponding author. Email: mingpei.you@uwa.edu.au
Functional Plant Biology 38(12) 984-992 https://doi.org/10.1071/FP11137
Submitted: 9 June 2011 Accepted: 20 October 2011 Published: 17 November 2011
Abstract
Productivity of Phaseolus vulgaris L. (common bean) is often limited by diseases such as seedling blight and root and stem rot caused by the fungus Macrophomina phaseolina and by abiotic stresses such as salinity. This paper reports controlled environment studies examining the interaction of biotic (M. phaseolina) and abiotic (NaCl) stresses. Studies were conducted at 32°C. On potato dextrose agar, the growth of two isolates of M. phaseolina (M1, M2) was differentially stimulated by 40 mM NaCl with 1 mM CaSO4. M. phaseolina was applied as either soil-borne inoculum or directly injected into P. vulgaris hypocotyls. For direct hypocotyl inoculation experiments, there was no difference in disease severity resulting from the two isolates. However, when soil inoculation was undertaken, isolate M2 caused more disease than M1. Addition of 40 mM NaCl to the soil increased disease development and severity (evident 4 days after inoculation), particularly as demonstrated in the hypocotyl inoculation tests, suggesting that salinity stress predisposes plants to infection by this pathogen. Plants infested by M. phaseolina showed increased tissue concentrations of Na+ and Cl– but decreased K+ concentration. Hypocotyls generally contained higher Na+ concentrations than shoots. Inoculated plants had higher Na+ and lower K+ concentrations than uninoculated plants. Our studies indicate that M. phaseolina will be a more severe disease threat where P. vulgaris is cultivated in areas affected by soil salinity.
Additional keywords: ashy grey stem, biotic-abiotic interactions charcoal rot, Macrophomina phaseolina.
Introduction
Phaseolus vulgaris L. (common bean) is the world’s most important food legume for direct human consumption, especially in Latin America and in eastern and southern Africa. Some 12 million metric tons are produced annually worldwide, of which ~8 million tons are from Latin America and Africa (FAO 2005).
P. vulgaris productivity is often limited by diseases (Schwartz and Pastor Corrales 1989). Macrophomina phaseolina (charcoal rot, ashy grey stem) causes seedling blight, root rot and stem rot of more than 500 cultivated and wild plant species (Sinclair 1982; Mihail and Taylor 1995; Srivastava et al. 2001) including common bean (Dhingra and Sinclair 1987). This pathogen is a problem in North and South America including the Dominican Republic (Sanchez 1989) and Puerto Rico (Echavez-Badel and Beaver 1987), in Asia, Africa, Europe, and also in Australia (Watson 2009). Generally, it is economically most important in subtropical and tropical countries with a semiarid climate (Wrather et al. 1997; Wrather et al. 2001) and causes severe or even complete losses in arid regions where P. vulgaris experiences water deficits (Mayek-Perez et al. 2001). In Australia, it can be a serious problem on P. vulgaris and commonly reduces both yield and quality, with grain yield dramatically reduced by heavy infestations of M. phaseolina during the reproductive phase in combination with hot and dry conditions (Redden et al. 1997). Thus, there is a strong association between the occurrence of drought and susceptibility to M. phaseolina (Gray et al. 1991; Manici et al. 1995; Mayek-Perrez et al. 1997).
In P. vulgaris, disease caused by M. phaseolina is characterised in young plants by black, irregular lesions which form at the base of the cotyledons and extend to the hypocotyl and stem causing strangulation and death. In adult plants, wilting and blockage of the vascular system occurs with production of black or grey microsclerotia. Salinity is one of the major factors affecting agricultural productivity worldwide, especially in the arid and semiarid areas. Even in the fertile Crescent of Jordan, Palestine, Lebanon, Syria and Iraq, and along the Nile Valley (including Egypt and Sudan), where P. vulgaris is a major vegetable crop, ~20–30% of the P. vulgaris production areas are affected by soil salinity (Bayuelo-Jiménez et al. 2002b), resulting in low yields as P. vulgaris is extremely sensitive to salinity and suffers yield losses at soil salinity levels less than 2 dS m–1 (Läuchli 1984). The physiological responses of P. vulgaris to salinity stress are widely documented and vary significantly between P. vulgaris genotypes (Gama et al. 2007), when exposed to salinity at germination, seedling stage (Bayuelo-Jiménez et al. 2002a) and early vegetative growth (Bayuelo-Jiménez et al. 2002b). Salinity retards plant growth as it reduces the ability of plants to take up water, and when Na+ and Cl– accumulate to high concentrations in tissues this interferes with plant metabolic processes, with direct toxicity or nutrient imbalance because Na+ competes with K+ for binding sites essential for cellular function (Tester and Davenport 2003; Munns and Tester 2008).
The idea of developing P. vulgaris cultivars with resistance to both biotic (e.g. disease) and abiotic (e.g. drought, salinity) stress over a broad range of environments has been canvassed by breeders (Acosta-Gallegos 1998) and the need for selection of P. vulgaris genotypes with resistance to pathogens and adaptation to variable environments is now more widely recognised (Mayek-Perrez et al. 2003). Moreover, higher concentrations of salts in irrigation water have been linked to increased susceptibility to M. phaseolina of sunflower (El Mahjoub et al. 1979) and melon (Nischwitz et al. 2002), suggesting that salinity could be an important factor in the increased incidence of disease from M. phaseolina generally in crops.
This paper reports experiments that demonstrate salinity does increase severity of disease caused by M. phaseolina, and that salinity interacts with M. phaseolina to increase pathogen growth rate on agar plates and disease severity when in soil. This study also shows that infection by M. phaseolina can result in further increases in tissue Na+ and Cl–, and decreased K+, in P. vulgaris in saline conditions.
Materials and methods
Cultivars and pathogen isolates
The Phaseolus vulgaris L. cultivars used were Borlotti, Brown Beauty, Gourmet Delight and Pioneer. Two isolates of Macrophomina phaseolina were used; these were isolated from strawberry (M1 and M2; Fang et al. 2011) in an area where legume crops including peas (Pisum sativum L.) had been severely affected by M. phaseolina.
Experimental conditions
Laboratory tests of growth of isolates M1 and M2 of the fungal pathogen M. phaseolina on potato dextrose agar (PDA) were conducted in an incubator at 32°C and without lighting. The PDA used was technical grade Difco PDA. Controlled environment experiments for plant growth were all carried out in controlled environment rooms where air temperature was maintained at 32 ± 1°C and a 12-h photoperiod with PAR of 372 µmol m–2 s–1. This temperature was selected as it mimics that commonly experienced in the field when disease caused by M. phaseolina becomes evident (e.g. Fang et al. 2011). Plants were grown in drained pots (two plants per 20 × 18 cm pot) containing University of Western Australia potting mix (per 5 m3 consisting of 2.5 m3 fine composted pine bark, 1 m3 coco peat, 1.5 m3 brown river sand; with additional ingredients per 5 m3 of 5 kg superphosphate, 10 kg extra fine limestone, 1.5 kg potassium sulfate, 1 kg macromin trace elements, 5 kg ammonium nitrate, 10 kg dolomite (CalMag), 2.5 kg iron sulfate (hepta) ferrous), the mix having been aerated and steam treated for 90 min at 65°C before use. All experiments were repeated at least once.
Inoculum production and use
For each isolate of M. phaseolina, 7-day-old colonies growing on PDA maintained at 22°C were used to inoculate millet seeds. Prior to inoculation, millet seeds were first soaked in deionised water (DI) water over night. Excess water was drained and seed autoclaved (in 250 mL flasks with ~150 mL of millet per flask for 3 × 20 min over 3 consecutive days). Autoclaved millet seeds were inoculated with the 7-day-old M. phaseolina-colonised PDA plugs (2 × 2 mm), mixed, and then incubated for 1 week at 22°C and shaken daily.
For soil inoculations, colonised millet seed was applied as a mixture (0.5% w/w) with potting mix and allowed to incubate for 2 weeks before sowing the P. vulgaris seeds.
For hypocotyl inoculations, ~0.5 g of colonised millet seeds were placed into 20 mL DI water and agitated by magnetic stirrer for 15 min to free the microsclerotia from colonised millet. Nylon mesh was used to filter out and discard the seeds. The concentration of the inoculum was adjusted to 5000 microsclerotia mL–1 (only microsclerotia >80 µm diameter were counted in subsamples of the inoculum using a microscope). The desired concentration was obtained by adding either more DI water or colonised millet seeds. Four days after direct sowing, each plant hypocotyl was injected with 10 microsclerotia per plant using a pipette with 10 µL disposable plastic tip and piercing directly into the hypocotyl ~0.5 mm, 2 µL of the inoculum (i.e. 10 microsclerotia plant–1) was injected into each hypocotyl 3 cm above the ground. To ensure uniform distribution of the microsclerotia in the inoculum solution, the flask containing the inoculum was constantly shaken by hand.
Salt treatment
Salinity was imposed by watering treated pots with DI water containing 40 mM NaCl and 1 mM CaSO4, whereas non-saline control pots were watered with DI water containing only 1 mM CaSO4. All treatments commenced from time of sowing and continued throughout the life of each experiment. Plants were watered to free draining with the appropriate solutions daily. The potting mix contained some fertiliser (as detailed above). The same salt solutions were used for the agar growth rate tests for the two isolates (M1 and M2) of M. phaseolina to mimic NaCl and CaSO4 conditions the fungus faced in the soil.
M. phaseolina growth assessments
Growth of the two M. phaseolina isolates was assessed at 14 h after subculturing a 2 mm plug onto the centre of freshly made PDA plates, by measuring the colony diameter with a ruler.
Disease assessments
For soil inoculations, disease assessments were made at 19 and 25 days after sowing, as this was the time normally required for symptoms to develop after soil inoculation. For the hypocotyl inoculations, disease assessments were made at 4 and 8 days after hypocotyl inoculation as symptoms normally appeared in 3–4 days. For either inoculation type, the plants were assessed for incidence/severity of disease using the same 0 to 5 scale for lesions and discoloration on the hypocotyls where: 0 = no disease; 1 = ≤1 cm lesion/discoloration; 2 = >1 to ≤1.5 cm; 3 = >1.5 –≤3 cm; 4 = >3 to ≤ 5 cm lesion/discoloration or plant collapsed from disease; 5 was where the plant had died from the infection.
Plant tissue ion analyses
Tissues were oven-dried at 60°C for 2 days, weighed, and then ground using a Sunbeam Coffee Multi Grinder (Model: EM0400). Tissue subsamples of ~0.1 g (exact weights were recorded) were extracted in 10 mL of 0.5 M HNO3 in plastic vials placed on a shaker at 20°C for 2 days. The extract was diluted appropriately (1/100 for K+ and 1/25 for Na+) and then K+ and Na+ were measured using a flame photometer (Jenway Ltd, model PFP7). Chloride was determined using a Slamed chloridometer (Chloridometer 50cl, SLAMED Laboratory Instruments, Frankfurtam, Main, Germany). To validate the reliability of these tissue ion determinations, a certified plant tissue reference material was also analysed in the same batch as the experimental samples, with recovery being 106% for Na+, 99% for K+ and 103% for Cl–. No adjustments were made to the data presented.
Experimental design and statistical analyses
There were eight two-plant replicates for each treatment (two plants per 20 × 18 cm pot) arranged in a completely randomised design, and all the experiments were repeated at least once. Analyses of variance were conducted using Genstat (11th edn, Lawes Agricultural Trust, Rothamsted Experimental Station, UK) to determine the effects of the different treatments and Fisher’s least significant differences (l.s.d.) at P < 0.05 were used to test the differences between treatment means.
Results
Hypocotyl inoculation – disease severity
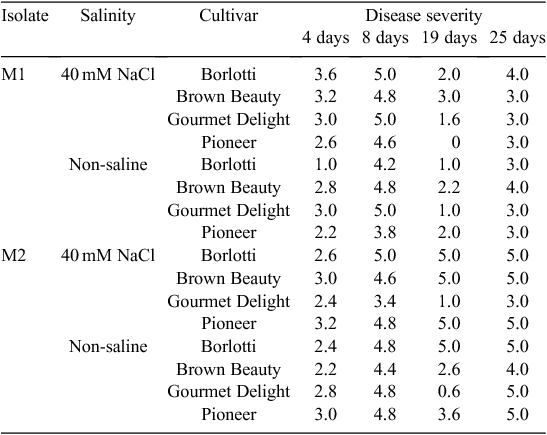
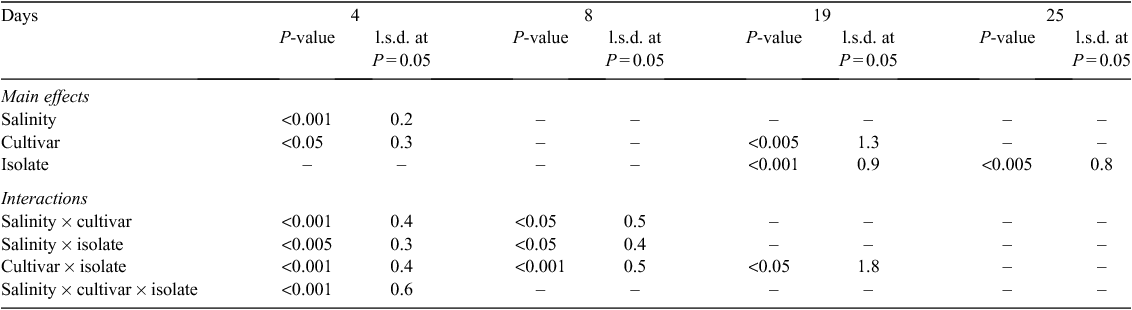
Salinity increased the disease severity at the first sampling time at 4 days after hypocotyl inoculation (8 days after sowing) (Tables 1 and 2). Disease severities of cultivars were different, where Borlotti had less disease. There was no difference in disease severity caused by the two isolates at the first sampling time. Cultivars Borlotti and Brown Beauty had more severe disease in the NaCl treatment than in the non-saline control. Isolate M1 with NaCl treatment caused more severe disease on Borlotti and Gourmet Delight. Pioneer suffered more severe disease from isolate M2 with NaCl than with the combination of isolate M1 with NaCl. Pioneer showed more severe disease from M2 than M1 but the other three cultivars did not show this difference towards the two isolates. Borlotti showed the most severe disease from isolate M1 in combination with NaCl. In contrast, Borlotti suffered least disease with M1 in absence of NaCl, and there was no difference in disease severity on this variety when comparing isolate M2 with or without NaCl. Brown Beauty had more severe disease from M2 with NaCl than without NaCl (Tables 1 and 2).
|
|
There were no differences in disease severity between cultivars, isolates or salinity at 8 days after hypocotyl inoculation (12 days after sowing), by this stage all cultivars suffered disease scores above 3.5. Borlotti with NaCl treatment showed more severe disease than both Gourmet Delight with NaCl and Pioneer without NaCl. Gourmet Delight showed the least disease with NaCl treatment. Gourmet Delight showed most and Pioneer least severe disease with isolate M1. In contrast, Gourmet Delight showed less severe disease with isolate M2 than other three cultivars, whereas Pioneer had the most severe disease. There were no significant three-way interactions between cultivars, salinity and isolates (Tables 1 and 2).
Macrophomina growth response to salinity
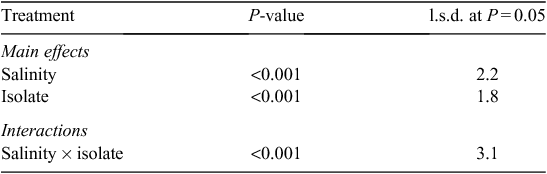
M. phaseolina showed increased growth when on PDA with 40 mM NaCl plus 1 mM CaSO4 or where only 1 mM CaSO4 had been added, compared with growth on normal PDA. Further, growth differed between the two isolates, with M2 growing faster than M1 (Tables 3 and 4).

|
|
Soil inoculation – disease
There were differences in relation to disease severity amongst the four cultivars as well as between the two isolates 19 days after sowing in the soil inoculation test. Gourmet Delight showed the least disease severity, Borlotti and Brown Beauty showed more severe disease. Isolate M1 caused less disease than isolate M2. NaCl had no effect on disease severity. There was a significant interaction between cultivars and isolates where M2 caused more severe disease on Borlotti but less disease on Gourmet Delight. Gourmet Delight showed least disease for both isolates. There were no significant interactions between all other tested factors (Tables 1 and 2).
Isolate M2 caused more severe disease than M1 at 25 days after sowing. There were no differences in relation to disease severity between cultivars, salinity or their interactions at this time period, with all cultivars having severe disease (3–5 disease score range) (Tables 1 and 2).
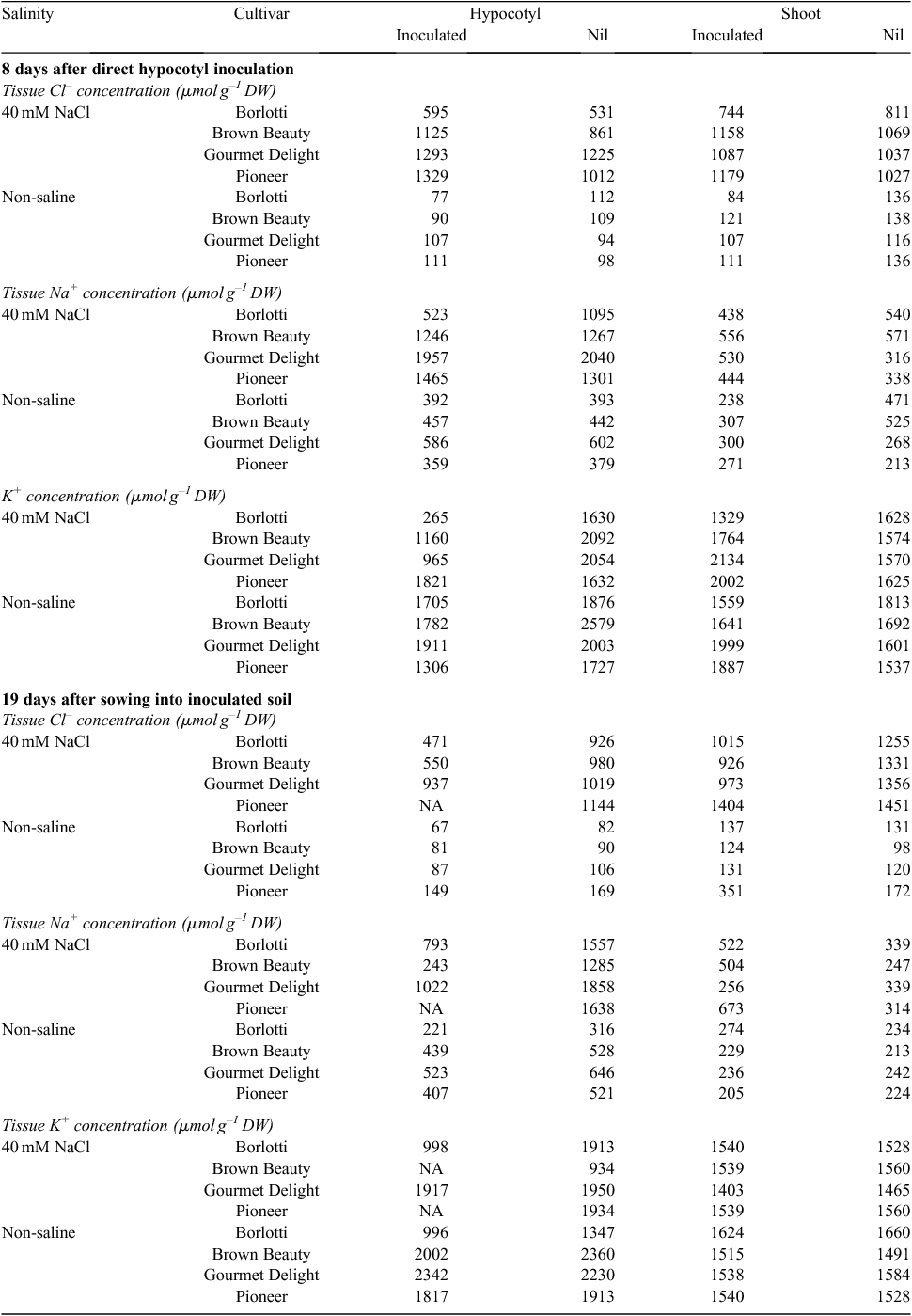
Hypocotyl inoculation – tissue ion concentrations
There were no significant effects of NaCl, cultivar, plant tissue (i.e. hypocotyl or shoot), or isolates, nor their interactions in relation to Cl–, Na+ or K+ concentrations in plant tissues sampled at 8 days after sowing (4 days after hypocotyl inoculation) (data not shown).
The NaCl treatment resulted in increased Cl– concentrations in plant tissues 8 days after inoculation. Cultivars Gourmet Delight and Pioneer had the highest Cl– concentrations and Borlotti the lowest. Pathogen inoculation had no effect on Cl– concentration in the plants (Tables 5 and 6).

|
Salinity also resulted in increased plant tissue Na+ concentrations, and hypocotyls had significantly higher Na+ than shoots (Tables 5 and 6, and Table S1 available as an Accessory Publication to this paper). The interaction of salinity and plant tissue type was significant; with NaCl treatment, hypocotyls contained more Na+ than shoots. The interaction of cultivar and tissue type also was significant; hypocotyls of Gourmet Delight had the highest Na+ concentration and the shoots of Pioneer and Gourmet Delight had the least.
In the case of K+, salinity and inoculation individually reduced K+ concentrations in plant tissues. The interaction of salinity and tissue type and the interaction of plant tissue and inoculation with the pathogen both had a significant but separate impact on K+ in plants; hypocotyls treated with NaCl or inoculated with the pathogen had least K+, whereas hypocotyls of plants without NaCl or pathogen had the highest K+ concentrations (Tables 5 and 6, and Table S2).
Soil inoculation – tissue ion concentrations
Salinity increased plant tissue Cl– concentrations and shoots had higher Cl– than hypocotyls 19 days after sowing. Inoculated plants had lower Cl– concentrations than uninoculated plants. The interaction of salinity and inoculation significantly impacted on Cl– concentration; where plants grown in soil treated with NaCl had the highest Cl– concentration (and the control plants had lowest Cl– concentration). The interaction of salinity and cultivar significantly impacted on Cl– concentration; where Pioneer without NaCl had the highest Cl– concentration whereas Borlotti treated with NaCl had the least Cl– concentration (Tables 5 and 6).
Salinity increased the Na+ concentration in the plant. Hypocotyls had higher Na+ concentration than shoots. Inoculated plants had lower Na+ concentration. The hypocotyls of plants in soil treated with NaCl had highest Na+ concentration and the shoots of plants in soil without NaCl the least. Plants in soil treated with NaCl but without inoculation had higher Na+ concentrations than plants in inoculated soil. Hypocotyls of uninoculated plants showed the highest Na+ concentration whereas the shoots of uninoculated plants showed the lowest Na+ concentration. The hypocotyls of plants in NaCl treated soil but uninoculated showed the highest Na+ concentration whereas the shoots of plants without both inoculation and NaCl showed the lowest Na+ concentration (Tables 5 and 6).
Salinity and inoculation significantly and separately decreased K+ concentration in plants. Hypocotyls of plants with NaCl treatment showed lowest K+ and those of plants without NaCl added to the soil showed highest K+ concentration. Hypocotyls of Gourmet Delight had highest K+ and the hypocotyls of Borlotti had the lowest K+ concentration. Plants in soil treated with NaCl and inoculated with the pathogen had the lowest K+ whereas plants in soil without NaCl but with the pathogen had the highest K+ concentration. Hypocotyls of non-inoculated plants had the highest K+ and the hypocotyls of inoculated plants had the lowest K+ concentration. However, shoots of inoculated and non-inoculated plants showed no significant differences in K+ concentration. Hypocotyls of plants in soil without NaCl treatment but with the pathogen showed the highest K+ concentration whereas hypocotyls of plants in soil without NaCl treatment but with the pathogen showed the lowest K+ concentration (Tables 5 and 6).
Analyses of Cl– and Na+ in tissues sampled at 25 days (Tables S1, S3) showed that salinity increased plant tissue Cl– and Na+ concentrations, but differences between plants in inoculated and uninoculated soil were less evident than at 19 days (described above). Hypocotyls contained higher Cl– and Na+ than shoots. By contrast with the earlier sampling, at 25 days cultivar impacted on Cl– concentration in the plants; where Pioneer had highest and Borlotti the lowest Cl– concentration. Similarly, at 25 days, tissue Na+ concentration also differed between cultivars; where Pioneer had highest Na+ concentration and Borlotti the lowest Na+ concentration. The interaction of salinity and cultivar also had significant effects on tissue Cl– and Na+ at 25 days. The interactions of salinity and inoculation, as well as cultivar and inoculation, were both significant for both Cl– and Na+ concentrations in the plant; and so was the three-way interaction between salinity, inoculation, and cultivar. These significant interactions highlight the complex nature of tissue ion regulation by the plants when under combined salinity and pathogen stresses.
Tissue K+ data at the 25 days sampling (Table S2) showed responses in addition to those seen at the 19 days sampling (described above). Whereas at 19 days the main effects, with the exception of inoculated versus non-inoculated, were not significant, by 25 days the main effects as well as the numerous interactive effects (of which several were seen also at 19 days) were evident. At 25 days, salinity decreased tissue K+ concentrations, and cultivars differed in this response. Borlotti had highest K+ concentration and Brown Beauty had the lowest. Hypocotyls had higher K+ concentration than shoots. Plants from inoculated soil had lower K+ concentrations compared with those from uninoculated soil. In addition, there were several interactions between the main effects of salinity, cultivar, tissue type, and inoculation, as shown in Table S2. Of these, the interaction of cultivar and inoculation significantly impacted on the K+ concentrations in plants, so although inoculation reduced tissue K+ the magnitude of the reduction differed between cultivars; however, no three-way interaction on tissue K+ concentration was seen for the combination of cultivar, inoculation and salinity.
Discussion
Results of the present study showed that although there was no difference between isolates of M. phaseolina in terms of disease severity when making hypocotyl inoculations, there were differences between the two isolates when soil inoculation was undertaken. M2 caused more severe disease than isolate M1 following soil-borne inoculations and combined with salinity. Soil inoculation best approximates what occurs under field conditions and in the soil the pathogen was directly exposed to NaCl in the salinity treatment. The M. phaseolina isolates in direct contact with NaCl in the saline soil might have responded in a similar, positive way as to the NaCl treated PDA. Moreover, salt stress predisposes plants to infection by soil-borne pathogens, leading to increased root rot severity (Swiecki 1984; El-Abyad et al. 1988). In contrast, for hypocotyl inoculation, the pathogen was growing inside of the plant hypocotyls where the effect of NaCl would be expected to be more directly on the plant rather than on the pathogen, which was demonstrated by our two isolates showing no differences in relation to disease severity following hypocotyl inoculation. Nevertheless, as NaCl is known to affect plant cell survival, division, and growth (Blumwald 2000; Hasegawa et al. 2000; Zhu 2003; Munns and Tester 2008), the adverse effects on salinity on the plants could, in turn, enhance plant susceptibility to pathogen attack. In our study using hypocotyl inoculation, the interaction of NaCl and the pathogen (both isolates) significantly reduced plant growth (dry weight when 12 days old; data not shown).
The hypocotyl inoculation method, in contrast to the soil inoculation method, provides a quick, reliable and repeatable way for assessing germplasm resistance. It largely eliminates other environmental influences, for example, soil nutrient elements and pH, and other microbes in the soil on M. phaseolina and focuses on direct pathogen – host interactions and relationships. For hypocotyl inoculations, the fact that salinity increased disease severity as the disease was still developing (4 days after inoculation) but had no significant impact on disease severity once disease had advanced (8 days after inoculation) suggests that assessments of this biotic-abiotic interaction are best done while disease symptoms are still developing. In our study, M. phaseolina showed increased growth when 40 mM NaCl plus 1 mM CaSO4 was added to the PDA, compared with growth on normal PDA. However, adding 1 mM CaSO4 alone also improved growth, so this response may primarily have been to CaSO4, but at least NaCl did not diminish this faster growth. Isolate M2 grew faster than isolate M1 on PDA and the observation that isolate M2 caused more disease than M1 in soil inoculation test may relate to M2 being able to grow and multiply faster therefore increasing the amount of inoculum for infecting plants than could M1. M2 also tended to respond more to salinity in PDA plates than M1. The work of Bouchibi et al. (1990), who found that salinity increased sporangium production by Phytophthora parasitica, supports this conclusion. Fungi have an absolute requirement for K+, but K+ may be partially replaced by Na+. For example, Na+ uptake in Ustilago maydis and Pichia sorbitophila can be rapid (Benito et al. 2004).
The four common bean cultivars we tested showed significantly different responses in relation to disease caused by the two isolates of M. phaseolina, by the saline environment and by the interaction between isolates and saline environment. For example, in soil inoculated plants, Borlotti had less disease in the early stage (8 days old) but disease progressed in the later stage (12 days, 19 days) and to where it became more diseased from isolate M2, whereas by 19 days Gourmet Delight showed a much lower disease level. In general, M2 was more pathogenic than M1 and Gourmet Delight was more robust than other cultivars tested. There are known differences between cultivars in many different crops in responding to NaCl and to pathogens, as well as in cultivar responses at different plant development stages. Natural host resistance to a pathogen is where a pathogen is less able to cause disease on one genotype or host compared with another. To this end, such resistance can be a consequence of several distinct phenomena that can operate simultaneously or at different phases of infection and disease development (Collinge et al. 2010). For example, resistance can be expressed at the penetration stage by determining the ability of a fungal pathogen to assimilate enough nutrients to be able to proliferate in the tissues or sporulate or spread. Host resistance can be constitutive or induced, and it has been demonstrated in several plant species that induced resistance can be regulated by different signalling pathways (Tyagi et al. 2008). The present study shows that host resistance expression (in terms of disease severity) is also impacted by interactive effects of salinity, at least for common bean.
Common bean in NaCl treated soil showed increased Cl– and Na+ concentrations (e.g. 12, 19 and 25 days old) in comparison with control plants in our study. High tissue Na+ and Cl– in shoots of common bean is consistent with observations (Boursier et al. 1987) that salt-sensitive plants generally have a poor capacity to restrict accumulation of these ions in shoots. Our study showed that plant hypocotyls generally had higher Na+ concentration than shoots at tested plant ages of 12, 19 and 25 days. The increased ion concentrations in hypocotyls may be of consequence for disease development, as the pathogen mostly either commences at the hypocotyls or spreads towards hypocotyls and since the pathogen grows faster in a moderately saline environment at 32°C (PDA plate experiment).
Our study showed that K+ concentration in the plants grown in NaCl treated soil and in the inoculated plants was significantly lower than for plants grown in non-saline soil and for uninoculated plants at all tested growth stages (viz. 12, 19 and 25 days). Even in the needle inoculated treatment, inoculation reduced K+ dramatically. However, Gourmet Delight, which presented the lowest disease scores at 19 days (Table 3), was also the only cultivar to maintain its K+ concentration in hypocotyls under inoculation (Tables 5 and 6). K+ is the preferred inorganic cation of plant cells, being essential for several basic physiological functions such as protein synthesis and enzyme activation, and must actively be taken up by ion transporters (Rodriguez-Navarro 2000). Excess external Na+ can, however, impair K+ acquisition and accumulation of Na+ results in a low K+ : Na+ ratio that impairs cellular functioning (Pardo and Quintero 2002). We noted that inoculated plants in our study had significantly lower K+ concentrations than uninoculated plants, which could have also influenced disease severity. The prevailing view is that a high K+ status decreases the incidence of many diseases. The beneficial effect of K+ is most obvious for fungal and bacterial diseases where up to 70% of studies report a decrease of disease incidence (Amtmann et al. 2008). Our study showed that the diseased plants had reduced K+ concentrations, suggesting in some way that the disease (pathogen) had reduced K+ concentration, slowing down enzyme activity and further influencing cellular functions.
Saline soil poses both water deficit and ion toxicity challenges that can have complex consequences for both the pathogen and the host plant (Snapp et al. 1991). Osmotic stress, ion imbalance in cells (especially lower concentrations of K+), and Na+ and/or Cl– toxicity (Tavakkoli et al. 2011) could all weaken plants, as well as encouraging growth of pathogens, resulting in more severe disease occurrence. Our study showed that the inoculated common bean had significantly higher tissue Na+ and lower K+ concentrations than uninoculated plants. This demonstrates the importance of maintaining the appropriate balance between K+ and Na+ in areas where M. phaseolina is a serious pathogen. Further, our study showed hypocotyls from inoculated plants contained less K+ and more Na+ than shoots and that pathogen spread within hypocotyl tissues was rapid following hypocotyl inoculation.
Acknowledgements
We thank the Worldwide University Network and the School of Plant Biology at the University of Western Australia for funding this research and the Department of Agriculture and Food Western Australia for providing half the salary of Martin Barbetti.
References
Acosta-Gallegos J (1998) Mejoramiento genetico del frijol en Mexico. In ‘Memoria del Taller Internacional de Majoramiento Genetico de Frijol Negro Mesoamericano’. (Ed. Lepiz-Ildefenso) pp. 5–12. (Profrijol: Veracruz, Mexico)Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiologia Plantarum 133, 682–691.
| The effect of potassium nutrition on pest and disease resistance in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit7s%3D&md5=bb71f6245909221787fb12385d0edd5bCAS |
Bayuelo-Jiménez J, Craig R, Lynch J (2002a) Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Science 42, 1584–1594.
| Salinity tolerance of Phaseolus species during germination and early seedling growth.Crossref | GoogleScholarGoogle Scholar |
Bayuelo-Jiménez J, Debouck D, Lynch J (2002b) Salinity tolerance of Phaseolus species during early vegetative growth. Crop Science 42, 2184–2192.
| Salinity tolerance of Phaseolus species during early vegetative growth.Crossref | GoogleScholarGoogle Scholar |
Benito B, Garciadeblás B, Schreier P, Rodríguez-Navarro A (2004) Novel P-Type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryotic Cell 3, 359–368.
| Novel P-Type ATPases mediate high-affinity potassium or sodium uptake in fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtlKqsr4%3D&md5=4aa0722fc36ae7392a9953a390e561a8CAS |
Blumwald E (2000) Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology 12, 431–434.
| Sodium transport and salt tolerance in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlt1Srtbg%3D&md5=d1c0b6ba7b4401cb324c339a56e3e30fCAS |
Bouchibi N, Van Brugcen AHC, MacDonald JC (1990) Effect of ion concentration and sodium: calcium ratio of a nutrient solution on phytophthora root of tomato and zoospore motility and viability of Phvtophthora parasitica. Phytopathology 80, 1323–1336.
| Effect of ion concentration and sodium: calcium ratio of a nutrient solution on phytophthora root of tomato and zoospore motility and viability of Phvtophthora parasitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhtFyksrY%3D&md5=7d3160aca6bb1df622bfa0b77a0471fdCAS |
Boursier P, Lynch J, Lauchli A, Epstein E (1987) Chloride partitioning in leaves of salt stressed sorghum, maize, wheat and barley. Australian Journal of Plant Physiology 14, 463–473.
| Chloride partitioning in leaves of salt stressed sorghum, maize, wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlt1Sq&md5=f277f1e6a2b0eb63563849fef96bfef8CAS |
Collinge DB, Jørgensen HJL, Lund OS, Lyngkjær MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annual Review of Phytopathology 48, 269–291.
| Engineering pathogen resistance in crop plants: current trends and future prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Wgt7%2FI&md5=2023c6a2b26126800188a3816256a88cCAS |
Dhingra O, Sinclair J (1987) ‘Biology and pathology of Macrophomina phaseolina.’ (Universidad Federal de Viçosa: Viçosa, Brazil)
Echavez-Badel R, Beaver JS (1987) Dry bean genotypes and Macrophomina phaseolina (Tassi) Goid in inoculated and noninoculated field plots. Journal of Agriculture of the University of Puerto Rico 71, 385–390.
El-Abyad MS, Hindorf H, Rizk MA (1988) Impact of salinity stress on soil-borne fungi of sugarbeet. I. Pathogenicity implications. Plant and Soil 110, 27–32.
| Impact of salinity stress on soil-borne fungi of sugarbeet. I. Pathogenicity implications.Crossref | GoogleScholarGoogle Scholar |
El Mahjoub M, Bouzaidi A, Jouhri A, Hamrouni A, Beji EL (1979) Influence de la salinité des eaux d’irrigation sur la sensibilité du Tournesol au Macrophomina phaseoli (Maubl.) Ashby. Annales de Phytopathologie 11, 61–67.
Fang X, Phillips D, Li H, Sivasithamparam K, Barbetti MJ (2011) Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in Western Australia with special reference to the effect of temperature. Scientia Horticulturae 131, 39–48.
| Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in Western Australia with special reference to the effect of temperature.Crossref | GoogleScholarGoogle Scholar |
FAO (2005) FAO Stat statistical database. Food and Agriculture Organization of the United Nations. Available at http://faostat.fao.org/default.htm [Verified 27 October 2011]
Gama P, Inanaga S, Tanaka K, Nakazawa R (2007) Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. African Journal of Biotechnology 6, 79–88.
Gray F, Mihail J, Lavigne R, Porter P (1991) Incidence of charcoal rot of sorghum and soil populations of Macrophomina phaseolina associated with sorghum and native vegetation in Somalia. Mycopathologia 114, 145–151.
| Incidence of charcoal rot of sorghum and soil populations of Macrophomina phaseolina associated with sorghum and native vegetation in Somalia.Crossref | GoogleScholarGoogle Scholar |
Hasegawa P, Bressan R, Zhu J-K, Bohnert H (2000) Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51, 463–499.
| Plant cellular and molecular responses to high salinity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlsVymt7s%3D&md5=6cb239a13a0581221e65204097647e08CAS |
Läuchli A (1984) Salt exclusion: an adaptation of legumes for crops and pastures under saline conditions. In ‘Salinity tolerance in plants. Strategies for crop improvement’. pp. 171–187. (John Wiley & Sons: New York)
Manici LM, Caputo F, Cerato C (1995) Temperature responses of isolates of Macrophomina phaseolina from different climatic regions of sunflower production in Italy. Plant Disease 79, 834–838.
Mayek-Perez N, Lopez-Castaneda C, Gonzalez-Chavira M, Garcia-Espinosa R, Acosta-Gallegos J, Martinez De La Bega O, Simpson J (2001) Variability of Mexican isolates of Macrophomina phaseolina based on the pathogenesis and AFLP genotype. Physiological and Molecular Plant Pathology 59, 257–264.
| Variability of Mexican isolates of Macrophomina phaseolina based on the pathogenesis and AFLP genotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovV2mtrY%3D&md5=1a89542b7ae9e00b153c1ff0ef124d90CAS |
Mayek-Perrez N, Lopez-Castaneda C, Acosta-Gallegos J (1997) Variacion en caracteristicas culturales in vitro de aislamientos de Macrophomina phaseolina y su virulencia en frijol. Agrociencia 31, 187–195.
Mayek-Perrez N, Lopez-Castaneda C, Lopez-Salinas E, Cumpian-Gutierrez J, Joaquin-Torres I, Padilla-Ramirez J, Acosta-Gallegos J (2003) Effect of Macrophomina phaseolina (Tassi) Goid. on grain yield of common beans (Phaseolus vulgaris L.) and its relationship with yield stability parameters. Revista Mexicana de Fitopatologia 21, 168–175.
Mihail J, Taylor S (1995) Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production, and chlorate utilization. Canadian Journal of Botany 73, 1596–1603.
| Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production, and chlorate utilization.Crossref | GoogleScholarGoogle Scholar |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=4ff74227fb3898bf12208db44bd191acCAS |
Nischwitz C, Olsen M, Rasmussen S (2002) Influence of salinity and root-knot nematode as stress factors in charcoal rot of melon. 2002 Vegetable Report. University of Arizona College of Agriculture and Life Sciences. Available at http://ag.arizona.edu/pubs/crops/az1292/ [Verified 27 October 2011]
Pardo JM, Quintero FJ (2002) Plants and sodium ions: keeping company with the enemy. Genome Biology 3, 1014–1017.
| Plants and sodium ions: keeping company with the enemy.Crossref | GoogleScholarGoogle Scholar |
Redden R, Tompkins W, Usher T (1997) Growth interactions of navy bean varieties with sowing date and season. Australian Journal of Experimental Agriculture 37, 213–221.
| Growth interactions of navy bean varieties with sowing date and season.Crossref | GoogleScholarGoogle Scholar |
Rodriguez-Navarro A (2000) Potassium transport in fungi and plants. Biochimica et Biophysica Acta 1469, 1–30.
Sanchez A (1989) ‘Catastro sobre incidencia de Macrophomina phaseolina en la zona de San Juan, R.D. Informe final.’ (Regional Suroeste: SEA, Reptiblica Dominicana)
Schwartz H, Pastor Corrales M (1989) ‘Bean production problems in the tropics.’ 2nd edn. (CIAT: Cali, Colombia)
Sinclair J (1982) ‘Compendium of soybean diseases.’ 2nd edn. (American Phytopathological Society: St Paul, MN, USA)
Snapp SS, Shennan C, Van Bruggen AHC (1991) Effects of salinity on severity of infection by Phytophthora parasitica Dast, ion concentrations and growth of tomato, Lycopersicon esculentum Mill. New Phvtologist 119, 275–284.
| Effects of salinity on severity of infection by Phytophthora parasitica Dast, ion concentrations and growth of tomato, Lycopersicon esculentum Mill.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhvVGgug%3D%3D&md5=414cb33376d615c96f7eac9c335f5b57CAS |
Srivastava A, Singh T, Jana T, Arora D (2001) Microbial colonization of Macrophomina phaseolina and suppression of charcoal rot of chickpea. In ‘Microbes and plants’. (Ed. A Sinha) pp. 269–319. (Vedams eBooks Pty Ltd: New Delhi)
Swiecki TJ (1984) Interactionsbetween salinity and Phytophthora root rots of chrysanthemum and tomato. PhD Thesis, Plant Pathology Department, University of California, Davis, CA.
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl– ions on barley growth under salinity stress. Journal of Experimental Botany 62, 2189–2203.
| Additive effects of Na+ and Cl– ions on barley growth under salinity stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFyju74%3D&md5=f38691f864065ee74a6719661f466f15CAS |
Tester M, Davenport R (2003) Na+ tolerance and Na transportation in higher plants. Annals of Botany 91, 503–527.
| Na+ tolerance and Na transportation in higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsVyisbk%3D&md5=1d347e97621c3429b50d3bd9471ffd5eCAS |
Tyagi H, Rajasubramaniam S, Rajam M, Dasgupta I (2008) RNA-interference in rice against rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. Transgenic Research 17, 897–904.
| RNA-interference in rice against rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtValt7vL&md5=d1fe871cd4e68d827ce50c397bce6109CAS |
Watson A (2009) Soil-borne diseases of beans. In ‘Primefact 586’. (Department of Industry & Investment: Ourimbah, NSW)
Wrather J, Anderson T, Arsyad D, Gai J, Ploper L, Porta-Puglia A, Ram H, Yorinori J (1997) Soybean disease loss estimates for the top 10 soybean producing countries in 1994. Plant Disease 81, 107–110.
| Soybean disease loss estimates for the top 10 soybean producing countries in 1994.Crossref | GoogleScholarGoogle Scholar |
Wrather J, Anderson T, Arsyad D, Tan Y, Ploper L, Porta-Puglia A, Ram H, Yorinori J (2001) Soybean disease loss estimates for the top 10 soybean producing countries in 1998. Canadian Journal of Plant Pathology 23, 115–121.
| Soybean disease loss estimates for the top 10 soybean producing countries in 1998.Crossref | GoogleScholarGoogle Scholar |
Zhu J (2003) Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology 6, 441–445.
| Regulation of ion homeostasis under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntVKhsbs%3D&md5=7e54c59bd255571504f5829ff758b75fCAS |