The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon
Donald F. Gaff A C and Melvin Oliver BA School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
B USDA-ARS, Plant Genetics Research Unit, 205 Curtis Hall – UMC, Columbia, MO 65211, USA.
C Corresponding author. Email: don.gaff@monash.edu
Functional Plant Biology 40(4) 315-328 https://doi.org/10.1071/FP12321
Submitted: 28 October 2012 Accepted: 10 January 2013 Published: 22 February 2013
Abstract
In a minute proportion of angiosperm species, rehydrating foliage can revive from airdryness or even from equilibration with air of ~0% RH. Such desiccation tolerance is known from vegetative cells of some species of algae and of major groups close to the evolutionary path of the angiosperms. It is also found in the reproductive structures of some algae, moss spores and probably the aerial spores of other terrestrial cryptogamic taxa. The occurrence of desiccation tolerance in the seed plants is overwhelmingly in the aerial reproductive structures; the pollen and seed embryos. Spatially and temporally, pollen and embryos are close ontogenetic derivatives of the angiosperm microspores and megaspores respectively. This suggests that the desiccation tolerance of pollen and embryos derives from the desiccation tolerance of the spores of antecedent taxa and that the basic pollen/embryo mechanism of desiccation tolerance has eventually become expressed also in the vegetative tissue of certain angiosperm species whose drought avoidance is inadequate in micro-habitats that suffer extremely xeric episodes. The protective compounds and processes that contribute to desiccation tolerance in angiosperms are found in the modern groups related to the evolutionary path leading to the angiosperms and are also present in the algae and in the cyanobacteria. The mechanism of desiccation tolerance in the angiosperms thus appears to have its origins in algal ancestors and possibly in the endosymbiotic cyanobacteria-related progenitor of chloroplasts and the bacteria-related progenitor of mitochondria. The mechanism may involve the regulation and timing of the accumulation of protective compounds and of other contributing substances and processes.
Additional keywords: abscisic acid, Borya, Craterostigma, gene expression, modular evolution, proteome, protoplasmic drought tolerance, Sporobolus, Tortula, Xerophyta.
Introduction
In the 8000 or so years that crops have been cultivated, drought has been a major scourge. The failure and death of a crop reinforced the general observation that poorly hydrated plants do not grow and that dry plants are dead plants. The angiosperm (flowering) plants that we depend on totally for our food are overwhelmingly sensitive to desiccation. Early in the 20th century, reports appeared of rare angiosperm species with a spectacular ability to recover from dryness (Dinter 1919; Table 1). Kurt Dinter, a plant collector in south-west Africa, observed the dry season small bushes of Myrothamnus flabellifolia Welw. that were desiccated, with hard dark brown shrunken leaves folded against the dehydrated stem. The foliage was dry enough to snap and rub into a powder between his fingers. Completely dead to all appearances, but after a single day of rain the leaves and plants became green healthy plants — not a mere semblance of recovery but a real return to active life, as the plants resumed normal growth. Myrothamnus flabellifolia well deserves its vernacular name, the ‘resurrection bush’.
|
Salient features of this phenomenon of desiccation tolerance are the extreme degree of dryness tolerated, the rapidity of rehydration and recovery, the multiple cycles of dehydration and rehydration endured, the stability of the dry tissue and its longevity in the dry state.
Actively growing plants have relative water contents (RWC) of ~85–100%. Crop plants die as water content falls from 59 to 30% RWC (Höfler et al. 1941). Air-dry desiccation-tolerant plants in the field are literally as dry as straw; their water contents are only 5–13% RWC and they survive even further drying to ~1% RWC in the laboratory (Gaff 1977).
Desiccation-tolerant angiosperm plants retain most of their water, until the soil moisture has been exhausted; the water content of desiccation-tolerant plant then falls rapidly until the plant reaches air-dryness in 2–4 days (Gaff 1977). The 1 cm plants of Chamaegigas intrepidus Dinter ex Heil dry extremely rapidly, ~1.8 h after the soil dries. Rehydration is usually more rapid than dehydration. Again Chamaegigas intrepidus is exceptional, rehydrating fully after only 1.5 h in water (Gaff and Giess 1986). A rainfall of 10 mm is sufficient for full rehydration of the air-dry desiccation-tolerant angiosperm plants (Gaff 1977). Less rain may produce partial rehydration, usually of the lower leaves whereas the upper leaves remain airdry. Full rehydration often occurs within 24 h of a rainfall in the field, but may take longer depending on the particular species or if the temperature is low.
Desiccation-tolerant angiosperm plants may traverse several cycles of dehydration and rehydration in a year, especially after isolated rainfalls at the beginning and the end of the dry season in the subtropics, e.g. 17 cycles in a year in the perennial Chamaegigas intrepidus (Gaff and Giess 1986). There appears to be no limit on the number of such cycles they can endure, provided there are periodic opportunities for photosynthesis. Many desiccation-tolerant species are long-lived perennials: the desiccation-tolerant velloziad Xerophyta equisetoides Baker with stems 2.6 m tall would exceed 200 years, based on the height of 40-year-old greenhouse-plants at Monash University. Dead leaf bases form a velloziad trunk that supports a tuft of live leaves – approaching an epiphytic state (Raven and Andrews 2010).
The air-dry state is both static and surprisingly stable. As plants dry, growth stops and metabolism slows until it ceases or nearly so; the plant is in a state of suspended animation or ‘anabiosis’. The walls of dry cells are often folded, indicative of a plasticity that is thought to avoid mechanical damage to the drying wall, the protoplast and the contact between them (Farrant 2007). The plasticity would also assist the rapid growth seen in rehydrated resurrection grasses (Blomstedt et al. 2010). The airdry leaves tolerate temperatures approaching 60°C and subfreezing temperatures of −80°C. The time-span over which air-dry leaves remain viable is impressive. Myrothamnus flabellifolia plants survive 6–10 months, depending on the ecotype (Farrant and Kruger 2001). Most angiosperm species examined survive air-dryness for ~2 years and leaves of Borya constricta Churchill recovered when rehydrated after 5 years of storage air-dry (Gaff 1981). The longevity of anabiotic tissue varies greatly with the humidity of the storage air, the storage temperature, the species and the plant organ. Air-dry desiccation-tolerant leaves stored at high humidity may lose their viability within a few days, whereas storage at 20–40% RH, leading to tissue water potentials of approximately –215 MPa to –123.6 MPa, generally allows the longest survival times (Gaff 1980).
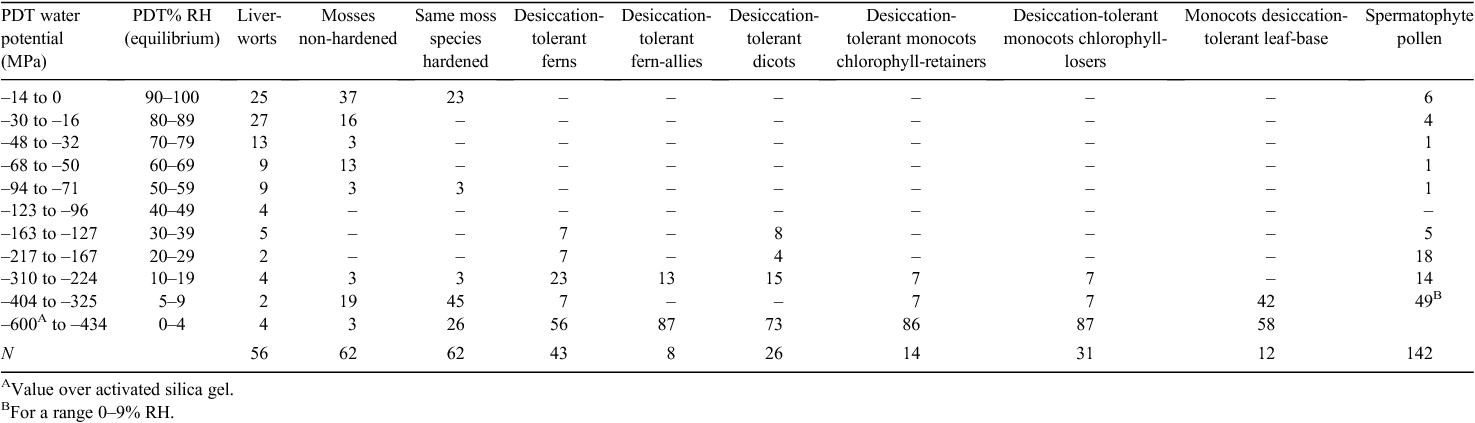
In the past 50 years, the number of angiosperm plant species that have been reported to possess desiccation-tolerant vegetative tissue has risen from 10 species to 135 species. Desiccation tolerance has been quantified in 83 of these species in terms of the lowest water potential survived (Table 2) and in a further 33 species in terms of the lowest relative water content survived.
|
The evolution of an ecological assemblage
Desiccation-tolerant ‘resurrection plants’ commonly grow on shallow soils (often only 1 cm) overlying rock slabs or collecting in depressions on rock outcrops (Gaff 1977; Porembski and Barthlott 2000). Their desiccation tolerance allows them to persist during the periodic dehydration to which such shallow soils are prone. In this situation, the low growing resurrection plants colonise the bare soils and by trapping further soil and detritus allow taller desiccation-tolerant plant species to follow them. Eventually taller desiccation-sensitive species overshadow and out-compete the desiccation-tolerant plants. Granitic outcrops and inselbergs in particular offer numerous soil pans suitable for desiccation-tolerant colonisers. Whereas desiccation-tolerant species exploit a much wider range of habitats beyond rock outcrops, the shallow soils in rock pans may be central to the origin of desiccation-tolerant angiosperms from non-tolerant ancestors and a major influence on their further evolution.
The evolution of a cuticle resisting water loss of intercellular spaces and stomata regulating water vapor loss and of a xylem, which enables long-range transport of water from moist soil to foliage, led in the angiosperms to a general evolutionary thrust towards improved drought-avoidance mechanisms that can be very effective preventing injuriously low plant water contents developing (i.e. homoiohydry). Most desiccation-tolerant angiosperm species have one or more mechanisms retarding transpiration: for example, xeromorphy, inrolling curling or folding leaves, stomata that are confined to grooves, densely-hairy leaf surfaces and ephemeralism. These mechanisms of drought avoidance and evasion are functionally compatible with desiccation tolerance. Desiccation-sensitive species with well-developed avoidance/evasion mechanisms can colonise shallow soils. Any inadequacy of the avoidance mechanisms during dry spells subjects the species to selective pressure for improved protoplasmic drought tolerance (PDT), leading to the extreme case – desiccation tolerance – a definite advantage for pioneer species.
Selection on the rock pan site influences the evolution of associated characteristics of desiccation-tolerant species. The periodic dryness of shallow rock-pan soils prevents tall-growing desiccation-sensitive species from establishing and limits the growth of drought-resistant non-resurrection species. Selection pressure on the desiccation-tolerant plants, for height and dry-mass productivity therefore is weakened; selection focuses on plant survival, reproduction and dispersal. The majority of desiccation-tolerant angiosperm species have short plants, mostly 1–15 cm, with short internodes (Gaff 1972). Myrothamnus spp. (50–100 cm tall) and species in the Velloziaceae (up to 1–2 m) are notable exceptions. Desiccation-tolerant dicot plants are frequently rosettes and most non-velloziad monocot plants have numerous shoots forming a tight clump (a caespitose habit). In both growth habits the shoot meristems are on or under the soil partially protected from direct sunlight and from wildfires. As a result, resurrection plants in burnt areas shoot again after rain, often rapidly. A thick tightly-packed mass of persistent leaf bases surround the aerial stems and apical meristems of velloziads; fire lightly chars only the surface of this pseudostem, leaving the true stem and its meristems unharmed.
In many resurrection grass species, phenotypic shortness of the vegetative shoots has become largely genetically determined; a few however respond dramatically to transfer from shallow soils to deep soils, e.g. the desiccation-tolerant grasses Microchloa caffra Nees and Eragrostis invalida Pilger grow 10–50 cm taller respectively. Rock pans also limit the space available to desiccation-tolerant plants. Root systems of most desiccation-tolerant angiosperms form dense much-branched masses that bind the limited soil resource firmly against erosion. Root systems of plants transferred to deep soil grow extensively.
Many desiccation-tolerant angiosperm plants spread across a rock pan by vegetative reproduction as well as from seed. The numerous seed released from the infructescences of desiccation-tolerant angiosperms are small to minute and so are well adapted for long-distance dispersal by wind and water and on animals to remote rock outcrops.
Whilst predominant environments for desiccation-tolerant angiosperms can be discerned, the plants are found over most of the range available – tropics to polar zones, altitudes of 0–5000 m, on rocks to in deep soils on trees to swamps and stony soil to clay (D F Gaff, unpubl. data).
Distribution of desiccation-tolerant foliage in the angiosperms
Taxonomic
Desiccation-tolerant vegetative tissue occurs in 13 mainly unrelated angiosperm families: implying it has arisen separately at least 13 times during angiosperm evolution. The phenomenon is poorly represented in the families that are in the more direct lineages from the ancestral angiosperms where it occurs only in the corms and tubers of two species in the dicot family Ranunculaceae and in the foliage of only Borya in the monocot Anthericaceae (or Liliaceae sensu lato; Table 1). In the family Velloziaceae, every tested species is desiccation tolerant, suggesting an early appearance in this large monocot family. The largest number of genera containing desiccation-tolerant species are found in two families Poaceae and Cyperaceae, in which the desiccation-tolerant species make up only a small proportion of their genus and these genera only a small fraction of the family. The late appearance of the phenomenon in apparently early-evolved families and its early appearance and representation mainly in some later-evolved families suggests a relatively ‘mid-stream’ rather than an early appearance of desiccation tolerant vegetative tissue in the angiosperms. In regard to phylogeny, information given later in this paper indicates that desiccation tolerance occurs right across the Angiosperm families in general: of the thousands of species examined, all species without exception have desiccation-tolerant seed or pollen. That is the total phylogeny of the angiosperms possesses desiccation tolerance. Nevertheless, desiccation tolerance is expressed in the foliage of only a few angiosperm families that are not in a linear phylogenetic sequence one to the other. These families appear to be predisposed to express desiccation tolerance – but usually only in a small proportion of the species in the family. At an ecological level, a factor in this predisposition may be as simple as the production of numerous minute seed that can readily be dispersed over long distances to suitable shallow-soil sites. The natures of other factors that may contribute to the predisposition at a physiological or molecular level are as yet not clear.
Quantitative: protoplasmic drought tolerance of angiosperms
Desiccation tolerance is the extremity of protoplasmic drought tolerance (PDT) being the lowest water potential of the cell protoplast that the protoplast can survive. The PDT of angiosperm plants lie in the range from –22 to –2.7 MPa (derived from the 85–98% RH values reported by Levitt et al. 1960), except for ~0.01% of the angiosperm species whose PDT are all –162 MPa or less.
Desiccation tolerance is well developed in angiosperm resurrection plants: the proportions of dicot and monocot resurrection plants with the most extreme PDT values (below –400 MPa) range from 58 to 87% in the four angiosperm categories in Table 2. In the angiosperm data, no PDT values are found in the intermediate range of PDTs (–123 to –30 MPa), which lies above the PDT of resurrection plants and below the PDT of nonresurrection plants.
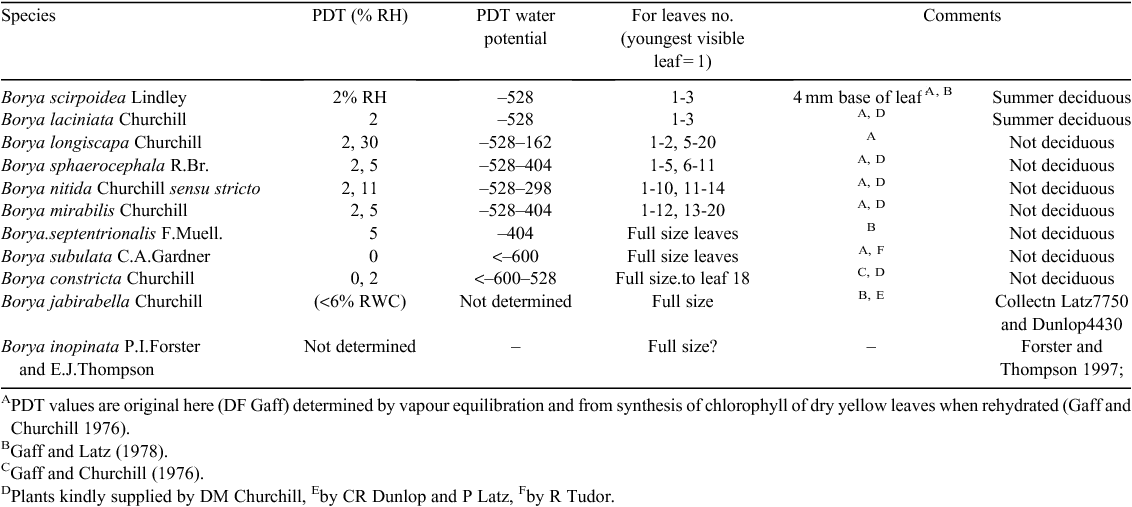
The situation in Borya is of interest here. Leaves of Borya constricta are not constitutively desiccation-tolerant. The mechanism for desiccation tolerance is activated when drying leaves are in the water potential range from –14 to –2.7 MPa for 2 days (Gaff and Churchill 1976). Desiccation tolerant vegetative tissue has not been found in related genera or families. In Borya scirpoides Lindley desiccation tolerance is restricted to the 4 mm base of the first three immature leaves (Table 3). In a series of other Borya species desiccation tolerance spreads to the full length of young leaves and to increasingly more mature leaves (but as in all resurrection plants, senescing tissues do not become desiccation tolerant). In all Borya species the PDT values are in the extreme range of –404 MPa and below, even in the most restricted case B. scirpoides. There is no evidence of leaf PDT evolution through a range of intermediate PDT values.
|
It appears that in the Borya spp. desiccation tolerance in foliage has evolved de novo by the expression of a fully-developed desiccation tolerance mechanism first in immature meristematic leaf tissue then evolving in other species in progressively more mature tissue.
The facultative induction of desiccation tolerance during drought stress seen in Borya constricta seems to occur in the few poikilohydrous angiosperm species examined to date, in all but one of which fully hydrated leaves are desiccation sensitive when detached (Gaff 1980). In the single exception, Myrothamnus flabellifolia, desiccation tolerance is constitutive, since leaves detached while hydrated survive subsequent airdrying. Drought-induced desiccation tolerance is not confined to angiosperm resurrection species. Such an induction is evident in 49% of moss species, compared with 26% of moss species with constitutive desiccation tolerance (Table 2). Constitutive desiccation tolerance has been studied intensively in the moss Tortula ruralis (Bewley 1979; Oliver 2007).
Desiccation tolerance in other angiosperm organs
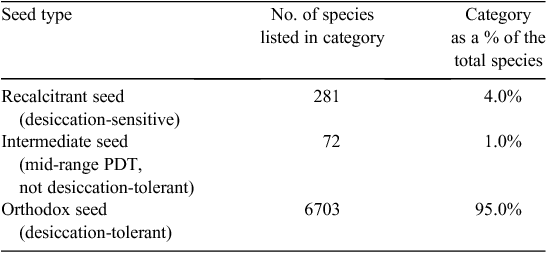
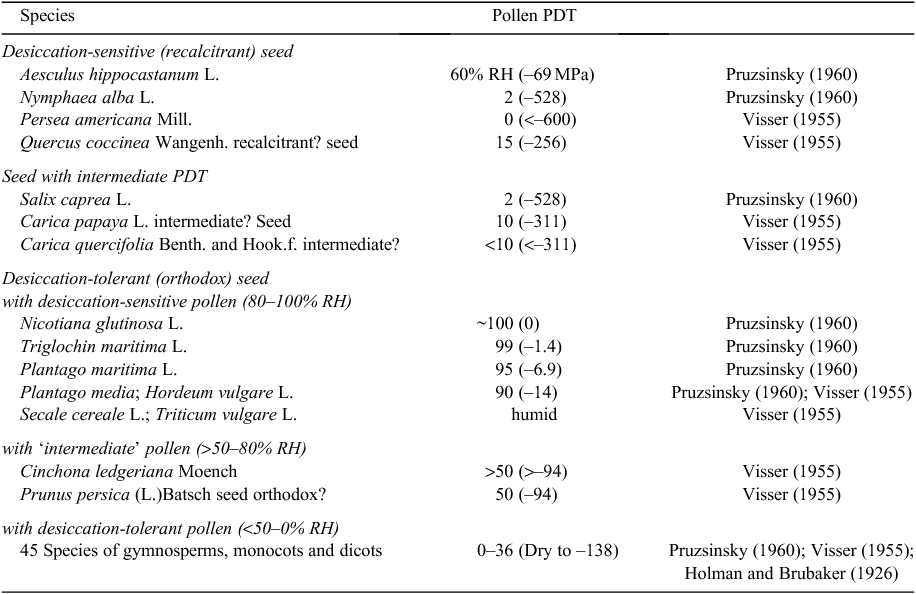
A few angiosperm species have vegetative organs that perennate in a desiccated state, e.g. corms of Limosella grandiflora Bentham in D.C., Philydrella pygmaea (R.Br.) Caruel and Stylidium petiolare Sond, rhizomes of Anenome coronaria L. and tubers of Ranunculus asiaticus L. (Pate and Dixon1982; Antipov and Romanyak 1983; Dixon et al. 1983; Gaff and Giess 1986). Desiccation tolerance in the angiosperms occurs most commonly in the reproductive organs, the seed embryos and the pollen. At least 15 species have embryos with the extreme PDT values of ~0% RH or –600 MPa (including the grasses Avena sativa L., Hordeum vulgare L., Triticum durum Desf., Triticum spelta L., Zea mays L., De Saussure 1827), Seed PDT values range up to –2.7 MPa (Gaff 1980), however, in most of species, seeds are considered ‘orthodox’, i.e. seed are desiccation-tolerant with long-term viability (95% of species, Table 4). Of spermatophyte species ~4% have ‘recalcitrant’ seed, i.e. their seed are desiccation-sensitive with only a brief period of viability, whereas only ~1% are ‘intermediate’. Pollen too is desiccation-tolerant in most spermatophyte species (~87% of spp. with PDT < –124 MPa, Table 2); 33% of these have PDT of approximately –600 MPa and ~57% have PDT < –310 MPa. ‘Intermediates’ (PDT = –93 to –30 MPa) constitute ~3.5% of all species. Desiccation-sensitive pollen occurs in ~10% of all species, including the important cereals barley, Hordeum vulgare L., and rye, Secale cereale L.
|
In 61 species, we have data on the PDT levels of both seed and pollen (Table 5). No species with recalcitrant or intermediate seed had desiccation-sensitive pollen; desiccation sensitive pollen is found only in species with desiccation-tolerant seed whereas the majority of the orthodox-seed species have desiccation-tolerant pollen. If these data are representative of angiosperms and gymnosperms in general, then virtually all seed-plant species have the genetic information for desiccation tolerance, which is expressed in their seed embryo or the pollen but in the foliage of relatively few species (resurrection plants). It is only partially expressed in the ‘intermediate’ seed and pollen of a small proportion of species.
|
Modules in evolution
An underlying aspect of biological evolution is that once a particularly successful module has evolved, with further evolution the module is replicated to form a multi-modular individual and later in evolution the various modules differentiate into a diversity of forms and functions. For example, unicellular algae, multicellular algae with undifferentiated cells, multicellular algae with some cells photosynthetic and other cells reproductive.
A single module of physiological-cum-biochemical assemblage may also evolve and differentiate to suit a variety of functions, e.g. chloroplasts with C3 photosynthesis, chloroplasts associated with CAM, and chloroplasts differentiated in sheath and mesophyll cells for C4 photosynthesis. The simplest working hypothesis of desiccation tolerance in angiosperms is that a single mechanism of desiccation tolerance evolved for the survival of reproductive units shed from the plant and consequently subject to air-drying (spores, seed and pollen). For vegetative angiosperm tissue also to show ‘resurrection’ behaviour, the mechanism controlling the coordinated gene-expression for the implementation of fully-developed desiccation tolerance, must itself undergo further evolution to develop specific expression in non-reproductive cells.
We propose that evolution extended expression of the desiccation tolerance module from the seed/pollen first to the young meristematic vegetative tissue, later to the immature leaf tissue, and lastly to the mature leaf tissue as exemplified in the Borya spp. (Table 3). The desiccation tolerance is fully developed even in the Borya species where only immature leaf tissue revives (PDT of –528 MPa). This supports the view that a fully developed seed/pollen desiccation tolerance mechanism is being invoked. This hypothesis, an extension of the suggestion that angiosperm vegetative desiccation tolerance derived the developmentally programmed seed tolerance mechanism, was first alluded to by Bewley (as reported by Bewley and Oliver 1992; and later discussed by Oliver et al. 2000, 2005; and more recently by Farrant and Moore 2011), and implies a common basic mechanism of desiccation tolerance from early in (or probably well before) the evolution of the angiosperms. Divergences in the mechanism of desiccation tolerance would be expected to arise between foliage, pollen and seed as evolution proceeded. Such divergences in organ specificity of the mechanism are likely to involve the regulatory elements rather than the elements implementing desiccation tolerance.
Several features are shared by desiccation tolerance in pollen, in seed and in resurrection-angiosperm foliage. The airdry cells are very stable. They tolerate temperatures below −70°C and high temperatures. They may remain viable for weeks to years, depending on the ambient conditions, the organ and the species. The dry cells contain substances opposing damage by free-radicals, carotenoids, catalase and peroxidase. Physically protective substances in the dry cells, particularly sucrose and LEA proteins, are thought to make a large contribution to desiccation tolerance. A large number of protein species are conserved in the dry tissues. Polysomes are not present in the dry cells, but ribosomes and mRNA (possibly as messenger ribonucleoprotein complexes) are present and so are available to support protein synthesis during rehydration. Mitochondria persist in dry cells and respiration recovers in the first 30–60 min of rehydration. Dry viable cells are injured by storage in air of >50% RH (–94 MPa), in which case the higher the humidity is, the more rapidly cells are damaged. In those desiccation-tolerant tissues which are prone to injury during storage in air of <50% RH, the lower the RH in the <50% RH range then the greater is the injury. Rehydration of dry anabiotic cells is swift (1–48 h).
Divergent features also exist between dry desiccation tolerant organs. Dry pollen contains starch, but there is little or no starch in resurrection plant foliage and embryos. Whereas dry resurrection plant leaves contain minor amounts of the protectant disaccharide trehalose, none are detectable in seed and pollen. Nitrogen content is low in embryos (~0.1 mg g–1 FW) compared with pollen and resurrection plant foliage (3–4 mg g–1 FW). Protein synthesis in pollen reaches maximum rates at 1 h of rehydration, embryos at ~4 h rehydration and resurrection plant foliage at ~16h. Longevity in the dry state covers 0.5 days to 3 years for pollen (Visser 1955), 0.5–5 years for resurrection plant leaves and <1 to approximately 1900 years for seed (1900 for date seed Phoenix dactilifera L. at Masada, Israel, Sallon et al.2008). The ecological need for longevity varies greatly from hours to days for pollen to reach a stigma, or 5–10 months of the subtropical dry season endured by dry resurrection foliage or years to centuries for seed to outlast climatic catastrophes or suppression by ecological succession. The surprising longevity of some pollens, compared with an ecological need of hours or days for pollen to reach a stigma, is explicable if (i) ecological selection for a long-lasting seed-bank sets the maximum dry-state longevity for the basic desiccation tolerance mechanism, and (ii) with lower selective pressure acting for dry-state longevity in foliage and pollen than in seeds, the expression of the potential longevity tends to decline at differing evolutionary rates in foliage and in pollen and in different species.
Desiccation-tolerance in other photosynthetic organisms
The major physiological and morphological features of the evolutionary antecedents of the angiosperms are exemplified by a series of extant plant groups. Tracing phylogenetically from the fern-allies, ferns, towards the mosses and liverworts, and algae, we pass from plants with mechanisms for controlling water loss (cuticles and stomata) and for replacing lost water (xylem and roots or rhizoids) to plants with little or no drought avoidance mechanisms and whose water potential fluctuate markedly with ambient water potentials (‘poikilohydrous’ plants). Poikilohydrous plants have much greater exposure to selection for desiccation tolerance than do drought-avoiding angiosperm species.
In the fern-allies desiccation-tolerance is met in the shoots of several species of Selaginella and in the corms in several Isoetes species. All of the investigated desiccation-tolerant Selaginella species have foliage PDT below –311 MPa. The PDT values of foliage of desiccation-tolerant fern species range more widely, from < –600 to –127 MPa, with 56% of desiccation-tolerant species in the –434 MPa and lower range. The proportion of desiccation-tolerant species (per total number of species in the taxon) is ~10 times greater in the ferns than in the angiosperms (Gaff 1972). In addition, recent work has determined that desiccation tolerance, with PDT values between –94.6 and –220MPa, is more widely distributed in the gametophytes of ferns (Watkins et al. 2007).
The PDT distribution of mosses is broadly bimodal with low representation in the mid PDT range (Table 2). Desiccation-tolerant moss species are common — 25% of species have PDT values below –220 MPa. A preliminary drought stress (equilibration to –5.5 MPa) improves the PDT in moss species and (as in the monocot Borya constricta) it induces desiccation tolerance in 49 of the tested species (Table 2). The distribution of thallus PDT among the species of liverworts (Hepaticae) is a continuum (as if desiccation tolerance were newly evolving in the taxon) with 10% of species having a PDT below –220 MPa. The PDT of seven species improved during dry months, suggesting a degree of facultative alteration in the PDT in these cases (Clausen 1952).
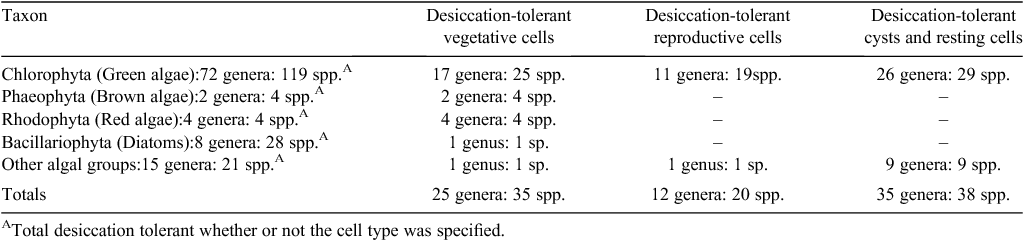
Among the algae, several species in the Phaeophyta (brown algae), Rhodophyta (red algae) and Bacillariophyta (diatoms) have desiccation tolerant vegetative tissue (Table 6). Desiccation-tolerant species are especially well represented in the Chlorophyta (green algae) whose habitats spread across marine, freshwater and terrestrial environments (Table 6). Desiccation tolerance occurs in vegetative cells of 25 species, in the reproductive cells of 19 species and in both cysts and resting cells in 29 species.
|
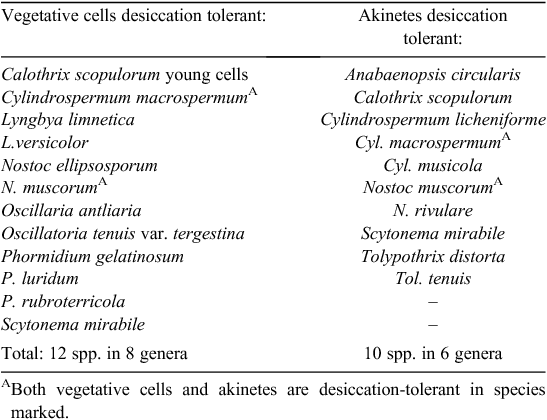
Desiccation tolerance was reported in Table 7 in 59 species of present day Cyanobacteria (blue-green bacteria), specifically in the vegetative cells in 12 species (eight genera). Evidence from DNA sequencing indicates that the chloroplasts of algae and of the other plant groups are derived from endosymbiotic cyanobacteria-related cells captured by the host and incorporated during evolution into eukaryote cells (Gould et al. 2008). It is conceivable then that the cyanobacterioid endosymbiont may have carried the genetic information for desiccation tolerance across with it into the evolving composite host-cum-symbiont cell. The same reasoning may apply also to the mitochondria derived from bacterioid endosymbionts. Neither of these possibilities necessarily precludes pre-existing desiccation tolerance in the host eukaryotoid organisms, which also were probably small and poikilohydrous.
|
In all the groups (fern-allies to hepatics to algae) spores rather of seeds are the major reproductive and dispersive structures. In species where long-distance dispersal depends on spore longevity, the aerial spores of terrestrial plants need to be desiccation tolerant. Desiccation-tolerant spores have been reported from pteridophytes (e.g. 33 fern species with nonchlorophyllous spores and five fern species with chlorophyllous spores; Pence 2000) and from the distantly related sphenophyte Equisetum hyemale L. (spore PDT approximately –525 MPa, Lebkuecher 1997). Desiccation-tolerant spores are common in the mosses dispersed across oceans (Van Zanten 1978). Among the algae, 20 species are known to possess desiccation-tolerant reproductive cells (Table 6). We note that desiccation-tolerant oospores are produced by all eight of the species studied in the aquatic genus Chara (Ch. canescens Desvaux and L.Deslongschamps, Ch. contraria A.Braun and Kützing, Ch. evoluta Allen, Ch. globularis Thuill., Ch. rusbyana Howe, Ch. sejuncta A.Braun, Ch. zeylanica Klein; Davis 1972). The chlorophyte Order Charales is close to the progenitors of the bryophytes and of the vascular plants (McCourt et al. 2004). Beyond the algae, at least 10 species of cyanobacteria have desiccation-tolerant akinetes (Table 7).
The molecular biology of desiccation tolerance
As desiccation-tolerant angiosperms dry, they accumulate compounds that can physically protect cell membranes and protein against the disorganising effects of high ion concentrations and dehydration. Marked accumulation of sucrose and lesser accumulations of other known protectants (e.g. raffinose and trehalose) are general in the desiccation-tolerant angiosperms (Ghasempour et al. 1998b). The main protectant, sucrose, in desiccation-tolerant angiosperm plants, is constitutively at a high content in the desiccation-tolerant moss Tortula ruralis (Bewley 1979). A wide variety of osmoregulatant solutes, including sucrose, are produced in algae and cyanobacteria in response to environmental stress (Grant et al. 1976; Reed et al. 1984).
Trehalose appeared to be the main protective sugar accumulated in the desiccation tolerant fern-allies Selaginella lepidophylla (Hook. and Grev.) Spring and Selaginella sartorii Hieron. (Iturriaga et al. 2000). However, recent work has demonstrated that trehalose also accumulates to higher levels in the desiccation sensitive species Selaginella moellendorffii than it does in Selaginella lepidophylla under the same conditions (Yobi et al. 2012). Thus it appears that trehalose accumulation is not necessarily required for desiccation tolerance and indeed in the same study it became clear that sucrose, as in other resurrection species, is the main protectant.
Protection by sucrose is augmented by its interaction with LEA-proteins that also accumulate in response to drought stress and in maturing seeds. The effectiveness of LEA-protein may be increased by its greater tertiary structure at the high solute concentrations in airdry tissue (Goyal et al. 2005a, 2005b). A LEA-like protein in Craterostigma plantagineum binds to two enzymes, stabilising their activity after repeated cycles of dehydration (Petersen et al. 2012). Wider roles than stabilisation of protein have been suggested for LEA4 proteins, including increased drought avoidance, membrane permeability and antioxidant enzyme activity (Liu et al. 2009). Concurrent action of the various LEA proteins may be needed for desiccation tolerance (Oliver 2007). LEA proteins accumulate late in the maturation of orthodox seed when the embryo is becoming desiccation tolerant. They also accumulate in other desiccation-tolerant organs, pollen and resurrection plant foliage, as they dry and in drought-stressed desiccation-sensitive angiosperms. LEA-like proteins are also found in some bacteria and in nematodes (Browne et al. 2002). In contrast to the induced adaptive desiccation of angiosperm resurrection species, the constitutive desiccation tolerance of Tortula ruralis does not involve accumulation of LEA during dehydration but is based on a constant high sucrose content and on synthesis of rehydrin-proteins encoded by RNA transcribed during dehydration and conserved in the dry moss plants (Oliver and Bewley 1997) and presumably other macromolecules essential for revival are also conserved. The constitutive levels of LEA proteins in this moss have not been determined.
Dry resurrection plants retain protective mechanisms against chemical injury by stress-related free-radicals, e.g. quenching by carotenoids and the antioxidant action of glutathione-ascorbate cycle enzymes and catalase (Gaff et al. 2009; Oliver et al. 2011b; Gechev et al. 2012; Dinakar et al. 2012). Rises in the contents of tocopherol, putrescine and agmatine, lipid anti-oxidants, may safeguard membrane integrity during drying of Sporobolus stapfianus (Oliver et al. 2011a). Reactive oxidants accumulate less as desiccation tolerance is induced in slow drying plants of the moss Fontinalis antipyretica L. ex Hedw., compared with plants damaged by rapid drying (Cruz de Carvalho et al. 2012). Anti-oxidant mechanisms are probably general in the eukaryotes. Anti-oxidants may trace back to the cyanobacterioid endosymbiont progenitor of the plant chloroplast. In the cyanobacterium Spirulina platensis (Gomont) Geitler high antioxidant activity stems from its contents of carotenoids, α-tocopherol and phenolic compounds (Abd El-Baky et al. 2009).
Dehydration-induced pigment binding ELIP proteins may protect plastids against injury from high-light induced free-radical generation and ultraviolet radiation (Bartels et al. 1992) and their induction is a common feature during dehydration of resurrection plants (Gechev et al. 2013; Dinakar et al. 2012). These proteins are thought to play a role in non-photochemical quenching of light energy and the protection and repair of photosystems during desiccation and appear to be an active participant in the drying induced accumulation of protective pigments in resurrection plants (Dinakar et al. 2012). ELIP proteins evolved early in the evolution of photosynthetic organisms and their ancestry and probable role in protection from light induced oxidative stress can be traced back to the cyanobacteria (Heddad and Adamska 2002).
In contrast with cryptogamic desiccation-tolerant species, many desiccation-tolerant vascular plants have physical barriers such as light-scattering epidermal hairs or scales and drought-induced folding or rolling-up of foliage that shade the mesophyll and meristems. These adaptations diminish the formation of radiation-induced free-radicals, as also do epidermal pigments in numerous dry angiosperm resurrection plants (Dinakar et al. 2012) and an absence of chlorophyll in many dry monocot examples (poikilochlorophylly) (Gaff and Hallam 1974).
Strikingly higher contents of asparagine, glutamine and allantoin in fully hydrated leaves of Sporobolus stapfianus than in Sporobolus pyramidalis P.Beauv. suggests the importance of spatial and metabolic mobility of a reserve of nitrogen in the desiccation-tolerant species (Oliver et al. 2011a). Their contents declined at intermediate RWCs in Sporobolus stapfianus. A later resurgence at 20% RWC in asparagine and glutamine levels may reflect detoxification of ammonia (produced below 30% RWC in this resurrection grass, Martinelli et al.2007) as well as providing a reserve of organic nitrogen compounds for metabolic recovery during rehydration. Among early-evolved organisms, some species of bacteria have long been noted for a versatile metabolism of inorganic and organic nitrogenous molecules.
The link between nitrogen metabolism, antioxidant pathways, and desiccation tolerance is highlighted by the significant accumulation of several gamma glutamyl amino acids (GGAA) as leaves of Sporobolus stapfianus dry below 30% RWC (Oliver et al. 2011a). These compounds are postulated to be involved in the replenishment of 5-oxyproline in the glutathione biosynthetic pathway but their exact role in desiccation tolerance is not known. That the accumulation of these compounds has significance in desiccation tolerance and its evolutionary journey is implied by the fact that these compounds also accumulate in the later stages of drying of Selaginella lepidophylla (Oliver et al. 2011a; Yobi et al. 2012) and the moss Tortula ruralis (Oliver et al. 2011a).
Chaperonins (some of which are stress-inducible) including some heat shock proteins (HSP) that associate with and protect other protein species against denaturation are found from the algae to angiosperm resurrection plants Boea hygrometrica F.Muell. and Sporobolus stapfianus (M Oliver, P Payton, unpubl. data). A lowered abundance of a large HSP (70 kDa) inhibitor of the synthesis of small HSPs probably allows HSP18 to accumulate (Kim and Schoffl 2002; Oliver et al. 2011b). Small HSPs are associated with induction of desiccation tolerance in plants of Craterostigma plantagineum Hochst. and in seeds (Alamillo et al. 1995; Wehmeyer and Vierling 2000). Overexpression of a sunflower-seed transcription factor HaHSFA9 in transgenic tobacco seedlings resulted both in ectopic expression of a seed-specific small HSP and in an increase in seedling survival of severe drought stress (–40MPa) that were not accompanied by increased levels of the protective sugars and LEA (Prieto-Dapena et al. 2008; Cushman and Oliver 2011).
Aquaporins act as channels for cross-membrane transfer of small molecules, including water and gas molecules. They occur in both resurrection plants and nonresurrection plants and are distributed widely in the plant and in animal kingdoms (Bartels et al. 2007; Danielson and Johanson 2008). Genes encoding them are highly expressed in drying resurrection plants, in which the encoded proteins may be important in hastening the intracellular movement of water and of respiratory gases (Neale et al. 2000).
Anabiotic leaves of angiosperms retain high contents of ATP, reflecting an excess production compared with usage during drying and constituting an energy reserve to support the resumption of cell metabolism early in rehydration (Gaff and Ziegler 1989). Although photosynthesis may be restricted at 30% RWC in Sporobolus stapfianus by stomatal closure and reduced abundance of the Rubisco large subunit, synthesis of ATP and NADPH is supported by increased abundance of cytoplasmic and plastidic enzymes of glycolysis, a process well established in algae and microorganisms (Oliver et al. 2011b). Lowered abundance of malate dehydrogenase may reduce metabolism of pyruvate in the TCA respiratory cycle (Oliver et al. 2011b).
Desiccation-tolerant Craterostigma plantagineum and Sporobolus stapfianus, as their water content falls, display major changes in the complement of proteins that reflect protein turnover even at very low water contents. Many seemingly novel proteins appear, several of which correlate well with the induction of desiccation tolerance. The main group of specific transcripts whose abundance increases during drying encode proteins putatively are involved in membrane protection or membrane function (Bartels et al. 1990; Gaff et al. 2009). Other ‘more-abundant’ transcripts encoded proteins that are implicated in protein turnover or have regulatory roles. Prominent in this regard is the protein initiation factor eIF1, whose abundance increases with moderate stress to reach a maximum at air-dryness (Neale et al. 2000; Oliver et al. 2011a, 2011b).
The phytohormone abscisic acid (ABA) induced desiccation tolerance in hydrated leaves of Borya constricta and in callus of Craterostigma plantagienum (Gaff and Loveys 1984; Bartels et al. 1990). ABA also activated strong expression of genes for some of the above transcripts, e.g. genes encoding the protective LEA group 2 (dehydrin) and LEA group 3 proteins and encoding the membrane-associated proteins aquaporin and a pore-like protein (Bartels et al. 1990; Gaff et al. 2009). Synthesis of LEA proteins appears to be universally inducible in plants by ABA. Immunological evidence indicates that abscisic acid is widely distributed in bryophytes and can evoke drought resistance in some mosses (Hartung et al. 1987; Werner et al. 1991). ABA is released under salt stress from the alga Dunaliella sp. and the cyanobacterium Trichormus variabilis (Kützing)Komárek and Anagnostidis (Tietz et al. 1989; Zahradníčková et al. 1991).
More information on the mechanisms of desiccation tolerance in cyanobacteria and in plants is presented in detail in the wide-ranging chapters by Lüttge et al. (2011).
Large comprehensive transcriptome profiling studies of drying and rehydration tissues have been reported for two dicot resurrection plants, Craterostigma plantagineum (Rodriguez et al. 2010) and Haberlea rhodopensis (Gechev et al. 2013), in an attempt to build a more comprehensive assessment of the cellular protection aspects of the molecular mechanisms of tolerance in these plants (reviewed by Gechev et al. 2012). These studies, in general, support the proteomic and metabolomic aspects of the desiccation responses as discussed earlier but they also point to novel aspects that offer new insights into the cellular protection mechanisms and the processes that are important for successful recovery upon rehydration. Of particular note are the transcription factor and signalling associated gene transcripts that respond to desiccation and rehydration. The genes these transcripts represent are targeted as the underlying genetic components that control the massive reprogramming of cellular activity that determines desiccation tolerance (Gechev et al. 2012). The larger transcriptome studies also support earlier more targeted gene expression profiling studies with the resurrection monocot Xerophyta humilis that offered the first transcriptomic evidence for the hypothesis that vegetative desiccation tolerance in the resurrection angiosperms derived from the genetic program that is operational in the seed (Illing et al. 2005). Recent studies centred on the dissection of the desiccation tolerance genetic program from that of seed development and germination in seed based systems also support this notion (Buitink et al. 2006; Maia et al. 2011).
Mechanisms involved in desiccation tolerance arose early in plant evolution
The major metabolites and proteins that are associated with desiccation tolerance are wide-spread in desiccation-sensitive as well as desiccation-tolerant angiosperms and throughout the plant kingdom, as are the tolerance-associated processes of ATP-production, protein turnover, DNA-repair and removal of free-radicals. Since the above substances and processes occur in both desiccation-sensitive and desiccation-tolerant species and tissues, their role in implementing desiccation tolerance presumably involves factors such as their concentration, their cellular location, their interactions and the timing of their accumulation or diminution in content. The metabolic assemblage implementing desiccation tolerance in angiosperms is subject to intense natural selection in the dehydrated seed or pollen of each generation, consequently much of the implementation mechanism is likely to be highly conserved. Just as different types of switches can be chosen to open and close an electrical circuit, it is conceivable that the mechanism which regulates the implementation of desiccation tolerance may vary from one plant taxon to another as the happenstances of discrete evolutionary events separated in geological time. The observation, that foliage desiccation tolerance appears to arise independently in the several angiosperm families endowed with such tolerance, supports this notion. A wide array of systems of transduction and regulation are available in plants. Regulatory differences also may be implied by the specificity of expression of desiccation tolerance in seed, pollen, leaves, corms or tubers, and to the stage of development (in meristematic, in young and mature leaves but never in senescent tissue). The different responses of PDT to exogenous ABA in diverse families supports the view that substantial differences in the regulatory mechanism of desiccation tolerance may exist. Exogenous ABA induced full desiccation tolerance in hydrated leaves of the monocot Borya constricta and in the hydrated callus of Craterostigma plantagineum (Scrophulariaceae). ABA treatment stimulated a small improvement in the desiccation tolerance of detached shoots of the dicot Myrothamnus flabellifolia, but evoked only a slight improvement in the PDT (which remained in the desiccation-sensitive range) of free cells of the grass Sporobolus stapfianus.
In addition to the ABA-regulated induction path for desiccation tolerance in Borya constricta, a complementary non-ABA pathway of gene activation for DT induction was postulated in this species (Gaff 1981). In Craterostigma plantagineum, a short interference RNA (derived from the ‘desiccation-tolerant gene’ CDT), together with calcium- and phospholipid-based transduction pathways are implicated in a non-ABA regulatory path, that complements the major ABA desiccation-tolerance-inductive pathway (Bartels 2005; Hilbricht et al. 2008). A non-ABA pathway dominates the regulation of desiccation tolerance induction in Sporobolus stapfianus (Gaff and Loveys 1993). In the drought-tolerant Boea crassifolia a drought-induced MYB gene, BcMYB1, appears to be implicated in a non-ABA pathway regulating plant responses to drought stress (Shen et al. 2004) as do some of the transcription factors upregulated by dehydration in Haberlea rhodopensis (Gechev et al. 2013).
Environmental stress induces ABA production in several cyanobacteria and algae, but the concentrations of exogenous ABA required to elicit observable effects exceed normal physiological levels (Hartung 2010). Adaptive effects of drought induced ABA synthesis arose with the colonisation of the terrestrial environment by bryophytes and with the evolution of stomata and of water conducting tissue in early embryophytes (Raven 2002; Hartung 2010). ABA is pre-eminent in the angiosperms in the drought stimulation of many drought avoidance mechanisms and in the induction of desiccation tolerance in some resurrection angiosperms. The greatest role of ABA in resurrection angiosperms is reached in the poikilochlorophyllous monocots, in which exogenous ABA induces desiccation tolerance in fully-hydrated leaves while it induces loss of their chlorophyll (Gaff and Churchill 1976).
Hartung’s (2010) review implies that, in the algae and cyanobacteria, any occurrence of drought-induced desiccation tolerance would probably involve non-ABA pathways. In the resurrection grass Sporobolus stapfianus jasmonate and brassinosteroids are implicated in non-ABA induction of desiccation tolerance (Ghasempour et al. 2001).
Plants of many drought-sensitive species die at ~30% RWC. Comparison of the leaf proteome of Sporobolus stapfianus plants at the critical 30% RWC with those of fully-hydrated plants disclosed altered abundances of several proteins that influence the structure and function of chromatin indicating that dehydration may affect these (Oliver et al. 2011b) (cf. two drought-upregulated proteins, one with a SNF2/helicase domain and a chloroplast RNA-binding protein, in desiccation-tolerant Xerophyta humilis (Baker) Dur and Schinz; Collett et al. 2004). Two such proteins in Sporobolus stapfianus included a protein able to bind phosphorylated protein and a protein receptor for a specific kinase that is required for the perception of brassinosteroid (BR) and the transduction of its signal (Oliver et al. 2011b). The latter observation is consistent with exogenous brassinosteroids’ ability to improve the PDT of free cells of this species and strengthens the case for involvement of brassinosteroids in the induction of its desiccation tolerance (Ghasempour et al. 2001). Brassinosteroids were detected in Sporobolus stapfianus leaves but drought-induced increases in the content (JM Sasse, DF Gaff, unpubl. data) were not confirmed in a second test. Treatment of free cell suspensions with methyl jasmonic acid (MJA) or brassinosteroid gave identical improvements in the PDT of this species (Ghasempour et al. 2001). MJA and BR (applied separately) altered the expression of several genes: some MJA-induced changes in the proteome were identical with BR-induced changes, other MJA-induced changes differed from BR-induced alterations (Ghasempour et al. 1998). Brassinosteroids and MJA are found across the plant taxa from algae to angiosperms (Yokota et al. 1987; Collén et al. 2006).
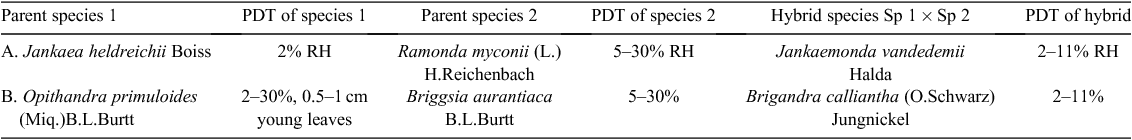
Despite the evolving complexities of the mechanism of desiccation tolerance and its regulation, the taxonomic variation of these in the angiosperms is small enough in the Gesneriaceae that hybrids of resurrection gesneriad species in different genera are as desiccation tolerant as their parent species (Table 8).
|
Phases in the induction of desiccation tolerance
Even before any stress arises the resurrection grass Sporobolus stapfianus is constitutively better prepared to face the initial stress than is the desiccation sensitive Sporobolus pyramidalis (Oliver et al. 2011a). Metabolome comparisons between these two grass species when both were fully hydrated, found higher concentrations of osmotically-active solute (osmoregulation and protection roles) and of nitrogen metabolites (retranslocation into young tissue and reserve roles) in Sporobolus stapfianus than in the sensitive species (Oliver et al. 2011a). Similar observations have recently been reported for a sister group comparison between Selaginella lepidophylla and Selaginella moellendorffii (Yobi et al. 2012).
Soluble protein extracts from detached leaves of Borya constricta showed three distinct phases while they were equilibrated to air of 96% RH to induce desiccation tolerance. Electrophoresis patterns (PAGE 1D) of the first and second phases differed markedly from each other and from the control (100% RH), whilst in the third phase (desiccation tolerant leaves) the pattern of the control was largely restored but with an increased abundance of low molecular weight proteins (probably including LEA proteins) (Daniel and Gaff 1980).
Two main phases of major proteomic changes occurred in drying Sporobolus stapfianus plants. In the first phase: (85–60% RWC) interchange of materials with the rest of the plant is important (hormonal signalling and import of remobilised carbohydrate, minerals and nitrogenous compounds from aging leaves) since leaves detached before 60% RWC do not become desiccation tolerant with further drying (Kuang et al. 1995). Metabolome studies indicate that this phase ‘instigates a metabolic shift towards the production of protective compounds’ (Oliver et al. 2011a) that was not seen in the desiccation sensitive Sporobolus pyramidalis. The antioxidant tocopherol accumulated only in Sporobolus stapfianus in this phase, a lipophilic compound that is important in protecting cell-membranes lipids from oxidative damage (Oliver et al. 2011a). Successful completion of the first phase allows the initiation of the second phase: (51–37% RWC, which is approaching the death point of desiccation-sensitive plants) a phase that proceeds in drying leaves independently of any connection with the plant. Protective sugars and LEA proteins accumulate rapidly, measures to prevent toxicity from oxidant free radicals and from ammonia are evident whilst nitrogenous compounds are remobilised ready to support recovery-metabolism in the event of subsequent leaf rehydration (Martinelli et al. 2007; Oliver et al. 2011a) – processes that are seen widely in resurrection species at these extreme water deficits (Illing et al. 2005; Farrant 2007; Peters et al. 2007).
The presence of the above phases and their complexity raise the possibility of a diversity of regulatory mechanisms (acting at different times as drying proceeds), which are the products of past evolution and are substrates for ongoing evolutionary change.
Conclusion
Our interpretation of the above information postulates that desiccation tolerance in the angiosperms had its distant evolutionary origins in poikilohydrous unicells that contributed to the formation of the eukaryotic plant cell. Desiccation tolerance was carried forward into the plant cell probably from cyanobacteria-related endosymbionts (evolving to chloroplasts), possibly also from other bacteria-related endosymbionts (to mitochondria) and possibly also from the host polychromosome-nucleate cells. The molecular species implementing desiccation tolerance in the angiosperm are largely present in the cyanobacteria and in the algae.
Desiccation tolerance is expressed in both reproductive and vegetative cells of many species of algae, bryophytes, ferns and fern allies. The seed or pollen are desiccation tolerant in almost all angiosperms, probably an extension into the angiosperm reproductive structures of the desiccation tolerance of the spores of non-seed-bearing ancestral taxa. We propose that desiccation tolerance of seed/pollen has become expressed in the vegetative angiosperm tissue of resurrection angiosperms.
Constitutive desiccation tolerance is probably appropriate for the rapid drying of poikilohydrous unicells, but it is found also in some bryophytes and angiosperms. An early evolutionary appearance of facultative adaptive desiccation tolerance in vegetative tissue is seen in some bryophytes and such facultative tolerance persists in the angiosperms — whose vascularisation and drought avoidance allow ample time for induction of desiccation tolerance during drying.
ABA emerges as ‘stress hormone’ in the bryophytes. In the angiosperms, ABA accumulation under drought stress stimulates many diverse mechanisms of plant drought resistance. Its role in inducing desiccation tolerance in foliage is maximised in the poikilochlorophyllous monocot resurrection plants such as Borya constricta, whereas in the homoiochlorophyllous grass Sporobolus stapfianus a non-ABA pathway regulates the induction of desiccation tolerance, albeit with concomitant activity of several ABA-responsive genes.
Desiccation tolerance in foliage arose independently in several angiosperm families. This raises the possibility that different pathways regulate the implementation of desiccation tolerance in the diverse families. If so, genetic alterations in, for example, a cereal species to induce the expression of its seed-mechanism of desiccation tolerance in its foliage would need an understanding of the regulatory mechanism in a related resurrection grass or in the embryo of that cereal species.
Acknowledgments
The authors are grateful to Dr Janet L Gaff for helpful suggestions in the preparation of the manuscript, to Dr Rana Munns for proposing it and for her encouragement, and to our colleagues Dr Cecilia K Blomstedt, John D Hamill and Dr Alan D Neale for helpful discussion. The authors’ research given here was supported by grants from the ARC (A19230441), the Meat Research Corporation (UMON.004) and the Deutsche Forschungsgemeinschaft (MU 94/58–14810/83).
References
Abd El-Baky H, El Baz F, El-Baroty G (2009) Enhancement of antioxidant production in Spirulina platensis under oxidative stress. Acta Physiologiae Plantarum 31, 623–631.| Enhancement of antioxidant production in Spirulina platensis under oxidative stress.Crossref | GoogleScholarGoogle Scholar |
Abel WO (1956) Die Austrocknungsresistenz der Laubmose. Sitzungberichte der Wien Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse, Abteilung I 165, 619–707.
Alamillo J, Almoguera C, Bartels D, Jordano J (1995) Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum. Plant Molecular Biology 29, 1093–1099.
| Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum.Crossref | GoogleScholarGoogle Scholar |
Antipov N, Romanyak A (1983) Poikilohydric vegetative organs of reproduction in some flowering plants. Zhurnal Obshchei Biologii 44, 446–450. [In Russian]
Bartels D (2005) Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum. Integrative and Comparative Biology 45, 696–701.
| Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum.Crossref | GoogleScholarGoogle Scholar |
Bartels D, Schneider K, Terstappen G, Piatowski D, Salamini F (1990) Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 181, 27–34.
| Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum.Crossref | GoogleScholarGoogle Scholar |
Bartels D, Hancke C, Schneider K, Michel D, Salamini F (1992) A desiccation-related ELIP-like protein from the resurrection plant Craterostigma plantagineum is regulated by light and ABA. EMBO Journal 11, 2771–2778.
Bartels D, Phillips J, Chandler J (2007) Desiccation tolerance: gene expression, pathways, and regulation of gene expression. In ‘Plant desiccation tolerance’. (Eds M Jenk, AJ Wood) pp. 115–150. (Blackwell Publishing: Ames, IA)
Bewley JD (1979) Physiological aspects of desiccation tolerance. Annual Review of Plant Physiology 30, 195–238.
| Physiological aspects of desiccation tolerance.Crossref | GoogleScholarGoogle Scholar |
Bewley JD, Oliver MJ (1992) Desiccation-tolerance in vegetative plant tissues and seeds: protein synthesis in relation to desiccation and a potential role for protection and repair mechanisms. In ‘Water and life: a comparative analysis of water relationships at the organismic, cellular and molecular levels’. (Eds CB Osmond, G Somero) pp. 141–160. (Springer-Verlag: Berlin)
Blomstedt CK, Griffiths CA, Fredericks DP, Hamill JD, Gaff DF, Neale AD (2010) The resurrection plant Sporobolus stapfianus; an unlikely model for engineering enhance plant biomass. Plant Growth Regulation 62, 217–232.
| The resurrection plant Sporobolus stapfianus; an unlikely model for engineering enhance plant biomass.Crossref | GoogleScholarGoogle Scholar |
Browne J, Tunnacliffe A, Burnell A (2002) Plant desiccation gene found in a nematode. Nature 416, 38
| Plant desiccation gene found in a nematode.Crossref | GoogleScholarGoogle Scholar |
Buitink J, Leger JJ, Guisle I, Vu BL, Wuillème S, Lamirault G, Le Bars A, Le Meur N, Becker A, Küster H, Leprince O (2006) Transcriptome profiling uncovers metabolic and regulatory processes occurring during the transition from desiccation-sensitive to desiccation-tolerant stages in Medicago truncatula seeds. The Plant Journal 47, 735–750.
| Transcriptome profiling uncovers metabolic and regulatory processes occurring during the transition from desiccation-sensitive to desiccation-tolerant stages in Medicago truncatula seeds.Crossref | GoogleScholarGoogle Scholar |
Clausen E (1952) Hepatics and humidity. Dansk Botansk Archiv 15, 1–80.
Collén J, Hervé C, Guisele-Marsollier I, Léger JL, Boyen C (2006) Expression profiling of Chondrus crispus (Rhodophyta) after exposure to methyl jasmonate. Journal of Experimental Botany 57, 3869–3881.
| Expression profiling of Chondrus crispus (Rhodophyta) after exposure to methyl jasmonate.Crossref | GoogleScholarGoogle Scholar |
Collett H, Shen A, Gardner M, Farrant JM, Denby KJ, Illing N (2004) Toward transcript profiling of desiccation tolerance in Xerophyta humilis: construction of a normalized 11 k X. humilis cDNA set and microarray expression analysis of 424 cDNAs in response to dehydration. Physiologia Plantarum 122, 39–53.
| Toward transcript profiling of desiccation tolerance in Xerophyta humilis: construction of a normalized 11 k X. humilis cDNA set and microarray expression analysis of 424 cDNAs in response to dehydration.Crossref | GoogleScholarGoogle Scholar |
Cruz de Carvalho R, Cartalá M, Marques da Silva J, Branquinho C, Barreno E (2012) The impact of dehyration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Annals of Botany 110, 1007–1016.
| The impact of dehyration rate on the production and cellular location of reactive oxygen species in an aquatic moss.Crossref | GoogleScholarGoogle Scholar |
Cushman JC, Oliver MJ (2011) Understanding vegetative desiccation tolerance using integrated functional genomics approaches within a comparative evolutionary framework. In ‘Plant desiccation tolerance’. (Eds U Lüttge, E Beck, D Bartels) pp. 307–338. (Springer: Heidelberg)
Daniel V, Gaff DF (1980) Desiccation-induced changes in the protein complement of soluble extracts from leaves of resurrection plants and related desiccation-sensitive species. Annals of Botany 45, 174–181.
Danielson JA, Johanson U (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BioMed Central Plant Biology 8, 45
Davis JS (1972) Survival records in the algae, and the survival role of certain algal pigments, fats and mucilaginous substances. Biologist 54, 52–93.
De Saussure T (1827) De l’influence du dessèchement sur la germination de plusieurs grains alimentaires. Annales des Sciences Naturelles 10, 68–93.
Dinakar C, Djilianovb D, Bartels D (2012) Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Plant Science 182, 29–41.
| Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense.Crossref | GoogleScholarGoogle Scholar |
Dinter K (1919) Pflanzenarten aus Deutsch-suedwestafrika. XLIII. Feddes Repertorium 16, 239–244.
| Pflanzenarten aus Deutsch-suedwestafrika. XLIII.Crossref | GoogleScholarGoogle Scholar |
Dixon K, Kuo J, Pate J (1983) Storage reserves of the seed-like, aestivating organs of geophytes inhabiting granite outcrops in south-western Australia. Australian Journal of Botany 31, 85–103.
| Storage reserves of the seed-like, aestivating organs of geophytes inhabiting granite outcrops in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Farrant JM (2007) Mechanisms of desiccation tolerance in angiosperm resurrection plants. In ‘Plant desiccation tolerance.’ (Eds MA Jenks, AJ Wood) pp. 51–90. (Blackwell Publishing: Ames, IA)
Farrant J, Kruger L (2001) Longevity of dry Myrothamnus flabellifolius in simulated field conditions. Plant Growth Regulation 35, 109–120.
| Longevity of dry Myrothamnus flabellifolius in simulated field conditions.Crossref | GoogleScholarGoogle Scholar |
Farrant J, Moore J (2011) Programming desiccation-tolerance: from plants to seeds to resurrection plants. Current Opinion in Plant Biology 14, 340–345.
| Programming desiccation-tolerance: from plants to seeds to resurrection plants.Crossref | GoogleScholarGoogle Scholar |
Forster PI, Thompson EJ (1997) Borya inopinata (Anthericaceae), a new species of resurrection plant from north Queensland. Austrobaileya 4, 597–600.
Gaff D (1972) Drought resistance of Welwitschia mirabilis Hook. fil. Dinteria 7, 3–8.
Gaff DF (1977) Desiccation tolerant vascular plants of southern Africa. Oecologia 31, 95–109.
| Desiccation tolerant vascular plants of southern Africa.Crossref | GoogleScholarGoogle Scholar |
Gaff DF (1980) Protoplasmic tolerance of extreme water stress. In ‘Adaptation of plants to water and high temperature stress’. (Eds NC Turner, PJ Kramer) pp. 207–230. (John Wiley & Sons: New York)
Gaff DF (1981) The biology of resurrection plants. In ‘The biology of Australian plants’. (Eds JS Pate, AJ McComb) pp. 114–146. (University of Western Australia Press: Nedlands, WA)
Gaff DF (1987) Desiccation tolerant plants in South America. Oecologia 74, 133–136.
| Desiccation tolerant plants in South America.Crossref | GoogleScholarGoogle Scholar |
Gaff DF, Churchill DM (1976) Borya nitida Labill. — an Australian species in the Liliaceae with desiccation-tolerant leaves. Australian Journal of Botany 24, 209–224.
| Borya nitida Labill. — an Australian species in the Liliaceae with desiccation-tolerant leaves.Crossref | GoogleScholarGoogle Scholar |
Gaff DF, Giess W (1986) Drought resistance in water plants in rock pools in southern Africa. Dinteria 18, 17–36.
Gaff DF, Hallam ND (1974) Resurrecting desiccated plants. Royal Society of New Zealand Bulletin 12, 389–393.
Gaff DF, Latz PK (1978) The occurrence of resurrection plants in the Australian flora. Australian Journal of Botany 26, 485–492.
| The occurrence of resurrection plants in the Australian flora.Crossref | GoogleScholarGoogle Scholar |
Gaff DF, Loveys BR (1984) Abscisic acid content and effects during dehydration of detached leaves of desiccation tolerant plants. Journal of Experimental Botany 35, 1350–1358.
| Abscisic acid content and effects during dehydration of detached leaves of desiccation tolerant plants.Crossref | GoogleScholarGoogle Scholar |
Gaff DF, Loveys BR (1993) Abscisic acid levels in drying plants of a resurrection grass. Transactions of the Malaysian Society of Plant Physiology 3, 286–287.
Gaff D, Ziegler H (1989) ATP and ADP contents in leaves of drying and rehydrating desiccation-tolerant plants. Oecologia 78, 407–410.
| ATP and ADP contents in leaves of drying and rehydrating desiccation-tolerant plants.Crossref | GoogleScholarGoogle Scholar |
Gaff D, Blomstedt C, Neale A, Le T, Hamill J, Ghasempour H (2009) Sporobolus stapfianus, a model desiccation-tolerant grass. Functional Plant Biology 36, 589–599.
| Sporobolus stapfianus, a model desiccation-tolerant grass.Crossref | GoogleScholarGoogle Scholar |
Gechev TS, Dinakar C, Benina M, Toneva V, Bartels D (2012) Molecular mechanisms of desiccation tolerance in resurrection plants. Cellular and Molecular Life Sciences: CMLS 69, 3175–3186.
| Molecular mechanisms of desiccation tolerance in resurrection plants.Crossref | GoogleScholarGoogle Scholar |
Gechev TS, Benina M, Obata T, Tohge T, Sujeeth N, Minkov I, Hille J, Temanni M-R, Marriot AS, Bergström E, Thomas-Oates J, Antonio C, Mueller-Roeber B, Schippers JHM, Fernie AR, Toneva V (2013) Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cellular and Molecular Life Sciences 70, 689–704.
| Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis.Crossref | GoogleScholarGoogle Scholar |
Ghasempour H, Anderson E, Gianello RD, Gaff D (1998) Growth inhibitor effects on the protoplasmic drought tolerance and protein synthesis in the leaf cells of the resurrection grass Sporobolus stapfianus. Plant Growth Regulation 24, 179–183.
| Growth inhibitor effects on the protoplasmic drought tolerance and protein synthesis in the leaf cells of the resurrection grass Sporobolus stapfianus.Crossref | GoogleScholarGoogle Scholar |
Ghasempour HR, Gaff DF, Williams RP, Gianello RD (1998b) Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses. Plant Growth Regulation 24, 185–191.
| Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses.Crossref | GoogleScholarGoogle Scholar |
Ghasempour H, Anderson E, Gaff D (2001) Effects of growth substances on the protoplasmic drought tolerance of leaf cells of the resurrection grass Sporobolus stapfianus. Australian Journal of Plant Physiology 28, 1115–1120.
Gould S, Waller R, McFadden G (2008) Plastid evolution. Annual Review of Plant Biology 59, 491–517.
| Plastid evolution.Crossref | GoogleScholarGoogle Scholar |
Goyal K, Walton L, Browne J, Burnell A, Tunnacliffe A (2005a) Molecular anhydrobiology: identifying molecules implicated in invertebrate anhydrobiosis. Integrative and Comparative Biology 45, 702–709.
| Molecular anhydrobiology: identifying molecules implicated in invertebrate anhydrobiosis.Crossref | GoogleScholarGoogle Scholar |
Goyal K, Walton L, Tunnacliffe A (2005b) LEA proteins prevent protein aggregation due to water stress. The Biochemical Journal 388, 151–157.
| LEA proteins prevent protein aggregation due to water stress.Crossref | GoogleScholarGoogle Scholar |
Grant B, Howard R, Gayler K (1976) Isolation and properties of chloroplasts from the siphonous green alga Caulerpa simpliciuscula. Australian Journal of Plant Physiology 3, 639–651.
Halda J (1979) A new intergeneric hybrid in the family Gesneraceae. Preslia, Praha 51, 375–376.
Hartung W (2010) The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Functional Plant Biology 37, 806–812.
| The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen.Crossref | GoogleScholarGoogle Scholar |
Hartung W, Weiler EW, Volk OH (1987) Immunological evidence that abscisic acid is produced by several species of Anthocerotae and Marchantiales. The Bryologist 90, 393–400.
| Immunological evidence that abscisic acid is produced by several species of Anthocerotae and Marchantiales.Crossref | GoogleScholarGoogle Scholar |
Heddad M, Adamska I (2002) The evolution of light stress proteins in photosynthetic organisms. Comparative and Functional Genomics 3, 504–510.
| The evolution of light stress proteins in photosynthetic organisms.Crossref | GoogleScholarGoogle Scholar |
Hilbricht T, Varotto S, Sgaramella V, Bartels D, Salamini F, Furini A (2008) Retrotransposons and siRNA have a role in the evolution of desiccation tolerance leading to resurrection in the plant Craterostigma plantagineum. New Phytologist 179, 877–887.
| Retrotransposons and siRNA have a role in the evolution of desiccation tolerance leading to resurrection in the plant Craterostigma plantagineum.Crossref | GoogleScholarGoogle Scholar |
Höfler K (1943) Über die Austrocknungsfähigkeit des Protoplasmas. Berichte der deutschen botanischen Geselschaft 60, 94–106.
Höfler K, Migsch H, Rottenburg W (1941) Über die Austrocknungresistenz landwirtschaftlicher Kulturpflanzen. Forschungsdienst 12, 50–61.
Holman R, Brubaker F (1926) On the longevity of pollen. University of California Publications in Botany 13, 179–204.
Illing N, Denby K, Collet H, Shen A, Farrant J (2005) The signature of seeds in resurrection plants: a molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissue. Integrative and Comparative Biology 45, 771–787.
| The signature of seeds in resurrection plants: a molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissue.Crossref | GoogleScholarGoogle Scholar |
Iturriaga G, Gaff D, Zentella R (2000) New desiccation-tolerant plants, including a grass, in the Central Highlands of Mexico, accumulate trehalose. Australian Journal of Botany 48, 153–158.
| New desiccation-tolerant plants, including a grass, in the Central Highlands of Mexico, accumulate trehalose.Crossref | GoogleScholarGoogle Scholar |
Jäger K, Bergman B (1991) Localization of a multifunctional chaperonin (GroEL protein) in nitrogen-fixing Anabaena PCC 7120. Planta 183, 120–125.
| Localization of a multifunctional chaperonin (GroEL protein) in nitrogen-fixing Anabaena PCC 7120.Crossref | GoogleScholarGoogle Scholar |
Kim BH, Schoffl F (2002) Interaction between Arabidopsis heat shock transcription factor 1 and 70kDa heat shock proteins. Journal of Experimental Botany 53, 371–375.
| Interaction between Arabidopsis heat shock transcription factor 1 and 70kDa heat shock proteins.Crossref | GoogleScholarGoogle Scholar |
Kuang J, Gaff D, Gianello R, Blomstedt C, Neale A, Hamill J (1995) Changes in in vivo protein complements in drying leaves of the desiccation-tolerant grass Sporobolus stapfianus and the desiccation-sensitive grass Sporobolus pyramidalis. Australian Journal of Plant Physiology 22, 1027–1034.
| Changes in in vivo protein complements in drying leaves of the desiccation-tolerant grass Sporobolus stapfianus and the desiccation-sensitive grass Sporobolus pyramidalis.Crossref | GoogleScholarGoogle Scholar |
Lebkuecher JG (1997) Desiccation-time limits of photosynthetic recovery in Equisetum hyemale (Equisetaceae) spores. American Journal of Botany 84, 792–797.
| Desiccation-time limits of photosynthetic recovery in Equisetum hyemale (Equisetaceae) spores.Crossref | GoogleScholarGoogle Scholar |
Levitt J, Sullivan CY, Krull E (1960) Some problems in drought resistance. Bulletin of the Research Council of Israel 8D, 177–179.
Liu K, Eastwood RJ, Flynn S, Turner RM, Stuppy WH (2008) ‘Seed information database. (Release 7.1, May 2008)’ Available at http://www.kew.org/data/sid.
Liu X, Wang Z, Wang L, Wu R, Phillips J, Deng X (2009) LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. Plant Science 176, 90–98.
| LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco.Crossref | GoogleScholarGoogle Scholar |
Lüttge U, Beck E, Bartels D, Eds (2011) ‘Plant desiccation tolerance.’ (Springer: Heidelberg, Germany)
Maia J, Dekkers BJW, Provart NJ, Ligterink W, Hilhorst HWM (2011) The re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome. PLoS ONE 6, e29123
| The re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome.Crossref | GoogleScholarGoogle Scholar |
Martinelli T, Whittaker A, Bochicchio A, Vazzana C, Suzuki A, Masclaux-Daubresse C (2007) Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: a comparison between desiccation-sensitive and desiccation-tolerant leaves. Journal of Experimental Botany 58, 3037–3046.
| Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: a comparison between desiccation-sensitive and desiccation-tolerant leaves.Crossref | GoogleScholarGoogle Scholar |
McCourt RM, Delwiche CF, Karol KG (2004) Charophyte algae and land plant origins. Trends in Ecology & Evolution 19, 661–666.
| Charophyte algae and land plant origins.Crossref | GoogleScholarGoogle Scholar |
Neale A, Blomstedt C, Bronson P, Le T-N, Guthridge K, Evans J, Gaff D, Hamill J (2000) The isolation of genes from the resurrection grass Sporobolus stapfianus which are induced during severe stress. Plant, Cell & Environment 23, 265–277.
| The isolation of genes from the resurrection grass Sporobolus stapfianus which are induced during severe stress.Crossref | GoogleScholarGoogle Scholar |
Oliver MJ (2007) Lessons on dehydration tolerance from desiccation-tolerant plants. In ‘Plant desiccation tolerance’. (Eds MA Jenk, AJ Wood) pp. 11–50. (Blackwell: Ames, IA)
Oliver MJ, Bewley JD (1997) Desiccation tolerance of plant tissues: a mechanistic overview. Horticultural Reviews 18, 171–214.
Oliver MJ, Tuba Z, Mishler B (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecology 151, 85–100.
| The evolution of vegetative desiccation tolerance in land plants.Crossref | GoogleScholarGoogle Scholar |
Oliver MJ, Velten J, Mishler B (2005) Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integrative and Comparative Biology 45, 788–799.
| Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats?Crossref | GoogleScholarGoogle Scholar |
Oliver MJ, Guo L, Alexander DC, Ryals JA, Wone BWM, Cushman JC (2011a) A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. The Plant Cell 23, 1231–1248.
| A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus.Crossref | GoogleScholarGoogle Scholar |
Oliver MJ, Jain R, Balbuena TS, Agrawal G, Gasulla F, Thelen JJ (2011b) Proteome analysis of leaves of the desiccation-tolerant grass, Sporobolus stapfianus, in response to dehydration. Phytochemistry 72, 1273–1284.
| Proteome analysis of leaves of the desiccation-tolerant grass, Sporobolus stapfianus, in response to dehydration.Crossref | GoogleScholarGoogle Scholar |
Pate JS, Dixon KW (1982) ‘Tuberous, cormous and bulbous plants — biology of an adaptive strategy.’ (University of Western Australia Press: Nedlands, WA)
Pence VC (2000) Survival of chlorophyllous and nonchlorophyllous fern spores through exposure to liquid nitrogen. American Fern Journal 90, 119–126.
| Survival of chlorophyllous and nonchlorophyllous fern spores through exposure to liquid nitrogen.Crossref | GoogleScholarGoogle Scholar |
Peters S, Mundree SG, Thomson JA, Farrant JM, Keller F (2007) Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. Journal of Experimental Botany 58, 1947–1956.
| Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit.Crossref | GoogleScholarGoogle Scholar |
Petersen J, Eriksson SK, Harryson P, Pierog S, Colby T, Bartels D, Röhrig H (2012) The lysine-rich motif of intrinsically disordered stress protein CdeT11–24 from Craterostigma plantagineum is responsible for phosphatidic acid binding and protection of enzymes from damaging effects caused by desiccation. Journal of Experimental Botany 63, 4919–4929.
| The lysine-rich motif of intrinsically disordered stress protein CdeT11–24 from Craterostigma plantagineum is responsible for phosphatidic acid binding and protection of enzymes from damaging effects caused by desiccation.Crossref | GoogleScholarGoogle Scholar |
Porembski S, Barthlott W (2000) Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation tolerant plants. Plant Ecology 151, 19–28.
| Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation tolerant plants.Crossref | GoogleScholarGoogle Scholar |
Prieto-Dapena P, Castaño R, Almoguera C, Jordano J (2008) The ectotopic overexpression of a seed-specific transcription factor, HaHSFA9, confers tolerance to severe dehydration in vegetative organs. The Plant Journal 54, 1004–1014.
| The ectotopic overexpression of a seed-specific transcription factor, HaHSFA9, confers tolerance to severe dehydration in vegetative organs.Crossref | GoogleScholarGoogle Scholar |
Pruzsinsky S (1960) Über Trocken- und Feuchtluftresistenz des Pollen. Osterreichische Akademie der Wissenschaften: Mathematik.-natur Klass., Abteilung 1 169, 43–100.
Raven JA (2002) Selection pressure on stomatal evolution. New Phytologist 153, 371–386.
| Selection pressure on stomatal evolution.Crossref | GoogleScholarGoogle Scholar |
Raven JA, Andrews M (2010) Evolution of tree nutrition. Tree Physiology 30, 1050–1071.
| Evolution of tree nutrition.Crossref | GoogleScholarGoogle Scholar |
Reed R, Richardson DL, Warr S, Stewart WDP (1984) Carbohydrate accumulation and osmotic stress in cyanobacteria. Journal of General Microbiology 130, 1–4.
Rodriguez MCS, Edsgärd D, Hussain SS, Alquezar D, Rasmussen M, Gilbert T, Nielsen BH, Bartels D, Mundy J (2010) Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum. The Plant Journal 63, 212–228.
| Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum.Crossref | GoogleScholarGoogle Scholar |
Sallon S, Solowey E, Cohen Y, Korchinsky R, Egli M, Woodhatch I, Simchoni O, Kislev M (2008) Germination, genetics, and growth of an ancient date seed. Science 320, 1464
| Germination, genetics, and growth of an ancient date seed.Crossref | GoogleScholarGoogle Scholar |
Shen Y, Tang M-J, Hu Y-L, Lin Z-P (2004) Isolation and characterization of a dehydrin-like gene from drought-tolerant Boea crassifolia. Plant Science 166, 1167–1175.
Thompson MD, Paavola CD, Lenvik TR, Gantt JS (1995) Chlamydomonas transcripts encoding three divergent plastid chaperonins are heat-inducible. Plant Molecular Biology 27, 1031–1035.
| Chlamydomonas transcripts encoding three divergent plastid chaperonins are heat-inducible.Crossref | GoogleScholarGoogle Scholar |
Tietz A, Ruttkowski U, Köhler R, Kasprik W (1989) Further investigations on the occurrence and the effects of abscisic acid in algae. Biochemie und Physiologie der Pflanzen 184, 259–266.
Van Zanten B (1978) Experimental studies on the trans oceanic long-range dispersal of moss spores in the southern hemisphere. Journal of the Hattori Botanical Laboratory 44, 455–482.
Visser T (1955) Germination and storage of pollen. Meded Landbouwhogeschool Wageningen 55, 1–68.
Watkins JE, Mack MC, Sinclair TR, Mulkey SS (2007) Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176, 708–717.
| Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes.Crossref | GoogleScholarGoogle Scholar |
Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiology 122, 1099–1108.
| The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance.Crossref | GoogleScholarGoogle Scholar |
Werner O, Ros-Espin R, Bopp M, Atzorn R (1991) Abscisic acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186, 99–103.
| Abscisic acid-induced drought tolerance in Funaria hygrometrica Hedw.Crossref | GoogleScholarGoogle Scholar |
Yobi A, Wone BWM, Xu W, Alexander DC, Guo L, Ryals JA, Oliver MJ, Cushman JC (2012) Comparative metabolic profiling between desiccation sensitive and desiccation tolerant species of Selaginella reveals novel insights into the resurrection trait. The Plant Journal 72, 983–999.
| Comparative metabolic profiling between desiccation sensitive and desiccation tolerant species of Selaginella reveals novel insights into the resurrection trait.Crossref | GoogleScholarGoogle Scholar |
Yokota T, Kim SK, Fukui Y, Takahashi N, Takeuchi Y, Takematsu T (1987) Brassinosteroids and sterols from a green alga Hydrodictyon reticulatum: Configuration at C-24. Phytochemistry 26, 503–506.
| Brassinosteroids and sterols from a green alga Hydrodictyon reticulatum: Configuration at C-24.Crossref | GoogleScholarGoogle Scholar |
Zahradníčková H, Marðálek B, Poliðenská M (1991) High-performance thin-layer chromatographic and high-performance liquid chromatographic determination of abscisic acid produced by cyanobacteria. Journal of Chromatography. ,A 555, 239–245.
| High-performance thin-layer chromatographic and high-performance liquid chromatographic determination of abscisic acid produced by cyanobacteria.Crossref | GoogleScholarGoogle Scholar |


