Cyanogenesis in the Australian tropical rainforest endemic Brombya platynema (Rutaceae): chemical characterisation and polymorphism
Rebecca E. Miller A B , Judy Simon A and Ian E. Woodrow AA School of Botany, The University of Melbourne, Parkville, Vic. 3010, Australia.
B Corresponding author. Email: rem@unimelb.edu.au
C This paper originates from a presentation at ECOFIZZ 2005, North Stradbroke Island, Queensland, Australia, November 2005.
Functional Plant Biology 33(5) 477-486 https://doi.org/10.1071/FP05305
Submitted: 14 December 2005 Accepted: 23 February 2006 Published: 2 May 2006
Abstract
This study examined two aspects of cyanogenesis in Brombya platynema F. Muell. (Rutaceae), a subcanopy tree endemic to tropical rainforest in far north Queensland, Australia. First, cyanogenic glycosides in foliage were fractionated and identified. The rare meta-hydroxylated cyanogenic glycoside, holocalin, was identified as the principal cyanogen, and traces of prunasin and amygdalin were detected. This is the first characterisation of cyanogenic constituents within the genus, and to the authors’ knowledge, only the third within the Rutaceae, and the order Rutales. Second, variation in cyanogenic glycoside content within a population of B. platynema in lowland tropical rainforest was quantified. Both qualitative and quantitative polymorphism for cyanogenesis was identified. Interestingly, ~57% of individuals were considered acyanogenic, with concentrations of cyanogenic glycosides less than 8 μg CN g–1 DW. Among cyanogenic individuals there was substantial quantitative variation in cyanogenic glycoside concentration, which varied from 10.5 to 1285.9 μg CN g–1 DW. This high frequency of acyanogenic individuals is contrasted with the apparent absence of the acyanogenesis among populations of other tropical rainforest tree species. In the high herbivory environment of the tropical rainforest, this frequency of acyanogenesis among cyanogenic tropical tree taxa is unique.
Keywords: amygdalin, Australia, β-glucosidase, Brombya platynema, cyanogenesis, cyanogenic glycoside, defence, holocalin, polymorphism, prunasin, Rutaceae, tropical rainforest.
Introduction
Brombya platynema (Rutaceae) is a subcanopy tree species endemic to tropical rainforest in far north Queensland, Australia, where it occurs in well developed forest (sea level to 1100 m) (Hyland et al. 2003). B. platynema was first found to be cyanogenic in a large survey for cyanogenesis in Australian tropical rainforests (Miller et al. 2006a). Plant cyanogenesis is the release of toxic hydrogen cyanide (HCN) from endogenous cyanide-containing compounds, and requires the presence of either an unstable cyanohydrin, or of a stable cyanogen, usually a cyanogenic glycoside, and its degradative enzymes (Seigler 1991). Cyanogenesis typically occurs upon tissue disruption, due to mechanical damage or ingestion by herbivores, for example (Seigler 1991), which enables mixing of enzymes and cyanogenic substrates (Wajant et al. 1994). Owing largely to its affinity for the terminal cytochrome oxidase in the mitochondrial respiratory pathway, cyanide is toxic to most living organisms (Jones 1988; McMahon et al. 1995). Indeed cyanogenesis, which is a constitutive defence, is highly effective in reducing tissue loss to generalist herbivores (e.g. Hruska 1988; Jones 1988; Gleadow and Woodrow 2002a). In addition, both cyanogenesis and the specific cyanogen present have proven useful in systematic studies at all taxonomic levels — within orders, families and genera (e.g. Maslin et al. 1988; Spencer and Seigler 1985; Olafsdottir et al. 1989; Seigler 2003).
Miller and co-workers’ (2006a) survey was the first report of cyanogenesis in the genus Brombya, which consists of two species endemic to Australia (Hyland et al. 2003). The Rutaceae is a large cosmopolitan family (150 genera, 1500 spp.), although it is more common in tropical and temperate regions of the southern hemisphere (Morley and Toelken 1983; Mabberley 1990; Hyland et al. 2003). The family includes many strongly scented shrubs and trees, and rutaceous species are known for their terpenoids and alkaloids (Price 1963; Gibbs 1974; Everist 1981). Cyanogenesis is rare in Rutaceae, and had only been reported in Boronia bipinnate Lindl. (Rosenthaler 1919), Zieria spp. (Hurst 1942; Gibbs 1974; Fikenscher and Hegnauer 1977), Zanthoxylum fagara (Adsersen et al. 1988) and Loureira cochinchinensis Meissa (Gibbs 1974). Even within the order Rutales, cyanogenesis is rare, with only a few additional definitive reports of cyanogenesis in the Tremandraceae family (Gibbs 1974). The characterisations of cyanogenic glycosides from leaves of two Zieria spp. (Finnemore and Cooper 1936; Fikenscher and Hegnauer 1977) are the only reports detailing cyanogens in the Rutaceae.
The present study of B. platynema was initiated because of the rarity of cyanogenesis in the Rutaceae family, and because a preliminary analysis of a population of B. platynema had indicated a high frequency of acyanogenic individuals (Miller 2004). This latter finding is in stark contrast to those for populations of other tropical rainforest tree species studied to date, which show only quantitative variation in cyanogenic glycoside content, and indicate the absence or rarity of the acyanogenic phenotype (Miller et al. 2004, 2006a; Webber 2005). For example, in a survey of over 1000 individuals (trees and seedlings) of Ryparosa javanica, Webber (2005) failed to detect a single acyanogenic individual. Mixtures of cyanogenic and acyanogenic individuals are, however, more common in temperate ecosystems (e.g. Cooper-Driver and Swain 1976; Ellis et al. 1977; Urbanska 1982; Conn et al. 1985; Hughes 1991; Aikman et al. 1996; Goodger et al. 2002; Woodrow et al. 2002).
Here we characterise two aspects of cyanogenesis in B. platynema. First, we identify the structures of the foliar cyanogens. Second, we describe both qualitative and quantitative polymorphism for cyanogenesis within a single population of B. platynema in lowland tropical rainforest.
Materials and methods
Site description — Lowland rainforest
The study population of Brombya platynema F. Muell. was in lowland rainforest in the Daintree World Heritage Area, near Cape Tribulation (Fig. 1). The site was near to Thompson and Myall Creeks (16°06.2′ S, 145°26.9′ E; altitude 40 m above sea level; slope 10–20°). The forest is complex mesophyll vine forest (Type 1a; Tracey 1982), the canopy is irregular, from 25 to 33 m in height, and supports a great diversity of species and life forms, including many palms and lianas. The floristic composition is patchy, with considerable variation in canopy and understorey dominants over small distances (Webb et al. 1972; Tracey 1982). The soil is relatively nutrient-poor red clay loam podsol derived from metamorphic substrate.
|
Climate
The climate in far north Queensland is characterised by a marked wet season from December to April. In the coastal lowland tropical rainforest near Cape Tribulation, mean daily temperatures range from 28°C in January to 22°C in July, and temperatures may reach mid- to high 30 s during the summer months. Average annual rainfall is also high, at 3928 mm recorded at Cape Tribulation (based on 65 years of records from the Queensland Bureau of Meteorology).
Qualitative detection of cyanogenesis — Feigl–Anger papers
During the preliminary survey, the presence of cyanogenic compounds in fresh field samples was determined with Feigl–Anger (FA) indicator papers (Feigl and Anger 1966). FA test papers were prepared according to Brinker and Seigler (1989). Refer to Miller et al. (2006a) for full description of the screening methodology. Fresh leaves (approximately 1–2 g FW) were crushed in duplicate screw top vials. Old and young foliage samples were tested separately. To facilitate cyanogenesis, 0.5 mL of water was added to one of the vials, and pectinase from Rhizopus spp. (0.4 g L–1; Macerase® Pectinase, 441201 Calbiochem®, Calbiochem-Novabiochem Corp., San Diego, CA) in 0.1 m Tris–HCl (pH 6.8) was added to the other. Pectinase has non-specific β-glycosidase activity (Brimer et al. 1995) and therefore, in the absence of sufficient endogenous β-glycosidase, enables tests for presence of cyanogenic glycosides to be made. Tissue of known cyanogenic species, Prunus turneriana or Ryparosa javanica, was used as a positive control. An individual was considered cyanogenic if a positive, repeatable result was obtained. The acyanogenic phenotype was confirmed by quantitative assay (see below).
Sample collection and handling
A population of B. platynema (n = 46) in lowland rainforest, near Cape Tribulation was sampled in July 2001. Within the rainforest B. platynema is highly patchy in its distribution, and individuals sampled did not form a discrete population. Large saplings and trees were sampled from within six randomly located 200-m2 plots within continuous forest (area < 1 ha). Whole-leaf samples (lamina and midrib; most recent fully expanded dark green leaves) were placed in sealed airtight bags and kept on ice for 2–6 h until snap frozen in liquid nitrogen. Leaves show a distinct colour and texture change following full expansion; hence it was possible to control broadly for leaf age. Owing to the logistical difficulties of sampling in the rainforest, it was not possible to control for aspect and height. There was no evidence of suckering. These initial samples were analysed for foliar nitrogen and cyanogenic glycoside content. Subsequently, a smaller number (n = 23) of trees within the same population, and ranging in cyanogenic glycoside content from 1.2 to 850 μg CN g–1 DW, were sampled in May 2005 for correlative analyses. In this case, leaf disks were sampled to enable determination of specific leaf area (SLA; the unit area leaf per mass). These samples were analysed for total phenolics and condensed tannins, in addition to cyanogenic glycoside and nitrogen content. All samples were transported to the laboratory on dry ice, freeze-dried, and stored on desiccant at –20°C before analysis. Freeze-dried samples were ground using either a cooled IKA Labortechnic A10 Analytical Mill (Janke and Kunkel, Stanfen, Germany) or, for smaller samples, an Ultramat 2 Dental Grinder (Southern Dental Industries Ltd, Bayswater, Vic.). Voucher specimens of B. platynema have been lodged at the Brisbane (specimen BRI AQ. 578818) and the University of Melbourne Herbaria (specimens MELU102283, 102313).
Quantitative determination of cyanogenesis
Cyanogenic glycoside concentration in plant material and in extracts was measured by hydrolysing the glycoside and trapping the evolved cyanide in a 1 m NaOH well (Gleadow et al. 1998; Brinker and Seigler 1989). Freeze-dried, ground plant tissue (15–20 mg) was incubated for 20 h at 37°C with 1 mL of 0.1 m citrate buffer–HCl (pH 5.5), conditions which allowed for complete conversion of the cyanogenic glycoside to cyanide (data not shown). To detect and quantify cyanogenic compounds during purification, glycosides were hydrolysed using β-glucosidase enzyme partially purified from the same B. platynema leaf tissue used for cyanogenic glycoside purification or by the addition of β-glucosidase from almonds [emulsin from Prunus amygdalus (L.) Bent. and Hook.; EC 3.2.1.21, Sigma G-0395] at the rate of 1.12 units mL–1 to the buffer (Gleadow et al. 1998). Preliminary experiments showed that in all cases, the almond emulsin was as effective as the extracted leaf enzyme in hydrolysing the cyanogens over 24 h. All subsequent assays therefore used almond emulsin. Cyanide in the NaOH well was determined by the method of Gleadow and Woodrow (2002b) adapted from Brinker and Seigler (1989) for use with a photometric microplate reader [Labsystems Multiskan® Ascent (Labsystems, Helsinki, Finland), with incubator].
Confirmation of acyanogenesis
To confirm the acyanogenic character of individuals producing negative results with FA papers, 100 mg of freeze-dried tissue was assayed quantitatively for the evolution of cyanide and compared with a non-cyanogenic species, Alstonia scholaris (L.) R.Br., as a baseline control for the assay. Based on the criteria of Adsersen et al. (1988), if less than 8 μg HCN g–1 DW was detected, then that individual was considered acyanogenic. The quantitative assay is highly sensitive, and as little as 1 μg CN g–1 DW can be reliably detected. A negative result indicated the absence of a cyanogenic glycoside, or of the specific cyanogenic β-glycosidase, or both. To determine whether functionally acyanogenic individuals had the cyanogen, but lacked the catabolic enzyme, acyanogenic individuals were also analysed with the addition of almond emulsin, known to cleave B. platynema glycosides.
Cyanogenic β-glucosidase purification
Cyanogenic β-glucosidase was partially purified from the same B. platynema leaf tissue used for identification of the cyanogenic glycosides (Brinker and Seigler 1989). Freeze-dried tissue (5 g) was extracted at 4°C in a protein extraction buffer (Gleadow et al. 1998), filtered, and centrifuged (20 min at 27 000 g) to remove remaining tissue. The supernatant was fractionated by adding solid ammonium sulfate and proteins precipitating between 35 and 90% ammonium sulfate saturation were collected and resuspended in a minimal amount of buffer [0.1 m citrate buffer–HCl (pH 5.5)] and desalted with a dialysis cassette (Slide-A-Lyzer® 3.5 K, MWCO 3500, Pierce, Rockford, IL) in 0.1 m citrate buffer–HCl (pH 5.5). Aliquots of the crude enzyme preparation were incubated and tested for cyanide to verify that no cyanogenic glycoside had been extracted in the protein preparation.
Identification of cyanogenic glycosides
Extraction and purification
Cyanogenic glycosides were extracted from leaf tissue of B. platynema by the procedure described by Goodger et al. (2002) and Gleadow et al. (2003). Leaf tissue was pooled from 10 trees, including several trees from a different area of forest not sampled in the population study. These individuals ranged in cyanogenic glycoside concentration from 82 to 615 μg CN g–1 DW. Homogenised, freeze-dried tissue (40 g) was de-fatted by five extractions with petroleum ether (solvent : tissue, 10 : 1 v / w), filtered (Whatman® 541 filter paper, Whatman Asia Pacific, San Centre, Singapore), and then twice extracted with cold methanol, and filtered. The filtrate volume was reduced by rotary evaporation (40°C), and an equivalent volume of CHCl3 was added, with sufficient H2O to facilitate phase separation. The methanol phase was collected and concentrated in vacuo and tested quantitatively for cyanogenic glycosides by the addition of β-glucosidase enzyme partially purified from the same leaf tissue. Both β-glucosidase from almonds (emulsin from Prunus amygdalus Batsch; E.C. 3.2.1.21, Sigma G-0395 added at 1.04 units mL–1), and pectinase from Rhizopus spp. (Macerase® Pectinase, E.C. 3.2.1.15; 441201 Calbiochem®, Calbiochem-Novabiochem Corp., added at 1.22 units mL–1) were also tested for activity. Emulsin was found to be as effective as the leaf protein extract in fully catabolising the cyanogens, and was therefore used for all subsequent assays. The methanol fraction was then fractionated isocratically by reverse-phase HPLC using 20% MeCN-H2O (2 mL min–1) through a Phenomenex Luna C18 column (250 mm × 10 mm × 5 μm particle size; Phenomenex, Pennant Hills, NSW). Fractions (1 min) were concentrated in vacuo and tested for the presence of cyanogenic glycoside.
Nuclear magnetic resonance
The 1H NMR spectrum of the purified cyanogen eluting at HPLC RT 10.6 min was obtained on a Bruker Avance 600 MHz LC / NMR (Bruker Biospin GmbH, Rheinstetten, Germany) using direct injection mode and a 3 mm LCSEI probe. The sample was dissolved in 50 µL of CD3OD with 30 µL injected into the probe using methanol as the elution solvent. The experiment used was an LC 1D 13C decoupled multiple-solvent suppression experiment. The methine singlet at around 5.8 ppm was referenced to 5.82 ppm for direct comparison to the literature values of DellaGreca et al. (2000).
GC analysis of TMS derivatives and LC-ESI / MS
To determine whether prunasin and / or sambunigrin were present, the minor cyanogenic region RT 17–18 min was analysed by liquid chromatography electrospray ionisation mass spectrometry (LC-ESI / MS Finnigan Surveyor LC with LCQ Deca XP MAX mass spectrometer; Thermo Electron Corporation, San Jose, CA). The methanol extract was dissolved in 1% NH3 in 80% methanol (v / v) and fractionated using reverse-phase HPLC (0.5 mL min–1, 20% MeCN-H20 through a 150 mm × 4.6 mm Phenomonex Luna C18 5 μm column). Negative ion ESI / MS analysis confirmed a compound with MW (m/z 294 [M-H]-) and fragmentation (MS / MS) pattern identical to that of authentic prunasin (Sigma M–0636), but also sambunigrin. To clarify the configuration at the chiral carbon, tri-methylsilyl (TMS)-derivative of the fractions collected at RT 17–18 min were analysed by GC. The TMS-ethers of epimers (R)-prunasin and (S)-sambunigrin are resolved by GC (Nahrstedt 1981; Seigler 1991). The cyanogenic fraction was derivatised using Tri-Sil® Reagent (HMDA : TMCS : Pyridine, 2 : 1 : 10) (Pierce, Rockford, IL) and analysed by GC (Autosystem XL GC-FID; Perkin Elmer, Melbourne, Vic.). Aliquots (1 μL) were injected at 310°C onto a column (BPX5; 30 m × 0.25 mm × 0.25 μm; SGE, Ringwood, Vic.) and separated using a temperature program increasing from 200 to 315°C, with a gradient of 5°C min–1 and a flow rate of 1.3 mL min–1 (see Buhrmester et al. 2000; Gleadow et al. 2003). The spectrum was analysed and compared with authentic prunasin (Sigma M–0636).
Other chemical analyses
Foliar nitrogen concentration
Total nitrogen concentration of 5–10 mg of freeze-dried tissue was determined with a Perkin Elmer 2400 Series II CHNS / O Analyser (Perkin-Elmer Pty Ltd, Melbourne, Vic.) calibrated with the organic analytical standard acetanilide (Perkin-Elmer #0240–1121).
Total phenolics and condensed tannins
Finely ground, freeze-dried leaf tissue (~20 mg) was extracted in 1 mL cold 50% acetone (v / v) by vortexing for 20 s. Samples were left at 4°C for 5 min, vortexed, and following centrifugation (26 000 g for 5 min), the supernatant was removed to pre-weighed tubes. The tissue was extracted a further three times (3 × 1 mL), and the four supernatants pooled into the pre-weighed tubes (see Gleadow and Woodrow 2002b). Aliquots of the same extract were used for determination of total phenolic and condensed tannin content. To avoid degradation, condensed tannin concentration was determined immediately after extraction. Condensed tannins were determined by the vanillin–HCl method described by Cork and Krockenberger (1991). An aliquot of the 50% acetone was analysed for condensed tannin concentration with (+)-catechin as a standard (Julkunen-Tiitto 1985).
Total phenolic concentration was determined with Folin-Ciocalteau’s phenol reagent (Fluka #47641, Neu-Ulm, Switzerland), according to the method of Cork and Krockenberger (1991). Gallic acid (GA) was the standard. This method quantifies the total concentration of phenolic hydroxyl groups in the extract, including lignin, tannins, phenolic acids and flavonoids (see Singleton and Rossi 1965; Cork and Krockenberger 1991).
Statistical analyses
Statistical analyses (regression analyses) were conducted with Minitab Release 14 (Minitab, Pasadena, CA). The normality of distributions of cyanogenic glycoside concentrations within populations was tested by the Ryan–Joiner (Shapiro–Wilks) normality tests, which were also computed with Minitab Release 14.
Results
Identification of cyanogenic glycosides
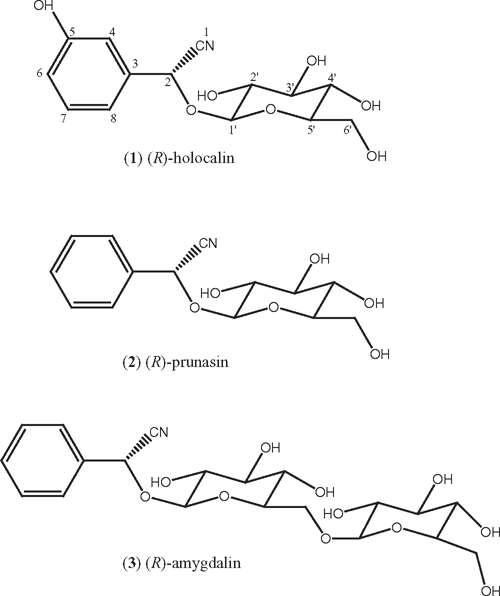
The principal cyanogenic glycoside, accounting for 94% of total cyanide in the leaf methanol extract, eluted at HPLC RT 10.6 min and was purified for analysis. ESI / MS indicated a MW of 311 amu, 16 mass units — the MW of a hydroxyl group (-OH) — more than the MW of the cyanogenic glycosides prunasin / sambunigrin, and the same as the MW of three known cyanogenic glycosides, zierin, holocalin and dhurrin (see Lechtenberg and Nahrstedt 1999). 1H NMR analysis identified the m-hydroxylated cyanogenic glycoside holocalin (Fig. 2; 1), by comparison with the published spectrum (DellaGreca et al. 2000). In addition, a second minor cyanogen eluting at RT 17–18 min, accounting for 4% total cyanogen, was in all respects (HPLC RT, LC-ESI / MS, TMS-ether) identical to authentic (R)-prunasin (Fig. 2; 2). LC-ESI / MS determined a MW (295 amu) and fragmentation pattern (MS / MS) identical to prunasin and its S epimer sambunigrin, and the (R)-configuration at the chiral carbon was confirmed by GC analysis of TMS-derivative. Finally, a trace amount of a third cyanogenic glycoside, the di-glycoside amygdalin (Fig. 2; 3) was also detected. The RP-HPLC elution time of 3 was 10.3 min, closely associated with holocalin (RT 10.6 min). This minor third cyanogen was therefore not resolved during CN assays of initial HPLC fractions; however, during LC-ESI / MS analysis of the leaf methanol extract, trace amounts of a compound with identical RT, MW (457 amu) and fragmentation (MS / MS) to authentic amygdalin was detected.
|
Qualitative and quantitative intra-population variation in cyanogenesis
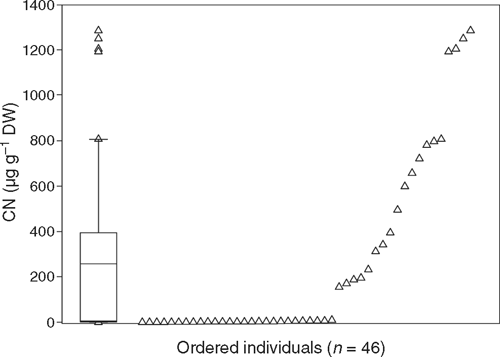
Within a population of B. platynema (n = 46), both qualitative and quantitative variation in cyanogenic glycoside content was identified (Fig. 3). Approximately 57% of individuals tested produced negative FA test paper results for both young and old leaves, and subsequent quantitative analysis of the most recent fully expanded leaf confirmed the acyanogenic nature of these individuals, which had cyanogenic glycoside concentrations in the range 0.6–6.8 μg CN g–1 DW (n = 26) (Fig. 3). Concentrations less than 2 μg CN g–1 DW were measured in 10 individuals. Based on the criteria of Adsersen et al. (1988), plants with less than 8 μg CN g–1 DW were considered to be acyanogenic. There was strong correlation between FA paper results and the quantitative assay, with only one individual being reclassified following quantitative analysis. The addition of buffered pectinase during testing did not alter the qualitative FA paper test result for any individual. Furthermore, neither the addition of cyanogenic β-glucosidase enzyme extracted from leaves of cyanogenic individuals of B. platynema, nor the addition of the cyanogenic β-glucosidase from almonds (emulsin) resulted in the release of more cyanide from acyanogenic individuals. Among cyanogenic individuals of B. platynema, the concentrations of cyanogenic glycosides varied over two orders of magnitude from 10.5 to 1285.9 μg CN g–1 DW (Fig. 3). The frequency distribution of cyanogenic glycoside concentrations was not normally distributed, but right skewed (Shapiro–Wilks P<0.01). Across the whole population, the mean cyanogenic glycoside concentration was 258.5 ± 58.3 μg CN g–1 DW (±1 s.e.).
|
Consistent with this large variation in cyanogenic glycoside concentration, the proportion of leaf N allocated to cyanogenic glycosides (CN-N / N%) in B. platynema was also highly variable. Among cyanogenic individuals, the proportion of N allocated to cyanide-based defence ranged from 0.004 to 4.7% (for n = 31; 2001 population). There was no correlation between foliar N concentration and the concentration of cyanogenic glycosides for populations sampled in 2001 or 2005 (data not shown). In addition, there was no correlation between cyanogenic glycoside content and specific leaf area (SLA; the unit area leaf per mass).
Total phenolics and condensed tannins
Variation in other secondary metabolites, specifically, total phenolics and condensed tannins was investigated for half of the population, covering a range of cyanogenic glycoside content from 1.2 – 850 μg CN g–1 DW. Mean total phenolic content was 15.5 ± 0.6 mg g–1 gallic acid equivalents (±1 s.e. for n = 23), while mean condensed tannin content was 6.08 ± 0.4 mg g–1 (+)-catechin equivalents (±1 s.e.). Total phenolics ranged from 12.9 to 24.4 mg g–1 DW, while condensed tannins ranged from 4.5 to 11.6 mg g–1 DW. There was no relationship between cyanogenic glycoside concentration and either total phenolics or condensed tannins (data not shown). The carbon-based secondary metabolites were, unlike the cyanogenic glycosides, related to foliar N content. In both instances, while only significant at the 10% level, there was a trend towards increasing phenolics (TP) and tannins (CT) with decreased foliar N [TP = 0.044–1.26 N, P = 0.055; CT = 0.021–0.612 N, P = 0.08].
Discussion
Cyanogenic glycosides in B. platynema
The cyanogenic glycoside holocalin (Fig. 2; 1), identified as the principal cyanogenic glycoside in leaves of B. platynema, has previously only been reported in a few species. First isolated from the seeds of Holocalyx balansae (Fabaceae; Gmelin et al. 1973), holocalin has since been isolated from the leaves of the common elder, Sambucus nigra (Caprifoliaceae; Jensen and Nielsen 1973), leaves, stems and flowers of Oxyanthus speciosus subsp. gerrardii (Rubiaceae; Rockenbach et al. 1992) and leaves of Chlorophytum comosum (Anthericaceae; van Valen 1978). In H. balansae, holocalin was restricted to the seeds, while prunasin was the sole cyanogen in leaves (Nahrstedt 1976). By contrast, in S. nigra leaves, the two m-hydroxylated diastereomers (R)-holocalin and (S)-zierin were found to co-occur with their unsubstituted homologues, (R)-prunasin and (S)-sambunigrin, but not in all individuals (Jensen and Nielsen 1973). Similarly, in B. platynema leaves, holocalin (Fig. 2; 1) co-occurred with small quantities of prunasin (Fig. 2; 2). In addition, preliminary LC-ESI / MS results suggested trace amounts of a third glycoside (R)-amygdalin (Fig. 2; 3) in the B. platynema leaf extract. This is the first reported co-occurrence of holocalin and amygdalin.
Holocalin belongs to a small group of cyanogenic glycosides with a m-hydroxyl attached to the aromatic ring. In addition to (S)-zierin, the epimer of (R)-holocalin, the other two members of this group are zierinxyloside and the more complex acylated cyanogenic trisaccharide, xeranthin (Hübel et al. 1982; Schwind et al. 1990), both of which also have the S configuration at the chiral carbon of the cyanohydrin.
The repeated co-occurrence of m-hydroxylated aromatic cyanogenic glycosides with non-hydroxylated (phenylalanine-derived) homologues (e.g. zierin and zierinxyloside with sambunigrin and epilucumin; Schwind et al. 1990) led to the assumption that the former may be biogenetically derived from phenylalanine (see also Jensen and Nielsen 1973; Nahrstedt 1976). The co-occurrence here in B. platynema of holocalin with phenylalanine-derived prunasin and amygdalin is consistent with this hypothesis. Feeding experiments with several amino acids subsequently demonstrated that phenylalanine is the precursor of zierin in the akenes of Xeranthemum cylindraceum (Asteraceae), in which zierin co-occurs with trace amounts of sambunigrin (Nahrstedt and Schwind 1992). Further studies of zierin and sambunigrin have indicated that m-hydroxylation probably occurs at the level of the intact cyanogenic glycoside (Lechtenberg and Nahrstedt 1999). Thus, it seems probable that holocalin results from the m-hydroxylation of intact prunasin.
In contrast to the rare holocalin, the cyanogenic glycoside prunasin is distributed widely in different plant tissues and unrelated plant families (e.g. Cooper-Driver et al. 1977; Fikenscher and Hegnauer 1977; Spencer and Seigler 1983; Maslin et al. 1988; Rockenbach and Nahrstedt 1990; Gleadow et al. 2003). Amygdalin, which has been isolated from a large number of species, is primarily restricted to the fruits of Rosaceae (Gomez et al. 1998; Møller and Seigler 1999; Dicenta et al. 2002). Indeed, with rare exception (e.g. Kofod and Eyjólfsson 1969; Miller et al. 2006b), cyanogenic di-glycosides are restricted to generative parts of the plants, the fruits and seeds. Small quantities of amygdalin have, however, previously been reported from foliage (e.g. Santamour 1998).
The only previous reports of cyanogenic glycosides in the family, and even the order Rutales, involved the isolation of phenylalanine-derived glycosides prunasin and sambunigrin and of zierin from leaves of two Zieria spp. (Finnemore and Cooper 1936; Fikenscher and Hegnauer 1977). These data, and the findings here for B. platynema, suggest the possibility that species in this family have cyanogenic glycosides biosynthetically derived from the amino acid phenylalanine.
Quantitative and qualitative polymorphism for cyanogenesis
Within a population of the tropical rainforest tree species B. platynema, we have identified a high proportion of acyanogenic individuals (Fig. 3). In contrast to a range of other studies, which have reported a greater frequency of cyanogenesis when testing young foliage, shoots (or reproductive structures) compared with old leaves (e.g. Gibbs 1974; Aikman et al. 1996; Thomsen and Brimer 1997; Buhrmester et al. 2000; Mali and Borges 2003; Miller et al. 2006a), cyanogenesis in B. platynema did not vary qualitatively with leaf age, based on indicator paper tests. Both young and old leaves from the same individuals of B. platynema were acyanogenic. Moreover, the acyanogenic character of individuals was verified by a quantitative assay for CN; based on the criteria of Adsersen et al. (1988), 57% of individuals were determined to be acyanogenic with concentrations of cyanogenic glycosides substantially less than the 8 μg CN g–1 DW threshold. Concentrations of between 0 and 2 μg CN g–1 DW were found in at least 10 individuals (Fig. 3). It is important to acknowledge that these small quantities of CN may, in fact, result from very minor quantities of cyanogenic glycosides in these individuals; alternatively, they may result from free CN in leaf tissue.
Within a single population of B. platynema, both qualitative and quantitative polymorphism for cyanogenesis was identified. Notably, 57% of individuals were acyanogenic — the first characterisation of true (i.e. qualitative) polymorphism for cyanogenesis in a tropical rainforest species — while among the cyanogenic individuals, there was substantial variation in the concentration of cyanogenic glycosides (i.e. quantitative polymorphism) from 10.5 to 1285.9 μg CN g–1 DW (Fig. 3).
A large number of studies have identified acyanogenic individuals within naturally occurring populations of herbaceous and woody species (e.g. Urbanska 1982; Hughes et al. 1988; Hughes 1991; Aikman et al. 1996; Pederson et al. 1996). These include reports of acyanogenic individuals in populations of the tropical shrub Turnera ulmifolia (Schappert and Shore 1995) and Australian species of Acacia (Conn et al. 1985) and Eucalyptus (Goodger et al. 2002; Woodrow et al. 2002; but see Goodger and Woodrow 2002; Neilson et al. 2006). The frequency of cyanogenesis varied substantially between different populations of the same species. For example, in bracken (Pteridium aquilinum; Cooper-Driver and Swain 1976), elderberry (Sambucus canadensis; Buhrmester et al. 2000) and Turnera ulmifolia (Schappert and Shore 1995), the frequency of cyanogenic phenotype among different naturally occurring populations ranged from 0 to 100%. In a study of 48 woody and non-woody species, Aikman et al. (1996) reported 14 species that lacked a population with a frequency of cyanogenesis greater than 50%.
Although the frequency of acyanogenesis observed within the population of B. platynema in this study is not unusual among temperate taxa, it is highly unusual among tropical rainforest taxa, in which the frequency of cyanogenesis among conspecific individuals is high. For example, no acyanogenic individuals were identified among extensive populations of Ryparosa javanica from the same lowland rainforest, in which more or less continuous quantitative variation in cyanogenic glycoside concentration was found (n > 800; Webber 2005). Similarly, no acyanogenic individuals were found among progeny of four Prunus turneriana parent trees (Miller 2004; Miller et al. 2004). Furthermore, in a survey for cyanogenesis, all individuals of most cyanogenic species were cyanogenic, albeit with low concentrations of cyanogenic glycosides in some instances (Miller et al. 2006a). There are, however, some indications of qualitative polymorphism in some species from the central and South American rainforest. Kaplan et al. (1983) and Thomsen and Brimer (1997) both detected potentially acyanogenic forms of several species with CN indicator papers. These papers are not as sensitive as the FA papers and quantitative assay used here, and it remains to be seen if these plants were truly acyanogenic.
It has been widely assumed that long-term exposure to elevated herbivory favours high levels of investment in chemical defence (Feeny 1976; Levin 1976; Levin and York 1978; Langenheim 1983; Marquis 1984; Coley and Aide 1991; Coley and Barone 1996). Thus, the high frequency of the cyanogenic phenotype in Ryparosa javanica (Webber 2005), Prunus turneriana (Miller et al. 2004) and other species (Miller 2004; Miller et al. 2006a) has been suggested as being consistent with selection against acyanogenesis in the high-herbivory environment of the tropical rainforest.
The forces shaping the pattern of qualitative and quantitative variation cyanogenesis in B. platynema and other cyanogenic plants are likely to be more complex than this. A range of genetic and environmental factors have been identified which can regulate the costs (e.g. reduced reproductive output; Kakes 1989) and benefits (e.g. reduced herbivory; Viette et al. 2000) of cyanogenesis at the species level (see Foulds and Grime 1972; Cooper-Driver et al. 1977; Urbanska 1982; Gorz et al. 1986; Briggs and Schultz 1990; Briggs 1991; Noitsakis and Jacquard 1992; Kakes and Chardonnens 2000; Schappert and Shore 2000). Moreover, qualitative variation in cyanogenesis in B. platynema does not necessarily imply that acyanogenic individuals are poorly defended. In fact the prevalence of acyanogenic individuals among B. platynema under high-herbivory conditions, suggests that the acyanogenic individuals are ecologically fit. Thus, it will be important to consider intra-population variation in concentrations of other secondary metabolites, such as phenolics, condensed tannins, and terpenoids (common in Rutaceae). While overall, low levels of both total phenolics and condensed tannins were found in B. platynema in comparison with a range of other tropical rainforest taxa (e.g. Coley 1983), and neither tannins nor total phenolics co-varied with cyanogenic glycoside content, these ‘quantitative’ defences (sensu Feeny 1976) were present in all individuals. B. platynema also contains a complex mix of sesquiterpenes (Brophy et al. 2004), and their relationship to cyanogenesis and other chemical defences remains to be identified. In addition, for individual species, interactions with specialist herbivores may be more important in determining defence characteristics (including the degree of polymorphism) over evolutionary time (Janzen 1973; Levin and York 1978; Coley and Barone 1996), particularly in the tropical rainforest where there are a large number of specialists (Janzen 1973; Coley and Barone 1996).
Acknowledgments
We thank Dr Michael Stewart and Prof. Robert Capon, The Institute of Molecular Biosciences, University of Queensland for conducting 1H NMR analysis. We are grateful to Rigel Jensen for identification of the species in the field, and Alan Curtis, for collecting extra foliage samples for chemical analysis. We also thank Kyatt Dixon and Pascal Stroh for assistance in the field. We also acknowledge the Rainforest Canopy Crane Facility, Cape Tribulation for allowing access to rainforest on the site. We thank Damian Callahan, School of Chemistry, The University of Melbourne, for preliminary LC-ESI / MS analysis of the crude methanol extract. Fieldwork was conducted under Queensland Parks and Wildlife Service Scientific Purposes Permits F1 / 000270 / 99 / SAA and WITK01167103, and was in part supported by an Individual Award from the Queen’s Trust for Young Australians to RE Miller.
Adsersen A,
Adsersen H, Brimer L
(1988) Cyanogenic constituents in plants from the Galapagos Islands. Biochemical Systematics and Ecology 16, 65–77.
| Crossref | GoogleScholarGoogle Scholar |

Aikman K,
Bergman D,
Ebinger J, Seigler D
(1996) Variation of cyanogenesis in some plant species of the Midwestern United States. Biochemical Systematics and Ecology 24, 637–645.
| Crossref | GoogleScholarGoogle Scholar |

Briggs MA
(1991) Influence of herbivory and nutrient availability on biomass, reproduction and chemical defences of Lotus corniculatus L. Functional Ecology 5, 780–786.

Briggs MA, Schultz JC
(1990) Chemical defense production in Lotus corniculatus L. II. Trade-offs among growth, reproduction and defense. Oecologia 83, 32–37.
| Crossref | GoogleScholarGoogle Scholar |

Brimer L,
Cicalini AR,
Federici F, Petruccioli M
(1995) Beta-glycosidase as a side activity in commercial pectinase preparations of fungal origin. The hydrolysis of cyanogenic glycosides. Italian Journal of Food Sciences 4, 387–394.

Brinker AM, Seigler DS
(1989) Methods for the detection and quantitative determination of cyanide in plant materials. Phytochemical Bulletin 21, 24–31.

Brophy JJ,
Goldsack RJ, Forster PI
(2004) Leaf oils of the genus Brombya (Rutaceae). Journal of Essential Oil Research 16, 402–404.

Buhrmester RA,
Ebinger JE, Seigler DS
(2000) Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. (Caprifoliaceae). Biochemical Systematics and Ecology 28, 689–695.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Coley PD
(1983) Herbivory and defensive characteristics of tree species in a lowland tropical rainforest. Ecological Monographs 53, 209–233.
| Crossref |

Coley PD, Barone JA
(1996) Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics 27, 305–335.
| Crossref | GoogleScholarGoogle Scholar |

Conn EE,
Maslin BR,
Curry S, Conn ME
(1985) Cyanogenesis in Australian species of Acacia. Survey of herbarium specimens and living plants. Western Australian Herbarium Research Notes 10, 1–13.

Cooper-Driver GA, Swain T
(1976) Cyanogenic polymorphism in bracken in relation to herbivore predation. Nature 260, 604.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cooper-Driver GA,
Finch S, Swain T
(1977) Seasonal variation in secondary plant compounds in relation to the palatability of Pteridium aquilinum. Biochemical Systematics and Ecology 5, 177–183.
| Crossref | GoogleScholarGoogle Scholar |

Cork SJ, Krockenberger AK
(1991) Methods and pitfalls of extracting condensed tannins and other phenolics from plants: insights from investigations into Eucalyptus. Journal of Chemical Ecology 17, 123–134.
| Crossref | GoogleScholarGoogle Scholar |

DellaGreca M,
Fiorentino A,
Monaco P,
Previtera L, Simonet AM
(2000) Cyanogenic glycosides from Sambucus nigra. Natural Product Letters 14, 175–182.

Dicenta F,
Martinez-Gomez P,
Grane N,
Martin ML,
Leon A,
Canovas JA, Berenguer V
(2002) Relationship between cyanogenic compounds in kernels, leaves, and roots of sweet and bitter kernelled almonds. Journal of Agricultural and Food Chemistry 50, 2149–2152.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ellis WM,
Keymer RJ, Jones DA
(1977) The defensive function of cyanogenesis in natural populations. Experimentia 33, 309–311.
| Crossref | GoogleScholarGoogle Scholar |

Feigl F, Anger V
(1966) Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. The Analyst 91, 282–284.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fikenscher LH, Hegnauer R
(1977) Cyanogenese bei den Cormophyten. 12. Mitteilung. Chaptalia nutans, eine stark cyanogene Planze Brasiliens. Planta Medica 31, 266–269.
| PubMed |

Finnemore H, Cooper JM
(1936) Cyanogenetic glucosides in Australian plants. Part 4. Zieria laevigata. Journal of the Royal Society of New South Wales 70, 175–182.

Foulds W, Grime JP
(1972) The influence of soil moisture on the frequency of cyanogenic plants in populations of Trifolium repens and Lotus corniculatus. Heredity 28, 143–146.

Gleadow RM, Woodrow IE
(2002a) Constraints on effectiveness of cyanogenic glycosides in herbivore defence. Journal of Chemical Ecology 28, 1301–1313.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gleadow RM, Woodrow IE
(2002b) Defense chemistry of cyanogenic Eucalyptus cladocalyx seedlings is affected by water supply. Tree Physiology 22, 939–945.
| PubMed |

Gleadow RM,
Foley WJ, Woodrow IE
(1998) Enhanced CO2 alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant, Cell & Environment 21, 12–22.
| Crossref | GoogleScholarGoogle Scholar |

Gleadow RM,
Vecchies AC, Woodrow IE
(2003) Cyanogenic Eucalyptus nobilis is polymorphic for both prunasin and specific β-glucosidases. Phytochemistry 63, 699–704.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gmelin R,
Schüler M, Bordas E
(1973) Holocalin: ein neues cyanogenes glykosid aus Holocalyx balansae. Phytochemistry 12, 457–461.
| Crossref | GoogleScholarGoogle Scholar |

Gomez E,
Burgos L,
Soriano C, Marin J
(1998) Amygdalin content in the seeds of several apricot cultivars. Journal of the Science of Food and Agriculture 77, 184–186.
| Crossref | GoogleScholarGoogle Scholar |

Goodger JQD, Woodrow IE
(2002) Cyanogenic polymorphism as an indicator of genetic diversity in the rare species Eucalyptus yarraensis (Myrtaceae). Functional Plant Biology 29, 1445–1452.
| Crossref | GoogleScholarGoogle Scholar |

Goodger JQD,
Capon RJ, Woodrow IE
(2002) Cyanogenic polymorphism in Eucalyptus polyanthemos Schauer subsp. vestita L. Johnson and K. Hill (Myrtaceae). Biochemical Systematics and Ecology 30, 617–630.
| Crossref | GoogleScholarGoogle Scholar |

Gorz HJ,
Gaskins FA, Vögel KP
(1986) Inheritance of dhurrin content in mature sorghum leaves. Crop Science 26, 65–67.

Hruska AJ
(1988) Cyanogenic glucosides as defense compounds. A review of the evidence. Journal of Chemical Ecology 14, 2213–2217.
| Crossref | GoogleScholarGoogle Scholar |

Hübel W,
Nahrstedt A,
Fikenscher LH, Hegnauer R
(1982) Zierinxyloside, a new cyanogenic glycoside from Xeranthemum cylindraceum. Planta Medica 44, 178–180.

Hughes MA
(1991) The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66, 105–115.

Jensen SR, Nielsen BJ
(1973) Cyanogenic glucosides in Sambucus nigra L. Acta Chemica Scandanavia 27, 2661–2662.

Julkunen-Tiitto R
(1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agricultural and Food Chemistry 33, 213–217.
| Crossref | GoogleScholarGoogle Scholar |

Kakes P
(1989) An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theoretical and Applied Genetics 77, 111–118.
| Crossref | GoogleScholarGoogle Scholar |

Kakes P, Chardonnens AN
(2000) Cyanotypic frequencies in adjacent and mixed populations of Trifolium occidentale Coombe and Trifolium repens L. are regulated by different mechanisms. Biochemical Systematics and Ecology 28, 633–649.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kaplan MAC,
Figueiredo MR, Gottlieb OR
(1983) Variation in cyanogenesis in plants with season and insect pressure. Biochemical Systematics and Ecology 11, 367–370.
| Crossref | GoogleScholarGoogle Scholar |

Kofod H, Eyjólfsson R
(1969) Cyanogenesis in species of the fern genera Cystopteris and Davallia. Phytochemistry 8, 1509–1511.
| Crossref | GoogleScholarGoogle Scholar |

Levin DA
(1976) Alkaloid-bearing plants: an ecogeographic perspective. American Naturalist 110, 261–284.
| Crossref | GoogleScholarGoogle Scholar |

Levin DA, York BM
(1978) The toxicity of plant alkaloids: an ecogeographic perspective. Biochemical Systematics and Ecology 6, 61–76.
| Crossref | GoogleScholarGoogle Scholar |

Mali S, Borges RM
(2003) Phenolics, fibre, alkaloids, saponins, and cyanogenic glycosides in a seasonal cloud forest in India. Biochemical Systematics and Ecology 31, 1221–1246.
| Crossref | GoogleScholarGoogle Scholar |

Marquis RJ
(1984) Leaf herbivores decrease fitness of a tropical plant. Science 226, 537–539.

Maslin BR,
Dunn JE, Conn EE
(1988) Cyanogenesis in Australian species of Acacia. Phytochemistry 27, 421–428.
| Crossref | GoogleScholarGoogle Scholar |

McMahon JM,
White WLB, Sayre RT
(1995) Cyanogenesis in cassava (Manihot esculenta Crantz). Journal of Experimental Botany 46, 731–741.

Miller RE,
Gleadow RM, Woodrow IE
(2004) Cyanogenesis in tropical Prunus turneriana: characterisation, variation and response to low light. Functional Plant Biology 31, 491–503.
| Crossref | GoogleScholarGoogle Scholar |

Miller RE,
Jensen R, Woodrow IE
(2006a) Frequency of cyanogenesis in tropical rainforests of far north Queensland, Australia. Annals of Botany In press ,
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Miller RE,
McConville MJ, Woodrow IE
(2006b) Cyanogenic glycosides from the rare Australian endemic rainforest tree Clerodendrum grayi (Lamiaceae). Phytochemistry 67, 43–51.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nahrstedt A
(1976) Prunasin in Holocalyx balansae. Phytochemistry 15, 1983–1984.
| Crossref | GoogleScholarGoogle Scholar |

Nahrstedt A, Schwind P
(1992) Phenylalanine is the biogenetic precursor of meta-hydroxylated zierin, the aromatic cyanogenic glucoside of unripe akenes of Xeranthemum cylindraceum. Phytochemistry 31, 1997–2001.
| Crossref | GoogleScholarGoogle Scholar |

Neilson EH,
Goodger JQD, Woodrow IE
(2006) Novel aspects of cyanogenesis in Eucalyptus camphora subsp. humeana. Functional Plant Biology 33, 487–496.
| Crossref | GoogleScholarGoogle Scholar |

Noitsakis B, Jacquard P
(1992) Competition between cyanogenic and acyanogenic morphs of Trifolium repens. Theoretical and Applied Genetics 83, 443–450.
| Crossref | GoogleScholarGoogle Scholar |

Olafsdottir ES,
Andersen JV, Jaroszewski JW
(1989) Cyanohydrin glycosides of Passifloraceae. Phytochemistry 28, 127–132.
| Crossref | GoogleScholarGoogle Scholar |

Pederson GA,
Fairbrother TE, Greene SL
(1996) Cyanogenesis and climatic relationships in US white clover germplasm collection and core subset. Crop Science 36, 427–433.

Rockenbach J, Nahrstedt A
(1990) New and known cyanogenic glycosides from the Rubiaceae. Planta Medica 56, 591–592.

Rockenbach J,
Nahrstedt A, Wray V
(1992) Cyanogenic glycosides from Psydrax and Oxyanthus species. Phytochemistry 31, 567–570.
| Crossref | GoogleScholarGoogle Scholar |

Rosenthaler L
(1919) Die Verbreitung der Blausäure im Pflanzenreich. Journal Suisse de Pharmacie 57, 279–346.

Santamour FS
(1998) Amygdalin in Prunus leaves. Phytochemistry 47, 1537–1538.
| Crossref | GoogleScholarGoogle Scholar |

Schappert PJ, Shore JS
(1995) Cyanogenesis in Turnera ulmifolia L. (Turneraceae). I. Phenotypic distribution and genetic variation for cyanogenesis on Jamaica. Heredity 74, 392–404.

Schappert PJ, Shore JS
(2000) Cyanogenesis in Turnera ulmifolia L. (Turneraceae). II. developmental expression, heritability and cost of cyanogenesis. Evolutionary Ecology Research 2, 337–352.

Schwind P,
Wray V, Nahrstedt A
(1990) Structure elucidation of an acylated cyanogenic triglycoside, and further cyanogenic constituents from Xeranthemum cylindraceum. Phytochemistry 29, 1903–1911.
| Crossref | GoogleScholarGoogle Scholar |

Seigler DS
(2003) Phytochemistry of Acacia — sensu lato. Biochemical Systematics and Ecology 31, 845–873.
| Crossref | GoogleScholarGoogle Scholar |

Singleton VL, Rossi JA
(1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16, 144–158.

Spencer KC, Seigler DS
(1983) Cyanogenesis of Passiflora edulis. Journal of Agricultural and Food Chemistry 31, 794–796.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Spencer KC, Seigler DS
(1985) Cyanogenic glycosides and the systematics of the Flacourtiaceae. Biochemical Systematics and Ecology 13, 421–431.
| Crossref | GoogleScholarGoogle Scholar |

Thomsen K, Brimer L
(1997) Cyanogenic constituents in woody plants in natural lowland rain forest in Costa Rica. Biological Journal of the Linnean Society 121, 273–291.

Urbanska K
(1982) Polymorphism of cyanogenesis in Lotus alpinus in Switzerland. I. Small-scale variability in phenotypic frequencies upon acidic silicate and carbonate. Bericht des Geobotanischen Institutes ETH. 49, 35–55.

van Valen F
(1978) Contribution to the knowledge of cyanogenesis in angiosperms. 3. Communication. Cyanogenesis in Liliaceae. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. Series C. 81, 132–140.

Viette M,
Tettamanti C, Saucy F
(2000) Preference for acyanogenic white clover (Trifolium repens) in the vole Arvicola terrestris. II. Generalization and further investigations. Journal of Chemical Ecology 26, 101–122.
| Crossref | GoogleScholarGoogle Scholar |

Wajant H,
Riedel D,
Benz S, Mundry K-W
(1994) Immunocytological localization of hydroxynitrile lyases from Sorghum bicolor L. and Linum usitatissimum L. Plant Science 103, 145–154.
| Crossref | GoogleScholarGoogle Scholar |

Webb LJ,
Tracey JG, Williams WT
(1972) Regeneration and pattern in the sub-tropical rainforest. Journal of Ecology 60, 675–695.

Woodrow IE,
Slocum DJ, Gleadow RM
(2002) Influence of water stress on cyanogenic capacity in Eucalyptus cladocalyx. Functional Plant Biology 29, 103–110.
| Crossref | GoogleScholarGoogle Scholar |



