Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis
Abby J. Cuttriss A , Alexandra C. Chubb A , Ali Alawady A B , Bernhard Grimm B and Barry J. Pogson A CA ARC Centre of Excellence in Plant Energy Biology, School of Biochemistry and Molecular Biology, The Australian National University, Canberra, ACT 0200, Australia.
B Institute of Biology/Plant Physiology, Humboldt University, Philippstrasse 13 Building 12, 10115 Berlin, Germany.
C Corresponding author. Email: barry.pogson@anu.edu.au
Functional Plant Biology 34(8) 663-672 https://doi.org/10.1071/FP07034
Submitted: 14 February 2007 Accepted: 9 May 2007 Published: 23 July 2007
Abstract
Carotenoids are critical for photosynthetic function in chloroplasts, and are essential for the formation of the prolamellar body in the etioplasts of dark-grown (etiolated) seedlings. They are also precursors for plant hormones in both types of plastids. Lutein is one of the most abundant carotenoids found in both plastids. In this study we examine the regulation of lutein biosynthesis and investigate the effect of perturbing carotenoid biosynthesis on the formation of the lattice-like membranous structure of etioplasts, the prolamellar body (PLB). Analysis of mRNA abundance in wildtype and lutein-deficient mutants, lut2 and ccr2, in response to light transitions and herbicide treatments demonstrated that the mRNA abundance of the carotenoid isomerase (CRTISO) and epsilon-cyclase (ϵLCY) can be rate limiting steps in lutein biosynthesis. We show that accumulation of tetra-cis-lycopene and all-trans-lycopene correlates with the abundance of mRNA of several carotenoid biosynthetic genes. Herbicide treatments that inhibit carotenoid biosynthetic enzymes in wildtype and ccr2 etiolated seedlings were used to demonstrate that the loss of the PLB in ccr2 mutants is a result of perturbations in carotenoid accumulation, not indirect secondary effects, as PLB formation could be restored in ccr2 mutants treated with norflurazon.
Introduction
Carotenoids are synthesised, and accumulate exclusively in the plastids of plants where they provide yellow and red pigmentation to many fruits and flowers (Cuttriss et al. 2006). They are essential components of the photosynthetic apparatus and thus accumulate in the chloroplasts of photosynthetic tissue (Cunningham and Gantt 1998; Goodwin and Britton 1988). Typically, four carotenoids accumulate in the chloroplasts of higher plants: (listed in decreasing order of abundance) lutein, β-carotene, violaxanthin and neoxanthin (DellaPenna and Pogson 2006). The four main carotenoids plus others, such as zeaxanthin, have a mix of unique, complementary and sometimes redundant functions (Pogson et al. 2005). The biosynthetic enzymes for the main pathway in chloroplasts are listed in Fig. 1. The first committed step is catalysed by phytoene synthase (PSY). Phytoene is dehydrogenated to form lycopene by the two desaturases, which introduce a series of four double bonds in a cis-configuration. The cis-bonds are isomerised to the all-trans-conformations by CRTISO. All-trans-lycopene is cyclised by epsilon cyclase (ϵLCY) and β-cyclase (βLCY) to form the cyclic carotenes, which are subjected to a series of oxygenation reactions to produce the xanthophylls and with further modifications to produce abscisic acid (ABA) (DellaPenna and Pogson 2006).
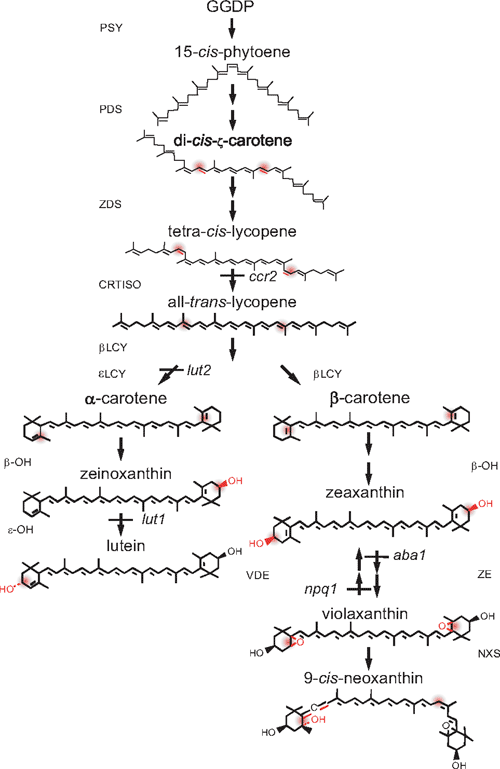
|
The chloroplastic location of enzymes whose genes are nuclear encoded necessitates chloroplast–nuclear communication. Carotenoid biosynthesis in the chloroplast must be tightly regulated throughout the life of the plant, so that the composition of the carotenoid pool best suits the prevailing environmental conditions, for example, incident light intensity. Increases in carotenoid accumulation in chromoplasts, be it during fruit ripening, flower development or production of stress-induced carotenoids in algae have coincided with increased transcript abundance of some, but not all, key steps in the pathway (von Lintig et al. 1997; Cunningham and Gantt 1998; Grunewald et al. 2000; Welsch et al. 2000; Hirschberg 2001). Carotenoid biosynthetic genes are redox sensitive in the green alga Haematococcus pluvialis (Steinbrenner and Linden 2003), which produces the high-value carotenoid, astaxanthin. Changes in transcript abundance are particularly evident during morphogenic changes from chloroplast to chromoplast of many fruits and flowers (Bramley 2002). Differences in transcript levels of almost all carotenoid biosynthetic genes in marigolds are suggested to be responsible for the dramatic differences in carotenoid accumulation between cultivars (Moehs et al. 2001). However, regulation is not simply transcriptional. A recent study has identified a mutant form of the plastid-associated DNAJ protein that affects carotenoid accumulation during plastid differentiation into chromoplasts (Lu et al. 2006).
With respect to chloroplasts and germinating seedlings there is less information about the regulation of carotenoid accumulation (Cunningham and Gantt 1998; Cunningham 2002). To avoid extensive photo-oxidative damage, the synthesis of carotenoids and chlorophylls and their subsequent binding to pigment-binding proteins must be precisely balanced. Indeed, carotenoid biosynthesis is known to be tightly coordinated with synthesis of chlorophyll and the pigment-binding proteins of the photosynthetic apparatus (Herrin et al. 1992). Likewise, carotenoid biosynthesis must be tightly regulated during de-etiolation, when the seedling emerges from the soil and functional photosystems are assembled (Hoober and Eggink 1999). Thus, the pathway is tightly regulated during development and in response to environmental stimuli (Young 1993; Pogson et al. 1996; Welsch et al. 2000; Matsubara et al. 2003). The biosynthetic pathway is, at least in part, regulated by changes in gene transcription during photomorphogenesis (Welsch et al. 2003; Woitsch and Römer 2003) as PSY and PDS are up-regulated via a phytochrome-mediated pathway from basal levels in etioplasts (Welsch et al. 2000). Light intensity also affects transcription abundance of βLCY, PSY, PDS, and βOH, a trend that was eradicated by DCMU application (3-(3,4-dichlorophenyl)-1,1-dimethylurea; oxidised plastoquinone pool) and exacerbated by DBMIB treatment (2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone; reduced plastoquinone pool) (Steinbrenner and Linden 2003).
Several lutein-deficient Arabidopsis mutants were utilised in this study (Pogson et al. 1996; Park et al. 2002). The ϵLCY mutant, lut2, makes no lutein at all, and ccr2 exhibits substantial reductions in lutein accumulation. ccr2 is a carotenoid isomerase mutant and as such, has a different carotenoid composition when grown in the dark, when it accumulates poly-cis-carotenes, predominantly tetra-cis-lycopene. Photoisomerisation is thought to convert the cis-carotenes into all-trans forms. It had been expected that once the chloroplast pigments have been photoisomerised, flux should be divided between the subsequent steps as seen in wildtype tissue. This is not the case, as ccr2 accumulates only 10–20% of wildtype lutein levels (Park et al. 2002). Dark-grown ccr2 seedlings lack the prolamellar body (PLB), the defining lattice-like structure of etioplasts. Whether this reflects the accumulation of cis-carotenes or is a secondary effect due to the loss of CRTISO activity or protein is not known.
PLBs are formed in etiolated tissue in the presence of all-trans and/or 15Z-phytoene that accumulate during growth in the presence of the phytoene desaturase inhibitor, norflurazon (NFZ) (Axelsson et al. 1982; Sundqvist and Dahlin 1997; Park et al. 2002; Denev et al. 2005). Whether all-trans-lycopene permits the formation of PLBs is less clear. Amitrole is a herbicide that prevents PLB formation and has been reported to cause lycopene accumulation (Rascio et al. 1996; Moro et al. 2004). However, a study has examined the accumulation of all carotenoids in response to amitrole treatment (Barry and Pallett 1990), not just those that absorb in the visible spectrum (Rascio et al. 1996; Dalla Vecchia et al. 2001). First, lycopene is just 0.6% of the acyclic carotene pool that accumulates in response to amitrole treatment in dark-grown barley, whereas phytoene forms 86.5%, phytofluene 10% and ζ-carotene 0.8% of the precursor pool (Barry and Pallett 1990). Second, xanthophyll and β-carotene do accumulate in amitrole-treated tissue, albeit at lower levels (Barry and Pallett 1990; Dalla Vecchia et al. 2001). In fact, lycopene is present at lower levels than one of its products, lutein (Barry and Pallett 1990). Thus, the herbicide does not completely block the pathway and there is no evidence that amitrole preferentially affects cyclisation. The primary effect, if any, is on phytoene desaturation. Furthermore, whether amitrole is even specific for carotenoid biosynthesis is debated. Amitrole alters root growth (Heim and Larrinua 1989), inhibits histidine biosynthesis by acting on imidazolegylcerolphosphate dehydratases from Arabidopsis and wheat (Tada et al. 1995), alters lipid composition (Di Baccio et al. 2002) and may alter catalase activity (Bouvier et al. 1998). One report notes that substantially less amitrole is needed to inhibit root growth than carotenoid biosynthesis (Heim and Larrinua 1989).
There is an herbicide that is known to directly inhibit the lycopene cyclase activity, namely 2-(4-methylphenoxy)-triethylamine hydrochloride (MPTA) (Bramley 1993; Ronen et al. 1999; Phillip and Young 2006). MPTA causes a substantial increase in all-trans-lycopene that largely compensates for loss of cyclic carotenoids and only low levels of other precursors accumulate. Authors have calculated the I50 values of MPTA for the β-cyclase and ϵ-cyclase as 6.0 and 13.5 μM, respectively, in tomato (Ronen et al. 1999). Consequently, in this study we used NFZ and MPTA to block the carotenoid biosynthetic pathway to study the effects on PLB formation in ccr2 and wildtype etiolated seedlings. The overall aims of this study were to investigate the transcriptional control of lutein accumulation, determine why there is less lutein in ccr2 plants and to investigate the roles of carotenoids in formation of the prolamellar body in etioplasts.
Materials and methods
Plant growth conditions
Arabidopsis thaliana L. seeds, ecotype Columbia (Col) were planted on sterilised soil (3 : 1, soil : vermiculite), vernalised for 2 days at 4°C, then transferred to a growth chamber at 21°C with a 16 h day, 150 μmol photons m–2 s–1 unless otherwise indicated. Light treatments are described in results and figure legends. Mature plants were fertilised regularly with half-strength Hoaglands solution (Hoaglands and Arnon 1950). Alternatively, surface-sterilised seeds were germinated on 1–2% sucrose supplemented MS media (Gibco BRL Gaithersberg, MD) at 80 μmol photons m–2 s–1 under constant light. For etiolated tissue, seedlings were germinated in the dark on MS media and harvested under dim green light before freezing in liquid nitrogen. Media was supplemented with carotenoid biosynthetic inhibitors as required: 10 μm norflurazon, from a 10 mm ethanol stock (Solicam DF herbicide, Syngenta Crop Protection, Pendle Hill, NSW) or 7.5, 75 and 150 μg mL–1 MPTA [2-(4-methylphenoxy)-triethylamine hydrochloride] from a 75 mg mL–1 stock in water. MPTA kindly provided by Achim Trebst (Ruhr Universität Bochum, Germany).
Pigment analyses
Pigments were extracted from plant tissue in 500 μL acetone : ethyl acetate 3 : 2 (v/v). Addition of water (400 μL) and subsequent centrifugation produced an ethyl acetate pigment phase that was analysed by HPLC (Pogson et al. 1996). A total of 20 μL was injected and separated by reverse-phase HPLC analysis on a Spherisorb ODS2 5-micron, C18, 4.6 × 250 mm column (Alltech, Columbia, MD) using an ethyl acetate gradient in acetonitrile : water : triethylamine 9 : 1 : 0.01 (v/v) at 1 mL min–1 unless otherwise stated according to the following timetable (0–1 min, 0% ethyl acetate; 1–31 min 0–66.7%, 31–31.2 min 66.7–100%, 31.2–32 min 100%). Carotenoids were identified by comparison with known standards using retention time and absorption spectra for individual peaks detected with an inline diode array detector (Beckman Gold, Beckman Coulter, Gladesville, NSW, or Agilent HPLC 1100, Agilent, Santa Clara, CA) (Pogson et al. 1996; Park et al. 2002). HPLC peak areas at 440 nm were integrated and individual pigments expressed as a proportion of the total carotenoid pool on a molar or molecular weight basis (Pogson et al. 1996, 1998; Rissler and Pogson 2001). Experiments were done in triplicate, unless otherwise noted.
Expression analyses
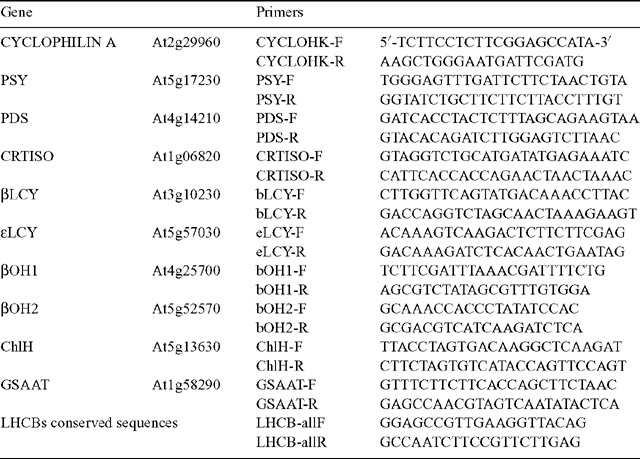
RNA was extracted from ~100 mg snap-frozen plant tissue using an RNeasy Plant Mini Kit (Qiagen, Santa Clarite, CA) according to the manufacturer’s instructions. Following quantification (Nanodrop ND-1000 spectrophotometer, NanoDrop Technologies, Wilmington, DE), the relative transcript abundance was quantified using a one step QuantiTect SYBR Green RT–PCR Kit that used the same primers for the reverse transcriptase reaction as the amplification so spliced and unspliced mRNA was detectable (Qiagen). Real Time RT–PCR was performed with a minimum of three biological replicates per experiment and typically three technical replicates per sample on a RotorGene 2000 (Corbett Research). Data was analysed using the ‘relative quantitation’ method using cyclophilin a as the house-keeper control gene (Rossel et al. 2002). Primers used in this study are shown in Table 1.
|
Vector construction and plant transformation
Gateway vectors were constructed according to the manufacturer’s instructions, using pDONR 221 and pDONR/Zeo entry vectors (Invitrogen, Carlsbad, CA) and pMDC32 binary vector (Curtis and Grossniklaus 2003). For CRTISO overexpression a 3328-bp genomic fragment was amplified using primers CRTISO-F (5′-GTTCTCTGAGAGAGTTGACCCTAGGAAG-3′) and CRTISO-R (5′-CTAGCCCCAACACACTCCATGGTCCT-3′) using high fidelity Platinum Pfx Taq Polymerase (Invitrogen) according to the manufacturer’s instructions. The PCR product was recombined into pMDC32, which contains the dual 35S constitutive promoter (Curtis and Grossniklaus 2003) via a pDONR/Zeo entry clone (Invitrogen). The CRTISO gene recombined between the attB1 and attB2 recombination sites. Electrocompetent and chemically-competent DH5α and DB3.1 cells were produced and transformed and transformation mixes were plated onto selective media containing spectinomycin (100 μg mL–1; Sigma-Aldrich, St Louis, MO). The transgene vector was sequenced and subsequently transformed into Agrobacterium tumefaciens strain LBA4404 containing the Ti helper plasmid either by the freeze/thaw method or electroporation. Selection was 100 μg mL–1 spectinomycin. Agrobacterium-mediated transformation of Arabidopsis was performed according to the floral-dip method (Clough and Bent 1998). Transformants were selected by plating sterilised seeds on MS media containing either 30 μg mL–1 hygromycin (Invitrogen) or 50 μg mL–1 kanamycin (Sigma-Aldrich). Transgenics were analysed for lutein content by HPLC.
Electron microscopy
Five-day-old etiolated tissue samples were harvested in the dark under a dim-green safe light and fixed overnight at 4°C in 2.5% glutaraldehyde in Sorenson’s phosphate buffer (NaH2PO4 + Na2HPO4 0.1 m, pH 7). Samples were rinsed three times (10 min) in Sorenson’s buffer (pH 7), post-fixed for 1 h (1% OsO4 in H2O) and washed three times in H2O (10 min each). This was followed by an ethanol series: 50, 70, 90, 95 and 2 × 100% for 30 min each. After dehydration, samples were infiltrated with propylene oxide (60 min), then propylene oxide : resin (3 : 1) overnight (resin = epon/araldite), (1 : 1) for 10 h and finally (1 : 3) overnight. Samples were dried for 6 h (room temperature), transferred to fresh resin overnight, followed by 2 × 2 h incubations in fresh resin. Finally, the tissue samples were transferred to fresh resin in moulds and baked (60°C overnight). Extra resin was cut away and the samples sectioned using an ultramicrotome (glass knife). Sections were placed on copper grids, stained with uranyl acetate, rinsed in H2O and imaged using a transmission electron microscope (Hitachi H7100FA 125 kV, Pleasanton, CA).
Results
Carotenoid biosynthesis in response to different light regimes
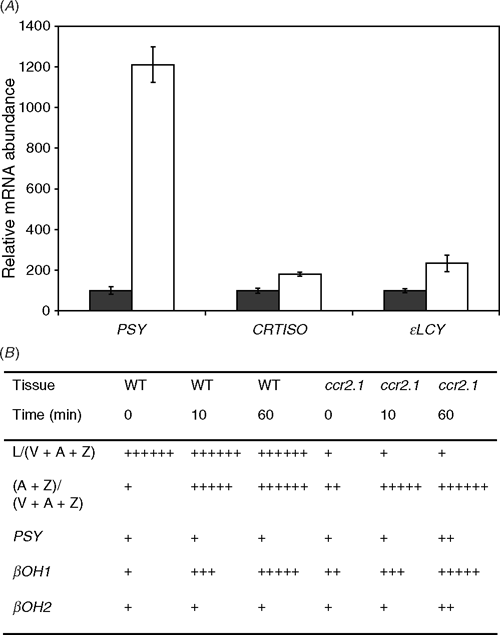
Dark–light transitions yield a large increase in PSY mRNA levels (Fig. 2; Welsch et al. 2003). Both CRTISO and ϵLCY also show increased transcript abundance on light transition (1.8- and 2.2-fold, respectively), but only to a minor extent compared to PSY (12-fold). In addition to dark–light transition carotenoid biosynthetic genes might also respond to other light regimes. High light activates the xanthophyll cycle, reducing the proportion of violaxanthin to antheraxanthin and zeaxanthin. It does not, however, appear to affect apportioning of metabolic flux between lutein and the xanthophyll cycle pigments (Fig. 2B). The ratio of L/(V + A + Z) stays the same for each genotype, irrespective of treatment. All genotypes have activated xanthophyll cycles after just 10 min of high light. The βOH1 gene also shows increased transcript abundance after just 10 min, and very high expression after an hour. In contrast, βOH2 barely responds at all, with only a slight increase in transcript in ccr2 after an hour. A similar experiment was conducted to compare transcript levels after growth at very low light (~10 μmol photons m–2 s–1) compared to moderate light. We did not observe any changes in lutein content under very low light growth conditions in Arabidopsis (data not shown).
|
Role of ϵLCY, CRTISO and lycopene in lutein regulation
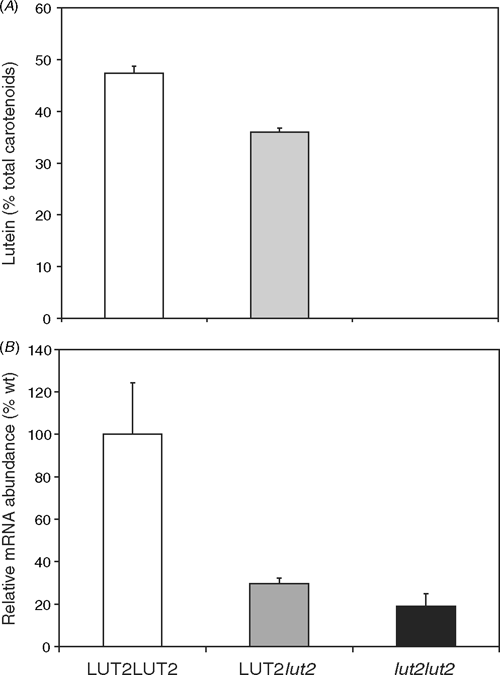
The relative activities of the ϵ- and β-cyclases could be important in apportioning flux between the two branches of the biosynthetic pathway, which lead either to the production of lutein or the xanthophyll cycle pigments. The lut2 plants homozygous for a non-functional ϵLCY accumulate no lutein, and heterozygous mutants accumulate intermediate levels of lutein compared with wildtype plants (Fig. 3A). There is a marked reduction in ϵLCY mRNA in the heterozygous LUT2lut2 F1 plants that have one wildtype and one mutated ϵLCY gene (Fig. 3B). The homozygous lut2lut2 mutant has an 80% reduction in ϵLCY mRNA abundance compared to wild type and no lutein because, in addition to less mRNA, the lut2 mutant gene encodes a non-functional enzyme (Pogson et al. 1996).
|
The reduction of the lutein content in ccr2 leaves could reflect incomplete photoisomerisation altering substrate specificity of the two cyclases. However, no intermediates are detected in green leaves (Park et al. 2002) and during photomorphogenesis the cis-carotenes are isomerised within hours, but lutein content is less than wildtype throughout the life cycle of the plant. Further, the reduced lutein levels in ccr2 correlates with a reduction in ϵLCY transcript by 70% compared to wildtype levels (Fig. 4A). Wildtype and ccr2 plants were transformed with a CRTISO transgene, driven by a strong constitutive promoter (35S). In a ccr2 background the 35S:CRTISO transgene can restore lutein levels to wildtype (Fig. 4B).
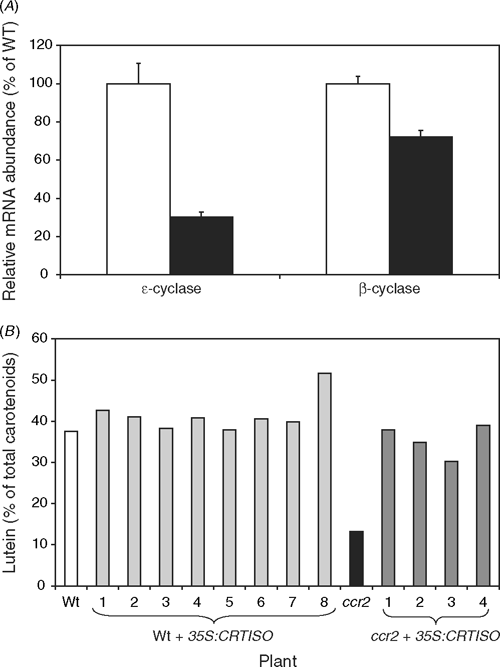
|
The possibility of substrate feedback regulation on the biosynthetic pathway in ccr2 resulting in changes to the mRNA abundance was investigated by treating plants with MPTA, which blocks the pathway at the cyclases resulting in the accumulation of lycopene (Fig. 5A). The subsequent mRNA changes were quantified by real time RT–PCR. Fig. 5B compares the transcriptional effect of all-trans-lycopene accumulation (wildtype tissue treated with 75 μg mL–1 MPTA) to that of tetra-cis-lycopene (ccr2.1 plus or minus MPTA as it acts after ccr2) and untreated wildtype tissue that accumulates predominantly lutein, violaxanthin and trace neoxanthin. Experiments were undertaken with etiolated tissue to ensure no contribution from phytochromes, ROS and chloroplastic redox poise on mRNA abundance.
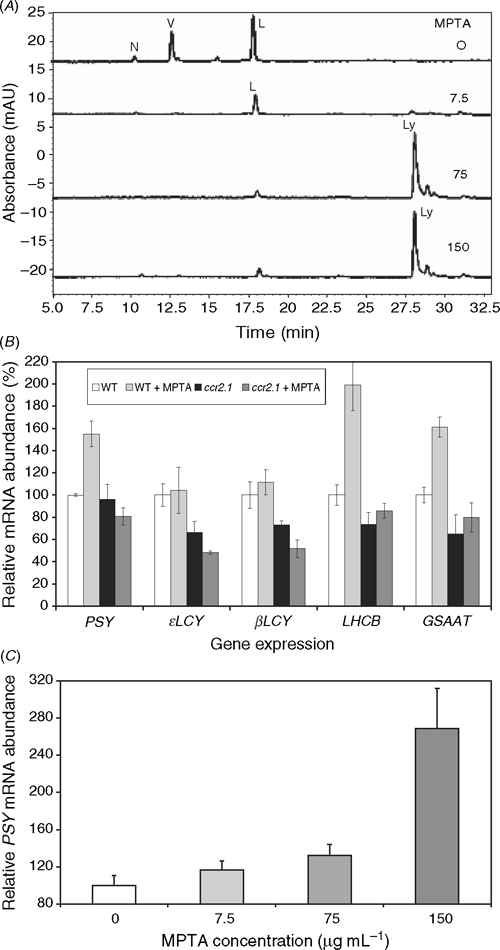
|
The effect of MPTA on ϵLCY and βLCY mRNA levels in wildtype was not markedly different to the untreated controls (Fig. 5B). Thus, accumulation of all-trans-lycopene is not sufficient to phenocopy the effect of ccr2 on ϵLCY and βLCY. This indicates an effect of the cis-carotenes and/or the loss of CRTISO. However, tissue accumulating all-trans-lycopene shows a consistent, significant increase in PSY, GSAAT and LHCB transcript accumulation (Fig. 5B). GSAAT (glutamate-1-semialdehyde aminotransferase) catalyses an early step in the tetrapyrrole biosynthetic pathway, producing δ-aminolevulinic acid and is known to be light-induced (Herman et al. 1999). CHLH encodes the H-subunit of Mg chelatase, which converts protoporphyrin IX to Mg protoporphyrin (Jensen et al. 1996). Both CHLH and its product are crucial for the integrity of the GUN plastid-nuclear signal (Strand et al. 2003), and, hence, plastid function. There is a little variability in CHLH transcript levels, but no consistent trend (data not shown). So beyond the transcriptional change, the effect on chlorophyll biosynthesis is likely to be negligible (Nogaj et al. 2005). No increase in PSY, GSAAT or LHCB transcript accumulation was seen in untreated ccr2 plants, which accumulate cis-carotenes, suggesting that this effect is unique to the all-trans-isomer. Neither was this effect due to non-specific MPTA interactions, as MPTA treated ccr2 seedlings did not differ to that of untreated ccr2 controls for PSY transcript levels (Fig. 5B). Confirmation of the PSY increase was achieved by treating plants with increasing concentrations of MPTA (Fig. 5C), which resulted in increasing levels of PSY mRNA.
Carotenoids and prolamellar body formation in etioplasts
The defining structure of etioplasts is the lattice-like prolamellar body (Fig. 6A). The ccr2 mutants do not form prolamellar bodies (PLB) in etioplasts (Park et al. 2002; Fig. 6D). The structural role of carotenoids in PLB formation was further investigated by blocking carotenoid biosynthesis with inhibitors and investigating alterations to plastid development by electron microscopy. Three herbicide treatments were undertaken on wild type and ccr2 etiolated seedlings, namely norflurazon which results in phytoene accumulation, MPTA which blocks cyclase activity and fosmidomycin which blocks the MEP pathway. A range of fosmidomycin treatments had no effect on carotenoid accumulation in etiolated tissue, so it was not pursued. Norflurazon treatment resulted in phytoene accumulation in wildtype and ccr2 etiolated tissue (Fig. 6G) and PLBs were detected in both (Fig. 6B, E). Wildtype and ccr2 etiolated tissue treated with MPTA also accumulated PLBs (Fig. 6C, F). Although MPTA acts after ccr2 and should not affect the carotenoid composition, there was a qualitative increase in the amount of phytoene in MPTA-treated ccr2 (Fig. 6G).
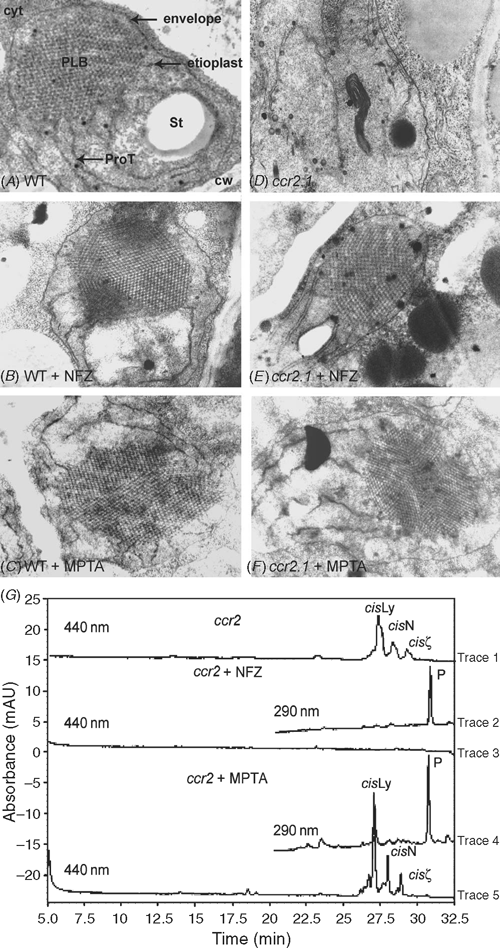
|
Discussion
Lutein regulation: ϵLCY and CRTISO
In general, carotenoid biosynthetic genes are expressed most highly in leaves and other photosynthetic tissue and expression is lowest in roots and other tissues with low pigment levels. The dark–light transition requires photoprotective carotenoids (lutein and violaxanthin) to be already in place in the dark-grown etioplast to protect the plastid from the abrupt increase in light exposure (Woitsch and Römer 2003). Furthermore, the rapid assembly of the photosynthetic apparatus during photomorphogenesis includes rapid accumulation of carotenoids concomitant with the build up of photosensitising chlorophyll molecules (Woitsch and Römer 2003). This data extends earlier observations (Woitsch and Römer 2003) on PSY transcript abundance by showing other genes, namely CRTISO and ϵLCY, increase during photomorphogenesis (Fig. 2), albeit to a much lesser extent.
Under low light plants absorb as much energy as possible to maintain photosynthesis. Photoprotective xanthophyll cycle pigments become less crucial and the proportion of ϵ-branch pigments, such as lutein increases (Johnson et al. 1993; Logan et al. 1996). In the converse situation, under high irradiance, plants dissipate excess energy and the xanthophyll cycle pigments (β,β-branch) accumulate at the expense of lutein (β,ϵ-branch) in some species (Johnson et al. 1993; Logan et al. 1996). In Arabidopsis we did not observe any changes in lutein content under very low light growth conditions nor did we observe a rapid shift in lutein to xanthophyll cycle pigments after 1 h of high light. However, such a shift would be expected to occur over a much longer time frame (Adams et al. 2002). But we did observe that genes, such as βOH1, involved in producing zeaxanthin are rapidly altered in response to high light (within 10 min). Acclimation to high light and protection from fluctuating light intensities is crucial for plant survival and zeaxanthin plays a critical role (Davison et al. 2002; Külheim et al. 2002). Thus, in addition to post-translation activation of the enzymes involved in zeaxanthin biosynthesis (Pogson et al. 2005), there are changes in levels of mRNA encoding proteins involved in zeaxanthin biosynthesis.
Altering activity of the branch point enzymes will modulate lutein accumulation (Pogson and Rissler 2000). The loss of one functional gene copy in the lut2 heterozygote plant is sufficient to reduce ϵLCY mRNA and lutein indicating a semi-dominant mutant phenotype and the rate-limiting step of the ϵLCY enzyme (Pogson et al. 1996). Thus, ϵLCY is involved in modulating metabolic flux down the two branches of the biosynthetic pathway. The marked reduction in mRNA for the heterozygote (greater than 50%) and homozygous lut2 plants likely reflects altered mRNA stability of the mutated gene.
The carotenoid isomerase, which catalyses the conversion of tetra-cis-lycopene to all-trans-lycopene, also has a role in apportioning metabolite flux through the two branches of the biosynthetic pathway. In light-grown CRTISO mutants (ccr2) photoisomerisation of poly-cis-carotenes to all-trans-lycopene should make the CRTISO enzyme functionally redundant in light-exposed photosynthetic tissues. Yet, it is conserved across higher plants, algae and cyanobacteria, indicating a selective pressure on the maintenance of this gene. One possible function for CRTISO in plants is maintaining the flux at the branch point of the pathway, evidenced in the reduction in lutein in photosynthetic tissue of ccr2 and the orthologuous tomato mutant, tangerine (Isaacson et al. 2002; Park et al. 2002). One possibility is that photoisomerisation in the absence of CRTISO produces lycopene isomers that are not optimal substrates for ϵLCY. Alternatively, it may be that the absence of the CRTISO protein or particular carotene isomers affects the expression or function of the other enzymes in the biosynthetic pathway. Indeed, there is a specific reduction in ϵLCY mRNA abundance in ccr2. Our experiments on lut2 heterozygotes and co-suppression of the ϵLCY (Pogson and Rissler 2000) indicate that reduced abundance of ϵLCY mRNA is sufficient to lower lutein accumulation. Thus, apparently ccr2 has reduced lutein as a consequence of limited ϵLCY mRNA.
The mechanism behind this phenomenon and the relationship between CRTISO and ϵLCY was investigated by comparing the relative transcriptional effect of all-trans-lycopene and tetra-cis-lycopene isomers. ϵLCY mRNA abundance is reduced in tetra-cis-lycopene accumulating plants (ccr2 ± MPTA) compared with those accumulating all-trans-lycopene (MPTA-treated wildtype) or lutein and violaxanthin (wildtype) (Fig. 5B). βLCY had a similar expression pattern (Fig. 5B), albeit more variable (data not shown). PSY is up-regulated in tissue accumulating all-trans-lycopene (in wildtype treated with MPTA), but not in tissue that accumulates tetra-cis-lycopene. This up-regulation of PSY is minor, but reproducible and increased in a dose dependant manner. This is mirrored by an increase in LHCB transcript abundance (Fig. 5) which is a traditional marker for alterations in plastid-nuclear signalling, such as in the ‘genome uncoupled’ (gun) mutants (Susek et al. 1993). Moreover, similar expression patterns for GSAAT, an enzyme at the beginning of chlorophyll biosynthesis suggests a specific effect feedback regulation of carotenoids and other genes by different isomers of lycopene on gene expression. Genetic manipulation of PSY altered mRNA abundance of carotenoid biosynthetic genes and other genes in potato (Ducreux et al. 2005), demonstrating that alterations in flux can also result in feedback regulation. The nature of feedback regulation at this stage is speculation, but could involve soluble cleavage products of carotenoids. This hypothesis is supported by the changes observed during light-dependent senescence. Carotenoids are maintained throughout the early stages of this process, presumably to protect the plant from potentially damaging photosensitising compounds, such as free and partially degraded chlorophylls (Matile et al. 1999). Although maintained, there are distinct changes in the carotenoid profile, including an increase in β-carotene relative to lutein (0.27–0.72 after 5 days in radish cotyledons (Suzuki and Shioi 2004)). Both ϵLCY and ϵOH are reduced in senescent leaf tissue (Zimmermann et al. 2004), which concurs with reduced lutein in such conditions. Many of the CCD genes are increased in senescing tissue corresponding to their role in carotenoid cleavage and catabolism.
Carotenoids and plastid development
Carotenoids are produced in almost all types of plastids, albeit only trace levels are observed in certain tissues (Cuttriss et al. 2006). The role of carotenoids in plastid development was investigated by blocking carotenoid biosynthesis at specific points and determining the consequent changes to etioplast structure by electron microscopy. Wildtype etioplasts are characterised by a uniform membrane lattice (PLB), which is perturbed in the poly-cis-carotenoid accumulating ccr2 mutant. Wildtype etioplasts were not altered by carotenoid biosynthetic herbicides, norflurazon or MPTA, confirming earlier observations that all-trans or 15Z-phytoene can form PLBs. Treatment with 75 µg mL–1 MPTA results in substantial accumulation of all-trans-lycopene replacing neoxanthin, violaxanthin and lutein as the predominant carotenoid absorbing in the visible spectrum (Fig. 5). Significantly, all-trans-lycopene accumulation permits PLB formation (Fig. 6C). Thus, the effect of amitrole on PLB formation (Rascio et al. 1996; Moro et al. 2004) is not due to all-trans-lycopene accumulation. So whether the amount of phytoene, lycopene, β-carotene and xanthophylls in amitrole-treated barley is insufficient to form a PLB or the effect is not related to carotenoids requires a quantitative analysis of all carotenoids in amitrole treated-tissue. This is because several different processes are known to alter PLB formation, such as altered lipid profile, changes in protochlorophyllide content or changes in protochlorophyllide oxidoreductase content (Selstam and Sandelius 1984; Sundqvist and Dahlin 1997; Sperling et al. 1998).
We noted that both norflurazon and MPTA treatments rescued PLB formation in the ccr2 mutant. MPTA acts after the ccr2 lesion, so to affect carotenoid accumulation requires feedback on the pathway. Indeed, we observed changes in phytoene levels that could have rescued PLB formation (Fig. 6B; Axelsson et al. 1982; Sundqvist and Dahlin 1997; Park et al. 2002; Denev et al. 2005). An alternative, untested explanation is that PLB formation could be due to a non-specific effect of MPTA. We applied the herbicides to determine whether the absence of the PLB in ccr2 reflects the accumulation of poly-cis-carotenes or a secondary effect independent of the carotenoid composition, such as the loss of CRTISO protein. Since norflurazon treatment rescued PLB formation in ccr2 then the absence of PLBs in ccr2 is not due to undefined effects of the absence of the CRTISO protein on PLB formation. Rather, it is the accumulation of poly-cis-carotenes at the expense of all-trans-carotenoids that apparently prevents the formation of PLBs. Clearly, the mechanism and nature of the interaction between carotenoids and PLBs warrants further investigation and a recent study by Sundqvist and colleagues (Denev et al. 2005) into the effect of carotenoids on the interaction of POR and lipids is a good step in this direction.
Acknowledgements
We acknowledge the support of the Australian Research Council via Centre of Excellence in Plant Energy Biology (CE0561495) to B.J.P.; D.E.S.T and D.A.A.D support to B.J.P. and B.G. and the A.N.U. Endowment of Excellence grant to A.J.C. We thank the ANU electron microscope unit and biomolecular resource facility for assistance with TEM and sequencing, respectively.
Adams WW,
Demmig-Adams B,
Rosenstiel TN,
Brightwell AK, Ebbert V
(2002) Photosynthesis and photoprotection in overwintering plants. Plant Biology 4, 545–557.
| Crossref | GoogleScholarGoogle Scholar |

Axelsson L,
Dahlin C, Ryberg H
(1982) The function of carotenoids during chloroplast development. 5. Correlation between carotenoid content, ultrastructure and chlorophyll-b to chlorophyll-a ratio. Physiologia Plantarum 55, 111–116.
| Crossref | GoogleScholarGoogle Scholar |

Barry P, Pallett KE
(1990) Herbicidal inhibition of carotenogenesis detected by HPLC. Zeitschrift Fur Naturforschung C. 45, 492–497.

Bouvier F,
Backhaus RA, Camara B
(1998) Induction and control of chromoplast-specific carotenoid genes by oxidative stress. Journal of Biological Chemistry 273, 30651–30659.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bramley PM
(2002) Regulation of carotenoid formation during tomato fruit ripening and development. Journal of Experimental Botany 53, 2107–2113.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Clough SJ, Bent AF
(1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cunningham FJ, Gantt E
(1998) Genes and enzymes of carotenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology 49, 557–583.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cunningham FX
(2002) Regulation of carotenoid synthesis and accumulation in plants. Pure and Applied Chemistry 74, 1409–1417.

Curtis M, Grossniklaus U
(2003) A Gateway TM cloning vector set for high-throughput functional analysis of genes in plants. Plant Physiology 133, 462–469.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dalla Vecchia F,
Barbato R,
La Rocca N,
Moro I, Rascio N
(2001) Responses to bleaching herbicides by leaf chloroplasts of maize plants grown at different temperatures. Journal of Experimental Botany 52, 811–820.
| PubMed |

Davison PA,
Hunter CN, Horton P
(2002) Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418, 203–206.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

DellaPenna D, Pogson BJ
(2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annual Review of Plant Biology 57, 711–738.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Denev ID,
Yahubyan GT,
Minkov IN, Sundqvist C
(2005) Organization of protochlorophyllide oxidoreductase in prolamellar bodies isolated from etiolated carotenoid-deficient wheat leaves as revealed by fluorescence probes. Biochimica et Biophysica Acta-Biomembranes 1716, 97–103.
| Crossref | GoogleScholarGoogle Scholar |

Di Baccio D,
Quartacci MF,
Dalla Vecchia F,
La Rocca N,
Rascio N, Navari-Izzo F
(2002) Bleaching herbicide effects on plastids of dark-grown plants: lipid composition of etioplasts in amitrole and norflurazon-treated barley leaves. Journal of Experimental Botany 53, 1857–1865.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ducreux LJM,
Morris WL,
Hedley PE,
Shepherd T,
Davies HV,
Millam S, Taylor MA
(2005) Metabolic engineering of high carotenoid potato tubers containing enhanced levels of beta-carotene and lutein. Journal of Experimental Botany 56, 81–89.
| PubMed |

Grunewald K,
Eckert M,
Hirschberg J, Hagen C
(2000) Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiology 122, 1261–1268.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Heim DR, Larrinua IM
(1989) Primary site of action of amitrole in Arabidopsis thaliana involves inhibition of root elongation but not of histidine or pigment biosynthesis. Plant Physiology 91, 1226–1231.
| PubMed |

Herman CA,
Im CS, Beale SI
(1999) Light-regulated expression of the GSA gene encoding the chlorophyll biosynthetic enzyme glutamate 1-semialdehyde aminotransferase in carotenoid-deficient Chlamydomonas reinhardtii cells. Plant Molecular Biology 39, 289–297.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Herrin DL,
Battey JF,
Greer K, Schmidt GW
(1992) Regulation of chlorophyll apoprotein expression and accumulation. Requirements for carotenoids and chlorophyll. Journal of Biological Chemistry 267, 8260–8269.
| PubMed |

Hirschberg J
(2001) Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology 4, 210–218.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hoaglands DR, Arnon DA
(1950) The water culture method of growing plants without soil. California Agricultural Experiment Station Bulletin , 1–39.

Hoober JK, Eggink LL
(1999) Assembly of light-harvesting complex II and biogenesis of thylakoid membranes in chloroplasts. Photosynthesis Research 61, 197–215.
| Crossref | GoogleScholarGoogle Scholar |

Isaacson T,
Ronen G,
Zamir D, Hirschberg J
(2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. The Plant Cell 14, 333–342.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jensen PE,
Gibson LCD,
Henningsen KW, Hunter CN
(1996) Expression of the chlI, chlD, and chlH genes from the cyanobacterium Synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. Journal of Biological Chemistry 271, 16662–16667.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Johnson GN,
Scholes JD,
Horton P, Young AJ
(1993) Relationships between carotenoid composition and growth habit in British plant species. Plant, Cell & Environment 16, 681–686.
| Crossref | GoogleScholarGoogle Scholar |

Külheim C,
Ågren J, Jansson S
(2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297, 91–93.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

von Lintig J,
Welsch R,
Bonk M,
Giuliano G,
Batschauer A, Kleinig H
(1997) Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. The Plant Journal 12, 625–634.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Logan BA,
Barker DH,
Demmig Adams B, Adams WW
(1996) Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant, Cell & Environment 19, 1083–1090.
| Crossref | GoogleScholarGoogle Scholar |

Lu S,
Van Eck JV,
Zhou X,
Lopez AB, O”Halloran DM , et al.
(2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. The Plant Cell 18, 3594–3605.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Matile P,
Hortensteiner S, Thomas H
(1999) Chlorophyll degradation. Annual Review of Plant Physiology and Plant Molecular Biology 50, 67–95.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Matsubara S,
Morosinotto T,
Bassi R,
Christian AL, Fischer-Schliebs E , et al.
(2003) Occurrence of the lutein-epoxide cycle in mistletoes of the Loranthaceae and Viscaceae. Planta 217, 868–879.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Moehs CP,
Tian L,
Osteryoung KW, DellaPenna D
(2001) Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Molecular Biology 45, 281–293.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Moro I,
Dalla Vecchia F,
La Rocca N,
Navari-Izzo F,
Quartacci MF,
Di Baccio D,
Rudiger W, Rascio N
(2004) Impaired carotenogenesis can affect organization and functionality of etioplast membranes. Physiologia Plantarum 122, 123–132.
| Crossref | GoogleScholarGoogle Scholar |

Nogaj LA,
Srivastava A,
van Lis R, Beale SI
(2005) Cellular levels of glutamyl-tRNA reductase and glutamate-1-semialdehyde aminotransferase do not control chlorophyll synthesis in Chlamydomonas reinhardtii. Plant Physiology 139, 389–396.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Park H,
Kreunen SS,
Cuttriss AJ,
DellaPenna D, Pogson BJ
(2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. The Plant Cell 14, 321–332.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Phillip DM, Young AJ
(2006) Preferential inhibition of the lycopene ϵ-cyclase by the substituted triethylamine compound MPTA in higher plants. Journal of Plant Physiology 163, 383–391.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pogson BJ, Rissler HM
(2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 355, 1395–1403.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pogson B,
McDonald K,
Truong M,
Britton G, DellaPenna D
(1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. The Plant Cell 8, 1627–1639.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pogson BJ,
Niyogi KK,
Bjorkman O, DellaPenna D
(1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proceedings of the National Academy of Sciences USA 95, 13324–13329.
| Crossref | GoogleScholarGoogle Scholar |

Rascio N,
DallaVecchia F,
Agnolucci L,
Barbato R,
Tassani V, Casadoro G
(1996) Amitrole effects on barley etioplasts. Journal of Plant Physiology 149, 295–300.

Rissler HM, Pogson BJ
(2001) Antisense inhibition of the beta-carotene hydroxylase enzyme in Arabidopsis and the implications for carotenoid accumulation, photoprotection and antenna assembly. Photosynthesis Research 67, 127–137.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ronen G,
Cohen M,
Zamir D, Hirschberg J
(1999) Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant delta. The Plant Journal 17, 341–351.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rossel JB,
Wilson IW, Pogson BJ
(2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiology 130, 1109–1120.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Selstam E, Sandelius AS
(1984) A comparison between prolamellar bodies and prothylakoid membranes of etioplasts of dark-grown wheat concerning lipid and polypeptide composition. Plant Physiology 76, 1036–1040.
| PubMed |

Sperling U,
Franck F,
van Cleve B,
Frick G,
Apel K, Armstrong GA
(1998) Etioplast differentiation in Arabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. The Plant Cell 10, 283–296.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Steinbrenner J, Linden H
(2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Molecular Biology 52, 343–356.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Strand A,
Asami T,
Alonso J,
Ecker JR, Chory J
(2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421, 79–83.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sundqvist C, Dahlin C
(1997) With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Physiologia Plantarum 100, 748–759.
| Crossref | GoogleScholarGoogle Scholar |

Susek RE,
Ausubel FM, Chory J
(1993) Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74, 787–799.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Suzuki Y, Shioi Y
(2004) Changes in chlorophyll and carotenoid contents in radish (Raphanus sativus) cotyledons show different time courses during senescence. Physiologia Plantarum 122, 291–296.
| Crossref | GoogleScholarGoogle Scholar |

Tada S,
Hatano M,
Nakayama Y,
Volrath S,
Guyer D,
Ward E, Ohta D
(1995) Insect-cell expression of recombinant imidazoleglycerolphosphate dehydratase of Arabidopsis and wheat and inhibition by triazole herbicides. Plant Physiology 109, 153–159.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Welsch R,
Beyer P,
Hugueney P,
Kleinig H, von Lintig J
(2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211, 846–854.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Welsch R,
Medina J,
Giuliano G,
Beyer P, von Lintig J
(2003) Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta 216, 523–534.
| PubMed |

Woitsch S, Römer S
(2003) Expression of xanthophyll biosynthetic genes during light-dependant chloroplast differentiation. Plant Physiology 132, 1508–1517.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zimmermann P,
Hirsch-Hoffmann M,
Hennig L, Gruissem W
(2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiology 136, 2621–2632.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



