Goldacre paper: Recognition events and host–pathogen co-evolution in gene-for-gene resistance to flax rust
Peter Dodds A B and Peter Thrall AA CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
B Corresponding author. Email: peter.dodds@csiro.au
C This paper originates from the Peter Goldacre Award 2006 of the Australian Society of Plant Scientists, received by the author.
Functional Plant Biology 36(5) 395-408 https://doi.org/10.1071/FP08320
Submitted: 16 December 2008 Accepted: 12 March 2009 Published: 6 May 2009
Abstract
The outcome of infection of individual plants by pathogenic organisms is governed by complex interactions between the host and pathogen. These interactions are the result of long-term co-evolutionary processes involving selection and counterselection between plants and their pathogens. These processes are ongoing, and occur at many spatio-temporal scales, including genes and gene products, cellular interactions within host individuals, and the dynamics of host and pathogen populations. However, there are few systems in which host–pathogen interactions have been studied across these broad scales. In this review, we focus on research to elucidate the structure and function of plant resistance and pathogen virulence genes in the flax-flax rust interaction, and also highlight complementary co-evolutionary studies of a related wild plant–pathogen interaction. The confluence of these approaches is beginning to shed new light on host–pathogen molecular co-evolution in natural environments.
Additional keywords: avirulence, innate immunity, NB-LRR.
Introduction
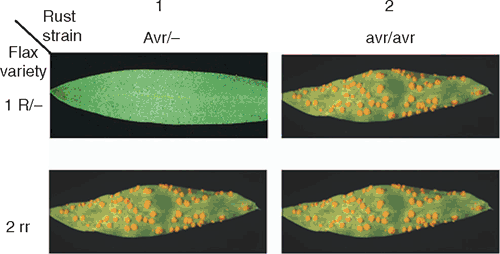
Plant diseases represent a continuing threat to agriculture and food supply around the world, as epidemics periodically cause devastating crop losses. For example, the recent emergence in East Africa of a new wheat rust strain, Ug99, which overcomes the major resistance genes used around the world, poses a major risk to global wheat production (http://www.globalrust.org, accessed 1 March 2009). Thus, it is of great importance to understand the constantly evolving interactions between pathogen infection strategies and plant resistance mechanisms. Plants have evolved a complex multi-layered defence system to prevent infection (Nurnberger et al. 2004; Chisholm et al. 2006), while successful pathogens have evolved means of circumventing host defences. The first layer of inducible defence responses in plants involves recognition of conserved structural components of potential pathogens (such as fungal chitin or bacterial flagellin) which are collectively known as pathogen associated molecular patterns (PAMPs), by cell surface receptors (Jones and Dangl 2006). This PAMP-triggered immunity (PTI) is effective at preventing infection by a broad range of microbes, and probably underlies non-host resistance mechanisms. However, pathogens that have the ability to cause infection on particular host plants have evolved means of suppressing PTI, largely through the use of effector proteins that are delivered into host cells to manipulate these signalling processes. This establishes a basic compatibility between the pathogen and host, but also exposes the pathogen to another layer of plant defence. The outcome of infection in these host-compatible interactions is usually controlled by the interaction of host plant resistance (R) genes and pathogen avirulence (Avr) genes. In this classic ‘gene-for-gene’ model the absence or lack of activity of either of these genes leads to pathogen establishment and disease expression (Flor 1971; Fig. 1).
|
In recent years, many R and Avr genes have been cloned from plants and their pathogens, respectively. It is now recognised that plant R genes encode the second layer of the plant immune system, and pathogen Avr genes encode the effector proteins whose normal function is to interfere with host plant cells to promote successful infection (Jones and Dangl 2006). R protein-mediated recognition of Avr proteins (for glossary of terms, see Appendix 1) leads to rapid activation of defence mechanisms, such as increased ion fluxes, extracellular oxidative burst, transcriptional responses near the infection sites, and a localised cell death termed the hypersensitive response (HR), which is thought to limit the spread of the pathogen from the infection site (Chisholm et al. 2006). Thus gene-for-gene resistance is now often referred to as effector-triggered immunity (ETI) and involves direct or indirect recognition of pathogen effector proteins by plant R proteins.
Pathogen effector proteins are highly diverse, representing the vast array of different molecules that could be adapted during evolution to interfere with host defence processes. Plant R proteins, on the other hand, are the recognition component of the plant immune system, and belong to a few conserved structural classes. The two main classes present a recognition domain to either the extra- or intra-cellular environment (Dangl and Jones 2001; Staskawicz et al. 2001). One class encodes membrane bound proteins with an extracellular leucine-rich-repeat (LRR) domain, either with or without an intracellular kinase domain. The corresponding Avr proteins are secreted into the apoplastic space during infection, where they may be detected. However, the majority of known R genes encode intracellular proteins with an LRR domain and a nucleotide-binding site (NBS) domain. These proteins are structurally related to animal Nod proteins, which play a role in PAMP recognition and subsequent induction of innate immunity responses in animals (Girardin et al. 2003). Many plant R proteins also contain TIR domains related to the intracellular signalling domain of the Drosophila Toll protein and mammalian Interleukin-1 receptor proteins. These animal proteins are part of the Toll-like receptor family involved in triggering innate immunity in response to extracellular PAMPs (Akira 2003).
Despite the many structural similarities between plant resistance and animal innate immunity systems, there are also several important differences. Animal innate immunity components recognise highly conserved PAMPs while R proteins respond to Avr proteins within the plant cell such as many of the effector proteins that bacterial pathogens deliver directly into the host cell cytoplasm via the type III secretion system (TTSS; Büttner and Bonas 2003). These effectors have various disease-related roles, such as degradation of specific host proteins (Orth et al. 2000; Axtell and Staskawicz 2003; Shao et al. 2003) or influencing host cell gene transcription (Büttner and Bonas 2003). Recent research indicates that fungal and oomycete pathogens also direct effector proteins to the plant cytoplasm, but the mechanism of transport is unknown (Ellis et al. 2007; Whisson et al. 2007; Panstruga and Dodds 2009; Tyler 2009).
The gene-for-gene paradigm has shaped much of our current thinking about host–pathogen co-evolution. In particular, the presence of corresponding R and Avr genes in host and pathogen populations implies the possibility of co-evolution driven by selection pressure on the pathogen to escape recognition by host R gene products, and concomitant pressure on the host to respond to new virulent strains of the pathogen. Many insights into these co-evolutionary processes have been derived from studies of the flax rust disease system and here we review recent progress in understanding this host–pathogen interaction.
The flax rust model system
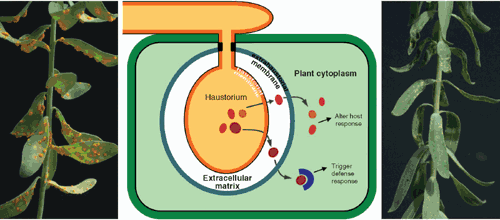
The flax (Linum usitatissimum L.) and flax rust (Melampsora lini) disease system has been an enduring model system for studies of plant disease resistance, having been the basis for Flor’s ‘gene-for-gene’ concept (Flor 1971; Lawrence et al. 2007). Rust fungi (basidiomycetes of the order Uredinales) are obligate biotrophs, meaning that they are completely dependent on nutritional resources obtained from living host cells for their growth and propagation. During infection of host plants, fungal hyphae grow in the intercellular spaces of the leaf, but form a close association with host cells through haustoria (Fig. 2). These specialised infection structures penetrate the plant cell wall and invaginate the plant cell plasma membrane. Rust fungi manipulate host cell metabolism through their haustoria, which are thought to be the primary site of nutrient acquisition from the plant (Hahn and Mendgen 2001; Voegele and Mendgen 2003). Immunocytochemical studies have revealed specialisations of the flax rust haustorium cell wall (Murdoch and Hardham 1998; Murdoch et al. 1998) and re-organisation of the host cell cytoskeleton during haustorium development, with the latter response playing an important role in host defence (Kobayashi et al. 1994, 1995, 1997). Haustoria are also the site of recognition in resistant plants, with the HR first observed in host cells containing an haustorium (Kobayashi et al. 1994; Heath 1997). Other obligate biotrophs, such as the downy mildews (oomycetes) and the powdery mildews (ascomycetes), also produce haustoria although these have probably evolved independently. Some hemibiotrophic pathogens, such as the oomycete Phytophthora, form haustoria early in infection, but later induce host cell death and enter a necrotrophic phase. Thus, the haustorium–host cell interface appears to mediate a dynamic interaction involving extensive trafficking of nutrients, and signalling and defence molecules.
|
Flax R genes and their products
Genetic studies of the interaction between the flax plant and flax rust have identified ~30 flax R genes, which occur as series of closely linked or allelic genes at five loci, and ~30 corresponding flax rust Avr genes that are mostly dispersed in the flax rust genome. A total of 19 different rust resistance genes have now been cloned from flax, including 11 allelic variants of the L locus, three at the M locus, three at the N locus and two at the P locus (Lawrence et al. 1995, 2009; Anderson et al. 1997; Ellis et al. 1999; Dodds et al. 2001a, 2001b). These genes all encode predicted cytosolic resistance proteins of the NBS-LRR class, with an N-terminal TIR domain, although the P locus proteins have an additional C-terminal domain of 150 amino acids downstream of the LRR region. Genes from the L and M loci are the most closely related (86% DNA identity) and these may represent homoeologous loci, since flax is an ancient tetraploid. Although the L locus contains only a single gene (Ellis et al. 1999), the M locus contains ~15 related genes arranged in tandem (Anderson et al. 1997). Like M, the N and P loci are also complex, with at least four related genes (Dodds et al. 2001a, 2001b).
The simple genetic structure of the L locus has made it possible to isolate and compare gene sequences of multiple allelic variants (L, L1, L2, … L11) of this resistance locus. These variants can be distinguished by their reaction to rust strains carrying different Avr genes. Most of the variation between sequences occurs in the region encoding the LRR domain of the proteins, and domain swap experiments have shown that this domain is critical for determining the specificity of R-Avr recognition processes (Ellis et al. 1999). For instance, chimeric genes encoding the L2 LRR domain fused to the N-terminal regions of L6 or L10 express the L2 resistance specificity; i.e. they confer resistance to rust strains carrying Avr-L2 but not those with Avr-L6 or Avr-L10. Similarly, six amino acid changes in the LRR were sufficient to convert the P2 resistance protein to the P resistance specificity (Dodds et al. 2001a). Further confirmation of the role of the LRR regions of NBS-LRR R proteins in Avr recognition comes from the work by Jia et al. (2000), who showed that the LRR domain of the rice Pita resistance protein interacts in a yeast two-hybrid assay with the corresponding Avr-Pita protein from Magnaporthe grisea.
However, there is also evidence that the TIR domain can influence recognition specificity and that intramolecular interactions between the TIR-NBS and LRR regions are important for the function of these proteins (Luck et al. 2000). Intramolecular interactions have also been implicated in the function of the Mi resistance protein in tomato (Hwang and Williamson 2003) and direct interaction has been demonstrated between these domains of Rx in potato, which are disrupted upon recognition of the corresponding Avr protein (Moffett et al. 2002). These observations have led to models of R protein function which suggest that the LRR is primarily responsible for recognition (direct or indirect) of the Avr product, which leads to conformational changes within the protein involving interactions between domains to allow signal propagation.
Avirulence genes in flax rust
Until recently, the isolation of Avr genes from biotrophic fungi and oomycetes has been difficult because these organisms cannot be readily cultured or transformed. However, four families of Avr genes, AvrL567, AvrM, AvrP123 and AvrP4 have now been identified in flax rust (Table 1; Dodds et al. 2004; Catanzariti et al. 2006). The first of these (AvrL567) was isolated by a subtractive hybridisation screen for rust genes expressed during infection, and these were mapped as restriction fragment length polymorphisms in a flax rust F2 mapping family segregating for 16 Avr specificities. One cDNA probe was found to co-segregate with the AvrL5, AvrL6 and AvrL7 cluster of avirulence genes. Two copies of this gene were present at the avirulence allele of this locus and a single copy at the virulence allele and there was a high level of sequence polymorphism between these genes. In planta expression of either of the avirulence allele genes caused R gene-dependent cell death specific to the corresponding L5, L6 or L7 genes, but expression of the virulence allele did not, consistent with induction of the typical HR during resistance. This effect was demonstrated in leaves of flax transiently transformed by Agrobacterium infiltration, as well as when the corresponding R and Avr genes were brought together by crossing transgenic flax expressing AvrL567 genes to resistant lines (Dodds et al. 2004). The AvrL567 genes encode small secreted proteins expressed in haustoria, and a subsequent screen used these characteristics to identify three further flax rust Avr genes. Flax rust haustoria were isolated by ConA-affinity chromatography (Hahn and Mendgen 1992) and used to construct a cDNA library. Sequencing of 822 cDNA clones from this library identified 20 that were predicted to encode secreted proteins. Amongst these 20 haustorially expressed secreted proteins (HESPs), one co-segregated with AvrM, another with AvrP4 and a third with the AvrP, AvrP1, AvrP2, AvrP3 cluster of avirulence genes (Catanzariti et al. 2006). Transient Agrobacterium expression assays in flax lines confirmed resistance gene specific avirulence function in all cases.

|
All four Avr gene families encode small secreted proteins that are expressed in haustoria and are apparently translocated into host cells during infection. Evidence for this translocation comes from the observation that transient expression of these Avr proteins as cytoplasmic proteins (i.e. lacking the signal peptide) in plants can trigger a defence response dependent on the corresponding R genes. This shows that Avr protein recognition occurs inside plant cells, implying that these proteins are translocated during infection. However, rust haustoria are separated from the host cytoplasm by a plant-derived outer membrane, so this result implies that, like the bacterial TTSS-dependent effectors, the flax rust Avr proteins cross the plant membrane and enter the host cell. Kemen et al. (2005) recently provided direct evidence for translocation of a bean rust (Uromyces fabae) haustorially-secreted protein, UfRTP1, into infected host cells. Immunolocalisation detected UfRTP1 in the plant cytoplasm adjacent to haustoria and within host cell nuclei, consistent with the presence of a predicted nuclear localisation signal in this protein. Thus, rust haustoria apparently secrete a suite of proteins (likely to include the other 20 or so flax rust Avr gene products) that are translocated into the plant cytoplasm and these probably represent a class of rust effector proteins that facilitate infection (Catanzariti et al. 2007; Fig. 2).
Biotrophic and hemibiotrophic oomycete pathogens have independently evolved a similar infection process involving haustoria formation (Perfect and Green 2001; Panstruga 2003) and several oomycete Avr genes encode secreted proteins that are recognised inside plant cells (Allen et al. 2004; Shan et al. 2004; Armstrong et al. 2005; Rehmany et al. 2005). These oomycete proteins are characterised by a conserved N-terminal RxLR motif that is related to a transport signal responsible for uptake of secreted proteins of the malaria parasite (Plasmodium falciparum) across the erythrocyte vacuolar membrane (Hiller et al. 2004; Marti et al. 2004; Bhattacharjee et al. 2006). Genomic analyses suggest that these pathogens secrete large arrays of RxLR effector proteins during infection (Kamoun 2006; Birch et al. 2008; Tyler 2009). Recent work indicates that the RxLR motif directs the transport of these proteins into host cells in the absence of the pathogen (Whisson et al. 2007; Dou et al. 2008). Likewise, rust Avr protein transport is apparently independent of the pathogen, and may utilise plant-derived transport mechanisms (Catanzariti et al. 2006). For example, transient expression of the AvrM protein with or without the signal peptide induces an M gene specific HR, but addition of the HDEL endoplasmic retention signal prevents recognition of the secreted but not the cytoplasmic version. This is consistent with recognition of the secreted form by the cytoplasmic M protein after secretion and re-entry into the plant cell. However, the limited numbers of rust Avr proteins identified to date do not contain any obviously conserved motif so it is not clear whether they could utilise the same uptake mechanism as oomycete effectors.
The molecular basis of Avr protein recognition and gene-for-gene specificity
The nature of the interactions between host recognition proteins and the corresponding pathogen molecules is critical for understanding how R and Avr genes co-evolve. There are essentially two current models to explain how plant R proteins respond to pathogen Avr proteins. First, they may interact directly with the Avr protein to trigger resistance, as has been observed for rice Pita and Magnaporthe grisea Avr-Pita proteins (Jia et al. 2000), and also for Arabidopsis RRS1 and Ralstonia solanacearum PopP2 (Deslandes et al. 2003). Second, they may detect Avr proteins indirectly by responding to changes induced in host target proteins. For example, the RPS2 and RPM1 resistance proteins in Arabidopsis recognise their corresponding Pseudomonas syringae Avr products by detecting changes induced in the host protein RIN4 by these Avr products (Mackey et al. 2002, 2003; Axtell and Staskawicz 2003). A modified version of this model, the decoy model, suggests that the guarded host target proteins may be co-opted in evolution for a dedicated role in pathogen recognition (van der Hoorn and Kamoun 2008).
In the flax rust system, the co-localisation of Avr and R proteins in the flax cytoplasm as well as the genetics of the gene-for-gene interactions are consistent with direct interaction between these proteins, and this has been confirmed by yeast two hybrid assays (Dodds et al. 2006). These experiments assayed interaction between three R proteins (L5, L6 and the chimeric construct L6-L11RV) and 12 AvrL567 variants (AvrL567-A to -L). Protein–protein interactions were detected in yeast for the same combinations of L and AvrL567 proteins as induced HR in transient expression assays in planta. The close correspondence between the detection of a protein interaction in yeast and the induction of HR in planta indicates that direct R-Avr protein interaction is the basis for recognition specificity. For example, L6 but not L5 interacts with AvrL567-D in yeast, and co-expression of L6 but not L5 with AvrL567-D induces HR in planta. Furthermore, the L6-L11RV chimera interacts with only AvrL567-J in yeast and again induces HR with only this Avr protein in planta. The observation that L6-L11RV and L6 differ only in the last 3 LRR units indicates that both the resistance and interaction specificities are controlled by the LRR domain. No interactions were detected in yeast between the resistance proteins and the proteins encoded by the virulence alleles that do not induce HR in flax lines.
Mutation in the P-loop ATP binding motif in the NBS domain of L6 eliminated both the yeast two hybrid interaction and HR response in plants (Dodds et al. 2006). This suggests that although the LRR is clearly the determinant of specificity, the presence of ATP or ADP bound to the NBS domain is required for a protein conformation capable of binding Avr proteins. Yeast two hybrid experiments with N- and C-terminal deletions of L6 have shown that the TIR domain is not necessary for R-Avr interactions and that the minimum interacting deletions include both NBS and LRR domains (P. N. Dodds, unpubl. data). These results are similar to those reported by Ueda et al. (2006) for the interaction between the TIR-NBS-LRR N protein of tobacco and the p50 fragment of the tobacco mosaic virus replicase protein.
In the flax system, the observation of direct interaction between L5 and L6 proteins and corresponding Avr proteins has now been extended to M and AvrM (P. N. Dodds, unpubl. data). However, whereas M is ~80% identical to L5 and L6, the AvrL567 and AvrM proteins are unrelated. Similarly, although L6 and L11 differ by only 32 LRR polymorphisms, their corresponding Avr proteins are also apparently unrelated. In addition, all the other distinct L alleles interact with genetically independent avirulence genes and these are not sufficiently related in DNA sequence to be detected by AvrL567 DNA probes. Thus, the emerging picture is that the LRR region is highly flexible in an evolutionary sense with the capacity to recognise diverse pathogen ligands by direct interaction when coupled with the NBS domain.
Evidence for co-evolution of flax R genes and flax rust Avr genes
The highly polymorphic nature of the flax resistance loci and flax rust avirulence loci raises the question of how these genes have evolved different recognition capacities and how these variants are maintained in host and pathogen populations. Analysis of nucleotide variation has shown evidence for diversifying selection at R loci in flax (Dodds et al. 2000, 2001a, 2001b) as well as Avr loci in rust (Dodds et al. 2004; Catanzariti et al. 2006). This is exemplified by an excess of nucleotide changes at non-synonymous sites compared with synonymous sites within the coding regions of these genes, indicating that selection has favoured the accumulation of amino acid variation (Hughes and Nei 1988). Observations of diversifying selection are predominantly in host genes involved in pathogen recognition – such as plant R genes (Parniske et al. 1997; Mondragon-Palomino et al. 2002) and mammalian MHC and TLR genes (Hughes and Nei 1988; White et al. 2003) and also in pathogen genes encoding proteins that may be targets of host recognition (Endo et al. 1996).
In flax R genes, the signature of diversifying selection is seen predominantly in the region encoding the LRR domain, particularly in the predicted solvent exposed region, consistent with the hypothesised role of this region in specificity (Dodds et al. 2000, 2001a, 2001b). In contrast, the N-terminal domains, thought to be involved in signalling, generally show evidence of purifying selection, although diversifying selection was detected in a small variable section of the TIR region of L genes, which also influences specificity in some cases (Luck et al. 2000). Similar observations of diversifying selection have been made for many other plant resistance loci (Parniske et al. 1997; Mondragon-Palomino et al. 2002), suggesting that positive selection of novel R protein sequence variants is a general phenomenon, presumably because these encode new recognition specificities that enhance fitness in natural disease situations. In addition to diversifying selection, a second major evolutionary process influencing the generation of new R gene recognition specificities involves extensive sequence exchange events as a result of meiotic chromosomal recombination or gene conversion, that shuffle this variation between related genes. This process occurs between allelic variants, such as the L locus genes (Ellis et al. 1999), as well as duplicated genes at complex loci such as N or P (Dodds et al. 2001a, 2001b). However, at complex loci, the accumulation of sequence differences due to mutation and divergent selection leads to a decline in sequence exchange between paralogs (Dodds et al. 2001b) which may then evolve largely in isolation as suggested by the ‘birth and death’ model proposed for vertebrate MHC genes (Nei et al. 1997) and some plant R genes (Michelmore and Meyers 1998).
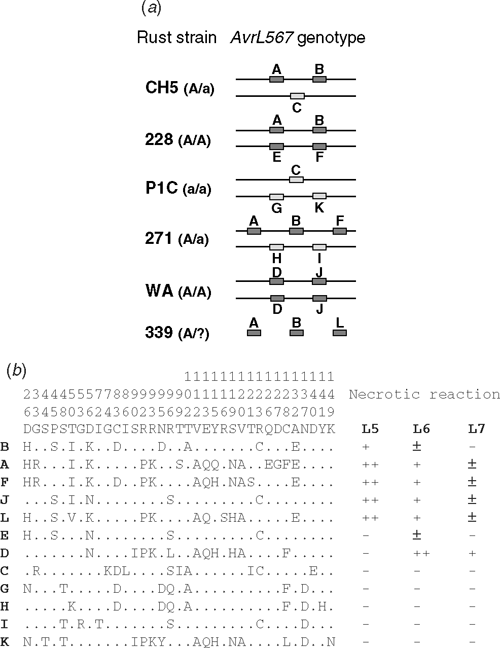
Evolution of the AvrL567 genes may have been driven by selective pressure for the pathogen to escape recognition by host R genes, but at same time maintain the pathogenicity function of these effector proteins. This is suggested by the observation that in flax rust numerous amino acid sequence variants of these proteins have evolved with altered recognition properties in preference to deletion or gene inactivation to overcome resistance. The AvrL567 genes are highly variable, with 12 different sequence variants (A–L) found in six rust strains of diverse origin, which contain at least eight different haplotypes for this locus (Fig. 3). The 127 amino acid sequence of the mature AvrL567 proteins contains 35 polymorphic sites, with nine sites showing multiple polymorphisms. Amino acid differences between the AvrL567 protein variants have resulted from diversifying selection as discussed above, and these differences result in changes to recognition specificity (Dodds et al. 2006; Fig. 3b). These observations suggest a strong co-evolutionary relationship between the flax rust AvrL567 genes and the corresponding L genes in flax, consistent with an ‘arms race’ model of host–pathogen co-evolution driven by selection for resistance in the host and virulence in the pathogen (Bergelson et al. 2001).
|
Biochemical analysis of Escherichia coli produced AvrL567 proteins shows that variants that escape recognition nevertheless maintain a conserved structure and stability, consistent with the maintenance of an as yet unknown pathogenicity function and suggesting that the amino acid sequence differences directly affect the R-Avr protein interaction (Dodds et al. 2006). The structures of AvrL567-A and -D have been determined by X-ray crystallography (Wang et al. 2007) and structural modelling indicates that avirulence and virulence variants of this protein have very similar structures and physical properties. All of the polymorphic residues map to the surface of the protein and polymorphisms in residues associated with recognition differences for the R proteins lead to significant changes in surface chemical properties. Analysis of single and multiple amino acid substitutions in AvrL567 proteins has confirmed the role of individual residues in conferring differences in recognition, but also suggest that the specificity results from the cumulative effects of multiple amino acid contacts. The fact that naturally occurring virulence forms are expressed and encode products highly related to the avirulence variants suggests that there has been selection for Avr variants that escape detection by R proteins but retain a selective value for the pathogen, most likely through a virulence effector function. AvrL567 proteins show no similarity to any known or predicted proteins in current databases and do not contain any known functional motifs, so the identification of their postulated virulence function is an important target of continuing research. Transgenic flax expressing AvrL567 proteins show no obvious phenotype in the absence of the corresponding resistance gene, and are not compromised in their expression of resistance to otherwise avirulent rust strains, which could have indicated a suppression of defence activity.
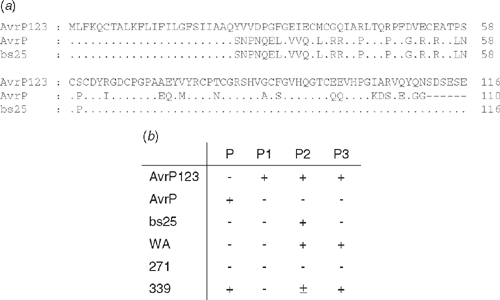
Diversifying selection is also evident in the other flax rust Avr genes, and most particularly the AvrP123 gene, which, like AvrL567, encodes an array of allelic variants with diverse recognition specificities for the corresponding P, P1, P2 and P3 resistance genes (Fig. 4). Of the two alleles identified in rust strain CH5, one is recognised by the P resistance gene alone, and the other is recognised by P1, P2 and P3. Lawrence et al. (1981) identified a putative recombinant allele derived from CH5 that expressed avirulence only to P2. Sequence analysis confirmed that this rust strain contains a recombinant AvrP123 gene, encoding a protein whose N-terminal 60 amino acids are identical to AvrP, and the remainder is identical to AvrP123 (Fig. 4a). Expression of this protein in flax plants confirmed that it was exclusively recognised by the P2 resistance gene (Fig. 4b), indicating that amino acid differences in the N-terminal region influence recognition by the P, P1 and P3 genes while P2 recognition depends on polymorphisms in the C-terminal region. Additional AvrP123 variants isolated from other rust strains also show diverse recognition spectra with the corresponding R genes (Fig. 4b). Co-expression of the AvrP123 alleles with the P or P2 resistance genes in tobacco shows a conservation of the recognition and HR induction in this heterologous host (P. N. Dodds, unpubl. data), which is also consistent with a direct recognition event that does not require conservation of other host recognition factors. Thus it seems likely that direct R-Avr protein recognition prevails in this disease system, which contrasts with Arabidopsis–Pseudomonas disease resistance interactions. Part of the evolutionary explanation for this difference may lie in the obligate parasitic and narrow host range characteristics of flax rust compared with bacterial pathogens. Mechanistically, the rust effectors may influence host target proteins through binding interactions rather than enzymatic modifications that can be detected indirectly.
|
A pre-requisite for such a co-evolutionary outcome is that the pathogen Avr gene must be able to accumulate mutations that affect recognition without imposing a significant fitness cost by impairing an important pathogenicity function. However, R proteins that confer resistance by detecting changes in host proteins modified by the effector function of their corresponding Avr proteins, impose selection against this function. Theoretical modelling has suggested that indirect recognition can lead to stable long-term resistance, but direct recognition is likely to lead to relatively rapid evolution of new virulence phenotypes (van der Hoorn et al. 2002; Ellis and Dodds 2003). This is because Avr recognition is not directly related to effector function, so it is theoretically possible for mutations to arise that abolish recognition while retaining effector function with little or no fitness penalty to the pathogen. Thus direct R-Avr recognition provides a molecular basis that can explain a plant–parasite arms race leading to extensive diversification in corresponding R and Avr genes, as is observed in the flax rust system. In contrast, the Arabidopsis Rpm1, Rps2 and Rps5 resistance proteins use indirect recognition mechanisms and these loci are characterised by low levels of genetic diversity and the presence of ancient polymorphisms, suggesting that simple balanced polymorphisms for functional and non-functional alleles have been maintained over long evolutionary time scales (Stahl et al. 1999; Mauricio et al. 2003). It is likely that direct recognition associated with high genetic diversity at corresponding R and Avr loci and indirect recognition associated with simple balanced polymorphisms for functional and non-functional R and Avr genes are alternative outcomes of plant–pathogen co-evolution. However, these alternatives may represent two extremes of a broad spectrum of possible R-Avr interaction mechanisms and evolutionary outcomes.
Interactions between M. lini and its wild host L. marginale in natural populations
Although molecular analysis of cloned R and Avr genes in flax rust (M. lini) and cultivated flax provides strong evidence for co-evolution, we have little knowledge of how variation at the molecular level influences the population and genetic dynamics of disease in nature. Likewise, we do not know how population level ecological processes influence the evolution of host resistance and pathogen avirulence genes. These issues are now being addressed in natural populations of flax rust. In addition to cultivated flax, M. lini also infects flax (L. marginale), a perennial herb endemic to southern Australia, and this host–pathogen system almost certainly represents a long-standing co-evolutionary association rather than a recent introduction (Lawrence and Burdon 1989; Barrett et al. 2008a). This interaction is related to but evolutionarily isolated from that between the pathogen and its agricultural host (L. usitatissimum). A major focus of studies of the L. marginale–M. lini association has been to investigate the dynamics of coevolved genetic polymorphisms across natural landscapes over time (Burdon et al. 1999, 2002; Thrall et al. 2001). Studies of this system have provided important insights into how co-evolution between host resistance and pathogen virulence is affected by metapopulation structure, gene-flow, and costs of virulence (Burdon et al. 1999; Thrall et al. 2001; Thrall and Burdon 2003). Much of this work has centred on a single well defined metapopulation (a group of discrete populations occurring within a local area) in the Kiandra plain, part of the subalpine region of southern New South Wales (Jarosz and Burdon 1991; Burdon and Thrall 2000).
Linum marginale shows significant variation in outcrossing rates across its range, but within the Kiandra metapopulation, plants are strongly inbreeding (Burdon et al. 1999). M. lini is a macrocyclic rust capable of repeated cycles of asexual reproduction (urediospores) or of initiating a process of sexual recombination (Fig. 5), although in the Kiandra region no evidence for sexual recombination has been detected (Burdon and Roberts 1995). Instead, in this region (referred to as the Mountains), plants overwinter as underground rootstocks with or without a few short shoots protected from frost by the surrounding vegetation. The pathogen overwinters as limited numbers of dormant uredial infections on the occasional small green shoots. With the coming of spring, fresh shoots develop and plants flower in mid- to late-summer. During the growing period, the pathogen is visible on living host tissue as localised (non-systemic), orange-coloured uredial lesions. The wind-dispersed urediospores can infect either the same or different plants. This stage of the pathogen’s life cycle is asexual, with 6–8 uredial generations following one another in quick succession, leading to local epidemics. However, following the first autumn frosts, host plants die back and abrupt crashes in pathogen numbers occur.
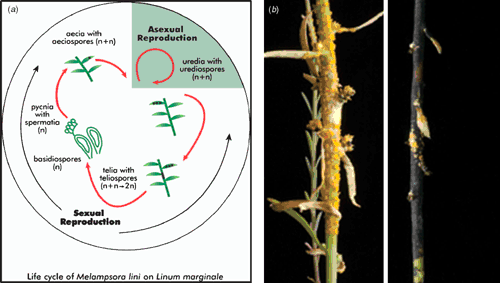
|
Phenological and epidemiological patterns in the Linum–Melampsora interaction in the Mountains are distinctly different to those occurring further west on the inland Plains. In the latter location, very hot dry summers and mild winters result in a virtual reversal of the pattern described above. Epidemics start earlier, often reach higher levels, and last longer in Plains populations with a slower decline than in the Mountains, implying greater disease risk in Plains than in mountain populations, and the potential for quite different selection regimes. In the Plains environment, telia (these spores are capable of surviving long periods of harsh conditions and are the precursor stage to the sexual cycle) are produced in large quantities as shoots dry out during the summer drought. We have identified sexual spore stages (aecia and pycnia; Fig. 5) in Plains populations, confirming that recombination occurs in this region (P. Thrall and L. G. Barrett, unpubl. data). Ongoing studies are quantifying the extent and importance of this process in structuring pathogen populations in both the Plains and Mountains regions. Thus, recent studies have shown that Plains pathogen populations are significantly more diverse, both genotypically and with regard to virulence expression, than the asexual Mountains populations (Barrett et al. 2008b).
Molecular analyses have also demonstrated that the pathogen populations in the Mountains region belong to a lineage that is genetically distinct from those occurring in the Plains (Barrett et al. 2007). Importantly, the Mountains lineage appears to be of hybrid origin, which directly influences a range of critical life history traits that determine disease development. Not only do these lineages appear to differ in both mating system and average virulence, but broader surveys of isolates from across Australia indicate that these lineages occur in physically different environments; one being primarily found in cool-temperate areas, typically with annual rainfall above 880 mm (e.g. Mountains populations) and the other confined to hotter drier inland locations (e.g. Plains populations) where the average annual rainfall is 640 mm or less. Ongoing studies are aimed at elucidating the impact of life-history differences on molecular diversity and patterns of selection in host resistance and pathogen virulence genes.
Like the cultivated flax system, disease outcomes in the wild host–pathogen association are governed by gene-for-gene interactions, and genetic analyses have demonstrated the occurrence of a minimum of 17 different genes or alleles for resistance in L. marginale (Burdon 1994). Populations of L. marginale are frequently composed of many different host resistance phenotypes (Burdon and Jarosz 1991; Thrall et al. 2001), and sympatric pathogen populations are also typically diverse although not infrequently they may be dominated by just a few pathotypes. Year-to-year fluctuations in the incidence and frequency of different pathotypes have provided strong circumstantial evidence for the effects of population bottlenecks and subsequent genetic drift (Burdon 1997). Detailed monitoring of disease incidence and severity on individual hosts over a series of years, where disease ranged from absent to epidemic levels, found high host mortality (70–80%) to be associated with severe epidemic conditions. In years of low disease, plant mortality was minimal (Jarosz and Burdon 1992). Thus, M. lini has the potential to impose strong selection on patterns of host resistance. Following the impact of a substantial epidemic, a large decline in overall host population size was also associated with a marked shift in host resistance structure such that the post-epidemic population was no longer dominated by just a few resistance phenotypes. Resistance phenotypes at high frequency in the pre-epidemic population declined significantly in the post-epidemic population, resulting in a post-epidemic population that was more genetically diverse (Burdon and Thompson 1995). In turn, among-population variation in host resistance is a major determinant of the severity of pathogen epidemics (Thrall and Burdon 2000).
Although these results demonstrate the basis for selective changes in host and pathogen populations, and thus the existence of a co-evolutionary interaction, the focus of this work was primarily on events occurring within individual populations. Surveys of multiple host and pathogen populations have revealed significant spatial structure in the distribution of resistance. Thus, within the Kiandra Mountains metapopulation, host populations in close proximity were more closely related with regard to the resistance phenotypes that were present than more distantly placed ones (Thrall et al. 2001). In contrast, reflecting the pathogen’s greater mobility, relatively little structure was detected at the same spatial scale in the virulence phenotypes present in pathogen populations. Glasshouse inoculation trials have demonstrated strong local adaptation by Melampsora to its host populations, with this effect being greatest at regional spatial scales, as predicted from the broader dispersal of M. lini relative to L. marginale (Thrall et al. 2002).
Results from these studies further demonstrate how co-evolutionary interactions between hosts and pathogens can be influenced by the resistance structure of local host populations with marked differences seen in the virulence structure of pathogen populations. Evidence from a range of populations indicates a strongly curvilinear relationship between average resistance and average virulence (Thrall and Burdon 2003) such that pathogen populations attacking more susceptible host populations are less virulent (carry more avirulence phenotypes) than those occurring on more resistant populations, a finding that is consistent with predictions derived from theoretical models of gene-for-gene interactions. At the same time, the high mobility of M. lini might be expected to result in all host populations being dominated by the most virulent pathogen strains. The fact that this is not the case suggests the possibility of an evolutionary trade-off between virulence and aggressiveness in this system (both are important determinants of whether pathogens can invade and spread) with more uniform host populations favouring the evolution of pathotypes of low virulence but high aggressiveness. Glasshouse inoculation studies confirmed a negative relationship between virulence and spore production such that pathotypes able to attack a broader range of resistance genes generally produced fewer spores/pustule (Thrall and Burdon 2003). This is likely due to a role of Avr genes (such as described above) in facilitating infection, such that loss or alteration of these genes has fitness costs for the pathogen. Although trade-offs between virulence and aggressiveness clearly have important implications for evolutionary dynamics and patterns of local adaptation in pathogen populations, these results further emphasise the importance of integrating population studies with molecular work on gene structure and function.
At larger geographic scales, differences in partitioning of host resistance within and among populations between the Plains and Mountains regions have also been detected (Burdon et al. 1999; Table 1). These appear to be associated with large-scale environmental (see above) and host mating system variability. For example, Linum populations in the Plains region exhibit significant levels of outcrossing while those in the Mountains are almost completely inbred. The consequences of such differences in host life history and genetic structure for disease epidemiology (and selection for sexual recombination in the pathogen) have yet to be assessed but may well reflect variation in the intensity and persistence of selective pressures leading to distinct hot and cold spots of co-evolutionary activity (Thompson 1994, 1999).
Overall, this work has demonstrated considerable variability in the resistance and virulence structure of host and pathogen populations and provides clear evidence for the selective force that pathogens may exert on host populations. It is likely that such within and among population processes play an important role in generating the observed patterns in the Linum–Melampsora system, and this is likely to also be the case for many other plant–pathogen interactions.
Molecular analysis of natural rust populations
Recently a cluster analysis of molecular markers (nine microsatellite plus amplified fragment length polymorphism (AFLP) markers) in 39 rust isolates collected from L. marginale hosts covering the host’s geographic range across Australia revealed that two genetically distinct lineages of M. lini occur on L. marginale (AA and AB; Barrett et al. 2007). Lineage AA isolates have both low genetic diversity and very low microsatellite heterozygosity at multiple loci. In contrast, hybrid lineage AB isolates show a fixed pattern of heterozygosity (i.e. a different allele at each of the two nuclei) at corresponding microsatellite loci. Most of the unique AFLP markers also occurred in this lineage. These lineage AB isolates consistently have one allele in common with lineage AA isolates, but also carry a second divergent allele not found in lineage AA. No intermediate genotypes have been identified to date. This suggests that this lineage is of hybrid origin between an AA strain and a highly divergent BB strain (now extinct or not yet identified). The high levels of fixed heterozygosity in the AB lineage suggest that sexual recombination (which would be expected to produce some progeny homozygous for the molecular markers) is not occurring in isolates of this lineage (Barrett et al. 2007). Geographically, lineage AB isolates mostly occur in southerly and coastal areas, reflecting an apparent preference for cooler and wetter areas than lineage AA isolates. AB isolates, which the molecular data suggest do not undergo sexual recombination, do form teliospores, but less frequently and at a slower rate both in the field and in controlled glasshouse tests compared with AA isolates. Furthermore, the two lineages have largely disjunct geographic distributions, and lineage AB isolates are on average nearly 20% more virulent than lineage AA isolates.
The availability of cloned Avr genes from M. lini now makes possible molecular population analysis of these genes in the natural infection system. All four Avr genes occur in wild isolates of M. lini and recent analysis shows evidence for selection acting on some of these genes in natural populations. The exception to this is AvrM, which appears to be fixed in these populations. All of the AA isolates contain a single AvrM allele closely related to variants seen in rust isolates from cultivated flax, while AB isolates also carry one additional more divergent AvrM gene variant, probably derived from the B genome (P. N. Dodds and P. Thrall, unpubl. data). Thus it is likely that no corresponding R gene exists in L. marginale that responds to this Avr gene.
In contrast, several variants of AvrL567 have been detected in different wild rust isolates, suggesting the possibility of selection operating at this locus, but the multicopy nature of this locus confounds genetic characterisation at the population level. However, the AvrP123 and AvrP4 loci each contain a single functional Avr gene and have been examined in more detail across M. lini populations (L. G. Barrett, P. H. Thrall, P. N. Dodds, M. van der Merwe, C. C. Linde, G. H. Lawrence, J. J. Burdon, unpubl. data). Extensive sequence variation at these avirulence loci was found across the range of isolates collected from L. marginale and these showed a significant excess of non-synonymous compared with synonymous polymorphism, suggesting that positive selection has contributed to the observed sequence diversity. Furthermore, comparative analyses among different genic regions revealed high levels of haplotype diversity and nucleotide variation at the Avr loci compared with neutral loci (β-tubulin intron and rRNA intergenic spacer sequences). We further characterised patterns of nucleotide variation at AvrP123 and AvrP4 in 10 local populations of M. lini infecting the wild host L. marginale. Populations were significantly and strongly differentiated in terms of allelic representation at the Avr loci, suggesting the possibility of local selection maintaining distinct genetic structures between populations. Transient expression assays showed that variants of both the AvrP123 and AvrP4 genes can induce an HR response in certain differential lines of L. marginale, indicating that corresponding R genes exist in this host which could apply selection pressure on these Avr loci. Expression assays have also showed that AvrP4 and AvrP123 recognition phenotypes vary between local populations of wild flax providing a potential driver of differences between local pathogen populations (P. Thrall and P. N. Dodds, unpubl. data). Together, these results imply that positive diversifying selection imposed by host resistance and acting across a broad range of scales is generating and maintaining virulence diversity in populations of M. lini. Further studies examining temporal changes in the frequency of resistance genes in the same L. marginale populations is needed to fully document the signature of co-evolution in this gene-for-gene host–pathogen interaction.
Concluding remarks
The flax rust disease system has been an excellent model for both molecular and population level studies of host–pathogen interaction. The identification of numerous corresponding R and Avr genes has yielded significant insights into the genetic and molecular basis of rust resistance and virulence mechanisms. Likewise the selective impact of the flax rust pathogen (Melampsora lini) on the survival and reproduction of individual host populations of Australian native flax (Linum marginale), and conversely the impact of host resistance diversity on the evolution of pathogen virulence has been clearly established. The combination of these molecular and population studies now has the potential to lead to exciting insights into the co-evolution of host resistance and pathogen virulence that encompasses the molecular basis of recognition events underlying phenotypic variability in host resistance and pathogen virulence, as well as the interplay of genetics and epidemiology at a population level. Insights from the flax rust system are also proving valuable for application to economically import rust diseases, such as wheat stem rust, where we are now able to predict the effector gene complement based on genome and haustorial cDNA sequences. This will facilitate Avr gene identification and understanding of how new virulent races arise in the field.
Akira S
(2003) Mammalian Toll-like receptors. Current Opinion in Immunology 15, 5–11.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Allen RL,
Bittner-Eddy PD,
Grenville-Briggs LJ,
Meitz JC,
Rehmany AP,
Rose LE, Beynon JL
(2004) Host-parasite co-evolutionary conflict between Arabidopsis and downy mildew. Science 306, 1957–1960.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Anderson PA,
Lawrence GJ,
Morrish BC,
Ayliffe MA,
Finnegan EJ, Ellis JG
(1997) Inactivation of the flax rust resistance gene M associated with loss of a repeated unit within the leucine-rich repeat coding region. The Plant Cell 9, 641–651.
|
CAS |
Crossref |
PubMed |

Armstrong MR,
Whisson SC,
Pritchard L,
Bos JI, Venter E , et al.
(2005) An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proceedings of the National Academy of Sciences of the United States of America 102, 7766–7771.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Axtell MJ, Staskawicz BJ
(2003) Initiation of RPS2-specified resistance in Arabidopsis is coupled to AvrRpt2-directed elimination of RIN4. Cell 112, 369–377.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Barrett LG,
Thrall PH, Burdon JJ
(2007) Evolutionary diversification through hybridization in a wild host–pathogen interaction. Evolution 61, 1613–1621.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Barrett LG,
Thrall PH,
Burdon JJ, Linde C
(2008a) Pathogen life-history and the evolutionary potential of populations. Trends in Ecology & Evolution 23, 678–685.
| Crossref | GoogleScholarGoogle Scholar |

Barrett LG,
Thrall PH,
Burdon JJ,
Nicotra AB, Linde CC
(2008b) Population structure and diversity in sexual and asexual populations of the pathogenic fungus Melampsora lini. Molecular Ecology 17, 3401–3415.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bergelson J,
Kreitman M,
Stahl EA, Tian D
(2001) Evolutionary dynamics of plant R-genes. Science 292, 2281–2285.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bhattacharjee S,
Hiller NL,
Liolio K,
Win J,
Kanneganti T-D,
Young C,
Kamoun S, Haldar K
(2006) The malarial host-targeting signal is conserved in the Irish potato famine pathogen. PLoS Pathogens 2, e50.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Birch PRJ,
Boevink PC,
Gilroy EM,
Hein I,
Pritchard L, Whisson SC
(2008) Oomycete RXLR effectors: delivery, functional redundancy and durable disease resistance. Current Opinion in Plant Biology 11, 373–379.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Burdon JJ
(1994) The distribution and origin of genes for race specific resistance to Melampsora lini in Linum marginale. Evolution 48, 1564–1575.
| Crossref |

Burdon JJ, Jarosz AM
(1991) Host–pathogen interactions in natural populations of Linum marginale and Melampsora lini: I. Patterns of resistance and racial variation in a large host population. Evolution 45, 205–217.
| Crossref | GoogleScholarGoogle Scholar |

Burdon JJ, Roberts JK
(1995) The population genetic structure of the rust fungus Melampsora lini as revealed by pathogenicity, isozyme and RFLP markers. Plant Pathology 44, 270–278.
| Crossref | GoogleScholarGoogle Scholar |

Burdon JJ, Thompson JN
(1995) Changed patterns of resistance in a population of Linum marginale attacked by the rust pathogen Melampsora lini. Journal of Ecology 83, 199–206.
| Crossref | GoogleScholarGoogle Scholar |

Burdon JJ, Thrall PH
(2000) Co-evolution at multiple spatial scales: Linum marginale-Melampsora lini from the individual to the species. Evolutionary Ecology 14, 261–281.
| Crossref | GoogleScholarGoogle Scholar |

Burdon JJ,
Thrall PH, Brown AHD
(1999) Resistance and virulence structure in two Linum marginale – Melampsora lini host–pathogen metapopulations with different mating systems. Evolution 53, 704–716.
| Crossref | GoogleScholarGoogle Scholar |

Burdon JJ,
Thrall PH, Lawrence GJ
(2002) Co-evolutionary patterns in the Linum marginale–Melampsora lini association at a continental scale. Canadian Journal of Botany 80, 288–296.
| Crossref | GoogleScholarGoogle Scholar |

Büttner D, Bonas U
(2003) Common strategies of plant and animal pathogenic bacteria. Current Opinion in Plant Biology 6, 312–319.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Catanzariti A-M,
Dodds PN,
Lawrence GJ,
Ayliffe MA, Ellis JG
(2006) Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. The Plant Cell 18, 243–256.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Catanzariti A-M,
Dodds PN, Ellis JG
(2007) Avirulence proteins from haustoria-forming pathogens. FEMS Microbiology Letters 269, 181–188.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chisholm ST,
Coaker G,
Day B, Staskawicz BJ
(2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dangl JL, Jones JDG
(2001) Plant pathogens and integrated defence responses to infection. Nature 411, 826–833.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Deslandes L,
Olivier J,
Peeters N,
Feng DX,
Khounlotham M,
Boucher C,
Somssich I,
Genin S, Marco Y
(2003) Physical interaction between RRS1-R-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proceedings of the National Academy of Sciences of the United States of America 100, 8024–8029.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dodds PN,
Lawrence GJ, Ellis JG
(2001a) Contrasting modes of evolution acting on the complex N locus for rust resistance in flax. The Plant Journal 27, 439–453.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dodds PN,
Lawrence GJ,
Pryor AJ, Ellis JG
(2001b) Six amino acid changes confined to the leucine-rich repeat β-strand/β-turn motif determine the difference between the P and P2 rust resistance specificities in flax. The Plant Cell 13, 163–178.
|
CAS |
Crossref |
PubMed |

Dodds PN,
Lawrence GJ,
Catanzariti A-M,
Ayliffe MA, Ellis JG
(2004) The Melampsora lini AvrL567 avirulence genes are expressed in haustoria and their products are recognized inside plant cells. The Plant Cell 16, 755–768.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Dodds PN,
Lawrence GJ,
Catanzariti A-M,
Teh T,
Wang CA,
Ayliffe MA,
Kobe B, Ellis JG
(2006) Direct protein interaction underlies gene-for-gene specificity and co-evolution of the flax resistance genes and flax rust avirulence genes. Proceedings of the National Academy of Sciences of the United States of America 103, 8888–8893.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Panstruga R, Dodds PN
(2009) Terrific protein traffic: the mystery of how filamentous pathogens deliver effectors into host plant cells. Science in press. ,
| PubMed |

Dou D,
Kale SD,
Wang X,
Jiang RHY,
Bruce NA,
Arredondo FD,
Zhang X, Tyler BM
(2008) RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen encoded machinery. The Plant Cell 20, 1930–1947.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ellis JG, Dodds PN
(2003) Plant disease resistance: monitoring a pathogen targeted host protein. Current Biology 13, R400–R402.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ellis JG,
Lawrence GJ,
Luck JE, Dodds PN
(1999) Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. The Plant Cell 11, 495–506.
|
CAS |
Crossref |
PubMed |

Ellis JG,
Dodds PN, Lawrence GJ
(2007) The role of secreted proteins in diseases of plants caused by rusts, powdery mildew and smut fungi. Current Opinion in Microbiology 10, 326–331.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Endo T,
Ikeo K, Gojobori T
(1996) Large scale search for genes on which positive selection may operate. Molecular Biology and Evolution 13, 685–690.
|
CAS |
PubMed |

Flor HH
(1971) Current status of the gene-for-gene concept. Annual Review of Phytopathology 9, 275–296.
| Crossref | GoogleScholarGoogle Scholar |

Girardin SE,
Travassos LH,
Nerve M,
Blanot D,
Boneca IG,
Philpott DJ,
Sansonetti PJ, Mengin-Lecreulx D
(2003) Peptidoglycan molecular requirements allowing detection by Nod 1 and Nod 2. Journal of Biological Chemistry 278, 41702–41708.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hahn M, Mendgen K
(1992) Isolation by ConA binding of haustoria from different rust fungi and comparison of their surface qualities. Protoplasma 170, 95–103.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hahn M, Mendgen K
(2001) Signal and nutrient exchange at biotrophic plant–fungus interfaces. Current Opinion in Plant Biology 4, 322–327.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Heath MC
(1997) Signalling between pathogenic rust fungi and resistant or susceptible host plants. Annals of Botany 80, 713–720.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hiller NL,
Bhattacharjee S,
van Ooij C,
Liolios K,
Harrison T,
Lopez-Estraño C, Haldar K
(2004) A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306, 1934–1937.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hughes AL, Nei M
(1988) Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335, 167–170.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hwang C-F, Williamson VM
(2003) Leucine-rich repeat-mediated intramolecular interactions in nematode recognition and cell death signalling by the tomato resistance protein Mi. The Plant Journal 34, 585–593.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jarosz AM, Burdon JJ
(1991) Host–pathogen interactions in natural populations of Linum marginale and Melampsora lini: II Local and regional variation in patterns of resistance and racial structure. Evolution 45, 1618–1627.
| Crossref | GoogleScholarGoogle Scholar |

Jarosz AM, Burdon JJ
(1992) Host–pathogen interactions in natural populations of Linum marginale and Melampsora lini: III Influence of pathogen epidemics on host survivorship and flower production. Oecologia 89, 53–61.
| Crossref | GoogleScholarGoogle Scholar |

Jia Y,
McAdams SA,
Bryan GT,
Hershey HP, Valent B
(2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO Journal 19, 4004–4014.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jones JDG, Dangl JL
(2006) The plant immune system. Nature 444, 323–329.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kamoun S
(2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annual Review of Phytopathology 44, 41–60.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kemen E,
Kemen AC,
Rafiqi M,
Hempel U,
Mendgen K,
Hahn M, Voegele RT
(2005) Identification of a protein from rust fungi transferred from haustoria into infected plant cells. Molecular Plant–Microbe Interactions 18, 1130–1139.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kobayashi I,
Kobayashi Y, Hardham AR
(1994) Dynamic reorganisation of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195, 237–247.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kobayashi I,
Murdoch LJ,
Kunoh H, Hardham AR
(1995) Cell biology of early events in the resistance response to infection by pathogenic fungi. Canadian Journal of Botany 73, 418–425.
| Crossref | GoogleScholarGoogle Scholar |

Kobayashi I,
Kobayashi Y, Hardham AR
(1997) Inhibition of rust-induced hypersensitive response in flax cells by the microtubule inhibitor oryzalin. Australian Journal of Plant Physiology 24, 733–740.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lawrence GJ, Burdon JJ
(1989) Flax rust from L. marginale: variation in a natural host–pathogen interaction. Canadian Journal of Botany 66, 3192–3198.

Lawrence GJ,
Shepherd KW, Mayo GME
(1981) Fine structure of genes controlling pathogenicity in flax rust, Melampsora lini. Heredity 46, 297–313.
| Crossref | GoogleScholarGoogle Scholar |

Lawrence GJ,
Finnegan EJ,
Ayliffe MA, Ellis JG
(1995) The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. The Plant Cell 7, 1195–1206.
|
CAS |
Crossref |
PubMed |

Lawrence GJ,
Dodds PN, Ellis JG
(2007) Rust of flax and linseed caused by Melampsora lini. Molecular Plant Pathology 8, 349–364.
| Crossref | GoogleScholarGoogle Scholar |

Lawrence GJ,
Anderson PA,
Dodds PN, Ellis JG
(2009) Relationships between rust resistance genes at the M locus in flax. Molecular Plant Pathology , in press.

Luck JE,
Lawrence GJ,
Dodds PN,
Shepherd KW, Ellis JG
(2000) Regions outside of the leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. The Plant Cell 12, 1367–1378.
|
CAS |
Crossref |
PubMed |

Mackey D,
Holt BF,
Wiig A, Dangl JL
(2002) RIN4 interacts with Pseudomonas syringae type III efector molecules and is required for RPM1-mediated resistance in Arabidopisis. Cell 108, 743–754.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mackey D,
Belkhadir Y,
Alonso JM,
Ecker JR, Dangl JL
(2003) Arabidopsis RIN4 is a target of the type III effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Marti M,
Good RT,
Rug M,
Knuepfer E, Cowman AF
(2004) Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306, 1930–1933.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mauricio R,
Stahl EA,
Korves T,
Tian D,
Kreitman M, Bergelson J
(2003) Natural selection for polymorphism in the disease resistance gene RPS2 of Arabidopsis thaliana. Genetics 163, 735–746.
|
CAS |
PubMed |

Michelmore RW, Meyers BC
(1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Research 8, 1113–1130.
|
CAS |
PubMed |

Moffett P,
Farnham G,
Peart J, Baulcombe DC
(2002) Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO Journal 21, 4511–4519.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mondragon-Palomino M,
Meyers B,
Michelmore RW, Gaut BS
(2002) Patterns of positive selection in the coplete NBS-LRR family of Arabidopsis thaliana. Genome Research 12, 1305–1315.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Murdoch LJ, Hardham AR
(1998) Components in the haustorial wall of the flax rust fungus, Melampsora lini, are labelled by three anti-calmodulin monoclonal antibodies. Protoplasma 201, 180–193.
| Crossref | GoogleScholarGoogle Scholar |

Murdoch LJ,
Kobayashi I, Hardham AR
(1998) Production and characterisation of monoclonal antibodies to cell wall components of the flax rust fungus. European Journal of Plant Pathology 104, 331–346.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Nei M,
Gu X, Sitnikova T
(1997) Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proceedings of the National Academy of Sciences of the United States of America 94, 7799–7806.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Nurnberger T,
Brunner F,
Kemmerking B, Piater L
(2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Reviews 198, 249–266.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Orth K,
Xu Z,
Mudgett MB,
Bao ZQ,
Palmer LE,
Bliska JB,
Mangel WF,
Staskawicz BJ, Dixon JE
(2000) Disruption of signalling by Yersinia effector YopJ, A ubiquitin-like protein protease. Science 290, 1594–1597.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Panstruga R
(2003) Establishing compatibility between plants and obligate biotrophic pathogens. Current Opinion in Plant Biology 6, 320–326.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Parniske M,
Hammond-Kosack KE,
Golstein C,
Thomas CM,
Jones DA,
Harrison K,
Wulff BBH, Jones JDG
(1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated gene at the Cf-4/9 locus of tomato. Cell 91, 821–832.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Perfect SE, Green JR
(2001) Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Molecular Plant Pathology 2, 101–108.
| Crossref | GoogleScholarGoogle Scholar |

Rehmany AP,
Gordon A,
Rose LE,
Allen RL,
Armstrong MR,
Whisson SC,
Kamoun S,
Tyler BM,
Birch PRJ, Beynon JL
(2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. The Plant Cell 17, 1839–1850.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Shan W,
Cao M,
Leung D, Tyler BM
(2004) The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Molecular Plant-Microbe Interactions 17, 394–403.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Shao F,
Golstein C,
Ade J,
Stoutemyer M,
Dixon JE, Innes RW
(2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301, 1230–1233.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Stahl EA,
Dwyer G,
Mauricio R,
Kreitman M, Bergelson J
(1999) Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400, 667–671.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Staskawicz BJ,
Mudgett MB,
Dangl JL, Galan JE
(2001) Common and contrasting themes of plant and animal diseases. Science 292, 2285–2289.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thompson JN
(1999) Specific hypotheses on the geographic mosaic of co-evolution. American Naturalist 153, S1–S14.
| Crossref | GoogleScholarGoogle Scholar |

Thrall PH, Burdon JJ
(2000) Effects of resistance variation in a natural plant host–pathogen metapopulation on disease dynamics. Plant Pathology 49, 767–773.
| Crossref | GoogleScholarGoogle Scholar |

Thrall PH, Burdon JJ
(2003) Evolution of virulence in a plant host–pathogen metapopulation. Science 299, 1735–1737.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thrall PH,
Burdon JJ, Young AG
(2001) Variation in resistance and virulence among demes of a plant host–pathogen metapopulation. Journal of Ecology 89, 736–748.

Thrall PH,
Burdon JJ, Bever JD
(2002) Local adaptation in the Linum marginale–Melampsora lini host–pathogen interaction. Evolution 56, 1340–1351.
| PubMed |

Tyler BM
(2009) Entering and breaking: virulence effector proteins of oomycete plant pathogens. Cellular Microbiology 11, 13–20.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ueda H,
Yamaguchi Y, Sano H
(2006) Direct Interaction between the tobacco mosaic virus helicase domain and the ATP-bound resistance protein, N factor during the hypersensitive response in tobacco plants. Plant Molecular Biology 61, 31–45.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

van der Hoorn RAL, Kamoun S
(2008) From guard to decoy: a new model for perception of plant pathogen effectors. The Plant Cell 20, 2009–2017.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

van der Hoorn RAL,
de Wit PJGM, Joosten MHAJ
(2002) Balancing selection favors guarding resistance proteins. Trends in Plant Science 7, 67–71.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Voegele RT, Mendgen K
(2003) Rust haustoria: nutrient uptake and beyond. New Phytologist 159, 93–100.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Wang C-IA,
Gunčar G,
Forwood JK,
Teh T, Catanzariti A-M , et al.
(2007) Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. The Plant Cell 19, 2898–2912.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Whisson SC,
Boevink PC,
Moleleki L,
Avrova AO, Morales JG , et al.
(2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450, 115–118.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

White SN,
Taylor KH,
Abbey CA,
Gill CA, Womack JE
(2003) Haplotype variation in bovine Toll-line receptor 4 and computational prediction of a positively selected ligand-binding domain. Proceedings of the National Academy of Sciences of the United States of America 100, 10364–10369.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



