Evans Review: Cell wall biosynthesis and the molecular mechanism of plant enlargement
John S. BoyerCollege of Marine and Earth Studies and College of Agriculture and Natural Resources, University of Delaware, Lewes, DE 19958, USA. Email: boyer@udel.edu
Functional Plant Biology 36(5) 383-394 https://doi.org/10.1071/FP09048
Submitted: 27 February 2009 Accepted: 24 March 2009 Published: 6 May 2009
Abstract
Recently discovered reactions allow the green alga Chara corallina (Klien ex. Willd., em. R.D.W.) to grow well without the benefit of xyloglucan or rhamnogalactan II in its cell wall. Growth rates are controlled by polygalacturonic acid (pectate) bound with calcium in the primary wall, and the reactions remove calcium from these bonds when new pectate is supplied. The removal appears to occur preferentially in bonds distorted by wall tension produced by the turgor pressure (P). The loss of calcium accelerates irreversible wall extension if P is above a critical level. The new pectate (now calcium pectate) then binds to the wall and decelerates wall extension, depositing new wall material on and within the old wall. Together, these reactions create a non-enzymatic but stoichiometric link between wall growth and wall deposition. In green plants, pectate is one of the most conserved components of the primary wall, and it is therefore proposed that the acceleration-deceleration-wall deposition reactions are of wide occurrence likely to underlie growth in virtually all green plants. C. corallina is one of the closest relatives of the progenitors of terrestrial plants, and this review focuses on the pectate reactions and how they may fit existing theories of plant growth.
Additional keywords: calcium, Chara corallina, gel, growth, irreversible deformation, pectate, pectin, tension, turgor pressure.
Introduction
The ability to grow larger is one of the most important activities of plants. All of development depends on it because cells divide as development occurs, and growth is part of the division cycle. After division, young plant cells generally enlarge further by 10- to 1000-fold before they mature. The latter enlargement controls most of the growth and shape of organs, allowing plants to reach the light, acquire nutrients and water, and develop reproductive structures. Reproductive success thus depends on the underlying enlargement process, giving it a primary significance for evolution.
This review considers plant enlargement in the knowledge of recently discovered reactions linking the process to cell wall biosynthesis. The reactions depend on unique chemistry, and the aim is to explore ways the reactions might fit the wider contexts of plant biology. Helpful early reviews describe physiological and biochemical aspects of enlargement and cell wall structure (Northcote 1969; Cleland 1971; Preston 1974; Taiz et al. 1981; Taiz 1984; Ray 1992; Carpita and Gibeaut 1993). Later reviews focus on gene expression, and understanding the formation of wall precursors (Carpita et al. 1996; Darley et al. 2001; Scheible and Pauly 2004; Seifert 2004; Somerville et al. 2004; Saxena and Brown 2005; Bacic 2006; Farrokhi et al. 2006; Lerouxel et al. 2006; Somerville 2006). Excellent overviews are given by Kutschera (2001), Fry (2004) and Cosgrove (2005). But mostly missing from these treatments are the enigmatic later steps in wall assembly and the specific chemistry controlling rates of enlargement. How does the solid composite wall extend while more polymeric material is integrated into itself? What are the specific chemical structures controlling the extension? Answers to these questions are important, and are beginning to emerge.
Background
Cells enlarge under the control of the tough cell wall, termed the primary wall, which is extended by cell wall tension produced by the turgor pressure (P) inside the cell. Normally, P is rather high, around 0.5 MPa or more, which is equivalent to two to three times the pressure in an automobile tyre. When P is sufficiently high, the wall extends both irreversibly and reversibly, which enlarges the cell compartment. The irreversible extension is considered to be ‘growth’. While the extension occurs, new material is simultaneously synthesised and deposited in the wall. Maintaining wall thickness is essential because enlargement of 10- to 1000-fold would make the wall too thin to hold P. In most plant cells, the thickness remains the same within a factor of about two (Taiz 1984; Kutschera 1990; and references therein; Bret-Harte et al. 1991). Consequently, in the living cell, wall enlargement and wall biosynthesis go together.
The primary wall is an intricate, largely noncovalent network of polymers. Cellulose microfibrils are cross-linked by hydrogen bonds to a matrix of hemicellulose intercalated with pectins and a small amount of structural protein (e.g. Preston 1974; Carpita and Gibeaut 1993; McCann and Roberts 1994; Fry 2004; Cosgrove 2005). The microfibrils have a high tensile strength, and are often laid down in an orientation perpendicular to the long axis of the cell. With this orientation, lateral extension is inhibited and the wall extends mostly lengthwise (Probine and Preston 1962; Baskin 2001, 2005). The longitudinal extension stretches the matrix between the microfibrils, spreading the microfibrils apart. The orientation of the microfibrils thus controls organ shape while the matrix controls growth rate.
The later steps in this process have remained enigmatic in part because of the difficulty in controlling P. In terrestrial plants, enlarging cells are small, densely packed, and considerably distant from a vascular supply. Gradients in water potential and P are required to move water and solutes from the distant vessels to the enlarging cells to feed the growth process (Boyer and Silk 2004). Because the source of the gradients is in the enlarging cells, the gradients are considered growth-induced (Boyer 2001). If the tissues are then excised, P can change and alter the gradients, creating an uncontrolled variable affecting enlargement. P-dependent enlargement is obscured by these changes.
New approaches
Because of this situation, single algal cells have proven useful for enlargement studies. Water and solute surround the cells, causing growth-induced water potentials to be negligible (Zhu and Boyer 1992). P can be controlled by inserting the tip of a microcapillary into the cell and injecting or withdrawing cell solution without altering any other property in or around the live cell (Zhu and Boyer 1992). An important feature is the ability to conduct manipulations in the culture medium, including isolating the wall, mounting it on a microcapillary, and supplying or injecting diverse molecules (Proseus and Boyer 2005, 2006a). All metabolic and enzymatic functions of the live cell or isolated wall are preserved with this approach.
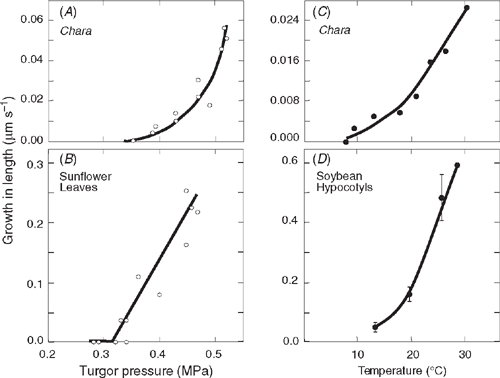
P-dependent elongation becomes obvious with this technique in a single cell of the large-celled green alga Chara corallina (Klien ex. Willd., em. R.D.W.) (Fig. 1A). The elongation resembles the growth of leaves on intact plants (Fig. 1B). Elongation begins above a critical P and increases when P increases further. Growth occurs over a similar range of P in both organisms. Likewise, the temperature response of growth in a single cell of Chara (Fig. 1C) resembles that of terrestrial species such as soybean (Glycine max L. Merr.; Fig. 1D). Growth decreases similarly in both species as temperature is lowered. These similarities probably occur because charophytes are the closest relatives of the algal progenitors of terrestrial plants (Chapman 1985; Graham 1985; Niklas 1992; Scherp et al. 2001). Note, however, that the terrestrial plants grow 5–20 times faster than Chara when P and T are favourable (cf. rates on the y-axes of Fig. 1).
|
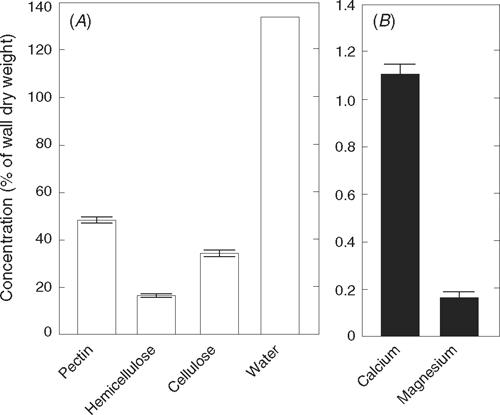
The quantities of pectin, hemicellulose, and cellulose in primary walls of charophytes (Fig. 2A) resemble those in terrestrial plants (Anderson and King 1961; Preston 1974). The cellulose (β-1,4-glucan) is in crystalline microfibrils surrounded by a matrix of pectin and hemicellulose that is gel-like. The pectin of charophytes is composed of polygalacturonic acid (pectate, i.e. α-1,4-linked galacturonic acid with small amounts of rhamnose) and minor quantities of polyglucuronic acid (Preston 1974; Morrison et al. 1993; Popper and Fry 2003). Few, if any, of the carboxyl groups are methylesterified, leaving them free to be weak acids (Anderson and King 1961; Morrison et al. 1993). Ca2+ forms cross-links with these carboxyl groups, and provided there are regions of at least 10–20 consecutive unmethylated galacturonic carboxyls in neighbouring pectate molecules, clusters of two or possibly four molecules will form non-covalent interpolymer associations with divalent cations (Grant et al. 1973; Morris et al. 1982; Powell et al. 1982; Jarvis 1984; Jarvis and Apperley 1995; Ralet et al. 2001; Willats et al. 2001). The associations create strong junction zones if Ca2+ is the cross-linking ion, and a gel can result (Jarvis 1984; Jarvis and Apperley 1995; Willats et al. 2001). Mg2+ does not form a gel (Proseus and Boyer 2006c). Calcium is abundant in Chara walls, and magnesium is detected (Fig. 2B). In primary walls of charophytes, if calcium is removed, the wall begins to disassemble (Gillet and Liners 1996).
|
The pectin rhamnogalactan II that complexes boron in terrestrial walls appears to be absent in Chara (Matsunaga et al. 2004; O’Neill et al. 2004). Also in contrast to the primary walls of terrestrial plants, Chara walls lack isoprimeverose (xylosyl-α-(1→6)-glucose) and thus a fundamental unit of xyloglucans (β-1,4-linked glucan with α-D-xylosyl side branches) (Popper and Fry 2003). Instead, the hemicellulose contains xylose-rich polymers whose structures remain to be elucidated.
The crystalline cellulose does not contain water, but there is considerable water in the interstices of the gel-like matrix (Fig. 2A). Whether in Chara (Shepherd and Goodwin 1989; Proseus and Boyer 2005) or terrestrial species (Read and Bacic 1996), the water-filled interstices have diameters of ~4–5 nm. Small molecules like glucose (0.42 nm) or calcium (hydrated diameter of 0.88 nm) readily pass through, but most polysaccharide polymers are much larger and do not enter unless P is present (Proseus and Boyer 2005). Taken together, these observations suggest that the structure of the charophyte wall is a simplified version of the terrestrial wall (Anderson and King 1961; Preston 1974; Morrison et al. 1993; Popper and Fry 2003).
Nature of growth-controlling bonds
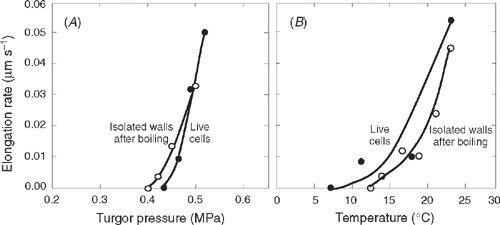
When primary cell walls are isolated from Chara, filled with oil in place of cytoplasm, and pressurised to the P normally found in the live cells (0.5 MPa), they grow nearly as rapidly as the live cells for 1–2 h (Proseus and Boyer 2006b). Even when the isolated walls are boiled for 10 min and cooled before pressurisation, their growth rates are similar to those in the live cells (Fig. 3). Since wall enzymes are highly unlikely to survive this treatment [except in extremophiles, see Van den Burg et al. (1998)], it seems safe to conclude that growth is controlled by a non-enzymatic reaction in the wall itself. The reaction depends on P (Fig. 3A) and T (Fig. 3B) in the same fashion as in the live cell (Fig. 3) and in terrestrial species (Fig. 1B, D). P and T responses of the live cell are thus controlled by the P and T responses in the wall without the activity of enzymes. This remarkable result indicates that growth is a non-enzymatic activity in Chara, and the cytoplasm and wall enzymes appear to play no part other than to determine wall structure (Proseus and Boyer 2006b).
|
Another consequence of this T-responsive growth is that it resembles the T response of metabolism or enzymes. Indeed, as recently as 2000, Proseus et al. (2000) used the T response of live Chara cells to conclude that growth was under metabolic control. It is now clear that this was likely incorrect and the T response is an inherent property of a non-enzymatic bond in the wall structure (Proseus and Boyer 2006b). The bond is weaker than covalent or electrostatic ones that require enzymes to lower their activation energy for metabolic reactions. The activation energy for the growth-controlling reaction is inherently moderate and allows reasonable rates of reaction without enzyme mediation.
Others have reported supporting results. Metraux and Taiz (1977) and Taiz et al. (1981) in Nitella, and Tepfer and Cleland (1979) in the chlorophyte alga Valonia, report no effect of boiling the walls on the ability of acidic media to promote elongation. But oat coleoptiles that were boiled (Tepfer and Cleland 1979) or maize coleoptiles that were exposed to metabolic inhibitors or destruction of the protoplast (Ding and Schopfer 1997) lost their responses to acid. Boiling cucumber hypocotyls for 15 min or exposing them to proteases irreversibly inhibited wall extension (Cosgrove 1989). It seems that enzyme activity may affect the growth of terrestrial species, perhaps because there are wall bonds not present in charophytic algae.
What is the nature of the non-enzymatic but growth-controlling bond in Chara? The rapid growth of isolated Chara walls is not sustained beyond 1–2 h, and soon becomes slow while the live cells continue to grow rapidly (Proseus and Boyer 2006b). Evidently, the cytoplasmic contribution to wall structure is necessary for sustained wall growth. The cytoplasm supplies precursors for wall biosynthesis, and when Proseus and Boyer (2006b) added pectate to the culture medium around pressurised walls of Chara, it rejuvenated growth. The rejuvenating action lasted for several h and required only a low pectate concentration (35 µm K-pectate having a molecular weight of 170 kDa, supplied to the culture medium at pH 7).
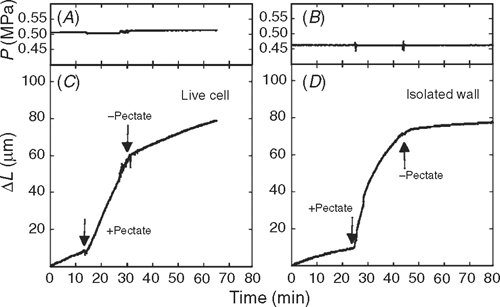
Because pectate is a prominent wall constituent, the cytoplasm supplies it to the wall for wall biosynthesis, and the rejuvenation is likely to occur in vivo. Consistent with this prediction, pectate supplied to live cells caused their growth to accelerate (Fig. 4A, B). Similar behaviour was seen in isolated walls (Fig. 4C, D). The effect required pectate because removing pectate and returning the original medium caused growth to revert to its original rate (Fig. 4). These results indicate that the cytoplasm sustains the growth-controlling reaction in the wall by providing a supply of precursors, specifically pectate, for wall biosynthesis.
|
But how could such a system work? Did the new pectate intercalate within the structure of the existing wall, taking up space between the polymers and thus expanding the matrix? This is unlikely because Proseus and Boyer (2005) report no spontaneous polysaccharide entry into the wall if the solution is at atmospheric pressure and the molecular weights are ~38 kDa or larger, as in this experiment. This rules out intercalation (i.e. intussusception) as a mechanism of pectate action. Also, because enzymes had no effect on wall growth, enzymatic mechanisms are ruled out. Without intussusception or enzyme activity, it seems possible that pectate might act chemically, directly on the bonds controlling the P and T responses of the cells in Fig. 3.
The latter possibility is supported by the action of newly supplied pectate on the calcium and magnesium in the wall (Proseus and Boyer 2006c). New pectate removed these elements from the wall, and similar but larger losses occurred when the wall was exposed to low concentrations of EGTA (2.5 mm, pH 7). Because EGTA is a strong chelator of calcium, pectate appeared to be acting as a chelator. Indeed, growth accelerated with EGTA in the same way as with the pectate (Proseus and Boyer 2006c). Moreover, the EGTA completely removed magnesium from the wall without loss in wall integrity. Some calcium remained, suggesting that wall magnesium was inactive in wall growth while calcium was important.
If so, re-supplying Ca2+ without pectate should replace the lost calcium in the wall and cause growth to revert to the original rate before new pectate was supplied. As shown in the experiment in Fig. 4, the fresh culture medium washed away the pectate, and Ca2+ normally in the medium caused the growth to revert to its original rate. The culture medium had a concentration of 0.61 mm Ca2+, and, therefore, only a low concentration of Ca2+ was necessary to replace that lost from the wall. Similar results were obtained with EGTA in live cells (Proseus and Boyer 2006c). Hence, pectate newly supplied by the cytoplasm acts to remove calcium from the growth-controlling bond in the cell wall, and the culture medium acts to re-supply it.
During biosynthesis, the new pectate is in the cytoplasm and exposed only to low Ca2+ concentrations, typically sub-micromolar around 0.1 µm (Clarkson and Hanson 1980; Hepler and Wayne 1985). In Chara, the calcium pump secretes Ca2+ at a rate of 0.078 nmol m−2 s−1 (Reid and Smith 1992), or ~1% of the rate of uronic acid delivery to the periplasm as pectate (~7.2 nmol m−2 s−1, calculated from Appendix 2 by Proseus and Boyer 2006c). Consequently, pectate is delivered to the wall essentially without Ca2+, perhaps as a K+ salt.
The wall is where new pectate first meets Ca2+ in significant concentrations. Sentenac and Grignon (1981) calculate a high concentration of Ca2+ in the interstitial water of cell walls. Newly synthesised pectate would bind this Ca2+ moderately strongly, with a pKd for dissociation of 3.5 (Van Cutsem and Gillet 1983; Jarvis 1984). Because of the dissociation, the new pectate would exchange calcium continually with the interstitial Ca2+ and the bound wall calcium. This exchange would cause the gel structure of the wall matrix to be dynamic, able to re-orient, and have a tendency to slip when placed under sufficient tension from P.
Tension and the molecular mechanism of cell enlargement
Given that calcium pectate seems to be the molecular site controlling the growth rate of these cells, it becomes important to consider how P affects the site. When P rises in cells, it puts wall polymers in tension. Proseus and Boyer (2006c) filled isolated walls with oil where the cytoplasm had been and created tension by pressurising the oil to P of 0.5 MPa. When as much as 95% of the wall calcium was removed, the walls grew rapidly but remained intact. This indicated that only 5% of the calcium pectate bonds were capable of bearing the entire tension from P in the wall matrix. If more than 95% of the calcium was removed, massive elongation occurred and the isolated wall soon broke, leaking the oil to the outside (Proseus and Boyer 2006c). P fell to zero. Without these few bonds, wall integrity was lost. Because they could bear most of the load from P, it follows that they also controlled irreversible wall deformation by P and thus growth. New pectate must have acted on them.
When the tension was varied by varying P in isolated Chara walls, newly supplied pectate was active only when P was above a critical value (Proseus and Boyer 2007). If P was below 0.2 MPa, newly supplied pectate had no growth-accelerating activity. In other words, tension appeared to make the load-bearing bonds susceptible to calcium loss. Removing their calcium would decrease the cross-bridging between neighbouring pectate molecules, weaken the load-bearing cross bridges, and allow polymer slippage that irreversibly deformed the wall.
Proseus and Boyer (2007) suggested that the ladder-like structure of pectate might explain this action (Fig. 5A). The rungs of the ladder are galacturonic acid and the single side rail is the α-(1–4) oxygenic bond connecting the rungs (Ridley et al. 2001). The side rail alternates from one side of the ladder to the other between rungs. The carboxyl groups also alternate. As a result, two anti-parallel ladders create an ‘egg-box’ structure that appears to sequester Ca2+ with coordination bonds as in Fig. 5B, shown for two calcium cross-links (Grant et al. 1973; Morris et al. 1982; Powell et al. 1982; Jarvis 1984; Jarvis and Apperley 1995; Ralet et al. 2001; Willats et al. 2001). When 10–20 of these calcium cross-links are adjacent on the ladder, a junction zone forms that is strong enough to create a gel.
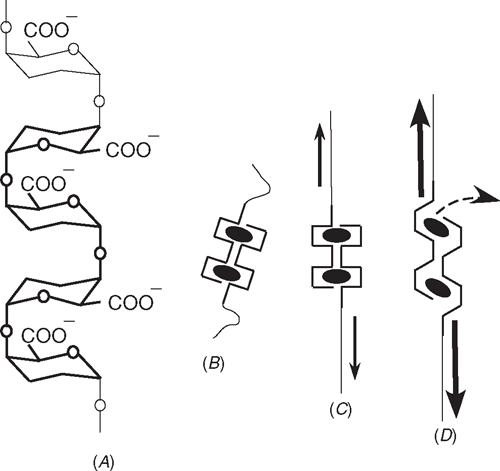
|
Proseus and Boyer (2007) suggested that the ladder structure would be susceptible to distortion by P. At low P, the ladder would straighten (Fig. 5C). Beyond a critical P, distortion would increase the distance between adjacent galacturonic residues as in Fig. 5D and lengthen the coordination bonds with calcium. The lengthening would weaken them and reduce the pKd for dissociation below 3.5. In thermodynamic terms, calcium in the wall would exist in a mixed population of undistorted (pKd 3.5) and a few distorted calcium pectate bonds (pKd lower than 3.5). Calcium would tend to migrate towards the undistorted bonds because of their higher affinity for calcium. Newly-supplied pectate is undistorted, so it would remove calcium most easily from the distorted bonds having low pKd. The number of distorted bonds would decrease per unit area of wall, causing growth to accelerate.
This distortion model generates several predictions. First, there should be two kinds of calcium pectate bonds in the cell wall at normal P, a few bonds that are distorted (Fig. 5D) and many that are not (Fig. 5B, C). Proseus et al. (1999) and Proseus and Boyer (2006b) detected two kinds of wall bonds when P stretched the wall: weak ones that controlled coiling and orientation of wall polymers (reversible elastic), and strong ones that controlled irreversible deformation of the wall (irreversible growth). The strong ones inevitably required a critical tension (critical P) before they could be broken, as in Fig. 3A.
A second prediction is that a fraction of the pectate should become oriented when charophyte walls are placed under tension. Using optical methods, Morikawa and Senda (1974) and Morikawa et al. (1974) found that some of the wall pectate aligned parallel to the long axis of the cell if tension was applied to isolated walls of Nitella. Richmond et al. (1980) similarly observed increasing order in Nitella walls when placed under tension.
A third prediction is that growth acceleration should be progressive. More acceleration should be observed when tension increases incrementally, because more of the calcium pectate bonds should become distorted. This would create a progressively steeper growth response as P increased, which was observed (Figs 1A, 3A) and also seen when new pectate was supplied (Proseus and Boyer 2007). Thus, higher P weakens more cross-links.
A fourth prediction is that wall polymers distorted by tension relax when calcium is removed. After relaxing, the polymers would remove calcium from other distorted chains. Wall calcium would migrate throughout the pectate matrix. This auto-catalytic action would cause acceleration to occur for long times, as observed by Proseus and Boyer (2006b).
Finally, Proseus and Boyer (2006c) found that auto-catalysis by new pectate could be quenched by high Ca2+ concentrations. This demonstrated that flooding the new pectate with externally supplied Ca2+ could stop removal of calcium from distorted wall pectate. Basically, the Ca2+ bound to the 170 kDa pectate, preventing its accelerating action but also causing it to gel in and around the wall. This 2-fold action of high Ca2+ concentrations revealed that newly-supplied pectate acted on distorted wall pectate and accelerated growth and, after binding Ca2+ and gelling, the new pectate also decelerated growth. Indeed, when saturated with Ca2+, the junction zones were so numerous that they could prevent growth.
Tension-dependent pectate cycle
The rate of wall biosynthesis determines how rapidly new pectate is supplied to the wall. Assuming each load-bearing junction zone contains 10 Ca2+ ions bound to two pectate molecules, the overall biosynthesis reaction for junction zones in wall pectate would be:

where the substrates are soluble pectate from the cytoplasm (probably as acid or potassium salt) and Ca2+ ions from the culture medium. The product is two anti-parallel chains of pectate cross-linked by calcium in the wall to form a junction zone.
But the junction zone also is the site of the P-dependent removal of calcium from load-bearing bonds. The overall reaction (1) thus includes several subreactions when P is high enough to distort the junction zones (i.e. above 0.2–0.3 MPa in Chara). Exposing the distorted pectate to new, undistorted pectate gives subreaction 1:
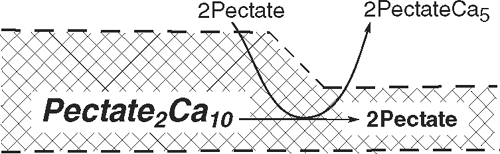
where a distorted junction zone (Pectate2Ca10) gives up its calcium to new pectate from the cytoplasm (2Pectate outside of the crosshatched wall). The junction zone in the wall dissociates to 2Pectate, and loses its distortion as it relaxes. Wall growth accelerates because the number of load-bearing (distorted) junction zones has decreased, weakening (loosening) the wall and allowing slippage under tension from P. This wall deformation is irreversible, and the wall becomes thinner.
The relaxed 2Pectate is able to bind calcium more tightly, and Ca2+ is absorbed from the external medium leading to subreaction 2:
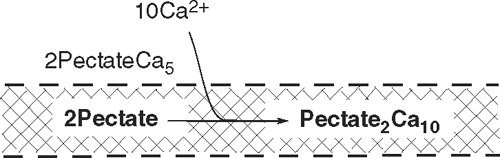
which re-forms the junction zone and re-tightens the structure. Growth decelerates, but the junction zones are now spread over a larger wall area because of the previous wall deformation, and the wall remains thin.
At the same time, the newly formed 2PectateCa5 of subreactions 1 and 2 (outside of the wall) can bind to the wall in subreaction 3:
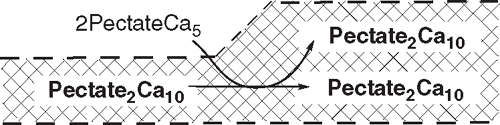
This new wall material adds a junction zone, returning the density of junction zones to the original level, and returning the wall to its original strength and thickness.
The acceleration-deceleration-biosynthesis sequence of these subreactions sum to the overall reaction (1) and, in reality, probably occur in a continuum while the wall extends, driven by P. With extension, new junction zones would become distorted, and the subreactions would repeat to create a P-dependent pectate cycle (Fig. 6).
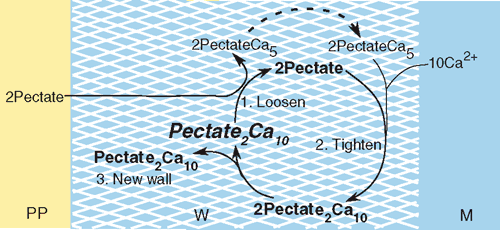
|
The cycle shows a strict stoichiometry between the supply of new pectate from the cytoplasm, wall extension, and the deposition of new wall. But the stoichiometry depends on P, and subreaction 1 ceases when P decreases below 0.2 or 0.3 MPa in Chara, blocking the entire cycle. For this reason, low P blocks not only growth, but also wall biosynthesis simultaneously, as reported by Cleland (1967) and Proseus and Boyer (2006a). But Proseus and Boyer (2006a) found that exocytosis continued unabated, and the pectate cycle predicts an accumulation of pectate at the inner wall face under these conditions. With a return to normal P, the enlarged pectate pool would remove calcium from the wall more rapidly than normal and the subreactions would speed up until the pool would be consumed. Growth would appear to have been ‘stored’ while P was low. Proseus and Boyer (2008) simulated this effect by supplying pectate to isolated walls at low P as though the cytoplasm had continued to supply it. Although the isolated walls did not normally show evidence of stored growth, they did so when P was returned to normal in the presence of the pectate. This provides strong evidence for the P-dependent pectate cycle in the wall, and also identifies wall precursors, especially new pectate, as an important cause of stored growth.
The cycle predicts that faster growth occurs with faster release of pectate by the cytoplasm, or lower molecular weight of the pectate (fewer junction zones), or more blockage of the free carboxyl groups (perhaps by methyesterification to give fewer junction zones). Also, faster growth would occur without external Ca2+ for a time, but this is likely to be lethal eventually. Without Ca2+, the wall would fail to strengthen as it was being stretched irreversibly, and would lose integrity at normal P or might fail to adequately support the plasma membrane.
Other factors could interrupt the stoichiometry of the cycle as well. If the molecular weight of pectate is too small to form junction zones (seen in fig. 6 by Proseus and Boyer 2006c), accelerated extension might occur without wall tightening, creating runaway extension. If the cells were starved photosynthetically, the pool of precursors might be reduced while the wall continued to extend at normal P, creating thin walls. If the plant was exposed to cations other than Ca2+, the chelation chemistry of pectate might be interrupted. Perhaps these effects account for some of the previously noted difficulties in linking wall deposition to plant growth rates (Taiz 1984; Kutschera 1990).
Cell wall biosynthesis
If pectate controls wall extension but ends up as part of the wall, the polymers must be integrated into the existing structure. Matrix polysaccharides, including pectate, are synthesised in Golgi-derived vesicles and released to the wall by exocytosis (Northcote and Pickett-Heaps 1966; Robinson et al. 1976; Moore et al. 1991; Perrin et al. 2001), where they are deposited around the cellulose microfibrils at the inner wall face. But many find their way into the existing wall structure (Northcote and Pickett-Heaps 1966; Ray 1967). It is difficult to explain how such large polymers enter the wall. Talbott and Ray (1992) report that the molecular weights of matrix polysaccharides in pea (Pisum sativum L.) stems are in the range from 40 to 1000 kDa, which would give them diameters of ~9 nm to more than 20 nm when in solution. Interstices in the wall are only ~5 nm in diameter (Read and Bacic 1996) and too small for spontaneous entry of wall polymers in this size range.
In agreement with this observation, Proseus and Boyer (2005) found in Chara that polysaccharides such as dextran (α-1,6-glucan) in the 9 to 20 nm range do not enter the wall spontaneously. At low P, they remain in a layer adjacent to the inner wall face. But with normal P of 0.5 MPa, the polymers became concentrated against the inner wall, and those with diameters of 9–11 nm readily moved into and through the wall (see cover photograph). Thus, the end of the polymer appeared to enter one of the interstices, and P provided the energy to push the rest through while uncoiling it. The polymer moved snake-like into the matrix (reptation) where it would come into intimate contact with polymers already in the wall. For calcium pectate, this would produce a seamless integration of new wall material into the old wall structure. Supporting evidence for this mechanism comes from plasmolysed cells, which synthesise wall precursors but fail to deposit them in the wall (Robinson and Cummins 1976; Robinson et al. 1976; Ueda and Yoshioka 1976; Kroh and Knuiman 1985). When the cells are de-plasmolysed, deposition begins (Robinson and Cummins 1976). New matrix polysaccharides thus appear to enter the wall by a P-driven mechanism, allowing large polymers to become part of an existing polymeric structure.
Very large polymers of ~500 kDa (diameters larger than 20 nm) failed to enter the wall even with P of 0.5 MPa (Proseus and Boyer 2005, 2006a). Figure 7 indicates that they remained at the inner wall where the osmotic potential of the polymer solution equilibrated with P. At an osmotic potential of –0.5 MPa, dextrans developed concentrations around 50% (weight of polymer/weight of water) while 170 kDa pectate reached 14% (Proseus and Boyer 2006a). These concentrations are similar to those in the gel-like matrix of the existing wall (matrix ~37% in charophytes, calculated from Fig. 2). At these concentrations, pectate begins to gel spontaneously (Proseus and Boyer 2006a). No gelling occurred if P decreased to 0.4 MPa, because the concentration of large dextran decreased to 45% and pectate to 11% when in equilibrium with the diminished P (Proseus and Boyer 2006a). Figure 8 indicates that live cells respond to this diminished P by depositing less wall than at 0.5 MPa. They also decreased their growth as shown in Fig. 1. These results indicate that, in addition to its effects on growth, P is a major contributor to wall assembly by concentrating matrix polymer solutions and pushing some of the polymers into the existing wall. Through this mechanism, new wall appears to be assembled in the pectate cycle.
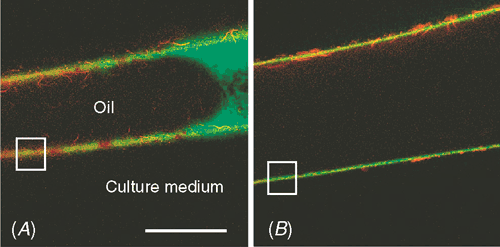
|
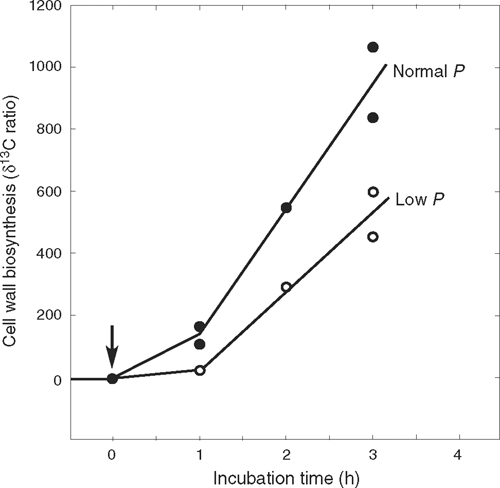
|
Terrestrial plants
Pectate is present in the primary walls of all terrestrial plants where it embeds the other wall components and forms the middle lamella (Carpita and Gibeaut 1993; Popper and Fry 2003, 2005). It is present in most green algae, and pectate relatives such as polymannuronic acid and polyguluronic acid are often found in other algal forms (brown and red algae, Kloareg and Quatrano 1988). During the evolution of green plants, pectate, together with cellulose, was one of the most conserved wall components (Carpita and Gibeaut 1993; Popper and Fry 2003, 2005). Calcium pectate chemistry was probably similarly conserved. This view suggests that the pectate cycle could underlie the growth control of virtually all green plants.
Because the junction zones have sufficient strength to bear the load of P or even prevent growth completely (Proseus and Boyer 2006c), Chara can control its growth rate by controlling the number of junction zones per unit of wall. In agreement with this concept, terrestrial species such as oat (Avena sativa L.) experienced a growth-accelerating action of auxin, which had increased the supply of pectin (and other matrix precursors) to the walls of the coleoptiles (Baker and Ray 1965; Ray and Baker 1965). Ca2+ quenched part of the acceleration. Ca2+ appeared to alter the biochemical wall-loosening in these structures (Cleland and Rayle 1977). Also like Chara, calcium chelators enhanced deformation of tomato (Lycopersicon esculentum Miller) epidermis when under tension, and Ca2+ abolished the effect (Thompson 2005).
In Arabidopsis thaliana (L.) Heynh, a substantial portion of the wall pectate is methylesterified. Derbyshire et al. (2007) used Arabidopsis mutants for gibberellic acid and transformants for certain pectin methylesterases to vary the degree of esterification in the walls. The cells grew faster when methylesterification was more prevalent. They conclude that esterification of the carboxyl groups blocks their ability to bind calcium, decreasing the number of junction zones and making pectate more extensible by P. In effect, the esterification loosens the walls.
It is easy to see why this might happen. P elongates the cells by pushing the end walls apart, and the force on the end walls decreases dramatically as their area decreases (P is force/area). Because the radius of an Arabidopsis cell (12 µm) is ~3% that of a Chara cell (375 µm), the end wall has only ~0.09% of the area in Chara and the force is correspondingly less at the same P. With so little force, Arabidopsis cytoplasm supplies pectate with only a few chemically reactive carboxyl groups, making it easier for P to deform the walls irreversibly. As the cells mature, pectinmethylesterase appears to reduce esterification, allowing more calcium binding and making the walls less deformable. It is worth noting that the middle lamella consists mostly of non-esterified pectate whose high strength with calcium may be necessary to hold the cells together and create a tissue. Taken together, these findings indicate that growth is likely to be controlled by calcium pectate not only in Chara but also in multicellular terrestrial species.
Xyloglucans in the wall matrix have also been suggested to control growth in terrestrial species. The xyloglucans readily form hydrogen bonds with cellulose, and Passioura and Fry (1992), Passioura (1994) and Fry (2004) proposed that the xyloglucans might act as tethers between microfibrils that could control wall extension. In pea stems supplied with exogenous xyloglucan, the cell walls decreased their growth rates as though the xyloglucans might have a load-bearing function (Takeda et al. 2002). But their absence in Chara excludes them for general growth control. In a terrestrial species, Cavalier et al. (2008) demonstrated that xyloglucan-less double mutants of Arabidopsis are able to grow. Without wall xyloglucan, these authors attribute growth control to other wall components such as pectins.
Xyloglucans are thought to cross-bridge between pectins and cellulose microfibrils with intertwining segments of the molecules or with hydrogen bonds (Passioura and Fry 1992; Passioura 1994; Fry 2004) or covalent bonds (Thompson and Fry 2000). A substantial fraction of xyloglucan is covalently linked to pectin in terrestrial species (Thompson and Fry 2000; Popper and Fry 2005, 2008). This suggests that, when present in the wall, xyloglucans contribute to load-bearing by pectins but are not essential. Instead, the xyloglucan portions of the xyloglucan-pectate might bind to the microfibrils by hydrogen bonding while the pectate portions might bind to adjacent pectate by calcium cross-bridges, creating a cross-linked xyloglucan-pectin matrix with tethers between microfibrils.
Several theories of growth control involve enzymes that cleave wall polysaccharides (endoglucanases) (Goldberg. 1980; Huber and Nevins 1981; Fry 1989), or rearrange interpolymeric bonds (e.g. xyloglucan endotransglycosylases) (Fry et al. 1992; Nishitani and Tominaga 1992). Other putative proteins have been implicated in wall extension but are of unknown identity (‘osmiophilic deposits’) (Kutschera 2001) or function (expansins that might disrupt non-covalent polysaccharide bonds in the wall [McQueen-Mason and Cosgrove 1995; Cosgrove 1998; Cosgrove 2005)]. In Chara, expansins are apparently absent (Cosgrove 2000), and although there is evidence for each of these theories, Fry (2004) points out that the presence of an enzyme of known activity is no assurance that it controls growth rate. Another difficulty is that enzymes would need to weaken wall structure at normal P but cease to function when P decreases and growth ceases. No enzyme has shown this P-dependent activity. Instead, it seems reasonable that some of the enzyme-based enlargement mechanisms could co-exist and interact with a non-enzymatic pectate cycle. An example is the pectin methylesterase studied by Derbyshire et al. (2007). The enzyme-based component of enlargement might then account for the rapid growth of cells of terrestrial plants compared with the slower growth of a typical Chara internode (Fig. 1).
A prominent enzyme-based theory is the acid growth hypothesis involving plasma membrane H+-ATPases that could acidify the wall and cause the wall to extend (Hope and Walker 1975; Metraux et al. 1980; Rayle and Cleland 1992). In live charophyte cells, the plasma membrane normally maintains a pH between 4.5 and 5.0 at the inner wall face (Hope and Walker 1975; Metraux et al. 1980). With the medium at pH 7 or 8, a pH gradient probably exists across these walls. Van Cutsem and Gillet (1983) found that H+ could displace wall calcium in charophytes, giving a rationale for growth control by the acid. However, Proseus and Boyer (2006c) varied the pH of the culture medium with little effect on Chara enlargement perhaps because substantial Ca2+ was always in the medium, and wall calcium displaced by H+ was replaced by Ca2+ from the medium. Pectate remained active in accelerating growth from pH 3.5 to pH 11 in the live cells. Therefore, the pH gradient appeared to play no significant role in growth under culture conditions.
Chemical inhibitors also have been used to determine whether enlargement is metabolically or enzymatically mediated (Cleland 1971; Taiz 1984). But in Chara, extensive tests indicated their inhibition was always accompanied by a collapse in P (Proseus and Boyer 2006b). In terrestrial species, P is rarely measured, so the interpretation of inhibitor action remains uncertain.
In terrestrial plants, gradients in growth-induced water potentials complicate these concepts. Substantial gradients move water into the enlarging cells in multicellular tissues (Boyer and Silk 2004), and P can be diminished while P drives enlargement in the same cells. Given this situation, the osmotic potential of the cells must generate sufficient P to allow the lowered P in the gradient while sustaining sufficient P for wall extension. When the growth-induced water potential varied diurnally in the growing region of maize leaves, the osmotic potential kept pace and also varied diurnally (Westgate and Boyer 1984; Tang and Boyer 2008). P was thus maintained. In this way, P-driven enlargement appears to co-exist with the water potential gradient. The cell osmotic potential may be an important feature of growth control in multicellular tissues.
Remaining issues
Although the experimental evidence focuses on pectate for growth control, a growth accelerating action of pectate has not been reported in terrestrial species to date. The difficulty may lie with P, which was rigorously controlled in single cells of Chara but cannot be so easily controlled in multicellular tissues. Lacking P control, accelerated growth often is accompanied by diluting cell contents and decreasing P that would counter the growth acceleration. Also, growing tissues have numerous cell walls and plasma membranes. The entry of exogenous pectate becomes uncertain with these structures, and supplying pectate for long times might be necessary to overcome this problem. But the pectate binds Ca2+, and Ca2+ deprivation for long periods of time can have potentially inhibitory or lethal effects for plant cells. In live cells of Chara, long exposure to Ca2+ deprivation was not necessary, and normal growth returned when normal concentrations of Ca2+ returned in the fresh culture medium. Brief calcium deprivation thus had no permanent effect.
The Chara experiments involved large amounts of exogenous pectate of low concentration while the cytoplasm usually supplies small amounts of pectate at high concentration. Professor Lincoln Taiz (personal communication) indicates that when the lumen of isolated Nitella walls was filled with pectate solution and pressurised, the walls extended faster than controls but soon lost wall integrity. This confirms pectate’s activity but also indicates that large amounts of pectate may be problematic when concentrated by P. More work on these effects seems warranted.
Also needing work is signal transduction between the wall and the cytoplasm. According to the pectate cycle (Fig. 6), when pectate (among other wall precursors) is supplied more rapidly, growth and wall deposition become more rapid at normal P. But the supply could also take the form of changes in molecular weight or altered chemical form of the pectate (e.g. methylesterified carboxyl groups) that would require control signals between the wall and cytoplasm. Also, when P is low and pectate and other wall precursors accumulate at the inner wall, feedback signalling would be expected to control further biosynthesis. Subtle metabolic mechanisms would be required for these signals.
Conclusions
The primitive wall of Chara contains calcium cross-linked with pectate which, when under tension from P, appears to be the primary growth-controlling bond in this plant. The growth-controlling feature of the bond is an immediate calcium loss to newly synthesised pectate on its way to the wall. Growth accelerates as a result, and then decelerates as the new pectate (now calcium pectate) is deposited in the wall. Despite the non-enzymatic character of these reactions, this chemistry acts in walls of live cells. The chemistry creates P and T responses like those in terrestrial plants, and it is proposed that the chemistry is the underlying growth mechanism in virtually all green plants. Xyloglucan, rhamnogalactan II, and methylesterification are often present in species other than Chara, and probably play modifying roles in this chemistry.
The activity of newly synthesised pectate is traced to its ability to act as a stronger calcium chelator than the load-bearing pectate in the wall. The load-bearing pectate bonds appear to be weakened by distortion of the pectate structure by tension generated from P. The weak bonds lose calcium, which decreases the number of load-bearing cross-links and accounts for the accelerated growth. Having removed calcium, the new calcium pectate is able to bind to pectate in the existing wall, accounting for growth deceleration and new wall deposition.
P concentrates the new pectate because the wall acts as an ultrafilter. Although most matrix polysaccharides are too large to enter the wall spontaneously, many enter with P and come into intimate contact with existing wall polymers. The high concentration and contact with existing polymers helps to create a tough, seamlessly-synthesised gel enclosing cellulose microfibrils and other components of the primary wall.
It is proposed that these reactions occur in sequence in a pectate cycle that links wall biosynthesis to growth in a P-dependent way. Although the cytoplasm supplies pectate and other wall precursors, the pectate cycle occurs entirely in and around the wall, and represents critical terminal steps in wall growth and assembly. Consequently, in plants, growth during wall biosynthesis may be determined to a large extent by the rate at which new polymers with chemically reactive carboxyl groups are supplied to the wall.
Acknowledgement
I thank Professor Lincoln Taiz for so generously sharing his unpublished experiments with pressurised pectate solutions in the lumen of isolated walls of Nitella. Special thanks are also due to Dr John Passioura and Professor Stephen Fry for many thoughtful discussions, and Professor JKE Ortega for helpful comments on the manuscript.
Anderson DMW, King NJ
(1961) Polysaccharides of the Characeae. III. The carbohydrate content of Chara australis. Biochimica et Biophysica Acta 52, 449–454.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bacic A
(2006) Breaking an impasse in pectin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 103, 5639–5640.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Baker DB, Ray PM
(1965) Relation between effects of auxin on cell wall synthesis and cell elongation. Plant Physiology 40, 360–368.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Baskin TI
(2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215, 150–171.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Baskin TI
(2005) Anisotropic expansion of the plant cell wall. Annual Review of Cell and Developmental Biology 21, 203–222.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Boyer JS
(1993) Temperature and growth-induced water potential. Plant, Cell & Environment 16, 1099–1106.
| Crossref | GoogleScholarGoogle Scholar |

Boyer JS
(2001) Growth-induced water potentials originate from wall yielding during growth. Journal of Experimental Botany 52, 1483–1488.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Boyer JS, Silk WK
(2004) Hydraulics of plant growth. Functional Plant Biology 31, 761–773.
| Crossref | GoogleScholarGoogle Scholar |

Bret-Harte MS,
Baskin TI, Green PB
(1991) Auxin stimulates both deposition and breakdown of material in the pea outer epidermal cell wall, as measured interferometrically. Planta 185, 462–471.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Carpita NC, Gibeaut DM
(1993) Structural models of primary cell walls in the flowering plants: consistency of molecular structure with the physical properties of walls during growth. The Plant Journal 3, 1–30.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Carpita NC,
McCann M, Griffing LR
(1996) The plant extracellular matrix: news from the cell’s frontier. The Plant Cell 8, 1451–1463.
|
CAS |
Crossref |
PubMed |

Cavalier DM,
Lerouxel O,
Neumetzler L,
Yamauchi K, Reinecke A , et al.
(2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell 20, 1519–1537.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Clarkson DT, Hanson JB
(1980) The mineral nutrition of higher plants. Annual Review of Plant Physiology 31, 239–298.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Cleland RE
(1967) A dual role of turgor pressure in auxin–induced cell elongation in Avena coleoptiles. Planta 77, 182–191.
|
CAS |

Cleland RE
(1971) Cell wall extension. Annual Review of Plant Physiology 22, 197–222.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Cleland RE, Rayle DL
(1977) Reevaluation of the effect of calcium ions on auxin-induced elongation. Plant Physiology 60, 709–712.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cosgrove DJ
(1989) Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta 177, 121–130.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cosgrove DJ
(1998) Cell wall loosening by expansins. Plant Physiology 118, 333–339.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cosgrove DJ
(2000) Expansive growth of plant cell walls. Plant Physiology and Biochemistry 38, 109–124.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cosgrove DJ
(2005) Growth of the plant cell wall. Nature Reviews 6, 850–861.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Darley CP,
Forrester AM, McQueen-Mason SJ
(2001) The molecular basis of plant cell wall extension. Plant Molecular Biology 47, 179–195.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Derbyshire P,
McCann MC, Roberts K
(2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biology 7, 31.
| Crossref |
PubMed |

Ding BL, Schopfer PK
(1997) Metabolic involvement in acid-mediated extension growth of maize coleoptiles. Journal of Experimental Botany 48, 721–728.
|
CAS |
Crossref |

Farrokhi N,
Burton RA,
Brownfield L,
Hrmova M,
Wilson SM,
Bacic A, Fincher GB
(2006) Plant cell wall biosynthesis: genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnology Journal 4, 145–167.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Fry SC
(1989) Cellulases, hemicellulases and auxin-stimulated growth: a possible relationship. Physiologia Plantarum 75, 532–536.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Fry SC
(2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist 161, 641–675.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Fry SC,
Smith RC,
Renwick KF,
Martin DJ,
Hodge SK, Matthews KJ
(1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal 282, 821–828.
|
CAS |
PubMed |

Gillet C, Liners F
(1996) Changes in distribution of short pectic polysaccharides induced by monovalent ions in the Nitella cell wall. Canadian Journal of Botany 74, 26–30.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Goldberg R
(1980) Cell wall polysaccharidase activities and growth processes: a possible relationship. Plant Physiology 94, 411–416.

Graham LE
(1985) The origin of the life cycle of land plants. American Scientist 73, 178–186.

Grant GT,
Morris ER,
Rees DA,
Smith PJC, Thom D
(1973) Biological interactions between polysaccharides and divalent cations: the egg box model. FEBS Letters 32, 195–198.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hepler PK, Wayne RO
(1985) Calcium and plant development. Annual Review of Plant Physiology 36, 397–439.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Huber DJ, Nevins DJ
(1981) Partial purification of endo- and exo-β-D-glucanase enzymes from Zea mays seedlings and their involvement in cell-wall autohydrolysis. Planta 151, 206–214.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Jarvis MC
(1984) Structure and properties of pectin gels in plant cell walls. Plant, Cell & Environment 7, 153–164.
|
CAS |

Jarvis MC, Apperley DC
(1995) Chain conformation in concentrated pectic gels: evidence from 13C NMR. Carbohydrate Research 275, 131–145.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kloareg B, Quatrano RS
(1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography and Marine Biology: an Annual Review 26, 259–315.

Kroh M, Knuiman B
(1985) Exocytosis in non-plasmolysed and plasmolysed tobacco pollen tubes. Planta 166, 287–299.
| Crossref | GoogleScholarGoogle Scholar |

Kutschera U
(1990) Cell-wall synthesis and elongation growth in hypocotyls of Helianthus annuus L. Planta 181, 316–323.
| Crossref | GoogleScholarGoogle Scholar |

Kutschera U
(2001) Stem elongation and cell wall proteins in flowering plants. Plant Biology 3, 466–480.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lerouxel O,
Cavalier DM,
Liepman AH, Keegstra K
(2006) Biosynthesis of plant cell wall polysaccharides – a complex process. Current Opinion in Plant Biology 9, 621–630.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Matsunaga T,
Ishii T,
Matsumoto S,
Higuchi M,
Darvill A,
Albersheim P, O’Neill MA
(2004) Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiology 134, 339–351.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Matthews MA,
Van Volkenburgh E, Boyer JS
(1984) Acclimation of leaf growth to low water potentials in sunflower. Plant, Cell & Environment 7, 199–206.

McCann MC, Roberts K
(1994) Changes in cell wall architecture during cell elongation. Journal of Experimental Botany 45, 1683–1691.
|
CAS |

McQueen-Mason SJ, Cosgrove DJ
(1995) Expansin mode of action on cell walls. Plant Physiology 107, 87–100.
|
CAS |
PubMed |

Metraux J-P, Taiz L
(1977) Cell wall extension in Nitella as influenced by acids and ions. Proceedings of the National Academy of Sciences of the United States of America 74, 1565–1569.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Metraux J-P,
Richmond P, Taiz L
(1980) Control of cell elongation in Nitella by endogenous cell wall pH gradients. Multiaxial extensibility and growth studies. Plant Physiology 65, 204–210.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Moore PJ,
Swords KMM,
Lynch MA, Staehelin LA
(1991) Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. Journal of Cell Biology 112, 589–602.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morikawa H, Senda M
(1974) Oriented structure of matrix polysaccharides in and extension growth of Nitella cell wall. Plant & Cell Physiology 15, 1139–1142.
|
CAS |

Morikawa H,
Tanizawa K, Senda M
(1974) Infrared spectra of Nitella cell walls and orientation of carboxylate ions in the walls. Agricultural and Biological Chemistry 38, 343–348.
|
CAS |

Morris ER,
Powell DA,
Gidley MJ, Rees DA
(1982) Conformation and interactions of pectins. I. Polymorphism between gel and solid states of calcium polygalacturonate. Journal of Molecular Biology 155, 507–516.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morrison JC,
Greve LC, Richmond PA
(1993) Cell wall synthesis during growth and maturation of Nitella internodal cells. Planta 189, 321–328.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Nishitani K, Tominaga R
(1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyses transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry 267, 21058–21064.
|
CAS |
PubMed |

Nonami H,
Boyer JS, Steudle ES
(1987) Pressure probe and isopiestic psychrometer measure similar turgor. Plant Physiology 83, 592–595.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Northcote DH, Pickett-Heaps JD
(1966) A function of the Golgi apparatus in polysaccharide synthesis and transport in root-cap cells of wheat. The Biochemical Journal 98, 159–167.
|
CAS |
PubMed |

O’Neill MA,
Ishii T,
Albersheim P, Darvill AG
(2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall polysaccharide. Annual Review of Plant Biology 55, 109–139.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Passioura JB
(1994) The physical chemistry of the primary cell wall: implications for the control of expansion rate. Journal of Experimental Botany 45, 1675–1682.
|
CAS |

Passioura JB, Fry SC
(1992) Turgor and cell expansion: beyond the Lockhart equation. Australian Journal of Plant Physiology 19, 565–576.

Perrin R,
Wilkerson C, Keegstra K
(2001) Golgi enzymes that synthesize plant cell wall polysaccharides: finding and evaluating candidates in the genomic era. Plant Molecular Biology 47, 115–130.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Popper ZA, Fry SC
(2003) Primary cell wall composition of bryophytes and charophytes. Annals of Botany 91, 1–12.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Popper ZA, Fry SC
(2005) Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Annals of Botany 96, 91–99.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Popper ZA, Fry SC
(2008) Xyloglucan-pectin linkages are formed intra-protoplasmically, contribute to wall-assembly, and remain stable in the cell wall. Planta 227, 781–794.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Powell ER,
Morris ER,
Gidley MJ, Rees DA
(1982) Conformations and interactions of pectins. II. Influence of residue sequence on chain association in calcium pectate gels. Journal of Molecular Biology 155, 517–531.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Probine MC, Preston RD
(1962) Cell growth and the structure and mechanical properties of the wall in internode cells of Nitella opaca. II. Mechanical properties of the walls. Journal of Experimental Botany 13, 111–127.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Proseus TE, Boyer JS
(2005) Turgor pressure moves polysaccharides into growing cell walls of Chara corallina. Annals of Botany 95, 967–979.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Proseus TE, Boyer JS
(2006a) Periplasm turgor pressure controls wall deposition and assembly in growing Chara corallina cells. Annals of Botany 98, 93–105.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE, Boyer JS
(2006b) Identifying cytoplasmic input to the cell wall of growing Chara corallina. Journal of Experimental Botany 57, 3231–3242.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE, Boyer JS
(2006c) Calcium pectate chemistry controls growth rate of Chara corallina. Journal of Experimental Botany 57, 3989–4002.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE, Boyer JS
(2007) Tension required for pectate chemistry to control growth in Chara corallina. Journal of Experimental Botany 58, 4283–4292.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE, Boyer JS
(2008) Calcium pectate chemistry causes growth to be stored in Chara corallina: a test of the pectate cycle. Plant, Cell & Environment 31, 1147–1155.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE,
Ortega JKE, Boyer JS
(1999) Separating growth from elastic deformation during cell enlargement. Plant Physiology 119, 775–784.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Proseus TE,
Zhu G-L, Boyer JS
(2000) Turgor, temperature, and the growth of plant cells: using Chara corallina as a model system. Journal of Experimental Botany 51, 1481–1494.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ralet M-C,
Dronnet V,
Buchholt HC, Thibault J-F
(2001) Enzymatically and chemically de-esterified lime pectins: characterization, polyelectrolyte behavior and calcium binding properties. Carbohydrate Research 336, 117–125.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ray PM
(1967) Radioautographic study of cell wall deposition in growing plant cells. Journal of Cell Biology 35, 659–674.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ray PM, Baker DB
(1965) The effect of auxin on synthesis of oat coleoptile cell wall constituents. Plant Physiology 40, 353–360.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rayle DL, Cleland RE
(1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiology 99, 1271–1274.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Read SM, Bacic A
(1996) Cell wall porosity and its determination. Modern Methods of Plant Analysis 17, 63–80.
|
CAS |

Reid RJ, Smith FA
(1992) Measurement of calcium fluxes in plants using 45Ca. Planta 186, 558–566.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Richmond PA,
Metraux J-P, Taiz L
(1980) Cell expansion patterns and directionality of wall mechanical properties in Nitella. Plant Physiology 65, 211–217.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ridley BL,
O’Neill MA, Mohnen D
(2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Robinson DG, Cummins WR
(1976) Golgi apparatus secretion in plasmolysed Pisum sativum L. Protoplasma 90, 369–379.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Robinson DG,
Eisinger WR, Ray PM
(1976) Dynamics of the Golgi system in wall matrix polysaccharide synthesis and secretion by pea cells. Berichte der Deutschen Botanischen Gesellschaft 89, 147–161.
|
CAS |

Saxena IM, Brown RM
(2005) Cellulose biosynthesis: current views and evolving concepts. Annals of Botany 96, 9–21.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Scheible W-R, Pauly M
(2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Current Opinion in Plant Biology 7, 285–295.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Scherp P,
Grotha R, Kutschera U
(2001) Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Reports 20, 143–149.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Seifert GJ
(2004) Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Current Opinion in Plant Biology 7, 277–284.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sentenac H, Grignon C
(1981) A model for predicting ionic equilibrium concentrations in cell walls. Plant Physiology 68, 415–419.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Shepherd VA, Goodwin PB
(1989) The porosity of permeabilised Chara cells. Australian Journal of Plant Physiology 16, 213–219.

Somerville C
(2006) Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology 22, 53–78.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Somerville C,
Bauer S,
Brininstool G,
Facette M, Hamann T , et al.
(2004) Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Taiz L
(1984) Plant cell expansion: regulation of cell wall mechanical properties. Annual Review of Plant Physiology 35, 585–657.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Takeda T,
Furuta Y,
Awano T,
Mizuno K,
Mitsuishi Y, Hayashi T
(2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proceedings of the National Academy of Sciences of the United States of America 99, 9055–9060.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Talbott LD, Ray PM
(1992) Changes in molecular size of previously deposited and newly synthesized pea cell wall matrix polymers. Plant Physiology 98, 369–379.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tang AC, Boyer JS
(2008) Xylem tension affects growth-induced water potential and daily elongation of maize leaves. Journal of Experimental Botany 59, 753–764.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tepfer M, Cleland RE
(1979) A comparison of acid-induced cell wall loosening in Valonia ventriculos and in oat coleoptiles. Plant Physiology 63, 898–902.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thompson DS
(2005) How do cell walls regulate plant growth? Journal of Experimental Botany 56, 2275–2285.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thompson JE, Fry SC
(2000) Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 211, 275–286.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ueda K, Yoshioka S
(1976) Cell wall development of Micrasterias americana, especially in isotonic and hypertonic solutions. Journal of Cell Science 21, 617–631.
|
CAS |
PubMed |

Van Cutsem P, Gillet C
(1983) Proton-metal cation exchange in the cell wall of Nitella flexilis. Plant Physiology 73, 865–867.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Van den Burg B,
Vriend G,
Veltman OR,
Venema G, Eijsink VGH
(1998) Engineering an enzyme to resist boiling. Proceedings of the National Academy of Sciences of the United States of America 95, 2056–2060.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Westgate ME, Boyer JS
(1984) Transpiration- and growth-induced water potentials in maize. Plant Physiology 74, 882–889.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Willats WGT,
McCartney L,
Mackey W, Knox JP
(2001) Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology 47, 9–27.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhu G-L, Boyer JS
(1992) Enlargement in Chara studied with a turgor clamp: growth rate is not determined by turgor. Plant Physiology 100, 2071–2080.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



