Sexual and asexual (apomictic) seed development in flowering plants: molecular, morphological and evolutionary relationships
Matthew R. Tucker A and Anna M. G. Koltunow A BA CSIRO Plant Industry, PO Box 350, Glen Osmond, SA 5064, Australia.
B Corresponding author. Email: anna.koltunow@csiro.au
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 36(6) 490-504 https://doi.org/10.1071/FP09078
Submitted: 7 April 2009 Accepted: 21 April 2009 Published: 1 June 2009
Abstract
Reproduction in the flowering plants (angiosperms) is a dynamic process that relies upon the formation of inflorescences, flowers and eventually seed. Most angiosperms reproduce sexually by generating gametes via meiosis that fuse during fertilisation to initiate embryo and seed development, thereby perpetuating the processes of adaptation and evolution. Despite this, sex is not a ubiquitous reproductive strategy. Some angiosperms have evolved an alternate form of reproduction termed apomixis, which avoids meiosis during gamete formation and leads to the production of embryos without paternal contribution. Therefore, apomixis results in the production of clonal progeny through seed. The molecular nature and evolutionary origin of apomixis remain unclear, but recent studies suggest that apomixis evolved from the same molecular framework supporting sex. In this review, we consider physical and molecular relationships between the two pathways, with a particular focus on the initial stages of female reproduction where apomixis deviates from the sexual pathway. We also consider theories that explain the origin of apomictic processes from sexual progenitors. Detailed characterisation of the relationship between sex and apomixis in an evolutionary and developmental sense is an important step towards understanding how apomixis might be successfully integrated into agriculturally important, but currently sexual crops.
Additional keywords: apomixis, evolution, Hieracium, ovule, reproduction.
Introduction
The angiosperms, or flowering plants, make up the largest number of species in the plant kingdom and dominate most terrestrial environments. They span diverse body plans and growth forms and are generally distinguished from other seed bearing plants (gymnosperms) by several key features. These include the presence of flowers, which comprise a perianth of attractive structures (e.g. petals) around the reproductive organs, and ovules that are enclosed in carpels (female sporophylls that after fertilisation of the ovule form part of the fruit) rather than lying bare on the surface of a cone. Many angiosperm flowers are hermaphroditic, or bisexual, meaning that the female and male reproductive organs are united in one structure instead of being located in different structures. Furthermore, during seed formation, double fertilisation leads to the production of the embryo that is the progenitor of the seedling, and also the triploid endosperm, which is often considered to be an important feature of the angiosperms since it provides additional nutrition to the developing embryo.
The reason for the success of the angiosperms, however, has been a remarkably controversial topic, mainly because it is still unclear when and from what ancient plant lineages they evolved (Theissen and Melzer 2007; Crepet and Niklas 2009). Interestingly, Darwin considered this was an ‘abominable mystery’ (Darwin 1903), and it is yet to be solved despite many years of research and numerous hypotheses. Although the most favoured hypothesis suggests that angiosperm diversification and success is linked to the relationship between flowering plants and the animal species that facilitate pollination or seed/fruit dispersal, there are alternatives, elegantly summarised by Crepet and Niklas (2009). These consider features such as vegetative and reproductive attributes, phenotypic plasticity and combinations of structural, genetic and chemical characteristics. In any case, the advent of angiosperms and their subsequent ecological success most likely reflects the synergy among many functional traits that allowed them to break free from the developmental constraints experienced by their gymnosperm progenitors (Crepet and Niklas 2009).
Seed development in the angiosperms requires a series of defined steps. First, a transition from a vegetative plant growth mode to a reproductive phase results in the formation of the floral organs such as petals, and the male and female reproductive organs, the stamens and carpels (Fig. 1). Meiosis and gamete formation occur in the ovules of the carpels and anthers of the stamen. Fertilisation begins with pollination of the stigma and concludes with double fertilisation within the embryo sac of the ovule. In this process, two sperm cells are transmitted to the ovule via a pollen tube and released into the embryo sac (female gametophyte) where they fuse with the egg cell to give rise to the embryo and with the central cell to produce the endosperm (Fig. 1).
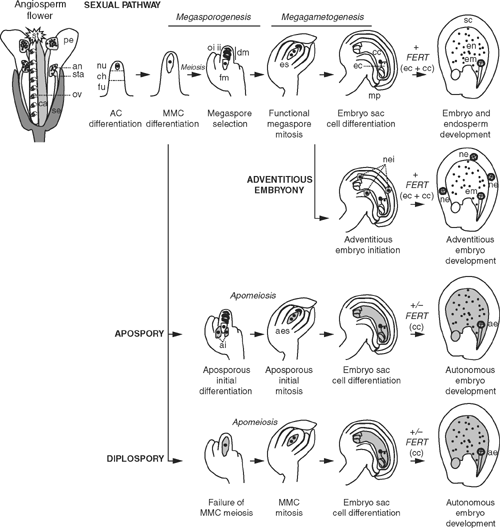
|
Of all the structures involved in seed development, the ovule is probably the most vital, since it is physically connected to the maternal plant and thus a supply of hormones and nutrients, and contains specific tissues called the integuments, which eventually form the seed coat and protect the seed during its development and dispersal. The ovule also supports growth of the embryo sac, which is formed from a specific ovule cell that transits through multiple developmental states including cell differentiation, meiotic and mitotic division, cell selection and cell death (megasporogenesis and megagametogenesis; Fig. 1). Most angiosperms follow the Polygonum-type of embryo sac development to produce a seven-celled, eight-nucleate embryo sac (Willemse and van Went 1984), and although the details may vary between species, the basic sexual pathway requiring meiosis and fertilisation for seed development is highly conserved. Sexual reproduction supports the generation of diverse progeny through meiotic recombination and the union of male and female gametes. Combined with the evolution of flowers that help to attract pollinators, this is likely to be another contributing factor to the success of the angiosperms.
While the majority of flowering plants rely upon the events of sexual reproduction to form a seed, over 400 species from 40 different families including both monocots and eudicots, have evolved an asexual form of seed formation termed apomixis. Despite deviating significantly from the sexual pathway, apomixis occurs without compromising seed viability. During apomictic reproduction, the embryo is formed from a somatic cell, independently of meiosis and fertilisation, thus resulting in seedling progeny that are identical to the maternal plant. Several types of apomixis have been identified in plants that differ based on the origin and location of the somatic cell that gives rise to the embryo (Fig. 1). The embryo can form within an embryo sac produced entirely from mitotic events (apospory or diplospory) or directly from somatic cells surrounding the developing sexual embryo sac (adventitious embryony). In some cases, fertilisation is required for endosperm development and subsequent seed viability, but in others it is not, and the endosperm, similar to the embryo, can develop autonomously. Furthermore, most apomictic plants are facultative, meaning that sexual reproduction is not completely eliminated. Apomixis and sexual reproduction can take place in the same plant, and even in the same ovule, giving rise to both maternal apomictic and hybrid progeny. The occurrence of apomixis in different forms and in unrelated families suggests that the apomictic pathway has evolved multiple times during plant evolution.
Apomixis is also a trait of considerable interest for agriculture, since in contrast to sexual reproduction, which typically results in the segregation of traits, apomixis results in the production of clonal progeny through seed. Theoretically, if apomixis could be employed in hybrid seed production it could fix hybrid vigour and allow for the stable fixation of many advantageous traits over many seed generations. Apomixis is noticeably absent from most crop plants, and strategies aimed at integrating apomixis into crops via hybridisation from the few known wild relatives have failed. Therefore, the molecular characterisation of the apomictic process in natural species has attracted considerable interest.
Recent reviews have focussed on the genetic inheritance of apomixis (Ozias-Akins and van Dijk 2007), the polyploid nature of most apomictic plants (Mogie et al. 2007) and the costs and benefits of apomixis and sexual reproduction from ecological perspectives (van Dijk 2007). Other reviews have focussed on the maternal and paternal genomic contributions to sexual seed formation and viability and speculated on how apomixis might overcome these requirements (Grossniklaus et al. 2001; Köhler and Grossniklaus 2005). Here, we consider apomixis and its origin by comparing the morphological and molecular relationships of sexual and apomictic pathways from a developmental perspective. We will focus our attention predominantly towards the earliest stages of sexual and apomictic reproduction in the ovule. These are the stages in sexual reproduction where the cells competent to undergo meiosis differentiate and the embryo sac, which houses the egg cell, is formed and fertilised, thus perpetuating the processes of angiosperm evolution and adaptation. At these same ovule stages in most apomictic plants, reproductive development is diverted towards the mitotic production of embryo sacs from somatic cells and the differentiation of eggs that are identical in genotype to the maternal plant; steps that are essential for the generation of clonal progeny.
Defining the early events in the female reproductive pathway during sexual reproduction
In the case of Arabidopsis, a sexually reproducing model angiosperm, the ovules are aligned in linear files within the developing carpels and are relatively small, easily accessible to mechanical dissection and compatible with simple clearing methods (Schneitz et al. 1995). Ovule structure often varies between species, but typically consists of only a few pattern elements or cell types on the proximal-distal axis. In Arabidopsis, these include the nucellus, which gives rise to the germ-cell progenitors, the chalaza (meaning ‘hard lump’ or ‘hailstone’ in Greek), which separates the nucellus from the funiculus and gives rise to the inner and outer integuments, and the funiculus, which is a stalk-like structure that contains vascular strands and connects the ovule to the placenta of the flower (Fig. 1). Similarly in the model monocot rice (Oryza sativa L.), two integuments form from the chalaza, but the funiculus is rudimentary and the chalaza seems to be attached directly to one side of the ovary wall (Itoh et al. 2005). A recent review summarises early stages of ovule development and its evolutionary conservation (Colombo et al. 2008).
Differentiation of the megaspore mother cell, meiosis and megaspore selection
The first stage of female reproductive development takes place within the ovule when an archesporial cell differentiates from sub-epidermal cells of the nucellus (Fig. 1). In the majority of angiosperms, this cell can be recognised by its elongated size relative to the surrounding cells, a large nucleus and vacuole and its position neighbouring the epidermis. Although a distinction is made between the archesporial cell and megasporocyte/megaspore mother cell (MMC), in Arabidopsis the archesporial cell directly functions as the MMC (Webb and Gunning 1990; Reiser and Fischer 1993). This is in contrast to the majority of angiosperms where the archesporial cell often undergoes a periclinal division, and subsequently the inner cell differentiates into the MMC.
Once the MMC has differentiated from the archesporial cell, it continues to expand and, in the case of Arabidopsis, eventually occupies much of the space at the apex of the nucellus. Prior to the initiation of meiosis, the cell exhibits a large nucleus and polarisation of organelles, such as the plastids, which are predominantly located at the chalazal end of the cell, and the rough endoplasmic reticulum, which are predominantly located in the cytoplasm at the micropylar end of the cell (Bajon et al. 1999). Although the reason for this polarisation is unclear, it may lead to the differential partitioning of cellular components in the megaspores after meiosis, hence influencing their survival (see below). Intimate details of female meiosis in Arabidopsis have been described in several studies (Webb and Gunning 1990, 1991, 1994). During meiosis I, the MMC nucleus divides transversely giving rise to a binucleate intermediate, which depending on the species and type of embryo sac development (Bouman 1984), typically comprises two dyad cells. Meiosis II proceeds rapidly and leads to the production of four haploid megaspores that can be arranged in a linear (Fig. 1) and/or multiplanar tetrad. In Arabidopsis, which develops the most common form of embryo sac structure found in angiosperms, the monosporic Polygonum-type, the spatial arrangement of megaspores varies between ovules, but in all cases the megaspore located closest to the chalazal region of the ovule enlarges while the other three rapidly degenerate. At maturity, the functional megaspore is tear-shaped, rich in organelles that are dispersed throughout the cytoplasm and contains small vacuoles (Webb and Gunning 1991). By contrast, the degeneration of the non-functional megaspores is accompanied by an accumulation of ribosomes, dicotysomes and lipid granules, vesicles and autophagic vacuoles and low cellular activity (Willemse and De Boer-De Jeu 1981). Expansion of the functional megaspore, sometimes referred to as the one-nucleate embryo sac, progressively displaces the degenerating spores towards the micropylar end of the ovule before initiating the first mitotic division of megagametogenesis (Fig. 1).
Molecular control of megasporogenesis
Most molecular studies of ovule development have focussed on dicot species such as Arabidopsis and Petunia, and these have identified several genes associated with primordium differentiation and ovule identity (for a summary, see reviews by Grossniklaus and Schneitz 1998; Skinner et al. 2004; Colombo et al. 2008). More recently, studies in rice have revealed novel details of the female reproductive pathway in monocots. Combined, results from these different systems have elaborated specific details of early ovule development and reproductive cell identity (Fig. 2).
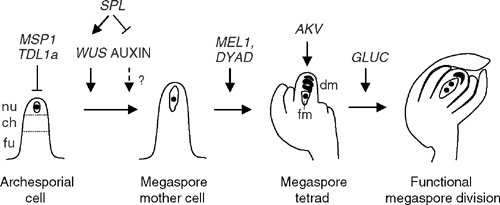
|
Megaspore mother cell (MMC) differentiation
Development of the MMC appears to be under the control of several pathways that either restrict or promote its differentiation. In Arabidopsis mutants lacking function of the SPOROCYTELESS/NOZZLE (SPL) gene, a proportion of ovules fail to produce a mature MMC from the archesporial cell (Schiefthaler et al. 1999; Yang et al. 1999), suggesting that SPL acts to promote MMC differentiation. SPL encodes a plant-specific nuclear protein and is expressed in various plant tissues, including the nucellus, archesporial cell and MMC. Since spl mutant ovules also show defects in nucellar identity and integument growth (Schiefthaler et al. 1999; Balasubramanian and Schneitz 2000; Sieber et al. 2004), it is unclear whether SPL functions in multiple independent pathways or primarily in ovule patterning thereby influencing MMC specification. Interestingly, ovules lacking function of the WUSCHEL (WUS) gene, which encodes a homeodomain transcription factor that acts downstream of SPL, also display defects in MMC differentiation and integument growth, but in contrast to spl ovules, appear to develop a normal nucellus (Groß-Hardt et al. 2002; Sieber et al. 2004). Therefore, WUS, which is a key regulator of cell-differentiation in meristems and anthers (Laux et al. 1996; Deyhle et al. 2007), may act as a specific component of a MMC differentiation pathway promoted by SPL. Recent studies of a dominant spl mutant (spl-D) suggest that SPL also acts to repress expression of YUCCA genes, which encode for flavin mono-oxygenases and are key players in synthesis of the plant hormone auxin (Li et al. 2008). Although this function is yet to be shown in ovules, auxin responses have previously been shown to influence ovule integument growth and female fertility (Schruff et al. 2006; Wu et al. 2006). Thus, a link is emerging between known regulators of cell differentiation and region-specific auxin responses that might influence reproductive cell fate during ovule development.
In angiosperm ovules, usually only one cell differentiates from the nucellus to undergo meiosis and give rise to the embryo sac. Recent studies in rice suggest that a signalling mechanism acts to restrict MMC competence to this one cell. The multiple sporocyte (msp1) mutant was identified as a male sterile mutant and found to contain an insertion of the Tos17 endogenous retrotransposon in a gene encoding for a leucine-rich repeat receptor–like protein kinase (LRR-RLK; Nonomura et al. 2003). msp1 mutants display an increased number of both male and female sporocytes, which in the case of the ovule can give rise to multiple disorganised, but occasionally viable, female gametophytes that produce germinable seeds after fertilisation with wild-type pollen. In the ovule, MSP1 mRNA is detected in cells surrounding the MMC, but not in the MMC itself, suggesting it is part of an intercellular signalling mechanism regulating sporogenic cell fate. This mechanism may be conserved in other monocots, since the male sterile multiple archesporial cell (mac1) mutant in maize (Zea mays L.) also leads to the production of extra sporocytes in both ovules and anthers (Sheridan et al. 1996; Sheridan et al. 1999), which in the case of the ovule, are capable of undergoing meiosis and embryo sac development. The identity of the mac1 gene is yet to be reported, but the recessive, sporophytic nature of the mutation suggests that it is also likely to act as part of a signalling mechanism from surrounding tissues that restricts MMC fate. Homologues of MSP1 (EXTRA SPOROGENOUS CELLS/EXCESS MICROSPOROCYTES1; EXS/EMS1; Canales et al. 2002; Zhao et al. 2002) have also been identified in the Arabidopsis genome and were shown to regulate aspects of microspore fate during pollen development similar to that reported in maize and rice. However, it is not known if these mutants display a multiple-MMC phenotype. Therefore, the conservation of RLK/MSP1-like signalling pathways regulating multiple MMC formation in monocot and eudicot species remains to be determined.
How might the MSP1 signalling mechanism function? LRR-RLKs are highly represented in plants (Shiu and Bleecker 2001) and are identified as transmembrane proteins with putative N-terminal extracellular domains and carboxyl-terminal intracellular kinase domains. RLKs are usually the starting point of a complex array of signalling pathways that depend on the binding of a ligand to the LRR extracellular domain, and have been shown to function in diverse developmental events including cell differentiation and pathogen defence (Diévart and Clark 2004). One well characterised example is the Arabidopsis CLAVATA1 (CLV1) LRR-RLK that binds the stem cell specific CLAVATA3 (CLV3) signal peptide and regulates meristem development (Brand et al. 2000; Schoof et al. 2000; Ogawa et al. 2008). In the absence of CLV1 function, or that of its CLV3 ligand, the size of the Arabidopsis meristem increases drastically due to an over-accumulation of stem cells and increased activity of WUSCHEL (Brand et al. 2000; Schoof et al. 2000). In Arabidopsis, the EXS/EMS RLK protein binds the putative ligand TAPETUM DETERMINANT (TPD1; (Ma 2005; Jia et al. 2008) and functions to restrict the number of male sporocytes. A recent report suggests that the homologue of TPD1 in rice, TAPETUM DETERMINANT-LIKE (TDL1A), may be the ligand for MSP1, since MSP1 and TDL1A interact in yeast-2-hybrid and bimolecular fluorescence experiments and TDL1A RNAi lines show a similar phenotype to msp1 mutants in the ovule (Zhao et al. 2008). TDL1A mRNA is also detected in the ovule cells surrounding the developing MMC.
Collectively, these findings identify the importance of inter-regional communication in specification of the MMC. At least two pathways appear to influence MMC specification in angiosperm ovules, one of which promotes the differentiation of an MMC from the archesporial cell and another that restricts sporogenous identity to a single nucellar cell (Fig. 2). The latter, in monocot rice and possibly maize, appears to comprise a LRR-RLK signalling pathway. Further details of the components of this signalling mechanism, particularly the final downstream targets and whether they are expressed in the MMC, will be useful in characterising the first step of sporogenous development. Although this pathway has yet to be extended to eudicot ovules, the observation that multiple MMCs can occasionally be detected in Arabidopsis ovules (U. Grossniklaus, pers. comm.) suggests that a similar mechanism may be in place.
Megaspore development (megasporogenesis)
Once the MMC has differentiated in the nucellus, it enters a phase of meiotic division leading to the production of four reduced megaspores. Although it is unclear on a molecular level why the MMC alone enters a meiotic pathway, several genes have been described that influence the progression of megasporogenesis. Most of these also play a role during early pollen development (microsporogenesis), suggesting they play a conserved role during meiotic reduction. Accordingly, some show homology to factors important for meiosis in yeast, but others are plant specific, and recent reviews provide a detailed summary (Yang 2006; Mercier and Grelon 2008). Several genes that function during megasporogenesis in rice and Arabidopsis are of particular interest, based on the identity of the proteins they encode and their loss-of-function phenotypes.
The MEIOSIS ARRESTED AT LEPTOTENE1 (MEL1) gene from rice was identified in a screen for seed-sterile rice plants (Nonomura et al. 2007). In mel1 mutants, both female and male sporogenesis is disrupted; pollen mother cells (PMCs) arrest during meiosis I and MMCs arrest at the pre-meiosis, meiosis or tetrad stages. In the ovule, MEL1 mRNA is first detected in a pool of hypodermal cells within the nucellus before becoming restricted to the MMC, but is notably absent during meiosis. Similar expression is observed in the anthers. Therefore, the initial expression of MEL1 marks a group of cells that, taken together with the MSP1 data discussed above, are likely to have competence to initiate sporogenous development. Interestingly, based on sequence homology, MEL1 encodes a putative member of the ARGONAUTE (AGO) protein family, which is conserved in plants and animals and has been implicated in many developmental processes. AGO proteins function to bind small RNA (sRNA) molecules and regulate expression of complementary RNA targets through either mRNA cleavage, translational repression or chromatin modification (Vaucheret 2008). The rice genome encodes 18 AGO proteins and the Arabidopsis genome encodes 10, of which AGO5 shows highest homology to MEL1. Publicly available microarray data (Arabidopsis eFP browser; http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi) show that AGO5 expression is restricted mainly to reproductive tissues in Arabidopsis, suggesting that it may have a similar function to MEL1. Although the developmental function and expression of AGO5 on the cellular level remain unknown, two closely related characterised Arabidopsis genes are AGO1, which regulates leaf polarity, resistance against pathogens and shoot development (Bohmert et al. 1998) and AGO10/ZWILLE/PINHEAD (Moussian et al. 1998; Lynn et al. 1999), which regulates stem cell maintenance by promoting WUS function from embryonic vascular cells (Tucker et al. 2008). Based on the nature of the MEL1 protein and the phenotype of mel1 mutants, it is likely that MEL1 regulates the expression of mRNA targets via small RNAs in young sporogenous tissues and this is critical for the completion of normal sporogenesis and meiosis. The further characterisation of MEL1, its targets and the sRNA molecules through which it functions will provide novel insight into the mechanisms of megasporogenesis in plants.
Another gene required for megasporogenesis (and microsporogenesis) is the Arabidopsis gene DYAD/SWITCH1 (SWI1), which is expressed in female and male meiotic cells and functions in sister chromatid cohesion and centromere organisation (Mercier et al. 2001). In swi1 mutants, a single equational division of the MMC occurs in place of normal female meiosis, but subsequent development is aborted. In the majority of swi1 alleles, pollen development is also disrupted leading to male sterility. In this respect, the SWI1 gene is not so different from other factors that are crucial for the progression of normal plant meiosis. However, one specific allele of swi1, called dyad, shows a less extreme phenotype and while female meiosis is similar to that in other swi1 alleles, male gamete formation is normal and female gametes can be produced at low frequency (Siddiqi et al. 2000). This leads to the production of viable seeds in a few cases. Subsequent analysis of these seeds showed that a small proportion of the few functional female gametes produced in dyad do so without meiotic reduction, as evidenced by the presence of triploid plants amongst the progeny of selfed dyad mutants and maintained parental heterozygosity (Ravi et al. 2008). This phenotype is particularly interesting because the bypass of meiosis and production of unreduced gametes resembles a mechanism called diplospory found in some natural apomictic plants (see below; Koltunow et al. 1995a; Koltunow and Tucker 2008).
Other factors influencing megaspore fate
Although megasporogenesis has been the topic of many morphological and ultrastructural studies, the regulation of megaspore selection and fate is not clearly understood, mainly due to the inaccessible nature of the megaspores in the young ovule. In the majority of angiosperms, only one of the megaspores produced during meiosis continues to develop and form an embryo sac while the others degenerate. One report suggests that megaspore survival is influenced by function of the ANTIKEVORKIAN (AKV) gene in Arabidopsis, because akv mutants show a proportion of ovules (~10%) with all four megaspores surviving (Yang and Sundaresan 2000). However, the molecular identity of the AKV gene has yet to be revealed.
Ultrastructural studies have shown that cellular components such as organelles and plastids are polarised during MMC development and meiosis (Bajon et al. 1999), which may contribute to differential patterning of the megaspore tetrad during, or even before meiosis. Alternatively, the functional megaspore may simply reside in a privileged position within the ovule, thereby gaining access to higher concentrations of nutritional or regulatory molecules that enhance its survival. In either case, some positional information seems to play an important role.
It has also been suggested that the cause of megaspore degeneration is a type of programmed cell death (PCD) or apoptosis (Wu and Cheung 2000; Yang and Sundaresan 2000). Plant cells undergoing PCD show distinguishing features such as cell shrinkage, nuclear DNA degradation, loss of mitochondrial membrane potential, cytochrome c release from mitochondria and induction of caspase-like activity (García-Heredia et al. 2008). The three degrading megaspores show some of these characteristics. TUNEL (TdT-mediated dUTP nick-end labelling) assays are a common method for detecting DNA fragmentation resulting from apoptotic signalling cascades (Gavrieli et al. 1992). In alfalfa (Medicago sativa L.) ovules, TUNEL assays showed that DNA fragmentation was mainly localised to the degenerating megaspores during megasporogenesis (Citterio et al. 2005). In the same study, several MPS-ONE-BINDER (MOB1) genes that encode cell-cycle associated proteins were identified and found to be expressed in the degenerating megaspores (Citterio et al. 2005). In Drosophila and mammalian cells, MOB-like proteins regulate several factors that are involved in cell proliferation and apoptosis (Hirabayashi Nakagawa et al. 2008). Another protein associated with cell death during reproduction and disease resistance is aspartic protease (Xia et al. 2004; Ge et al. 2005). In rice ovules, expression of the S5 aspartic protease is detected in the megaspores and nucellar cells adjoining the megaspores, and loss of function leads to a reduction in female fertility (Chen et al. 2008), suggesting that S5 may regulate megaspore fate.
Changes in dynamic calcium (Ca2+) concentrations have also been implicated in PCD of plant and animal cells (Yamaguchi et al. 1999; Canzoniero et al. 2004). To determine the relationship between calcium levels and megaspore death in lettuce (Lactuca sativa L.) ovules, free Ca2+ levels were examined by histological staining with potassium pyroantimonate (Qiu et al. 2008). Calcium precipitates were rarely detected in the cytoplasm and nucleus of the MMC, but accumulated to similar levels in the linear tetrad of megaspores after meiosis. Interestingly, during the sequential degeneration of the three most micropylar megaspores, calcium precipitates were observed in the cytoplasm but not the nucleus of the degenerating spore, followed by an increase in calcium levels in the nucleolar vacuole of the adjoining megaspores. After degeneration of the last non-functional megaspore, numerous calcium precipitates were noted in the cytoplasm and nucleus of the functional megaspore (Qiu et al. 2008). Although the functional relevance of these changes in calcium distribution is uncertain, the flux of free calcium into the degenerating spores appears to foreshadow imminent degeneration, suggesting that Ca2+ may play a role in signalling between the megaspores.
Finally, one of the most distinguishing morphological features observed during megasporogenesis in the majority of flowering plants is the accumulation of callose (β-1,3-glucan), a plant polysaccharide, in the cell walls of the MMC and megaspores. Callose deposition patterns vary between species (Rodkiewicz 1970) but in most cases, callose accumulates at one pole of the mature MMC and then in the transverse walls that separate the megaspores during meiosis. After the completion of meiosis, callose tends to persist in the part of the cell wall of the functional megaspore that is closest to the degenerating megaspores and diminishes elsewhere, possibly due to the activity of β-1,3-glucanase enzymes that target callose for degradation (Hird et al. 1993; Tucker et al. 2001; Levy et al. 2007). Whether the presence of callose or its deposition in a particular pattern around the megaspores influences their development remains unclear. In the case of pollen development, callose also accumulates around the developing microspores, and alterations in callose deposition due to the precocious activity of β-1,3-glucanase enzymes affect pollen viability but not necessarily meiosis or cell division (Worrall et al. 1992; Scott et al. 2004). Since callose is also deposited in plasmodesmata, pollen tubes and other plant cell types it is not a specific marker for the events of meiosis (Stone and Clarke 1992; Donofrio and Delaney 2001; Rinne et al. 2001), but it may play a common role by acting as a physical barrier to reduce the free space available for the passage of molecules and/or microbes (Stone and Clarke 1992; Radford et al. 1998). In the ovule, callose may act as a molecular or nutritional filter decreasing the permeability of the cell wall, thus enabling the megaspores to embark upon an independent course of development compared with diploid sporophytic surrounding tissues (Bouman 1984). If this is the case, what are the molecules that callose acts to restrict? Is it keeping signals contained within the megaspores or acting to prevent sporophytic signals from gaining entry? Finally, if callose was not present around the MMC or megaspores, what effect would this have on the fate of the megaspores and/or adjoining cells? These questions have yet to be answered in any detail, but molecular techniques now make it possible to modify callose deposition patterns in a temporally and spatially restricted manner and to characterise the downstream effects.
Apomixis: asexual seed formation
So far in this review, we have considered the initial steps of the female sexual pathway from a morphological and molecular perspective. Although it is clear that many regulatory details of this pathway are missing, considerable progress has been made towards identifying key signalling processes that influence reproductive cell fate. These details are of particular interest from a developmental perspective because they provide starting points in the search for upstream regulators and downstream targets. They are also of interest because in several cases, the loss of these signalling pathways leads to phenotypes that resemble early steps in apomictic seed development. It is possible that similar genetic changes may facilitate asexual seed development in wild apomicts. In the next sections of this review, we will address the apomictic process by asking several basic questions: what are the key events that take place during the initiation of apomixis, what is the relationship between apomictic and sexual reproduction and what did apomixis evolve from?
The different types of apomixis and their relationships with sexual reproduction
In the simplest form of apomixis, referred to as adventitious embryony, sexual seed development initiates normally in the ovule and leads to the production of an embryo sac via meiosis and mitosis. However, concomitant with sexual development, multiple somatic nucellar or integumentary cells surrounding the embryo sac spontaneously initiate embryogenesis. Growth of these embryos to maturity appears to be dependent upon fertilisation of the sexual embryo sac, possibly because the fertilised endosperm provides important nutrients and/or growth signals (Lakshmanan and Ambegaokar 1984; Asker and Jerling 1992; Naumova 1993; Koltunow et al. 1995b). Therefore, seeds produced in this manner in plants such as mango and citrus often contain multiple embryos of both maternal and hybrid origin.
Species such as Taraxacum officinale L. (van Dijk et al. 1999), Boechera holboellii Hornem. (Schranz et al. 2005), Tripsacum dactyloides L. (Grimanelli et al. 1998) and Erigeron annus L. (Noyes 2000) follow the type of apomixis referred to as diplospory. In these plants, female sexual development appears to initiate normally with the differentiation of a MMC from the nucellus. However, dependent on the species, this MMC fails to initiate the events of meiosis and immediately begins mitosis (referred to as mitotic diplospory; e.g. T. dactyloides) or enters the first steps of meiosis before aborting and restarting development on a mitotic program (referred to as meiotic diplospory; e.g. E. annus). In both cases, meiotic crossovers between chromosomes are avoided and mitotic divisions of the MMC or its product give rise to an embryo sac without meiosis (apomeiosis) containing an egg-like cell that initiates embryogenesis without fertilisation. Endosperm development in diplosporous species is either autonomous, meaning that it initiates without fertilisation, or more commonly pseudogamous, meaning that it requires fertilisation of the central cell. Because diplospory initiates from cells that were previously ‘sexual’ in origin, there is essentially no interaction between sexual and apomictic cell types in diplosporous plants. However, this does not preclude diplosporous apomicts from producing seeds via the sexual pathway; facultative apomixis is clearly observed in diplosporous Tripsacum and Boechera species, presumably because apomixis fails to initiate in some ovules (Grimanelli et al. 1998; Schranz et al. 2005).
A third type of apomixis, referred to as apospory, occurs in species such as Ranunculus auricomus L. (Nogler 1984), Pennisetum squamulatum Fresen. (Roche et al. 1999) and Hieracium piloselloides Vill. (Koltunow et al. 1998). In ovules from aposporous apomicts, the sexual process typically initiates with differentiation of the MMC and, in the case of Hieracium, often continues to the completion of meiosis. Concurrent with these early stages of sexual development, one or more somatic cells in close proximity to the MMC or megaspores differentiate into aposporous initial (AI) cells, bypass meiosis and directly undergo mitosis (apomeiosis) to give rise to an embryo sac. Similar to diplospory, egg-like cells within these embryo sacs initiate embryogenesis without fertilisation and, depending on the species, the endosperm can initiate with or without fertilisation. In aposporous apomicts, AI cells can appear at different times and with different frequencies during ovule development. In apomictic H. piloselloides for example, between one and four aposporous initial cells differentiate at the chalazal end of the ovule during late meiosis and megaspore selection, and are clearly distinguished by their large nucleus, dense cytoplasm and their close proximity to the sexual structures (Koltunow et al. 1998, 2000). By contrast, in apomictic Hieracium aurantiacum L., multiple AI cells can be detected in ovules at the same time as the MMC, indicating that although the site of AI formation is similar, the timing of AI initiation may be influenced by species specific genetic modifiers. The presence of multiple AI cells, and in some cases the functional megaspore, can lead to the production of more than one embryo sac in ovules of aposporous apomicts. For example, in some apomictic species of Brachiaria (Araujo et al. 2000, 2005) many ovules contain both sexual and aposporous embryo sacs, resulting in the production of seeds containing both hybrid and maternal embryos. In other aposporous apomictic species such as Pennisetum and Hieracium, however, the development of the sexual embryo sac usually terminates at the megaspore mother cell or megaspore stage and is replaced by development of aposporous embryo sac (Bray 1978; Koltunow et al. 1998). Therefore, communication between the aposporous and sexual cell types is likely to influence the fate of the sexual process.
In summary, the common features shared by the different types of apomixis are the ability to generate a cell capable of forming an embryo without having first undergone meiosis (apomeiosis), the capacity to produce an embryo without fertilisation, and the capacity to either produce an endosperm independent of fertilisation (autonomously) or to utilise an endosperm derived from fertilisation. Although the specific details of apomictic reproduction vary from one species to another, all three types of apomixis interact with the sexual process. Diplospory directly replaces the sexual process, adventitious embryony depends on the sexual process for viability and apospory appears to interact with the sexual process on a cellular level to allow for the co-existence or degeneration of sexual cells. In all cases, the events of sexual reproduction appear to initiate in ovules before development deviates towards apomixis. This suggests that the initiation of sexual reproduction may be a prerequisite for apomixis and also that the apomictic process may be superimposed over a basic sexual framework (Fig. 3).
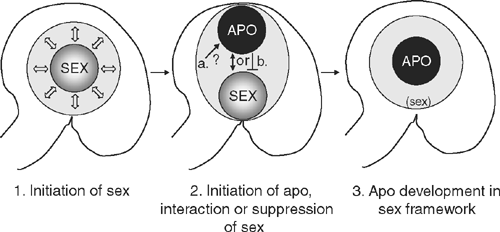
|
Developmental deviations that accompany the initiation of apomixis: a modified sexual pathway?
During the initiation of diplospory, reproductive development deviates from the normal sexual processes when meiosis of the MMC is inhibited and mitosis is precociously stimulated from an unreduced, previously meiotic cell. Although several meiotic mutants have been identified in sexual plants, only the dyad mutant of Arabidopsis has been reported to fail meiosis yet continue development to produce unreduced female gametes, suggesting that the inhibition of meiosis alone is not sufficient to stimulate apomeiosis or that unreduced gametes in other mutants have been overlooked. The elongate1 mutant of maize also produces unreduced female gametes by omitting the second meiotic division, but progeny are genetically diverse due to the success of meiotic crossovers in meiosis I (Barrell and Grossniklaus 2005). In dyad mutants (see above), normal meiosis is inhibited and an unreduced mitotic product of the MMC gives rise to an unreduced embryo sac, albeit at low frequency, containing a functional unreduced egg cell. After fertilisation, dyad eggs can give rise to viable embryos that retain parental heterozygosity. This suggests that dyad is a unique mutant that allows for a breakdown of meiosis while also allowing the precocious stimulation of mitosis from an unreduced cell. Since the phenotype of dyad appears superficially similar to that of diplospory, further studies of this mutant might be helpful in identifying factors that control the transition from meiotic to mitotic development. It is also possible that DYAD function, an important component of the sexual process in Arabidopsis, is altered in diplosporous apomicts thereby supporting development of the apomictic process. However, since apomixis appears to be controlled by one or few dominant loci in the majority of apomicts assessed to date, and the dyad mutation is recessive, it is unlikely that a similar mutation is the key dominant regulator of the diplosporous pathway in wild apomicts. Nonetheless, the characterisation of DYAD function in diplosporous Boechera, a relative of Arabidopsis may be informative. Furthermore, dyad may represent a useful tool for the de novo synthesis of apomixis.
During the initiation and progression of apospory, there are several features that bear a resemblance to modified sexual events. Morphologically, AI cells appear similar to both MMCs and functional megaspores, since they expand to a large size and contain a large nucleus and dense cytoplasm. In apomictic Hieracium, AI cells do not accumulate callose in their cell walls and unlike sexual Hieracium species fail to express MMC marker genes such as AtSPL:GUS and DMC1, and therefore are unlikely to share MMC identity (Tucker et al. 2001, 2003; Okada et al. 2007). However, this may not be the case in all aposporous species. The differentiation of multiple cells capable of giving rise to an embryo sac during apospory is somewhat similar to the ‘extra sporogenous cell’ phenotype of msp1, TDL1A-RNAi and mac1 mutants of rice and maize, respectively. Although this phenotype differs from apospory in that AI cells do not undergo meiosis, it identifies a region of reproductive competence in the developing ovule that surrounds the developing sexual structures. In aposporous apomicts, AI differentiation is predominantly restricted to cells in this zone, suggesting that a similar signalling mechanism may be compromised in these plants.
The development of AI cells and their subsequent morphogenesis into embryo sacs at the expense of the sexual cells in some aposporous apomicts such as Hieracium and Pennisetum bears a striking resemblance to the death of the three non-selected spores during normal sexual development. Termination of the sexual pathway may be promoted by AI cells inducing programmed cell death responses in the sexual cells, or the sexual cells may be crushed and physically displaced by the expanding aposporous embryo sac(s). Studies of megasporogenesis in apomictic Hieracium suggest that degeneration of the megaspores is not simply due to mechanical displacement (Tucker et al. 2001), because megasporogenesis occurs to completion in at least 96% of the ovules from H. piloselloides, which sets >97% apomictic seed. Furthermore, analysis of a H. aurantiacumloss of apospory1 (loa1) mutant (Okada et al. 2007) where the AI is defective in function in that it differentiates in a position removed from cells undergoing sexual events and frequently grows away from the sexual cells, meiosis occurs normally and mitotic events of sexual embryo sac formation proceed in the majority of ovules. Therefore at least in the case of Hieracium, an active mechanism induced by the presence of AI cells is likely to influence the fate of the sexual megaspores. Is this the same mechanism used by the functional megaspore? Molecular evidence indicates that apomictic and sexual reproduction are closely related pathways in Hieracium, since reproductive marker genes that are expressed in the MMC, embryo and endosperm show similar expression in apomictic and sexual plants (Tucker et al. 2003). This suggests that sexual and apomictic pathways in Hieracium share common molecular regulatory features, indicating that they are not distinct pathways. Rather, it seems that apomixis hijacks the process of sexual reproduction at key stages, thereby diverting the normal genetic program of sexual development towards apomictic processes and the production of maternal seed (Fig. 3).
How and why did apomixis evolve?
The benefits of sex and recombination in promoting fitness and adaptation to changing environments are well documented (Otto 2003); how then is it possible for an aberrant process such as apomixis that avoids recombination to arise and be successful in over 40 different angiosperm families? There must be some ecological advantage over sex, and this is supported by the fact that asexual reproduction is not restricted only to the angiosperms but also occurs in other multicellular organisms such as fungi, aphids and sharks (Taylor et al. 1999; Delmotte et al. 2001; Chapman et al. 2007). Theories suggest that asexual reproduction may have benefits when a population is rapidly expanding in a stable environment, because it requires less energy than sexual reproduction (i.e. no fusion of gametes), but sexual reproduction holds the advantage in rapidly changing environments by offering organisms exposure to genetic variation and hence adaptation (Engelstädter 2008; Blachford and Doebeli 2009). Why then are all plants not facultative apomicts, enabling them to experience the best of both worlds? Along with the short-term benefits, there must be some underlying problems associated with apomixis, even if it is facultative, since most apomictic lineages are short-lived and sexually reproducing species are far more common in nature. These concepts are pursued in greater detail in recent reviews (van Dijk 2007; Whitton et al. 2008).
Several models and hypotheses have been proposed that explain the genetic basis and origin of apomixis. Considering that apomixis has been identified in various forms and in over 400 plant species, it is likely that apomixis has evolved multiple times and via multiple paths from sexual ancestors. Therefore, models explaining its appearance may not necessarily be mutually exclusive.
Did apomixis arise through mutation of sexual genes?
The close relationship between sexual and apomictic processes combined with the finding that apomixis is typically under the control of one or few dominant loci (Ozias-Akins and van Dijk 2007) has led to the hypothesis that apomixis may have evolved from mutation(s) in key sexual genes (Peacock et al. 1995). This is supported by the identification of factors in sexual species that show loss- or gain-of-function phenotypes similar to those seen in apomictic plants. For example, the msp1, TDL1A:RNAi rice and Arabidopsis dyad mutants (described above) show similar ovule phenotypes to plants initiating apospory or diplospory, while ectopic expression of various genes including WUSCHEL, BABYBOOM, SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK), LEAFY COTTYLEDON1 or LEAFYCOTYLEDON2 (reviewed in Curtis and Grossniklaus 2007) induces the formation of somatic embryos from vegetative tissues and could possibly act in a similar way during adventitious embryony or autonomous development of unreduced egg cells. Furthermore, Arabidopsis mutants of the FERTILISATION INDEPENDENT SEED (FIS) class can show initiation of embryo development without fertilisation (see multicopy suppressor of ira (msi1) mutants; Guitton and Berger 2005) or endosperm development without fertilisation (medea, fertilisation-independent endospern and fis2; Ohad et al. 1996; Chaudhury et al. 1997; Grossniklaus et al. 1998), similar to that seen in autonomous apomicts. Whether any of these genes play a primary role during the initiation of apomixis, autonomous embryogenesis or endosperm development in wild apomicts is sometimes difficult to assess, since time-consuming functional analysis is often required in complex polyploid sexual and apomictic systems to determine their relevance. In some cases, results are promising, such as those from diposporous Boechera and aposporous Poa pratensis L., where SERK homologues were shown to be differentially expressed between sexual and apomictic ovules, specifically in the cells initiating apomixis (Albertini et al. 2005; Sharbel et al. 2009). In other cases, results are not so promising, such as that of the Arabidopsis FERTILISATION INDEPENDENT ENDOSPERM (FIE) FIS-class gene that represses endosperm development in the absence of fertilisation. fie mutations in Arabidopsis lead to autonomous proliferation of the central cell, but in apomictic Hieracium, which naturally initiates endosperm without fertilisation, a functional FIE orthologueue is critical for the initiation of endosperm development and embryo viability (Rodrigues et al. 2008). Although these candidate genes may have different functions and relevance to apomixis in other species and may yet be useful for the de novo synthesis of apomixis in crops, the identification of ‘apomixis’ genes through mutagenesis or profiling of dicot sexual systems seems to be somewhat unpredictable. Further studies in monocot crops such as rice may prove to be more revealing.
To date, no single mutant has been identified in a mutagenesis screen that displays an intact form of functional apomixis. Despite this, the simple mutation hypothesis seems feasible in species such as Brachiaria brizantha Stapf. (Pessino et al. 1998) and P. squamulatum (Ozias-Akins et al. 1998) where apomeiosis and autonomous embryogenesis are controlled by a single dominant locus. However, it is difficult to rationalise when multiple independent loci control the different components, which is the case for species such as T. officinale (van Dijk et al. 1999), E. annus (Noyes 2005) and H. caespitosum (Catanach et al. 2006). The chance that two mutations could occur almost simultaneously in a plant or population and subsequently give rise to viable apomictic progeny is highly unlikely (Asker and Jerling 1992). Even if a single mutation occurred in a sexual plant that resulted in the formation of unreduced gametes, this would presumably lead to an increase in progeny ploidy and the formation of odd seed ploidy levels that may not be able to support viable seed formation in the F1 generation. Furthermore, if a single mutation inducing parthenogenesis (the capacity to form an embryo autonomously from a reduced egg) occurred without apomeiosis in a sexual plant, then each subsequent reproductive cycle would lead to a halving of the genomic DNA content and severely reduced fitness. One factor that may enhance the longevity of such mutations is the predisposition of some plant families to support apomixis-like phenomena. Evidence to support this comes from the observation that the majority (~75%) of diplosporous and aposporous apomicts are restricted to three families: Asteraceae, Poaceae and Rosaceae. The reason for this bias is unknown, but may relate to the existence of reproductive features and/or genetic modifiers that are compatible with the stable induction of apomixis. Although apomixis typically appears to be controlled by few loci (Ozias-Akins and van Dijk 2007), a detailed flow cytometric seed screen in Paspalum determined that five loci control apomixis (Matzk et al. 2005). Interestingly, all five loci are not essential for apomixis, but different combinations can lead to a higher penetrance of apomictic seed set in a particular plant. Therefore, some of these loci may represent so-called ‘modifiers’ that, while not being essential for the apomictic process, enhance its progression at the expense of sexuality (Koltunow et al. 2000). It seems feasible that some of these loci may already be present in sexual plants, but without the complete set of ‘apomictic’ loci lead only to a base level of reproductive abnormalities.
Interspecific hybridisation may lead to the initiation of apomixis
The observation that virtually all natural apomicts are polyploid and highly heterozygous has led to the suggestion that hybridisation may play a role in the evolution of apomixis (Ernst 1918). Both hybridisation and polyploidy are important processes for the evolution of angiosperms because they have significant effects on genome organisation and gene expression that can increase adaptive potential (Chen 2007; Rieseberg and Willis 2007). The relative contributions of hybridisation and polyploidy to the evolution of apomixis, however, have been difficult to separate. Apomixis can be induced by the synthetic induction of polyploidy from sexual plants (Quarin and Hanna 1980; Quarin et al. 2001), suggesting that in some systems polyploidisation could initiate and maintain asexuality (Grimanelli et al. 2001). This might occur via genomic interactions, rearrangements or epigenetic phenomena that stabilise the genome and allow the manifestation of apomixis. However, apomixis can also occur in wild and synthetically induced diploid plants (Bicknell 1997; Schranz et al. 2005), suggesting that polyploidy is not necessarily a prerequisite but could be a consequence of asexual reproduction that confers some form of genomic stability.
One theory to explain the appearance of apomixis is referred to as the ‘hybridisation theory’ (Carman 1997, 2001, 2007). In this theory, the hybridisation of two sexual ecotypes or related species with different reproductive characters contributes to the induction of apomictic phenomena. On a mechanistic level, this results from hybrid plants containing two sets of parental genes that are involved in female embryo sac development; the asynchronous expression of these duplicated genes leads to precocious embryo sac initiation and embryogenesis at aberrant sites and times during reproduction. The attractive feature of the hybridisation model is that it relies only upon the additive effect of native gene expression, rather than mutations in genes involved in sexual reproduction, and thus overcomes the problems discussed above for independent mutations that have no immediate benefit to reproduction.
Support for this hypothesis comes from the analysis of gene expression in polyploids, where genes show asynchronous expression on a temporal and spatial level depending on the parental genome of origin (Adams et al. 2003; Gu et al. 2004), the appearance of apomictic traits in hybrids of related sexual plants, allo-polyploids or paleo-polyploids (for example, Antennaria, Sorghum and Arabidopsis; see Carman 2001, 2007), and the close morphological and molecular relationships observed during sexual and apomictic processes. Recent support also comes from a study of apomixis in Boechera where transcriptomic profiling was used to compare allele-specific gene expression in ovules from related sexual and apomictic plants (Sharbel et al. 2009). The results from this study show that several genes conserved in sexual and apomictic Boechera species are heterochronously expressed in the apomicts, and that altered levels of expression in the apomict are linked to gene duplication and parent-of-origin effects. Although further studies are required to assess the functional relevance of these expression changes, this study highlights the advantages of high-throughput transcriptomic approaches for the cross-species characterisation of apomixis and its evolutionary origin. If the hybridisation theory is correct, then the genomic regions controlling the initiation of apomixis might simply reflect key regulators of the sexual pathway that are mis-expressed in time and space. This may be an important point to consider as mapping and mutagenesis approaches in Pennisetum, Hieracium and Taraxacum and other apomictic species approach the core regions controlling apomixis in these plants (Vijverberg et al. 2004; Akiyama et al. 2005; Catanach et al. 2006).
Conclusions
In this review, we have explored morphological and molecular details of early stages in sexual and apomictic reproduction in flowering plants. The identification of novel apomixis-like mutants in sexual plants, the initiation of sexual reproduction preceding apomixis in most apomicts and the similar expression of reproductive marker genes in some sexual and apomictic species provide support for the theory that apomixis evolved and is manifested as a modified form of sexual reproduction, instead of an entirely novel reproductive pathway (Fig. 3). Whether this occurred via a combination of mutations in sexual genes and/or hybridisation of related sexual plants or by some other means remains unclear. Further characterisation of the sexual reproductive process, wild type apomicts and mutants lacking apomixis, combined with the identification of the genomic controlling regions and species- and allele-specific changes in gene expression on a genome wide scale will help to solve this puzzle, and bring understanding of apomixis one step closer to reality.
Acknowledgements
We apologise to the many investigators who could not be cited in this review due to space restrictions. We especially thank John Bennett, Steve Swain, Susan Johnson and other members of the Koltunow laboratory for helpful comments and discussions. Research in the Koltunow laboratory is supported by a Department of Education, Science and Training (DEST) Australia–India grant.
Adams KL,
Cronn R,
Percifield R, Wendel JF
(2003) Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proceedings of the National Academy of Sciences of the United States of America 100, 4649–4654.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Akiyama Y,
Hanna W, Ozias-Akins P
(2005) High-resolution physical mapping reveals that the apospory-specific genomic region (ASGR) in Cenchrus ciliaris is located on a heterochromatic and hemizygous region of a single chromosome. Theoretical and Applied Genetics 111, 1042–1051.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Albertini E,
Marconi G,
Reale L,
Barcaccia G,
Porceddu A,
Ferranti F, Falcinelli M
(2005) SERK and APOSTART. Candidate genes for apomixis in Poa pratensis. Plant Physiology 138, 2185–2199.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Araujo ACG,
Mukhambetzhanov S,
Pozzobon MT,
Santana EF, Carneiro VTC
(2000) Female gametophyte development in apomictic and sexual Brachiaria brizantha (Poaceae). Revue de Cytologie et Biologie vegetales-Le Botaniste 23, 13–26.

Araujo A,
Nobrega J,
Pozzobon M, Carneiro V
(2005) Evidence of sexuality in induced tetraploids of Brachiaria brizantha (Poaceae). Euphytica 144, 39–50.
| Crossref | GoogleScholarGoogle Scholar |

Bajon C,
Horlow C,
Motamayor J,
Sauvanet A, Robert D
(1999) Megasporogenesis in Arabidopsis thaliana L.: an ultrastructural study. Sexual Plant Reproduction 12, 99–109.
| Crossref | GoogleScholarGoogle Scholar |

Balasubramanian S, Schneitz K
(2000) NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule development in Arabidopsis thaliana. Development 127, 4227–4238.
|
CAS |
PubMed |

Barrell P, Grossniklaus U
(2005) Confocal microscopy of whole ovules for analysis of reproductive development: the elongate1 mutant affects meiosis. II. The Plant Journal 43, 309–320.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bicknell RA
(1997) Isolation of a diploid, apomictic plant of Hieracium aurantiacum. Sexual Plant Reproduction 10, 168–172.
| Crossref | GoogleScholarGoogle Scholar |

Blachford A, Doebeli M
(2009) On luck and sex. Evolution 63, 40–47.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bohmert K,
Camus I,
Bellini C,
Bouchez D,
Caboche M, Benning C
(1998) AGO1 defines a novel locus of Arabidopsis controlling leaf development. The EMBO Journal 17, 170–180.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Brand U,
Fletcher JC,
Hobe M,
Meyerowitz EM, Simon R
(2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289, 617–619.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bray RA
(1978) Evidence for facultative apomixis in Cenchrus ciliaris. Euphytica 27, 801–804.
| Crossref | GoogleScholarGoogle Scholar |

Canales C,
Bhatt AM,
Scott R, Dickinson H
(2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Current Biology 12, 1718–1727.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Canzoniero LM,
Babcock DJ,
Gottron FJ,
Grabb MC,
Manzerra P,
Snider BJ, Choi DW
(2004) Raising intracellular calcium attenuates neuronal apoptosis triggered by staurosporine or oxygen-glucose deprivation in the presence of glutamate receptor blockade. Neurobiology of Disease 15, 520–528.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Carman JG
(1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society. Linnean Society of London 61, 51–94.
| Crossref | GoogleScholarGoogle Scholar |

Catanach AS,
Erasmuson SK,
Podivinsky E,
Jordan BR, Bicknell R
(2006) Deletion mapping of genetic regions associated with apomixis in Hieracium. Proceedings of the National Academy of Sciences of the United States of America 103, 18 650–18 655.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chapman D,
Shivji M,
Louis E,
Sommer J,
Fletcher H, Prodohl P
(2007) Virgin birth in a hammerhead shark. Biology Letters 3, 425–427.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chaudhury AM,
Ming L,
Miller C,
Craig S,
Dennis ES, Peacock WJ
(1997) Fertilization-independent seed development in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 94, 4223–4228.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chen ZJ
(2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annual Review of Plant Biology 58, 377–406.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chen J,
Ding J,
Ouyang Y,
Du H, Yang J , et al.
(2008) A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proceedings of the National Academy of Sciences of the United States of America 105, 11 436–11 441.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Citterio S,
Albertini E,
Varotto S,
Feltrin E,
Soattin M,
Marconi G,
Sgorbati S,
Lucchin M, Barcaccia G
(2005) Alfalfa Mob 1-like genes are expressed in reproductive organs during meiosis and gametogenesis. Plant Molecular Biology 58, 789–807.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Colombo L,
Battaglia R, Kater MM
(2008) Arabidopsis ovule development and its evolutionary conservation. Trends in Plant Science 13, 444–450.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Crepet W, Niklas K
(2009) Darwin’s second “Abominable mystery”: why are there so many angiosperm species? American Journal of Botany 96, 366–381.
| Crossref | GoogleScholarGoogle Scholar |

Delmotte F,
Leterme N,
Bonhomme J,
Rispe C, Simon J
(2001) Multiple routes to asexuality in an aphid species. Proceedings of the Royal Society of London. Series B. Biological Sciences 268, 2291–2299.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Deyhle F,
Sarkar AK,
Tucker EJ, Laux T
(2007) WUSCHEL regulates cell differentiation during anther development. Developmental Biology 302, 154–159.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Diévart A, Clark SE
(2004) LRR-containing receptors regulating plant development and defense. Development 131, 251–261.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Donofrio NM, Delaney TP
(2001) Abnormal callose response phenotype and hypersusceptibility to Peronospoara parasitica in defence-compromised Arabidopsis nim1–1 and salicylate hydroxylase-expressing plants. Molecular Plant–Microbe Interactions 14, 439–450.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Engelstädter J
(2008) Constraints on the evolution of asexual reproduction. BioEssays 30, 1138–1150.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

García-Heredia J,
Hervas M,
De la Rosa M, Navarro J
(2008) Acetylsalicylic acid induces programmed cell death in Arabidopsis cell cultures. Planta 228, 89–97.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gavrieli Y,
Sherman Y, Ben-Sasson SA
(1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of Cell Biology 119, 493–501.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ge X,
Dietrich C,
Matsuno M,
Li G,
Berg H, Xia Y
(2005) An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Reports 6, 282–288.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Grimanelli D,
Leblanc O,
Espinosa E,
Perotti E,
De Leon DG, Savidan Y
(1998) Mapping diplosporous apomixis in tetraploid Tripsacum: one gene or several genes? Heredity 80, 33–39.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Grimanelli D,
Leblanc O,
Perotti E, Grossniklaus U
(2001) Developmental genetics of gametophytic apomixis. Trends in Genetics 17, 597–604.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Grossniklaus U, Schneitz K
(1998) The molecular and genetic basis of ovule and megagametophyte development. Seminars in Cell & Developmental Biology 9, 227–238.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Grossniklaus U,
Vielle-Calzada JP,
Hoeppner MA, Gagliano WB
(1998) Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 280, 446–450.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Grossniklaus U,
Spillane C,
Page DR, Kohler C
(2001) Genomic imprinting and seed development: endosperm formation with and without sex. Current Opinion in Plant Biology 4, 21–27.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Groß-Hardt R,
Lenhard M, Laux T
(2002) WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes & Development 16, 1129–1138.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gu YQ,
Coleman-Derr D,
Kong X, Anderson OD
(2004) Rapid genome evolution revealed by comparative sequence analysis of orthologous regions from four Triticeae genomes. Plant Physiology 135, 459–470.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Guitton A, Berger F
(2005) Loss of function of MULTICOPY SUPPRESSOR OF IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Current Biology 15, 750–754.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hirabayashi S,
Nakagawa K,
Sumita K,
Hidaka S,
Kawai T,
Ikeda M,
Kawata A,
Ohno K, Hata Y
(2008) Threonine 74 of MOB1 is a putative key phosphorylation site by MST2 to form the scaffold to activate nuclear Dbf2-related kinase 1. Oncogene 27, 4281–4292.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hird D,
Worrall D,
Hodge R,
Smartt S,
Paul W, Scott R
(1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6-gene displays similarity to beta-1,3-glucanases. The Plant Journal 4, 1023–1033.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Itoh J,
Nonomura K,
Ikeda K,
Yamaki S,
Inukai Y,
Yamagishi H,
Kitano H, Nagato Y
(2005) Rice plant development: from zygote to spikelet. Plant & Cell Physiology 46, 23–47.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jia G,
Liu X,
Owen HA, Zhao D
(2008) Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proceedings of the National Academy of Sciences of the United States of America 105, 2220–2225.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Köhler C, Grossniklaus U
(2005) Seed development and genomic imprinting in plants. Progress in Molecular and Subcellular Biology 38, 237–262.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koltunow AM, Tucker MR
(2008) Functional embryo sac formation in Arabidopsis without meiosis – one step towards asexual seed formation (apomixis) in crops? Journal of Biosciences 33, 309–311.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koltunow AM,
Bicknell RA, Chaudhury AM
(1995a) Apomixis: molecular strategies for the generation of genetically identical seeds without fertilization. Plant Physiology 108, 1345–1352.
|
CAS |
PubMed |

Koltunow AM,
Soltys K,
Nito N, Mcclure S
(1995b) Anther, ovule seed, and nucellar embryo development in Citrus sinensis cv. Valencia. Canadian Journal of Botany 73, 1567–1582.
| Crossref | GoogleScholarGoogle Scholar |

Koltunow AM,
Johnson SD, Bicknell RA
(1998) Sexual and apomictic development in Hieracium. Sexual Plant Reproduction 11, 213–230.
| Crossref | GoogleScholarGoogle Scholar |

Koltunow AM,
Johnson SD, Bicknell RA
(2000) Apomixis is not developmentally conserved in related, genetically characterized Hieracium plants of varying ploidy. Sexual Plant Reproduction 12, 253–266.
| Crossref | GoogleScholarGoogle Scholar |

Laux T,
Mayer KF,
Berger J, Jurgens G
(1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 87–96.
|
CAS |
PubMed |

Levy A,
Erlanger M,
Rosenthal M, Epel B
(2007) A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. The Plant Journal 49, 669–682.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Li LC,
Qin GJ,
Tsuge T,
Hou XH, Ding MY , et al.
(2008) SPOROCYTELESS modulates YUCCA expression to regulate the development of lateral organs in Arabidopsis. New Phytologist 179, 751–764.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lynn K,
Fernandez A,
Aida M,
Sedbrook J,
Tasaka M,
Masson P, Barton MK
(1999) The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 126, 469–481.
|
CAS |
PubMed |

Ma H
(2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annual Review of Plant Biology 56, 393–434.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Matzk F,
Prodanovic S,
Baumlein H, Schubert I
(2005) The inheritance of apomixis in Poa pratensis confirms a five locus model with differences in gene expressivity and penetrance. The Plant Cell 17, 13–24.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mercier R, Grelon M
(2008) Meiosis in plants: ten years of gene discovery. Cytogenetic and Genome Research 120, 281–290.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mercier R,
Vezon D,
Bullier E,
Motamayor JC,
Sellier A,
Lefevre F,
Pelletier G, Horlow C
(2001) SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes & Development 15, 1859–1871.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Moussian B,
Schoof H,
Haecker A,
Jurgens G, Laux T
(1998) Role of the ZWILLE gene in the regulation of central shoot meristem cell fate during Arabidopsis embryogenesis. The EMBO Journal 17, 1799–1809.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Nonomura K,
Miyoshi K,
Eiguchi M,
Suzuki T,
Miyao A,
Hirochika H, Kurata N
(2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. The Plant Cell 15, 1728–1739.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Nonomura K,
Morohoshi A,
Nakano M,
Eiguchi M,
Miyao A,
Hirochika H, Kurata N
(2007) A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. The Plant Cell 19, 2583–2594.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Noyes RD
(2000) Diplospory and parthenogenesis in sexual × agamospermous (apomictic) Erigeron (Asteraceae) hybrids. International Journal of Plant Sciences 161, 1–12.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Noyes RD
(2005) Inheritance of apomeiosis (diplospory) in fleabanes (Erigeron, Asteraceae). Heredity 94, 193–198.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ogawa M,
Shinohara H,
Sakagami Y, Matsubayashi Y
(2008) Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319, 294.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ohad N,
Margossian L,
Hsu Y,
Williams C,
Repetti P, Fischer RL
(1996) A mutation that allows endosperm development without fertilization. Proceedings of the National Academy of Sciences of the United States of America 93, 5319–5324.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Okada T,
Catanach A,
Johnson S,
Bicknell R, Koltunow A
(2007) An Hieracium mutant, loss of apomeiosis 1 (loa1) is defective in the initiation of apomixis. Sexual Plant Reproduction 20, 199–211.
| Crossref | GoogleScholarGoogle Scholar |

Otto S
(2003) The advantages of segregation and the evolution of sex. Genetics 164, 1099–1118.
| PubMed |

Ozias-Akins P, van Dijk PJ
(2007) Mendelian genetics of apomixis in plants. Annual Review of Genetics 41, 509–537.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ozias-Akins P,
Roche D, Hanna W
(1998) Tight clustering and hemizygosity of apomixis-linked molecular markers in Pennisetum squamulatum genetic control of apospory by a divergent locus that may have no allelic form in sexual genotypes. Proceedings of the National Academy of Sciences of the United States of America 95, 5127–5132.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Pessino S,
Evans C,
Ortiz J,
Armstead I,
Do Valle C, Hayward M
(1998) A genetic map of the apospory-region in Brachiaria hybrids: identification of two markers closely associated with the trait. Hereditas 128, 153–158.
| Crossref | GoogleScholarGoogle Scholar |

Qiu Y,
Liu R,
Xie C,
Russell S, Tian H
(2008) Calcium changes during megasporogenesis and megaspore degeneration in lettuce (Lactuca sativa L.). Sexual Plant Reproduction 21, 197–204.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Quarin CL, Hanna WW
(1980) Effect of three ploidy levels on meiosis and mode of reproduction in Paspalum hexastachyum. Crop Science 20, 69–75.

Quarin CL,
Espinoza F,
Martinez EJ,
Pessino SC, Bovo OA
(2001) A rise of ploidy level induces the expression of apomixis in Paspalum notatum. Sexual Plant Reproduction 13, 243–249.
| Crossref | GoogleScholarGoogle Scholar |

Radford J,
Vesk M, Overall R
(1998) Callose deposition at plasmodesmata. Protoplasma 201, 30–37.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Ravi M,
Marimuthu MP, Siddiqi I
(2008) Gamete formation without meiosis in Arabidopsis. Nature 451, 1121–1124.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Reiser L, Fischer RL
(1993) The ovule and the embryo sac. The Plant Cell 5, 1291–1301.
| Crossref |
PubMed |

Rieseberg LH, Willis JH
(2007) Plant speciation. Science 317, 910–914.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rinne PL,
Kaikuranta PM, van der Schoot C
(2001) The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. The Plant Journal 26, 249–264.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Roche D,
Cong PS,
Chen ZB,
Hanna WW,
Gustine DL,
Sherwood RT, Ozias-Akins P
(1999) An apospory-specific genomic region is conserved between Buffelgrass (Cenchrus ciliaris L.) and Pennisetum squamulatum Fresen. The Plant Journal 19, 203–208.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rodkiewicz B
(1970) Callose in cell walls during megasporogensis in angiosperms. Planta 93, 39–47.
| Crossref | GoogleScholarGoogle Scholar |

Rodrigues JC,
Tucker MR,
Johnson SD,
Hrmova M, Koltunow AM
(2008) Sexual and apomictic seed formation in Hieracium requires the plant polycomb-group gene FERTILIZATION INDEPENDENT ENDOSPERM. The Plant Cell 20, 2372–2386.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Schiefthaler U,
Balasubramanian S,
Sieber P,
Chevalier D,
Wisman E, Schneitz K
(1999) Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 96, 11 664–11 669.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Schneitz K,
Hulskamp M, Pruitt RE
(1995) Wild-type ovule development in Arabidopsis thaliana – a light-microscope study of cleared whole-mount tissue. The Plant Journal 7, 731–749.
| Crossref | GoogleScholarGoogle Scholar |

Schoof H,
Lenhard M,
Haecker A,
Mayer KFX,
Jürgens G, Laux T
(2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Schranz M,
Dobes C,
Koch M, Mitchell-Olds T
(2005) Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae). American Journal of Botany 92, 1797–1810.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Schruff MC,
Spielman M,
Tiwari S,
Adams S,
Fenby N, Scott RJ
(2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133, 251–261.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Scott RJ,
Spielman M, Dickinson HG
(2004) Stamen structure and function. The Plant Cell 16(Suppl), S46–S60.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sharbel TF,
Voigt ML,
Corral JM,
Thiel T,
Varshney A,
Kumlehn J,
Vogel H, Rotter B
(2009) Molecular signatures of apomictic and sexual ovules in the Boechera holboellii complex. The Plant Journal in press. ,
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sheridan WF,
Avalkina NA,
Shamrov II,
Batygina TB, Golubovskaya IN
(1996) The mac1 gene: controlling the commitment to the meiotic pathway in maize. Genetics 142, 1009–1020.
|
CAS |
PubMed |

Sheridan WF,
Golubeva EA,
Abrhamova LI, Golubovskaya IN
(1999) The mac1 mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics 153, 933–941.
|
CAS |
PubMed |

Shiu SH, Bleecker AB
(2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Science STKE 113, RE22.

Siddiqi I,
Ganesh G,
Grossniklaus U, Subbiah V
(2000) The DYAD gene is required for progression through female meiosis in Arabidopsis. Development 127, 197–200.
|
CAS |
PubMed |

Sieber P,
Gheyselinck J,
Gross-Hardt R,
Laux T,
Grossniklaus U, Schneitz K
(2004) Pattern formation during early ovule development in Arabidopsis thaliana. Developmental Biology 273, 321–334.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Skinner DJ,
Hill TA, Gasser CS
(2004) Regulation of ovule development. The Plant Cell 16(Suppl), S32–S45.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Taylor J,
Jacobson D, Fisher M
(1999) The evolution of asexual fungi: reproduction, speciation and classification. Annual Review of Phytopathology 37, 197–246.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Theissen G, Melzer R
(2007) Molecular mechanisms underlying origin and diversification of the angiosperm flower. Annals of Botany 100, 603–619.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tucker M,
Paech N,
Willemse M, Koltunow A
(2001) Dynamics of callose deposition and beta-1,3-glucanase expression during reproductive events in sexual and apomictic Hieracium. Planta 212, 487–498.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tucker M,
Araujo A,
Paech N,
Hecht V,
Schmidt E,
Rossell J,
de Vries S, Koltunow A
(2003) Sexual and apomictic reproduction in Hieracium subgenus Pilosella are closely interrelated developmental pathways. The Plant Cell 15, 1524–1537.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tucker M,
Hinze A,
Tucker E,
Takada S,
Jurgens G, Laux T
(2008) Vascular signalling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 135, 2839–2843.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

van Dijk PJ,
Tas ICQ,
Falque M, Bakx-Schotman T
(1999) Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83, 715–721.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vaucheret H
(2008) Plant ARGONAUTES. Trends in Plant Science 13, 350–358.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Vijverberg K,
Van der Hulst R,
Lindhout P, Van Dijk P
(2004) A genetic linkage map of the diplosporous chromosomal region in Taraxacum officinale (common dandelion; Asteraceae). Theoretical and Applied Genetics 108, 725–732.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Webb MC, Gunning BES
(1990) Embryo sac development in Arabidopsis thaliana. I. Megasporogenesis, including the microtubular cytoskeleton. Sexual Plant Reproduction 3, 244–256.
| Crossref |

Webb MC, Gunning BES
(1991) The microtubular cytoskeleton during development of the zygote, proembryo and free-nuclear endosperm in Arabidopsis thaliana (L.) Heynh. Planta 184, 187–195.
| Crossref | GoogleScholarGoogle Scholar |

Webb MC, Gunning BES
(1994) Embryo sac development in Arabidopsis thaliana II: The cytoskeleton during megagametogenesis. Sexual Plant Reproduction 7, 153–163.
| Crossref | GoogleScholarGoogle Scholar |

Whitton J,
Sears C,
Baack E, Otto S
(2008) The dynamic nature of apomixis in the angiosperms. International Journal of Plant Sciences 169, 169–182.
| Crossref | GoogleScholarGoogle Scholar |

Willemse MTM, De Boer-De Jeu MJ
(1981) Megasporogenesis and early megagametogenesis. Acta Societatis Botanicorum Poloniae 50, 111–120.

Worrall D,
Hird DL,
Hodge R,
Wyatt P,
Draper J, Scott R
(1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. The Plant Cell 4, 759–771.
|
CAS |
Crossref |
PubMed |

Wu H, Cheung A
(2000) Programmed cell death in plant reproduction. Plant Molecular Biology 44, 267–281.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wu MF,
Tian Q, Reed JW
(2006) Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133, 4211–4218.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Xia Y,
Suzuki H,
Borevitz J,
Blount J,
Guo Z,
Patel K,
Dixon RA, Lamb C
(2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. The EMBO Journal 23, 980–988.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Yamaguchi Y,
Yamamoto Y, Matsumoto H
(1999) Cell death process initiated by a combination of aluminium and iron in suspension-cultured tobacco cells (Nicotiana tabacum): apoptosis-like cell death mediated by calcium and proteinase. Soil Science and Plant Nutrition 45, 647–657.
|
CAS |

Yang W, Sundaresan V
(2000) Genetics of gametophyte biogenesis in Arabidopsis. Current Opinion in Plant Biology 3, 53–57.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Yang WC,
Ye D,
Xu J, Sundaresan V
(1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes & Development 13, 2108–2117.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhao DZ,
Wang GF,
Speal B, Ma H
(2002) The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes & Development 16, 2021–2031.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhao X,
de Palma J,
Oane R,
Gamuyao R,
Luo M,
Chaudhury A,
Herve P,
Xue Q, Bennett J
(2008) OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. The Plant Journal 54, 375–387.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



