Viewing leaf structure and evolution from a hydraulic perspective
Tim J. Brodribb A E , Taylor S. Feild B D and Lawren Sack CA School of Plant Science, University of Tasmania, Private Bag 55, Hobart, Tas. 7001, Australia.
B Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 37996, USA.
C UCLA Ecology and Evolutionary Biology, 621 Charles E. Young Drive South, Los Angeles, CA 90095-1606, USA.
D Present address: School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
E Corresponding author. Email: timothyb@utas.edu.au
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 37(6) 488-498 https://doi.org/10.1071/FP10010
Submitted: 12 January 2010 Accepted: 6 March 2010 Published: 20 May 2010
Abstract
More than 40 000 km3 year–1 of water flows through the intricate hydraulic pathways inside leaves. This water not only sustains terrestrial productivity, but also constitutes nearly 70% of terrestrial evapotranspiration, thereby influencing both global and local climate (Chapin et al. 2002). Thus, the central role played by leaf vascular systems in terrestrial biology provides an important context for research into the function and evolution of water transport in leaves. Significant progress has been made recently towards understanding the linkages between anatomy and water transport efficiency in leaves, and these discoveries provide a novel perspective to view the evolution of land plants.
Additional keywords: photosynthesis, vein density, xylem.
Leaf hydraulics and gas exchange
Photosynthesis in air affords considerable benefits over photosynthesis in the aquatic zone because CO2 diffuses into and through the leaf 10 000 times faster in air than in water (Nobel 2005). However, the cost of rapid CO2 diffusion into the leaf is a counter-flow of water vapour from the leaf to atmosphere, exposing photosynthetic tissue to potentially lethal desiccation. Vascular plants offset the risk of drying out by investing in a hydraulic system – the xylem – that irrigates the photosynthetic tissue to limit the development of damaging water deficits during photosynthetic gas exchange. However, because the capacity of xylem to supply water is finite and the synthesis of xylem tissue is costly (6.5 and 11.8 mmol glucose per g of cellulose and lignin, respectively (Lambers and Poorter 1992)) selection should favour plants that tailor hydraulic investment to fit the likely evaporational demand of a leaf (Sperry 2003). Clear evidence of the adaptive link between the gas-exchange capacity and hydraulic efficiency comes from studies of leaves (Sack et al. 2003; Brodribb et al. 2005), stems (Brodribb and Feild 2000; Santiago et al. 2004), roots (Becker et al. 1999) and whole plants (Sperry 2000; Meinzer 2002). The contrasting functional and architectural demands upon these different plant organs affect the way evolution has moulded the structure of their water transport systems. The following discussion focuses on the hydraulic systems in leaves, examining how different configurations of hydraulic and photosynthetic tissue are able to satisfy the competing demands of water delivery, light capture and economic cost.
Leaf hydraulics and water supply to the photosynthetic surface
Leaves not only create the demand for water transport in plants, but also represent a major resistor in plant hydraulic system (Tyree 2002). The fact that the water transport pathway in leaves constitutes at least 30% (Sack and Holbrook 2006) of the whole-plant resistance to water flow (while representing only a very small fraction of the whole-plant transport distance) indicates the inherent complexity of irrigating a surface at which evaporation is occurring. Once water enters from the stem into the leaf lamina, there is a dramatic shift in functional and architectural demands upon the hydraulic system. Unlike stems, the hydraulic transport distances in leaves are typically relatively short, but hydraulic transport is complicated by the fact that most leaves are highly flattened to optimise light harvesting efficiency (Smith et al. 1997). As a result, leaf hydraulic systems are generally required to uniformly irrigate a flat surface that is often complex in shape. Maximum hydraulic transport capacity would be achieved by a network of irrigation whereby xylem tubes were plumbed directly into all transpiring cells. Such an extravagant investment in xylem tissue never occurs because the cost to the plant in terms of construction and displacement of photosynthetic tissue would always exceed the benefits in photosynthetic performance. An evolutionary trade-off between the antagonistic demands of maximising photosynthesis relative to structural investment has yielded a great diversity in the hydraulic and morphological character of leaves. Here we will demonstrate how, amidst this diversity, a limited set of optimal solutions have led to broad convergences in leaf structure and morphology in land plants.
The evaporative pathway
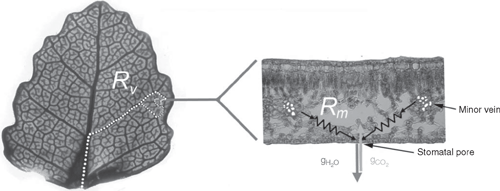
The leaf hydraulic pathway extends from the point of water entry into the leaf (typically the petiole) to the final site of evaporation before water vapour diffuses from the leaf as transpiration through the stomatal pore. Evaporation from the huge surface area of mesophyll cells beneath the epidermis probably accounts for the majority of water loss from leaves, but these living cells have a very limited capacity to transport water. Indeed, the low conductivity of living cells is the reason why specialised water conducting tissue provided such an adaptive advantage to early vascular plants (Raven 1977). In leaves, efficient water transport requires differentiated (non-living) xylem cells to deliver water very close to the sites of evaporation in the mesophyll, thereby providing a hydraulic detour around inefficient transport pathways through living cells. Xylem must, therefore, branch and taper (Coomes et al. 2008) within the leaf lamina, a process that leads to the xylem itself contributing a significant resistance to the hydraulic transport pathway in the leaf. The relative contributions of xylem and mesophyll tissue to the total hydraulic resistance of the leaf can vary across species and can change with microhabitat, since light, desiccation and temperature differentially affect xylem and mesophyll tissues (Sack et al. 2002; Brodribb and Holbrook 2006; Scoffoni et al. 2008). A possible involvement of aquaporins in modulating the extra-xylary hydraulic resistance of leaves has also been demonstrated (Cochard et al. 2007; Kaldenhoff et al. 2008; Heinen et al. 2009).The bulk of evidence indicates that the resistance to water flow through leaves acclimated to light is split evenly between the leaf xylem network and the mesophyll apoplast (Sack et al. 2004; Nardini et al. 2005; Sack and Holbrook 2006).
The precise location of evaporation within the leaf from the liquid to vapour phase is not well understood and cannot yet be visualised or measured directly. Different indirect measurements and models place the site of evaporation either close to the veins (Boyer 1985), evenly throughout the mesophyll (Farquhar and Raschke 1978) or close to the stomata (Meidner 1976; Tyree and Yianoulis 1980). In fact, the specific locations and tissues from which water evaporates within the leaf may vary according to leaf anatomy, because some leaves have huge airspaces such that substantial evaporation might occur inside the leaf far from the stomata, whereas others have tightly packed cells with the only open spaces near the substomatal cavities. Morphological variation even between closely related species can be high and the extremes in mesophyll packing may even be found among species of a single genus, e.g. the Hawaiian Chamaesyce Euphorbias; (Pearcy et al. 1982). Other structures such as bundle sheath extensions that may guide water from the veins to epidermis (Wylie 1952; Zwieniecki et al. 2007) and waxy cuticle coating the inside of the epidermis (Wullschleger and Oosterhuis 1989; Pesacreta and Hasenstein 1999) are likely to modify the passage of water through the leaf. The impacts of such anatomical diversity on the topology and termini of the hydraulic pathways outside of the xylem requires further study.
A general model for hydraulic flow in leaves
In a similar fashion to stems, the hydraulic pathway through a leaf can be considered as analogous to an electrical circuit (van den Honert 1948); where the transpiration stream, flowing through leaf tissues behaves equivalently to current in an electrical circuit. Different parts of the hydraulic pathway in the leaf impose different resistances to water flow, and as the transpiration stream moves through the leaf its water potential (Ψ) declines as a function of the resistivity of the tissue, the length of the pathway and the evaporative flux (Eqn 1). Unlike stems, where water flows through highly specialised non-living tubes, the leaf hydraulic pathway includes an important non-xylem component where water flows out of the vein and traverses the living mesophyll (probably through the apoplast) before evaporating. Eqn 1 shows how the very low conductivity of the mesophyll tissue (κm) has a major influence over the water potential at the sites of evaporation in the leaf. The last term in Eqn 1 represents the hydraulic conductance of the pathway between the leaf vein termini and the evaporation sites furthest from the xylem veinlet. Notwithstanding the uncertainties mentioned above, the length of hydraulic path through the mesophyll apoplast would theoretically have a major influence in determining the total hydraulic conductance of the leaf (Kleaf).

where ΔΨleaf is water potential drop across the whole leaf, E is evaporation flux, Kv is hydraulic conductance of the xylem vein network; dmax is the longest distance from minor vein terminals to the sites of evaporation, κm is the hydraulic conductivity of mesophyll tissue.
The importance of the mesophyll hydraulic pathway was recently demonstrated in a diverse sample of plants ranging in complexity from mosses to angiosperms (Fig. 1; Brodribb et al. 2007). In their broad sample of leaves Brodribb et al. (2007) found that Kleaf was strongly correlated with the distance from the terminals of the leaf xylem network to the inner surface of the epidermis adjacent to the stomatal pores (Fig. 2). This discovery provides a basis for predicting both Kleaf and photosynthetic performance from leaf anatomical parameters that can easily be assessed. Mesophyll hydraulic path length is calculated from the distances between veins (an inverse correlate of vein density) and the vertical distance from vein to epidermis. Thus, the maximal efficiency of water transport in leaves under optimum conditions is predictably related to vein density and the length of the hydraulic pathway through the mesophyll, which themselves are related (Sack and Frole 2006; Brodribb et al. 2007; Noblin et al. 2008). Data by Brodribb et al. (2007) suggests that hydraulic resistivity of leaf mesophyll tissue is conservative across species under given conditions and hence that Kleaf can be estimated from the vein density, or the distance water in the transpiration stream must traverse as it travels from the termini of the xylem network to sites of evaporation. Combined with the close relationship across diverse species between Kleaf and the maximum rate of photosynthesis Pmax, this general model of leaf hydraulic flow provides an important link between the hydraulic architecture of leaves and their capacity for CO2 uptake.
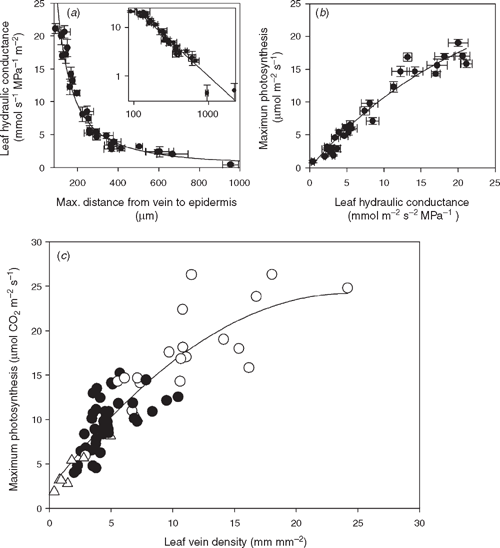
|
|
Leaf morphology and hydraulic performance
The maximum photosynthetic rate of leaves growing under conditions of high light is often limited by the ability of the leaf hydraulic system to maintain leaves sufficiently hydrated for stomata to remain fully open (Nardini and Salleo 2003; Brodribb et al. 2005). Plants growing in full sun, therefore, have only limited architectural options for photosynthetic structures that can satisfy the multiple biophysical demands of water supply light capture and high rates of photosynthetic gas exchange. Examining the spectrum of land plant foliar morphology reveals that over the ~400 million year history of leaves, three recurrent patterns emerge as successful hydraulic architectures in canopies exposed to full sun. The features and functions of these three leaf categories are discussed below.
Needles and scales
The simplest leaf hydraulic architecture observed in vascular plants involves photosynthetic tissue arranged around a central vein. Modern examples of such architectures are the needle and scale leaves common to most temperate conifers. Single-vein leaf hydraulic architectures can sustain leaves with moderate photosynthetic rates per area (Brodribb and Feild 2008) under substantial evaporative load. However, the earliest examples of single vein leaves were unlikely to have been so efficient. Amongst the first endohydric land plants were the Silurian Rhyniophytes, which produced bifurcating axes that approximated needles as photosynthetic structures (~400 million years ago). Although superficially similar to the needles of modern plants, close examination of the photosynthetic structures of plants like Cooksonia indicate that the hydraulic configuration of these early plants was distinctly different from the needles of modern sun-dwelling species. Unlike modern needles, the cross-sectional detail of typical rhiniophyte foliage indicates that water had to flow long distances from the vein through the mesophyll before evaporating from the epidermis (Edwards 1980). This anatomy suggests that the hydraulic efficiency and photosynthetic capacity of these early plants was low.
In the case of leaves with a single vein, the maximum length of the mesophyll hydraulic pathway is determined by the width of the leaf, which would thus be highly constrained (Zwieniecki et al. 2004a). According to Eqn 1 the water potential (Ψ) of the transpiration stream falls rapidly as water exits the leaf vein and traverses the mesophyll apoplast enroute to the stomata. Assuming fully hydrated soil, the final Ψ at the sites of evaporation will be largely determined by the transpiration rate and the distance from the vein to the evaporating surfaces of the leaf (Fig. 3).
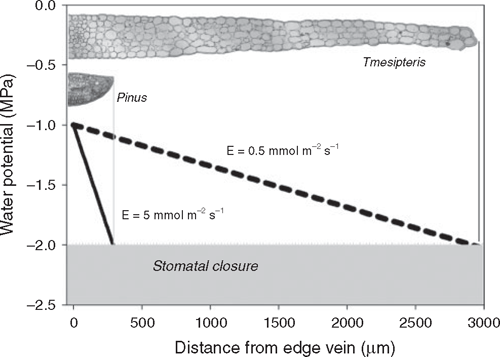
|
Conservatism in the functioning of guard cells of most terrestrial plants prevents leaf water potentials from falling below –2 to –3 MPa before stomata initiate closure (Brodribb and Holbrook 2003; Jacobsen et al. 2008). Thus, the need for stomata to remain open for photosynthesis would constrain the functional maximum distance between veins and transpiring cells (Fig. 3). In single-vein leaves this translates into a constraint upon leaf width because the length of the radial (non-xylem) hydraulic pathway from vein to leaf margin largely determines Kleaf and, hence, the water potential gradient during transpiration. Plants derive little benefit from elaborating leaves that generate much more than 1 MPa of water potential drop during photosynthetic gas exchange because stomata located too far from the midrib would be unable to remain open during the day even when soil water is abundant. Assuming that selection favours plants that do not wastefully allocate carbon or nitrogen (Givnish 1986; Goldberg et al. 2008) it follows that plants should limit the width of needle or scale leaves to enable the stomata of non-water-stressed plants to remain open during the day. Therefore, species that grow in full sun and that attain high photosynthetic rates (and, hence, high transpirational fluxes) would be expected to produce very narrow needles or scale-leaves in which the xylem tissue (or transfusion tissue, see below) is separated only from the stomata by a few cells (Zwieniecki et al. 2004a; Brodribb et al. 2007). This is indeed the case for needles and scale leaves of many productive conifers such as Cedrus, Larix and Pinus (Pinaceae) and Callitris, Cupressus and Juniperus (Cupressaceae). By contrast, single-vein species that grow under low light intensities, with low stomatal densities and low capacity for photosynthesis can attain much greater leaf widths. Single-vein leaves of some ferns and lycopods that are adapted to low light habitats can reach widths up to 8 mm (Tmesipteris; Fig. 3). However, such a long hydraulic pathway through mesophyll tissue renders Kleaf very low, thereby limiting gas exchange to extremely low rates to prevent the development of damaging water deficits.
The needles and scale leaves of conifers are successful in high light but there are clear limits on the utility of this hydraulic design where competition for light is intense in the forest understorey. A general trend of increased leaf size in plants grown under low light (Givnish 1987; Abrams and Kubiske 1990) suggests that the economic benefits of large leaf size for reducing the proportion of mechanical support costs (Westoby and Wright 2003; Niinemets et al. 2007a) become important in the understorey (Saldaña et al. 2007). Because of the hydraulic constraint on maximum width, needles can increase in size only by increasing length. However, mechanical support costs increase disproportionally with leaf length (Niklas 1992) and when combined with the requirement for hydraulic tapering along the leaf (Zwieniecki et al. 2006), long needles would become an expensive means of increasing leaf area in the understorey. By contrast, high light conditions tend to favour leaves with high gas exchange rates rather than large leaf areas, which have reduced capacity for convective cooling; (Vogel 2009). Under these conditions narrow needles represent an important hydraulic architecture.
High vein density
The size limitation of single vein leaves was overcome early during the evolution of land plants by the production of a branched network of veins to irrigate the leaf lamina (Roth-Nebelsick et al. 2001). Branching leaf venation largely removes the hydraulic constraint on leaf width because the mesophyll hydraulic pathway is defined by the distance between veins rather than the distance from the vein to the leaf margin (Fig. 2). As a result, the hydraulic conductivity of multi-vein leaves is dependent upon the density of veins (Dv) per unit area (mm mm–2) in the lamina (Sack and Frole 2006; Brodribb et al. 2007). The relationship between Dv and Kleaf can be determined in a similar fashion to single-vein leaves, by calculating the mean distance from vein termini to the evaporative surface. Higher Dv yields shorter mesophyll distances and hence higher Kleaf, which, in turn, enables leaves to produce greater stomatal conductance and higher photosynthetic rates. In the case of multi-vein leaves an approximation of the maximum mesophyll hydraulic pathway can be calculated from the vein density in one dimension and the thickness of the leaf from the vein endings to the stomatal surface in the other (Figs 1, 2).
The first multi-vein leaves likely possessed open dichotomising venation systems (Boyce 2005) that allowed leaf size to increase substantially beyond the limits of needle and scale morphologies. Open dichotomous venation is not a complete solution to leaf water supply however, because vein density gradients in the leaf are inevitable, leading to non-homogeneous water transport and leaf water potential during transpiration (cf. Roth et al. 1995). The most common open dichotomous leaf vein systems today are seen in ferns, where higher order veins are typically parallel at the leaf margin. Moving from the midrib towards the margin in this type of leaf the number of veins doubles with each hierarchical step in vein order (Fig. 4), and maximum Dv is achieved at the leaf margin. The internal parts of these leaves have lower Dv and all else being equal, would, therefore, have a much lower capability for water transport and photosynthetic performance. Leaf dissection reduces the vein density gradient across leaves and many sun-loving ferns with open dichotomising veins do produce highly dissected leaves (Wagner 1979). However, even with highly divided leaves, the maximum hydraulic conductance and photosynthetic rates observed in leaves with dichotomous venation falls well short of the maxima achieved by leaves with higher density reticulate vein architectures. Additionally, open venation systems may be more affected by damage to veins, as water cannot flow around sites of damage as it can in reticulate systems (Wagner 1979; Sack et al. 2008).
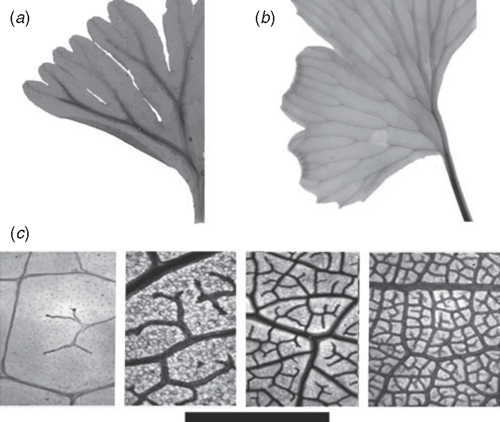
|
Reticulate vein systems evolved early in the diversification of land plants and are found in most major clades of plants both living and extinct (Boyce 2005). The advantage of reticulate plumbing in the leaf is that multiple orders of veins can be developed without generating a gradient in Dv across the leaf. Larger leaves can achieve high vein densities by adding vein orders, thereby removing the relationship between leaf size and Dv. Thus, reticulate venation theoretically enables plants to produce large entire leaves without compromising hydraulic performance (McKown et al. 2010). In plants with reticulate vein leaves, the maximum Kleaf and photosynthetic capacity of individual leaves is a function of the mean density of veins in the leaf lamina (Sack and Frole 2006; Brodribb et al. 2007). Under the current atmospheric conditions only very high Dv (>5 mm mm–2) can supply sufficient water to support the CO2 assimilation rates typically found in productive herbs and trees (Fig. 4c; Brodribb and Feild 2010). Such an observation is particularly interesting in view of the recent realisation that angiosperms represent the only known lineage to have ever evolved high vein densities (Boyce et al. 2009; Brodribb and Feild 2010). Dv values of a few isolated non-angiosperms peak at nearly 6.5 mm mm–2 in some tropical Gnetum species and 5.5 mm mm–2 in dipterid ferns, but angiosperms from highly productive temperate and tropical habitats possess Dv values from 6 to 23 mm mm–2 (Boyce et al. 2009; T. S. Feild, unpubl. data), i.e. 0.6 to 2.3 m of vein cm–2 of leaf. This massive length is equivalent to the highest thread density that can be woven into single-ply fabrics – highlighting both the investment in leaf hydraulic supply and the intricacy of the development process underlying the construction of angiosperm venation architecture.
In modern angiosperm-dominated vegetation, high Dv leaves are the norm. However, terrestrial angiosperms evolved high Dv only following a significant phase of diversification with low Dv leaves and, therefore, low transpirational and photosynthetic capacities (Feild et al. 2009; Brodribb and Feild 2010). Based on the phylogenetic distribution of Dv sampled from a broad diversity of basal lineages for all of the major phylogenetic lineages within extant angiosperms, the five major successive divergences at the base of angiosperm phylogeny were reconstructed as having ancestrally low Dv values in a narrow range between 1 mm mm–2 and 5 mm mm–2 characterising ferns and most non-angiosperm seed plants (Brodribb and Feild 2010). After 40–60 million years of diversification at low vein density (i.e. by the mid-Cretaceous), Dv of angiosperm leaves increased relatively rapidly to high values far above Dv maxima found across non-angiosperms, both living and extinct. Extant phylogeny also suggested that high Dv evolution occurred across major angiosperms clades, with high Dv leaves perhaps appearing multiple times within the angiosperms, specifically within eudicot, magnoliid, and monocot lineages (Brodribb and Feild 2010).
The profound rise in angiosperm Dv during the Late Cretaceous enabled photosynthetic capacity in angiosperms to rise substantially above the static level characteristic of the previous 280 million years of leaf evolution. The reconstructed rise in gas exchange capacity accompanying the appearance of densely-veined leaves pushed photosynthetic rates up from low values to levels equivalent to modern tree dominants and crop plants. The high rates of carbohydrate formation and associated transpiration typical of angiosperm canopies have come to underpin fundamental terrestrial ecosystem processes operating over much of the planet’s vegetation (Boyce et al. 2009). Furthermore, the diversification of Dv in angiosperms is also associated with the occupation of a very wide range of habitats, as the Dv requirement tends to be low for shade species, and higher for exposed species, and highest for C3 and C4 plants of dry areas (Haberlandt 1884; Sack and Frole 2006; Dunbar-Co et al. 2009).
From a hydraulic perspective, many questions remain about why angiosperms were the plant group to evolve high Dv. Current evidence points to the coincidence of environmental opportunity and hydraulic innovation. For example, it is has been posited that declining atmospheric CO2 ([CO2]atm) during the Cretaceous as angiosperms radiated provided a critical environmental opportunity for increased vein branching. As [CO2]atm falls, photosynthesis becomes increasingly substrate limited and hence leaves must produce many more stomata to retain the same photosynthetic rate. More stomata mean more water loss and hence hydraulic efficiency exerts increasing leverage over the rate of photosynthesis (Sperry 2003; Brodribb and Feild 2010). However, the geological record of venation systems and palaeoatmospheric composition indicates that low CO2 cannot be the whole story. For example, Gigantopteridales were probable seed plants from the Permian that produced astonishingly varied multi-vein reticulated vascular systems, with some closely approximating angiosperm leaves (Glasspool et al. 2004). Moreover, giganopterids diversified continuously under low [CO2]atm conditions for nearly 40 million years, before their termination at the Permian-Triassic boundary (Berner 1994; Beerling 2002). At this time, despite apparently appropriate environmental conditions and a gross-approximation of angiosperm venation architecture, there is no evidence that high Dv evolved among the Gigantopterids (Glasspool et al. 2004).
Something unique about angiosperm hydraulic structure appears likely to have interacted with environmental pressure such as low [CO2]atm to enable the evolution of very high Dv in the clade. It is has been suggested that the ability in angiosperms to develop xylem vessels throughout the leaf venation may afford an economical means of expanding the hierarchical organisation and density of the minor vein system (Brodribb and Feild 2010). Hydraulic modelling of reticulate vein architectures reveals that high vein density only becomes economically viable when it is supported by a highly conductive low order venation (McKown et al. 2010). It may be that non-angiosperms lacking leaf vessels cannot build sufficiently high hydraulic conductivity in low vein orders to achieve multi-order hierarchy in the leaf architecture. Indeed, amongst the vesselless angiosperms there are no examples of leaves with Dv > 6 (T. S. Feild and D. S. Chatelet, unpubl. data) and most of these species are confined to understorey or cold environments where transpirational demand remains low (Feild et al. 2004).
Whatever the causes of high Dv evolution in the angiosperms, it is clear that the global transformation of biodiversity during the emergence of the angiosperm epoch is probably as much about veins as it is about flowers and seeds.
Extra-xylary sclereids and tracheids
A third mechanism that enabled plants to evolve leaves with high hydraulic conductivities involves water-conducting cells in the mesophyll beyond the venation system to increase the efficiency of water flow through living tissue. The presence of lignified cells outside the vein system is common throughout land plant phylogeny and is typically associated with support tissue that forms around the central axes of plant organs. However, instances of sclereids apparently engaged in water transport in leaves are more restricted, probably first appearing in Cordaite gymnosperms. Leaf sclereids in extant species are found in both single and multi-vein leaves and have varying degrees of connectivity with the xylem tissue. Here we identify three types of sclereid-enhanced hydraulic systems in leaves and discuss their functional significances.
Accessory transfusion tissue
Transfusion tissue is widespread among gymnosperms incorporating densely-pitted water conducting cells that extend the efficient radial transport of water in single-veined leaves. Restricted to single-vein gymnosperms, this is probably the most sophisticated non-xylem leaf water transport system. The term ‘accessory transfusion tissue’ (ATT) was coined by (Worsdell 1897) to describe a layer of tracheids that extends radially from the midrib of a gymnosperm leaf towards the margin (Fig. 5). The ATT consists of a highly interconnected matrix of lignified, hollow pitted tubes that form a layer sandwiched between the palisade and spongy tissue of the leaf (Fig. 5), and is most commonly found in genera of Podocarpaceae (Podocarpus, Afrocarpus, Acmopyle and Falcatifolium). Almost identical anatomies are also present in leaves of Cycas species (Fig. 5c) and the large (40 mm wide) understorey leaves of the Taxaceae genera Austrotaxus and Amentotaxus.
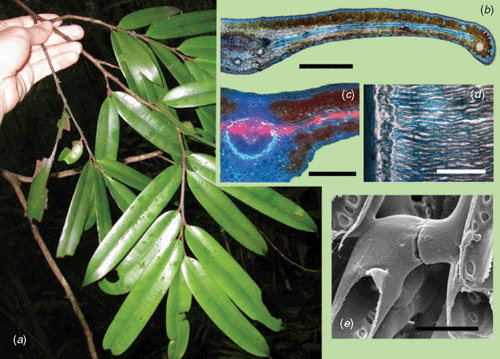
|
Apart from the anatomical inference of a water-transport function of ATT, several other lines of evidence support the functionality of ATT as an extra-xylary hydraulic pathway. Dye experiments on Cycas and Podocarpus leaves clearly demonstrate that water moves rapidly from the midrib of the leaf or pinna out into the ATT thereby allowing efficient radial water transport towards the margin (Fig. 5). Direct measurements of Kleaf in species with ATT have also showed high hydraulic transport efficiency despite the very large mesophyll hydraulic distances involved, implying that ATT was functioning as a transport tissue in the mesopyll (Brodribb et al. 2007). Another study showed ATT cells were water filled and collapsed under tension (Brodribb and Holbrook 2005) in a similar fashion to the xylem tracheids of Pinus species (Cochard et al. 2004). However, the connection between ATT and the xylem does not appear as efficient as a tracheid to tracheid connection in the xylem because the density of tracheids in leaves with ATT is numerically similar to very high vein density (Fig. 5d), yet the Kleaf for ATT-bearing gymnosperms was shown to be lower than that of angiosperms with high Dv. A lower hydraulic efficiency of ATT relative to veins is likely due to the development of the xylem and ATT from different ground tissues and thus an indirect connection between the two can be achieved only late during leaf expansion (Griffith 1957). Rather than being connected through shared pits in cell walls like xylem cells, the ATT may interface with xylem through a layer of living cells, which would markedly reduce hydraulic efficiency.
Isolated sclerieds
Similar but less sophisticated than ATT, the placement of sclereids scattered more or less randomly throughout the mesophyll is very commonly observed in gymnosperms and angiosperms alike. Although these cells often appear to serve non-hydraulic functions (like astrosclereids in Nymphaeales that reinforce aerenchyma channels in the leaf and petioles) there are also many cases where the shape and positioning of sclereids in leaves must confer a substantial hydraulic advantage. Many conifers possess a layer of sclereids in an array similar to ATT (e.g. Prumnopitys, Pseudotsuga) except that the sclereids are isolated in the mesophyll. The hydraulic advantages of these lignified cells are likely to derive from their function as a hydraulic ‘shortcut’ through the mesophyll apoplast and as collapsible water storage elements that increase leaf capacitance.
The most extreme development of hydraulic sclereids occurs in leaves with reticulate veins and a mass of tubular or filiform sclereids that extend throughout the mesophyll and often the palisade tissue directly contacting the majority of cells. This leaf anatomy is widespread and occurs in diverse species, from the scleromorphic leaves of Australian family Proteaceae (Rao et al. 1985) and mediterranean Olea (Arzee 1953) to dry tropical Simaroubiaceae (Foster 1956; Franceschinelli and Yamamoto 1993) and mesic Thea (Zhang et al. 2009). Even amongst gymnosperms there is a representative: the broad leaves of Gnetum known to produce dense, water conducting thread-like filiform fibres in the mesophyll (Tomlinson and Fisher 2005; Feild and Balun 2008). High densities of leaf sclereids are associated with high hydraulic efficiency in leaves (Brodribb and Holbrook 2004), and tend to occur in species growing in high light, and in the case of Olea and Simarouba at high vapour pressure deficit. An advantage of filiform sclereids over increased vein density is that they do not tend to displace mesophyll or palisade tissues, rather tending to meander between cells. However, their construction cost may be high.
Terminal sclereids
The development of sclereids intimately connected with the vein endings was first noted by (Foster 1946) who labelled them ‘terminal sclereids’ as distinct from the previously described isolated sclereids. Terminal sclereids differentiate early during leaf expansion and intrude into the spongy and palisade layers of the leaf after xylem development is complete (Foard 1959). The unusual ‘apical growth’ of terminal sclereids has attracted attention from developmental anatomists (Foster 1947; Rao and Singaray 1968), but the hydraulic potential of these structures has not been investigated. According to the relationship between Dv and Kleaf (Fig. 1), if terminal sclereids possessed equivalent hydraulic transport properties to tracheids, they should substantially improve Kleaf. Indeed, the branching of terminal sclereids close to stomatal crypts (Foster 1947), indicates the potential for water delivery very close to the sites of evaporation in leaves.
The facultative production of terminal sclereids may provide a means of readily adjusting leaf hydraulics to suit ambient growth conditions. Hormones such as auxins (Altalib and Torrey 1961; Rao and Singaray 1968) as well as a high osmotic concentration of growth medium (Foard 1959) have been shown to reduce sclereid development in leaves, whereas surgical manipulation can alter sclereid distribution (Foard 1959). Such plasticity would potentially enable Kleaf to be modified even late in leaf expansion, independently of leaf size or vein density.
Hydraulic supply and leaf economics
Interpretation of leaf hydraulic structure at the community scale requires an understanding of the competitive trade-offs associated with adaptive modifications. Several studies have shown correlations between leaf hydraulic efficiency and the availability of irradiance, water, nutrients and CO2, and vapour pressure deficit (VPD), both within and among species (Sack and Frole 2006; Domec et al. 2009; Gortan et al. 2009). These correlations indicate that investment in venation occurs to the extent that it improves the plant carbon budget. We currently lack an explicit model for calculating benefits against costs, and the degree that given parameters can be altered by genetic and phenotypic modification. Plasticity in vein architecture seems the optimal solution to balance vein investment with environmental demand. Although some plants have a demonstrated capacity to modify the conductivity and density of venation in leaves from exposed and shaded habitats, and from the bottom to top of the canopy (Haberlandt 1884; Maximov 1929; Wylie 1951; Zwieniecki et al. 2004b; Woodruff et al. 2008), in general, it appears that in many clades of land plants the vein branching topology is conservative and slow to evolve (Sack et al. 2008). In general it appears that total vein density varies over a maximum range of 2- to 3-fold within given dicotyledonous genera (Dunbar-Co et al. 2009; Brodribb and Feild 2010).
Modelling of the construction costs related to leaf hydraulic performance have shown that traits that increase Kleaf, such as higher vein conductivities and densities, also tend to increase Kleaf relative to construction costs. Explicit determination of the construction costs of venation (cf. Niinemets et al. 2006, 2007a, 2007b) should be a priority for future work on leaf hydraulics. Formulating the details of cost v. benefit as it applies to hydraulic investment and photosynthetic gain will provide a basis for interpreting patterns of leaf hydraulic architecture in extant natural vegetation as well as in fossil floras. In this latter respect there is considerable potential for using fossil leaf vein characteristics as a tool for reconstructing atmospheric conditions over geological time. Similar to stomatal indices, leaf veins provide insight into the gas exchange characteristics of leaves as well as potentially indicating atmospheric humidity and CO2 concentrations (Uhl and Mosbrugger 1999; Brodribb and Feild 2010). Recent work has demonstrated that leaf veins may in fact be a more sensitive measure of the leaf physiology than stomatal characters, due to the complex influence of stomatal and cuticular architecture upon actual versus modelled gas-exchange rates in leaves (T. S. Feild, D. Chatelet and T. J. Brodribb, unpubl. data).
Conclusions
Leaf hydraulic science provides an informative perspective from which the function and evolution of leaf structure can be interpreted. Strong patterns of leaf hydraulic convergence across all lineages of land plants have led to the recurrence of only relatively few hydraulic configurations capable of supporting leaf gas exchange under high irradiance. Such global hydraulic convergence emphasises the central importance of leaf hydraulic architecture in plant evolution. As discussed above, the processes of water flow and diffusion provide a physical basis for predicting how anatomy constrains the maximum limits of plant hydraulic and gas-exchange performance. Leaf hydraulic traits have the potential to greatly enhance the utility of other commonly cited leaf performance indicators, such as the ‘leaf economic spectrum’, i.e. the general positive correlation of photosynthetic rates per mass with nitrogen per mass, with both negatively correlated with leaf mass per area and leaf lifespan (Wright et al. 2004; Royer et al. 2007). The relationships that link hydraulic capacity, anatomy and gas exchange rates per area, representing a constellation of ‘flux-related traits’ (Sack et al. 2003; Brodribb et al. 2007) appear sufficiently robust as to allow reasonably precise reconstruction of hydraulic and gas-exchange capacity. Beyond these general correlations, however, many other aspects of plant form, function and ecology are likely to interact to determine the evolution of leaf hydraulic architecture within any given lineage, and the set of architectures that assemble and persist in given plant communities (cf. Kraft et al. 2008; Cavender-Bares et al. 2009; Cornwell and Ackerly 2009).
Many challenges remain before we are able to completely understand the anatomical, physiological and evolutionary complexities of leaf water transport. At the molecular scale, the roles of aquaporins and the genetic coordination of hydraulic, stomatal, and photosynthetic systems rank as high priority for research (Maherali et al. 2008). Anatomically there are many questions about where water evaporates in the leaf and the importance of mesophyll structure on water transport. Compartmentalisation in leaf anatomy could dramatically influence water flow and evaporation inside the leaf (Zwieniecki et al. 2007), potentially modifying the relationship between vein architecture and leaf hydraulic conductance. At the scale of whole leaves the hierarchy of unknowns expands due to interactions between leaf function and the rest of the plant and its environment. However, in the light of many recent advances, these challenges can be viewed as achievable goals. Future research promises to decipher the depth of information contained in leaf hydraulic architecture as we move towards a critical understanding of leaf design.
Acknowledgements
Support from the Australian Research Council in the form of a fellowship to TJB is gratefully acknowledged. TSF was supported by US National Science Foundation grant (IOB-0714156) and LS by NSF Grant IOB-0546784.
Abrams MD, Kubiske ME
(1990) Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin: influence of light regime and shade-tolerance rank. Forest Ecology and Management 31, 245–253.
| Crossref | GoogleScholarGoogle Scholar |

Altalib KH, Torrey JG
(1961) Sclereid distribution in leaves of Pseudotsuga under natural and experimental conditions. American Journal of Botany 48, 71–79.
| Crossref |

Arzee T
(1953) Morphology and ontogeny of foliar sclereids in Olea europaea. 1. Distribution and structure. American Journal of Botany 40, 680–687.
| Crossref |

Becker P,
Tyree MT, Tsuda M
(1999) Hydraulic conductances of angiosperms versus conifers: similar transport efficiency at the whole-plant level. Tree Physiology 19, 445–452.
| PubMed |

Beerling DJ
(2002) Low atmospheric CO2 levels during the Permo-Carboniferous glaciation inferred from fossil lycopsids. Proceedings of the National Academy of Sciences of the United States of America 99, 12567–12571.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Berner RA
(1994) GEOCARB II: a revised model of atmospheric CO2 over Phanerozoic time. American Journal of Science 294, 56–91.

Boyce CK
(2005) Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies. Paleobiology 31, 117–140.
| Crossref | GoogleScholarGoogle Scholar |

Boyce CK,
Brodribb TJ,
Feild TS, Zwieniecki MA
(2009) Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proceedings of the Royal Society of London. Series B. Biological Sciences 276, 1771–1776.
| Crossref | GoogleScholarGoogle Scholar |

Boyer JS
(1985) Water transport. Annual Review of Plant Physiology 36, 473–516.
| Crossref | GoogleScholarGoogle Scholar |

Brodribb TJ, Feild TS
(2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell & Environment 23, 1381–1388.
| Crossref | GoogleScholarGoogle Scholar |

Brodribb TJ, Feild TS
(2008) Evolutionary significance of a flat-leaved Pinus in Vietnamese rainforest. New Phytologist 178, 201–209.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb TJ, Feild TS
(2010) Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecology Letters 13, 175–183.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb TJ, Holbrook NM
(2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132, 2166–2173.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb TJ, Holbrook NM
(2004) Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant, Cell & Environment 27, 820–827.
| Crossref | GoogleScholarGoogle Scholar |

Brodribb TJ, Holbrook NM
(2005) Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology 137, 1139–1146.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb TJ, Holbrook NM
(2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell & Environment 29, 2205–2215.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb TJ,
Holbrook NM,
Zwieniecki MA, Palma B
(2005) Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist 165, 839–846.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brodribb T,
Feild T, Jordan G
(2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cavender-Bares J,
Kozak KH,
Fine PVA, Kembel SW
(2009) The merging of community ecology and phylogenetic biology. Ecology Letters 12, 693–715.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cochard H,
Froux F,
Mayr S, Coutard C
(2004) Xylem wall collapse in water-stressed pine needles. Plant Physiology 134, 401–408.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cochard H,
Venisse JS,
Barigah TS,
Brunel N,
Herbette S,
Guilliot A,
Tyree MT, Sakr S
(2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology 143, 122–133.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Coomes DA,
Heathcote S,
Godfrey ER,
Shepherd JJ, Sack L
(2008) Scaling of xylem vessels and veins within the leaves of oak species. Biology Letters 4, 302–306.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cornwell WK, Ackerly DD
(2009) Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs 79, 109–126.
| Crossref | GoogleScholarGoogle Scholar |

Domec JC,
Palmroth S,
Ward E,
Maier CA,
Therezien M, Oren R
(2009) Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N fertilization. Plant, Cell & Environment 32, 1500–1512.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dunbar-Co S,
Sporck MJ, Sack L
(2009) Leaf trait diversification and design in seven rare taxa of the Hawaiian plantago radiation. International Journal of Plant Sciences 170, 61–75.
| Crossref | GoogleScholarGoogle Scholar |

Edwards DS
(1980) Evidence for the sporophytic status of the Lower Devonian plant Rhynia gwynne-vaughanii Kidston and Lang. Review of Palaeobotany and Palynology 29, 177–188.
| Crossref | GoogleScholarGoogle Scholar |

Farquhar GD, Raschke K
(1978) Resistance to transpiration of sites of evaporation within leaf. Plant Physiology 61, 1000–1005.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Feild TS, Balun L
(2008) Xylem hydraulic and photosynthetic function of Gnetum (Gnetales) species from Papua New Guinea. New Phytologist 177, 665–675.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Feild TS,
Arens NC,
Doyle JA,
Dawson TE, Donoghue MJ
(2004) Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30, 82–107.
| Crossref | GoogleScholarGoogle Scholar |

Feild TS,
Chatelet DS, Brodribb TJ
(2009) Ancestral xerophobia: a hypothesis on the whole-plant ecophysiology of early angiosperms. Geobiology 7, 237–264.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Foard DE
(1959) Pattern and control of sclereid formation in the leaf of Camellia japonica. Nature 184, 1663–1664.
| Crossref | GoogleScholarGoogle Scholar |

Foster AS
(1946) Ontogeny of the foliar sclereids in Mouriria huberi Cogn. American Journal of Botany 33, 820.

Foster AS
(1947) Structure and ontogeny of the terminal sclereids in the leaf of Mouriria huberi Cogn. American Journal of Botany 34, 501–514.
| Crossref | GoogleScholarGoogle Scholar |

Foster AS
(1956) Plant idioblasts – remarkable examples of cell specialization. Protoplasma 46, 184–193.
| Crossref | GoogleScholarGoogle Scholar |

Franceschinelli EV, Yamamoto K
(1993) Taxonomic use of leaf anatomical characters in the genus Simarouba (Simaroubaceae). Flora 188, 117–123.

Givnish TJ
(1987) Comparative-studies of leaf form – assessing the relative roles of selective pressures and phylogenetic constraints. New Phytologist 106, 131–160.

Glasspool IJ,
Hilton J,
Collinson ME,
Wang S-J, Sen L-C
(2004) Foliar physiognomy in Cathaysian gigantopterids and the potential to track Palaeozoic climates using an extinct plant group. Palaeogeography, Palaeoclimatology, Palaeoecology 205, 69–110.
| Crossref | GoogleScholarGoogle Scholar |

Goldberg D,
Wildova R, Herben T
(2008) Consistency vs. contingency of trait-performance linkages across taxa. Evolutionary Ecology 22, 477–481.
| Crossref | GoogleScholarGoogle Scholar |

Gortan E,
Nardini A,
Gasco A, Salleo S
(2009) The hydraulic conductance of Fraxinus ornus leaves is constrained by soil water availability and coordinated with gas-exchange rates. Tree Physiology 29, 529–539.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Griffith MM
(1957) Folar ontogeny of Podocarpus macrophyllus with special reference to transfusion tissue. American Journal of Botany 44, 705–715.
| Crossref | GoogleScholarGoogle Scholar |

Heinen RB,
Ye Q, Chaumont F
(2009) Role of aquaporins in leaf physiology. Journal of Experimental Botany 60, 2971–2985.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

van den Honert TH
(1948) Water transport in plants as a catenary process. Discussions of the Faraday Society 3, 146–153.
| Crossref | GoogleScholarGoogle Scholar |

Jacobsen AL,
Pratt RB,
Davis SD, Ewers FW
(2008) Comparative community physiology: nonconvergence in water relations among three semi-arid shrub communities. New Phytologist 180, 100–113.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kaldenhoff R,
Ribas-Carbo M,
Flexas J,
Lovisolo C,
Heckwolf M, Uehlein N
(2008) Aquaporins and plant water balance. Plant, Cell & Environment 31, 658–666.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kraft NJB,
Valencia R, Ackerly DD
(2008) Functional traits and niche-based tree community assembly in an amazonian forest. Science 322, 580–582.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lambers H, Poorter H
(1992) Inherent variation in growth-rate between higher-plants – a search for physiological causes and ecological consequences. Advances in Ecological Research 23, 187–261.
| Crossref | GoogleScholarGoogle Scholar |

Maherali H,
Sherrard ME,
Clifford MH, Latta RG
(2008) Leaf hydraulic conductivity and photosynthesis are genetically correlated in an annual grass. New Phytologist 180, 240–247.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

McKown AD,
Cochard H, Sack L
(2010) Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. The American Naturalist 175, 447–460.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Meidner H
(1976) Water vapor loss from a physical model of a substomatal cavity. Journal of Experimental Botany 27, 691–694.
| Crossref | GoogleScholarGoogle Scholar |

Meinzer FC
(2002) Co-ordination of vapour and liquid phase water transport properties in plants. Plant, Cell & Environment 25, 265–274.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nardini A, Salleo S
(2003) Effects of the experimental blockage of the major veins on hydraulics and gas exchange of Prunus laurocerasus L. leaves. Journal of Experimental Botany 54, 1213–1219.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nardini A,
Gortan E, Salleo S
(2005) Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Functional Plant Biology 32, 953–961.
| Crossref | GoogleScholarGoogle Scholar |

Niinemets U,
Portsmuth A, Tobias M
(2006) Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytologist 171, 91–104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Niinemets U,
Portsmuth A,
Tena D,
Tobias M,
Matesanz S, Valladares F
(2007a) Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Annals of Botany 100, 283–303.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Niinemets U,
Portsmuth A, Tobias M
(2007b) Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation? Functional Ecology 21, 28–40.
| Crossref | GoogleScholarGoogle Scholar |

Noblin X,
Mahadevan L,
Coomaraswamy IA,
Weitz DA,
Holbrook NM, Zwieniecki MA
(2008) Optimal vein density in artificial and real leaves. Proceedings of the National Academy of Sciences of the United States of America 105, 9140–9144.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pearcy RW,
Osteryoung K, Randall D
(1982) Carbon-dioxide exchange characteristics of C4 Hawaiian Euphorbia species native to diverse habitats. Oecologia 55, 333–341.
| Crossref | GoogleScholarGoogle Scholar |

Pesacreta TC, Hasenstein KH
(1999) The internal cuticle of Cirsium horridulum (Asteraceae) leaves. American Journal of Botany 86(7), 923–928.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rao AN, Singaray M
(1968) Controlled differentiation of foliar sclereids in Fagraea fragrans. Experientia 24, 298–299.
| Crossref | GoogleScholarGoogle Scholar |

Rao TA,
Nayak P, Chakraborti S
(1985) Foliar sclereids in Persoonia R.Br. ex Knight (Proteaceae). Current Science 54, 350–353.

Raven JA
(1977) Evolution of vascular land plants in relation to supracellular transport processes. Advances in Botanical Research 5, 153–219.
| Crossref | GoogleScholarGoogle Scholar |

Roth A,
Mosbrugger V,
Belz G, Neugebauer HJ
(1995) Hydrodynamic modelling study of angiosperm leaf venation types. Botanica Acta 108, 121–126.

Roth-Nebelsick A,
Uhl D,
Mosbrugger V, Kerp H
(2001) Evolution and function of leaf architecture: a review. Annals of Botany 87, 553–566.
| Crossref | GoogleScholarGoogle Scholar |

Royer DL,
Sack L,
Wilf P,
Lusk CH, Jordan GJ ,
et al
.
(2007) Fossil leaf economics quantified: calibration, Eocene case study, and implications. Paleobiology 33, 574–589.
| Crossref | GoogleScholarGoogle Scholar |

Sack L, Frole K
(2006) Leaf structural diversity is related to hydraulic capacity in tropical rainforest trees. Ecology 87, 483–491.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sack L, Holbrook NM
(2006) Leaf hydraulics. Annual Review of Plant Physiology and Molecular Biology 57, 361–381.

Sack L,
Melcher PJ,
Zwieniecki MA, Holbrook NM
(2002) The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany 53, 2177–2184.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sack L,
Cowan PD,
Jaikumar N, Holbrook NM
(2003) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell & Environment 26, 1343–1356.
| Crossref | GoogleScholarGoogle Scholar |

Sack L,
Streeter C, Holbrook NM
(2004) Hydraulic analysis of water flow through sugar maple and red oak. Plant Physiology 134, 1824–1833.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sack L,
Dietrich EM,
Streeter CM,
Sanchez-Gomez D, Holbrook NM
(2008) Leaf palmate venation and vascular redundancy confer tolerance of hydraulic disruption. Proceedings of the National Academy of Sciences of the United States of America 105, 1567–1572.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Saldaña A,
Lusk CH,
Gonzáles WL, Gianoli E
(2007) Natural selection on ecophysiological traits of a fern species in a temperate rainforest. Evolutionary Ecology 21, 651–662.
| Crossref | GoogleScholarGoogle Scholar |

Santiago LS,
Goldstein G,
Meinzer FC,
Fisher JB,
Machado K,
Woodruff D, Jones T
(2004) Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140, 543–550.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Scoffoni C,
Pou A,
Aasamaa K, Sack L
(2008) The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell & Environment 31, 1803–1812.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Smith WK,
Vogelmann TC,
DeLucia EH,
Bell DT, Shepherd KA
(1997) Leaf form and photosynthesis. Bioscience 47, 785–793.
| Crossref | GoogleScholarGoogle Scholar |

Sperry JS
(2000) Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology 104, 13–23.
| Crossref | GoogleScholarGoogle Scholar |

Sperry JS
(2003) Evolution of water transport and xylem structure. International Journal of Plant Sciences 164, S115–S127.
| Crossref | GoogleScholarGoogle Scholar |

Tomlinson PB, Fisher JB
(2005) Development of nonlignified fibers in leaves of Gnetum gnemon (Gnetales). American Journal of Botany 92, 383–389.
| Crossref | GoogleScholarGoogle Scholar |

Tyree MT, Yianoulis P
(1980) The site of water evaporation from substomatal cavities, liquid path resistances and hydroactive stomatal closure. Annals of Botany 46, 175–193.

Uhl D, Mosbrugger V
(1999) Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeography, Palaeoclimatology, Palaeoecology 149, 15–26.
| Crossref | GoogleScholarGoogle Scholar |

Vogel S
(2009) Leaves in the lowest and highest winds: temperature, force and shape. New Phytologist 183, 13–26.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wagner WH
(1979) Reticulate veins in the systematics of modern ferns. Taxon 28, 87–95.
| Crossref | GoogleScholarGoogle Scholar |

Westoby M, Wright IJ
(2003) The leaf size-twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135, 621–628.
| PubMed |

Woodruff DR,
Meinzer FC, Lachenbruch B
(2008) Height-related trends in leaf xylem anatomy and shoot hydraulic characteristics in a tall conifer: safety versus efficiency in water transport. New Phytologist 180, 90–99.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Worsdell WC
(1897) On ‘transfusion tissue’: its origin and function in the leaves of gymnospermous plants. Transactions of the Linnean Society London; Botanical Series II 5, 301–319.
| Crossref | GoogleScholarGoogle Scholar |

Wright IJ,
Reich PB,
Westoby M,
Ackerly DD, Baruch Z ,
et al
.
(2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wullschleger SD, Oosterhuis DM
(1989) The occurrence of an internal cuticle in cotton (Gossypium hirsutum L.) leaf stomates. Environmental and Experimental Botany 29, 229–235.
| Crossref | GoogleScholarGoogle Scholar |

Wylie RB
(1951) Principles of foliar organization shown by sun-shade leaves from 10 species of deciduous dicotyledonous trees. American Journal of Botany 38, 355–361.
| Crossref | GoogleScholarGoogle Scholar |

Wylie RB
(1952) The bundle sheath extension in leaves of dicotyledons. American Journal of Botany 39, 645–651.
| Crossref | GoogleScholarGoogle Scholar |

Zhang W,
Hu YX,
Li ZY,
Wang PS, Xu M
(2009) Foliar sclereids in tea and its wild allies, with reference to their taxonomy. Australian Systematic Botany 22, 286–295.
| Crossref | GoogleScholarGoogle Scholar |

Zwieniecki MA,
Boyce CK, Holbrook NM
(2004a) Functional design space of single-veined leaves: role of tissue hydraulic properties in constraining leaf size and shape. Annals of Botany 94, 507–513.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zwieniecki MA,
Boyce CK, Holbrook NM
(2004b) Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell & Environment 27, 357–365.
| Crossref | GoogleScholarGoogle Scholar |

Zwieniecki MA,
Stone HA,
Leigh A,
Boyce CK, Holbrook NM
(2006) Hydraulic design of pine needles: one-dimensional optimization for single-vein leaves. Plant, Cell & Environment 29, 803–809.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zwieniecki MA,
Brodribb TJ, Holbrook NM
(2007) Hydraulic design of leaves: insights from rehydration kinetics. Plant, Cell & Environment 30, 910–921.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



