Development of a novel semi-hydroponic phenotyping system for studying root architecture
Ying L. Chen A D , Vanessa M. Dunbabin B , Art J. Diggle C , Kadambot H. M. Siddique D and Zed Rengel A D EA Soil Science and Plant Nutrition, School of Earth and Environment (M087), University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Tasmanian Institute of Agricultural Research, University of Tasmania, Private Bag 54, Hobart, Tas. 7001, Australia.
C Department of Agriculture and Food, Western Australia, Locked Bag 4, Bentley, WA 6983, Australia.
D University of Western Australia Institute of Agriculture, University of Western Australia (M082), 35 Stirling Highway, Crawley, WA 6009, Australia.
E Corresponding author. Email: zed.rengel@uwa.edu.au
Functional Plant Biology 38(5) 355-363 https://doi.org/10.1071/FP10241
Submitted: 13 December 2010 Accepted: 20 March 2011 Published: 2 May 2011
Abstract
A semi-hydroponic bin system was developed to provide an efficient phenotyping platform for studying root architecture. The system was designed to accommodate a large number of plants in a small area for screening genotypes. It was constructed using inexpensive and easily obtained materials: 240 L plastic mobile bins, clear acrylic panels covered with black calico cloth and a controlled watering system. A screening experiment for root traits of 20 wild genotypes of narrow-leafed lupin (Lupinus angustifolius L.) evaluated the reliability and efficiency of the system. Root architecture, root elongation rate and branching patterns were monitored for 6 weeks. Significant differences in both architectural and morphological traits were observed among tested genotypes, particularly for total root length, branch number, specific root length and branch density. Results demonstrated that the bin system was efficient in screening root traits in narrow-leafed lupin, allowing for rapid measurement of two-dimensional root architecture over time with minimal disturbance to plant growth and without destructive root sampling. The system permits mapping and digital measurement of dynamic growth of taproot and lateral roots. This phenotyping platform is a desirable tool for examining root architecture of deep root systems and large sets of plants in a relatively small space.
Additional keywords: bin system, branching, Lupinus angustifolius, narrow-leafed lupin, root length, root morphology, root system architecture, root traits, RSA.
Introduction
Quantifying the architecture of root systems is essential because crop productivity is almost always influenced by the availability and accessibility of water and nutrients that are heterogeneously distributed in the soil (Miller et al. 2003; Cichy et al. 2009; Gregory et al. 2009). Root architecture directly impacts the capture of such soil resources and is, therefore, fundamental to crop productivity (Lynch 1995; Casson and Lindsey 2003; Malamy 2005; Watt et al. 2006; Dunbabin 2007; Fita et al. 2008; Gregory et al. 2009). Changes in root system architecture and exploitation of soil water influence the accumulation of crop biomass (Hammer et al. 2009).
Progress in root measurement methodology has enhanced our ability to visualise, quantify and conceptualise root architecture and its relationship to plant productivity (Lynch 1995). Numerous approaches have been developed for the study of root architecture with the support of advanced optical recording techniques. However, reliable approaches for phenotyping large numbers of plants are still being developed. Several non soil-filled methods for evaluating roots are available, including hydroponic, aeroponic and agar-plate systems (Gregory et al. 2009). The soil-filled root observation chambers (Manschadi et al. 2008) or rhizotrons (Wiese et al. 2005), and growth pouches (Bonser et al. 1996; Liao et al. 2004) are often used in studying roots. More recently, an improved pouch system was developed by Hund et al. (2009) for rapid measurement of maize (Zea mays L.) root systems during the first days of lateral root growth. In this system, roots grew on the surface of blotting paper facilitating the two-dimensional observation of root growth over time. However, phenotyping large sets of genotypes beyond very early growth stages remains problematic, particularly for mapping studies of quantitative trait loci (QTL).
The main objective of this work was to design and construct an inexpensive, space-saving, high-throughput phenotyping system for quantifying root architecture for large numbers of plants. This system was evaluated through a screening trial with 20 wild genotypes of narrow-leafed lupin (Lupinus angustifolius L.). This paper describes the construction of the bin system, along with a summary of the data that was collected in the screening experiment using this bin system.
Materials and methods
The phenotyping system
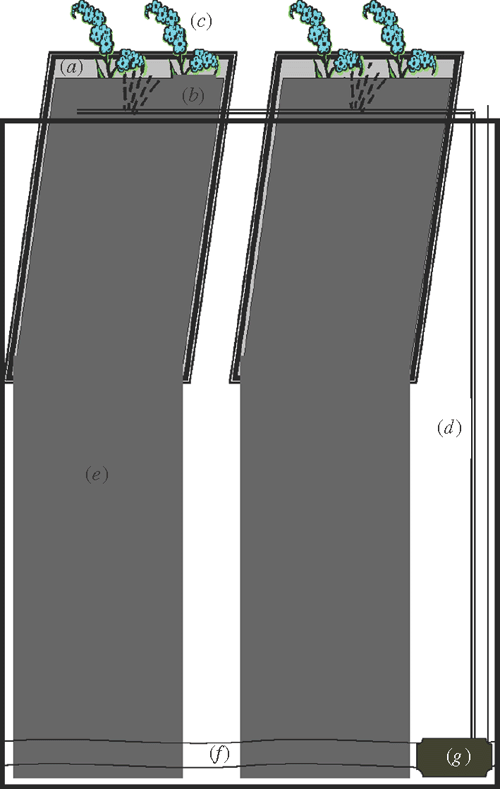
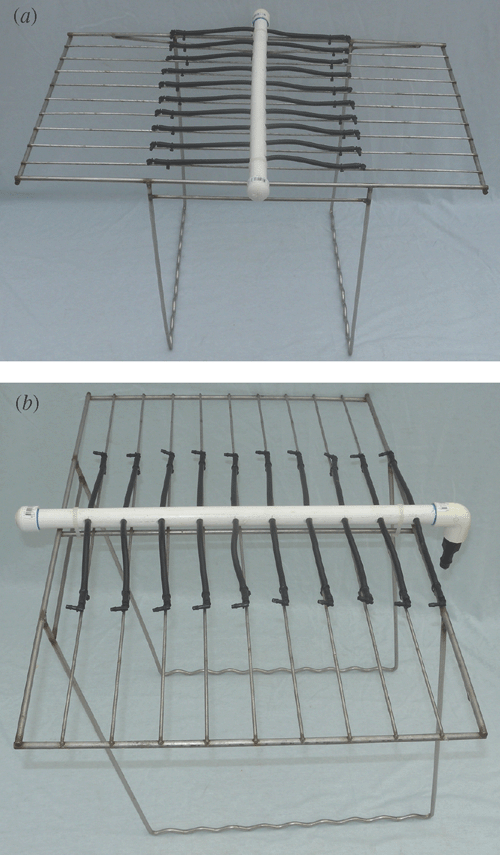
Fig. 1 shows photographs of the operational system. Each unit of the system consisted of a plastic mobile bin, support framework, 20 growth units, and a controlled watering system (Figs 2, 3). A commonly-used mobile rubbish bin (240 L, top 751× 580 mm, height 1080 mm; Silverlock Packaging, Perth, WA, Australia) was chosen. The size of this bin allows for studying deep root systems (up to 1.2 m) and many genotypes within a limited space (40 plants in ~0.5 m2). Also, the bin is mobile (on wheels), which helps in moving and repositioning during the course of the experiment.

|
|
|
Support framework
The stainless steel supporting framework consisted of a top frame (400 × 600 mm) connected perpendicularly with two U-shaped support bars (420 mm deep) (Fig. 3). Within the top rectangular frame there were 20 areas divided equally by nine parallel rods and one central dividing rod for inserting the growth units. The bottom wires of the support bars were bent in a zig-zag to hold the growth units and separate them evenly.
Growth unit
Each plant growth unit contained a 5 mm thick acrylic panel (260 × 480 mm) covered with black calico cloth (570 × 900 mm). Four 15 mm fold-back butterfly clips were used to attach the cloth to the panel on both vertical sides. The lower part of the cloth over each panel was cut in half to make two columns for root training.
Irrigation system
Each bin system was equipped with a watering system consisting of a fountain pump (Pond Max PM1000F, pump flow rate 550 L h–1) and drippers (Philmac Dripper True Drip, Philmac Pty Ltd, Adelaide, SA, Australia) connected with rigid and flexible PVC tubing. The rigid PVC tubing was secured to the middle bar of the support frame with cable ties, and the flexible tubes branched from the rigid tubing onto each dividing bar (Fig. 3). A timer was attached to the pump system for periodic water supplying.
Growth unit assembly
The growth units were positioned in the chambers against the horizontal rods and sited on the zig-zagged wire frame at the base (20 growth units per bin). The drippers located in the middle of the growth units were adjusted to face the growth unit. The wrapped acrylic panel with cloth formed pouches for seedlings to grow, with moisture maintained by irrigation. The growth unit allowed easy imaging down to the bottom of the plate, and the extensions of the cloth allowed room for a total length of root growth of ~1.2 m.
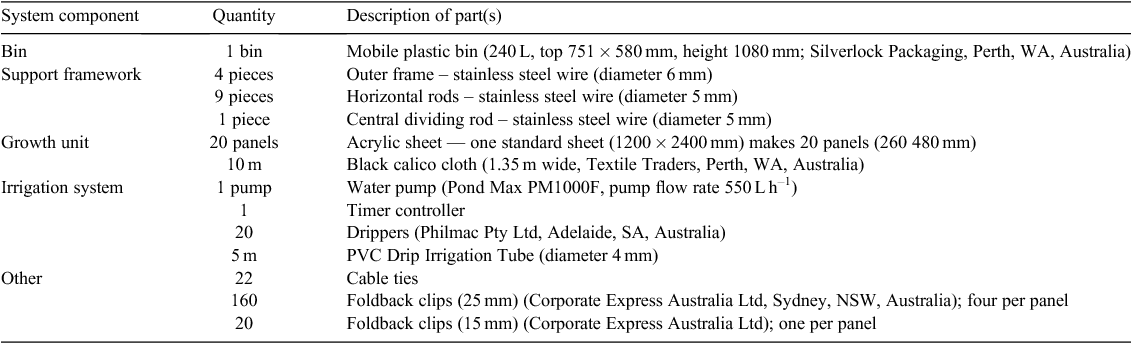
A list of equipment and materials needed to construct one single bin system is provided in Table 1.
|
System trial and evaluation
Plant materials and growth conditions
A screening experiment used two bin systems in a controlled-temperature glasshouse in June–July (20/15°C day/night temperatures) at The University of Western Australia (31°58′S, 115°49′E). Twenty wild genotypes of narrow-leafed lupin (Lupinus angustifolius L.) were randomly chosen, as representatives, from a subset of wild germplasm containing 111 genotypes to be screened for genetic intrinsic of root traits as part of an on-going research project. The 111 genotypes were previously selected using Diversity Array Technology (DArT) method which can detect DNA variability in hundreds of loci simultaneously from a large germplasm pool (1301 genotypes). Seeds were scarified by scalpel and sown in pots filled with washed river sand in a glasshouse. Seeds were germinated for 4 days and four germinated seeds of each genotype were randomly transplanted into growth units of two bins (two plants per bin). Bins were filled with 30 L solution containing (in μM): K (1220), P (20), S (1802), Ca (600), Mg (200), Cu (0.2), Zn (0.75), Mn (0.75), B (5), Co (0.2), Na (0.06), Mo (0.03), Fe (20) and N (1000). The nutrient solution was supplied to the plant growth units by the automatic irrigation system through a timer controller. The periodic pumping was set as 10 min on–5 min off during the daytime (0600–1800 hours), and 5 min on–10 min off during the night time (1800–0600 hours). The solution was refreshed weekly.
Data collection
Root growth was monitored and measured weekly. The pattern of root growth on each plate was photographed by removing the cloth, and an early pattern of root growth (up to 3 weeks) was traced onto a clear panel using a marker pen. After 3 weeks, taproots of most genotypes were beyond the lower base of the plate and the length of taproot was measured. Plants were harvested 6 weeks after transplanting. The roots were subsampled by cutting into 20 cm sections along the taproot. Dry mass of shoot and roots was determined.
Image analysis
Root subsamples from harvest were scanned in greyscale at 300 dpi using a desktop scanner (Epson Expression Scan 1680, Epson America, Long Beach, Canada). Scanned images were analysed in WinRHIZO (V.2009 Pro, Regent Instruments, Montreal, QC, Canada) for root morphological and architectural studies. The debris removal filter of WinRHIZO was set to remove objects with an area smaller than 1 cm2 and a length : width ratio lower than 10. Diameter classes were set to 0.17 mm (equivalent to two pixels) with 15 equal intervals. The program distinguished the taproot, lateral roots and root hairs in the images and generated root data of several traits, indicating that the image quality was appropriate. Root length data computed in WinRHIZO were compared with measurements by the Tennant’s (1975) method and by manually tracing root images, with all three methods producing consistent results (data not shown).
Statistical analyses
One-way ANOVAs were performed on root traits using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). Non-significant differences were found on most of root traits between bins (95% confidence, paired-samples t-test) and, therefore, four plants were treated as four replicates in the analysis. The multivariate standard errors of skewness and kurtosis were 0.29 and 0.58, respectively, when all parameters were included in the analysis, indicating no significant departure from multivariate normality in the dataset. General correlations between parameters were examined with Pearson’s correlation coefficients. Correlations were considered statistically significant and are indicated at *, P ≤ 0.05 or **, P < 0.01. Selected traits were subjected to principal components analysis (PCA; eigenvalue >1, Tabachnik and Fidell 1996) and k-means clustering analysis to compare traits with similar functions among genotypes.
Results
Root development in bin system
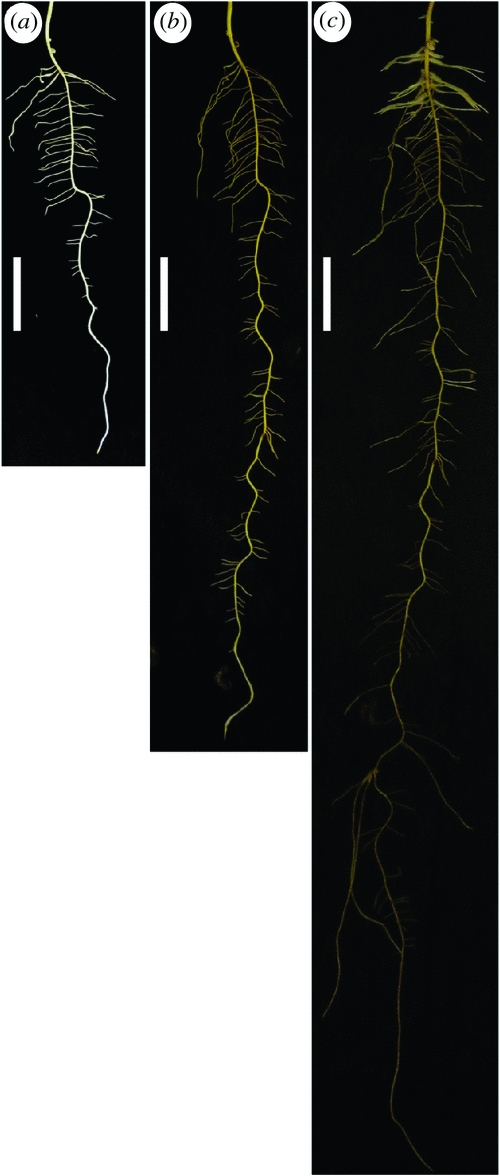
Growth of both shoots and roots was vigorous in the system tested. The morphology of lupin root systems was recorded photographically by removing cover cloth. Genotypic variation in root growth dynamics was apparent. The root systems of all genotypes tested primarily consisted of a taproot and first-order branches in the first 3 weeks (Fig. 4) and second-order branches were also observed for some genotypes in the later growth stage (Fig. 5a).
|

|
By the end of week 6, there was genotypic variation in the density of first-order branches, and in the abundance of second-order branches. It is notable that several genotypes produced occasional fast-growing geotropic first-order branches that resembled taproots to some extent. A few genotypes also had dense root hairs usually on the first-order branches, creating an increased surface area (Fig. 5a, b).
Root trait variation
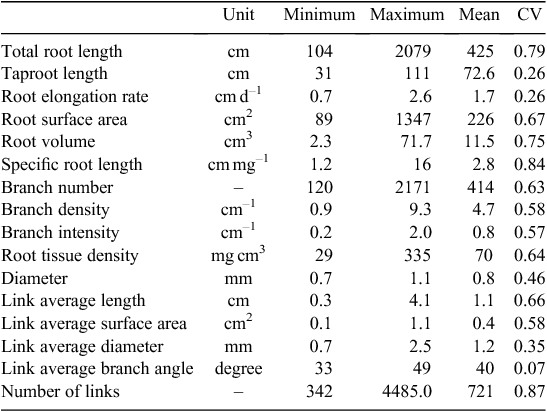
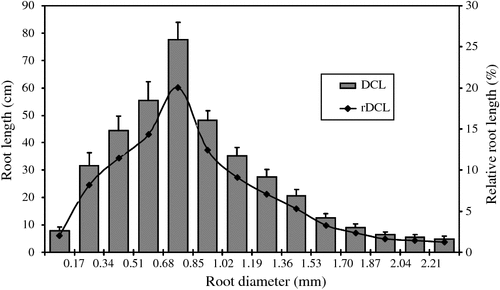
Phenotyping of 20 wild genotypes of narrow-leafed lupin produced root systems that were readily imaged and evaluated. Variations in some root traits between the tested genotypes were large (Table 2). Greatest variations were observed in the number of links, specific root length, total root length and root volume (coefficient variation, CV = 0.87, 0.84, 0.79 and 0.75, respectively). Root area, link length, root tissue density, branch number and density, link area and branch intensity also differed significantly between genotypes (CV = 0.67, 0.66, 0.64, 0.63, 0.58, 0.58 and 0.57, respectively). Mean root diameter showed moderate variation (CV = 0.46). A large proportion of root length occurred in diameter classes of 0.34 to 1.19 mm with 67% in relative diameter-class length (Fig. 6). A diameter class of 0.68–0.85 mm had the longest root length (20% of the total length).
|
|
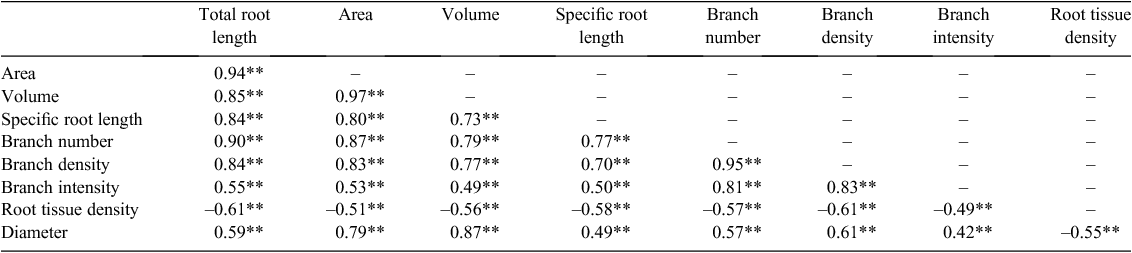
Pearson’s correlation coefficient analysis revealed strong correlations among morphological (e.g. root length, specific root length, root area, average diameter) and architectural traits (branch density and branch intensity) (Table 3). In particular, the total root length was highly correlated with root area (r = 0.94), branch number (0.90), root volume (0.85) and branch density (0.84) (each at the 0.01 level of significance). Specific root length increased as numbers of branches, branch density and branch intensity increased (r = 0.77, 0.70 and 0.50, respectively, P < 0.01). Greater branch density was strongly correlated with increasing branch intensity and root diameter (r = 0.83 and 0.61, respectively, P < 0.01), and decreasing root tissue density (r = −0.61, P < 0.01).
|
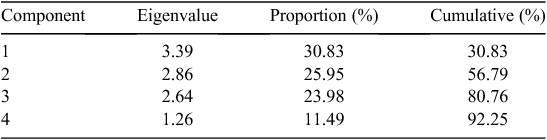
Eleven root traits with large variations (e.g. CV > 0.50, see Table 2 for CV values) were included in the PCA analysis, which showed that 92% of the observed variation of root architecture was captured by four principal components (Tables 4, 5). The first and strongest principal component was associated with genotypes differing primarily in total root length and specific root length representing 31% of the variability. PC2 consisted mostly of root diameter and volume and accounts for 26% of the variability. PC3 and PC4 add another 24% and 11% of the variability, respectively, and were strongly influenced by branch intensity (PC3) and taproot length (PC4).
|

|
Trait variation among genotypes
The tested genotypes varied significantly in the development of taproots and branches. Taproot length ranged from 31 to 111 cm with an average of 73 cm (Table 2). The total root length differed by up to 19-fold among genotypes. Clustering algorithms were used to automatically assign genotypes in terms of variability among root traits. The k-means clustering analysis was performed on the global data of 10 primary traits of each genotype. The 20 genotypes tested were generally separated into two major clusters, with nine and 11 genotypes in each. Branch number, total root length and branch density were the most important contributors to this separation (F = 56, 42 and 37, respectively, P < 0.001; Table 6). In contrast, taproot length and root diameter had little influence on the separation. The value of the final cluster centre indicated genotypes in cluster 1 possessed larger root systems with more root length, branch numbers, and branch density than those in cluster 2.
Discussion
The reason we developed an efficient phenotyping system was to examine genetic intrinsic variations in root traits among wild genotypes of narrow-leafed lupin and to provide basis for root models to constructing three-dimensional root architecture for selecting superior root traits for breeding program. The evaluation experiment using wild narrow-leafed lupin genotypes was to test efficiency of the established phenotyping system and the performance of wild lupin in the system. Therefore, this preliminary investigation has provided information for a follow-up large screening experiment with the same species. The rationale for the choice of narrow-leafed lupin genotypes was also because of the availability of a large germplasm pool of wild narrow-leafed lupin from landraces and diverse climatic and geographic locations (Clements and Cowling 1991; JC Clements, pers. comm.).
Care is needed when handling growth units for observation and photography. This is because lupin roots are relatively fragile and root damage may occur. During root observations, roots and cloth remain moist, but exposure time for observation should be minimised to prevent roots from drying out. The irrigation system can be amended, e.g. by adding another opening for each unit if competition for water occurs between neighbouring plants. The volume of water or solution in the bin can be adjusted depending on the frequency of refreshment and duration of the experiment. Watering frequency and water flow can be optimised with a timer controller attached to the pump system.
Plants of wild narrow-leafed lupin genotypes were tap-rooted confirming previous observations (Clements et al. 1993). Each wild lupin genotype displayed a unique root system architecture when cultivated in our phenotyping system. The root system of each genotype retained similar morphological pattern, particularly in the proximal part, in terms of the number of the first order branches and branch angles during the 6 week growth period. We compared weekly root images and data and concluded that the 6 week root systems presented larger diversity of root traits than the early roots, although variation could be seen as early as 2 weeks after planting. Differences in root architecture among the tested genotypes primarily include taproot length, branch number and branch density. For example, some genotypes developed deeper taproot, or substantially high branch density in the upper root. Root architectural differences have significant impact on the efficient acquisition of water and nutrients in soils (e.g. Nibau et al. 2008; Rengel and Damon 2008; Rose et al. 2009), and, therefore, have relationship to plant productivity (Lynch 1995).
During the preparation of this report, we have obtained comparable data from two consequent phenotyping experiments using the same semi-hydroponic system developed in this study. The large screening experiment examined root trait variation among 111 wild genotypes of narrow-leafed lupin, followed by a repeat experiment with 10 selected genotypes undertaken in the same phenotyping system under the similar grown conditions. There was no significant difference between bins (replications) (P ≤ 0.05). Consistent rankings of genotypes in each root trait between these two separate experiments confirmed the value of the approach for screening large numbers of genotypes using our designed semi-hydroponic phenotyping system. These screening experiments demonstrated a reliable and efficient phenotyping platform for the development of lupin roots. Further examinations are required to explore the potential for evaluation of root system architecture of other crop species on the phenotyping system, and to investigate the biological effects of the specific adaptations of selected genotypes with novel root traits.
Numerous rooting parameters can be collected using this bin-based system coupled with a digital photography and image analysis software (e.g. WinRHIZO). The root architectural traits achieved can be divided into two general categories: topological properties (describing the pattern of root branching) and geometric properties (such as the growth angle of root axes) (Manschadi et al. 2008). Both traits are critical in studying root architecture (Lynch and Brown 2001; Kato et al. 2006; Manschadi et al. 2008). According to Fitter (1991), five types of data are required to reconstruct a three-dimensional model of a root: the number of internal and external links (without and with top meristem, respectively); the length of the links; the distribution of branches within the root, i.e. the topology; the branching angles; and the diameter per link. If one is interested in total size, rather than three-dimensional distribution, the branching angles can be left out. For the total length, rather than volume or weight, the diameters can be excluded and only the first three types of information are needed (van Noordwijk et al. 1994). Data collected from our constructed phenotyping system can be used to simulate a three-dimensional model using root modelling software such as ROOTMAP (Diggle 1988; Dunbabin et al. 2002) and SimRoot (Lynch et al. 1997).
To help understand genotypic variation in root architecture and functions, routine data transformation of some traits were performed to normalise the disparity between plants of different sizes. For example, specific root length (calculated as root length divided by root mass) is an important measure of how root biomass can be used for nutrient acquisition, and is widely used in studying root architecture and functions (Ostonen et al. 2007). Root branch density (number of branches per unit taproot length) and branch intensity (number of branches per unit root length) govern exploration of the soil matrix and may also affect nutrient acquisition (Fitter 1991).
Unlike other hydroponic or aeroponic phenotyping systems (reviewed by Gregory et al. 2009), our bin system is semi-hydroponic and partly aeroponic. It is notable that the bin system significantly reduces environmental stresses such as hypoxia and drought effects by optimising water supply with the equipped automatic irrigation system with a controller. This system using nutrient solution has the advantage of being faster and more accurate for analysing a range of root traits, since it eliminates the challenges of soil contamination, and root loss during washing. Because the supply of water and nutrient can be adjusted for each individual bin system, there is a potential to use this system in studying root plasticity in morphological and physiological responses to water and nutrients.
To conclude, our constructed phenotyping system made from inexpensive readily-available materials was efficient in screening root traits in wild narrow-leafed lupin. It can be used to investigate relatively deep root systems and phenotype root architecture in many genotypes in a relatively small space. Furthermore, the range of root architectures observed in our phenotyping platform can be simulated using root modelling software, i.e. ROOTMAP and SimRoot, for rapid and reliable reconstruction of root systems grown in a particular environment.
Acknowledgements
This research was supported by the Australian Research Council (DP0988193). We acknowledge Dr Jon Clements from the Centre for Legumes in Mediterranean Agriculture, The University of Western Australia, and The Department of Agriculture and Food of Western Australia for providing lupin seed for this work. We are grateful to Professor Jonathan Lynch and Mr Jouke Postma of Penn State University, and Dr Jairo Palta of CSIRO for their advice and comments. Mr Ray Scott of The University of Western Australia provided assistance in constructing the bin system. We also extend our thanks to the two anonymous reviewers for their constructive and helpful comments, by which the content of this paper was much improved.
References
Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist 132, 281–288.| Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnlvFOjtg%3D%3D&md5=87d6675e68df47b4ca2c5c8ee3da42e8CAS | 11541132PubMed |
Casson SA, Lindsey K (2003) Genes and signaling in root development. New Phytologist 158, 11–38.
Cichy KA, Snapp SS, Blair MW (2009) Plant growth habit, root architecture traits and tolerance to low soil phosphorus in an Andean population. Euphytica 165, 257–268.
| Plant growth habit, root architecture traits and tolerance to low soil phosphorus in an Andean population.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVWht7%2FM&md5=e2058d86c20140527fcbb7ea4ce5717eCAS |
Clements JC, Cowling WA (1991) Catalogue of the Australian lupin collection including field evaluation data for wild, semi-domesticated and fully domesticated accessions. Research Report 3/91, Department of Agriculture Western Australia.
Clements JC, White PF, Buirchell BJ (1993) The root morphology of Lupinus angustifolius in relation to other Lupinus species. Australian Journal of Agricultural Research 44, 1367–1375.
| The root morphology of Lupinus angustifolius in relation to other Lupinus species.Crossref | GoogleScholarGoogle Scholar |
Diggle AJ (1988) ROOTMAP – a model in three-dimensional co-ordinates of the growth and structure of fibrous root systems. Plant and Soil 105, 169–178.
| ROOTMAP – a model in three-dimensional co-ordinates of the growth and structure of fibrous root systems.Crossref | GoogleScholarGoogle Scholar |
Dunbabin VM (2007) Simulating the role of rooting traits in crop–weed competition. Field Crops Research 104, 44–51.
| Simulating the role of rooting traits in crop–weed competition.Crossref | GoogleScholarGoogle Scholar |
Dunbabin VM, Diggle AJ, Rengel Z, van Hugten R (2002) Modelling the interactions between water and nutrient uptake and root growth. Plant and Soil 239, 19–38.
| Modelling the interactions between water and nutrient uptake and root growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktVGhtbw%3D&md5=ac9005eaa26733ef90fb2c969c5736a2CAS |
Fita A, Pico B, Monforte AJ, Nuez F (2008) Genetics of root system architecture using near-isogenic lines of melon. Journal of the American Society for Horticultural Science 133, 448–458.
Fitter AH (1991) Characteristics and functions of root systems. In ‘Plant roots: the hidden half’. (Eds Y Waisel, A Eshel, U Kafkafi) pp. 3–25. (Marcel Dekker: New York)
Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young M (2009) Root phenomics of crops: opportunities and challenges. Functional Plant Biology 36, 922–929.
| Root phenomics of crops: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Science 49, 299–312.
| Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt?Crossref | GoogleScholarGoogle Scholar |
Hund A, Trachsel S, Stamp P (2009) Growth of axile and lateral roots of maize. I: Development of a phenotyping platform. Plant and Soil 325, 335–349.
| Growth of axile and lateral roots of maize. I: Development of a phenotyping platform.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSrt7vI&md5=09fa0807116a281710bd93a4cc731b5eCAS |
Kato Y, Abe J, Kamoshita A, Yamagishi J (2006) Genotypic variation in root growth angle in rice and its association with deep root development in upland fields with different water regimes. Plant and Soil 287, 117–129.
| Genotypic variation in root growth angle in rice and its association with deep root development in upland fields with different water regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCntb3N&md5=30a683663c05f4da9eac73c959e01b2fCAS |
Liao H, Yan XL, Rubio G, Beebe SE, Blair MW, Lynch JP (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Functional Plant Biology 31, 959–970.
| Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXosFSqu7s%3D&md5=44220cfe5c0fa1e69cc7c98fc0a20415CAS |
Lynch J (1995) Root architecture and plant productivity. Plant Physiology 109, 7–13.
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237, 225–237.
| Topsoil foraging – an architectural adaptation of plants to low phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVWltA%3D%3D&md5=71bc932d0f826182691b51c3a1545899CAS |
Lynch J, Nielsen K, Davis R, Jablokow A (1997) SimRoot: modeling and visualization of botanical root systems. Plant and Soil 188, 139–151.
| SimRoot: modeling and visualization of botanical root systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjvFymsrc%3D&md5=3f8c959f0a981347f435f40e475fb6f8CAS |
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell & Environment 28, 67–77.
| Intrinsic and environmental response pathways that regulate root system architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsVGqtLg%3D&md5=2304d7791881cee23328eadb6054301fCAS | 16021787PubMed |
Manschadi AM, Hammer GL, Christopher JT, de Voil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant and Soil 303, 115–129.
| Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Shtbg%3D&md5=1a581516a38cfef5eeb321a9eea23032CAS |
Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology 30, 973–985.
| Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVWnsrk%3D&md5=98a85d3b0efe3c84a32f3aa436b6aaaeCAS |
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions, the control of root architecture by lateral root formation. New Phytologist 179, 595–614.
| Branching out in new directions, the control of root architecture by lateral root formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVKns7zN&md5=76b87b0aca58bc16956028edacb4ab9aCAS | 18452506PubMed |
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosystems 141, 426–442.
| Specific root length as an indicator of environmental change.Crossref | GoogleScholarGoogle Scholar |
Rengel Z, Damon PM (2008) Crops and genotypes differ in efficiency of potassium uptake and use. Physiologia Plantarum 133, 624–636.
| Crops and genotypes differ in efficiency of potassium uptake and use.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit78%3D&md5=afd39867683d0d42ad0a7c335ed312adCAS | 18397208PubMed |
Rose TJ, Rengel Z, Ma Q, Bowden JW (2009) Crop species differ in root plasticity response to localised P supply. Journal of Plant Nutrition and Soil Science 172, 360–368.
| Crop species differ in root plasticity response to localised P supply.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFGqurc%3D&md5=775a1582e540c65bd3e6ffe1c5c8274fCAS |
Tabachnik BG, Fidell LS (1996) ‘Using multivariate statistics.’ (Harper Collins: New York)
Tennant D (1975) A test of a modified line interest method for estimating root length. Journal of Ecology 63, 995–1001.
| A test of a modified line interest method for estimating root length.Crossref | GoogleScholarGoogle Scholar |
van Noordwijk M, Spek LY, de Willigen P (1994) Proximal root diameter as predictor of total root size for fractal branching models. I: Theory. Plant and Soil 164, 107–117.
| Proximal root diameter as predictor of total root size for fractal branching models. I: Theory.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXitlyktbo%3D&md5=5dfa04907d69507098a8f398b05054ffCAS |
Watt M, Kirkegaard JA, Passioura JB (2006) Rhizosphere biology and crop productivity. Australian Journal of Soil Research 44, 299–317.
| Rhizosphere biology and crop productivity.Crossref | GoogleScholarGoogle Scholar |
Wiese AH, Riemenschneider DE, Zalesny RS (2005) An inexpensive rhizotron design for two-dimensional, horizontal root growth measurements . Tree Planters’ Notes 51, 40–46.



