Large root systems: are they useful in adapting wheat to dry environments?
Jairo A. Palta A E , Xing Chen A B , Stephen P. Milroy A , Greg J. Rebetzke C , M. Fernanda Dreccer D and Michelle Watt CA CSIRO Plant Industry, Private Bag No. 5, Wembley, WA 6913, Australia.
B Triticeae Research Institute, Sichuan Agricultural University, Yaan, Sichuan 625014, China.
C CSIRO Plant Industry, PO Box 1600, Canberra, ACT 2601, Australia.
D CSIRO Plant Industry, Cooper Laboratory, Warrego Highway, Gatton, Qld 4343, Australia.
E Corresponding author. Email: jairo.palta@csiro.au
This paper was presented at the 2nd International Workshop on Ecosystem Assessment and Management on ‘Climate Change and Agricultural Ecosystem Management in Dry Areas’ held from 19 to 25 July 2010 at Lanzhou University, Lanzhou, China.
Functional Plant Biology 38(5) 347-354 https://doi.org/10.1071/FP11031
Submitted: 28 January 2011 Accepted: 24 March 2011 Published: 2 May 2011
Abstract
There is little consensus on whether having a large root system is the best strategy in adapting wheat (Triticum aestivum L.) to water-limited environments. We explore the reasons for the lack of consensus and aim to answer the question of whether a large root system is useful in adapting wheat to dry environments. We used unpublished data from glasshouse and field experiments examining the relationship between root system size and their functional implication for water capture. Individual root traits for water uptake do not describe a root system as being large or small. However, the recent invigoration of the root system in wheat by indirect selection for increased leaf vigour has enlarged the root system through increases in root biomass and length and root length density. This large root system contributes to increasing the capture of water and nitrogen early in the season, and facilitates the capture of additional water for grain filling. The usefulness of a vigorous root system in increasing wheat yields under water-limited conditions maybe greater in environments where crops rely largely on seasonal rainfall, such as the Mediterranean-type environments. In environments where crops are reliant on stored soil water, a vigorous root system increases the risk of depleting soil water before completion of grain filling.
Additional keywords: root biomass, root length, root length density, root system size, water capture.
Introduction
This paper questions whether a large root system contributes to improved adaptation in wheat (Triticum aestivum L.) to dry environments. A large root system in wheat is described as one that has large biomass, length and root length density (Hamblin and Tennant 1987). The first account of the role played by a root system with large biomass and root length in adapting wheat to dry environments was given by Aamodt and Johnston (1936). A major finding of their study was that the large root system of the wheat cultivar Pelissier was important in the avoidance of damage by drought in a dry season on the Canadian prairies. Since that time, there has been little consensus in the value of large root systems despite most assessments of the adaptation of crops to water-limited environments having the implicit idea that a large root system is able to collect more water, and produce more growth and yield. Reviews by Kramer (1969), Hurd (1974) and Jackson et al. (2000) have suggested that a deep, wide-spreading and much-branched root system is essential in the design of drought-tolerant crops. On the other hand, Passioura (1983) suggested that small root systems could provide benefits in water-limited situations through improved water use efficiency. He reasoned that there would be an optimum root : shoot ratio above which further increases in root size would provide limited benefits but would also impose a cost on shoot growth by consuming biomass. This was demonstrated by Ma et al. (2008) in root pruning experiments in which water use efficiency was increased and a shift in water consumption to the post-anthesis period provided additional benefit through an increase in the harvest index.
There are several reasons for the contrasting conclusions on the value of root system size. The primary reason is that water supply in dryland farming systems is variable among environments and seasons, and imposes water stress at different stages of crop development. For crops which grow on soil water accumulated before sowing or early in growth, there is a significant benefit in conserving water for the reproductive phase (Richards and Passioura 1989; Morison et al. 2008). Alternatively, for crops growing under conditions of more uniform rainfall distribution, the ability to capture water and use it quickly may be beneficial (Turner and Nicolas 1987; Moeller et al. 2009). Severely water-stressed crops leave substantial amounts of available water in the subsoil at maturity and few would argue that the root system was too small to capture soil water. However, for crops growing on stored soil water, there may be benefit if the root system expands slowly to allow soil water to be used later during the grain filling. In a controlled environment study, Passioura (1977) showed that the proportion of water consumed before and after anthesis was correlated with harvest index and hence yield. The benefit of using subsoil moisture late in the season has been demonstrated in the field by (Kirkegaard et al. 2007). Of course, the influence of the rainfall pattern interacts strongly with soil type in terms of its water holding capacity, the depth at which water is stored in the soil and the depth of soil that can be penetrated by the roots. Thus, a genotype that performs well in one water stress environment may not necessarily perform well in another environment or season. In terms of selection and breeding, this means that the target environment and dominant pattern of moisture stress needs to be defined and understood before an appropriate root morphology can be considered.
In addition to aspects related to the adaptation value of the root systems, several experimental issues can also influence the conclusions. A major problem is that the difference between a ‘large’ and ‘small’ root system has not been described well. Parameters such as maximum rooting depth, spread of the root system, root length density, rate of root descent, number of axes or total root DW could all be taken as indication of root system size. Many of these parameters are often correlated across genotypes but this is not always the case (O’Brien 1979; Sanguineti et al. 2007) and the conclusions drawn will depend on which measures are used.
Furthermore, the capacity to assess the value of the size of the root system has often been limited by a restricted genotypic range in the size of the root system of the varieties assessed (Løes and Gahoonia 2004). The root systems of ‘green revolution’ wheat genotypes were smaller than earlier genotypes and landraces (Waines and Ehdaie 2007), a suite of Rht genes being shown to have a significant effect on root growth (Wojciechowski et al. 2009). Landraces of barley (Hordeum vulgare L.) have also been shown to have larger root systems and greater seminal root length than cultivars (Grando and Ceccarelli 1995; Wahbi and Gregory 1995), with a historical analysis of Nordic barley breeding programs showing a long-term downward trend in seedling root weight (Bertholdsson and Brantestam 2009). In light of these results, comparisons between relatively contemporary cultivars may not capture the full genetic variation available within the species. The problem is compounded by environmental factors affecting root growth, such as acid subsoils, compaction layers, root diseases and predation (Tang et al. 2003; Higginbotham et al. 2004; Botwright Acuña et al. 2007). These factors can either limit the expression of root system size or differentially effect genotypes, compromising the capacity to assess the value of potentially different root systems.
Lessons from individual root traits for water capture
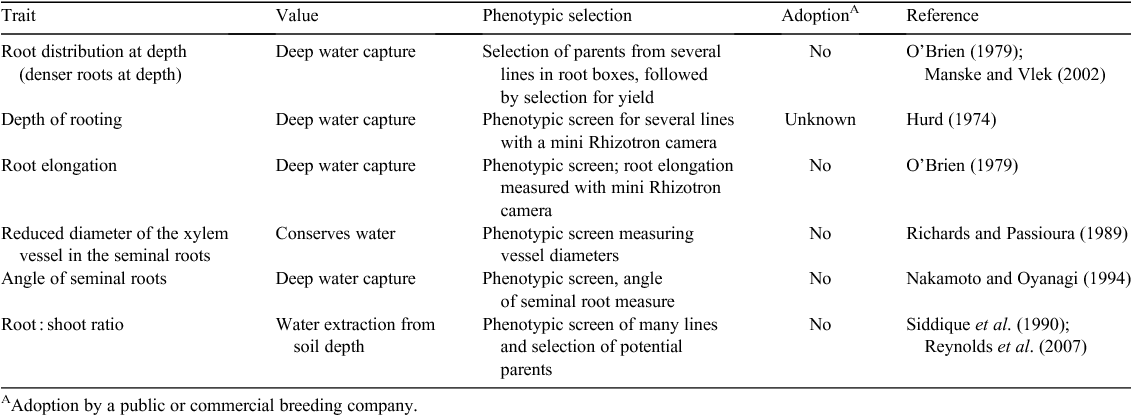
Since the O’Toole and Bland (1987) review, it has become clear that genotypic variation for root system characteristics and their function can be exploited to improve grain yields of crops when grown in environments prone to water deficit (Palta and Watt 2009). During the last 20–25 years, interest in identifying and evaluating root system traits that adapt wheat to water-limited environments has increased. Potential traits include increasing root distribution at depth to improve deep water capture (O’Brien 1979; Manske and Vlek 2002), depth of rooting to extract water from full soil depth (Hurd 1974), fast root elongation rates for deep water capture (O’Brien 1979), reducing the diameter of the xylem vessel in the seminal roots to conserve soil water (Richards and Passioura 1989), angle of seminal roots for extracting water from full soil depth (Nakamoto and Oyanagi 1994; Manschadi et al. 2006) and improve the root : shoot dry matter for improve water capture across the soil profile (Siddique et al. 1990; Reynolds et al. 2007) (Table 1).
|
Phenotypic screening for these potential traits has been conducted and, in the case of denser root distribution at depth and reduced diameter of the xylem vessel, extreme lines have been selected and grown in the field alongside the parents to estimate grain yield. However, none of these traits has been adopted by a commercial or public breeding organisation to date. There are three major reasons for the poor adoption of these potential traits. First, an important limitation to the utilisation of root traits is that very few have been rigorously validated under field conditions to demonstrate their capacity to increase water capture and grain yield. Field validation is critical for morphological root traits because the water and soil conditions in the field during the growing season are extremely difficult to replicate under controlled environment conditions such as in pots, hydroponics or root growth boxes. This will alter both the expression of root traits and their influence on plant performance. For example, it is known that plant growth and morphology, including both roots and shoots, can be modified by the volume available for root growth, independent of nutrition or moisture stress (e.g. Krizek et al. 1985; Peterson et al. 1991a, 1991b). The difficulties are accentuated when exploring root system traits that are expressed or become important later in the life cycle, such as around flowering or during grain filling, because of the limitations imposed by soil volume. As indicated earlier, the value of a trait will vary depending on the pattern of water deficit that the crop experiences and field validation thus needs to occur in the target environment both in terms of climate and soil type.
A second reason why potential traits have not been adopted is that the genetics of many individual root traits, such as the heritability and inheritance patterns, are poorly understood. This presents a significant impediment to the effective incorporation of individual root traits into breeding programs. Progress is being made (e.g. Sharma and Lafever 1992; Camargo and Ferreira-Filho 2005; Sanguineti et al. 2007; Sharma et al. 2010). However, while there are indications that many morphological individual root traits are controlled by single genes, others, including total root system size, are controlled by complex polygenic systems and are influenced by other plant growth traits such as growth rate, duration of the vegetative phase and dry partitioning of dry matter (Monyo and Whittington 1970; Lynch 2007).
Thirdly, for implementation in breeding programs, rapid and simple screening procedures are needed. Screening for individual root traits under field conditions is labour-intensive, subject to high variability and inefficient (Lynch 2007). However, screening methods used in controlled environments must yield results that are well correlated with performance under field conditions. As discussed above, this can be problematic. For example, Wojciechowski et al. (2009) showed that the effect of Rht genes on root growth was strongly positive in gel cultures but strongly negative in soil. Potential approaches for efficient phenotyping of root traits under controlled conditions have recently been reviewed by Gregory et al. (2009), who indicated significant emerging capability. However, field screening remains difficult. Surrogate approaches such as screening for differences in canopy temperature may prove important (Slafer et al. 2005). Marker assisted selection offers promise under both controlled and field conditions, although limitations exist for its use with complex traits (Reynolds et al. 2005; Slafer et al. 2005).
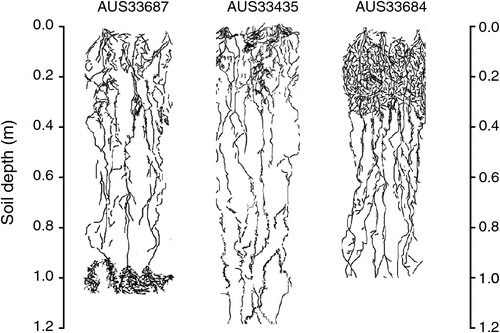
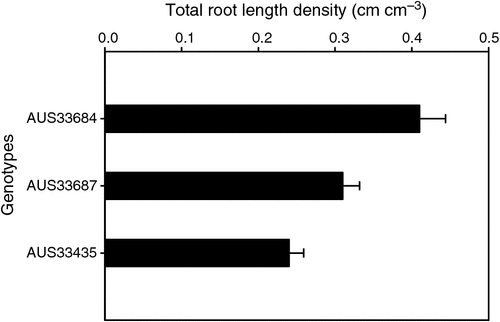
Regardless of the interest in these individual morphological traits and their functional implications for water capture and increased yields in water-limited environments, they do not describe a root system as large or small. For instance, the denser roots at depth characteristic of the root system of the Australian synthetic derivative wheat AUS33687 and the deeper seminal roots of AUS33435, another Australian synthetic derivative wheat, measured at ear emergence (Z59 in Zadoks’ growth scale for cereals; Zadoks et al. 1974) when grown on a deep sandy soil at Wongan Hills, Western Australia (WA), do not describe these systems as large when compared with the root system of AUS33684 (Fig. 1). The root system of AUS33684 neither had seminal roots as deep as those of AUS33435 nor as dense a root at depth as AUS33687, but its root system had a profuse branching in the top 0.35 m of the soil profile. This root proliferation in AUS33684 led to a higher total root length density (in the 0.0–1.2 m soil layers) than in the other two genotypes (Fig. 2). Total root length density is considered to be directly related to the amount of water uptake and to indicate the size of the root system.
|
|
Invigorating the root system in wheat is increasing its size
Vigour in the root system of wheat has recently been increased in novel germplasm through indirect selection for greater leaf size (Richards and Lukacs 2002). Here greater ‘early vigour’ describes faster leaf area development to increase shoot biomass (Rebetzke and Richards 1999). The value of greater shoot vigour has primarily been thought to increase water use efficiency in Mediterranean environments through shading of the soil surface to reduce water loss through soil evaporation (Botwright et al. 2002).
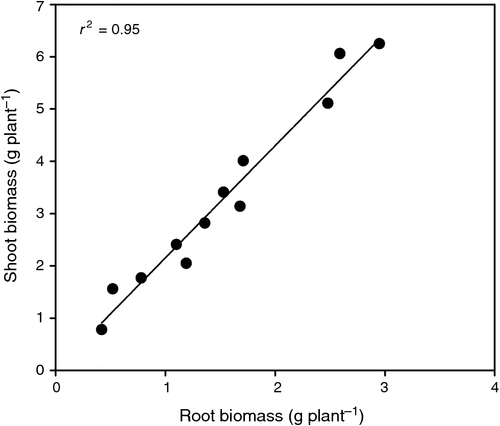
More recent evidence from recurrent selection lines for greater leaf vigour suggests that greater shoot vigour may contribute partly or fully to greater root vigour (X. Chen 2010, unpubl. data). The characteristics of a vigorous root system are early and fast extension, early and profuse proliferation, high root biomass and high root length density (Liao et al. 2006; Palta and Watt 2009). So it is not surprising that leaf and root vigour are related. When 12 genotypes of wheat, including current commercial cultivars and breeding lines differing in leaf vigour, were grown in glass-walled root boxes, differences in shoot biomass at stem elongation (Z31) were closely related to variation in root biomass (R2 = 0.95; y = 0.0088 + 2.14x) (Fig. 3). This suggests that increasing vigour in the shoot can also increase vigour in the root system and therefore, shoot biomass potentially offers the possibility of being a surrogate characteristic of vigour in the root system.
|
Total root length density and root biomass (Waines and Ehdaie 2007; Ehdaie et al. 2010) are indicators of the size of a root system. A vigorous root system which has large biomass, length and root length density is then considered as a large root system. The early bred lines with vigorous root systems such as Vigor18 and B18, selected by Drs R. Richards and G. Rebetzke at CSIRO Plant Industry (Rebetzke and Richards 1999; Richards and Lukacs 2002), had 50–70% more root biomass and 33–83% greater root length, and captured 42–60% more nitrogen than non-vigorous commercial cultivars at stem elongation (Z31; Liao et al. 2006). However, the root elongation rates of the seminal roots and hence the depth of rooting was similar to the non-vigorous commercial cultivars (Liao et al. 2006). This indicates that the main advantage of the vigorous root system of these lines was early in the season, facilitating crop establishment and growth, and improving the capture of water and nitrogen (Liao et al. 2006; Palta et al. 2007; Palta and Watt 2009). Early in the season, capture of water and nitrogen is important in improving grain yields in wheat grown on deep sandy soils in Mediterranean-type climates where end-of-season drought (terminal drought) often occurs (Palta and Fillery 1995). Less advantage was seen around flowering and grain filling when deeper rooting was required to capture additional water, particularly when watering was withheld from 50% anthesis to simulated terminal drought (J. Palta, unpubl. data). This is not surprising, since the root system of wheat often fails to take up all the available deep soil water because root growth stops owing to the lack of available time (Passioura 1983). Root growth runs out of time because it often slows down when less photosynthetic fixed carbon is invested in root growth from ear initiation (Davidson et al. 1990; Palta and Gregory 1997) and ceases from flowering when grain filling becomes the major sink for carbon from current assimilation and stem reserves (Gregory et al. 1978). Consequently, root proliferation and elongation of the seminal roots before flowering is critical in increasing the capture of available deep water, although post-flowering root growth in wheat has been observed in the cultivar SeriM82 (Manschadi et al. 2006).
Improving the elongation rate of seminal roots
An S1 recurrent selection program was initiated by Dr G. Rebetzke at CSIRO Plant Industry to accumulate favourable alleles for early vigour and indirectly for root vigour, from across 20 international lines with unrelated pedigrees and thereby assumed to contain different alleles for greater early vigour. Briefly, the process followed intermating of the original parents at random for two cycles before allowing specimens to self-pollinate. Approximately 6000 progeny, representing 60 random crosses, were sized to a common seed weight and then sown under favourable conditions. When seedlings developed four leaves, they were measured for leaf width, and the largest 50% (~3000 lines) were selected for transplanting to small pots. Plants were then allowed to grow under favourable conditions until maturity. Harvested seed from all 3000 lines were sized to a common weight and then sown in a replicated study. Seedlings were grown under favourable conditions until the fourth-leaf stage, when leaf widths were measured. The two most vigorous lines in each cross were retained and allowed to grow until flowering, whereupon they were intermated at random to initiate the second cycle of recurrent selection for greater early vigour. This process was repeated four times to generate Cycle four progeny. The 400 most vigorous selected lines during Cycle four were seed-sized to a common weight before sowing in 50 cm deep tubes containing a 80 : 20 sand : compost soil mix. These were then grown in favourable conditions until emergence of the fourth leaf, when they were harvested for leaf area and root determination. Four lines (37–6, 38–19, 50–4 and 92–11) were selected as producing very vigorous shoot and root growth (G. Rebetzke, unpubl. data).
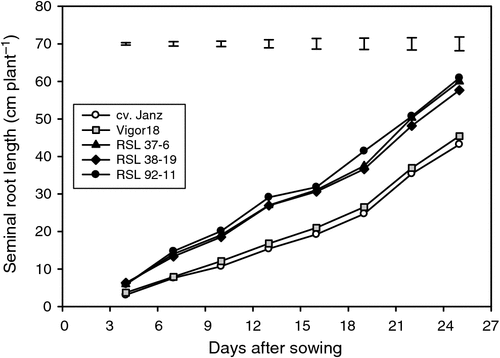
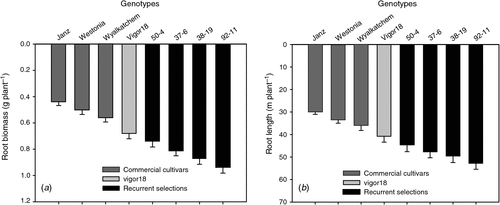
The four recurrent selection lines (RSL) were grown together with Vigor18 and three current commercial cultivars in glass-walled boxes filled to a depth of 1.0 m with soil obtained from a field site, packed to a bulk density of ~1.53 g cm–3 for comparison of their root systems. The mean axis length of the seminal roots measured by root mapping every 3 days, beginning at 4 days after sowing (DAS), was greater in the RSLs than in Vigor18 (one of the original parents used in the recurrent selection program) and the cultivar Janz (Fig. 4). The rate of elongation of the axes was 2.05–2.16 cm d–1 in Janz and Vigor18, and 2.74–290 cm d–1 in the RSLs. Total root length at stem elongation (Z45) in the RSLs was 47–78% greater than in the three current commercial cultivars and 9.3–29% greater than Vigor18 (Fig. 5). Total root biomass of the RSLs was 68–113% greater than the commercial cultivars and 8.8–38% greater than Vigor18 (Fig. 5). This indicates that elongation rates of the seminal roots and, presumably, the depth of rooting was improved as the root system of wheat was enlarged by genetically increasing leaf vigour.
|
|
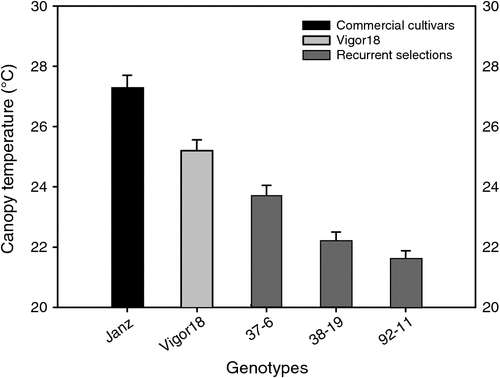
Canopy temperature measured by infrared thermometry is a method for estimating crop water and heat stress in wheat (Blum et al. 1989; Reynolds et al. 1998). Under conditions of drought-stress, wheat genotypes with lower midday canopy temperatures had a better plant water status (Blum et al. 1989). Average canopy temperature measured between 1200 hours and 1400 hours at anthesis was 1.5–3.6°C lower in the RSLs than in Vigor18 and 3.6–5.7°C lower than in the cultivar Janz (Fig. 6). The genotypes were grown in rain-fed conditions and by the time the RSLs were at anthesis, the top 0.7 m of the soil profile was already dry. Therefore it is likely that the low canopy temperature of the RSLs, particularly 92–11 and 38–19, reflected water extraction at deeper layers compared to the other genotypes. Preliminary analysis indicates that the lines 92–11 and 38–19 accessed 9.5 and 7.2 mm more soil water, respectively, than the cultivar Janz, and yielded 0.50 and 0.35 t ha–1, respectively, more than Janz (X. Chen, unpubl. data). In a recent crop simulation model exercise, Lilley and Kirkergaard (2010) quantified the predicted benefits of modifying wheat root systems in the variable Australian climate for a range of soils and crop management scenarios. They demonstrated that wheat varieties with faster and more efficient root growth provided significant yield benefits (0.3 to 0.4 t ha–1) at all of the sites tested and such traits would rarely result in yield reduction.
|
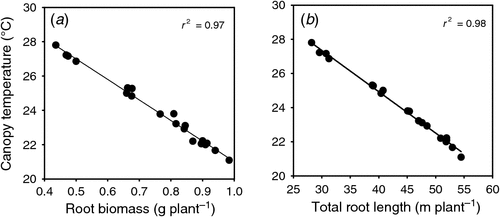
The measured canopy temperature in the field at anthesis was closely related to root biomass (R2 = 0.97; y = 32.99 – 1.198x) and total root length (R2 = 0.98; y = 34.61 – 24.22x) measured at booting (Z45) in plants grown in glass-walled root growth boxes (Fig. 7). Increasing the vigour of the root system and hence its size lowered the canopy temperature because the crop had a better plant water status, as the roots were accessing more water. Canopy temperature potentially offers the possibility of phenotyping wheat lines non-invasively in the field for the size of the root system.
|
The contribution of vigorous root systems in wheat to increased yield under water-limited conditions depends on the pattern of development of the water deficits in the target environment. For instance, in environments where wheat is mostly grown on stored soil water, such as in north-eastern Australia, vigorous root systems run the risk of exhausting the soil water before completing grain filling and hence reducing yield. In a quantitative analysis of root adaptive traits for southern Queensland, Australia, Manschadi et al. (2006) demonstrated that the wheat genotype SeriM82 was tolerant to terminal drought because its compact, uniform and deep root architecture reduced water use early in the season and increased access to water from deeper soil layers during the grain filling. In Mediterranean-type environments where crops depend mostly on in-crop rainfall, vigorous root systems are critical for increasing growth, pre-anthesis water use and yield if they also have deep seminal roots.
Conclusions
Despite the increasing interest in individual morphological root traits and their functional implications for water capture under water-deficient conditions, there is still little consensus on whether a large root system contributes to wheat adaptation in water-limited environments. This is because the individual root traits, which include the depth of rooting, root elongation rate, root distribution at depth, diameter of the xylem vessel, angle of seminal roots and root : shoot dry matter ratio, do not adequately describe a root system as large or small. We believe that increasing root vigour in wheat will increase the total size of the root system, as greater vigour encapsulates early and fast rates of root extension, and early and profuse root proliferation correlates to an increase in root biomass and root length density. Furthermore, a vigorous or large root system contributes to adaptation in dry environments and dry seasons where crop growth depends on seasonal rainfall. However, a large root system may be of less value in environments where crop growth is dependent on stored soil water where access to more soil water runs the risk of exhausting soil water before completing grain filling.
Acknowledgements
We thank Professor Neil C. Turner and Dr Helen Bramley for critically reading the manuscript.
References
Aamodt S, Johnston WH (1936) Studies on drought resistance in spring wheat. Canadian Journal of Research 14, 122–152.Bertholdsson NO, Brantestam AK (2009) A century of Nordic barley breeding – effects on early vigour root and shoot growth, straw length, harvest index and grain weight. European Journal of Agronomy 30, 266–274.
| A century of Nordic barley breeding – effects on early vigour root and shoot growth, straw length, harvest index and grain weight.Crossref | GoogleScholarGoogle Scholar |
Blum A, Shpiler L, Golan G, Mayer J (1989) Yield stability and canopy temperature of wheat genotypes under drought-stress. Field Crops Research 22, 289–296.
| Yield stability and canopy temperature of wheat genotypes under drought-stress.Crossref | GoogleScholarGoogle Scholar |
Botwright TL, Condon AG, Rebetzke GJ, Richards RA (2002) Field evaluation of early vigour for genetic improvement of grain yield in wheat. Australian Journal of Agricultural Research 53, 1137–1145.
| Field evaluation of early vigour for genetic improvement of grain yield in wheat.Crossref | GoogleScholarGoogle Scholar |
Botwright Acuña TL, Pasuquin E, Wade LJ (2007) Genotypic differences in root penetration ability of wheat through thin wax layers in contrasting water regimes and in the field. Plant and Soil 301, 135–149.
| Genotypic differences in root penetration ability of wheat through thin wax layers in contrasting water regimes and in the field.Crossref | GoogleScholarGoogle Scholar |
Camargo CE de O, Ferreira-Filho AWP (2005) Genetic control of wheat seedling root growth. Scientia Agricola 62, 325–330.
Davidson JL, Jones DB, Christian KR (1990) Winter feed production and grain yield in mixtures of spring and winter wheats. Australian Journal of Agricultural Research 41, 1–18.
| Winter feed production and grain yield in mixtures of spring and winter wheats.Crossref | GoogleScholarGoogle Scholar |
Ehdaie B, Merhaut DJ, Ahmadian S, Hoops AC, Khuong T, Layne AP, Waines JG (2010) Root system size influences water-nutrient uptake and nitrate leaching potential in wheat. Agronomy and Crop Science 196, 455–466.
| Root system size influences water-nutrient uptake and nitrate leaching potential in wheat.Crossref | GoogleScholarGoogle Scholar |
Grando S, Ceccarelli S (1995) Seminal root morphology and coleoptile length in wild (Hordeum vulgare ssp. spontaneum) and cultivated (Hordeum vulgare ssp. vulgare) barley. Euphytica 86, 73–80.
| Seminal root morphology and coleoptile length in wild (Hordeum vulgare ssp. spontaneum) and cultivated (Hordeum vulgare ssp. vulgare) barley.Crossref | GoogleScholarGoogle Scholar |
Gregory PJ, McGowan M, Biscoe PV (1978) Water relations of winter wheat: 1. Growth of the root system. The Journal of Agricultural Science 91, 103–116.
| Water relations of winter wheat: 1. Growth of the root system.Crossref | GoogleScholarGoogle Scholar |
Gregory PJ, Glyn Bengough A, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM (2009) Root phenomics of crops: opportunities and challenges. Functional Plant Biology 36, 922–929.
| Root phenomics of crops: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Hamblin A, Tennant D (1987) Root length density and crop water uptake: how well are they correlated? Australian Journal of Agricultural Research 38, 513–527.
| Root length density and crop water uptake: how well are they correlated?Crossref | GoogleScholarGoogle Scholar |
Higginbotham RW, Paulitz TC, Campbell KG, Kidwell KK (2004) Evaluation of adapted wheat cultivars for tolerance to pythium root rot. Plant Disease 88, 1027–1032.
| Evaluation of adapted wheat cultivars for tolerance to pythium root rot.Crossref | GoogleScholarGoogle Scholar |
Hurd EA (1974) Phenotype and drought tolerance in wheat. Agricultural Meteorology 14, 39–55.
| Phenotype and drought tolerance in wheat.Crossref | GoogleScholarGoogle Scholar |
Jackson RB, Sperry JS, Dawson TE (2000) Root water uptake and transport: physiological processes in global predictions. Trends in Plant Science 5, 482–488.
| Root water uptake and transport: physiological processes in global predictions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FmtFSmtA%3D%3D&md5=1453d2f4fc9f5a7cf527e488bf2af31aCAS | 11077257PubMed |
Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315.
| Impact of subsoil water use on wheat yield.Crossref | GoogleScholarGoogle Scholar |
Kramer PJ (1969) ‘Plant and soil water relationships: a modern synthesis.’ (McGraw-Hill: New York)
Krizek DT, Carmi A, Mirecki RM, Snyder FW, Bunce JA (1985) Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological response of soybean (Glycine max L. Merr.). Journal of Experimental Botany 36, 25–38.
| Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological response of soybean (Glycine max L. Merr.).Crossref | GoogleScholarGoogle Scholar |
Liao M, Palta JA, Fillery IRP (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Australian Journal of Agricultural Research 57, 1097–1107.
| Root characteristics of vigorous wheat improve early nitrogen uptake.Crossref | GoogleScholarGoogle Scholar |
Lilley JM, Kirkergaard JA (2010) Benefits of increased soil exploration by wheat roots. In ’Food security from sustainable agriculture. Proceedings of the 15th Australian Agronomy Conference, Lincoln, New Zealand’. Available at: http://www.regional.org.au/au/asa/2010/crop-production/soil-water/7176_lilleyjm.htm [Verified 30 March 2011]
Løes AK, Gahoonia TS (2004) Genetic variation in specific root length in Scandinavian wheat and barley accessions. Euphytica 137, 243–249.
| Genetic variation in specific root length in Scandinavian wheat and barley accessions.Crossref | GoogleScholarGoogle Scholar |
Lynch JP (2007) Roots of the second green revolution. Australian Journal of Botany 55, 493–512.
| Roots of the second green revolution.Crossref | GoogleScholarGoogle Scholar |
Ma SC, Xu BC, Li FM, Liu WZ, Huang ZB (2008) Effects of root pruning on competitive ability and water use efficiency in winter wheat. Field Crops Research 105, 56–63.
| Effects of root pruning on competitive ability and water use efficiency in winter wheat.Crossref | GoogleScholarGoogle Scholar |
Manschadi AM, Christopher J, de Voil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837.
| The role of root architectural traits in adaptation of wheat to water-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVClsbY%3D&md5=7022839cebfa04fc5169e6d738f15c13CAS |
Manske GGB, Vlek PLG (2002) Root architecture –wheat as a model. In ‘Plant roots: the hidden half’. (Eds Y Waisel, A Eshel) pp. 249–259. (Marcel Dekker, Inc: New York)
Moeller C, Asseng S, Berger J, Milroy SP (2009) Plant available soil water at sowing in Mediterranean environments – is it a useful criterion to aid nitrogen fertiliser and sowing decisions? Field Crops Research 114, 127–136.
| Plant available soil water at sowing in Mediterranean environments – is it a useful criterion to aid nitrogen fertiliser and sowing decisions?Crossref | GoogleScholarGoogle Scholar |
Monyo JR, Whittington WJ (1970) Genetic analysis of root growth in wheat. The Journal of Agricultural Science 74, 329–338.
| Genetic analysis of root growth in wheat.Crossref | GoogleScholarGoogle Scholar |
Morison JIL, Baker NR, Mullineaux PM, Davies WJ (2008) Improving water use in crop production. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 639–658.
| Improving water use in crop production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFClsrs%3D&md5=72df4005fd709f04334fda9d667d1cceCAS | 17652070PubMed |
Nakamoto T, Oyanagi A (1994) The direction of growth of seminal roots of Triticum aestivum L. an experimental modification thereof. Annals of Botany 73, 363–367.
| The direction of growth of seminal roots of Triticum aestivum L. an experimental modification thereof.Crossref | GoogleScholarGoogle Scholar |
O’Brien L (1979) Genetic variability of root growth in wheat (T. aestivum L.). Australian Journal of Agricultural Research 30, 587–595.
| Genetic variability of root growth in wheat (T. aestivum L.).Crossref | GoogleScholarGoogle Scholar |
O’Toole JC, Bland WL (1987) Genotypic variation in crop plant root systems. Advances in Agronomy 41, 91–145.
| Genotypic variation in crop plant root systems.Crossref | GoogleScholarGoogle Scholar |
Palta JA, Fillery IRP (1995) N application increases pre-anthesis contribution of dry matter to grain yield in wheat grown on a duplex soil. Australian Journal of Agricultural Research 46, 507–518.
| N application increases pre-anthesis contribution of dry matter to grain yield in wheat grown on a duplex soil.Crossref | GoogleScholarGoogle Scholar |
Palta JA, Gregory PJ (1997) Drought affects the fluxes of carbon to roots and soil in 13C, pulse-labelled plants of wheat. Soil Biology and Biochemistry 29, 1395–1403.
| Drought affects the fluxes of carbon to roots and soil in 13C, pulse-labelled plants of wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtVCgt7k%3D&md5=1f63fc900fe2896becf97a60c4fb90c2CAS |
Palta JA, Watt M (2009) Crop roots systems form and function: improving the capture of water and nutrients with vigorous root systems. In ‘Crop physiology: applications for genetic improvement and agronomy’.(Eds V Sadras, D Calderini) pp. 309–325. (Academic Press: San Diego)
Palta JA, Fillery IRP, Rebetzke GJ (2007) Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crops Research 104, 52–59.
| Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake.Crossref | GoogleScholarGoogle Scholar |
Passioura JB (1977) Grain yield, harvest index and water use of wheat. Journal of the Australian Institute of Agricultural Science 43, 117–120.
Passioura JB (1983) Roots and drought resistance. Agricultural Water Management 7, 265–280.
| Roots and drought resistance.Crossref | GoogleScholarGoogle Scholar |
Peterson TA, Reinsel MD, Krizek DT (1991a) Tomato (Lycopersicon esculentum Mill cv. Better Bush) plant response to root restriction I. Alteration of plant morphology. Journal of Experimental Botany 42, 1233–1240.
| Tomato (Lycopersicon esculentum Mill cv. Better Bush) plant response to root restriction I. Alteration of plant morphology.Crossref | GoogleScholarGoogle Scholar |
Peterson TA, Reinsel MD, Krizek DT (1991b) Tomato (Lycopersicon esculentum Mill cv. Better Bush) plant response to root restriction II. Root respiration and ethylene generation. Journal of Experimental Botany 42, 1241–1249.
| Tomato (Lycopersicon esculentum Mill cv. Better Bush) plant response to root restriction II. Root respiration and ethylene generation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhvVCnsA%3D%3D&md5=22cad4da45153a799a55896a5c00c634CAS |
Rebetzke GJ, Richards RA (1999) Genetic improvement of early vigour in wheat. Australian Journal of Agricultural Research 50, 291–301.
| Genetic improvement of early vigour in wheat.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Singh RP, Ibrahim A, Ageeb OAA, Larqué-Saavedra A, Quick JS (1998) Evaluating physiological traits to complement empirical selection for wheat in warm environments. Euphytica 100, 85–94.
| Evaluating physiological traits to complement empirical selection for wheat in warm environments.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Mujeeb-Kazi A, Sawkins M (2005) Prospects for utilising plant adaptive mechanisms to improve wheat and other crops in drought- and salinity-prone environments. The Annals of Applied Biology 146, 239–259.
| Prospects for utilising plant adaptive mechanisms to improve wheat and other crops in drought- and salinity-prone environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktFagtLc%3D&md5=33895aa0aeaea7b34e5efebf86c2d662CAS |
Reynolds MP, Saint Pierre C, Saad ASI, Vargas M, Condon AG (2007) Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Science 47, 172–189.
| Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress.Crossref | GoogleScholarGoogle Scholar |
Richards RA, Lukacs Z (2002) Seedling vigour in wheat – sources of variation for genetic and agronomic improvement. Australian Journal of Agricultural Research 53, 41–50.
| Seedling vigour in wheat – sources of variation for genetic and agronomic improvement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhsVGrtL0%3D&md5=ed7cb9a1f673f48f1d068be92375f25cCAS |
Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 40, 943–950.
| A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments.Crossref | GoogleScholarGoogle Scholar |
Sanguineti M, Li S, Maccaferri M, Corneti S, Rotondo F, Chiari T, Tuberosa R (2007) Genetic dissection of seminal root architecture in elite durum wheat germplasm. The Annals of Applied Biology 151, 291–305.
| Genetic dissection of seminal root architecture in elite durum wheat germplasm.Crossref | GoogleScholarGoogle Scholar |
Sharma RC, Lafever NN (1992) Variation for root traits and their genetic control in spring wheat. Euphytica 59, 1–8.
Sharma RC, Morgounov AI, Braun HJ, Akin B, Keser M, Bedoshvili D, Bagci A, Martius C, van Ginkel M (2010) Identifying high yielding stable winter wheat genotypes for irrigated environments in Central and West Asia. Euphytica 171, 53–64.
| Identifying high yielding stable winter wheat genotypes for irrigated environments in Central and West Asia.Crossref | GoogleScholarGoogle Scholar |
Siddique KHM, Belford RK, Tennant D (1990) Growth, development and light interception of old and modern wheat cultivars in a Mediterranean-type environment. Australian Journal of Agricultural Research 40, 473–487.
| Growth, development and light interception of old and modern wheat cultivars in a Mediterranean-type environment.Crossref | GoogleScholarGoogle Scholar |
Slafer G, Araus J, Royo C, Morol LG (2005) Promising ecophysiological traits for genetic improvement of cereal yields in Mediterranean environments. Annals of Biology 146, 61–70.
| Promising ecophysiological traits for genetic improvement of cereal yields in Mediterranean environments.Crossref | GoogleScholarGoogle Scholar |
Tang C, Rengel Z, Diatloff E, Gazey C (2003) Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Field Crops Research 80, 235–244.
| Responses of wheat and barley to liming on a sandy soil with subsoil acidity.Crossref | GoogleScholarGoogle Scholar |
Turner NC, Nicolas ME (1987) Drought resistance of wheat for light-textured soils in a Mediterranean climate. In ’Drought tolerance in winter cereals‘. (Eds JP Srivastava, E Porceddu, E Acevedo, S Varma) pp. 203–216. (John Wiley: Chichester)
Wahbi A, Gregory PJ (1995) Growth and development of young roots of barley (Hordeum vulgare L.) genotypes. Annals of Botany 75, 533–539.
| Growth and development of young roots of barley (Hordeum vulgare L.) genotypes.Crossref | GoogleScholarGoogle Scholar |
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Annals of Botany 100, 991–998.
| Domestication and crop physiology: roots of green-revolution wheat.Crossref | GoogleScholarGoogle Scholar | 17940075PubMed |
Wojciechowski T, Gooding MJ, Ramsay L, Gregory PJ (2009) The effects of dwarfing genes on seedling root growth of wheat. Journal of Experimental Botany 60, 2565–2573.
| The effects of dwarfing genes on seedling root growth of wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntlGit7o%3D&md5=1a900a1a10c31777c1157c617fcd984eCAS | 19439763PubMed |
Zadoks JC, Chang TT, Konzak CF. (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |


