Polyols as biomarkers and bioindicators for 21st century plant breeding
Andrew Merchant A D and Andreas A. Richter B CA Faculty of Agriculture Food and Natural Resources, University of Sydney, Suite 401 Biomedical Building, 1 Central Avenue, Australian Technology Park, Everleigh, NSW 2015, Australia.
B Department of Chemical Ecology and Ecosystem Research, University of Vienna. Althanstrasse 14 1090 Vienna, Austria.
C School of Earth and Environment, Ecosystems Research Group, University of Western Australia, Perth, WA, Australia.
D Corresponding author. Email: andrew.merchant@sydney.edu.au
Functional Plant Biology 38(12) 934-940 https://doi.org/10.1071/FP11105
Submitted: 28 April 2011 Accepted: 10 August 2011 Published: 5 October 2011
Abstract
Characterising changes in the plant metabolome is central to understanding adaptive responses to environmental change. New and improved quantitative and qualitative technologies have enabled the characterisation of plant metabolism at unprecedented scales and precision. New frontiers have therefore emerged for improving our understanding of the adaptability of plant metabolic networks. However, despite these advances, outcomes for ‘in field’ plant management remain largely based on subsets of plant metabolism due to broader scale network complexity. The synthesis and occurrence of polyols offer considerable promise as bioindicators of plant health and biomarkers for use as selective traits for plant improvement. Polyols are polyohydroxy compounds that may be either open chain (acyclic) alditols or cyclic compounds (cyclohexan-hexols), usually termed cyclitols or inositols. Here we highlight the functions of polyols in stress acclimation or amelioration and as sinks for carbon and indicate their potential for the development of integrated measures of plant function using new technologies in 21st century plant breeding.
Additional keywords: alditol, carbon partitioning, cyclitol, metabolites.
Introduction
Global change, comprising of climate change and additional pressures arising from increased human population, pose major challenges for plants and plant breeding strategies. Principal among these challenges are increased environmental variability and homeostatic compensation for higher concentrations of atmospheric carbon dioxide. Changes in plant metabolism are undoubtedly a major component of adaptive traits to mitigate such conditions. The development of integrative tools to monitor and promote adaptive strategies of metabolic networks are therefore of great importance to agriculturalists and plant breeders.
The carbon status of leaves is considered to reflect plant growth across a range of temporal and spatial scales and is a prominent candidate for the development of tools to monitor plant processes. Recent advances in high-throughput extraction, separation and identification of plant compounds have enabled broad scale qualification and quantification of large swathes of the plant metabolome (e.g. Fiehn 2004; Trethewey 2004). Rapid expansion of ‘omics’ technologies and efforts to combine transcriptomic, proteomic and metabolomic data (e.g. Fiehn 2001, 2002; Sweetlove et al. 2008; Leakey et al. 2009) aim to enable ‘predictive metabolic engineering’ (e.g. Sweetlove et al. 2003). Interpretation and use of this knowledge is limited because of the complexity of plant metabolic networks with few applications developed for ‘in field’ plant management.
It is widely recognised that research relevant to plant breeders and landscape managers has been limited across a range of disciplines (see Passioura 2007; McClean et al. 2011). Similarly, large gaps remain between the molecular level science and the interpretation and application of this knowledge at the whole-plant level in the field (Araus et al. 2007). Development of biomarkers based on a range of quantitative morphometric, and chemical traits have led to the identification of powerful quantitative trait loci (QTL) that form the basis of plant breeding programs. Of equal importance is the development of bioindicators that reflect plant physiological status supporting plant management decisions. High-throughput technologies to monitor subsets of plant metabolism, sometimes termed ‘targeted metabolic profiling’, offer an alternative to complex broad scale metabolomic characterisations. Targeting subsets of plant metabolism within or close to the core metabolic reactions of the cell, for example carbohydrates and derived metabolites, may offer significant insight into alterations in plant metabolism in response to environmental change.
The synthesis and accumulation of polyols is common to many plant tissues with discrete patterns identified among both plant taxa (Plouvier 1963; Lewis and Smith 1967; Bieleski 1982) and environments (Merchant et al. 2006a; Monson et al. 2006). Several functions of polyols in plant tissues have been identified, however, despite their close association with primary metabolism they are often overlooked in plant research. Here we highlight the importance of polyol synthesis in plant tissues, their physiochemical properties and how their occurrence is well suited for use as biomarkers and bioindicators of plant function. We discuss future research directions from the cellular to the plant scale and emphasise the applicability of such suggestions to broader efforts to develop metabolite based biomarkers and bioindicators of plant health.
Polyols are a major carbon sink
The immediate fate of carbon exiting the Calvin cycle has been well studied, with much of the biosynthetic pathways well characterised (for a concise review see Stitt et al. 2010). To reduce oxidative damage to cellular components during transport and storage, carbon exported from the Calvin Benson cycle must be ‘packaged’ into highly reduced forms. Major sinks for carbon exiting the cycle are starch synthesis in the chloroplast and cytosolic sucrose synthesis after export of DHAP across the thylakoid membrane. In model plants, allocation of carbon between these two competing pathways is well characterised (Sulpice et al. 2009; Stitt et al. 2010) leading to the suggestion of integrated measures of plant growth (Sulpice et al. 2009).
Second only to sucrose and its derivatives, polyols represent a major highly reduced sink in which plants may store and transport carbon. Polyols have been isolated from plant tissues up to 8.9% leaf DW (Richter and Popp 1992; Streeter et al. 2001; Monson et al. 2006; Merchant et al. 2007) up to 90% of phloem sap carbon (Moing et al. 1997) and often more than 50% of the carbon in the xylem sap (Richter and Popp 1992; Popp and Smirnoff 1995; Popp et al. 1997). The synthesis of polyols follows strong taxonomical (Bieleski and Briggs 2005; Merchant et al. 2007) and ecotypic (Pfundner 1993) patterns and these concentrations fluctuate in response to environmental cues (Vernon and Bohnert 1992; Wanek and Richter 1997; Streeter et al. 2001; Merchant et al. 2006b). Such properties suggest that the capacity to synthesise polyols under various stress conditions is ideal for use as selection criterion in plant breeding programs for enhanced drought or salinity tolerance.
Polyols are either open chain (acyclic) compounds with the general formula HOCH2[CH(OH)]nCH2OH, or cyclic compounds (cyclohexan-hexols or -pentols), usually termed cyclitols or inositols. Although not regarded as primary metabolites, biosynthesis of polyols stems directly from glucose-6-phsophate (G-6-P). Alditols such as sorbitol (e.g. Prunus spp., Nadwodnik and Lohaus 2008) or mannitol (e.g. Apium graveolens, Sickler et al. 2007) are chemically reduced forms of either glucose or fructose (Fig. 1) whose synthesis are well characterised (e.g. Loescher and Everard 2000) and subject to many attempts to engineer stress tolerance (Tarczynski et al. 1993; Prabhavathi et al. 2002; Maheswari et al. 2010). Although these attempts lead to increased stress tolerance of the engineered plants, it has been questioned whether this can lead to a significant increase of whole-plant tolerance under field conditions (Bohnert and Shen 1998). In addition to alditols, a great diversity of cyclitols is commonly isolated from plant tissues due to their many substituted and dehydroxylated forms (Drew 1984). Knowledge of cyclitol biosynthesis in plants is largely based upon radio-labelling experiments by Kindl and Hoffmann-Ostenhof in the 1960s (e.g. Kindl and Hoffmann-Ostenhof 1966; Hofmann et al. 1969) suggesting that myo-inositol (a cyclitol itself) is a common precursor to all cyclitols with only one characterised exception where G-6-P is converted to muco-inositol via a phosphorylated intermediate in a Zannichellian seagrass (Drew 1984). Several authors have shown evidence suggesting some cyclitols (notably the cyclohexane-pentols) are derived directly from G-6-P (Kindl 1969; Popp et al. 1997), with further work required to determine the enzyme processes involved (Fig. 1).
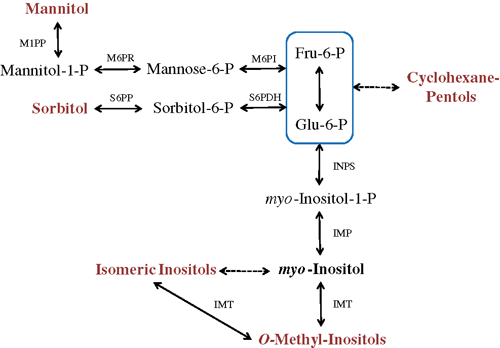
|
Plant carbohydrate metabolism is flexible and can be altered to enable the allocation of resources towards the synthesis of alternative metabolites (Vernon et al. 1993; Sheveleva et al. 1997). Despite a close association with primary metabolism and several physiochemical properties supporting their role as a carbon sink (see below), quantifying the flux of carbon to the polyol pool has received little attention. Relative to carbohydrates, polyols are highly stable, chemically inert compounds that are not readily metabolised (Paul and Cockburn 1989; Popp et al. 1997; Sheveleva et al. 1997), thus, do not undergo significant short-term fluctuations (Paul and Cockburn 1989). Further, polyol biosynthesis is reliant on the diversion of common intermediates of primary metabolism (Fig. 1) and is reliant only on carbon, oxygen and hydrogen as its final constituents. Relatively short metabolic pathways, closely aligned with a core set of metabolic reactions, may facilitate the rapid accumulation of polyols to high concentrations in plant cells.
Avoiding sugar-mediated downregulation of photosynthesis is thought to be significant characteristic enabling plants to tolerate changes in resource availability (Paul and Driscoll 1997; Chiou and Bush 1998; Paul and Foyer 2001) and impart ‘upstream influence on primary metabolism (e.g. Paul and Driscoll 1997; Halford and Paul 2003; Smith and Stitt 2007). With the exception of myo-inositol, many polyols are thought not to participate in primary metabolic reactions. Allocation of carbon to the polyol pool may assist plants to avoid sugar mediated downregulation of photosynthesis by removing carbon from the carbohydrate pool and avoiding allosteric feedback effects on Calvin cycle components (Stitt et al. 2010). Equally, the synthesis of polyols, specifically the alditols, are a means by which plants can store ‘reduction equivalents’ facilitating the regeneration of NADP+/NAD+ allowing the dark reactions of photosynthesis to continue thus protecting photosystems from oxidative damage. Although both of these processes clearly have an upper limit in their ability to avoid the negative effects of plant stress, both processes can aid in tolerating shorter term variation in resource availability for example across diurnal fluctuations. These processes, with the potential for significant repercussions on primary metabolism, will require an understanding of flux through metabolite networks.
Quantifying fluxes in complex pathways represents a new frontier to understanding metabolic networks, therefore, is the subject of several recent reviews (Rontein et al. 2002; Schwender et al. 2004; Schwender 2008; Sweetlove et al. 2008; Allen et al. 2009). In cases, such as for most polyols, where little information is available regarding the kinetics of the synthetic pathways, such approaches are limited by lack of data on the parameters that govern enzyme function (e.g. Fiehn et al. 2000; Fiehn 2002, 2004; Trethewey 2002, 2004). Alternatively, the use of metabolic flux analysis (MFA) enables the in vivo measurement of co-occurring flux rates among the metabolic network thus is ideal for use in detecting ‘switches’ in allocation of carbon among soluble pools. Changes in the allocation of carbon from carbohydrates to polyols most likely reflect alterations in plant growth strategies in response to changes in environmental conditions. In a well characterised example, Pattanagul and Madore (1999) observed allocation of carbon to the synthesis of O-methyl inositols at the expense of raffinose family oligosaccharide (RFO) synthesis in response to water deficit in Coleus. Major shifts in allocation of carbon at such a central point in plant metabolism have significant downstream consequences and require further attention for the study of plant acclimation to changes in resource availability.
Polyol chemistry and cellular functions
In addition to the function of polyols as a carbon sink, evidence for multiple roles as stress metabolites have been gleaned from investigations across the breadth of higher plants (Paul and Cockburn 1989; Williamson et al. 2002). The function of polyols as ‘compatible solutes’ (e.g. Paul and Cockburn 1989) in plant tissues is an established mechanism of coping with low external osmotic potentials (ψπ). As one example, the accumulation of the cyclitol D-pinitol is influenced by reduced water availability (drought, salinity, osmotic potential) among a range of crop and woody plant genera including Glycine max L. (Streeter 1985) Cicer arietinum L. (Orthen et al. 2000) Vigna spp. (Ford 1982; Wanek and Richter 1997) Pisum sativum L. (Streeter 1985) Mesembryanthemum crystallinum L. (Paul and Cockburn 1989; Vera-Estrella et al. 1999) coastal New Zealand species (Bieleski 1994) Actinidia A. Chev. (Klages et al. 1998, 1999) and transgenic tobacco (Vernon et al. 1993; Sheveleva et al. 1997) illustrating the potential for this group of compounds to influence the physiology among a range of herbaceous and woody plant species.
Some polyols are thought to possess protective properties towards protein structures, membranes and liposomes (Ortbauer and Popp 2008) through several modes of function. As discussed above, concentrations of many polyols do not significantly fluctuate in the short term, leading to suggestions that this improves their ability function in stabilising cellular structures (e.g. Paul and Cockburn 1989; Sheveleva et al. 1997). Many polyols are thought to promote the hydration of cellular structures via ‘preferential exclusion’ (e.g. Andersen et al. 2011) because of their high concentrations seemingly among all cellular components. Alternatively, methylation of many polyols incorporates a partial hydrophobicity and increases the size of the hydration shell of small zwitterionic molecules (Hare et al. 1998), which is thought to improve the capacity of the solute to interact with and preserve the tertiary structures of proteins.
Polyol synthesis may also assist to avoid the products of excessive photorespiration. For example, it is hypothesised that methylation of myo-inositol to form D-ononitol in plant tissues reduces H2O2 generation by photorespiration through increased demand on N5-methelenetetrahydrofolate (Hare et al. 1998). Additionally, the ability of various cyclitols to scavenge hydroxyl radicals in vitro has been shown (Orthen et al. 1994) and transgenic tobacco plants that accumulate mannitol in chloroplasts and that exhibited an increased hydroxyl radical scavenging capacity have been produced (Shen et al. 1997).
Although polyol accumulation undoubtedly influences ψπ and other cellular processes, the significance of subcellular compartmentalisation remains weakly characterised. Paul and Cockburn (1989) demonstrated for M. crystallinum, subcellular compartmentalisation of D-pinitol in the chloroplast up to 230 mol m–3, compared with cytosolic concentrations of 100 mol m–3. Equally, for some plants subcellular compartmentalisation of polyols may not be important or even possible due to high concentrations of solutes found in plant tissues (up to 8.9% leaf DW). Either way, significant concentrations of polyols in plant tissues would support the rapid establishment of osmotic potential under varying environmental conditions. Investigations of subcellular distributions of polyols face significant challenges owing to limited options for labelling attributable to the relatively inert properties and common constituents (C, N, O). The use of isotope labelling or cell fractionation is time consuming and hindered by the high levels of background carbon involved in plant metabolism. A promising alternative technique may be the use of quantum dots (e.g. Eggenberger et al. 2010) to resolve this question. Evaluation of this approach may provide significant insight into the transport and partitioning of plant solutes from the cellular to the plant scale.
The importance of polyol partitioning at the plant scale
Many applications of metabolite analysis focus on the cellular scale with relatively little attention placed on whole-plant metabolite distributions. Partitioning of polyols between plant tissues (e.g. Noiraud et al. 2001b) and excretion from roots (e.g. Timotiwu and Sakurai 2002) perform important roles for plant function and interaction with the environment. Polyols are highly mobile among plant tissues and are involved in rapid redistributions to satisfy physiological requirements such as bud burst (Popp et al. 1997) or the onset of physiological stress (Richter and Popp 1992; Guo and Oosterhuis 1995). In the only known study of its type, polyol synthesis has been shown to occur in plant tissues remote to the location of polyol accumulation (Wanek and Richter 1997), highlighting the importance of whole-plant analysis.
Up to 80% of the carbon acquired in photosynthesis is, at some stage, transported in the phloem sap (Chiou and Bush 1998), of which polyols are often a major constituent. Apoplastic and symplastic modes of loading outlined for sucrose and raffinose family oligosaccharides (RFOs) are well characterised (Turgeon 1996; Turgeon 2000) and although not discounting the likely symplastic movement of polyols in to the phloem in some cases, recent studies have now isolated several genes for apoplastic loading of polyols (Noiraud et al. 2001a; Gao et al. 2003; Ramsperger-Gleixner et al. 2004; Klepek et al. 2005; Pommerrenig et al. 2007). Changes in the concentrations of sugars, and RFOs are sensitive to environmental conditions along with indirect measures of phloem sap ψπ (Pate and Arthur 1998; Pate et al. 1998; Merchant et al. 2010), suggesting that the abundance of such compounds may be used as surrogate measures of plant physiological status. Despite such promise, movement of polyols via the phloem remains relatively unstudied with little information regarding flux. Nevertheless, movement of photoassimilates into the phloem is a recognised ‘bottleneck’ for carbon movement with direct consequences for the repression of photosynthesis (e.g. Adams et al. 2007). Uncovering the relative contributions of polyol, carbohydrate and RFO to the transport of carbon and their flexibility in response to environmental change may provide insight into the flexibility of assimilation under changing environmental conditions.
What are the priorities for future fundamental and applied research?
Polyols can ameliorate the effects of stressful conditions among a range of plant genera across a range of stress types. Although a great deal is known regarding the function of polyols in plant material, the development of tools for plant management remains unheralded. Uncovering genetic diversity in the capacity to accumulate polyols (e.g. Streeter et al. 2001) will be a significant advancement in the generation of selective traits for plant improvement. Underpinning such efforts will also require a more comprehensive understanding of the effects of polyol accumulation on yield in target species.
Identifying ‘switches’ in the allocation of carbon to polyols straddles an important concept for developing applied tools for use in plant management programs. The resilience of plant metabolism to withstand environmental change or rapid induction of polyol synthesis in response to environmental cues may represent valid reflections of fitness under particular conditions. Equally, preparedness for environmental change may be reflected in the preconditioning of plant tissues through the constitutive accumulation of polyols (e.g. Merchant et al. 2006b). Characterising such patterns under a range of environmental conditions using targeted analysis of metabolites (such as MFA) will be required to underpin the development of effective, usable tools for plant management programs.
Metabolic engineering to upregulate polyol content has been successfully achieved among a range of plant genera (e.g. Vernon et al. 1993; Nuccio et al. 1999; Sickler et al. 2007) leading to increased tolerance to a range of environmental conditions. Studies of this type play important roles in determining ‘proof of function’ for compounds involved in complex metabolic networks with the long-term objective of developing improved stress tolerance. Although metabolic engineering of polyol synthesis shows great promise, the close association of polyol synthesis with primary photosynthetic reactions and roles in long distance transport highlight the need to investigate the influence of changes in carbon allocation on plant development and yield (e.g. Teo et al. 2006).
Finally, a renewed focus must be made on plant-scale distributions of polyols and the transport mechanisms that underpin movement of polyols among plant tissues. The contribution of polyols to carbon transport for metabolism and the quantification of polyol exudation from root tissues and subsequent effects on root-associated micro-organisms remain relatively unstudied. Such information, although not immediately applicable to plant management programs, may provide a mechanistic basis for future breeding objectives.
Acknowledgements
The authors would like to thank the Worldwide Universities Network (WUN) for supporting a workshop entitled ‘Improving Bean Yield in the Tropics’ held at CIAT, Columbia. A.M. is supported by the Australian Research Council (DP 0988731).
References
Adams WW, Watson AM, Mueh KE, Amiard V, Turgeon R, Ebbert V, Logan BA, Combs AF, Demmig-Adams B (2007) Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynthesis Research 94, 455–466.| Photosynthetic acclimation in the context of structural constraints to carbon export from leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2ks7bE&md5=59f88d82f1230c958a6eecf8919ffddbCAS |
Allen DK, Libourel IGL, Shachar-Hill Y (2009) Metabolic flux analysis in plants: coping with complexity. Plant, Cell & Environment 32, 1241–1257.
| Metabolic flux analysis in plants: coping with complexity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFGhtrfJ&md5=7847e3664b0ba4351b9d771eece358f0CAS |
Andersen HD, Wang CH, Arleth L, Peters GH, Westh P (2011) Reconciliation of opposing views on membrane–sugar interactions. Proceedings of the National Academy of Sciences of the United States of America 108, 1874–1878.
| Reconciliation of opposing views on membrane–sugar interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhslyjsLw%3D&md5=df3e5ff850dbe238a93f8c504a205759CAS |
Araus J, Blum A, Nguyen HT, Parry MAJ, Tuberosa R (2007) Integrated approaches to sustain and improve plant production under drought stress – preface. Journal of Experimental Botany 58, iv
| Integrated approaches to sustain and improve plant production under drought stress – preface.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOltLg%3D&md5=99cef76782f444041ac069f38b061cdbCAS |
Bieleski RL (1982) Sugar alcohols. In ‘Encyclopedia of plant physiology’. (Eds FA Loewus, W Tanner) (Springer-Verlag: New York)
Bieleski RL (1994) Pinitol is a major carbohydrate in leaves of some coastal plants indigenous to New Zealand. New Zealand Journal of Botany 32, 73–78.
Bieleski RL, Briggs BG (2005) Taxonomic patterns in the distribution of polyols within the Proteaceae. Australian Journal of Botany 53, 205–217.
| Taxonomic patterns in the distribution of polyols within the Proteaceae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVGjtLo%3D&md5=f458476d9e8a2ec52f37db65a29ebe2aCAS |
Bohnert HJ, Shen B (1998) Transformation and compatible solutes. Scientia Horticulturae 78, 237–260.
| Transformation and compatible solutes.Crossref | GoogleScholarGoogle Scholar |
Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences of the United States of America 95, 4784–4788.
| Sucrose is a signal molecule in assimilate partitioning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXis1OgtL4%3D&md5=417deab7e78597f5f3b39489fde9541bCAS |
Drew DH (1984) Physiology and metabolism of cyclitols. In ‘Storage carbohydrates in vascular plants’. (Ed. DH Lewis) pp. 133–155. (Cambridge University Press: Cambridge, UK)
Eggenberger K, Frey N, Zienicke B, Siebenbrock J, Schunck T, Fischer R, Brse S, Birtalan E, Nann T, Nick P (2010) Use of nanoparticles to study and manipulate plant cells. Advanced Engineering Materials 12, B406–B412.
| Use of nanoparticles to study and manipulate plant cells.Crossref | GoogleScholarGoogle Scholar |
Fiehn O (2001) Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comparative and Functional Genomics 2, 155–168.
| Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsVCntbw%3D&md5=5bea1283b657f85a4d1e421525aad4bbCAS |
Fiehn O (2002) Metabolomics – the link between genotypes and phenotypes. Plant Molecular Biology 48, 155–171.
| Metabolomics – the link between genotypes and phenotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1Kqtr0%3D&md5=c8350e85a932ce8187c3a03479c44651CAS |
Fiehn O (2004) High-throughput metabolite profiling for functional genomics. Plant & Cell Physiology 45, S7–S7.
Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L (2000) Metabolite profiling for plant functional genomics. Nature Biotechnology 18, 1157–1161.
| Metabolite profiling for plant functional genomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotVSmtL0%3D&md5=5b76fc2dc6ebf00146707b816fef3fa5CAS |
Ford CW (1982) Accumulation of O-methyl-inositols in water stressed Vigna species. Phytochemistry 21, 1149–1151.
| Accumulation of O-methyl-inositols in water stressed Vigna species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlsF2ktLg%3D&md5=4b165defdf094ce7a15f5ecda09fdd7dCAS |
Gao ZF, Maurousset L, Lemoine R, Yoo SD, van Nocker S, Loescher W (2003) Cloning, expression, and characterisation of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiology 131, 1566–1575.
| Cloning, expression, and characterisation of sorbitol transporters from developing sour cherry fruit and leaf sink tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1SqsrY%3D&md5=e3d80fc88ddc5ca5e40ae4630fa03b86CAS |
Guo CX, Oosterhuis DM (1995) Pinitol occurrence in soybean plants as affected by temperature and plant growth regulators. Journal of Experimental Botany 46, 249–253.
| Pinitol occurrence in soybean plants as affected by temperature and plant growth regulators.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXktVOrsLY%3D&md5=5e12c6255877657551e99bd4adb1ef3fCAS |
Halford NG, Paul MJ (2003) Carbon metabolite sensing and signalling. Plant Biotechnology Journal 1, 381–398.
| Carbon metabolite sensing and signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvVyisr8%3D&md5=7aa7325c6c4d1341dc6e5febf7d5097dCAS |
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant, Cell & Environment 21, 535–553.
| Dissecting the roles of osmolyte accumulation during stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltl2hu7s%3D&md5=c2f11517bfc0a34c59bd80b0ab511120CAS |
Hofmann H, Wagner I, Hoffmann O (1969) Studies on biosynthesis of cyclitols. 24. A soluble enzyme from Vinca rosea methylating myo-inositol to L-bornesitol. Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie 350, 1465–1468.
| Studies on biosynthesis of cyclitols. 24. A soluble enzyme from Vinca rosea methylating myo-inositol to L-bornesitol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXhvVKmug%3D%3D&md5=53632fb646e423bfdfb7c75c9627e5a4CAS |
Kindl H (1969) Biosynthesis of epimers of myo-inositol, cyclohexanepentols, cyclohexenetetrols, and C-methyl inositols. Annals of the New York Academy of Sciences 165, 615–623.
Kindl H, Hoffmann-Ostenhof O (1966) Cyclite: biosynthese, stoffwechsel und vorkommen. Fortschritte der Chemie organischer Farbstoffe 24, 313–316.
Klages K, Boldingh H, Donnison H, MacRae E (1998) Myo-inositol is the major sugar in Actinidia arguata during early fruit development. Australian Journal of Plant Physiology 25, 61–67.
| Myo-inositol is the major sugar in Actinidia arguata during early fruit development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisl2qt70%3D&md5=bfc9de3cfe37c673e0f709179e66b2eeCAS |
Klages K, Boldingh H, Smith GS (1999) Accumulation of myo-inositol in Actinidia seedlings subjected to salt stress. Annals of Botany 84, 521–527.
| Accumulation of myo-inositol in Actinidia seedlings subjected to salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXms1yhu7o%3D&md5=75f0336fa5ea05b2ef96348bd5af286aCAS |
Klepek YS, Geiger D, Stadler R, Klebl F, Landouar-Arsivaud L, Lemoine R, Hedrich R, Sauer N (2005) Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol and ribosele. The Plant Cell 17, 204–218.
| Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol and ribosele.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXotlantg%3D%3D&md5=9c63751da5122d0347f68a868874807bCAS |
Leakey ADB, Ainsworth EA, Bernard SM, Markelz RJC, Ort DR, Placella SA, Rogers A, Smith MD, Sudderth EA, Weston DJ, Wullschleger SD, Yuan S (2009) Gene expression profiling: opening the black box of plant ecosystem responses to global change. Global Change Biology 15, 1201–1213.
| Gene expression profiling: opening the black box of plant ecosystem responses to global change.Crossref | GoogleScholarGoogle Scholar |
Lewis DH, Smith DC (1967) Sugar alcohols (polyols) in fungi and green plants. 1. Distribution, physiology and metabolism. New Phytologist 66, 143–184.
| Sugar alcohols (polyols) in fungi and green plants. 1. Distribution, physiology and metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXksVSqur0%3D&md5=8fd09c7174efebce37d7d4cde9646463CAS |
Loescher W, Everard JD (2000) Regulation of sugar alcohol biosynthesis. In ‘Storage carbohydrates in vascular plants’. (Ed. DH Lewis) pp. 275–299. (Kluwer Academic: Dordrecht, The Netherlands)
Maheswari M, Varalaxmi Y, Vijayalakshmi A, Yadav SK, Sharmila P, Venkateswarlu B, Vanaja M, Saradhi PP (2010) Metabolic engineering using mtlD gene enhances tolerance to water deficit and salinity in sorghum. Biologia Plantarum 54, 647–652.
| Metabolic engineering using mtlD gene enhances tolerance to water deficit and salinity in sorghum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtF2lurzK&md5=1b6df0b562c6a7af360e80ac358cf1c8CAS |
McClean PE, Burridge J, Beebe S, Rao IM, Porch TG (2011) Crop improvement in the era of climate change: an integrated, multi-disciplinary approach for common bean (Phaseolus vulgaris). Functional Plant Biology 38, XXX–XXX.
| Crop improvement in the era of climate change: an integrated, multi-disciplinary approach for common bean (Phaseolus vulgaris).Crossref | GoogleScholarGoogle Scholar |
Merchant A, Adams MA, Richter A, Popp M (2006a) Targeted metabolite profiling provides a functional link among eucalypt taxonomy, physiology and evolution. Phytochemistry 67, 402–408.
| Targeted metabolite profiling provides a functional link among eucalypt taxonomy, physiology and evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVahug%3D%3D&md5=53a45e81c3b38a9a2766e3e9f16b6d8bCAS |
Merchant A, Tausz M, Arndt SK, Adams MA (2006b) Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant, Cell & Environment 29, 2017–2029.
| Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtF2gsrnP&md5=45c1620953d2f52323f35ad22a49df2cCAS |
Merchant A, Ladiges PY, Adams MA (2007) Quercitol links the physiology, taxonomy and evolution of 279 eucalypt species. Global Ecology and Biogeography 16, 810–819.
| Quercitol links the physiology, taxonomy and evolution of 279 eucalypt species.Crossref | GoogleScholarGoogle Scholar |
Merchant A, Tausz M, Keitel C, Adams MA (2010) Relations of sugar composition and δ13C in phloem sap to growth and physiological performance of Eucalyptus globulus (Labill). Plant, Cell & Environment 33, 1361–1368.
Moing A, Carbonne F, Zipperlin B, Svanella L, Gaudillere JP (1997) Phloem loading in peach: symplastic or apoplastic? Physiologia Plantarum 101, 489–496.
| Phloem loading in peach: symplastic or apoplastic?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnvFelt7Y%3D&md5=eeae369470991fd1d1ee39c145dea48eCAS |
Monson RK, Rosenstiel TN, Forbis TA, Lipson DA, Jaeger CH (2006) Nitrogen and carbon storage in alpine plants. Integrative and Comparative Biology 46, 35–48.
| Nitrogen and carbon storage in alpine plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVGltbg%3D&md5=91cb24c1d7db4f597b455751d9891597CAS |
Nadwodnik J, Lohaus G (2008) Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica and Apium graveolens. Planta 227, 1079–1089.
| Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica and Apium graveolens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsF2isLs%3D&md5=27e8f92192b2078d3395c273275654e0CAS |
Noiraud N, Maurousset L, Lemoine R (2001a) Identification of a mannitol transporter, AgMaT1, in celery phloem. The Plant Cell 13, 695–705.
Noiraud N, Maurousset L, Lemoine R (2001b) Transport of polyols in higher plants. Plant Physiology and Biochemistry 39, 717–728.
| Transport of polyols in higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvVKqsrc%3D&md5=ead45db902e0d63ce66cc8085e5c4732CAS |
Nuccio ML, Rhodes D, McNeil SD, Hanson AD (1999) Metabolic engineering of plants for osmotic stress resistance. Current Opinion in Plant Biology 2, 128–134.
| Metabolic engineering of plants for osmotic stress resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXivVGlsLo%3D&md5=35eff02d3c08ec58dcc3c08b4fadb10cCAS |
Ortbauer M, Popp M (2008) Functional role of polyhydroxy compounds on protein structure and thermal stability studied by circular dichroism spectroscopy. Plant Physiology and Biochemistry 46, 428–434.
| Functional role of polyhydroxy compounds on protein structure and thermal stability studied by circular dichroism spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFGksLs%3D&md5=9712f3496c5a85f179875e77443ce8d0CAS |
Orthen B, Popp M, Smirnoff N (1994) Hydroxyl radical scavenging properties of cyclitols. Proceedings of the Royal Society of Edinburgh Section B – Biological Sciences 102, 269–272.
Orthen B, Popp M, Barz W (2000) Cyclitol accumulation in suspended cells and intact plants of Cicer arietinum L. Journal of Plant Physiology 156, 40–45.
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany 58, 113–117.
| The drought environment: physical, biological and agricultural perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOlt7g%3D&md5=fdd92ce1cb19251aef960389caf812b8CAS |
Pate J, Arthur D (1998) Delta 13C analysis of phloem sap carbon: novel means of evaluating seasonal water stress and interpreting carbon isotope signatures of foliage and trunk wood of Eucalyptus globulus. Oecologia 117, 301–311.
| Delta 13C analysis of phloem sap carbon: novel means of evaluating seasonal water stress and interpreting carbon isotope signatures of foliage and trunk wood of Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar |
Pate J, Shedley E, Arthur D, Adams M (1998) Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus. Oecologia 117, 312–322.
| Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar |
Pattanagul W, Madore MA (1999) Water deficit effects on raffinose family oligosaccharide metabolism in coleus. Plant Physiology 121, 987–993.
| Water deficit effects on raffinose family oligosaccharide metabolism in coleus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXns12ntbY%3D&md5=cf7d787724f79853abfe6fc634040c26CAS |
Paul MJ, Cockburn W (1989) Pinitol, a compatible solute in Mesembryanthemum crystallinum L? Journal of Experimental Botany 40, 1093–1098.
| Pinitol, a compatible solute in Mesembryanthemum crystallinum L?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXjtlCisA%3D%3D&md5=9850783692a1e67b966cfb76b3a07f1dCAS |
Paul MJ, Driscoll SP (1997) Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source–sink imbalance. Plant, Cell & Environment 20, 110–116.
| Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source–sink imbalance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhsV2msLY%3D&md5=f6473595368e80f3d879a940e5ddae81CAS |
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. Journal of Experimental Botany 52, 1383–1400.
| Sink regulation of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVCmtLo%3D&md5=c02474b5cb5069b4b76d7137bd80c627CAS |
Pfundner G (1993) ‘Vergleichende Untersuchungen zum Inhaltsstoffmuster neuweltlicher Trocken- und Salzpflanzen.’ (Comparison of metabolite patterns of plants from arid or saline habitats of the new world). (University of Vienna: Vienna)
Plouvier V (1963) Distribution of aliphatic polyols and cyclitols. In ‘Chemical plant taxonomy’. (Ed. T Swain) pp. 313–336. (Academic Press: London)
Pommerrenig B, Papini-Terzi FS, Sauer N (2007) Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiology 144, 1029–1038.
| Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvValtLs%3D&md5=cad5ef64980cfa96c7619ef6b9175993CAS |
Popp M, Smirnoff N (1995) Polyol accumulation and metabolism during water deficit. In ‘Environment and Metabolism Flexibility and Acclimation – Environmental Plant Biology’. (Ed. N Smirnoff) pp. 199–214. (Bioscientific Publishers Ltd: Oxford)
Popp M, Lied W, Bierbaum U, Gross M, Grosse-Schulte T, Hams S, Oldenettel J, Schuler S, Wiese J (1997) Cyclitols – stable osmotica in trees. In ‘Trees – Contributions to Modern Tree Physiology’. (Eds H Rennenberg, W Eschrich, H Ziegler) pp. 257–270. (Backhuys Publishers Leiden, The Netherlands)
Prabhavathi V, Yadav JS, Kumar PA, Rajam MV (2002) Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene. Molecular Breeding 9, 137–147.
| Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xltlems7c%3D&md5=bc4121a7deea7301e14b99684138e797CAS |
Ramsperger-Gleixner M, Geiger D, Hedrich R, Sauer N (2004) Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiology 134, 147–160.
| Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVagur0%3D&md5=9affa4a6eba2561de0fd9be321821c91CAS |
Richter A, Popp M (1992) The physiological importance of accumulation of cyclitols in Viscum album L. New Phytologist 121, 431–438.
| The physiological importance of accumulation of cyclitols in Viscum album L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmsVymtLY%3D&md5=c27305f8a7692a72045ca24f64ccf2f4CAS |
Rontein D, Basset G, Hanson AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metabolic Engineering 4, 49–56.
| Metabolic engineering of osmoprotectant accumulation in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVSqsg%3D%3D&md5=899d1837afbc9491b1d1ab274509f16eCAS |
Schwender J (2008) Metabolic flux analysis as a tool in metabolic engineering of plants. Current Opinion in Biotechnology 19, 131–137.
| Metabolic flux analysis as a tool in metabolic engineering of plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslKis7k%3D&md5=6d8ef2b296039b5201faa67cc8144d87CAS |
Schwender J, Ohlrogge J, Shachar-Hill Y (2004) Understanding flux in plant metabolic networks. Current Opinion in Plant Biology 7, 309–317.
| Understanding flux in plant metabolic networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVamsLo%3D&md5=8f97f056675f3a2ca11cef440899cee4CAS |
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiology 113, 1177–1183.
| Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXis1Gltb8%3D&md5=b32876456660adf71b8f5497e5a7da3fCAS |
Sheveleva E, Chmara W, Bohnert HJ, Jensen RG (1997) Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabacum L. Plant Physiology 115, 1211–1219.
Sickler CM, Edwards GE, Kiirats O, Gao ZF, Loescher W (2007) Response of mannitol-producing Arabidopsis thaliana to abiotic stress. Functional Plant Biology 34, 382–391.
| Response of mannitol-producing Arabidopsis thaliana to abiotic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFOlur8%3D&md5=370fdab0c8004afafb0793eec82c6a23CAS |
Smith AM, Stitt M (2007) Co-ordination of carbon supply and plant growth. Plant, Cell & Environment 30, 1126–1149.
| Co-ordination of carbon supply and plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeiurrJ&md5=71d7d6930da1fb00b7c16b9f072f8eb5CAS |
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism – more than the icing on the cake. The Plant Journal 61, 1067–1091.
| Arabidopsis and primary photosynthetic metabolism – more than the icing on the cake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvFKntLw%3D&md5=d23d64c1b4a48997e82fa57e2dd45229CAS |
Streeter JG (1985) Identification and distribution of ononitol in nodules of Pisum sativum and Glycine max. Phytochemistry 24, 174–176.
| Identification and distribution of ononitol in nodules of Pisum sativum and Glycine max.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXpt12qtQ%3D%3D&md5=8b9c8b8a3c4e89371279b7c056828dc6CAS |
Streeter JG, Lohnes DG, Fioritto RJ (2001) Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant, Cell & Environment 24, 429–438.
| Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtFyjtLg%3D&md5=7440cc7d121a6627938819a5ed73a9f7CAS |
Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Conceição Piques M, Von Korff M, Steinhauser MC, Keurentjes JJB, Guenther M, Hoehne M, Selbig J, Fernie AR, Altmann T, Stitt M (2009) Starch as a major integrator in the regulation of plant growth. Proceedings of the National Academy of Sciences of the United States of America 106, 10348–10353.
| Starch as a major integrator in the regulation of plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXot1Giu7c%3D&md5=a071cce205f4e53ef5c4d5790d639d00CAS |
Sweetlove LJ, Last RL, Fernie AR (2003) Predictive metabolic engineering: a goal for systems biology. Plant Physiology 132, 420–425.
| Predictive metabolic engineering: a goal for systems biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslers7Y%3D&md5=b1f8fb922227ee9993f67a45da4e6090CAS |
Sweetlove LJ, Fell D, Fernie AR (2008) Getting to grips with the plant metabolic network. Biochemical Journal 409, 27–41.
| Getting to grips with the plant metabolic network.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVantLfP&md5=359800e27a7ff94fc46c3c9c85ed148eCAS |
Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259, 508–510.
| Stress protection of transgenic tobacco by production of the osmolyte mannitol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXpvVaktA%3D%3D&md5=d5a6549335afba22805cdb5faab06b2eCAS |
Teo G, Suziki Y, Uratsu SL, Lampinen B, Ormonde N, Hu WK, DeJong TM, Dandekar AM (2006) Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proceedings of the National Academy of Sciences of the United States of America 103, 18842–18847.
| Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlahtbrI&md5=852d31033de410ec54f7d6febd34ad76CAS |
Timotiwu PB, Sakurai N (2002) Identification of mono-, oligo-, and polysaccharides secreted from soybean roots. Journal of Plant Research 115, 77–85.
| Identification of mono-, oligo-, and polysaccharides secreted from soybean roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksFSktr4%3D&md5=1f4074ed7af15c3cfafbec37f774d479CAS |
Trethewey R (2002) Gene function discovery via high throughput metabolite profiling. Abstracts of Papers of the American Chemical Society 224, U91–U91.
Trethewey RN (2004) Metabolite profiling as an aid to metabolic engineering in plants. Current Opinion in Plant Biology 7, 196–201.
| Metabolite profiling as an aid to metabolic engineering in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhslChsrs%3D&md5=792b4ad2a381070450ff56a8f3374762CAS |
Turgeon R (1996) Phloem loading and plasmodesmata. Trends in Plant Science 1, 418–423.
| Phloem loading and plasmodesmata.Crossref | GoogleScholarGoogle Scholar |
Turgeon R (2000) Plasmodesmata and solute exchange in the phloem. Australian Journal of Plant Physiology 27, 521–529.
| Plasmodesmata and solute exchange in the phloem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlslarsrs%3D&md5=58d1569fcec0de69735e2efa665fc79aCAS |
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (1999) Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta 207, 426–435.
| Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtlOgtro%3D&md5=68bd33b2f5e45ad77f03508380b7f92fCAS |
Vernon DM, Bohnert HJ (1992) Increased expression of a myo-inositol methyl transferase in Mesembryanthemum crystallinum is part of a stress response distinct from crassulacean acid metabolism induction. Plant Physiology 99, 1695–1698.
| Increased expression of a myo-inositol methyl transferase in Mesembryanthemum crystallinum is part of a stress response distinct from crassulacean acid metabolism induction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmsVyls70%3D&md5=e3338ed5523693a3333694119d26fec0CAS |
Vernon DM, Tarczynski MC, Jensen RG, Bohnert HJ (1993) Cyclitol production in transgenic tobacco. The Plant Journal 4, 199–205.
| Cyclitol production in transgenic tobacco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhvVOhtrg%3D&md5=e0d81974fb9a1111c8b85dc45540299bCAS |
Wanek W, Richter A (1997) Biosynthesis and accumulation of D-ononitol in Vigna umbellata in response to drought stress. Physiologia Plantarum 101, 416–424.
| Biosynthesis and accumulation of D-ononitol in Vigna umbellata in response to drought stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmvVWmur8%3D&md5=2fdad536a96c11e2441a4aaa6d8f94b0CAS |
Williamson JD, Jennings DB, Guo WW, Pharr DM, Ehrenshaft M (2002) Sugar alcohols, salt stress, and fungal resistance: polyols – multifunctional plant protection? Journal of the American Society for Horticultural Science 127, 467–473.


