The art of growing plants for experimental purposes: a practical guide for the plant biologist
Hendrik Poorter A J , Fabio Fiorani A , Mark Stitt B , Uli Schurr A , Alex Finck B , Yves Gibon C , Björn Usadel D A , Rana Munns E F , Owen K. Atkin G , François Tardieu H and Thijs L. Pons IA Plant Sciences (IBG-2), Forschungszentrum Jülich, D-52425 Jülich, Germany.
B Max Planck Institute of Molecular Plant Physiology, Am Mühlenberg 1, D-14476 Golm, Germany.
C INRA, Univ. Bordeaux, UMR1332 Biologie du Fruit et Pathologie, 71 Avenue Edouard Bourlaux, F-33883 Villenave d’Ornon, France.
D RWTH Aachen, Worringer Weg 1, 52074 Aachen, Germany.
E CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
F School of Plant Biology, University of Western Australia, Crawley, WA 6009, Australia.
G Division of Plant Sciences, Research School of Biology, Australian National University, Canberra, ACT 0200, Australia.
H INRA, Laboratoire d’Ecophysiologie des Plantes sous Stress Environnementaux, 2 Place Viala, F-34820 Montpellier, France.
I Institute of Environmental Biology, Utrecht University, PO Box 800.84, 3508 TB Utrecht, The Netherlands.
J Corresponding author. Email: h.poorter@fz-juelich.de
Functional Plant Biology 39(11) 821-838 https://doi.org/10.1071/FP12028
Submitted: 24 January 2012 Accepted: 19 March 2012 Published: 15 June 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Every year thousands of experiments are conducted using plants grown under more-or-less controlled environmental conditions. The aim of many such experiments is to compare the phenotype of different species or genotypes in a specific environment, or to study plant performance under a range of suboptimal conditions. Our paper aims to bring together the minimum knowledge necessary for a plant biologist to set up such experiments and apply the environmental conditions that are appropriate to answer the questions of interest. We first focus on the basic choices that have to be made with regard to the experimental setup (e.g. where are the plants grown; what rooting medium; what pot size). Second, we present practical considerations concerning the number of plants that have to be analysed considering the variability in plant material and the required precision. Third, we discuss eight of the most important environmental factors for plant growth (light quantity, light quality, CO2, nutrients, air humidity, water, temperature and salinity); what critical issues should be taken into account to ensure proper growth conditions in controlled environments and which specific aspects need attention if plants are challenged with a certain a-biotic stress factor. Finally, we propose a simple checklist that could be used for tracking and reporting experimental conditions.
Additional keywords: controlled experiments, environmental conditions, glasshouse, growth chamber, plant growth, stress.
Introduction
Plant biologists rely on experimental setups in which plants are cultivated either under laboratory conditions, in experimental gardens or in agricultural and ecological settings. Such experiments enable researchers to determine the phenotypic responses of plants to defined experimental treatments and evaluate the performance of different genotypes or species in a given environment. Examples are experiments in which a given species is challenged with different levels of a particular environmental factor (e.g. Schortemeyer et al. 1999; Juurola 2003; Ghars et al. 2008) and comparative studies where species with different ecological optima (Grime and Hunt 1975; Poorter et al. 1990) or genotypes of a given species (Sulpice et al. 2009; Zhang et al. 2012) are compared in one standardised environment.
To enable generalisations across experiments, it is necessary that results are not only replicable, but also reproducible. Replication of results is achieved when the same researcher finds the same results when repeating an experiment in time (Drummond 2009). Reproducibility is accomplished if different laboratories are able to find the same results in independent experiments. In plant biology, achieving a high degree of replicability and reproducibility can be a challenge. It is well known from plant breeding that among genotypes screened for performance, the one that grows best in one year may display only average productivity in another year (Annicchiarico 2002). Such variability is generally ascribed to year-to-year differences in the environmental conditions. Replicability and reproducibility are expected to increase when environmental conditions are under stricter control, such as in growth chambers. However, the following two examples demonstrate that high reproducibility should not be taken for granted even under controlled conditions. The first example combines data from two independent experiments where genome-wide mRNA expression was measured in Arabidopsis thaliana (L.) Heynh. plants that were pre-grown at 20−22°C and subsequently switched to low temperatures (4−8°C; Vogel et al. 2005; Usadel et al. 2008). In both experiments, ~800–900 of the 25 000 genes were found to be significantly up- or downregulated, which, therefore, seems a reproducible result. However, when the analysis focuses on the identity of the transcripts, only a 30% overlap between the two experiments is found (Fig. 1a ). This low degree of overlap may reflect the fact that the experimental protocols used in the two studies were not exactly identical and neither was the accession used. That said, similar differences in the transcriptional responses were observed in other experiments, even when using the same Arabidopsis accession (see Morcuende et al. 2007; Kilian et al. 2012) and are also reported in the field of medical biology (Ioannides et al. 2009). General principles could be derived from focusing on the overlapping transcripts. Yet, the question remains: why was the response of all the other affected genes so different across experiments?
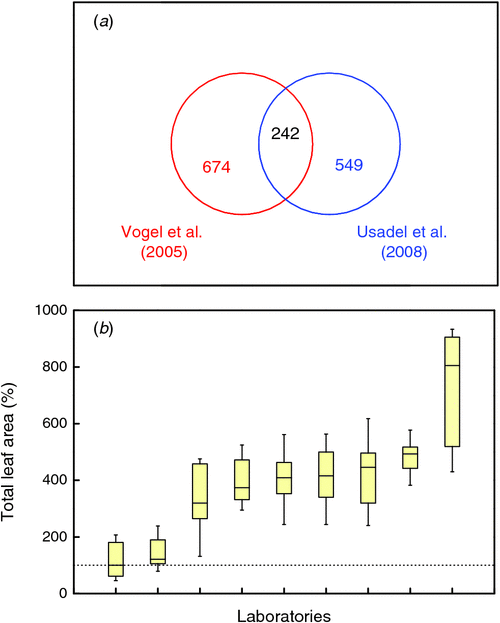
|
A second example relates to a consortium of nine laboratories that studied leaf growth of Arabidopsis (Massonnet et al. 2010). That consortium evaluated the extent to which the different research groups were able to reproduce the same phenotype. To this end, one of the laboratories with ample experience in plant growth devised an extended protocol for plant cultivation that was adopted by all partner laboratories. Each of the laboratories used the same Arabidopsis single-seed-descent seed lots and a quantity of the same soil and grew the plants to the same phenological stage, following the common protocol as far as possible. Plant performance was then evaluated by measuring the total leaf area of 10 individuals. Based on the median values, leaf area showed 8-fold variation across laboratories (Fig. 1b ). The full experiment was conducted with three different accessions and also revealed a significant laboratory × accession interaction.
Clearly, in the examples listed above, unexplained variation has an important influence on phenotypic responses. Large unexplained variation constitutes a problem, as independent reproduction of results by other scientists is one of the essential requirements for the progress of science (Popper 1959). The specific reasons for the lack of reproducibility across experiments can be manifold and include variation in the growth conditions, the extent to which they are controlled and the way a given stress is applied (Massonnet et al. 2010; Skirycz et al. 2011), as well as the number of replicates. Plants can be grown in a variety of ways, strongly influenced by the local traditions of the laboratories where students receive their education. On a positive note, one has to conclude that plants have amazing capabilities to survive and reproduce in a wide range of experimental conditions. However, the negative side of this plasticity may be that by overlooking a major pitfall in the way treatments are applied or plants are grown, researchers will obtain data that do not provide a full answer to their research question, or are difficult to reconcile with the findings of colleagues from the same laboratory or elsewhere. Given that the costs of these experiments can be considerable, but also because inadequate results may hinder scientific progress, it is of utmost importance that researchers develop and apply good practices for growing their plants. This requires an informed decision on the following fundamental questions before setting out on any experiment.
-
Where are the plants grown?
-
How are they grown?
-
How many replicates are necessary?
-
How are environmental treatments applied such that the experiment yields meaningful results?
The aim of our paper is to provide a pragmatic compendium capturing the minimum level of knowledge on how to grow plants either under so-called ‘optimal conditions’ or under conditions designed to simulate the effects of an abiotic stress factor. We first focus on the pros and cons of various growth facilities and discuss practical aspects relating to the choice of the rooting medium and pot size. We then briefly touch upon the required number of replicates in relationship to plant variability. Third, we cover general points concerning the eight most important a-biotic factors for plant growth. For each factor several suggestions are made on how to monitor and control them. Part of this paper is based on an online appendix to Hannemann et al. (2009).
Practical considerations for choosing appropriate methodologies
1. Where to conduct the experiment?
The cheapest option for conducting experiments with only a basic level of control is an outdoor experimental garden. Use of an experimental garden has the important advantage that conditions are relatively close to the natural environment that agricultural or wild species experience in the field, at least when considering the same climatic region. Experimental gardens have the additional advantage compared with the other options discussed below that they provide an opportunity to compare plants under conditions in which spatial heterogeneity (e.g. in light quantity) is relatively small. However, this comes at the cost of uncontrolled temporal variations in light, temperature and water supply and sometimes a relatively high chance of unwanted damage from influences such as foraging herbivores and hail. Another drawback of experimental gardens could be that various environmental conditions may change in concert (e.g. a period of high irradiance could come with high temperatures and low precipitation). If the aim of the experiment would be to separate the effects of these factors from each other, it is better to seek alternatives.
Glasshouses are such an alternative and offer better control of water supply and protection against too low temperatures. Additional lighting in the glasshouse may ensure a minimal daily irradiance and a fixed photoperiod while shade screens can protect against high light intensities in summer (Max et al. 2012). Thus, greenhouses provide more buffered conditions for growing plants; they often extend the period for experiments (a strong economic argument) and to some extent, allow the dissection of plant responses to different environmental conditions. In practical terms, plants grown in glasshouses will usually experience higher-than-outdoor air temperatures during nights and winters and lower irradiance because of shading. Most glasshouses have limited possibilities to reduce temperatures during periods of strong solar irradiance in summer. Air ventilation can control temperatures to some degree and the same applies to the evaporative cooling effect caused by the transpiration of a considerable amount of plants present in the glasshouse. In many greenhouses there are significant spatial heterogeneities in irradiance, due to shading by the greenhouse structure itself and often also by neighbouring buildings or other objects. Artificial lighting, although compensating for temporal variation in external irradiance, can also introduce new heterogeneities, including a marked vertical gradient in irradiance intensity (see below). Overall, diurnal and seasonal variation in growth conditions remain, but in a more buffered way compared with the experimental garden.
The most expensive option for investments and running costs is to grow plants in climate-controlled growth chambers. They offer the most sophisticated possibilities for environmental control and thereby allow (at least theoretically) for an orthogonal dissection of environmental factors and good replicability of experiments. Consequently, they are widely used by plant biologists. However, their application has some drawbacks as well. First, conditions in growth chambers are generally the furthest away from those in the field, not only because environmental values are often programmed within a relatively small diurnal range, but also with regard to the absolute values of, for example, light and temperature, at which they operate (Garnier and Freijsen 1994). Second, although growth chambers enable a strong temporal control over conditions, spatial variability is often larger than anticipated and higher than those measured in experimental gardens. For example, light intensity may vary from place to place in the growth chamber (Granier et al. 2006) and can be especially lower close to the walls (Fig. 2a ). Another often ignored gradient relates to the vertical light profile: the closer the plants grow to the lamps, the higher the light intensity. For rosette species like Arabidopsis this is not a point of concern, but erect species may experience substantial gradients, especially when they grow over 40 cm tall (Fig. 2b ). As growing taller is inevitably connected to development, plants that are older or in later stages of development often experience significantly higher light regimes than younger ones; despite this, differences in plant characteristics are then often associated with development and not with changed light conditions. Adjusting lamp height to a fixed distance relative to the top of the shoot may partly mitigate the fact that plants that grow faster because of, for example, a higher nutrient supply, will by consequence also grow faster because they experience higher light levels and higher temperatures. These examples illustrate that even in controlled environments it is not easy to separate different environmental/developmental responses from each other.
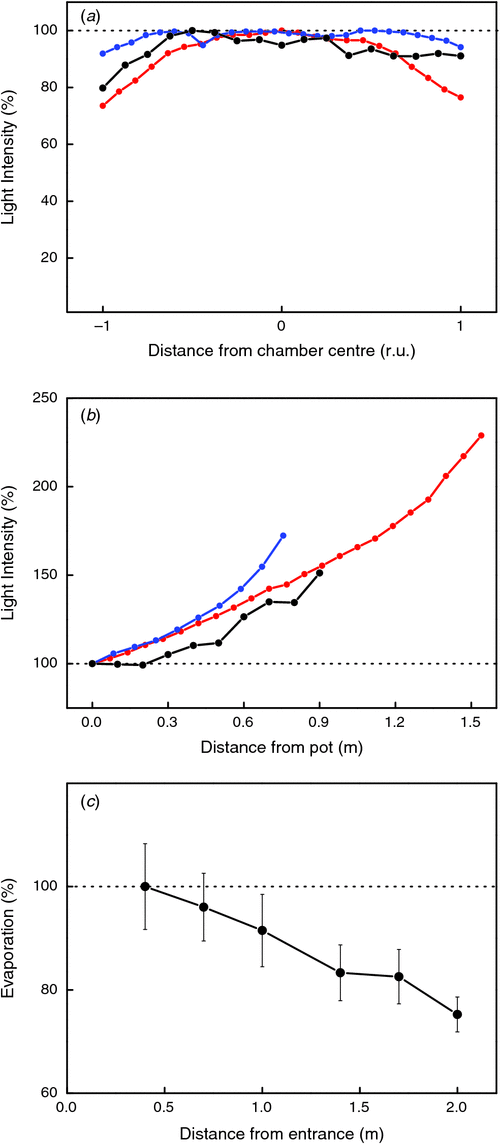
|
Gradients in air velocity may go unnoticed in growth chambers, although they can affect evaporative demand, as demonstrated by the rate of evaporation from glass beakers (Fig. 2c ). Variation in air circulation may be especially large when plant density is high or plants are placed in trays, which may block air circulation around the plants. Both too high and too low wind speeds are undesirable. A factor that may strongly vary in a temporal manner is the local atmospheric CO2 concentration: generally, CO2 levels in a building are higher than outside and, in addition, concentrations will peak strongly when humans are breathing freely during their presence in a growth chamber (see section 4C).
There are some additional points to be made concerning climate control. First, even in the case that a laboratory has well managed growth chambers with good climate regulation and even control of the atmospheric CO2 concentration, it may still be that the plants present in these facilities grow differently during different seasons (Hughes and Cockshull 1971). Second, although tight climate control sounds highly desirable, it may simultaneously reduce the so-called ‘external validity’ of the results (Richter et al. 2009). That is, by making the conditions highly specific, reproducibility across laboratories may actually decrease, with the result that generalisations are less easily made. Richter et al. (2009), therefore, advocate the use of a range of subplots within the experiment where plants are grown and measured at somewhat differently designed conditions. One useful experimental design in this respect is to undertake an experiment concurrently in more than one growth chamber, glasshouse or experimental garden using nested treatments within each replicated facility. In the end, repeating the experiment in time is probably the best way to filter out many spurious results.
2. What rooting medium to choose?
Although considerable attention is often paid to the aerial conditions and it is possible to measure and adjust important parameters like the irradiance and aboveground temperature, typically much less attention is paid to the environment in which the roots are growing. However, this may have at least as much impact on plant growth as the aerial environment.
A. Hydroponics
Roots provide nearly all the water and nutrients that a plant requires. If the aim is to design an experiment in which these two factors have the least limiting effect on growth, then hydroponics or aeroponics is the preferred choice (Waisel 2002; Gorbe and Calatayud 2010). Hydroponics systems can be either based on roots suspended in a water solution or in some solid medium such as sand, rockwool, or another relatively inert medium, which is continuously replenished with nutrient solution (Cooper 1979). Water-based systems have the advantage that they allow easy experimental access to the roots for physiological or biomass measurements. However, care has to be taken while roots are transferred from one solution to another, as breakage of roots may easily occur (Miller 1987). Frequently used nutrient solutions are described by, for example, Hoagland and Snijder (1933) and Hewitt (1966), although the truly optimal composition is species specific. There is also a need to take into account the composition of tap water when setting for the final composition. Because of the much higher mixing rate in soilless systems and the direct access of plant roots to the nutrients, the concentrations of nutrients that are needed to sustain supply are ~5–10 times lower than those required for plants growing on sand where there is an absence of continuous flow through. Therefore, on one hand, a researcher has to ensure that the concentration of macro- and especially micro-nutrients in a hydroponics system is not too high, as this will negatively affect plant growth or may even cause leaf senescence (Munns and James 2003). On the other hand, nutrient concentrations should not become too low either, as plants will otherwise deplete the available minerals. Regular replacement of nutrient solution is, therefore, necessary. This needs to take into account the fact that bigger plants usually need more nutrients and so the rate of replenishment must increase with plant size, unless the nutrient concentration itself is continuously monitored and adjusted. In the end, it is advisable to test growth versus the strength of the nutrient solution before starting experiments with a specific species. Good mixing of aerated nutrient solution is vital to avoiding depletion zones around the roots and anaerobic patches, but should not be too vigorous to avoid strong mechanical strains. In addition, specific uptake mechanisms like the release of chelating agents to increase Fe availability (Römheld 1991) or the release of organic acids by the root may be affected, since in nutrient solution there is hardly an option to form a defined rhizosphere zone, which is intrinsically necessary to make these processes efficient and effective. The pH of the hydroponic solution may increase or decrease, depending on whether nitrate or ammonium is present in the solution and the specific preference of a given species. For most plant species a pH of ~6 seems to be optimal, although specific species may deviate significantly. Monitoring and adjusting the pH of the solution at a regular basis is highly recommended, keeping in mind that pH changes are stronger in small volumes of nutrient solution and for roots with faster nitrogen uptake rates. It should also be checked that there is no accumulation of salts at the root : shoot junction over time, as this can damage the seedlings of some plant species.
B. Pots
An alternative to hydroponics is to grow plants in pots filled with an inert solid medium (e.g. sand, glass pearls, perlite) or soil and to water them regularly (that can be significantly more than once a day), or on demand. This allows more freedom in the choice of the location of the experiment and ensures easy handling and manipulation of the shoots of individual plants. Use of pots with a solid substrate may at least mimic the higher mechanical impedance to root growth that plants experience in soils and allows for a higher homogeneity and control of the nutrient and water conditions than in soil. However, contrary to hydroponics systems, it is less likely that nutrients and water will be present in non-limiting supply. Demands for water and nutrients increase strongly with the size of the plants, so the water and nutrient availability that are amply sufficient for small plants at an early life phase may become limiting at later developmental stages. Nutrient availability of commercially provided soil will vary among suppliers and even over time from soil batch to soil batch. Mixing of slow-release fertiliser with the soil or regular addition of nutrient solution may mitigate this problem to some extent. Root damage may occur if pots are black and warm up under direct solar radiation. Moreover, soil temperature per se and even gradients in soil temperature within single pots can affect plant growth and allocation (Füllner et al. 2012). Specific considerations on these issues are given in sections 4D,F,G.
The size of the rooting volume also requires careful attention. The smaller the pot, the more plants fit into a growth chamber or glasshouse, an undeniable advantage for nearly all laboratories where demand for space is high. At the same time, in most experiments smaller pots will also imply a lower availability of below-ground resources and, if pots are closely spaced, also a comparatively lower amount of irradiance available for each plant. Moreover, the smaller the pot the stronger roots become pot-bound, leading to undesirable secondary effects. In experiments in which rooting volume is varied, there is almost invariably a strong positive correlation between plant growth and pot volume (Fig. 3). Conditions obviously differ from experiment-to-experiment, but as a rule of thumb, pot size is certainly small if the total plant dry mass per unit rooting volume exceeds 2 g L–1 (Poorter et al. 2012).
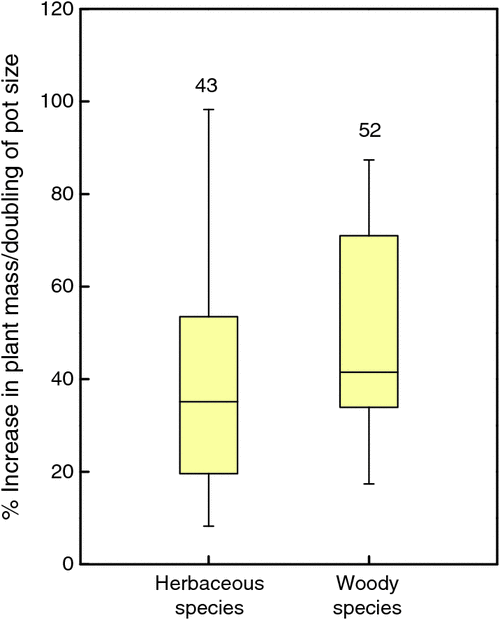
|
3. How many replicates per treatment?
Another aspect that requires attention is the number of plants that will be allocated to each treatment. In experiments in which a mapping population is used to detect quantitative trait loci (QTL), the rule is that it is better to minimise the number of replicates and maximise the number of genotypes. This will yield better QTL estimates (Zou et al. 2006). In experiments that are aimed at evaluating an overall genotypic or environmentally-induced difference in size between plants, the situation is different. The statistical power to detect such differences increases with the number of replicates and decreases with plant-to-plant variability. Harvesting big plants destructively is labour intensive, so sets a strong upper limit to the number of samples that can be processed. If other factors in addition to plant size are being analysed, it is vital to consider whether and how they are subject to diurnal or other temporal variation and to plan the harvest such that it is completed in a suitably short time interval. Accordingly, in most experiments the number of plants harvested per day and treatment usually does not exceed eight (Poorter and Garnier 1996). Imaging solutions using non-invasive approaches to evaluate plant performance are currently implemented (Jansen et al. 2009; Fiorani et al. 2012) and allow much higher densities of observations. However, most of the imaging methods to date do not yet reach the same precision for large plants as they do for small plants, simply because of overlapping leaves. In some rosette species this overlap may render more than 50% of the total leaf area invisible (Poorter et al. 1988).
The number of replicates necessary to statistically confirm a given difference between two treatments depends on the size of that difference and the variability within the two plant populations assigned to each treatment. If plant size is the phenotypic variable of interest, it pays off to reduce the initial variability in the plant material by removing the smallest and largest plants within the population just before the start of the treatment (Poorter and Garnier 1996). In case the variation itself is of interest, it is better to a priori group plants of similar size into subclasses and use these as subplots in the experimental design. There are two alternative options to minimise variability in growth caused by spatial environmental variation. Plants can be spatially rearranged during the experiment within an experimental layout either manually or mechanically. This is especially relevant when the location of plants is confounded with the treatment they receive, such as experiments with low and high CO2 concentrations for which only two chambers or glasshouses are available. The alternative is to fix the location of plants and include this location as subplot (block) in the statistical analysis. Blocks should be arranged such that the known variation in climate conditions (e.g. radiation, temperature, vapour pressure deficit, wind speed) among blocks is maximised and the variation within blocks is minimised. Finally, for all experimental conditions in which plants are grown at a density where plants mutually influence each other, it may be that plants grown at the edges experience an environment different from those that are surrounded by other plants. In such cases, edge plants are better excluded from the measurements and analyses.
Generally, plant size within a population of individually grown plants does not show a normal distribution, but rather a log-normal one, with relatively many small individuals and a few larger plants. A good metric to describe the variability in size is therefore the standard deviation of ln-transformed dry mass or leaf area (s.d.lnS). Comparing a range of growth studies, Poorter and Garnier (1996) found that size variability is low when this metric is <0.2 and high when it is >0.6. The consequence of plant-to-plant variation for the power of a statistical test is large. Fig. 4a shows that any difference in size between two populations that is larger than 50% will easily be considered as being statistically significant when s.d.lnS is 0.15, even when the number of replicates is low (4–5). However, with an s.d.lnS of 0.6, even 15 plants will not suffice to consistently find a significant difference, even when the true difference between the populations is 100% or more. If one is not only interested in testing for significance but also in a quantitative estimation of the difference, requirements on variability and replication become even more stringent. Suppose that there is a true size difference of 100% between two populations and that we require the estimated growth stimulation to be in the 80–120% range. At low population variability, four replicates will give an estimate in the desired range in 65% of the simulated experiments (Fig. 4b ). At high plant-to-plant variability, even 12 replicates will only suffice in 32% of the simulated experiments.
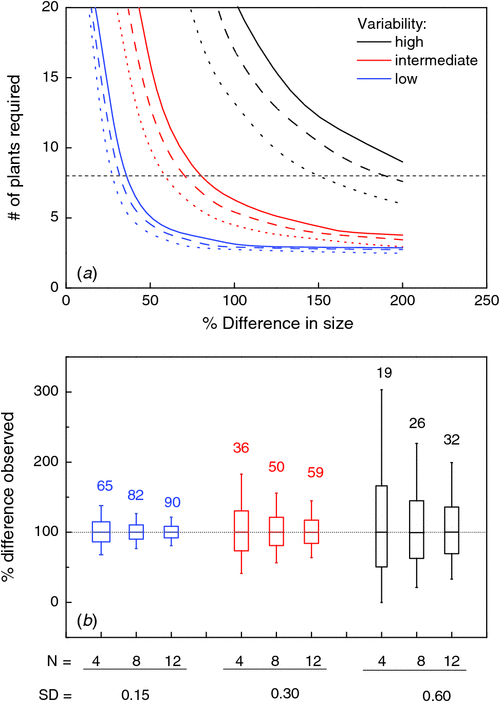
|
The necessary extent of replication also depends crucially on the parameter(s) that are under study. Take, for example, a study that is analysing the reason for the difference in growth in two 40-day-old plants that differ in biomass by 100%. Here, a significant difference in biomass can be relatively easily established. However, this difference in biomass is likely to be the result of sustained difference in growth rates over the previous 40 days. The actual change in growth rate (more technically, the relative growth rate, the increase in mass formed per initial starting mass per day) will be much smaller, of the order of 10%. A completely different dimension of replication will be needed to identify physiological, metabolic or molecular parameters that underlie such a small change in the rate of growth.
4. Environmental conditions
The subsequent sections outline how eight of the most important abiotic factors vary in nature and how they are best measured, controlled in growth chambers and reported. Furthermore, we discuss briefly some aspects to consider when these environmental conditions are used as a treatment and provide for each treatment a list with some of the most consistent and relatively easily measured changes in plants (Table 1). An excellent coverage of technical aspects of growth chambers and how to measure the various environmental factors can be found in Langhans and Tibbitts (1997).
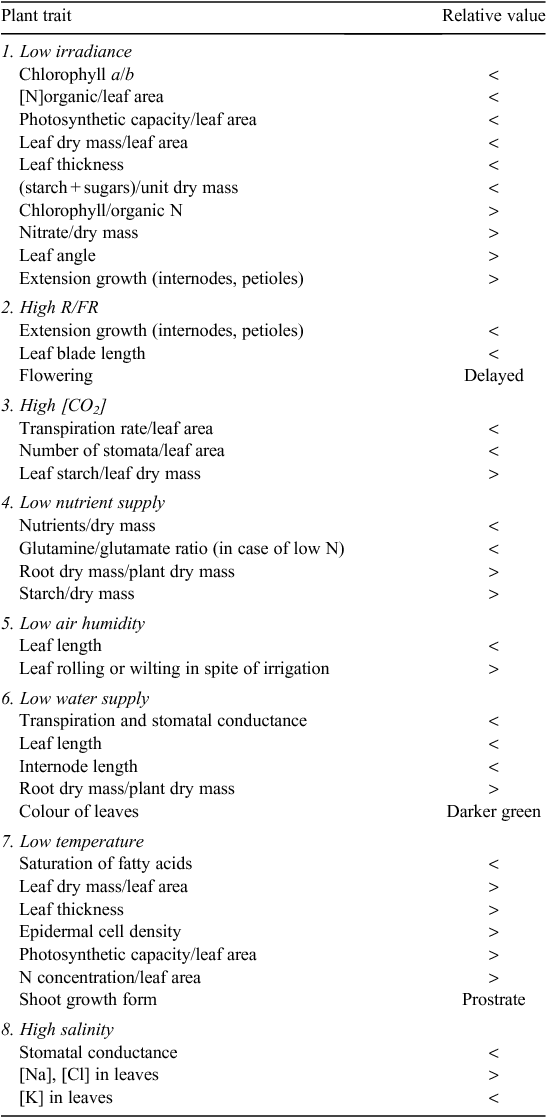
|
An accurate description of the experimental conditions (climate, expected and unexpected events) may greatly help in reproducing experiments elsewhere, or identify hidden factors with major effects on reproducibility in case of conflicting results. Table 2 provides a checklist with the type of information that we consider necessary for the ‘Materials and methods’ section of a publication and differentiates between a recommended minimum and an extended description. Note that this set of parameters is generally and collectively defined as ‘experimental meta data’. This list is inspired by the minimum guidelines to report on the environment in growth rooms, as provided by the ICCEG (2004). A program to document experimental conditions and design in a machine-readable format is described by Hannemann et al. (2009).
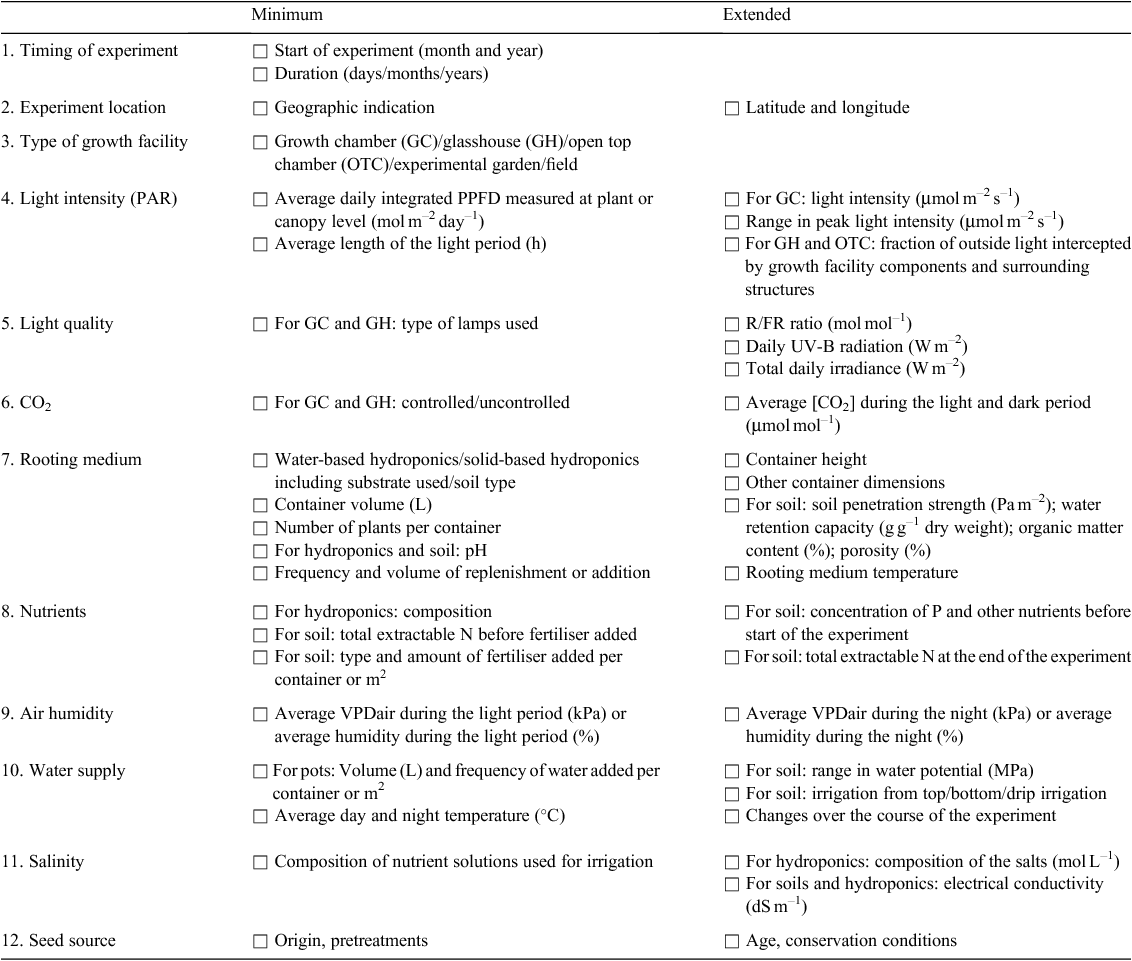
|
A. Light quantity
Background
Solar radiation comprises a wide range of wavelengths, of which only a relatively narrow range is used in photosynthesis (PAR 400–700 nm). Within the PAR region, blue-light quanta have 70% more energy than red light quanta, whereas both are equally effective in driving the photosynthetic reactions. Concerning photosynthesis and C-budgets, light availability for a plant is therefore best described by the PPFD, i.e. the number of quanta in the 400–700 nm range that hit a horizontal plane for a given amount of time (Björn and Vogelmann 1994). Under natural conditions this may vary from 0 at night till 2200 µmol m–2 s–1 in full sunlight at lower latitudes. The light intensity at which photosynthesis saturates strongly depends on the light availability at the location where the plants were grown, as well as other environmental conditions like temperature and nutrient supply. The relative growth rate (RGR) of the plant is not determined by the instantaneous light intensity as much as it is by the total amount of quanta integrated over the day (Granier and Tardieu 1998). For most individually-grown seedlings, RGR saturates at a light level of ~20 mol quanta m–2 day–1 (Poorter and Van der Werf 1998), but this value will be higher in larger plants and at high plant densities. In nature, daily quantum input varies from ~1 mol quanta m–2 day–1 in deep shade to more than 50 on clear days in summer and in (sub)tropical areas.
Controlled environments
In controlled-environment growth cabinets, light is provided by fluorescent tubes, high intensity discharge (HID) lamps or, in a recent development, light emitting diodes (LEDs). Several points are pertinent here. First, the question of how homogenously the light intensity is distributed over the growth room needs to be considered. This is particularly the case when point sources such as HID lamps are used, where variation in light intensity of more than 50% at plant level may occur locally. Densely spaced fluorescent tubes or LEDs may provide better distributed light levels.
The light intensity can drop by 20–50% close to the walls of a growth room (Fig. 2a ). This reduction is partly mitigated by using clean reflective mirrors on walls. A white coating may also help, but is less effective. Light intensity generally increases the closer plants grow to the light sources (i.e. with increasing plant height). This is especially relevant for plants that become taller than 40 cm (Fig. 2b ). Vertical gradients are more prominent closer to the lamps and when plants are not sufficiently spaced and shade each other. Finally, light output also decreases with the age of the lamps. Measuring at the beginning and the end of the experiment and at least once every two weeks in longer experiments over various positions in the growth room is recommended to characterise light quantity (ICCEG 2004).
In glasshouses, the intensity of the natural light varies with the geographical location. Glasshouse covers, frames and lamp fixtures hanging over the plants usually block 25–50% of the incoming radiation (Max et al. 2012). Still, PPFD inside can be over 1100 µmol m–2 s–1 on clear summer days. In winter or with cloudy weather intensities can go lower than 100 µmol m–2 s–1. Additional lamps can help to add extra quanta for plant growth and also extend the light period in the winter season to a defined duration. Although seemingly trivial, cleaning of the glasshouse cover from accumulated dirt helps to increase light availability as well. As mentioned before, peak irradiance is a poor characterisation of a glasshouse light environment and should at least be supplemented with a value for the daily quantum input averaged per month or over the experimental period.
Light quantity as a treatment
To use light quantity as a treatment, plants can be shaded by nettings. It is important that the netting intercepts all wavelengths to an equal extent; otherwise light quality will also be affected. Shade cloth can also introduce confounding effects when they block the air stream around the plants. Air circulation might also be an issue when light intensity is changed by positioning racks of lamps at different heights. Alternatively, the voltage of some types of lamps can be adjusted to alter light intensity, but it should be checked whether this solution does not affect light quality as well (Sager and McFarlane 1997; see section 4B). No matter which way the light level is changed, secondary effects will almost inevitably occur. For instance, a high-light environment will warm up the leaves, thereby increasing the rate at which development occurs. Leaf heating also increases the internal water vapour level. Together with a higher stomatal opening due to higher light this may increase the transpiration rate of the leaves, thereby increasing the risk for water stress. Over a longer time frame (days to weeks) more light accelerates a plant’s growth rate, which implies that those plants will have greater demands for water and nutrients than the smaller low-light grown plants. Thus, care must be taken to make sure that secondary limitations in the below-ground environment do not covary with daily irradiance.
Further reading
Technical: Björn and Vogelmann (1994), Sager and McFarlane (1997) and Jones et al. (2003).
Biological: McCree (1972), Evans et al. (1988), Poorter and Van der Werf (1998) and Evans and Poorter (2001).
B. Light quality
Background
The PAR part of the light spectrum (see section 4A) represents roughly half of the solar irradiance that reaches the Earth’s surface. The other half is partly ultraviolet (UV; 10–400 nm) but mainly infrared (700–3000 nm) radiation. Additional long-wave radiation is emitted from surrounding objects. Although photosynthetically inactive, this radiation can strongly affect the heat balance of the leaves and thereby leaf temperature (Lambers et al. 2008). As different light sources can show large differences in the intensity and distribution of irradiance in the UV and infrared regions of the spectrum, even at the same PPFD, it is advisable to characterise not only the amount of PAR, but the full light spectrum as well.
A strong modulator of plant morphology is the red (R; 660 nm) to far-red (FR; 730 nm) ratio. Sunlight has a R : FR ratio of 1.2 mol mol–1, except around sunrise and sunset, when values are lower. Plants modify this spectrum by selectively absorbing most of the PAR and UV and less of the infrared. Hence, light transmitted or reflected by green tissue has a R : FR ratio as low as 0.2 (Smith 1984). Phytochromes, a family of photoreceptors with a common chromophore, perceive the R : FR ratio of light and thus the proximity of neighbours, leading to a multitude of photomorphogenetic responses collectively called ‘shade avoidance’ (Franklin 2008). There are also other photoreceptors active in plants – cryptochrome and phototropin – that show absorption in the blue region of the spectrum (Jiao et al. 2007). These are probably more involved in the perception of light quantity than quality, but their absorption characteristics can make their action relevant in growth chambers with artificial lighting.
Controlled environments
Most lamps used in growth chambers have not been designed for imitating the daylight spectrum or optimising plant growth. Some are specifically adjusted for supplemental lighting in greenhouses where the spectrum is mixed with that of daylight. The most important differences in the emission spectra of these lamps compared with daylight are that their short-wave emission is largely in the PAR region, resulting in a much higher R : FR ratio than that of daylight. Their emission may be further depleted in red or blue and they show strong emission peaks at specific wavelengths. HID lamps in particular have a very high emission of long-wave infrared as a result of their high temperatures (Sager and McFarlane 1997). In growth rooms, excessive thermal radiation of HID and incandescent lamps may increase leaf and particularly shoot apex temperatures substantially above the controlled air temperature, especially with the low air turbulence present in most growth chambers (Cummings et al. 2007). This problem can be reduced by mounting the lamps in compartments separated by an infrared-absorbing but light-transparent filter. Such a filter does not, however, solve the problem completely, since re-radiation from its surface can still result in undesirably elevated leaf temperatures. Moreover, because of the small volume of the lamp compartment, it can be difficult to attain operational temperature stability. Temperature stability is an issue for fluorescent tubes, as their output strongly depends on temperature (Sager and McFarlane 1997).
Growth in light with a high R : FR ratio results in reduced extension growth and may alter daylength responses. Correction used to be done in the past by adding incandescent lamps; but because of their low efficiency a large wattage is required to raise the R : FR ratio to daylight values. Alternatively, end-of-day illumination with incandescent lamps has been applied. Recently, far-red emitting diodes have been used for the correction and manipulation of the R : FR ratio in growth chambers (Pierik et al. 2005). Such LEDs have the potential advantage of a high efficiency and wavelength precision. High output white LEDs are becoming available as well. LEDs have a higher efficiency and a reduced thermal radiation per unit of PAR (Massa et al. 2008). Their emission spectra can be adjusted and a mixture of LEDs with different emission spectra can be used for plant growth purposes.
Light quality as a treatment
When studying photomorphogenesis, spectral quality of the light is of particular concern. Its control should be more rigorous than for general plant growth purposes and it must be possible to manipulate it experimentally. Spectral quality can be manipulated by filters that absorb in specific spectral regions, or by supplementary lighting. Typically, irradiance of PAR should be kept constant between treatments, thus, avoiding an effect on photosynthetic rate. Filtering in the PAR region should be compensated by proportionally increasing irradiance. Use of filters for spectral manipulation can have the undesirable side effect of obstructing airflow. When the R : FR ratio is the experimental variable, PAR irradiance is most easily kept constant by manipulating irradiance in the far-red. Filtered incandescent lamps were used for this purpose in the past, but a high wattage is required to decrease the R : FR ratio, resulting in a thermal radiation problem that should be corrected for instance by a water filter. The far-red emitting diodes mentioned above are now the preferred choice. Similarly, other parts of the spectrum can be manipulated by specific LED’s as well.
Further reading
Technical: Smith (1984) and Sager and McFarlane (1997).
Biological: Smith (1984), Pierik et al. (2005), Cummings et al. (2007) and Jiao et al. (2007).
C. CO2 concentration
Background
Over geological time scales, the atmospheric CO2 concentration ranged from less than 200 to well over 2000 µL L–1. The CO2 level is currently ~390 µL L–1 and rises by 1–3 µL L–1 each year. There is variation over seasons, especially in the northern hemisphere, with values during summer being ~5–20 µL L–1 lower than during winter (Keeling et al. 1996). In some locations, short-term peaks may occur, for example, during periods of heavy traffic in proximity to roads or in cities on winter days when a temperature inversion is present. In the short term, atmospheric CO2 concentration limits photosynthesis and initial increases of up to 50% in CO2 fixation are found when the CO2 concentration around leaves is doubled. However, over longer terms, growth is less responsive than expected from the initial response of photosynthesis; individually-grown plants are on an average 30–60% heavier at elevated CO2 concentrations (600–800 µL L–1; Poorter and Navas 2003). Strong negative effects on growth are likely to occur when plants are grown at CO2 concentrations below 300 µL L–1 (Campbell et al. 2005).
Controlled environments
In many growth chambers, atmospheric CO2 concentrations are not controlled and even not measured, making this the most poorly-defined factor of the aerial environment in plant research. If a large amount of biomass is present in the growth room, CO2 concentrations will increase during the night and decrease during the day, sometimes to levels well below ambient. The extent to which this happens will partly depend on how ‘leaky’ the growth room is towards its outer environment. In buildings, the CO2 concentration is generally 50–200 µL L–1 higher than outside, due mainly to the human respiratory activity. Rush hours with a large intensity of traffic may have a ‘positive’ effect as well. With people working and respiring inside a growth room, the CO2 concentration may inadvertently increase to values over 1200 µL L–1, which leads to an unwanted and uncontrolled CO2 enrichment experiment. If phenotypic variables are being studied that respond rapidly (e.g. stomatal conductance and photosynthesis), it may therefore be necessary to exhale into a mask that leads the CO2 into an absorber or outside the chamber.
CO2 concentration as a treatment
Basic control in CO2 enrichment studies is conducted by measuring the CO2 concentration and adding CO2 by operating a valve on a CO2 cylinder. It is important to make sure that the CO2 source is not of biological origin, as otherwise ethylene may be present and cause unwanted growth-regulatory effects (Morison and Gifford 1984). Removal of CO2 from the air stream is more complicated. CO2 control is therefore easiest if considerable biomass is present in the growth room, as this causes a pronounced drawdown effect during the day. At the beginning of an experiment, when plants are small, CO2 control is more challenging and necessitates some CO2 scrubbing of the air. When subambient concentrations are of interest, sophisticated equipment for removal is necessary, such as molecular sieves that catch the CO2 in the ingoing air stream (Campbell et al. 2005).
High CO2 concentrations induce stomatal closure with the consequence that water availability is generally positively affected in high-CO2 treatments. The CO2 response is usually stronger at high nutrient availability (Geiger et al. 1999; Poorter and Navas 2003). As larger and fast-growing plants require more nutrients than smaller slow-growing plants, careful control over the nutrient supply is required.
Further reading
Technical: Romer (2001) and Flitcroft and Ingram (2002).
Biological: Stitt and Krapp (1999), Poorter and Perez-Soba (2001) and Körner (2006).
D. Nutrients
Background
Plants require a wide range of macro- and micronutrients for their growth. Almost all originally derive from the weathering of rocks, except for N, which is primarily of atmospheric origin. Biological N2 fixation is an important component of the N-cycle, especially in tropical areas >20 g m–2 year–1 can be fixed (Larcher 2003). Other parts of N-cycle are atmospheric N-deposition (usually not >3 g m–2 year–1) and partial mineralisation of organic material present in the soil (mostly up to 3 g m–2 year–1). Fertilisation of agricultural fields varies strongly as well, but can reach 30 g N m–2 year–1.
Plants can show a variety of deficiencies, with symptoms that can be element- as well as species-specific. Supra-optimal concentrations may occur as well, especially for micronutrients. The levels of nutrients such as N, P, or K should be regularly monitored in stocks of potting soils that are used for experiments. However, nutrient availability is not always equal to the concentration measured. Phosphorus, for example, may be present in the soil in rather large amounts, but can be bound so strongly to the soil substrate, that it is not available for plant growth (Lambers et al. 2008). In contrast, nitrate is easily leached by irrigation and its availability throughout a pot experiment can be assessed by analysing a small volume of water that is withdrawn from the pot by suction.
Controlled environments
De novo uptake by the roots is generally required only for the construction of new biomass. This implies that nutrient demand of a plant follows growth (Ingestad 1982) and as growth of vegetative plants studied in controlled environments is often close to exponential, it follows that nutrient demand increases exponentially over time as well. This consideration has to be kept in mind when choosing the type of nutrient supply for the experiments.
The simplest way to grow plants in pots is by using a solid substrate. Potting substrate mixtures generally contain peat, which mineralises very slowly. If this has not been done already by the supplier, it may be good to add a slow-release fertiliser, as it ensures nutrient delivery to the plants during a longer period of time. Nevertheless, even with the addition of slow-release fertiliser, the demand of exponentially-growing plants is not always met. The total requirement is probably easiest estimated from the expected maximum biomass of the plants and their nutrient concentration. For non-limiting conditions, double this quantity and apply it to the pots over time. Absence of deficiency symptoms is not the best criterion for optimal supply, as nutrient stress will negatively affect growth well before visual symptoms appear. Compounds like nitrate are very mobile and may easily leach out of pots when plants are watered. Placing pots in saucers may prevent this, but avoid standing water in the bottom of the pot. Generally, nutrient stress is less of an issue for experiments using sufficiently large pots (section 2B).
There are several ways to provide a somewhat more ‘controlled’ nutrient supply. Their usefulness depends on the plant and the biological question. Species like Arabidopsis are frequently grown on nutrient agar. The levels of solutions typically supplied in this medium, for example full strength MS (Murashige and Skoog 1962), are extremely high, but can also be varied in a precise manner. Nutrient agar can also be used to study the effect of local heterogeneities in nutrient supply (Zhang and Forde 1998). This growth system does not allow control of nutrient supply as the plants become larger. It may also negatively affect plants that are sensitive to the low O2 supply in agar. Another option is to grow plants in some inert medium, for example, sand and fertigate with a defined nutrient solution (see section 2). The sand needs to be chosen to provide airspaces, while also retaining enough water and nutrient solution to avoid dehydration and support plant growth. Typically, this can be achieved by mixing sand with at least two different particle size ranges. The nutrient solution is applied by filling the pot, allowing the nutrients to run through and repeating this 2–3 times, in order to fully replace the original depleted solution; if this is not done, nutrient concentrations can build up to unacceptably high levels, especially if evapotranspiration is high. These growth systems can be further refined by using a hydroponic approach with a nutrient supply that is continuous or based on computer-controlled drip irrigation. As an alternative to solid media, plants can be grown in a water-based hydroponic system (see section 2A). Although not present in most recipes, addition of silicon to water-based nutrient solutions is recommended (Epstein 1994), especially for grasses, sedges, horsetails and palms.
A potential problem can arise in experiments in which the treatment (e.g. changes in the amount of irradiance or temperature, or comparison of different genotypes) alters the growth rate substantially. Unless plants have free (unlimited) access to nutrients, the differing growth rates can result in secondary changes in the nutrient status of the plant modifying or even masking the effects of the original treatment. For example, elevated atmospheric CO2 concentrations lead to only marginal increases in growth but marked decreases in organic N if the nitrogen supply does not suffice for the additional growth. However, elevated CO2 concentrations increase growth but have little or no effect on the organic N concentration when plants are supplied with a very high level of N (Geiger et al. 1999).
Nutrient availability as a treatment
Nutrient deficiency is typically applied as an overall decrease of available nutrients, or with one specific element in short supply. The latter requires plants to receive nutrients in some form of nutrient solution. Different approaches have been applied to impose a nutrient stress. The first is a water-based hydroponic system with different concentrations of nutrients. Typically, the uptake rate of nitrate by the roots in a hydroponic system is virtually constant over a wide range of nitrate concentrations (between 10 µM and 10 mM; Freijsen and Otten 1984). This implies that plants will first experience a situation of luxury supply in all treatments, until almost all nutrients are taken up and then suddenly nutrient stress will hit the plants severely. The moment that this happens depends not only on the concentration of the applied nutrient solution, but also on the solution volume present per plant and the size or absolute growth rate of those plants. This is generally not the intended type of treatment. The second approach makes also use of a water-based hydroponic system, but imposes nutrient stress on plants in an approximately steady-state condition. To this end, plants are administered nutrients in an exponential manner, but at different rates of increase (Ingestad 1982). In this way, plant growth will reach a steady-state over longer periods of time, with constant nutrient concentrations and biomass allocation patterns. A characteristic of water-based hydroponic systems is that the medium is continuously stirred and consequently nutrients are brought to the roots. This is quite different from the situation in soils, where plants that allocate more biomass to roots will be able to explore a larger soil volume and are, therefore, likely to sequester more nutrients. To study the consequences of such a search strategy, it is better to use a third approach, where plants are grown in sand or another solid substrate and a limited amount of nutrient solution is administered to the pots at defined time intervals. One last possibility is to grow plants in soil to which a different amount of nutrients is added at the beginning of the experiment. In the last case, the choice of the appropriate soil volume is a particularly important issue (see section 2B).
Further reading
Technical: Cooper (1976), Berry and Knight (1997) and Spomer et al. (1997).
Biological: Ingestad (1982), Stitt and Krapp (1999), Lambers et al. (2008) and Forde (2002).
E. Air humidity/evaporative demand
Background
The maximum amount of water present in the gas phase of a given volume of air increases exponentially with temperature. Generally, humidity will be lower than this maximum, except when dew appears. A common way to characterise air humidity is by the relative humidity (RH), which is the ratio between the actual partial pressure of water vapour in the air and the pressure at saturation. For the functioning of plants this is not a particular relevant measure because evaporative demand depends on the absolute difference in vapour pressure rather than on RH. Humidity is, therefore, better characterised by the vapour pressure deficit (VPDair), the difference between the partial pressure at saturation and the actual water vapour pressure, at a given temperature (Jones 1992). This value ranges from 0 in water-saturated air to 4 kPa in dry and warm air (e.g. 30% RH at 35°C). The driving force for leaf transpiration is then given by the difference in water vapour pressure between the intercellular spaces (assuming 100% humidity at leaf temperature) and in the air around the leaf and is termed VPDla (VPD from leaf to air). This difference can easily be calculated from RH when air and leaf temperature are known.
Controlled environments
Humidity in a growth room generally increases with the amount of evapotranspiration of the plants and pots and thus depends on the size and number of plants present. Part of the transpired water will be removed by the ventilation system, but most of the air is re-circulated. Especially if the radiation load in the growth room is high, the re-circulated air will be strongly cooled before re-entering the growth chamber. This acts as a cold finger and will extract most of the water vapour out of the air. In that case an additional humidification step may be necessary for the incoming air stream. A very low humidity (VPDair > 3 kPa) causes stomatal closure of the leaves in some species, whereas too high humidity (VPDair < 1 kPa) decreases transpiration and may increase the risk of diseases. It can also cause damage to equipment in the growth chamber.
Although VPD is the relevant parameter, many measurement devices in growth chambers actually determine RH. A good measurement depends on both accurate gauging of temperature and RH in the air. Therefore, it is essential that the RH sensor is shaded and ventilated, otherwise it provides information about the sensor temperature rather than the air temperature. In case of doubt, it is preferable to measure air temperature with a separate thermometer and calculate the actual VPDair accordingly. As with all environmental sensors, regular maintenance and calibration is essential.
Many growth chambers are programmed to control for a constant RH. However, a RH of 30% corresponds to a VPD of 1.4 kPa at 18°C, 2.2 kPa at 25°C and 3.3 kPa at 32°C. Any change in temperature is therefore accompanied by considerable changes in evaporative demand. If one would like to avoid such confounding effects, RH has to be adjusted as well. Alternatively, change the control system of the chamber to steer for VPD rather than RH.
Air humidity as a treatment
Air VPD affects both transpiration rate and leaf expansion rate in most species. Under well-watered conditions, it can be an experimental way to manipulate transpiration or to affect leaf growth rate without changing the soil water status. In addition, it is a component of the water deficit, which is defined by both soil supply and evaporative demand (see 4F). An experiment with varying VPD usually requires chambers with a considerable air desiccation capability. Note that the outside air is drier during winter than during summer; consequently, low humidity are more easily obtained in winter. Alternatively, one could supplement the chambers with an air stream from pressurised air sources.
Further reading
Technical: Jones (1992).
Biological: Tardieu and Simonneau (1998) and Sadok et al. (2007).
F. Soil water
Background
Precipitation is the main source of water for most plants and varies – depending on geographic location – from <50 to >2000 mm year–1. Part of the precipitation may disappear as runoff, another part as evaporation from soil or transpiration by the vegetation. The amount of water that can be stored in the soil (after drainage) depends on its composition: Sandy soils can contain up to 0.12 g of water per g of soil, loamy soil 0.25, clay 0.40 and potting compost up to 1.5 g g–1. In plant biology, it is essential to characterise soil water status as soil water potential rather than soil water content, as this is the variable that determines water availability for plants. The relation between water content and water potential is a permanent characteristic of a soil, often named water release curve and can be measured in many laboratories of soil science.
Water deficit occurs for a plant when water uptake is insufficient to cope with the evaporative demand (see 4E). Apart from the available water in the soil and its osmotic potential (see section 4H), the aerial environment (VPD, wind speed) is, therefore, an essential determinant of water stress. A fourth component is the plant itself, which can reduce transpiration by stomatal closure. The variables involved in water deficit vary rapidly in fluctuating conditions (e.g. 4-fold variations in some minutes when light varies) and control each other by multiple feedbacks. Hence, an isolated measurement of plant water status is almost meaningless without accurate measurements of the environmental context.
Leaf water potential measured at the end of the night (predawn leaf water potential) gives a good indication of the soil water status as sensed by the plant because, in the absence of transpiration, soil and leaf water potentials are theoretically equal. In nature, soil water potential ranges from near-zero in saturated soil to –100 MPa in a fully air-dried soil, but plants of most species can only extract water in the range from 0 to –2 MPa. Stomatal control prevents leaves (and roots) from drying below this value, which is therefore a biological threshold for water extraction.
Controlled environments
The necessity to water plants not only depends on environmental conditions (VPD, irradiance, wind speed) but also on plant size, species, potting volume and substrate. Especially in the glasshouse at high irradiance, plants may experience mild water stress within a day if pots – and therefore the available water volume – are small. A good method for ensuring equal water status to all plants consists of weighing the pots and adding water until a pre-defined target weight is reached, corresponding to the target soil water content. Accidental leakage of water and thereby also of water-soluble nutrients, caused by an unequal flow of water through various parts of the pot can be mitigated by supplying pots with saucers. Subirrigation systems where a small layer of water is applied around the pots for a limited amount of time ensures a good supply, as long as the soil column is intact. In both types of irrigation, care has to taken to ensure that the bottom of the pot is not water-saturated and thereby anoxic. Especially in pots with a limited height of the soil column, water saturation can be a problem (Passioura 2006).
Not only quantity, but also water quality is of great importance and quality parameters such as electrical conductivity (EC) and pH should be monitored regularly. Tap water may have a high pH and can contain undesirable additives. In that case rain water or distilled water may be preferred. Water stored in a canister will ensure that its temperature is not too different from that of the soil. Some species watered daily with cold tap water have been found to remain 20–30% smaller than with warmed tap water (Brockwell and Gault 1976). Rewatering of completely dry pots can be difficult for peat-containing soil, as peat can be modified structurally when dried out (Schwärzel et al. 2002). As a consequence, water may not redistribute in the pot evenly.
Water supply as a treatment
Drought stress can be applied in a variety of ways, all with different outcomes. The approach followed strongly depends on the type of questions one wants to tackle. For plants growing in a solid substrate, water deficit can be applied by withholding irrigation from a certain point in time. In an experimental garden, this may be a useful way to mimic the onset of a dry season. For plants grown in pots the approach is less suitable. If large plants are present in relatively small pots under high evaporative demand this leads to a very quick depletion of the available water, giving hardly any time for the plant to acclimate physiologically and morphologically. If plants are small at the onset of the drought in relation to the available soil water and placed under conditions of low evaporative demand, this strategy may not work either, simply because plants will not draw down the available water in the pot enough to induce drought. Owing to the interaction with plant size, interpretation of performance differences between species or genotypes is complicated: larger plants that seemed to perform worse may have depleted the soil water more quickly because of their higher total transpiration, not because of any physiological or morphological acclimation.
A second approach is to re-water plants periodically, either to field capacity or with a fixed volume for each pot. This allows for an easy achievable protocol that can also be automated relatively simple. As with the first option, the drawback is that plants of different size experience different levels of water stress.
A third option is to re-water plants in such a way that soil water status is controlled individually for each pot. This implies that the amount of water that is provided will replace that taken up by the plants, keeping a constant and equal soil water status. The actual amount required can be determined gravimetrically, after correction for the increased plant biomass. The higher the frequency of these additions, the closer plants are to steady state. Soil water status can then be transformed into soil water potential via a water release curve (Groenevelt and Grant 2004).
A fourth option is to apply osmolytes such as polyethylene-glycol (PEG) or mannitol in water-based hydroponics that impose a constant and known water potential to the roots. This is best used for short-term experiments only (usually not more than 1–2 days), as they may have negative side effects; strongly reduced O2 supply to the roots being one (Munns et al. 2010). A last approach, where supply and demand are probably best in equilibrium and still different levels of drought stress can be invoked, is by placing water-conductive material below the pots, which then are placed in tubs with different levels of water. The plants with larger distance to the water level will experience stronger resistance to water flow (Fernández and Reynolds 2000). Good capillary contact between the water and the pot during the experiment is the key here.
Apart from coping with drought stress, plants in the field can (partly) avoid drought by adjusting their root architecture. If this enables them to grow into deeper soil layers they may exploit water sources which were otherwise unavailable to the plants. Any of the above-mentioned methods that uses water-based hydroponics or small pots is not suitable to pick up such a mechanism (cf. Lilley and Fukai 1994). Really large and especially deep pots or lysimeters are more appropriate here. Whatever method with soil is used, it is good to add a fixed mass of soil to each replicate pot rather than a fixed volume, as soil density may vary, especially for peat and thereby the amount of available water.
The minimum indication for characterising water deficit treatments should be soil water potential and evaporative demand at sampling time. Sampling at the end of night is usually preferred because it is reproducible, which is not necessarily the case for the light period. For experiments in soil, two strategies can be adopted. One, if the measuring capacity of the research group is limited, it may be preferable to impose a constant evaporative demand in a growth chamber and to maintain soil water content constant by daily irrigation after weighing pots. Two, fluctuating evaporative demand and naturally declining soil water content are closer to natural situations, but need a more careful design of the experiment and frequent measurements of plant, air and soil variables. Both strategies can be used in phenotyping platforms for analysing hundreds of plants (e.g. Granier et al. 2006; Sadok et al. 2007).
Further reading
Technical: Jones (1992).
Biological: Chaves and Oliveira (2004), Munns et al. (2010) and Tardieu and Tuberosa (2010).
G. Temperature
Background
In nature, plants frequently experience considerable temporal and spatial temperature variability, with profound consequences for their physiology, development and overall growth. Temperatures decline by ~5°C for each 10° increase in latitude and 6°C for every km increase in altitude. Within the plant, temperatures of leaves, stems and roots may differ substantially, with aboveground organs being sometimes more than 20°C warmer than roots during the day, but cooler during the night (Larcher 2003). Similarly, leaf temperatures can be up to 10°C higher or lower than that of the surrounding air, depending on irradiance, air movement and transpiration. Large daily and seasonal variation in temperature is also common in many habitats. Finally, temperatures are changing as a result of global climate change, with night-time temperatures raising more than daytime temperatures. Most species will actively grow somewhere in a window of daily mean temperatures of 5−35°C.
Controlled environments
As mentioned above, radiation can result in substantially higher shoot temperatures than that of the surrounding air. Exposure to solar radiation may also strongly affect the temperature of pots in glasshouses, especially when they are black. To minimise direct heating of pot surfaces in glasshouse-grown plants (thus, warming of roots), pots should be painted white or covered in reflective foil. In growth cabinets, heterogeneity of temperature from one side of the cabinet to another can be minimised by vertical air flow. This also helps reduce, but not eliminate, radiative heating of leaves at the top of canopy. In older cabinets, temperatures are often controlled using on-off system cycles, with the result that temperatures could vary by as much as 5°C above and below the set point temperature. In modern cabinets, this variability is reduced by matching the rate of heating or cooling to the extent to which the temperature has deviated from a set point temperature. Although it is often assumed that maximal growth rates are exhibited when air temperatures are 3–10°C higher during the day than night, past experiments suggest that the benefits of cooler nights can be negligible or even detrimental to whole plant growth, particularly at low growth irradiance (Rajan and Blackman 1975).
Temperature as a treatment
When using temperature as a treatment, consideration needs to be given to whether the aim is to achieve a set point leaf or air temperature. In the event that leaf temperature is of interest, then adjustments to air temperature and air flow rates may be necessary. This can be obtained by automatic control of the growth chamber using meristem or leaf temperature measured with thermocouples instead of air temperature for control. In treatments where cabinet day- and night-time temperatures differ, the temperature of the rooting media will lag behind that of the leaves. Where it is necessary to control shoot and root temperatures independently, set point root temperatures should be maintained separately (e.g. by inserting heat exchange tubing into or around solid rooting media). Also be aware that higher air temperatures increase evaporative demand (see section 4E). When growing plants at low temperature, ice needs to be removed from the cooling coils at regular intervals using a defrosting cycle; in some cabinets, defrosting may therefore result in a transient increase in air temperature. As maintaining low temperatures places a heavy load on refrigerating systems, it is advisable to select cabinets designed to maintain temperatures several degrees below the set point temperature, rather than expect reliable operation at the engineering limits of the cabinet.
Further reading
Technical: Hicklenton and Heins (1997).
Biological: Berry and Raison (1981), Stitt and Hurry (2002), Fitter and Hay (2002), Atkin et al. (2006), and Sadok et al. (2007).
H. Salinity
Background
Salts can be deposited in the soil directly by seawater, indirectly from wind and rain, as well as through the weathering of rocks. These processes, combined with the influence of climatic and landscape features (e.g. soil water fluxes and runoff) and the effects of human activities (e.g. irrigation practices), determine where salt accumulates in the landscape (Rengasamy 2006). Where the salt is derived from wind and rain, its origin is oceanic spray and so has the same composition as seawater. If derived by weathering of rock, Na+ and Cl– are still the predominant ions but there is a higher proportion of Ca2+, Mg2+ and SO4 2– compared with seawater. It is often accompanied by HCO3 – ions in which case it is alkaline and affects uptake of other ions (Rengasamy 2006).
Fresh rainwater generally contains negligible amounts of salts. Seawater has a concentration of ca 500 mM NaCl plus some minor quantities of other salts. The actual salinity level in a soil will vary with its water content and can in extreme cases with high evaporation go up to more than 1000 mM.
Controlled environments
Generally, salinity is not an issue for most experiments where this factor is not studied. However, high concentrations of ions may accumulate in pots, where evapotranspiration is substantial and nutrient solution is added frequently (see section 4D). In these cases, it can help to flush the pots every now and then with demineralised water and allow them to free-drain. Build-up of salt is easily measured by determining the electrical conductivity of a (soil) solution. From an agricultural perspective, a soil is considered to be saline when its saturated solution has an EC of 4 dS m–1 (USSL, 2011), equivalent to ~40 mM NaCl. Note that especially dicotyledonous halophytes require some salt (10–100 mM NaCl) to reach maximum growth (Flowers and Colmer 2008).
Salinity as a treatment
Three treatment approaches can be taken. The most common and convenient one is water- or solid-based hydroponics (see section 2A), adding NaCl to a complete nutrient solution. A second method is sand culture, when the sand is irrigated with nutrient solution to which NaCl is added. The sand must be coarse enough (or have a narrow particle distribution) to allow rapid drainage and large volumes of solution are needed to flush frequently. A third method is to use soil as the medium, which is likely to best mimic field conditions but most difficult to control, especially in small pots. In all cases build-up of salts may occur because of evapotranspiration and water has to be added to compensate for this. Be aware that this method may create pockets of low-salinity soil in a high-salinity background. Alternatively, soil can be flushed periodically with salt-nutrient solution to completely replace the soil solution.
In all types of experimental setups, a decision needs to be made about which salts to use, at which stage of plant development to start the salinity treatment and at what concentration. Experiments should be conducted over a sufficiently long period, as plant responses in the short term are primarily osmotic. Only in the longer term (days, weeks or months, depending on the species) does the salt rise to toxic concentrations in leaves and salt-specific effects are seen (Munns 2002). Glycophytes are generally germinated under non-saline conditions, with the salt concentration increased in gradual steps to avoid severe osmotic shock.
A protocol describing how to grow glycophytes in salt solution, to measure differences in the rate of salt accumulation in leaves, or differences in growth rates over time is provided in PrometheusWiki (see below). The method is suitable for screening a large number of genotypes for genetic variation in Na+ exclusion from leaves, or a smaller number of genotypes or species for differences in ‘salinity tolerance’, that is, biomass production in saline versus non-saline solution.
Further reading
Technical: Protocols in PrometheusWiki (http://www.publish.csiro.au/prometheuswiki).
Biological: Munns (2002), Flowers and Colmer (2008) and Munns and Tester (2008)
I. Confounding factors
There is a large variety of ways in which the growth of plants can be affected by unintended factors, thereby limiting the reproducibility of experiments. Ozone concentrations, for example, may develop strongly during the summer season in densely populated areas and will also affect the plants in growth chambers. Ozone concentrations can be obtained from technical services in towns. Several other air contaminants may have negative effects on growth and development. Therefore, it is a good idea to check the intake location of the fresh air that is used to replenish the air in growth chambers. Point sources close to a car park, inside the basement of a building, near the heating system, or close to the exhaust from a fume hood may contain relatively high concentrations of compounds that can affect the plants in erratic ways. Contamination can also come from materials or paints used to manufacture growth chambers (Tibbitts 1997). Another source of variation can be the sand or soil that is used, not only because the different amount and availability of nutrients, but also because of contaminations of various sorts. Some laboratories use sensitive indicator plants to timely recognise the presence of certain contaminations.
Conclusions
Phenotyping experiments with plants require careful planning. The most controlled growth environment is not necessarily always the best one, even though it has the best potential for ensuring within-laboratory replicability. A wide variety of measures can be taken to avoid the introduction of artefacts. Some of the most overlooked factors in that respect are pot size and the fact that nutrient and water supply strongly interact with plant size. Growing plants for experimental purposes remains an art, requiring in-depth knowledge of physiological responses to the environment together with proper gauging of the environmental parameters. We advocate the adoption of a simple and practical checklist to document and report a minimum set of information concerning the abiotic environment plants experienced during experiments. This checklist is found in Table 2, together with some suggestions for a more extended characterisation of the experimental conditions.
Acknowledgements
We thank Silvia Braun for conducting the evaporation measurements, Lew Ziska for providing data on light distribution in his growth chamber and Chryssa Dimaki, Nikolaos Ntagkas, Leo Marcelis and Jouke Postma for insightful comments on a previous version of this manuscript. Contributions of FT, YG and BU have been partly funded by the project DROPS (www.drops-project.eu), which received funding from the European Community’s Seventh Framework Program under the grant agreement no. FP7–244374.
References
Annicchiarico P (2002) Genotype × environment interaction: challenges and opportunities for plant breeding and cultivar recommendations. FAO Plant Production and Protection Paper 174.Atkin OK, Loveys BR, Atkinson LJ, Pons TL (2006) Phenotypic plasticity and growth temperature: understanding interspecific variability. Journal of Experimental Botany 57, 267–281.
| Phenotypic plasticity and growth temperature: understanding interspecific variability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XisFKnsg%3D%3D&md5=bc8d68dea1808b40ff98b85be7d7dd3fCAS |
Berry WL, Knight S (1997) Plant culture in hydroponics. In ‘Plant growth chamber handbook’. (Eds RW Langhans, TW Tibbitts) pp. 119–131. (Iowa State University: Ames, IA)
Berry JA, Raison JK (1981) Responses of macrophytes to temperature. In ‘Physiological plant ecology I. Responses to the physical environment’. (Eds OL Lange, PS Nobel, CB Osmond, H Ziegler) pp. 277–338. (Springer-Verlag: Berlin)
Björn LO, Vogelmann TC (1994) Quantification of light. In ‘Photomorphogenesis in plants’. 2nd edn. (Eds RE Kendrick, GHM Kronenberg) pp. 17–25. (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Brockwell J, Gault RR (1976) Effects of irrigation water temperature on growth of some legume species in glasshouses. Australian Journal of Experimental Agriculture and Animal Husbandry 16, 500–505.
| Effects of irrigation water temperature on growth of some legume species in glasshouses.Crossref | GoogleScholarGoogle Scholar |
Campbell CD, Sage RF, Kocacinar F, Way DA (2005) Estimation of the whole-plant CO2 compensation point of tobacco (Nicotiana tabacum L.) Global Change Biology 11, 1956–1967.
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany 55, 2365–2384.
| Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVOisr0%3D&md5=8510308201fe996b0f6a03f35fe0ccd7CAS |
Cooper A (1976) ‘Nutrient film technique of growing crops’. (Grower Books: London)
Cooper AJ (1979) ‘The ABC of NFT.’ (Grower Books: London)
Cummings IG, Reid JB, Koutoulis A (2007) Red to far-red ratio correction in plant growth chambers – growth responses and influences of thermal load on garden pea. Physiologia Plantarum 131, 171–179.
Drummond C (2009) Replicability is not reproducibility: nor is it good science. Proceedings of the Evaluation Methods for Machine Learning Workshop 26th International Conference for Machine Learning, Montreal, Quebec, Canada.
Epstein E (1994) The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences of the United States of America 91, 11–17.
| The anomaly of silicon in plant biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXntlehtA%3D%3D&md5=68ed3224c3c68bedb831852c505366a8CAS |
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell & Environment 24, 755–767.
| Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmsFCmurk%3D&md5=d5d21e3a2db4c2e5a3f591a51060bfbbCAS |
Evans JR, von Caemmerer S, Adams WWI (Eds) (1988) ‘Ecology of photosynthesis in sun and shade.’ (CSIRO Publishing: Melbourne)
Fernández RJ, Reynolds JF (2000) Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia 123, 90–98.
| Potential growth and drought tolerance of eight desert grasses: lack of a trade-off?Crossref | GoogleScholarGoogle Scholar |
Fiorani F, Jahnke S, Rascher U, Schurr U (2012) Imaging plants dynamics in heterogenic environments. Current Opinion in Biotechnology
| Imaging plants dynamics in heterogenic environments.Crossref | GoogleScholarGoogle Scholar | in press
Fitter AH, Hay RKM (2002) ‘Environmental physiology of plants.’ (Academic Press: London)
Flitcroft ID, Ingram KT (2002) Controlling CO2 in a commercial controlled environment plant growth chamber. International Journal of Biotronics 31, 73–84.
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqur%2FE&md5=72b4deb297e7aba3822bd35460a0cbe9CAS |
Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology 53, 203–224.
| Local and long-range signaling pathways regulating plant responses to nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVWhtbk%3D&md5=6c5f219caf5e774799cb4768a0811ad4CAS |
Franklin KA (2008) Shade avoidance. New Phytologist 179, 930–944.
| Shade avoidance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqur%2FK&md5=af57afbaf76617055189820bb1fc6fbdCAS |
Freijsen AHJ, Otten H (1984) The effect of nitrate concentration in a flowing solution system on growth and nitrate uptake of two Plantago species. Plant and Soil 77, 159–169.
| The effect of nitrate concentration in a flowing solution system on growth and nitrate uptake of two Plantago species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXktVKju7g%3D&md5=a593b9773cc81db40848e38b03fe840fCAS |
Füllner K, Temperton VM, Rascher U, Jahnke S, Rist R, Schurr U, Kuhn AJ (2012) Vertical gradient in soil temperature stimulates development and increases biomass accumulation in barley. Plant, Cell & Environment
| Vertical gradient in soil temperature stimulates development and increases biomass accumulation in barley.Crossref | GoogleScholarGoogle Scholar | In press.
Garnier E, Freijsen AHJ (1994) On ecological inference from laboratory experiments conducted under optimum conditions. In ‘A whole-plant perspective on carbon-nitrogen interactions’. (Eds J Roy, E Garnier) pp. 267–292. (SPB Academic Publishing: The Hague, The Netherlands)
Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M (1999) Influence of nitrate and ammonium nitrate supply on the response of photosynthesis, carbon and nitrogen metabolism, and growth to elevated carbon dioxide in tobacco. Plant, Cell & Environment 22, 1177–1199.
| Influence of nitrate and ammonium nitrate supply on the response of photosynthesis, carbon and nitrogen metabolism, and growth to elevated carbon dioxide in tobacco.Crossref | GoogleScholarGoogle Scholar |
Ghars MA, Parre E, Debez A, Bordenave M, Richard L, Leport L, Bouchereau A, Savouré A, Abdelly C (2008) Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation. Journal of Plant Physiology 165, 588–599.
| Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXls1Wgsr8%3D&md5=7247b0d3fe646376c641f3a7aa45cbbfCAS |
Gorbe E, Calatayud A (2010) Optimization of nutrition in soilless systems: a review. Advances in Botanical Research 53, 193–245.
| Optimization of nutrition in soilless systems: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovV2nsbo%3D&md5=69191cf55efd1638f91d4f496f11c7cfCAS |
Granier C, Tardieu F (1998) Is thermal time adequate for expressing the effects of temperature on sunflower leaf development? Plant, Cell & Environment 21, 695–703.
| Is thermal time adequate for expressing the effects of temperature on sunflower leaf development?Crossref | GoogleScholarGoogle Scholar |
Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux JJ, Rolland G, Bouchier-Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist 169, 623–635.
| PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Grime JP, Hunt R (1975) Relative growth rate: its range and significance in a local flora. Journal of Ecology 63, 393–422.
| Relative growth rate: its range and significance in a local flora.Crossref | GoogleScholarGoogle Scholar |
Groenevelt PG, Grant CD (2004) A new model for the soil–water retention curve that solves the problem of residual water contents. European Journal of Soil Science 55, 479–485.
| A new model for the soil–water retention curve that solves the problem of residual water contents.Crossref | GoogleScholarGoogle Scholar |
Hannemann J, Poorter H, Usadel B, Bläsing OE, Finck A, Tardieu F, Atkin OK, Pons T, Stitt M, Gibon Y (2009) Xeml Lab: a tool that supports the design of experiments at a graphical interface and generates computer-readable metadata files, which capture information about genotypes, growth conditions, environmental perturbations and sampling strategy. Plant, Cell & Environment 32, 1185–1200.
| Xeml Lab: a tool that supports the design of experiments at a graphical interface and generates computer-readable metadata files, which capture information about genotypes, growth conditions, environmental perturbations and sampling strategy.Crossref | GoogleScholarGoogle Scholar |
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical Communication no. 22. Commonwealth Bureau of Horticulture and Plantation Crops, Great Britain.
Hicklenton PR, Heins RD (1997) Temperature. In ‘Plant growth chamber handbook’. (Eds RW Langhans, TW Tibbitts) pp. 31–41. (Iowa State University: Ames, IA)
Hoagland DR, Snijder WC (1933) Nutrition of strawberry plants under controlled conditions. Proceedings of the American Society for Horticultural Science 30, 288–296.
Hughes AP, Cockshull KE (1971) The variation in response to light intensity and carbon dioxide concentration shown by two cultivars of Chrysanthemum morifolium grown in controlled environments at two times of year. Annals of Botany 35, 933–945.
ICCEG (2004) International Committee for Controlled Environment Guidelines. Minimum guidelines for measuring and reporting environmental parameters for experiments on plants in growth rooms and chambers. Available at http://www.controlledenvironments.org/Guidelines/minimum-guidelines.htm [Accessed 3 January 2012]
Ingestad D (1982) Relative addition rate and external concentration – driving variables used in plant nutrition research. Plant, Cell & Environment 5, 443–453.
| Relative addition rate and external concentration – driving variables used in plant nutrition research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXovVWisQ%3D%3D&md5=05b48187d69b82abc36791461f9de8a8CAS |
Ioannides JP, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, Falchi M, Furlanello C, Game L, Jurman G, Mangion J, Mehta T, Nitzberg M, Page GP, Petretto E, van Noort V (2009) Repeatability of published microarray gene expression analyses. Nature Genetics 41, 149–155.
| Repeatability of published microarray gene expression analyses.Crossref | GoogleScholarGoogle Scholar |
Jansen M, Gilmer F, Biskup B, Nagel KA, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, De Jaeger I, Metzlaff M, Schurr U, Scharr H, Walter A (2009) Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Functional Plant Biology 36, 902–914.
| Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7rF&md5=f5e29447ec332857f8a47803fa405c61CAS |
Jiao YL, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nature Reviews. Genetics 8, 217–230.
| Light-regulated transcriptional networks in higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhslOgtrY%3D&md5=16cd90b78d47fb58a5904e089ef459e9CAS |
Jones HG (1992) ‘Plants and microclimate, a quantitative approach to environmental plant physiology.’ (Cambridge University Press: Cambridge)
Jones HG, Archer N, Rotenberg E, Casa R (2003) Radiation measurement for plant ecophysiology. Journal of Experimental Botany 54, 879–889.
| Radiation measurement for plant ecophysiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXivFWms70%3D&md5=f1500c95d4a16b1fe070415d8bae4343CAS |
Juurola E (2003) Biochemical acclimation patterns of Betula pendula and Pinus sylvestris seedlings to elevated carbon dioxide concentrations. Tree Physiology 23, 85–95.
| Biochemical acclimation patterns of Betula pendula and Pinus sylvestris seedlings to elevated carbon dioxide concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXit1Srsb8%3D&md5=875a3c04b99dec4d2684c74c213f9c48CAS |
Keeling CD, Chin JFS, Whorf TP (1996) Increased activity of northern vegetation inferred from atmospheric CO2 measurements. Nature 382, 146–149.
| Increased activity of northern vegetation inferred from atmospheric CO2 measurements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XktlSktbg%3D&md5=b5090888e7bba3b285893fee9fcd5d57CAS |
Kilian J, Peschke F, Berendzen KW, Harter K, Wanke D (2012) Prerequisites, performance and profits of transcriptional profiling the abiotic stress response. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms 1819, 166–175.
| Prerequisites, performance and profits of transcriptional profiling the abiotic stress response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVWqsL8%3D&md5=80eb2a0d45362a506133c33c6bcb8c02CAS |
Körner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytologist 172, 393–411.
| Plant CO2 responses: an issue of definition, time and resource supply.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Pons TL, Chapin FS (2008) ‘Plant physiological ecology.’ (Springer-Verlag: Berlin)
Langhans RW, Tibbitts TW (Eds) (1997) ‘Plant growth chamber handbook’ (Iowa State University: Ames, IA)
Larcher W (2003) ‘Physiological plant ecology.’ (Springer-Verlag: Berlin)
Lilley JM, Fukai S (1994) Effect of timing and severity of water deficit on four diverse rice cultivars. II. Physiological responses to soil water deficit. Field Crops Research 37, 215–223.
| Effect of timing and severity of water deficit on four diverse rice cultivars. II. Physiological responses to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Massa GD, Kim HH, Wheeler RM, Mitchell CA (2008) Plant productivity in response to LED lighting. HortScience 43, 1951–1956.
Massonnet C, Vile D, Fabre J, Hannah MA, Caldana C, Lisec J, Beemster GTS, Meyer RC, Messerli G, Gronlund JT, Perkovic J, Wigmore E, May S, Bevan MW, Meyer C, Díaz SR, Weigel D, Micol JL, Buchanan-Wollaston V, Fiorani F, Walsh S, Rinn B, Gruissem W, Hilson P, Hennig L, Willmitzer L, Granier C (2010) Probing the reproducibility of leaf growth and molecular phenotypes: a comparison of three Arabidopsis accessions cultivated in ten laboratories. Plant Physiology 152, 2142–2157.
| Probing the reproducibility of leaf growth and molecular phenotypes: a comparison of three Arabidopsis accessions cultivated in ten laboratories.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVantrw%3D&md5=2075c4f26a95a08d353f63dd12317ee7CAS |
Max JFJ, Schurr U, Tantau HJ, Hofmann T, Ulbrich A (2012) Greenhouse cover technology. Horticultural Reviews 40, in press.
McCree KJ (1972) Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agricultural Meteorology 10, 443–453.
| Test of current definitions of photosynthetically active radiation against leaf photosynthesis data.Crossref | GoogleScholarGoogle Scholar |
Miller DM (1987) Errors in the measurement of root pressure and exudation volume flow rate caused by damage during the transfer of unsupported roots between solutions. Plant Physiology 85, 164–166.
| Errors in the measurement of root pressure and exudation volume flow rate caused by damage during the transfer of unsupported roots between solutions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnhslyisw%3D%3D&md5=73c86cb6986c6727574d39ec56aafbd9CAS |
Morcuende R, Bari RM, Gibon Y, Zheng W, Pant BD, Bläsing O, Usael B, Czechowski T, Udvardi MK, Stitt M, Scheible WR (2007) Genome-wide reprogramming of metabolism, protein synthesis, cellular processes and the regulatory networks of Arabidopsis in response to phosphate. Plant, Cell & Environment 30, 85–112.
| Genome-wide reprogramming of metabolism, protein synthesis, cellular processes and the regulatory networks of Arabidopsis in response to phosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitVCltb0%3D&md5=18f6f96c914d501080163de7dade3465CAS |
Morison JIL, Gifford RM (1984) Ethylene contamination of CO2 cylinders. Effects on plant growth in CO2 enrichment studies. Plant Physiology 75, 275–277.
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell & Environment 25, 239–250.
| Comparative physiology of salt and water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhslakurw%3D&md5=8558cb25c538a179db778e53069617f0CAS |
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil 253, 201–218.
| Screening methods for salinity tolerance: a case study with tetraploid wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVemsbc%3D&md5=70bdd8fd1e2a3bae5d69458170c6bba7CAS |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=74a9a391a4c4a1f572e0302bd42130f5CAS |
Munns R, James RA, Sirault XRR, Furbank RT, Jones HG (2010) New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany 61, 3499–3507.
| New phenotyping methods for screening wheat and barley for beneficial responses to water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVert7jJ&md5=626f450aea4d5653be21fc53f83e2d18CAS |
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bio assays with tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXksFKm&md5=653cbff4216b152d155a6572062308d2CAS |
Passioura JB (2006) The perils of pot experiments. Functional Plant Biology 33, 1075–1079.
| The perils of pot experiments.Crossref | GoogleScholarGoogle Scholar |
Pierik R, Millenaar FF, Peeters AJM, Voesenek LACJ (2005) New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana. Annals of Botany 96, 533–540.
| New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Pot CS, Lambers H (1988) The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major. Physiologia Plantarum 73, 553–559.
Poorter H, Garnier E (1996) Plant growth analysis: evaluation of experimental design and computational methods. Journal of Experimental Botany 47, 1343–1351.
| Plant growth analysis: evaluation of experimental design and computational methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmslWlsr4%3D&md5=51a9ae79d56d40042581fe9e6c1ee249CAS |
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist 157, 175–198.
| Plant growth and competition at elevated CO2: on winners, losers and functional groups.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Perez-Soba M (2001) The growth response of plants to elevated CO2 under non-optimal environmental conditions. Oecologia 129, 1–20.
| The growth response of plants to elevated CO2 under non-optimal environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Van der Werf A (1998) Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? A review of herbaceous species. In ‘Inherent variation in plant growth. Physiological mechanisms and ecological consequences’. (Eds Lambers H, Poorter H, Van Vuuren MMI) pp. 309–336. (Backhuys Publishers: Leiden, The Netherlands)
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology 94, 621–627.
| Carbon and nitrogen economy of 24 wild species differing in relative growth rate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXjsFOi&md5=03f92a1ce561154c21db6dd8b3b48e5dCAS |
Poorter H, Climent J, Van Dusschoten D, Bühler J, Postma J (2012) Pot size matters: a meta-analysis on the effects of rooting volume on plant growth. Functional Plant Biology 39, 839–850.
| Pot size matters: a meta-analysis on the effects of rooting volume on plant growth.Crossref | GoogleScholarGoogle Scholar |
Popper KR (1959) ‘The logic of scientific discovery.’ (Hutchinson: London)
R Development Core Team (2011) R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria). Available at http://www.R-project.org/
Rajan AK, Blackman GE (1975) Interacting effects of light and day and night temperatures on growth of 4 species in vegetative phase. Annals of Botany 39, 733–743.
Rengasamy P (2006) World salinization with emphasis on Australia. Journal of Experimental Botany 57, 1017–1023.
| World salinization with emphasis on Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xis1Gls74%3D&md5=538b5a4354070d331a8028017afdce46CAS |
Richter SH, Garner JP, Würbel H (2009) Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nature Methods 6, 257–261.
| Environmental standardization: cure or cause of poor reproducibility in animal experiments?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsl2ns7Y%3D&md5=ae5b76f4ee33d9b70b9ea1be5bfb81e5CAS |
Romer M (2001) Carbon dioxide within controlled environments; the commonly neglected variable. In ‘Proceedings of the international conference: controlled environments in the new millennium’. (The John Innes Centre: Norwich, UK)
Römheld V (1991) The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: an ecological approach. Plant and Soil 130, 127–134.
| The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: an ecological approach.Crossref | GoogleScholarGoogle Scholar |
Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F (2007) Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant, Cell & Environment 30, 135–146.
| Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions.Crossref | GoogleScholarGoogle Scholar |
Sager JC, McFarlane JC (1997) Radiation. In ‘Plant growth chamber handbook’. (Eds RW Langhans, TW Tibbitts) pp. 1–29. (Iowa State University: Ames, IA)
Schortemeyer M, Atkin OA, McFarlene N, Evans JR (1999) The impact of elevated atmospheric CO2 and nitrate supply on growth, biomass allocation, nitrogen partitioning and N2 fixation of Acacia melanoxylon. Australian Journal of Plant Physiology 26, 737–747.
| The impact of elevated atmospheric CO2 and nitrate supply on growth, biomass allocation, nitrogen partitioning and N2 fixation of Acacia melanoxylon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlt1Ogtg%3D%3D&md5=94f3e76d0638c0b431c822e5badd0a50CAS |
Schwärzel K, Renger M, Sauerbrey R, Wessolek G (2002) Soil physical characteristics of peat soils. Journal of Plant Nutrition and Soil Science 165, 479–486.
| Soil physical characteristics of peat soils.Crossref | GoogleScholarGoogle Scholar |
Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, Galbiati M, Tonelli C, Van Breusegem F, Vuylsteke M, Inzé D (2011) Survival and growth of Arabidopsis plants given limited water are not equal. Nature Biotechnology 29, 212–214.
| Survival and growth of Arabidopsis plants given limited water are not equal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFWktr8%3D&md5=7f57caa993d48c4bb79cfef0b4ff3bfbCAS |
Smith H ed. (1984) ‘Plants and the daylight spectrum.’ (Academic Press: London)
Spomer LA, Berry WL, Tibbitts TW (1997) Plant culture in solid media. In ‘Plant growth chamber handbook’. (Eds RW Langhans, TW Tibbitts) pp. 105–118. (Iowa State University: Ames, IA)
Stitt M, Hurry V (2002) A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology 5, 199–206.
| A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivVSmur0%3D&md5=089955de538f793d3154419a5ec1d340CAS |
Stitt M, Krapp A (1999) The molecular physiological basis for the interaction between elevated carbon dioxide and nutrients. Plant, Cell & Environment 22, 583–621.
| The molecular physiological basis for the interaction between elevated carbon dioxide and nutrients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksVartLo%3D&md5=45e16e67c9394a58d9bacb93533ee179CAS |
Sulpice R, Pyl ET, Trenkamp S, Steinfath M, Tschoep H, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques M, Von Korff M, Steinhauser MC, Guenther M, Hoehne M, Selbig J, Fernie AR, Altmann T, Stitt M (2009) Starch as a major integrator in the regulation of growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 104, 4759–4764.
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49, 419–432.
| Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours.Crossref | GoogleScholarGoogle Scholar |
Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Current Opinion in Plant Biology 13, 206–212.
| Dissection and modelling of abiotic stress tolerance in plants.Crossref | GoogleScholarGoogle Scholar |
Tibbitts TW (1997) Air contaminants. In ‘Plant growth chamber handbook’. (Eds RW Langhans, TW Tibbitts) pp. 81–86. (Iowa State University: Ames, IA)
Usadel B, Gibon Y, Bläsing OE, Poree F, Höhne M, Günter M, Trethewey R, Kamlage B, Poorter H, Stitt M (2008) Multilevel genomics analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of the temperature in the non-freezing range. Plant, Cell & Environment 31, 518–547.
| Multilevel genomics analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of the temperature in the non-freezing range.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlt1Cks7g%3D&md5=007ba9909cf348f2ea7d10c4474e1976CAS |
USSL (2011) United States Salinity Laboratory. Prometheus wiki. Available at http://www.publish.csiro.au/prometheuswiki [Accessed 3 January 2012]
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. The Plant Journal 41, 195–211.
| Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWitrc%3D&md5=0fd6f295979e69cbfbd10e450052e147CAS |
Waisel Y (2002) Aeroponics: a tool for root research under minimal environmental restrictions. In ‘Plant roots, the hidden half’. (Eds Y Waisel, A Eshel, U Kafkaki) pp. 323–332. (Marcel Dekker: New York)
Zhang HM, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409.
| An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmsVShtA%3D%3D&md5=a94980fe97fa4a7228721a3f06ac7ea2CAS |
Zhang X, Hause RJ, Borevitz JO (2012) Natural genetic variation for growth and development revealed by high throughput phenotyping in Arabidopsis thaliana. G3 Genetics in press.
Zou F, Xu Z, Vision T (2006) Assessing the significance of quantitative trait loci in replicable mapping populations. Genetics 174, 1063–1068.
| Assessing the significance of quantitative trait loci in replicable mapping populations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht12mur3O&md5=d98f6f8079cb359344d119d287928b2aCAS |


