Yield stability for cereals in a changing climate
Nicola Powell A , Xuemei Ji A , Rudabe Ravash A , Jane Edlington A and Rudy Dolferus A BA CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
B Corresponding author. Email: rudy.dolferus@csiro.au
Functional Plant Biology 39(7) 539-552 https://doi.org/10.1071/FP12078
Submitted: 9 March 2012 Accepted: 15 May 2012 Published: 15 June 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
The United Nations Food and Agriculture Organisation (FAO) forecasts a 34% increase in the world population by 2050. As a consequence, the productivity of important staple crops such as cereals needs to be boosted by an estimated 43%. This growth in cereal productivity will need to occur in a world with a changing climate, where more frequent weather extremes will impact on grain productivity. Improving cereal productivity will, therefore, not only be a matter of increasing yield potential of current germplasm, but also of improving yield stability through enhanced tolerance to abiotic stresses. Successful reproductive development in cereals is essential for grain productivity and environmental constraints (drought, cold, frost, heat and waterlogging) that are associated with climate change are likely to have severe effects on yield stability of cereal crops. Currently, genetic gains conferring improved abiotic stress tolerance in cereals is hampered by the lack of reliable screening methods, availability of suitable germplasm and poor knowledge about the physiological and molecular underpinnings of abiotic stress tolerance traits.
Additional keywords: abiotic stress, cereals, grain number, grain size, reproductive stage, sterility.
Introduction
By the year 2050 the world population is expected to have grown by 34%, with an additional 2.3 billion people to feed (FAO 2009). This increase will occur mainly in developing countries where the population is expected to be more affluent and predominantly urbanised. World food production is, therefore, required to increase by 70% with the demand for staple crops like cereals to rise by 43%, an increase of almost one billion tonnes. Global rice production, which feeds approximately half the world population, has to increase by 0.6–0.9% annually until 2050 to meet demand (Carriger and Vallee 2007).
Since the introduction of ‘Green Revolution’ wheat and rice varieties, yields have reached a plateau, suggesting that increased crop yield targets will not be reached (FAO 2002; Tilman et al. 2002; Rosegrant and Cline 2003; Edmeades et al. 2010). The average annual yield increase has steadily declined from 3.2% per annum in 1960, to 1.5% in 2000 as a result of limited genetic biodiversity and environmental factors. The genetic gain that can be obtained via technologies such as heterosis, molecular breeding and transgenics is currently estimated at 50%, falling short of the 70% yield increase required by 2050 (Edmeades et al. 2010). In Europe, climate change, rather than lack in genetic progress, is considered to be the main reason for decreasing yield growth rate in wheat (Brisson et al. 2010). Agriculture will be affected by climate change through higher temperatures (estimated to increase ±2°C by 2050), changing rainfall patterns and higher carbon dioxide (CO2) levels. A change in weeds, pests and disease pressure on crops will also be associated with these climatic changes (Jaggard et al. 2010).
An additional challenge is that increasing areas of farmland are being diverted to biofuel production, causing competition for food production. The rising global scarcity and insecurity regarding availability of fossil fuels has caused increased interest in converting grain into biofuel, resulting in an unprecedented insecurity in food supply (Young 2009; Banerjee 2011). The increasing diversion of food crops to biofuel production risks escalating food prices and provides an additional challenge to meet future food production targets.
Under the majority of environmental conditions crop productivity is limited by water availability, light, heat and nutrients. Although higher temperatures and CO2 levels can improve crop yields, the gain in productivity can be counteracted by other factors. For instance, free-air CO2 enrichment (FACE) studies have shown that rice crops become more sensitive to the damaging effect of cold temperatures, thereby neutralising the expected yield improvements obtained from CO2 enrichment (Shimono et al. 2008, 2009). Application of higher nitrogen levels to boost yields may also have a negative impact under certain environmental conditions. In rice, high nitrogen supply before and during the critical stage of pollen development exacerbates the effect of cold-induced pollen sterility (Williams and Angus 1994; Gunawardena et al. 2003). High nitrogen fertilisation levels also have an adverse effect on grain-filling and drought tolerance (Demotes-Mainard and Jeuffroy 2001; Ruuska et al. 2008).
In the United States the lack of adaptation to abiotic stresses is responsible for 71% of reduction in yield potential (Boyer 1982). There are opportunities to increase crop yield by closing the gap between actual yield and the genetic yield potential (Richards 2000; Araus et al. 2008). However, this strategy may be compromised by climate change. Extreme weather events have already become more frequent and have caused crop losses in many parts of the world (Vellinga and Van Verseveld 2000). Boosting future yield will not only be a case of increasing yield and yield potential per se, but it will also be a question of maintaining this higher productivity under adverse weather conditions. Improving abiotic stress tolerance will be crucial to achieve greater yield stability within a changing environment.
Improving abiotic stress tolerance through conventional breeding methods has so far been met with limited success. Detailed accounts of the problems generally associated with quantitative trait loci (QTL) mapping for abiotic stress tolerance in cereals have recently been published (Collins et al. 2008; Fleury et al. 2010; Dolferus et al. 2011). Breeding programs tend to focus on commercial factors such as high yield potential and grain quality, not on abiotic stress tolerance. This has caused a bias in the selection of breeding lines, which may so far have excluded those lines with superior abiotic stress tolerance (Forster et al. 2000). Reintroducing stress tolerant traits in current cereal germplasm is essential and will require a focussed effort. This paper will discuss the effect of abiotic stresses on reproductive development and grain productivity in cereals, focusing on the two temperature stresses (cold/frost and heat), and extremes of water availability (drought and waterlogging).
Vegetative versus reproductive stage abiotic stresses
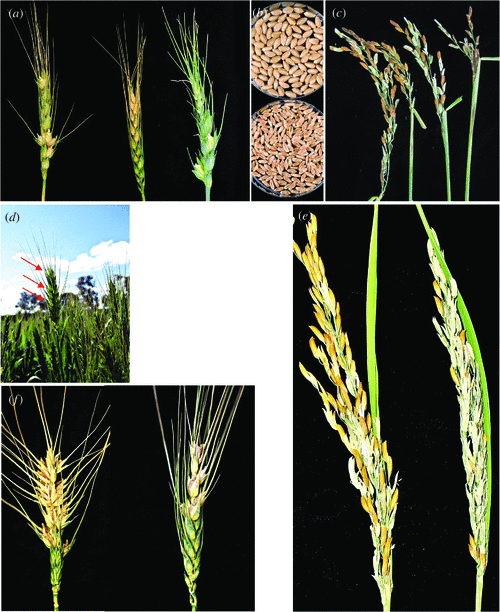
Plants are sessile organisms, so they must adapt their development continuously in function of the reigning environmental conditions. Abiotic stress stimuli affect both vegetative and reproductive development. Even though grain yield in cereals depends on successful reproductive development in a given environment, unrestrained development of the plant during the vegetative growth phase is critical. In cereals, ‘yield’ is measured as the amount of grain produced per surface area. At the plant level, grain yield is determined by both grain weight (hereafter referred to as grain size) and grain number. The timing of the stress stimulus in relation to reproductive development determines whether grain number or size will be affected. Grain number is affected by abiotic stresses such as drought mainly during the earlier stages of reproductive development and is widely considered to be the main contributor to yield losses (Fig. 1a–g; Savin and Slafer 1991; Fischer 1993; Abbate et al. 1995; Sayre et al. 1997; González et al. 2003). In contrast, the effect of drought on grain weight occurs from anthesis onwards (Fig. 1b) and during the grain maturation stage (Ji et al. 2010).
|
Abiotic stresses can affect tiller development and formation of spikes, as well as the number of spikelets per spike during floral meristem differentiation. Spikelets and florets also abort when stresses occur later during floral development (Dolferus et al. 2011). The fixation of grain number is, therefore, a dynamic process that is determined continuously by the environment throughout reproductive development. Several traits have been identified that improve vegetative stage tolerance of cereals to abiotic stress conditions (Blum 2005, 2011). For instance, in the case of drought stress these traits include yield potential, water use efficiency (WUE), harvest index (HI), deep root penetration (to access water and nutrients) and improved transpiration efficiency. These traits are ultimately important for reproductive development but they are not the focus of this paper.
In wheat, the ability to accumulate carbohydrates in the stem and leaf sheaths and remobilise these to the reproductive structures is important for the determination of grain number and size during reproductive development. Water-soluble carbohydrates are important for maintaining grain size, particularly under drought conditions when photosynthesis is arrested (Gebbing et al. 1999; Yang et al. 2001; Ruuska et al. 2006, 2008). Genetic variation in the ability to mobilise stem reserves to the developing grain has been identified and used for biochemical characterisation and QTL mapping (Yang et al. 2007; Ehdaie et al. 2008; Xue et al. 2008). The stay-green trait, which is characterised by delayed leaf senescence, is generally considered to improve tolerance at the vegetative stage to mid-season droughts (Thomas and Howarth 2000), but the trait may also interfere with carbohydrate mobilisation to the reproductive structures and affect grain-filling (Blum 1998; Sanchez et al. 2002; Collins et al. 2008; Blum 2011).
Drought stress
Drought stress is the most common cause of yield loss, with the affected area likely to double as a result of climate change, especially in tropical regions of the world (Isendahl and Schmidt 2006; IPCC 2007; Passioura 2007). Rice is a staple food for more than half of the global population; however, production uses 2–3 times more water than other cereal crops such as wheat or maize and uses 30% of the freshwater used for crops worldwide (Barker et al. 1999). Half of the world’s rice production is affected by water stress (Bouman et al. 2005; Tao et al. 2006; Yang and Zhang 2006).
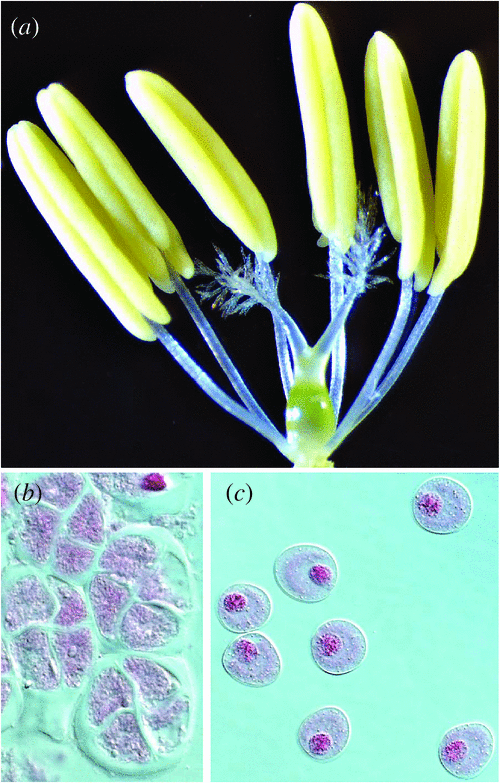
The effect of drought on reproductive processes in cereals has been extensively reviewed (Barnabás et al. 2008). Drought during the pre-anthesis stage of reproductive development has a dramatic effect on grain number (Fig. 1a–c; Bingham 1966; Fischer 1973; Ji et al. 2010). Even short, mild water stress periods at the young microspore stage of pollen development (Fig. 2b, c) cause sterility; the ovule appears to be more resilient (Ji et al. 2010). The higher sensitivity of pollen to drought stress may be due to the unique properties of the tapetum, the innermost layer of the anther wall. This specialised sporophytic secretion cell layer is dedicated to feeding the nascent microspores and the deposition of the pollen cell wall. These functions occur during meiosis and at the young microspore stage when the tapetum is most active (Clément et al. 1996). Drought stress in rice causes a pre-mature programmed cell death (PCD) response in the tapetum (Nguyen et al. 2009). PCD is a process that is also responsible for pollen abortion in cytoplasmic male sterile lines in rice (Li et al. 2004). The capacity of the tapetum to download sugars for pollen development is downregulated, consistent with repression in cell wall invertase gene expression in rice and wheat (Sheoran and Saini 1996; Koonjul et al. 2005; Ji et al. 2010). In drought-tolerant wheat, maintaining sugar transport to pollen and cell wall invertase expression is correlated with drought tolerance (Ji et al. 2010).
|
Drought stress at the young microspore stage causes abscisic acid (ABA) to accumulate in reproductive structures, resulting in pollen sterility. Pollen sterility can be mimicked by exogenous application of ABA (Morgan 1980; Zeng et al. 1985; Westgate et al. 1996; Ji et al. 2010). Application of sucrose to developing panicles reduces the detrimental effect of ABA, indicating that ABA acts via repression of sucrose metabolism (Waters et al. 1984). Under drought stress, the water potential in floral organs was maintained whereas it decreased in all other plant parts, suggesting that ABA is transported to the anthers from other plant parts (Morgan 1980; Saini and Aspinall 1982; Morgan and King 1984). However, ABA biosynthesis does also occur in the anthers and ABA can repress sugar transport to pollen by downregulating cell wall invertase expression. Downregulation of anther ABA levels using transgenic approaches resulted in improved stress-tolerance (Ji et al. 2011).
Post-anthesis drought stress has a strong effect on grain-filling and grain size (Artlip et al. 1995; Jamieson et al. 1995; Yang and Zhang 2006; Ji et al. 2010). Drought stress during early seed development reduces the rate of grain filling, induces early senescence and shortens the grain-filling and ripening period by 10 days (Nicolas et al. 1985; Westgate 1994). The process of grain-filling is supported by carbohydrate mobilisation from the stem of the plant. Cell wall invertase is also an important component in controlling sugar transport during grain filling in rice and maize (Miller and Chourey 1992; Hirose et al. 2002). Under drought conditions, ABA levels increased in developing barley seeds, resulting in induction of β-amylase genes and a reduction in starch accumulation and quality (Seiler et al. 2011). However, the fact that wheat germplasm that maintains grain number under young-microspore-stage drought conditions but does not maintain grain size when stressed at anthesis indicates that the genetic control may be different (Ji et al. 2010).
In maize, drought stress reduces kernel number as a result of ovary abortion. Drought-stressed ovules show decreased vacuolar and cell wall invertase activity, starch depletion and inhibited photosynthesis (Zinselmeier et al. 1999). The maize nucellus is supported by the pedicel and cell wall invertase is expressed in the placento-chalaza cell layer which separates the nucellus from the pedicel (Miller and Chourey 1992). Abortion of ovary development is associated with the induction of a PCD response and sugar flow to the nucellus is restricted (McLaughlin and Boyer 2004). These events, leading to ovary abortion in maize, are analogous to loss of pollen viability in rice and wheat under drought conditions.
Genes associated with tolerance to drought stress include dehydrins, late-embryogenesis abundant-like (LEA), aquaporins, heat shock proteins and several metabolic enzymes involved in osmolyte (glycinebetaine), sugar, antioxidant, lipid and amino acid (proline) biosynthesis are likely to be expressed throughout the plant (Ergen et al. 2009; Matsui et al. 2008). Extensive studies into the signal transduction and gene regulatory events associated with drought stress have also been conducted (Seki et al. 2007; Qin et al. 2011). The transcription factors of the DREB family (dehydration responsive element binding) play a central role in regulating the expression of ABA-independent-drought-inducible genes (Lata and Prasad 2011). Elaboration of the gene networks involved in response to drought is expected to be one of the important outputs as gene expression profiling using microarrays and deep sequencing technologies continue to be applied to cereals.
Cold and frost damage
Low temperatures, chilling (0–12°C) and freezing (<0°C), are another major yield limitation to cereal productivity. Plants growing in temperate regions have evolved a cold acclimation response that is triggered under mild chilling conditions (4–6°C), which enhances tolerance to more severe, sub-zero, temperatures (Guy 1999; Thomashow 1999). In contrast to drought stress, which establishes progressively over several weeks, yield losses due to chilling and frost conditions are often caused by short events at night (‘cold spells’).
Temperate climate cereals, such as wheat and barley, have the capability to sense and adapt to seasonal changes in temperatures and daylength. However, they show considerable variability in their ability to mount an acclimation response and survive freezing temperatures. Wheat and related temperate cereals that are grown under widely diverse conditions vary dramatically in their ability to withstand chilling and freezing conditions (Dubcovsky and Dvorak 2007), consistent with a broad genetic variability (Fowler and Gusta 1979; Monroy et al. 2007; Winfield et al. 2010). Throughout warm growing seasons plants have little capability to withstand freezing temperatures (below 0°C). However, as the year progresses, some are able to sense the change in environmental conditions that signal the coming winter. The gradual exposure to low non-freezing temperatures triggers an increase in freezing tolerance, known as cold acclimation (Guy 1999; Thomashow 1999). The temperature at which cold acclimation is initiated differs amongst the cereals. Acclimation in rye starts at warmer temperatures, but spring wheat and barley do not initiate acclimation until around 2°C (Fowler et al. 1999). There are also differences between wheat varieties in threshold temperatures at which cold acclimation is induced (Fowler 2008).
Cereals are most sensitive to freezing temperatures during the reproductive stage of development, in particular the young microspore stage (Fig. 2b, c). Non-freezing temperatures below 10°C are destructive at meiosis (Figs 1d, 2), causing male sterility (Langer and Olugbemi 1970; Downes and Marshall 1971; Qian et al. 1986; Demotes-Mainard et al. 1995, 1996; Subedi et al. 1998). Long-season varieties and delayed sowing can ensure that flowering is past the highest risk period for low temperatures, minimising the risk of yield loss. However, in some environments this can result in greater yield losses when flowering and grain filling is pushed to the hottest and driest periods. Some wheat varieties are quite chilling and frost hardy at the vegetative phase of development but show no tolerance at the reproductive stages, indicating that the genetic control is different (Fuller et al. 2007).
Winter and spring temperate cereals both exhibit a degree of chilling tolerance that is either induced by cold or constitutive (Jan et al. 2009). The response to cold has been extensively characterised in wheat and barley. Sugar accumulation in the vacuoles decreases the osmotic potential, causing increased ABA levels. Gene expression studies have revealed several cold-responsive genes, including signalling and transcription components, genes encoding putative protective components (cellular transport, cell membrane proteins, cryo-protectants and chaperones), as well as genes encoding metabolic, respiratory and photosynthetic components that are downregulated (Guy 1999; Thomashow 1999; Svensson et al. 2006; Monroy et al. 2007; Rapacz et al. 2008; Winfield et al. 2010). Some of these genes play an important regulatory function in the cold response (Tsuda et al. 2000; Iba 2002; Winfield et al. 2010).
Cold inducible promoters contain a C repeat/dehydration responsive element, which binds C repeat binding factors/dehydration responsive element binding proteins (CBFs/DREBs), as well as cis-elements binding bZIP transcription factors (basic leucine zipper; Thomashow 2001; Zhang et al. 2004). It has been documented that within 15 min of exposure to low temperatures CBF transcripts accumulate within the plant (Gilmour et al. 1998). In barley, 20 CBF genes have been identified; half of these are located in two tight clusters on the long arm of chromosome 5H in the same region as the Fr-H2 frost resistance locus (Francia et al. 2004; Skinner et al. 2006). A similar gene cluster at the orthologous region on chromosome 5A in diploid wheat (Triticum monococcum) is also located at the Fr-Am2 frost resistance QTL for the level of transcription of the cold-regulated gene COR14b at 15°C. (Snape et al. 2001; Vágújfalvi et al. 2003; Miller et al. 2006). The locus for frost tolerance was shown to be completely linked to the central gene cluster (Cbf-14, –15, –12; Sandve et al. 2011; Tondelli et al. 2011).
Flowering time in cereals is an important adaptation mechanism to protect sensitive reproductive structures against frost. Winter genotypes require a long period of cold exposure to accelerate the transition from the vegetative to the reproductive growth phase, a process called vernalisation (Trevaskis et al. 2007; Distelfeld et al. 2009). The requirement of periods of low but non-freezing temperatures is common to both vernalisation and cold-acclimation, suggesting that there is functional overlap between the two processes. The main vernalisation gene VRN-1 co-locates with the frost resistance QTL FR-1 on chromosome 5. When the reproductive phase has been reached in winter cereals the ability to maintain the expression of frost tolerance genes decreases and throughout the spring they de-acclimatise (Prášil et al. 2004). VRN-1 is induced during vernalisation and was shown to play a role in decreasing the cold acclimation ability during reproductive development (Limin and Fowler 2006). The correlation between winter habit (Vrn1) and frost tolerance (Fr1) could be a result of pleiotropic effects of Vrn1 loci. In spring wheat varieties the vernalisation pathway limits the expression of cold-responsive genes; expression of cold responsive genes is initially the same for spring and winter varieties but spring varieties are unable to sustain their expression (Monroy et al. 2007; Galiba et al. 2009). Further, the fact that QTL for copper tolerance were localised in the same position as the Vrn-A1 and Vrn-D1 alleles on chromosome 5A and 5D, respectively, suggests that the VRN1 gene may also play a role in other abiotic stress responses (Bálint et al. 2008). In wheat, a QTL for ABA accumulation on chromosome 5A was also found to coincide with the VRN1 gene (Quarrie et al. 1997). It is evident that response pathways to vernalisation and photoperiodism integrate a variety of other environmental cues (Distelfeld et al. 2009).
Some of our major cereal crops are of tropical origin (maize, rice and sorghum). Rice is increasingly grown in temperate climate zones but is not adapted to cold and does not have a cold acclimation response. In temperate climate zones rice is grown as a summer crop, but yields are compromised by cooler temperatures (Lin and Peterson 1975; Satake 1976; Board et al. 1980; Jacobs and Pearson 1994). The shorter temperate climate zone growing season confronts rice crops with cold conditions both at the start and end of the season. Currently, an estimated 7 million ha worldwide are prone to damage by cold at the reproductive stage (Sthapit and Witcombe 1998). In Australia, cold spells during the early booting stage cause an average yield reduction of 5–10% annually (A$44 million), making spikelet sterility the main yield-limiting factor.
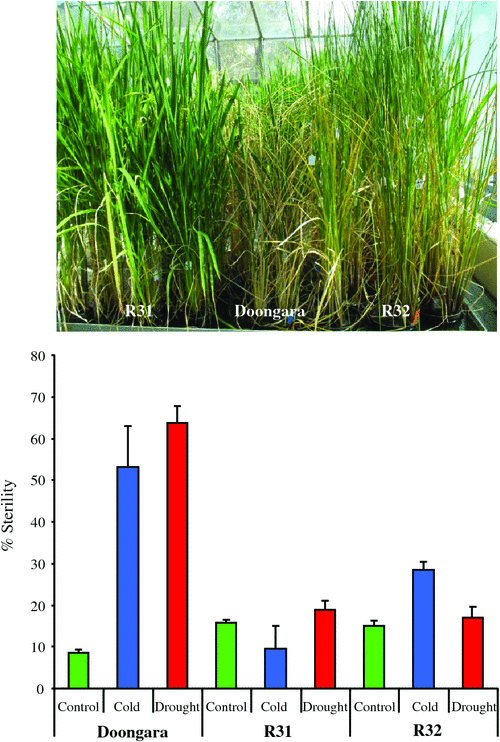
Cold-induced sterility in rice is due to pollen abortion (Fig. 1e). Pollen development is most sensitive to cold at the young microspore stage (Fig. 2b, c). The effect of cold is irreversible and cross-fertilisation with non-cold-stressed pollen results in seed production, suggesting that the ovule is not affected (Hayase et al. 1969). Cold stress in rice was shown to primarily affect the endoplasmatic reticulum (ER) in the tapetum layer (Gothandam et al. 2007). The ER plays a role in PCD of animal and plant cells (Zuppini et al. 2004). Physiological characterisations indicated that non-reducing sugars accumulate in cold-stressed panicles 12–24 h after cold treatment (Ito 1974); this is followed by tapetal hypertrophy (Nishiyama 1984). Cold stress induces a reduction in sink strength in anthers of sensitive rice lines; cell wall invertase activity and gene expression (OSINV4) are reduced and sugar transport to the pollen grains is repressed. Cold-tolerant rice maintains sink strength and pollen fertility (Oliver et al. 2005). ABA plays an important role in cold-induced sterility in rice. ABA accumulates in cold-sensitive but not cold-tolerant rice anthers and ABA treatments result in repression of anther cell wall invertase gene expression (Oliver et al. 2007). Reducing ABA accumulation in anthers by overexpressing the ABA catabolic gene ABA 8′-hydroxylase results in improved cold tolerance (Ji et al. 2011). Cold-induced sterility in sorghum shows the same stage-specificity; sterile pollen lacks starch and ovule development is not affected by cold stress. A high altitude sorghum line showed strong tolerance to cold at the young microspore stage (Brooking 1976, 1979). There is a striking similarity between cold and drought-induced pollen sterility. Rice germplasm that is tolerant to cold stress at the young microspore stage is also tolerant to drought stress (Fig. 3). This suggests that both stresses affect overlapping pathways and induce pollen abortion.
|
Heat stress
Accumulation of greenhouse gasses (carbon dioxide, methane and nitrous oxide) in the Earth’s atmosphere has caused annual average temperatures to rise by 0.35–1.13°C from 1979 to 2003 (Peng et al. 2004). The average global surface air temperature will increase by 1.8–4°C by the end of this century (IPCC 2007). In Europe, summer precipitation is predicted to decrease and heat waves will become more common and severe, placing heat ahead of drought in terms of overall effect on crop productivity (Semenov and Shewry 2011). Higher temperatures will exacerbate the problem of heat stress on crop yields. For instance, rice yields are estimated to be reduced by 41% by the end of this century (Ceccarelli et al. 2010). Similarly, wheat production in Australia is estimated to decrease by 50% when average growing season temperatures increase by 2°C (Semenov and Shewry 2011). It is estimated that around 9 million ha of wheat in tropical or subtropical areas experience yield losses due to high-temperature stress (Lillemo et al. 2005).
The response to heat stress involves physiological adaptations that are required to protect the cellular functions (compatible osmolytes such as glycinebetaine, γ-aminobutyric acid), changes in photosynthesis and assimilate partitioning, hormonal changes (ABA and ethylene) and accumulation of secondary metabolites (carotenoids, phenolics, isoprenoids; for reviews, see Kotak et al. 2007; Wahid et al. 2007; Barnabás et al. 2008; Krishnan et al. 2011). As with other abiotic stresses, heat stress induces a response to oxidative stress to protect against the damaging effect of activated oxygen species. Chaperone-like heat shock proteins are induced, as well as known drought-response proteins (late embryogenesis abundant, LEA and osmotin-like proteins). Heat stress is often combined with drought stress, with high temperatures leading to tissue dehydration. Under field conditions, selection for heat stress is often confounded by drought stress conditions and the regulatory system for both stresses may have co-evolved (Jagadish et al. 2011).
The effect of heat on the reproductive stage in rice ranges from pollen sterility induced at meiosis (Fig. 1f), poor anther dehiscence at anthesis, to reduced grain-filling and reduced grain quality (Stone and Nicolas 1994; Prasad et al. 2006; Jagadish et al. 2007; Shah et al. 2011). Research efforts on the effect of heat stress have often focussed on the effect on grain-filling and grain quality. In wheat, heat stress alters the high molecular weight gluten protein content in the grain (Gibson and Paulsen 1999; Yang et al. 2002a; Don et al. 2005; Wahid et al. 2007). Heat stress during grain development reduces grain weight in wheat (Wardlaw et al. 1989a). During the early stages of reproductive development the effect of heat was mainly on grain number. When heat-stressed at meiosis, most pollen grains were found to lack starch, causing high levels of sterility in barley (Sakata et al. 2000; Abiko et al. 2005). In rice, exposure to heat during the fertilisation process prevents anther dehiscence and reduces pollen shedding and germination (Matsui and Omasa 2002; Prasad et al. 2006). High temperatures at the young microspore stage induce pollen sterility in rice as a result of premature tapetum degeneration and abnormal vacuolation and persistence of the tapetum cells in rice and wheat (Wardlaw et al. 1989a, 1989b; Endo et al. 2009). This is similar to what was observed for drought conditions (Saini et al. 1984; Dorion et al. 1996; Lalonde et al. 1997; Ku et al. 2003). The young microspore stage of pollen development is also very sensitive to heat stress in Arabidopsis (Kim et al. 2001). Similar to cold and drought stress in rice and wheat, heat stress affects carbohydrate assimilation by the tapetum and young microspores. Cell wall invertase gene expression is repressed by heat in sorghum, and starch and sugar content in anthers is downregulated (Aloni et al. 2001; Pressman et al. 2002; Jain et al. 2007, 2010). The hormone ABA has also been implicated in the response to heat stress (Toh et al. 2008). This may be due to the fact that heat and drought stress often coincide. However, ABA can induce thermo-tolerance in maize and it can activate some genes encoding heat shock proteins (Wu et al. 1994; Gong et al. 1998). In addition, heat-resistant dwarf mutants can be made sensitive to heat stress by GA treatment (Barnabás et al. 2008). Treatment of wheat plants with an ethylene receptor inhibitor alleviates the effect of heat stress, suggesting that ethylene plays a role in inducing kernel abortion in wheat (Hays et al. 2007).
Genetic variability for heat tolerance has been identified in maize, wheat and rice (Maestri et al. 2002; Prasad et al. 2006; Spiertz et al. 2006). In wheat, QTL for yield stability at the early grain-filling stage and grain-filling duration were mapped, and QTL for pollen heat tolerance were identified in maize (Frova and Sari-Gorla 1994; Yang et al. 2002b; Mason et al. 2010).
Waterlogging
Waterlogging affects ~10% of the global land area and an estimated 10 million hectares of land in developing countries (Samad et al. 2011). Periodic flooding affects many cereal crops in high rainfall environments and under irrigation conditions. About 15–20% of the world’s wheat crops (10–15 million ha) are prone to periodic flooding every year (Sayre et al. 1994; Setter and Waters 2003). Climate change and rising sea levels are expected to affect the frequency and intensity of rainfall in some areas, thereby increasing the risk of floods. Waterlogging can cause a wide variety of symptoms that can affect yield either directly or indirectly, through affecting leaf senescence, tiller number and reduced plant height (Samad et al. 2011). Waterlogging causes a reduction in both grain number and size in wheat (Van Ginkel et al. 1992; Musgrave 1994), but also spikelet sterility. Spikelet sterility has been blamed on the combination of reduced light intensity (due to high cloud cover) and high humidity (Fischer 1985a, 1985b). Waterlogging also causes nutrient deficiencies and it has been suggested that flooding-induced spikelet sterility in wheat is caused by reduced boron uptake (Rawson et al. 1996; Saifuzzaman and Meisner 1996; Saifuzzaman et al. 2008). Boron plays an essential role in pollen cell wall biosynthesis and pollen tube growth (Iwai et al. 2006).
Selecting germplasm tolerant to abiotic stress
The difficulties associated with generating tolerant cereal lines using classic breeding approaches have been abundantly illustrated in other recent review papers (Collins et al. 2008; Fleury et al. 2010; Dolferus et al. 2011). Many abiotic stress tolerance QTL have so far been identified in plants (for a summary, see Plant Stress, http://www.plantstress.com, 22 May 2012; and Gramene website, http://www.gramene.org, accessed 22 May 2012). Suitable germplasm is available in some cereals for cold, drought and heat tolerance, but the focus of breeding programs on commercial traits (e.g. grain quality) may have led to exclusion of germplasm that is superior in terms of abiotic stress tolerance. What has hampered the quest for tolerant germplasm so far is the lack of reliable screening methods and the lack of control in the timing, severity and even occurrence of the stress stimulus under field conditions. The use of controlled environment conditions (growth chambers) or in the field using managed environment facilities (Rebetzke et al. 2012; e.g. use of irrigation and rainout shelters to control water stress conditions) are valuable developments for the establishment of reliable pre-screening methods.
Manipulation of abiotic stress tolerance using transgenic approaches
Transgenic approaches using overexpression of stress-responsive genes in model plants (e.g. Arabidopsis and rice) have identified several genes that contribute positively to abiotic stress tolerance, including several transcription factors and metabolic genes (see the Plant Stress website, address given above). The effect of very few of these genes has been investigated at the reproductive stage. Likely candidate genes for the improvement of reproductive stage abiotic stress tolerance are CBF/DREB1 transcription factors that are affected by cold and drought stress. Overexpression of CBF/DREB1 transcription factors under control of a strong constitutive promoter improves stress tolerance, but they lead to stunted growth and there is an adverse effect on yield (Oh et al. 2007; Morran et al. 2011). The use of an inducible promoter such as the drought-inducible rd29A promoter was shown to overcome the negative effect of DREB1A overexpression (Kasuga et al. 2004; Pellegrineschi et al. 2004). CBF/DREB1 transcription factors are normally expressed in the vascular parenchyma cells (Endo et al. 2008) and ectopic expression of these transcription factors may also have negative effects on yield in cereals. Recently, ABA levels were reduced by expressing the ABA catabolic gene ABA 8′-hydroxylase using a strong tapetum-specific promoter in rice anthers. This resulted in reduced anther ABA levels, maintenance of sugar supply to the pollen and improved spikelet fertility under cold conditions in rice (Ji et al. 2011). The choice of an anther-specific promoter was essential, because ABA plays a positive role in regulating water relationships and acclimation to abiotic stresses (Larosa et al. 1985; Lu et al. 2009). A better understanding of hormonal interactions involved in controlling pollen fertility may lead to identification of other target genes for transgenic approaches. Microarray studies have revealed that response to abiotic stresses such as cold, drought and heat shows a lot of similarity and many protective mechanisms are shared by these stresses. Genetic manipulations in rice and maize using the Escherichia coli cold shock proteins CspA and CspB have resulted in significant improvements in growth and grain yield under a variety of stress conditions (cold, drought and heat; Castiglioni et al. 2008). The E. coli cold shock proteins act as RNA chaperones and belong to a widespread class of proteins with homologous genes in plants. This study has illustrated that genetic manipulations can lead to crops with superior performance under field conditions.
Selection of germplasm with reproductive drought-tolerance has been based on grain yield-related traits, with selection often being conducted under field conditions. Several QTL with widely varying contribution to the grain yield phenotype under drought conditions were identified in wheat and rice (Kato et al. 2000; Lanceras et al. 2004; Wang et al. 2005; Bernier et al. 2007; Kirigwi et al. 2007; Kumar et al. 2007; Venuprasad et al. 2009). A major problem with drought tolerance selection is interference with avoidance/escape mechanisms (e.g. early flowering), especially under field conditions, where occurrence, timing, severity and length of water stress conditions cannot be controlled (Yue et al. 2006; Dolferus et al. 2011). Osmotic stress under controlled environmental conditions has been used as an alternative screening method to drought stress (Lilley et al. 1996; Zhang et al. 2001); this method has not been used at the reproductive stage to date. In maize, the anthesis-silking interval (ASI) is negatively associated with grain yield under drought conditions (Campos et al. 2004). By using marker assisted selection (MAS) QTL have been introduced in order to reduce the ASI (Boyer and Westgate 2004; Tuberosa and Salvi 2006). Despite the availability of tolerant germplasm, little progress has been made in breeding cereals with reproductive stage drought tolerance. Reliability of screening methods and availability of relevant and precisely defined traits remains a limitation.
Cold/frost tolerance in wheat and barley is a problem that requires a better physiological and molecular understanding. Flowering time and time of sowing can be exploited as effective avoidance mechanisms; however, then breeding will need to focus on germplasm that is better adapted to heat and drought stress. Although winter wheat and barley lines are able to survive cold and frost conditions at the vegetative stage, a lot still has to be learned about varietal differences in mounting an effective cold acclimation response that protects against cold spells and frost periods that occur during flowering in spring. Transgenic approaches using the E. coli cold shock proteins have shown that this technology can protect the reproductive structures (Castiglioni et al. 2008). In the case of cold-tolerance in rice breeding efforts have focussed on improving seedling vigour, shortening the growth season, and improving cold-tolerance at the booting stage (Andaya and Mackill 2003a, 2003b). Genetic variability has been identified for reproductive stage cold tolerance and this material has made it possible to identify cold-tolerance QTL (Saito et al. 2004; Oliver et al. 2005; Kuroki et al. 2009; Suh et al. 2010; Zhou et al. 2010). Two cold tolerance loci, Ctb-1 and qCTB7, have been fine-mapped (Saito et al. 2004; Zhou et al. 2010). Breeding for water-logging tolerance is complicated and needs to focus mainly on survival of the below-ground and vegetative plant parts, because the effects on reproductive development are secondary.
In conclusion, improvement of reproductive-stage abiotic stress tolerance in cereals is possible in the foreseeable future using either breeding or transgenic approaches. Critical for future achievements is defining the physiological and molecular basis of well defined abiotic stress tolerance traits at particular stages of reproductive development. This knowledge base will then provide the basis for the design of high throughput diagnostics to drive new advances in the selection of abiotic stress tolerance in our major cereal food crops.
Acknowledgements
The research presented in this paper was supported by the Australian Grains Research and Development Corporation (GRDC, grants CSP00130 and CSP00143).
References
Abbate PE, Andrade FH, Culot JP (1995) The effects of radiation and nitrogen on number of grains in wheat. The Journal of Agricultural Science 124, 351–360.| The effects of radiation and nitrogen on number of grains in wheat.Crossref | GoogleScholarGoogle Scholar |
Abiko M, Akibayashi K, Sakata T, Kimura M, Kihara M, Itoh K, Asamizu E, Sato S, Takahashi H, Higashitani A (2005) High-temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition. Sexual Plant Reproduction 18, 91–100.
| High-temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition.Crossref | GoogleScholarGoogle Scholar |
Aloni B, Peet M, Pharr M, Karni L (2001) The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112, 505–512.
| The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination.Crossref | GoogleScholarGoogle Scholar |
Andaya VC, Mackill DJ (2003a) Mapping of QTLs associated with cold tolerance during the vegetative stage in rice. Journal of Experimental Botany 54, 2579–2585.
| Mapping of QTLs associated with cold tolerance during the vegetative stage in rice.Crossref | GoogleScholarGoogle Scholar |
Andaya VC, Mackill DJ (2003b) QTLs conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica × indica cross. Theoretical and Applied Genetics 106, 1084–1090.
Araus JL, Slafer GA, Royo C, Serret MD (2008) Breeding for yield potential and stress adaptation in cereals. Critical Reviews in Plant Sciences 27, 377–412.
| Breeding for yield potential and stress adaptation in cereals.Crossref | GoogleScholarGoogle Scholar |
Artlip TS, Madison JT, Setter TL (1995) Water deficit in developing endosperm of maize: cell division and nuclear DNA endo-reduplication. Plant, Cell & Environment 18, 1034–1040.
| Water deficit in developing endosperm of maize: cell division and nuclear DNA endo-reduplication.Crossref | GoogleScholarGoogle Scholar |
Bálint AF, Vágújfalvi A, Szira F, Borner A, Cattivelli L, Dubcovsky , Galiba G (2008) QTLs and genes for abiotic stress tolerance in cereals: their general role in the environmental adaptation and their developmental-stage specificity. Options Méditerranéennes, Series A 81, 197–200.
Banerjee A (2011) Food, feed, fuel: transforming the competition for grains. Development and Change 42, 529–557.
| Food, feed, fuel: transforming the competition for grains.Crossref | GoogleScholarGoogle Scholar |
Barker R, Dawe D, Tuong TP, Bhuiyan SI, Guerra LC (1999) The outlook for water resources in the year 2020: challenges for research on water management in rice production. In ‘Assessment and Orientation towards the 21st century, Proceedings of the 19th session of the International Rice Commission, Cairo, Egypt, 7–9 September 1998’. pp. 96–109. (FAO: Rome)
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31, 11–38.
Bernier J, Kumar A, Ramaiah V, Spaner D, Atlin G (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Science 47, 507–516.
| A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice.Crossref | GoogleScholarGoogle Scholar |
Bingham J (1966) Varietal response in wheat to water supply in the field, and male sterility caused by a period of drought in a glasshouse experiment. Annals of Applied Biology 57, 365–377.
| Varietal response in wheat to water supply in the field, and male sterility caused by a period of drought in a glasshouse experiment.Crossref | GoogleScholarGoogle Scholar |
Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 100, 77–83.
| Improving wheat grain filling under stress by stem reserve mobilisation.Crossref | GoogleScholarGoogle Scholar |
Blum A (2005) Drought resistance, water-use efficiency, and yield potential – are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56, 1159–1168.
| Drought resistance, water-use efficiency, and yield potential – are they compatible, dissonant, or mutually exclusive?Crossref | GoogleScholarGoogle Scholar |
Blum A (2011) Drought resistance – is it really a complex trait? Functional Plant Biology 38, 753–757.
| Drought resistance – is it really a complex trait?Crossref | GoogleScholarGoogle Scholar |
Board JE, Peterson ML, Ng E (1980) Floret sterility in rice in a cool environment. The Astronomical Journal 72, 483–487.
Bouman BAM, Peng S, Castaoeda AR, Visperas RM (2005) Yield and water use of irrigated tropical aerobic rice systems. Agricultural Water Management 74, 87–105.
| Yield and water use of irrigated tropical aerobic rice systems.Crossref | GoogleScholarGoogle Scholar |
Boyer JS (1982) Plant productivity and environment. Science 218, 443–448.
| Plant productivity and environment.Crossref | GoogleScholarGoogle Scholar |
Boyer JS, Westgate ME (2004) Grain yields with limited water. Journal of Experimental Botany 55, 2385–2394.
| Grain yields with limited water.Crossref | GoogleScholarGoogle Scholar |
Brisson N, Gate P, Gouache D, Charmet G, Oury FX, Huard F (2010) Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crops Research 119, 201–212.
| Why are wheat yields stagnating in Europe? A comprehensive data analysis for France.Crossref | GoogleScholarGoogle Scholar |
Brooking IR (1976) Male sterility in Sorghum bicolor (L.) Moench induced by low temperature. I. Timing of the stage sensitivity. Australian Journal of Plant Physiology 3, 589–596.
| Male sterility in Sorghum bicolor (L.) Moench induced by low temperature. I. Timing of the stage sensitivity.Crossref | GoogleScholarGoogle Scholar |
Brooking IR (1979) Male sterility in Sorghum bicolor (L.) Moench induced by low temperature. II Genotypic differences in sensitivity. Australian Journal of Plant Physiology 6, 143–147.
| Male sterility in Sorghum bicolor (L.) Moench induced by low temperature. II Genotypic differences in sensitivity.Crossref | GoogleScholarGoogle Scholar |
Campos H, Cooper M, Habben JE, Edmeades GO, Schussler JR (2004) Improving drought tolerance in maize: a view from industry. Field Crops Research 90, 19–34.
| Improving drought tolerance in maize: a view from industry.Crossref | GoogleScholarGoogle Scholar |
Carriger S, Vallee D (2007) More crop per drop. Rice Today 6, 10–13.
Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoeker M, Abad M, Kumar G, Salvadore S, D’Ordine R, Navarro S, Back S, Fernandes M, Targolli J, Dasgupta S, Bonin C, Luethy MH, Heard JE (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology 147, 446–455.
| Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions.Crossref | GoogleScholarGoogle Scholar |
Ceccarelli S, Grando S, Maatougui M, Michael M, Slash M, Haghparast R, Rahmanian M, Taheri A, Al-Yassin A, Benbelkacem A, Labdi M, Mimoun H, Nachit M (2010) Plant breeding and climate changes. Journal of Agricultural Science 148, 627–637.
| Plant breeding and climate changes.Crossref | GoogleScholarGoogle Scholar |
Clément C, Burrus M, Audran JC (1996) Floral organ growth and carbohydrate content during pollen development in Lilium. American Journal of Botany 83, 459–469.
| Floral organ growth and carbohydrate content during pollen development in Lilium.Crossref | GoogleScholarGoogle Scholar |
Collins NC, Tardieu F, Tuberosa R (2008) Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiology 147, 469–486.
| Quantitative trait loci and crop performance under abiotic stress: where do we stand?Crossref | GoogleScholarGoogle Scholar |
Demotes-Mainard S, Jeuffroy MH (2001) Partitioning of dry matter and nitrogen to the spike throughout the spike growth period in wheat crops subjected to nitrogen deficiency. Field Crops Research 70, 153–165.
| Partitioning of dry matter and nitrogen to the spike throughout the spike growth period in wheat crops subjected to nitrogen deficiency.Crossref | GoogleScholarGoogle Scholar |
Demotes-Mainard S, Doussinault G, Meynard JM (1995) Effects of low radiation and low temperature at meiosis on pollen viability and grain set in wheat. Agronomie 15, 357–365.
| Effects of low radiation and low temperature at meiosis on pollen viability and grain set in wheat.Crossref | GoogleScholarGoogle Scholar |
Demotes-Mainard S, Doussinault G, Meynard JM (1996) Abnormalities in the male developmental programme of winter wheat induced by climatic stress at meiosis. Agronomie 16, 505–515.
| Abnormalities in the male developmental programme of winter wheat induced by climatic stress at meiosis.Crossref | GoogleScholarGoogle Scholar |
Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Current Opinion in Plant Biology 12, 178–184.
| Regulation of flowering in temperate cereals.Crossref | GoogleScholarGoogle Scholar |
Dolferus R, Ji X, Richards RA (2011) Abiotic stress and control of grain number in cereals. Plant Science 181, 331–341.
| Abiotic stress and control of grain number in cereals.Crossref | GoogleScholarGoogle Scholar |
Don C, Lookhart G, Naeem H, MacRitchie F, Hamer RJ (2005) Heat stress and genotype affect the glutenin particles of the glutenin macropolymer-gel fraction. Journal of Cereal Science 42, 69–80.
| Heat stress and genotype affect the glutenin particles of the glutenin macropolymer-gel fraction.Crossref | GoogleScholarGoogle Scholar |
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology 111, 137–145.
Downes RW, Marshall DR (1971) Low temperature induced male sterility in Sorghum bicolor. Australian Journal of Experimental Agriculture and Animal Husbandry 11, 352–356.
| Low temperature induced male sterility in Sorghum bicolor.Crossref | GoogleScholarGoogle Scholar |
Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316, 1862–1866.
| Genome plasticity a key factor in the success of polyploid wheat under domestication.Crossref | GoogleScholarGoogle Scholar |
Edmeades G, Fischer T, Byerlee D (2010) Can we feed the world in 2050? In ‘Food security from sustainable agriculture. Proceedings of the 15th Agronomy Conference 2010, 15–18 November 2010, Lincoln, New Zealand’. pp. 15–19.
Ehdaie B, Alloush GA, Waines JG (2008) Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Research 106, 34–43.
| Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat.Crossref | GoogleScholarGoogle Scholar |
Endo A, Koshiba T, Kamiya Y, Nambara E (2008) Vascular system is a node of systemic stress responses: competence of the cell to synthesize abscisic acid and its responsiveness to external cues. Plant Signaling & Behavior 3, 1138–1140.
| Vascular system is a node of systemic stress responses: competence of the cell to synthesize abscisic acid and its responsiveness to external cues.Crossref | GoogleScholarGoogle Scholar |
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperature cause male sterility in rice plants with transcriptional alterations during pollen development. Plant & Cell Physiology 50, 1911–1922.
| High temperature cause male sterility in rice plants with transcriptional alterations during pollen development.Crossref | GoogleScholarGoogle Scholar |
Ergen NZ, Thimmapuram J, Bohnert HJ, Budak H (2009) Transcriptome pathways unique to dehydration tolerant relatives of modern wheat. Functional & Integrative Genomics 9, 377–396.
| Transcriptome pathways unique to dehydration tolerant relatives of modern wheat.Crossref | GoogleScholarGoogle Scholar |
FAO (2002). World agriculture: towards 2015/2030. Available at http://www.fao.org. [Accessed 22 May 2012]
FAO (2009). How to feed the world in 2050. Available at http://www.fao.org. [Accessed 22 May 2012]
Fischer RA (1973) The effect of water on various stages of development on yield processes in wheat. In ‘Plant responses to climatic factors. Proceedings of the Uppsala Symposium 1970: Ecology and Conservation, 5’. pp. 233–241. (UNESCO: Paris)
Fischer RA (1985a) Number of kernels in wheat crops and the influence of solar radiation and temperature. The Journal of Agricultural Science 105, 447–461.
| Number of kernels in wheat crops and the influence of solar radiation and temperature.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (1985b) Physiological limitations to producing wheat in semitropical and tropical environments and possible selection criteria. In ‘Wheat for more tropical environments. Proceedings of the International Symposium’. pp. 209–230. (UNDP/CIMMYT: Mexico)
Fischer RA (1993) Irrigated spring wheat and timing and amount of nitrogen fertilizer. II. Physiology of grain yield response. Field Crops Research 33, 57–80.
| Irrigated spring wheat and timing and amount of nitrogen fertilizer. II. Physiology of grain yield response.Crossref | GoogleScholarGoogle Scholar |
Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany 61, 3211–3222.
| Genetic and genomic tools to improve drought tolerance in wheat.Crossref | GoogleScholarGoogle Scholar |
Forster BP, Ellis RP, Thomas WTB, Newton AC, Tuberosa R, This D, El-Enein RA, Bahri MH, Ben Salem M (2000) The development and application of molecular markers for abiotic stress tolerance in barley. Journal of Experimental Botany 51, 19–27.
| The development and application of molecular markers for abiotic stress tolerance in barley.Crossref | GoogleScholarGoogle Scholar |
Fowler DB (2008) Cold acclimation threshold induction temperatures in cereals. Crop Science 48, 1147–1154.
| Cold acclimation threshold induction temperatures in cereals.Crossref | GoogleScholarGoogle Scholar |
Fowler DB, Gusta LV (1979) Selection for winter hardiness in wheat. I. Identification of genotypic variability. Crop Science 19, 769–772.
| Selection for winter hardiness in wheat. I. Identification of genotypic variability.Crossref | GoogleScholarGoogle Scholar |
Fowler DB, Limin AE, Ritchie JT (1999) Low-temperature tolerance in cereals: model and genetic interpretation. Crop Science 39, 626–633.
| Low-temperature tolerance in cereals: model and genetic interpretation.Crossref | GoogleScholarGoogle Scholar |
Francia E, Rizza F, Cattivelli L, Stanca AM, Galiba G, Toth B, Hayes PM, Skinner JS, Pecchioni N (2004) Two loci on chromosome 5H determine low-temperature tolerance in a ‘Nure’ (winter) × ‘Tremois’ (spring) barley map. Theoretical and Applied Genetics 108, 670–680.
| Two loci on chromosome 5H determine low-temperature tolerance in a ‘Nure’ (winter) × ‘Tremois’ (spring) barley map.Crossref | GoogleScholarGoogle Scholar |
Frova C, Sari-Gorla M (1994) Quantitative trait loci (QTLs) for pollen thermotolerance detected in maize. Molecular & General Genetics 245, 424–430.
| Quantitative trait loci (QTLs) for pollen thermotolerance detected in maize.Crossref | GoogleScholarGoogle Scholar |
Fuller MP, Fuller AM, Kaniouras S, Christophers J, Fredericks T (2007) The freezing characteristics of wheat at ear emergence. European Journal of Agronomy 26, 435–441.
| The freezing characteristics of wheat at ear emergence.Crossref | GoogleScholarGoogle Scholar |
Galiba G, Vágújfalvi A, Li C, Soltész A, Dubcovsky J (2009) Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Science 176, 12–19.
| Regulatory genes involved in the determination of frost tolerance in temperate cereals.Crossref | GoogleScholarGoogle Scholar |
Gebbing T, Schnyder H, Kuhbauch W (1999) The utilization of pre-anthesis reserves in grain filling of wheat. Assessment by steady-state 13C–CO2/12C–CO2 labelling. Plant, Cell & Environment 22, 851–858.
| The utilization of pre-anthesis reserves in grain filling of wheat. Assessment by steady-state 13C–CO2/12C–CO2 labelling.Crossref | GoogleScholarGoogle Scholar |
Gibson LR, Paulsen GM (1999) Yield components of wheat grown under high temperature stress during reproductive growth. Crop Science 39, 1841–1846.
| Yield components of wheat grown under high temperature stress during reproductive growth.Crossref | GoogleScholarGoogle Scholar |
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. The Plant Journal 16, 433–442.
| Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression.Crossref | GoogleScholarGoogle Scholar |
Gong M, Li YJ, Chen SZ (1998) Abscisic acid induced thermotolerance in maize seedlings is mediated by Ca2+ and associated with antioxidant systems. Journal of Plant Physiology 153, 488–496.
| Abscisic acid induced thermotolerance in maize seedlings is mediated by Ca2+ and associated with antioxidant systems.Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2003) Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats. Field Crops Research 81, 17–27.
| Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats.Crossref | GoogleScholarGoogle Scholar |
Gothandam KM, Kim ES, Chung YY (2007) Ultrastructural study of rice tapetum under low-temperature stress. Journal of Plant Biology 50, 396–402.
| Ultrastructural study of rice tapetum under low-temperature stress.Crossref | GoogleScholarGoogle Scholar |
Gunawardena RA, Fukai S, Blamey FPC (2003) Low temperature induced spikelet sterility in rice. I. Nitrogen fertilisation and sensitive reproductive period. Australian Journal of Agricultural Research 54, 947–956.
| Low temperature induced spikelet sterility in rice. I. Nitrogen fertilisation and sensitive reproductive period.Crossref | GoogleScholarGoogle Scholar |
Guy C (1999) Molecular responses of plants to cold shock and cold acclimation. Journal of Molecular Microbiology and Biotechnology 1, 231–242.
Hayase H, Satake T, Nishiyama I, Ito N (1969) Male sterility caused by cooling treatment at the meiotic stage in rice plants, II. The most sensitive stage to cooling and the fertilising ability of pistils. Proceedings of the Crop-Science Society of Japan 38, 706–711.
| Male sterility caused by cooling treatment at the meiotic stage in rice plants, II. The most sensitive stage to cooling and the fertilising ability of pistils.Crossref | GoogleScholarGoogle Scholar |
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Science 172, 1113–1123.
| Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar.Crossref | GoogleScholarGoogle Scholar |
Hirose T, Takano M, Terao T (2002) Cell wall invertase in developing rice caryopsis: molecular cloning of OsCIN1 and analysis of its expression in relation to its role in grain filling. Plant & Cell Physiology 43, 452–459.
| Cell wall invertase in developing rice caryopsis: molecular cloning of OsCIN1 and analysis of its expression in relation to its role in grain filling.Crossref | GoogleScholarGoogle Scholar |
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology 53, 225–245.
| Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance.Crossref | GoogleScholarGoogle Scholar |
IPCC (2007) Summary for Policymakers. In ‘Climate change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds S Solomon, D Qin, M Manning, Z Chen, M Marquis, KB Averyt, M Tignor, HL Miller) pp. 1–18. (Cambridge University Press: Cambridge)
Isendahl N, Schmidt G (2006) Drought in the Mediterranean – WWF policy proposals. WWF Report, Madrid.
Ito N (1974) Change of carbohydrates in anthers cooled at the young microspore stage. Proceedings of the Crop Science Society of Japan 43, 179–180.
Iwai H, Hokura A, Oishi M, Chida H, Ishii T, Sakai S, Satoh S (2006) The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proceedings of the National Academy of Sciences of the United States of America 103, 16 592–16 597.
| The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization.Crossref | GoogleScholarGoogle Scholar |
Jacobs BC, Pearson CJ (1994) Cold damage and development of rice: a conceptual model. Australian Journal of Experimental Agriculture 34, 917–919.
| Cold damage and development of rice: a conceptual model.Crossref | GoogleScholarGoogle Scholar |
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). Journal of Experimental Botany 58, 1627–1635.
| High temperature stress and spikelet fertility in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar |
Jagadish KSV, Cairns JE, Kumar A, Somayanda IM, Craufurd PQ (2011) Does susceptibility to heat stress confound screening for drought stress in rice? Functional Plant Biology 38, 261–269.
| Does susceptibility to heat stress confound screening for drought stress in rice?Crossref | GoogleScholarGoogle Scholar |
Jaggard KW, Qi A, Ober ES (2010) Possible changes to arable crop yields by 2050. Philosophical Transactions of the Royal Society B, Biological Sciences 365, 2835–2851.
| Possible changes to arable crop yields by 2050.Crossref | GoogleScholarGoogle Scholar |
Jain M, Vara Prasad PV, Boote KJ, Hartwell AL, Chourey PS (2007) Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227, 67–79.
| Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench).Crossref | GoogleScholarGoogle Scholar |
Jain M, Chourey PS, Boote KJ, Allen LH (2010) Short-term high temperature growth conditions during vegetative-to-reproductive phase transition irreversibly compromise cell wall invertase-mediated sucrose catalysis and microspore meiosis in grain sorghum (Sorghum bicolor). Journal of Plant Physiology 167, 578–582.
| Short-term high temperature growth conditions during vegetative-to-reproductive phase transition irreversibly compromise cell wall invertase-mediated sucrose catalysis and microspore meiosis in grain sorghum (Sorghum bicolor).Crossref | GoogleScholarGoogle Scholar |
Jamieson PD, Martin RJ, Francis GS (1995) Drought influences on grain yield of barley, wheat and maize. New Zealand Journal of Crop and Horticultural Science 23, 55–66.
| Drought influences on grain yield of barley, wheat and maize.Crossref | GoogleScholarGoogle Scholar |
Jan N, Hussain M, Andrabi KI (2009) Cold resistance in plants: a mystery unresolved. Electronic Journal of Biotechnology 12, 1–15.
| Cold resistance in plants: a mystery unresolved.Crossref | GoogleScholarGoogle Scholar |
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, Richards RA, Dolferus R (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell & Environment 33, 926–942.
| Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat.Crossref | GoogleScholarGoogle Scholar |
Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R (2011) Control of ABA catabolism and ABA homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiology 156, 647–662.
| Control of ABA catabolism and ABA homeostasis is important for reproductive stage stress tolerance in cereals.Crossref | GoogleScholarGoogle Scholar |
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant & Cell Physiology 45, 346–350.
| A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer.Crossref | GoogleScholarGoogle Scholar |
Kato K, Miura H, Swada S (2000) Mapping QTLs controlling grain yield and its components on chromosome 5A of wheat. Theoretical and Applied Genetics 101, 1114–1121.
| Mapping QTLs controlling grain yield and its components on chromosome 5A of wheat.Crossref | GoogleScholarGoogle Scholar |
Kim SY, Hong CB, Lee I (2001) Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana. Journal of Plant Research 114, 301–307.
| Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Kirigwi FM, Van Ginkel M, Brown-Guedira G, Gill BS, Paulsen GM, Fritz AK (2007) Markers associated with a QTL for grain yield in wheat under drought. Molecular Breeding 20, 401–413.
| Markers associated with a QTL for grain yield in wheat under drought.Crossref | GoogleScholarGoogle Scholar |
Koonjul PK, Minhas JS, Nunes C, Sheoran IS, Saini HS (2005) Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat. Journal of Experimental Botany 56, 179–190.
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Current Opinion in Plant Biology 10, 310–316.
| Complexity of the heat stress response in plants.Crossref | GoogleScholarGoogle Scholar |
Krishnan P, Ramakrishnan B, Raja Reddy K, Reddy VR (2011) High-temperature effects on rice growth, yield, and grain quality. Advances in Agronomy 111, 87–206.
| High-temperature effects on rice growth, yield, and grain quality.Crossref | GoogleScholarGoogle Scholar |
Ku S, Yoon H, Suh HS, Chung YY (2003) Male sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217, 559–565.
| Male sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum.Crossref | GoogleScholarGoogle Scholar |
Kumar R, Venuprasad R, Atlin GN (2007) Genetic analysis of rain fed lowland rice drought tolerance under naturally occurring stress in eastern India: heritability and QTL effects. Field Crops Research 103, 42–52.
| Genetic analysis of rain fed lowland rice drought tolerance under naturally occurring stress in eastern India: heritability and QTL effects.Crossref | GoogleScholarGoogle Scholar |
Kuroki M, Saito K, Matsuba S, Yokogami N, Shimizu H, Ando I, Sato Y (2009) Quantitative trait locus analysis for cold tolerance at the booting stage in a rice cultivar, Hatsushizuku. Japan Agricultural Research Quarterly 43, 115–121.
| Quantitative trait locus analysis for cold tolerance at the booting stage in a rice cultivar, Hatsushizuku.Crossref | GoogleScholarGoogle Scholar |
Lalonde S, Beebe DU, Saini HS (1997) Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction 10, 40–48.
| Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit.Crossref | GoogleScholarGoogle Scholar |
Lanceras JC, Pantuwan G, Jongdee B, Toojinda T (2004) Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiology 135, 384–399.
| Quantitative trait loci associated with drought tolerance at reproductive stage in rice.Crossref | GoogleScholarGoogle Scholar |
Langer RHM, Olugbemi LB (1970) A study of New Zealand wheats. IV. Effects of extreme temperature at different stages of development. New Zealand Journal of Agricultural Research 13, 878–886.
| A study of New Zealand wheats. IV. Effects of extreme temperature at different stages of development.Crossref | GoogleScholarGoogle Scholar |
Larosa PC, Handa AK, Hasegawa PM, Bressan RA (1985) Abscisic acid accelerates adaptation of cultured tobacco cells to salt. Plant Physiology 79, 138–142.
| Abscisic acid accelerates adaptation of cultured tobacco cells to salt.Crossref | GoogleScholarGoogle Scholar |
Lata C, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. Journal of Experimental Botany 62, 4731–4748.
| Role of DREBs in regulation of abiotic stress responses in plants.Crossref | GoogleScholarGoogle Scholar |
Li S, Wan C, Kong J, Zhang Z, Li Y, Zhu Y (2004) Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Functional Plant Biology 31, 369–376.
| Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria.Crossref | GoogleScholarGoogle Scholar |
Lillemo M, van Ginkel M, Trethowan RM, Hernandez E, Crossa J (2005) Differential adaptation of CIMMYT bread wheat to global high temperature environments. Crop Science 45, 2443–2453.
| Differential adaptation of CIMMYT bread wheat to global high temperature environments.Crossref | GoogleScholarGoogle Scholar |
Lilley JM, Ludlow MM, McCouch SR, O’Toole JC (1996) Location of a QTL for osmotic adjustment and dehydration tolerance in rice. Journal of Experimental Botany 47, 1427–1436.
| Location of a QTL for osmotic adjustment and dehydration tolerance in rice.Crossref | GoogleScholarGoogle Scholar |
Limin AE, Fowler DB (2006) Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): response to photoperiod, vernalization, and plant development. Planta 224, 360–366.
| Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): response to photoperiod, vernalization, and plant development.Crossref | GoogleScholarGoogle Scholar |
Lin SS, Peterson ML (1975) Low temperature-induced floret sterility in rice. Crop Science 15, 657–660.
| Low temperature-induced floret sterility in rice.Crossref | GoogleScholarGoogle Scholar |
Lu S, Su W, Li H, Guo Z (2009) Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2 – and NO– induced antioxidant enzyme activities. Plant Physiology and Biochemistry 47, 132–138.
| Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2 – and NO– induced antioxidant enzyme activities.Crossref | GoogleScholarGoogle Scholar |
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Molecular Biology 48, 667–681.
| Molecular genetics of heat tolerance and heat shock proteins in cereals.Crossref | GoogleScholarGoogle Scholar |
Mason RE, Mondal S, Beecher FW, Pacheco A, Jampala B, Ibrahim AMH, Hays DB (2010) QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive heat stress. Euphytica 174, 423–436.
| QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive heat stress.Crossref | GoogleScholarGoogle Scholar |
Matsui T, Omasa K (2002) Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Annals of Botany 89, 683–687.
| Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics.Crossref | GoogleScholarGoogle Scholar |
Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, Satou M, Kim JM, Kobayashi N, Toyoda T, Shinozaki K, Seki M (2008) Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant & Cell Physiology 49, 1135–1149.
| Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array.Crossref | GoogleScholarGoogle Scholar |
McLaughlin JE, Boyer JS (2004) Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Annals of Botany 94, 75–86.
| Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems.Crossref | GoogleScholarGoogle Scholar |
Miller ME, Chourey PS (1992) The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. The Plant Cell 4, 297–305.
Miller AK, Galiba G, Dubcovsky J (2006) A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am2 in Triticum monococcum. Molecular Genetics and Genomics 275, 193–203.
| A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am2 in Triticum monococcum.Crossref | GoogleScholarGoogle Scholar |
Monroy A, Dryanova A, Malette B, Oren D, Ridha Farajalla M, Liu W, Danyluk J, Ubayasena L, Kane K, Scoles G, Sarhan F, Gulick P (2007) Regulatory gene candidates and gene expression analysis of cold acclimation in winter and spring wheat. Plant Molecular Biology 64, 409–423.
| Regulatory gene candidates and gene expression analysis of cold acclimation in winter and spring wheat.Crossref | GoogleScholarGoogle Scholar |
Morgan JM (1980) Possible role of abscisic acid in reducing seed set in water-stressed wheat plants. Nature 285, 655–657.
| Possible role of abscisic acid in reducing seed set in water-stressed wheat plants.Crossref | GoogleScholarGoogle Scholar |
Morgan JM, King RW (1984) Association between loss of leaf turgor, abscisic acid levels and grain set in two wheat cultivars. Australian Journal of Plant Physiology 11, 143–150.
| Association between loss of leaf turgor, abscisic acid levels and grain set in two wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, Ismagul A, Eliby S, Shirley N, Langridge P, Lopato S (2011) Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnology Journal 9, 230–249.
| Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors.Crossref | GoogleScholarGoogle Scholar |
Musgrave ME (1994) Waterlogging effects on yield and photosynthesis in eight winter wheat cultivars. Crop Science 34, 1314–1318.
| Waterlogging effects on yield and photosynthesis in eight winter wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Nguyen GN, Hailstones DL, Wilkes M, Sutton BG (2009) Drought-induced oxidative conditions in rice anthers leading to a programmed cell death and pollen abortion. Journal Agronomy & Crop Science 195, 157–164.
| Drought-induced oxidative conditions in rice anthers leading to a programmed cell death and pollen abortion.Crossref | GoogleScholarGoogle Scholar |
Nicolas ME, Gleadow RM, Dalling MJ (1985) Effect of post-anthesis drought on cell division and starch accumulation in developing wheat grains. Annals of Botany 55, 433–444.
Nishiyama I (1984) Climatic influence on pollen formation and fertilisation. In ‘Biology of rice’. (Eds S Tsunoda, N Takahashi) pp. 153–171. (Japanese Science Society Press: Tokyo)
Oh SJ, Kwon CW, Choi DW, Song SI, Kim JK (2007) Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnology Journal 5, 646–656.
| Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice.Crossref | GoogleScholarGoogle Scholar |
Oliver SN, Van Dongen JT, Alfred SC, Mamun EA, Zhao XC, Saini HS, Fernandes SF, Blanchard CL, Sutton BG, Geigenberger P, Dennis ES, Dolferus R (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell & Environment 28, 1534–1551.
| Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility.Crossref | GoogleScholarGoogle Scholar |
Oliver SN, Dennis ES, Dolferus R (2007) ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant & Cell Physiology 48, 1319–1330.
| ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice.Crossref | GoogleScholarGoogle Scholar |
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany 58, 113–117.
| The drought environment: physical, biological and agricultural perspectives.Crossref | GoogleScholarGoogle Scholar |
Pellegrineschi A, Reynolds M, Pacheco M, Brito RM, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D (2004) Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47, 493–500.
| Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions.Crossref | GoogleScholarGoogle Scholar |
Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Khush GS, Cassman KG (2004) Rice yields decline with higher night temperature from global warming. Proceedings of the National Academy of Sciences of the United States of America 101, 9971–9975.
| Rice yields decline with higher night temperature from global warming.Crossref | GoogleScholarGoogle Scholar |
Prasad PVV, Boote KJ, Allen LH, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research 95, 398–411.
| Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Crossref | GoogleScholarGoogle Scholar |
Prášil IT, Pránilová P, Pánková K (2004) Relationships among vernalization, shoot apex development and frost tolerance in wheat. Annals of Botany 94, 413–418.
| Relationships among vernalization, shoot apex development and frost tolerance in wheat.Crossref | GoogleScholarGoogle Scholar |
Pressman E, Peet MM, Pharr DM (2002) The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90, 631–636.
| The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers.Crossref | GoogleScholarGoogle Scholar |
Qian CM, Xu A, Liang GH (1986) Effects of low temperatures and genotypes on pollen development in wheat. Crop Science 26, 43–46.
| Effects of low temperatures and genotypes on pollen development in wheat.Crossref | GoogleScholarGoogle Scholar |
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant & Cell Physiology 52, 1569–1582.
| Achievements and challenges in understanding plant abiotic stress responses and tolerance.Crossref | GoogleScholarGoogle Scholar |
Quarrie SA, Laurie DA, Zhu J, Lebreton C, Semikhodskii A, Steed A, Witsenboer H, Calestani C (1997) QTL analysis to study the association between leaf size and abscisic acid accumulation in droughted rice leaves and comparisons across cereals. Plant Molecular Biology 35, 155–165.
| QTL analysis to study the association between leaf size and abscisic acid accumulation in droughted rice leaves and comparisons across cereals.Crossref | GoogleScholarGoogle Scholar |
Rapacz M, Wolanin B, Hura K, Tyrka M (2008) The effects of cold acclimation on photosynthetic apparatus and the expression of COR14b in four genotypes of barley (Hordeum vulgare) contrasting in their tolerance to freezing and high-light treatment in cold conditions. Annals of Botany 101, 689–699.
| The effects of cold acclimation on photosynthetic apparatus and the expression of COR14b in four genotypes of barley (Hordeum vulgare) contrasting in their tolerance to freezing and high-light treatment in cold conditions.Crossref | GoogleScholarGoogle Scholar |
Rawson HM, Rawson HM, Subedi KD (1996) Hypothesis for why sterility occurs in wheat in Asia. In ‘Sterility in wheat in subtropical Asia: extent, causes and solutions. ACIAR Proceedings No. 72’. (Ed. HM Rawson) pp. 132–134.
Rebetzke GJ, Moeller C, Biddulph B, Deery D, Mayer J, Bennett D, Chenu K, Rattey A (2012) Development of a multisite, managed environment facility for targeted trait and germplasm evaluation. Functional Plant Biology
Richards RA (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. Journal of Experimental Botany 51, 447–458.
| Selectable traits to increase crop photosynthesis and yield of grain crops.Crossref | GoogleScholarGoogle Scholar |
Rosegrant MW, Cline SA (2003) Global food security: challenges and policies. Science 302, 1917–1919.
| Global food security: challenges and policies.Crossref | GoogleScholarGoogle Scholar |
Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Jenkins C (2006) Genotypic variation for water soluble carbohydrate accumulation in wheat. Functional Plant Biology 33, 799–809.
| Genotypic variation for water soluble carbohydrate accumulation in wheat.Crossref | GoogleScholarGoogle Scholar |
Ruuska SA, Lewis DC, Kennedy G, Furbank RT, Jenkins CL, Tabe LM (2008) Large scale transcriptome analysis of the effects of nitrogen nutrition on accumulation of stem carbohydrate reserves in reproductive stage wheat. Plant Molecular Biology 66, 15–32.
| Large scale transcriptome analysis of the effects of nitrogen nutrition on accumulation of stem carbohydrate reserves in reproductive stage wheat.Crossref | GoogleScholarGoogle Scholar |
Saifuzzaman M, Meisner CA (1996) Wheat sterility in Bangladesh: An overview of the problem, research and possible solutions. In ‘Sterility in wheat in subtropical Asia: extent, causes and solutions. ACIAR Proceedings No. 72’. (Ed. HM Rawson) pp. 104–108.
Saifuzzaman M, Fattah QA, Islam MS (2008) Spikelet sterility of wheat in farmer’s field in Northwest Bangladesh. Bangladesh Journal of Botany 37, 155–160.
| Spikelet sterility of wheat in farmer’s field in Northwest Bangladesh.Crossref | GoogleScholarGoogle Scholar |
Saini HS, Aspinall D (1982) Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature: possible mediation by abscisic acid. Australian Journal of Plant Physiology 9, 529–537.
| Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature: possible mediation by abscisic acid.Crossref | GoogleScholarGoogle Scholar |
Saini HS, Sedgley M, Aspinall D (1984) Developmental anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid. Australian Journal of Plant Physiology 11, 243–253.
| Developmental anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid.Crossref | GoogleScholarGoogle Scholar |
Saito K, Hayano-Saito Y, Maruyama-Funatsuki W, Sato Y, Kato A (2004) Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theoretical and Applied Genetics 109, 515–522.
| Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice.Crossref | GoogleScholarGoogle Scholar |
Sakata T, Takahashi H, Nishiyama I, Higashitani A (2000) Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. Journal of Plant Research 113, 395–402.
| Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L.Crossref | GoogleScholarGoogle Scholar |
Samad A, Meisner CA, Saifuzzaman M, van Ginkel M (2011) Waterlogging tolerance. In ‘Plant breeding for water-limited environments’. (Ed. A Blum) pp. 136–144. (Springer: New York)
Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT (2002) Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Molecular Biology 48, 713–726.
| Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench).Crossref | GoogleScholarGoogle Scholar |
Sandve SR, Kosmala A, Rudi H, Fjellheim S, Rapacz M, Yamada T, Rognli OA (2011) Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates. Plant Science 180, 69–77.
| Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates.Crossref | GoogleScholarGoogle Scholar |
Satake T (1976) Determination of the most sensitive stage to sterile-type cool injury in rice plants. Research Bulletin of the Hokkaido National Agricultural Experiment Station 113, 1–40.
Savin R, Slafer GA (1991) Shading effects on the yield of an Argentinean wheat cultivar. The Journal of Agricultural Science 116, 1–7.
| Shading effects on the yield of an Argentinean wheat cultivar.Crossref | GoogleScholarGoogle Scholar |
Sayre KD, Van Ginkel M, Rajaram S, Ortiz-Monasterio I (1994) Tolerance to waterlogging losses in spring bread wheat: effect of time of onset on expression. Annual Wheat Newsletter 40, 165–171.
Sayre KD, Rajaram S, Fischer RA (1997) Yield potential progress in short bread wheats in northwest Mexico. Crop Science 37, 36–42.
| Yield potential progress in short bread wheats in northwest Mexico.Crossref | GoogleScholarGoogle Scholar |
Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N (2011) ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. Journal of Experimental Botany 62, 2615–2632.
| ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions.Crossref | GoogleScholarGoogle Scholar |
Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology 10, 296–302.
| Regulatory metabolic networks in drought stress responses.Crossref | GoogleScholarGoogle Scholar |
Semenov MA, Shewry PR (2011) Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Scientific Reports 1, 1–5.
| Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe.Crossref | GoogleScholarGoogle Scholar |
Setter TL, Waters I (2003) Reviews of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253, 1–34.
| Reviews of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats.Crossref | GoogleScholarGoogle Scholar |
Shah F, Huang J, Cui K, Nie L, Shah T, Chen C, Wang K (2011) Impact of high temperature stress on rice plant and its traits related to tolerance. Journal of Agricultural Science 149, 545–556.
| Impact of high temperature stress on rice plant and its traits related to tolerance.Crossref | GoogleScholarGoogle Scholar |
Sheoran IS, Saini HS (1996) Drought-induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sexual Plant Reproduction 9, 161–169.
| Drought-induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen.Crossref | GoogleScholarGoogle Scholar |
Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2008) Rice yield enhancement by elevated CO2 is reduced in cool weather. Global Change Biology 14, 276–284.
| Rice yield enhancement by elevated CO2 is reduced in cool weather.Crossref | GoogleScholarGoogle Scholar |
Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2009) Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group. Journal of Experimental Botany 60, 523–532.
| Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group.Crossref | GoogleScholarGoogle Scholar |
Skinner JS, Szucs P, von Zitzewitz J, Marquez-Cedillo L, Filichkin T, Stockinger EJ, Thomashow MF, Chen TH, Hayes PM (2006) Mapping of barley homologs to genes that regulate low temperature tolerance in Arabidopsis. Theoretical and Applied Genetics 112, 832–842.
| Mapping of barley homologs to genes that regulate low temperature tolerance in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Snape JW, Sarma R, Quarrie SA, Fish L, Galiba G, Sutka J (2001) Mapping genes for flowering time and frost tolerance in cereals using precise genetic stocks. Euphytica 120, 309–315.
| Mapping genes for flowering time and frost tolerance in cereals using precise genetic stocks.Crossref | GoogleScholarGoogle Scholar |
Spiertz JHJ, Hamer RJ, Xu H, Primo-Martin C, Don C, van der Putten PEL (2006) Heat stress in wheat (Triticum aestivum L.): effects on grain growth and quality traits. European Journal of Agronomy 25, 89–95.
| Heat stress in wheat (Triticum aestivum L.): effects on grain growth and quality traits.Crossref | GoogleScholarGoogle Scholar |
Sthapit BR, Witcombe JR (1998) Inheritance of tolerance to chilling stress in rice during germination and plumule greening. Crop Science 38, 660–665.
| Inheritance of tolerance to chilling stress in rice during germination and plumule greening.Crossref | GoogleScholarGoogle Scholar |
Stone PJ, Nicolas ME (1994) Wheat cultivars vary widely in their responses of grain yield and quality to short periods of post-anthesis heat stress. Australian Journal of Plant Physiology 21, 887–900.
| Wheat cultivars vary widely in their responses of grain yield and quality to short periods of post-anthesis heat stress.Crossref | GoogleScholarGoogle Scholar |
Subedi KD, Floyd CN, Budhathoki CB (1998) Cool temperature-induced sterility in spring wheat (Triticum aestivum L.) at high altitudes in Nepal: variation among cultivars in response to sowing date. Field Crops Research 55, 141–151.
| Cool temperature-induced sterility in spring wheat (Triticum aestivum L.) at high altitudes in Nepal: variation among cultivars in response to sowing date.Crossref | GoogleScholarGoogle Scholar |
Suh JP, Jeung JU, Lee JI, Choi YH, Yea JD, Virk PS, Mackill DJ, Jena KK (2010) Identification and analysis of QTLs controlling cold tolerance at the reproductive stage and validation of effective QTLs in cold-tolerant genotypes of rice (Oryza sativa L.). Theoretical and Applied Genetics 120, 985–995.
| Identification and analysis of QTLs controlling cold tolerance at the reproductive stage and validation of effective QTLs in cold-tolerant genotypes of rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar |
Svensson JT, Crosatti C, Campoli C, Bassi R, Stanca AM, Close TJ, Cattivelli L (2006) Transcriptome analysis of cold acclimation in barley albina and xantha mutants. Plant Physiology 141, 257–270.
| Transcriptome analysis of cold acclimation in barley albina and xantha mutants.Crossref | GoogleScholarGoogle Scholar |
Tao H, Brueck H, Dittert K, Kreye C, Lin S, Sattelmacher B (2006) Growth and yield formation for rice (Oryza sativa L.) in the water-saving ground cover rice production system (GCRPS). Field Crops Research 95, 1–12.
| Growth and yield formation for rice (Oryza sativa L.) in the water-saving ground cover rice production system (GCRPS).Crossref | GoogleScholarGoogle Scholar |
Thomas H, Howarth CJ (2000) Five ways to stay green. Journal of Experimental Botany 51, 329–337.
| Five ways to stay green.Crossref | GoogleScholarGoogle Scholar |
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology 50, 571–599.
| Plant cold acclimation: freezing tolerance genes and regulatory mechanisms.Crossref | GoogleScholarGoogle Scholar |
Thomashow MF (2001) So what’s new in the field of plant cold acclimation? Lots! Plant Physiology 125, 89–93.
| So what’s new in the field of plant cold acclimation? Lots!Crossref | GoogleScholarGoogle Scholar |
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418, 671–677.
| Agricultural sustainability and intensive production practices.Crossref | GoogleScholarGoogle Scholar |
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, Iuchi S, Kobayashi M, Yamaguchi S, Kamiya Y, Nambara E, Kawakami N (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology 146, 1368–1385.
| High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds.Crossref | GoogleScholarGoogle Scholar |
Tondelli A, Francia E, Barabaschi D, Pasquariello M, Pecchioni N (2011) Inside the CBF locus in Poaceae. Plant Science 180, 39–45.
| Inside the CBF locus in Poaceae.Crossref | GoogleScholarGoogle Scholar |
Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science 12, 352–357.
| The molecular basis of vernalization-induced flowering in cereals.Crossref | GoogleScholarGoogle Scholar |
Tsuda K, Tsvetanov S, Takumi S, Mori N, Atanassov A, Nakamura C (2000) New members of a cold-responsive group-3 Lea/Rab-related Cor gene family from common wheat (Triticum aestivum L.). Genes & Genetic Systems 75, 179–188.
| New members of a cold-responsive group-3 Lea/Rab-related Cor gene family from common wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends in Plant Science 11, 405–412.
| Genomics-based approaches to improve drought tolerance of crops.Crossref | GoogleScholarGoogle Scholar |
Vágújfalvi A, Galiba G, Cattivelli L, Dubcovsky J (2003) The cold-regulated transcriptional activator Cbf3 is linked to the frost tolerance locus Fr-A2 on wheat chromosome 5A. Molecular Genetics and Genomics 269, 60–67.
Van Ginkel M, Rajaram S, Thijssen M (1992) Waterlogging in wheat: Germplasm evaluation and methodology development. In ‘Seventh Regional Wheat Workshop for Eastern, Central and Southern Africa’. (Eds DG Tanner, W Mwangi) pp. 115–124. (CIMMYT: Nakuru, Kenya)
Vellinga P, Van Verseveld WJ (2000) ‘Climate change and extreme weather events.’ (World Wide Fund for Nature: Gland, Switzerland)
Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Sta Cruz MT, Amante M, Kumar A, Atlin GN (2009) Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theoretical and Applied Genetics 120, 177–190.
| Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis.Crossref | GoogleScholarGoogle Scholar |
Wahid A, Gelania S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environmental and Experimental Botany 61, 199–223.
| Heat tolerance in plants: an overview.Crossref | GoogleScholarGoogle Scholar |
Wang XS, Zhu J, Mansueto L, Bruskiewich R (2005) Identification of candidate genes for drought stress tolerance in rice by the integration of a genetic (QTL) map with the rice genome physical map. Journal of Zhejiang University. Science 6B, 382–388.
| Identification of candidate genes for drought stress tolerance in rice by the integration of a genetic (QTL) map with the rice genome physical map.Crossref | GoogleScholarGoogle Scholar |
Wardlaw IF, Dawson IA, Munibi P, Fewster R (1989a) The tolerance of wheat to high temperatures during reproductive growth: I. Survey procedures and general response patterns. Australian Journal of Agricultural Research 40, 1–13.
| The tolerance of wheat to high temperatures during reproductive growth: I. Survey procedures and general response patterns.Crossref | GoogleScholarGoogle Scholar |
Wardlaw IF, Dawson IA, Munibi P (1989b) The tolerance of wheat to high temperatures during reproductive growth: II. Grain development. Australian Journal of Agricultural Research 40, 15–24.
| The tolerance of wheat to high temperatures during reproductive growth: II. Grain development.Crossref | GoogleScholarGoogle Scholar |
Waters SP, Martin P, Lee BT (1984) Influence of sucrose and abscisic acid on the determination of grain number in wheat. Journal of Experimental Botany 35, 829–840.
| Influence of sucrose and abscisic acid on the determination of grain number in wheat.Crossref | GoogleScholarGoogle Scholar |
Westgate ME (1994) Water status and development of the maize endosperm and embryo during drought. Crop Science 34, 76–83.
| Water status and development of the maize endosperm and embryo during drought.Crossref | GoogleScholarGoogle Scholar |
Westgate ME, Passioura JB, Munns R (1996) Water status and ABA content of floral organs in drought-stressed wheat. Australian Journal of Plant Physiology 23, 763–772.
| Water status and ABA content of floral organs in drought-stressed wheat.Crossref | GoogleScholarGoogle Scholar |
Williams RL, Angus JF (1994) Deep floodwater protects high-nitrogen rice crops from low-temperature damage. Australian Journal of Experimental Agriculture 34, 927–932.
| Deep floodwater protects high-nitrogen rice crops from low-temperature damage.Crossref | GoogleScholarGoogle Scholar |
Winfield MO, Lu C, Wilson ID, Coghill JA, Edwards KJ (2010) Plant responses to cold: transcriptome analysis of wheat. Plant Biotechnology Journal 8, 749–771.
| Plant responses to cold: transcriptome analysis of wheat.Crossref | GoogleScholarGoogle Scholar |
Wu SH, Wong C, Chen J, Lin BC (1994) Isolation of a cDNA encoding a 70 kDa heat shock cognate protein expressed in the vegetative tissue of Arabidopsis. Plant Molecular Biology 25, 577–583.
| Isolation of a cDNA encoding a 70 kDa heat shock cognate protein expressed in the vegetative tissue of Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Xue GP, McIntyre CL, Jenkins CL, Glassop D, van Herwaarden AF, Shorter R (2008) Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiology 146, 441–454.
| Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat.Crossref | GoogleScholarGoogle Scholar |
Yang J, Zhang J (2006) Grain filling of cereals under soil drying. New Phytologist 169, 223–236.
| Grain filling of cereals under soil drying.Crossref | GoogleScholarGoogle Scholar |
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2001) Water deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agronomy Journal 93, 196–206.
Yang J, Sears RG, Gill BS, Paulsen GM (2002a) Genotypic differences in utilization of assimilate sources during maturation of wheat under chronic and heat shock stresses: utilization of assimilate sources by wheat under heat stresses. Euphytica 125, 179–188.
| Genotypic differences in utilization of assimilate sources during maturation of wheat under chronic and heat shock stresses: utilization of assimilate sources by wheat under heat stresses.Crossref | GoogleScholarGoogle Scholar |
Yang J, Sears RG, Gill BS, Paulsen GM (2002b) Quantitative and molecular characterization of heat tolerance in hexaploid wheat. Euphytica 126, 275–282.
| Quantitative and molecular characterization of heat tolerance in hexaploid wheat.Crossref | GoogleScholarGoogle Scholar |
Yang DL, Jing RL, Chang XP, Li W (2007) Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems. Genetics 176, 571–584.
| Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems.Crossref | GoogleScholarGoogle Scholar |
Young AL (2009) Finding the balance between food and biofuels. Environmental Science and Pollution Research 16, 117–119.
| Finding the balance between food and biofuels.Crossref | GoogleScholarGoogle Scholar |
Yue B, Xue WJ, Xiong LZ, Yu XQ, Luo LJ, Cui KH, Jin DM, Xing YZ, Zhang QF (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172, 1215–1228.
Zeng ZR, Morgan JM, King RW (1985) Regulation of grain number in wheat: genotype differences and responses to applied abscisic acid and to high temperatures. Australian Journal of Plant Physiology 12, 609–619.
| Regulation of grain number in wheat: genotype differences and responses to applied abscisic acid and to high temperatures.Crossref | GoogleScholarGoogle Scholar |
Zhang J, Zheng HG, Aarti A, Pantuwan G, Nguyen TT, Tripathy JN, Sarial AK, Robin S, Babu RC, Nguyen BD, Sarkarung S, Blum A, Nguyen HT (2001) Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species. Theoretical and Applied Genetics 103, 19–29.
| Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species.Crossref | GoogleScholarGoogle Scholar |
Zhang JZ, Creelman RA, Zhu JK (2004) From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiology 135, 615–621.
| From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops.Crossref | GoogleScholarGoogle Scholar |
Zhou L, Zeng Y, Zheng W, Tang B, Yang S, Zhang H, Li J, Li Z (2010) Fine mapping a QTL qCTB7 for cold tolerance at the booting stage on rice chromosome 7 using a near-isogenic line. Theoretical and Applied Genetics 121, 895–905.
| Fine mapping a QTL qCTB7 for cold tolerance at the booting stage on rice chromosome 7 using a near-isogenic line.Crossref | GoogleScholarGoogle Scholar |
Zinselmeier C, Jeong BR, Boyer JS (1999) Starch and the control of kernel number in maize at low water potentials. Plant Physiology 121, 25–36.
| Starch and the control of kernel number in maize at low water potentials.Crossref | GoogleScholarGoogle Scholar |
Zuppini A, Navazio L, Mariani P (2004) Endoplasmic reticulum stress-induced programmed cell death in soybean cells. Journal of Cell Science 117, 2591–2598.
| Endoplasmic reticulum stress-induced programmed cell death in soybean cells.Crossref | GoogleScholarGoogle Scholar |


